Abstract
Background and purpose:
δ-Opioid receptors (DOP receptors) could represent a novel target in the treatment of depressive disorders. To explore this new field of interest, the development of highly selective DOP receptor agonists is essential. UFP-512 [H-Dmt-Tic-NH-CH(CH2-COOH)-Bid], was recently shown to behave in vitro as a selective and potent DOP receptor agonist and to promote antidepressant- and anxiolytic-like effects in vivo (Vergura et al., 2007). Here, we have characterized the pharmacological properties of UFP-512 and established a link between desensitization and tolerance.
Experimental approach:
Studies were performed in the human neuroblastoma SK-N-BE cells to establish i) binding parameters for UFP-512 ii) signalling pathways activated after acute and chronic treatment iii) regulation (phosphorylation and trafficking) of human DOP (hDOP) receptors after sustained activation by UFP-512. In vivo, we studied UFP-512-induced antidepressant-like effects after acute or chronic treatment in the mouse forced swimming test.
Key results:
In vitro, UFP-512 was a high affinity agonist for DOP receptors. While UFP-512 induced marked phosphorylation of DOP receptors on Ser363, we observed a low desensitization of the cAMP pathway, associated with receptor endocytosis and recycling without any reduction on extracellular signal-regulated protein kinase 1/2 activation. In vivo, acute administration of UFP-512 produced an antidepressant-like effect, without any sign of tolerance after chronic administration.
Conclusions and implications:
There was a correlation between weak desensitization, significant internalization and recycling of the human DOP receptors and lack of tolerance to UFP-512. This suggests that this compound would be a promising drug prototype for exploring innovative treatments for mood disorders.
Keywords: UFP-512, morphine, human δ-opioid receptor (hDOP receptor), phosphorylation, desensitization, endocytosis, trafficking, antidepressant-like effect, opioid tolerance
Introduction
The opioid receptor family is composed of three major types, namely μ, δ and κ. Using knockout (KO) mice, μ-opioid (MOP) receptors were described as the mediators of morphine-induced analgesia (Matthes et al., 1996). The same group showed that δ-opioid (DOP) receptors were also involved in antinociception by cooperating with MOP receptors (Matthes et al., 1998). Moreover, several lines of evidence indicate that DOP receptors could also play an essential role in the regulation of emotional responses (that is, anxiety and depression), as demonstrated both by DOP receptor KO mice and in vivo pharmacological studies (see Jutkiewicz, 2006). In this new field of interest, DOP receptor activation would act as a positive regulator of the ‘mood state homeostasis'. In opposition, MOP receptors would negatively regulate the emotional responses, whereas κ-opioid (KOP) receptors would not be involved (Filliol et al., 2000). These data demonstrate the specific role of DOP receptors in mood control and underline the need for developing nonpeptidic DOP receptor agonists or highly selective and potent peptidic DOP receptor agonists, resistant to peptidase degradation.
Unfortunately, chronic or repeated opioid receptor activation invariably leads to tolerance limiting the therapeutic benefits. Opioid tolerance is a highly complex phenomenon resulting, in part, from opioid receptor desensitization. This latter process is defined by a decrease of the receptor's ability to transduce a signal (Marie et al., 2006). From the classical model based on the β-adrenoceptor, desensitization could be divided into the following three steps: receptor phosphorylation by G protein-coupled receptor (GPCR) kinases (GRKs), endocytosis and sorting (recycling or downregulation). By focusing on these interrelated molecular mechanisms, Whistler's group showed that endocytosis of opioid receptors, followed by their recycling, reduces desensitization and consequently tolerance (Finn and Whistler, 2001).
Thus, in vitro studies (that is, desensitization and receptor trafficking) could predict, at least in part, the ability of the newly synthesized DOP receptor agonists to promote tolerance.
We previously showed that some agonists (etorphine and enkephalins) promote a low desensitization rate, which is preferentially associated with hDOP receptor endocytosis followed by their recycling (Marie et al., 2003b; Lecoq et al., 2004). In contrast, a profound and rapid desensitization was observed with DOP-selective agonists, such as DPDPE (D-Pen2, D-Pen5]enkephalin) and deltorphin I (H-Tyr-D-Ala-Phe-Asp-Val-Val-Gly-NH2) (Marie et al., 2003b), AR-M1000390 (N,N-diethyl-4-(phenylpiperidin-4-ylidene-methyl)-benzamide) (Marie et al., 2003a) and SNC-80 ([(+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxy benzyl]-N,N-diethylbenzamide) (Lecoq et al., 2004). While AR-M1000390 was unable to induce hDOP receptor endocytosis, we observed an important internalization with a subsequent downregulation in the presence of DPDPE, deltorphin I and SNC-80.
Recently, a new selective DOP receptor agonist, UFP-512 (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid) (see Figure 1a), was synthesized on the basis of the Dmt-Tic pharmacophore (Balboni et al., 2002). UFP-512 displayed a high affinity for the hDOP receptor (pKi 10.2) associated with high selectivity over MOP (160-fold) and KOP (3500-fold) sites. In GTPγS experiments performed in membranes from CHO cells containing hDOP receptors and in the electrically stimulated mouse vas deferens preparation, UFP-512 behaves as a highly potent (pEC50 10.2 and 11.6, respectively) full agonist. In addition, this promising ligand was shown to promote naltrindole-sensitive antidepressant- and anxiolytic-like effects in mice (Vergura et al., 2007).
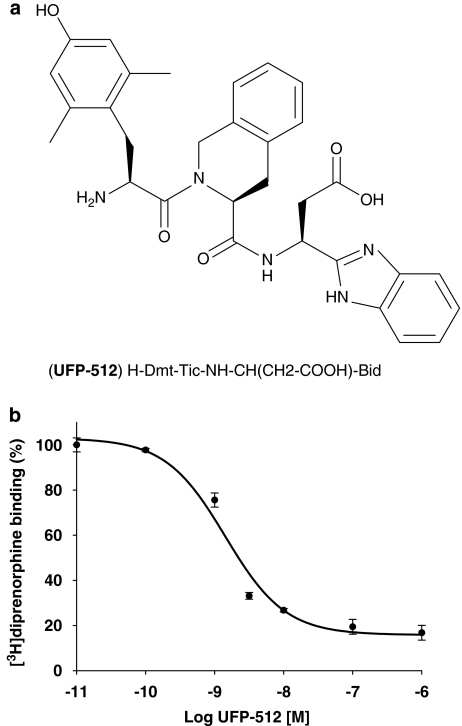
Figure 1.
Structure (a) and binding profile (b) of the novel human δ-opioid receptor (hDOP) receptor agonist UFP-512 (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid). The human neuroblastoma SK-N-BE cells stably expressing the FLAG-tagged-human DOP receptor (SK-N-BE-hDOP-R cells) were incubated with 10 nM [3H]diprenorphine (total binding) in the presence of increasing concentrations of UFP-512 (10 pM to 1 μM) or in the presence of 10 μM naltrindole (nonspecific binding) for 60 min at 4 °C. The competition between [3H]diprenorphine and UFP-512 binding was best fitted to a one-site model (F-test, P<0.0001). Data are representative of three separate experiments performed in triplicate (mean±s.e.mean).
In the present study, the in vitro and the in vivo pharmacological profiles of this compound were investigated in detail. All the in vitro experiments were performed in the human neuroblastoma SK-N-BE cells stably expressing the FLAG-tagged-human DOP receptor (SK-N-BE-hDOP-R cells, ∼2600 fmol mg−1 of protein) (Marie et al., 2003a). Indeed, in preliminary internalization studies (that is, immunolabelling and radioligand-binding experiments) conducted on hDOP receptors endogenously expressed in the SK-N-BE wild-type cells, we observed a specific but low-level signal, too weak to allow an accurate quantification. Thus, to overcome this problem, all experiments were carried out into the SK-N-BE-hDOP-R cells.
Taken together, binding affinity, functional (cAMP production and extracellular signal-regulated protein kinase Erk1/2 (Erk1/2) phosphorylation) and trafficking studies showed that UFP-512 is a high-affinity hDOP receptor agonist promoting a unique desensitization/trafficking profile compared to previously tested agonists. These latter characteristics were correlated with the lack of opioid tolerance to the antidepressant-like effect evoked by UFP-512 in the mouse forced swimming test (FST).
Methods
In vitro studies
Cell culture
SK-N-BE-hDOP-R cells (∼2600 fmol mg−1 of protein) (Marie et al., 2003a) were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% (v/v) fetal calf serum (Invitrogen, Carlsbad, USA), 1% (v/v) antibiotic–antimycotic mixture (Sigma, Saint-Louis, USA), 2 mM L-glutamine and 1 mg ml−1 geneticin (Sigma) at 37 °C, in a humidified atmosphere containing 5% CO2.
Competition-binding experiments on whole cells
SK-N-BE-hDOP-R cells were seeded into 24-well plates at a density of 75 000–100 000 cells per well. Cells were incubated with 10 nM [3H]diprenorphine (total binding), with the desired concentration (from 10 pM to 1 μM) of UFP-512, or in the presence of 10 μM naltrindole (nonspecific binding), during 60 min at 4 °C (to prevent hDOP receptor internalization) in ice-cold 50 mM Tris–HCl supplemented with 1% (w/v) BSA, pH 7.4. The reaction was stopped by removing the medium and by addition of 1 N NaOH. Bound radioactivity was measured after addition of 3 ml scintillation cocktail (PicoFluor-40, PerkinElmer, Waltham, USA), before assaying in a scintillation counter (PerkinElmer). All experiments were carried out in triplicate and repeated at least three times with similar results. IC50 values were obtained from curve fitting performed by the scientific program Sigma Plot software (Jandel Scientific, San Rafael, USA) using a sigmoidal regression analysis. The Ki was calculated according to the equation of Cheng and Prusoff (Ki=IC50/{1+[radioligand]/Kd}) (Cheng and Prusoff, 1973) where Kd for [3H]diprenorphine is 1.26 nM as previously determined (Marie et al., 2003b).
Measurement of cAMP
SK-N-BE-hDOP-R cells were seeded in 24-well plates at a density of 40 000 cells per well in a culture medium supplemented with 0.6 μCi [3H]adenine and incubated overnight. cAMP accumulation was determined in the presence of 0.5 mM isobutylmethylxanthine and 40 μM forskolin and in the absence or in the presence of agonists. After 5 min at 37 °C, the reaction was stopped by addition of 5% (w/v) trichloroacetic acid. The [3H]cAMP content of each well was isolated by chromatography on acid alumina columns, mixed with 8 ml of scintillation mixture (PicoFluor-40, PerkinElmer), before assaying in a scintillation counter (PerkinElmer). The percentage of inhibition was calculated according to the following formula: (1–(c.p.m. agonist–c.p.m. basal)/(c.p.m. forskolin–c.p.m. basal)) × 100, where c.p.m. basal was determined in a medium containing only isobutylmethylxanthine and c.p.m. forskolin in the presence of forskolin+isobutylmethylxanthine. ED50 values and maximal inhibitory levels of opioid agonists were determined by curve fitting of dose–response curves using SigmaPlot, and statistical analysis was realized using StatView software (Abacus Concepts, Berkeley, USA). All experiments were carried out in triplicate and repeated at least three times with similar results.
Study of mitogen-activated protein kinase signalling pathway
SK-N-BE-hDOP-R cells were cultured in 6-well plates until 90–100% confluence. Cells were placed in serum-free medium for 18 h both for phospho-JNK (Janus NH2 terminal kinase) and phospho-Erk1/2 mitogen-activated protein (MAP) kinase assays, but not for phospho-p38. The ability of UFP-512 to activate p38 and JNK pathway was tested at 10 μM and using 10-min ultraviolet treatment and hydrogen peroxide as positive controls, respectively. Activation of the Erk1/2 MAP kinase pathway by UFP-512 was tested from 100 pM to 1 μM in dose–response experiments, during 10 min at 37 °C, and using fetal calf serum as a positive control. In kinetic experiments, SK-N-BE-hDOP-R cells were treated for various times (0–60 min) with the maximal effective UFP-512 concentration.
For western blot analysis, reactions were stopped by removing the medium and by addition of 100 μl sodium dodecyl sulphate sample buffer (62.5 mM Tris–HCl, pH 6.8, 2% (w/v) sodium dodecyl sulphate, 10% (v/v) glycerol, 50 mM dithiothreitol, 0.01% (w/v) bromophenol blue). Samples were sonicated, centrifuged (14 000 g, 15 min, 4 °C) to remove the nonsolubilized material, and heated at 95 °C during 5 min. Proteins were separated using 12% (w/v) acrylamide gels and transferred on to nitrocellulose membranes. Membranes were incubated successively with the blocking buffer (5% (w/v) BSA or nonfat dry milk in Tris-buffered saline/0.1% (v/v) Tween 20 (TBS/T)) during 1 h, and then with a 1:1000 dilution of anti-phospho-Erk1/2, anti-phospho-p38 or anti-phospho-JNK (Cell Signalling, Danvers, USA) in TBS/T containing 5% (w/v) BSA overnight. After three washes in TBS/T, membranes were incubated with peroxidase-coupled secondary goat anti-rabbit antibody (1:2000; Cell signalling or Sigma-Aldrich) and immunoreactive proteins were visualized by enhanced chemiluminescence (Western Lightning Chemiluminescence Reagent Plus; PerkinElmer) using Fluor S MultiImager and Quantity One Software (Bio-Rad Laboratories, Hercules, USA). Then, membranes were stripped (Tris–HCl 62.5 mM pH 6.8, 2% (w/v) sodium dodecyl sulphate, β-mercaptoethanol 100 mM), then placed in the blocking buffer, and finally incubated with the Erk1/2 (1:4000; Upstate, Millipore, Billerica, USA), the p38 or the JNK antibody (1:1000; Cell Signalling). Immunoreactive proteins were detected following the same procedure as described previously. Immunoblots are representative of three independent experiments.
Phospho-Erk1/2, total Erk1 and total Erk2 were also quantified using R&D System ELISA quantification kits (R&D System, Minneapolis, USA). After treatment, reactions were stopped by removing the medium and by addition of 120 μl of lysis buffer (10 mM Tris–HCl, 1% (v/v) Nonidet P-40, 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 0.5% sodium dodecyl sulphate (w/v) and 10 mM orthovanadate, pH 7.5). Then, samples were sonicated and centrifuged (14 000 g, 15 min, 4 °C). ELISA assays were performed following recommendations of the supplier and were measured at 450 nm using the plate reader Wallac 1420 VICTOR3 (PerkinElmer). Results were expressed as the mean±s.e.mean of the following ratio: [(phospho-Erk1/2-treated cells)/(total (Erk1+Erk2)-treated cells)]/[(phospho-Erk1/2-control cells)/(total (Erk1+Erk2)-control cells)]. ED50 values and maximal activation of Erk1/2 by UFP-512 were determined by curve fitting of dose–response curves using SigmaPlot, and statistical analysis was realized using StatView software. All experiments were repeated at least four times with similar results.
Measurement of intracellular calcium concentrations ([Ca2+]i)
SK-N-BE-hDOP-R cells were loaded in an HEPES-buffered saline solution (116 mM NaCl, 5.4 mM KCl, 1.8 mM. CaCl2, 0.8 mM MgSO4, 1.3 mM NaH2PO4, 12 mM HEPES, 11 mM glucose, 25 mM NaHCO3) with 5 μM of Fura-2/AM plus 0.1% (w/v) pluronic F-127 (Invitrogen) and incubated for 45 min at 37 °C. Then, cells were rinsed in HEPES-buffered saline solution and experiments were performed at room temperature, on the stage of a Nikon Eclipse inverted microscope equipped with a 75-W mercury lamp and an oil immersion Nikon, Tokyo, Japan × 40 objective with 1.4 numerical aperture. Fura-2 (excitation: 340, 380 nm, emission: 510 nm) ratio images were acquired with a CCD camera (Princeton Instrument, Trenton, USA), and digitized (512 × 512) using Metafluor 4.11 software (Universal Imaging Corporation, Chester, USA). All experiments were carried out with at least 50 cells per experiment and repeated at least three times with similar results.
Confocal laser microscopy of FLAG-tagged-hDOP receptors
SK-N-BE-hDOP-R cells were grown on alcohol-cleaned glass coverslips in 24-well plates in a culture medium until 50–60% confluence. Then, cells were either exposed to vehicle (DMEM/20 mM HEPES), 10 nM UFP-512 or 10 μM morphine for 30 min at 37 °C in DMEM/20 mM HEPES. Cells were rinsed thoroughly with phosphate-buffered saline (PBS), and fixed using a fresh solution of 4% (w/v) paraformaldehyde for 15 min and subsequently permeabilized with 0.1% (w/v) saponin. Cells were rinsed thoroughly with PBS. After blocking with PBS/1% (w/v) BSA for 30 min at room temperature, cells were incubated with the monoclonal anti-FLAG M2 (Sigma) antibody (5 μg ml−1) in the blocking buffer for 2 h at room temperature. After washing with PBS, cells were incubated with a dilution of 1:300 of fluorescein isothiocyanate-conjugated anti-mouse IgG (Sigma) for 2 h at room temperature. Coverslips were washed with PBS and mounted. Images were obtained using an Olympus confocal laser microscope (Fluo View), lens × 60.
Quantitation of hDOP receptor internalization and recycling
SK-N-BE-hDOP-R cells were seeded into 24-well plates at a density of 100 000 cells per well. Monolayers of cells were treated or not in the presence of 10 nM UFP-512 or 10 μM morphine (15–60 min) at 37 °C in DMEM/20 mM HEPES. In recycling conditions, after 60 min of agonist pretreatment, cells were rinsed and incubated in agonist-free medium for 30–60 min. Before binding studies, cells were rinsed three times thoroughly with DMEM/20 mM HEPES. Then, cells were incubated for 60 min at 4 °C (to prevent further hDOP receptor recycling) with 10 nM [3H]-TICP[ψ] ([3H]-H-Tyr-Ticψ[CH2-NH]Cha-Phe-OH) in the presence (nonspecific binding) or in the absence (total binding) of 10 μM naltrindole, in 0.5 ml of 50 mM Tris–HCl/1% (w/v) BSA, pH 7.4. The reaction was stopped by removing the medium and by addition of 1 N NaOH. Bound radioactivity was measured as described above, and the percentage of hDOP receptor internalization was calculated according to the following formula: (1−(specific binding in UFP-512-treated cells)/(specific binding in cells exposed briefly to UFP-512)) × 100. Our preliminary experiments showed that our rinsing procedure was not totally effective in removing all UFP-512 molecules from cell surface, even in the presence of Na+. Indeed, this ligand seems to be ‘sticky', since ∼30% of specific binding disappeared after a brief UFP-512 exposure (<1 min at 4 °C). Thus, we considered as 100%, cells briefly exposed to this ligand (1 min at 4 °C). Each determination was carried out in triplicate, and all experiments were repeated at least three times with similar results. Statistical analysis was realized using StatView software.
hDOP receptor phosphorylation on Ser363
SK-N-BE-hDOP-R cells were treated or not with morphine (10 μM), UFP-512 (10 nM), NTI (1 μM) alone or in combination with UFP-512 for 15 min at 37 °C in DMEM/20 mM HEPES. After agonist treatment, cells were rapidly harvested in a detachment solution (137 mM NaCl, 3 mM KCl, 6 mM NaHPO4, 15 mM KH2PO4 and 0.5 mM. EDTA, pH 7.5) and collected by centrifugation (100 g for 5 min). After solubilization in lysis buffer (50 mM Tris–HCl, 0.1% (v/v) Nonidet P-40, pH 7.4) followed by sonication, total protein concentrations were determined by the Bradford assay. An amount of 75 μg of total proteins were resolved in a 10% (w/v) polyacrylamide gel and transferred on to a nitrocellulose membrane. hDOP receptor phosphorylation on Ser363 was assessed by western blot using a polyclonal antibody directed against phospho-Ser363 of hDOP receptors (1:1000, Cell Signalling) and a peroxidase-conjugated goat anti-rabbit secondary antibody (1:1000, Cell Signalling). Immunoreactive bands were revealed using the enhanced chemiluminescence system (Super-Signal West Pico Chemiluminescent Substrate; Pierce, Pierce Biotechnology, Rockford, USA).
In vivo studies
Animals
All animal procedures complied with the European Community Guidelines and the French Law on Animal Experimentation (personal authorization no. 14–17 for MB). Naive male NMRI mice (Centre d'Elevage Renι Janvier, Le Genest Saint Isle, France) weighing 25–30 g were used for all experiments. Mice were housed by groups of 10 animals in standard polycarbonate cages and maintained in a regulated environment (22±1 °C) under 12- to 12-h light/dark cycle (light on between 20 and 08 hours) with food and water freely available in the home cage. Behavioural tests were conducted during the dark phase of the cycle, between 10 and 16 h. Each animal was used only once.
Procedures for drug injection
Naltrindole was dissolved in saline and administered subcutaneously at 10 mg kg−1 in a volume of 10 ml kg−1. In all experiments, UFP-512 (dissolved in saline) was given i.p. 1 h before the test in a volume of 10 ml kg−1. In dose–response studies, UFP-512 was administered at 0.01, 0.1 or 1 mg kg−1. For tolerance studies, mice were injected with UFP-512 once daily at 1 mg kg−1 for 7 days and were then evaluated in the FST 60 min after the last injection. In another experiment, to investigate the involvement of hDOP receptors in the antidepressant-like effect of UFP-512 in the FST, mice were pretreated with naltrindole or vehicle, and then, they received UFP-512 (1 mg kg−1, i.p.) or vehicle injection before being tested in the FST after 1 h. Time injections for all the compounds used in this study was chosen according to the study of Vergura et al. (2007).
Forced swimming test
The FST (Porsolt et al., 1977) was carried out in mice individually forced to swim during 6 min in an open cylindrical container (diameter 12 cm, height 20 cm) with a water depth of 13 cm at 23–24 °C. The duration of immobility, after a delay of 2 min, was measured during the last 4 min. Each mouse was judged to be immobile when it ceased struggling and remained floating motionless in the water, making only those movements necessary to keep its head above water. Animals were not pretested.
Analysis of data
For in vitro experiments, results are expressed as the mean±s.e.mean of n experiments. ANOVA followed by the Bonferroni–Dunn test was used to determine the statistical significance (StatView). For in vivo studies, all experimental series were analysed separately as independent series with their own control groups. In all studies, results were expressed as means±s.e.mean. Values of P<0.05 were considered to be significant. Comparison between groups was made by using ANOVA. Post hoc comparisons were made by a post hoc multiple comparison test (protected least-significant difference (PLSD) of Fischer).
Materials
[3H]diprenorphine was purchased from PerkinElmer Life Sciences, morphine was a generous gift from Francopia, Paris, France, naltrindole was purchased from Sigma-Aldrich, UFP-512 was synthesized by Balboni et al. (2002) and [3H]-TICP[ψ] was synthesized as described by Szatmari et al. (1999).
Results
In vitro studies
Affinity of UFP-512 for hDOP receptors
The binding of the novel hDOP receptor agonist UFP-512 (Figure 1b) was measured in the human neuroblastoma SK-N-BE cells stably overexpressing the FLAG-tagged-hDOP receptor. The competition between [3H]diprenorphine and UFP-512 binding was best fitted to a one-site model (F-test, P<0.0001). UFP-512 significantly displaced [3H]diprenorphine at low concentrations (1 nM) but at high concentrations of this agonist, we did not observe a complete radioligand displacement (∼80–90%) (Figure 1b). Calculation revealed that UFP-512 had a high affinity for hDOP receptors with a pKi of 9.78.
Measurement of cAMP
Inhibition of cAMP accumulation by UFP-512 and morphine
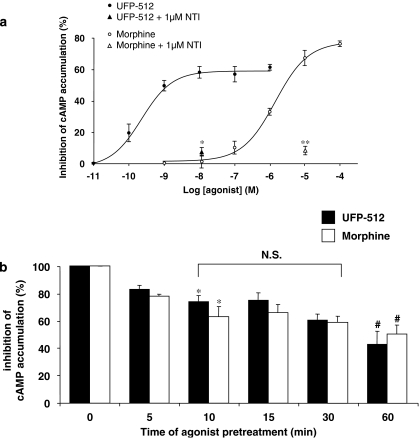
To further characterize the biological activity of the novel agonist UFP-512, we measured its ability to inhibit cAMP accumulation in the presence of forskolin in whole cells, by comparison with morphine. As shown in Figure 2a, UFP-512 inhibited cAMP accumulation in a dose-dependent manner from 100 pM to 10 nM with a pEC50 value of 9.40 and an efficacy of nearly 60%. Inhibition of adenylyl cyclase was totally prevented by coadministration of 10 nM UFP-512 with 1 μM naltrindole, a DOP-selective opioid antagonist (Figure 2a). Similarly, morphine inhibited cAMP accumulation in a dose-dependent manner from 100 nM to 10 μM (pEC50=5.7) (Figure 2a). The maximal inhibition of 70% obtained with 10 μM morphine was also abolished by naltrindole coadministration (Figure 2a). The concentrations of 10 nM UFP-512 and 10 μM morphine were selected for further experiments.
Figure 2.
Measurement of adenylyl cyclase inhibition. (a) Concentration-dependent inhibition of cAMP accumulation both by UFP-512 (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid) (1) and morphine (2). The human neuroblastoma SK-N-BE cells stably expressing the FLAG-tagged-human DOP receptor (SK-N-BE-hDOP-R cells) were incubated in the presence of 0.5 mM isobutylmethylxanthine, 40 μM forskolin to stimulate adenylyl cyclase and increasing concentrations of UFP-512 (10 pM to 1 μM) or morphine (1 nM to 100 μM) for 5 min at 37 °C. The specificity of the agonist-induced inhibition of adenylyl cyclase was checked by the addition of 1 μM naltrindole (NTI). The [3H]cAMP was collected as described in the Methods. Data are representative of four distinct experiments performed in triplicate (mean±s.e.mean; protected least-significant difference (PLSD) of Fischer, *P<0.05; **P<0.005). (b) Similar profile of human δ-opioid receptor (hDOP) receptor desensitization induced both by UFP-512 and morphine. SK-N-BE-hDOP-R cells were pretreated or not (0 min) with 10 nM UFP-512 or 10 μM morphine for various times (5–60 min). Then, the ability of each opioid agonist to inhibit forskolin-stimulated adenylyl cyclase activity was determined. Data are representative of three to four distinct experiments performed in triplicate (mean±s.e.mean, ANOVA followed by the Bonferroni–Dunn test, *P<0.05 as compared to the naive cells, #P<0.05 compared to the 10-min pretreated cells).
Effects of hDOP receptor desensitization on adenylyl cyclase pathway: comparison between UFP-512 and morphine
We studied the desensitization profile of hDOP receptors after UFP-512 or morphine exposure, via the inhibition of adenylyl cyclase. SK-N-BE-hDOP-R cells were pretreated or not during various times (5–60 min) at the concentrations promoting the maximal inhibition of cAMP accumulation. As shown in Figure 2b, both UFP-512 (10 nM) and morphine (10 μM) caused a weak but significant desensitization of about 20%, detected only after 10 min exposure to agonist. The inhibitory effects of these two opioid agonists progressively decreased in a time-dependent manner to reach a similar level (40–50% control), after a 60-min exposure period. Interestingly, whatever the time of exposure, we did not observe any significant difference between UFP-512- and morphine-pretreated cells (Figure 2b, ANOVA followed by the Bonferroni–Dunn test).
Subsequently, to gain a better understanding of the regulation of hDOP receptors by UFP-512, we studied two other signalling pathways: MAP kinase (Erk1/2, JNK and p38) activation and the rise of intracellular free calcium concentration ([Ca2+]i).
Study of the mitogen-activated protein kinase-signalling pathway
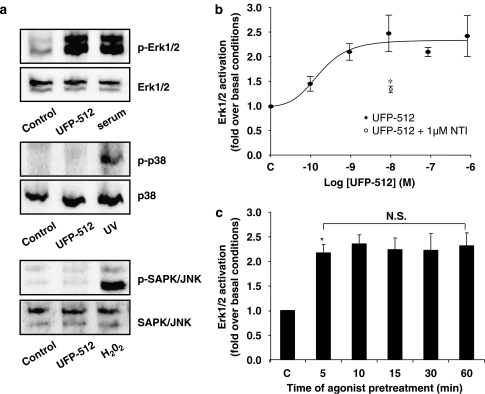
Like many members of the GPCR family, DOP receptors were shown to activate the MAP kinase cascade, including the extracellular signal-regulated protein kinase Erk1/2, p38 and JNK (Gutstein et al., 1997; Belcheva et al., 1998; Zhang et al., 1999b; Shahabi et al., 2003). In western blot experiments, treatment of the cells with either UFP-512 or fetal calf serum was able to activate the Erk1/2 MAP kinase pathway, but neither p38 nor JNK was activated even at a high concentration of UFP-512 (10 μM) (Figure 3a). This lack of activation is not due to a deficiency of these pathways since ultraviolet light and hydrogen peroxide were able to induce phosphorylation of p38 and JNK (Figure 3a). Activation of p42/p44 MAP kinase pathway was also studied in the SK-N-BE-hDOP-R cells by ELISA using anti-phospho-Erk1/2, total Erk1 and total Erk2. Results are expressed as the ratio of phospho-Erk1/2/total Erk1/2 in treated versus control cells. As shown in Figure 3b, UFP-512 activated the Erk1/2 pathway in a dose-dependent manner from 100 pM to 10 nM with a pEC50 value of 9.6 and an efficacy of 2.4±0.3-fold over basal conditions. Coadministration of 10 nM UFP-512 with 1 μM naltrindole significantly reduced Erk1/2 activation (1.3±0.1-fold over basal conditions). In kinetic studies, SK-N-BE-hDOP-R cells were exposed or not (control) for various periods (5–60 min) to 10 nM UFP-512. As shown in Figure 3c, UFP-512 caused a significant Erk1/2 activation, detectable only after 5-min agonist exposure, and this level of activation was maintained for up to 60-min exposure, suggesting a lack of desensitization in this signalling pathway.
Figure 3.
Measurement of mitogen-activated protein (MAP) kinase activation. (a) UFP-512 (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid) activates extracellular signal-regulated protein kinase Erk1/2 (Erk1/2) but not p38 and JNK (Janus NH2 terminal kinase) pathways. The human neuroblastoma SK-N-BE cells stably expressing the FLAG-tagged-human DOP receptor (SK-N-BE-hDOP-R cells) were incubated or not (c: control) with UFP-512 (10 μM for p38 and JNK or 1 μM for Erk1/2); ultraviolet exposure (for p38), hydrogen peroxide (for JNK) and fetal calf serum (serum) (for Erk1/2) were used as positive controls for the different kinase pathways. Phosphorylation of MAP kinases was evaluated by western blot as described in the Methods. Immunoblots are representative of three independent experiments. (b) Concentration-dependent activation of Erk1/2 induced by UFP-512. SK-N-BE-hDOP-R cells were incubated or not (c: control) with UFP-512 (100 pM to 1 μM). Level of phosphorylation was measured using ELISA assays (see the Methods). Results are expressed as the ratio of phospho-Erk1/2/total Erk1/2 in treated versus control cells. This effect was reversed by coadministration of 1 μM naltrindole (NTI). Data are representative of four separate experiments (mean±s.e.mean; PLSD of Fischer, *P<0.05). (c) Lack of desensitization on Erk1/2 pathway. SK-N-BE-hDOP-R cells were incubated or not (c: control) with 10 nM UFP-512 during various times (5–60 min) and Erk1/2 activation was quantified using ELISA assay (as described in the Methods). Data are representative of five separate experiments (mean±s.e.mean, ANOVA followed by the Bonferroni–Dunn test, *P<0.05 as compared to the naive cells). HDOP, human δ-opioid receptor; NS, nonsignificant.
Rise in [Ca2+]i
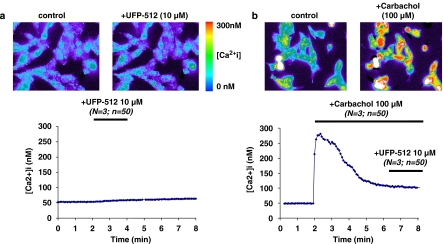
In SK-N-BE neuroblastoma cells, we have already demonstrated that etorphine-activated hDOP receptors were coupled to the increase of [Ca2+]i, through a ryanodine receptor-mediated mechanism (Allouche et al., 1996). Here, we studied UFP-512-induced calcium signalling at different concentrations. The first series of experiments showed no significant change in [Ca2+]i, even after high concentrations of UFP-512 (Figure 4a). Interestingly, Connor and Henderson (1996) showed in SH-SY5Y neuroblastoma cells that DPDPE only elevated [Ca2+]i during a concomitant activation of muscarinic acetylcholine receptors. Since these receptors are endogenously expressed in our cellular model (Allouche et al., 1996), we tested this hypothesis in a second series of experiments. SK-N-BE-hDOP-R cells were first exposed to 100 μM carbachol, a potent muscarinic acetylcholine receptor agonist, which induced a rapid increase of [Ca2+]i (from 50 to 275 nM), through the PLC-IP3-signalling pathway, and a subsequent decrease (Figure 4b). Then, in the presence of ongoing muscarinic acetylcholine receptor activity, 10 μM UFP-512 was coapplied, but we did not observe any significant change in [Ca2+]i (Figure 4b).
Figure 4.
Measurement of rise in [Ca2+]i. The human neuroblastoma SK-N-BE cells stably expressing the FLAG-tagged-human DOP receptor (SK-N-BE-hDOP-R cells) were split on 35-mm glass dishes and loaded with the fluorescent probe fura-2/AM. 10 μM UFP-512 alone (a) did not elevate [Ca2+]i, whereas 100 μM carbachol (b) produced a rapid and transient elevation of [Ca2+]i that fell back to baseline levels in the continued presence of this agonist. Thereafter, in the continuous presence of 100 μM carbachol, coapplication of 10 μM (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid) UFP-512 failed to elevate [Ca2+]i. Drugs were applied for the periods indicated by the bars. Data are representative of three separate experiments, each performed with approximately 50 cells. hDOP, human δ-opioid receptor.
Effects of UFP-512 on hDOP receptor trafficking
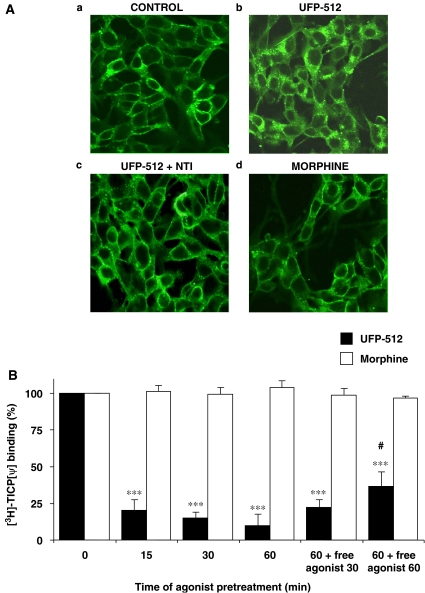
To explore hDOP receptor trafficking, we performed both immunofluorescence experiments using anti-FLAG antibody directed against the FLAG-tagged hDOP receptor and radioligand-binding experiments. As observed in naive cells by confocal microscopy (Figure 5A), the FLAG-tagged hDOP receptors were mainly expressed at the plasma membrane (a). This immunolabelling is specific since it was not observed in the wild-type SK-N-BE cells (Marie et al., 2003b). After a 30-min treatment, 10 nM UFP-512 (b), but not 10 μM morphine (d), induced a marked endocytosis of hDOP receptors, illustrated by the cytosolic localization of the immunolabelling, which was blocked by coadministration of 1 μM naltrindole (c).
Figure 5.
Human δ-opioid receptor (hDOP) receptor internalization. (A) Immunofluorescence studies. The human neuroblastoma SK-N-BE cells stably expressing the FLAG-tagged-human DOP receptor (SK-N-BE-hDOP-R cells) were incubated for 30 min in the absence of agonist (a) or in the presence of 10 nM UFP-512 (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid) alone (b), 10 nM UFP-512±1 μM naltrindole (NTI) (c) or 10 μM morphine (d). Localization of hDOP receptors was revealed, using anti-FLAG M2 antibody, by confocal microscopy. Images are representative of two separate experiments. (B) Kinetics of UFP-512-induced hDOP receptor endocytosis: comparison with morphine. SK-N-BE-hDOP-R cells were incubated briefly (<1 min) or during various times (5–60 min) in the presence of 10 nM UFP-512 or 10 μM morphine. For recycling experiments, agonist-pretreated cells were washed and left for 30 or 60 min in agonist-free medium. Then, cells were incubated with 10 nM [3H]-TICP[ψ] ([3H]-H-Tyr-Ticψ[CH2-NH]Cha-Phe-OH) alone (total binding) or plus 10 μM naltrindole (nonspecific binding). Data are representative of three to four separate experiments performed in triplicate (mean±s.e.mean, ANOVA followed by the Bonferroni–Dunn test, ***P<0.001 versus morphine-pretreated cells, #P<0.05 versus 60 min UFP-512-pretreated cells).
To quantify the disappearance of hDOP receptors from the plasma membrane, we measured the level of opioid receptors in [3H]-TICP(ψ)-binding experiments. This radioligand fulfilled the following two criteria necessary to specifically quantify cell surface hDOP receptors: (i) it is a peptidic ligand unable to cross the plasma membrane and (ii) it is an antagonist, thus unable to trigger hDOP receptor endocytosis.
SK-N-BE-hDOP-R cells were pretreated or not (control) with 10 nM UFP-512 or 10 μM morphine for various times (5–60 min). Since a brief exposure (<1 min) of cells to UFP-512 reduced by 30% the specific binding of [3H]-TICP(ψ) compared to nontreated cells (as indicated in the Methods section), the specific binding under this condition was considered as 100%. After only 15 min of 10 nM UFP-512 exposure, we observed the strongest level of internalization (about 80% internalization of hDOP receptors; Figure 5B). No further decrease of [3H]-TICP(ψ) binding decrease was observed after 30 or 60 min exposure. In contrast, 10 μM morphine was unable to trigger hDOP receptor endocytosis, at any time of exposure up to 60 min. After 60 min of agonist exposure, SK-N-BE-hDOP-R cells were thoroughly washed and maintained in an agonist-free medium for another 30 or 60 min to analyse receptor recycling (last two bars in Figure 5B). For cells pretreated with UFP-512, a significant reappearance of hDOP receptors at the cell surface was measured after 60-min incubation in agonist-free media, corresponding to about 30% of the [3H]-TICP(ψ) binding in naive cells. For morphine-pretreated cells, incubation in morphine-free media (‘recycling condition') did not alter the plasma membrane [3H]-TICP(ψ) binding.
hDOP receptor phosphorylation at Ser363
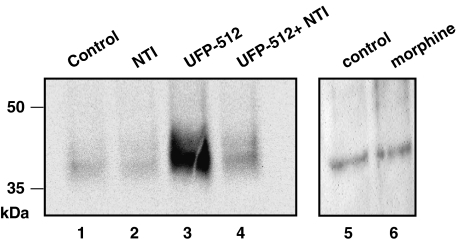
As we observed marked differences between UFP-512 and morphine in promoting hDOP receptor internalization, the next step was to explore the phosphorylation state of the opioid receptors after these agonist exposures. These experiments were carried out by using the anti-DOP receptor phospho-Ser363 antibody. As illustrated in Figure 6, both control (C) and naltrindole (N)-activated hDOP receptors displayed a weak basal phosphorylation level at Ser363 (lanes 1, 2 and 5). After a 15-min treatment, 10 nM UFP-512 (U, lane 3) induced a strong and specific hDOP receptor phosphorylation, as demonstrated by coadministration with 1 μM naltrindole (N+U, lane 4), which totally blocked phosphorylation of the opioid receptors. In contrast, 10 μM morphine was almost ineffective in increasing hDOP receptor phosphorylation (M, lane 6). The densities of the 35- to 50-kDa protein bands were quantified, using the quantity one software, and in these experiments, UFP-512 produced an increase in hDOP receptor phosphorylation by 4.6-fold over naive cell level.
Figure 6.
UFP-512 (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid), but not morphine, induced human δ-opioid receptor (hDOP) receptor phosphorylation on Ser363. The human neuroblastoma SK-N-BE cells stably expressing the FLAG-tagged-human DOP receptor (SK-N-BE-hDOP-R cells) were treated (lanes 2, 3, 4 and 6) or not (lanes 1 and 5) in DMEM/20 mM HEPES with opioid ligands for 15 min at 37 °C and samples were prepared as described in the Methods. Western blot analysis was performed using anti-DOP-receptor-phospho-Ser363 antibody (Cell Signalling). Almost no immunoreactivity was detected in naive (lanes 1 and 5), in naltrindole (NTI)-pretreated (lane 2) or in 10 μM morphine-treated cells (lane 6). Pretreatment during 15 min with 10 nM UFP-512 (lane 3) led to the phosphorylation of the hDOP receptor at Ser363. This phosphorylation was completely blocked by a concomitant incubation of cells with 1 μM NTI (lane 4). Immunoblot is representative of two distinct experiments.
In vivo studies
Forced swimming test after acute or chronic UFP-512 administration
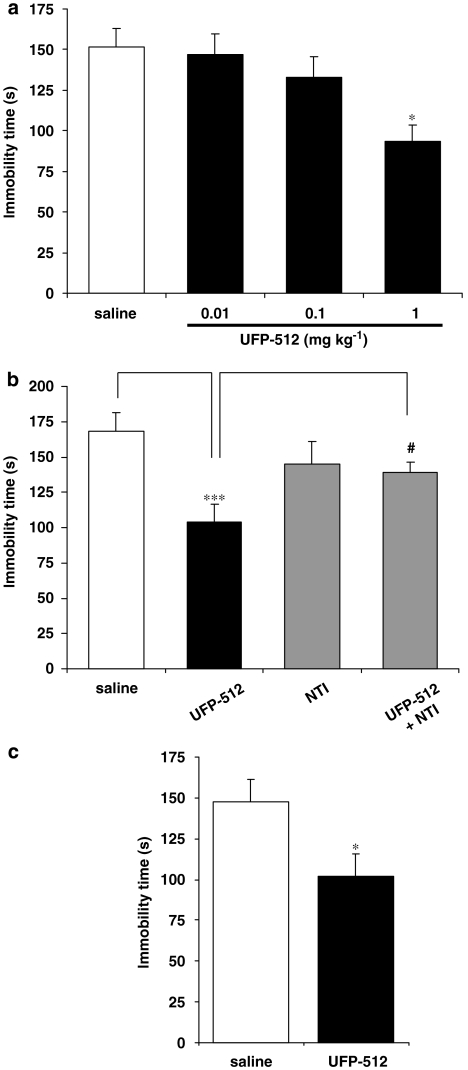
In the FST, acute i.p. injection of UFP-512 (60 min before the test) produced antidepressant-like activity in mice at 1 mg kg−1 (Figure 7a). A significant decrease of immobility time was observed with neither locomotor stimulation nor convulsive effects (data not shown). Therefore, the concentration of 1 mg kg−1 UFP-512 was selected for chronic injection experiments. This UFP-512-induced antidepressant-like effect was totally blocked by acute s.c. injection of 10 mg kg−1 naltrindole (Figure 7b: U+N) with no effect of the antagonist alone (N), demonstrating the involvement of DOP receptors. After 7 days of daily agonist administration, UFP-512 exhibited antidepressant-like effects similar to those observed in acutely treated mice, as illustrated by decreased immobility time in FST (Figure 7c), demonstrating a lack of tolerance to the antidepressant-like effects of UFP-512.
Figure 7.
Antidepressant-like effects after acute (a, b) or chronic (7 days) (c) UFP-512 (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid) administration, were assessed by the forced swimming test (FST). (a) Effect of increasing doses of UFP-512 (0.01, 0.1 and 1 mg kg−1). UFP-512 significantly reduced the immobility time at 1 mg kg−1, indicating an antidepressant-like effect. *P<0.005 (protected least-significant difference (PLSD) of Fischer). (b) Naltrindole (NTI) blocked the antidepressant-like effect of UFP-512. The effect of 1 mg kg−1 UFP-512 was totally blocked by concomitant administration with 10 mg kg−1 NTI, but NTI alone did not modify the duration of immobility. ***P<0.001 as compared to the control group. #P<0.05 versus UFP-512 group (PLSD of Fischer). (c) Lack of tolerance to the antidepressant-like effects of 1 mg kg−1 UFP-512 after 7 days of daily injection. The effect of 1 mg kg−1 UFP-512 on the immobility time, was still observed even after chronic treatment (PLSD of Fischer, *P<0.05 versus saline) and was not significantly different from that in acutely treated mice (PLSD of Fischer, NS: all results are expressed as mean±s.e.mean (n=10 animals for each group).
Discussion and conclusions
Among the different opioid receptors, the DOP receptors appear as an interesting target to treat anxiety and depression (Jutkiewicz, 2006), but to obtain this biological effect, selective agonists need to be developed. In the present paper, we attempted to define the pharmacological properties of a novel δ-selective ligand derived from the Dmt-Tic pharmacophore, UFP-512, towards hDOP receptors expressed in the SK-N-BE-hDOP-R cells. From the in vitro data, we also attempted to predict the ability of this agonist to induce antidepressant-like effects, without tolerance. This latter point was confirmed by in vivo studies using the FST.
Since the cloning of opioid receptors, pharmacological characterization of opioid ligands has essentially been conducted in nonneuronal cells (that is, Chinese hamster ovary or CHO cells, green monkey kidney or COS-7 cells and human embryonic kidney or HEK 293 cells). The main reason is the easy use of these models, in terms of transfection and cell culture. However, recent data indicate that such cellular models are far from ideal models of neuronal cells, endogenously expressing opioid receptors, and thus conflicting results have been observed. For instance, morphine failed to promote MOP receptor internalization in heterologous systems (Arden et al., 1995; Keith et al., 1996), while in primary cultures of rat striatal neurons, morphine was effective (Haberstock-Debic et al., 2005). So, to improve the characterization of newly synthesized opioid ligands, we decided to choose a human neuronal cell line, the SK-N-BE cells, which has already been used for studying DOP receptor regulation (Namir et al., 1997; Hasbi et al., 1998; Allouche et al., 1999; Hasbi et al., 2000; Marie et al., 2003a, 2003b; Lecoq et al., 2004; Aguila et al., 2006).
Binding experiments revealed that UFP-512 binds the hDOP receptor with high affinity (pKi of 9.8), which fits very well with the data obtained by Vergura et al. (2007) (pKi of 10.2 in CHO cells containing hDOP receptor cells). Functional experiments have shown that UFP-512 behaves as a potent agonist on adenylyl cyclase inhibition and Erk1/2 activation. Comparison with our previous data reveals that UFP-512 is up to 1000-fold more potent to compete with [3H]diprenorphine for binding to hDOP receptor than other DOP receptor selective (DPDPE, deltorphin I, DTLET ([D-Thr2]-Leu-Enkephalin-Thr), SNC-80 and AR-M1000390) and nonselective opioid agonists (Leu- (H-Tyr-Gly-Gly-Phe-Leu-OH) and Met- (H-Tyr-Gly-Gly-Phe-Met-OH) enkephalins and morphine) (Polastron et al., 1994; Allouche et al., 2000; Marie et al., 2003a; Lecoq et al., 2004). In the presence of a high concentration of UFP-512 (1 μM), [3H]diprenorphine binding was not completely displaced, and about 20% of specific binding remains. It is possible that UFP-512, which is a peptidic agonist, cannot freely cross the plasma membrane and compete with the lipophilic radioligand [3H]diprenorphine for the intracellular hDOP receptors.
We also explored the regulation of different signalling pathways by UFP-512, as a drug can be either a full, partial or inverse agonist depending on the second messenger studied (Galandrin and Bouvier, 2006). As expected from the binding data, UFP-512 was approximately 5500-fold more potent than morphine in inhibiting the forskolin-stimulated adenylyl cyclase, but these opioid agonists had a similar efficacy. UFP-512 was also able to regulate the Erk1/2 pathway and adenylyl cyclase with an identical potency (pEC50 of 9.6 and 9.4, respectively), and these effects were specifically mediated by the hDOP receptors as they were blocked by naltrindole. Our results correlate well with those obtained by Vergura et al. (2007) both in GTPγS experiments and in the electrically stimulated mouse vas deferens preparation (that is, pEC50 values of 10.2 and 11.6, respectively).
Conversely, UFP-512 was not able to increase [Ca2+]i either alone or after muscarinic activation by carbachol. These data contrasted with earlier studies performed with etorphine (Allouche et al., 1996) and suggested that UFP-512 was unable to activate the Pertussis toxin-insensitive G proteins responsible for the calcium rise mediated by etorphine. Furthermore, UFP-512 was not able to activate p38 and JNK, two other members of the MAP kinase family. This indicated that regulation of each MAP kinase module was mediated by different mechanisms. As UFP-512 regulated Erk1/2 proteins and cAMP synthesis with identical potency, it is probable that these effects were produced by the same G proteins.
Upon sustained activation either by UFP-512 or by morphine, we observed a rapid but moderate decrease in cAMP inhibition, suggesting that both agonists were able to cause similar hDOP receptor desensitization. Comparison with earlier data obtained in our laboratory revealed that the DOP-selective agonist UFP-512 promotes a weak hDOP receptor desensitization compared to DPDPE, deltorphin I, SNC-80 and AR-M1000390 (Allouche et al., 1999; Marie et al., 2003a; Lecoq et al., 2004). After 30 min of pretreatment with these latter agonists, a desensitization of about 80–90% was observed, while the challenge of SK-N-BE-hDOP-R cells with either UFP-512 or morphine reduced the adenylyl cyclase inhibition by only 40–50%. This observation invalidates our previous hypothesis, which indicated that all the DOP-selective agonists would produce strong hDOP receptor desensitization. This suggests that the DOP-selective agonists would bind not only to common, but also to specific, regions of the hDOP receptor to induce different conformations and exposing various amino acids responsible for the phosphorylation and desensitization. However, results obtained with UFP-512 and morphine show that phosphorylation and desensitization could be dissociated, in contrast to the well-accepted model proposed by Lefkowitz (Pitcher et al., 1999). This indicates that there are different ways to induce desensitization (Marie et al., 2006) and highlights the complexity of those mechanisms. Indeed, in phosphorylation experiments, we showed that UFP-512 produced a marked hDOP receptor phosphorylation on Ser363, while morphine, as previously reported (Zhang et al., 1999a), was ineffective.
Surprisingly and in contrast to the cAMP pathway, we were unable to observe desensitization of the effects on the Erk1/2 pathway. Indeed, even after 1 h of pretreatment with UFP-512, the level of Erk1/2 activation was the same as that observed in naive cells. This result contrasts with the time course of Erk1/2 activation by DPDPE observed both in CHO and SK-N-SH cells. Indeed, Trapaidze et al. (2000) showed that the phosphorylation level of these MAP kinases reached a maximum around 5 min, remained stable until 30 min and then decreased. The profound hDOP receptor sequestration, without reduction of Erk1/2 activation, after UFP-512 exposure suggested that the internalized receptors were able to transduce and activate MAPK/ERK kinase. From the model of GPCR regulation, it is possible that once phosphorylated by GRKs, the DOP receptor would recruit β-arrestins, which in turn would activate Ras/Raf proteins leading to Erk1/2 phosphorylation and activation. This would explain the long-lasting stimulation of Erk1/2 proteins, while the inhibition of cAMP, which relies on Gi/Go proteins, would be impaired after the uncoupling mediated by the β-arrestins.
In the third part of this work, we studied the putative relationships between desensitization of hDOP receptors and their intracellular trafficking. Qualitative and quantitative experiments provided evidence for a rapid and profound hDOP receptor sequestration by 80% after 15 min exposure to UFP-512, while morphine failed to promote DOP receptor sequestration as previously reported (Zhang et al., 1999a). Interestingly, whereas UFP-512 and morphine produced the same hDOP receptor desensitization profile on the cAMP pathway, these agonists displayed an opposite ability to induce receptor internalization. The fact that about 80% of cell surface receptors disappeared without marked desensitization suggests that hDOP receptors would be cointernalized with their cognate G proteins and adenylyl cyclase, allowing the signalling to continue. We can also hypothesize that UFP-512 is a potent full agonist requiring a limited receptor number to produce the maximal inhibition on the cAMP pathway. Even with the marked degree of phosphorylation, only a small number of hDOP receptors would then be inactivated and a weak desensitization would be observed. We can also suppose that the population of cell surface hDOP receptors contains spare receptors that could bind UFP-512 when agonist-activated receptors are internalized, thus limiting desensitization on the cAMP pathway. Recycling of dephosphorylated hDOP receptors to the cell surface would also maintain a population of active receptors and would counteract desensitization. Indeed, after 1 h in agonist-free medium, we observed a significant reappearance of hDOP receptors by about 30%.
According to Whistler's hypothesis (Finn and Whistler, 2001), our in vitro data would indicate that UFP-512, by promoting a weak desensitization coupled with an internalization and recycling of hDOP receptors, would have a low propensity to induce tolerance. To confirm this assumption, we studied the antidepressant-like effects of UFP-512 in mice, using the FST after acute or chronic treatment. Similar to other DOP-selective agonists (UFP-502, Vergura et al., 2006; BW373U86, Broom et al., 2002; and SNC-80, Jutkiewicz et al., 2003), we observed that UFP-512 mediates a specific hDOP receptor-dependent antidepressant-like response in naive animals. Moreover, this effect was fully maintained after a chronic treatment. This observation clearly demonstrates the absence of tolerance to this effect.
Among the new pharmacological ways to treat depression, DOP receptor activation seems to be an interesting tool, but requires the development of newly selective, effective and noninducing tolerance opioid drugs. Until now, to discover such a molecule, the main experimental strategy relied on in vivo experiments, which are expensive and time-consuming. To improve this screening and select the most promising drugs, we would propose an in vitro step based on complementary assays (that is, binding studies, functional experiments in acute and chronic conditions, internalization and recycling kinetics and phosphorylation measurement) in the SK-N-BE cell line. Then, the selected molecules could be further tested in assays for antidepressant activity.
Acknowledgments
We thank Professor Girolamo Calò (Department of Experimental and Clinical Medicine and Neuroscience Centre, University of Ferrara, Ferrara, Italy) for critical reading of the manuscript and Professor Michel Bouvier (Institute for Research in Immunology and Cancer, Universitι de Montrιal, Montrιal, Quιbec, Canada) for generously providing pcDNA3-FLAG-tagged-hDOR. This work was supported by the Conseil Rιgional de Basse-Normandie, France; the Association de Recherche contre le Cancer, France; OTKA 46434 (AB), grant from the Hungarian Scientific Research Fund and by RETDNT 2004 (GT and AB) from National Bureau of Research and Technology, Hungary. B Aguila is the recipient of a fellowship from the Conseil Regional Basse-Normandie.
Abbreviations
- AR-M1000390
N,N-diethyl-4-(phenylpiperidin-4-ylidene-methyl)-benzamide
- deltorphin I
H-Tyr-D-Ala-Phe-Asp-Val-Val-Gly-NH2
- DOP receptor
δ-opioid receptor
- DPDPE
[D-Pen2, D-Pen5]enkephalin
- DTLET
[D-Thr2]-Leu-Enkephalin-Thr
- Erk1/2
extracellular signal-regulated protein kinase Erk1/2
- FST
forced swimming test
- GPCR
G protein-coupled receptor
- KOP receptor
κ-opioid receptor
- Leu-enkephalin
H-Tyr-Gly-Gly-Phe-Leu-OH
- Met-enkephalin
H-Tyr-Gly-Gly-Phe-Met-OH
- MOP receptor
μ-opioid receptor
- SNC-80
[(+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxy benzyl]-N,N-diethylbenzamide
- [3H]-TICP[ψ]
[3H]-H-Tyr-Ticψ[CH2-NH]Cha-Phe-OH
- UFP-512
H-Dmt-Tic-NH-CH(CH2-COOH)-Bid
Conflict of interest
The authors state no conflict of interest.
References
- Aguila B, Roussel M, Jauzac P, Allouche S. High-purity selection and maintenance of gene expression in human neuroblastoma cells stably over-expressing GFP fusion protein application for opioid receptors desensitization studies. Brain Res. 2006;1114:11–18. doi: 10.1016/j.brainres.2006.07.069. [DOI] [PubMed] [Google Scholar]
- Allouche S, Hasbi A, Ferey V, Sola B, Jauzac P, Polastron J. Pharmacological delta1- and delta2-opioid receptor subtypes in the human neuroblastoma cell line SK-N-BE: no evidence for distinct molecular entities. Biochem Pharmacol. 2000;59:915–925. doi: 10.1016/s0006-2952(99)00404-9. [DOI] [PubMed] [Google Scholar]
- Allouche S, Polastron J, Jauzac P. The delta-opioid receptor regulates activity of ryanodine receptors in the human neuroblastoma cell line SK-N-BE. J Neurochem. 1996;67:2461–2470. doi: 10.1046/j.1471-4159.1996.67062461.x. [DOI] [PubMed] [Google Scholar]
- Allouche S, Roussel M, Marie N, Jauzac P. Differential desensitization of human delta-opioid receptors by peptide and alkaloid agonists. Eur J Pharmacol. 1999;371:235–240. doi: 10.1016/s0014-2999(99)00180-6. [DOI] [PubMed] [Google Scholar]
- Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E,JinsmaaY, et al. Potent delta-opioid receptor agonists containing the Dmt-Tic pharmacophore. J Med Chem. 2002;45:5556–5563. doi: 10.1021/jm020336e. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Vogel Z, Ignatova E, Avidor-Reiss T, Zippel R, Levy R, et al. Opioid modulation of extracellular signal-regulated protein kinase activity is ras-dependent and involves Gbetagamma subunits. J Neurochem. 1998;70:635–645. doi: 10.1046/j.1471-4159.1998.70020635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a non-peptidic delta-opioid receptor agonist is not required for its antidepressant-like effects in Sprague–Dawley rats. Psychopharmacology (Berl) 2002;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Connor M, Henderson G. delta- and mu-opioid receptor mobilization of intracellular calcium in SH-SY5Y human neuroblastoma cells. Br J Pharmacol. 1996;117:333–340. doi: 10.1111/j.1476-5381.1996.tb15195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- Gutstein HB, Rubie EA, Mansour A, Akil H, Woodgett JR. Opioid effects on mitogen-activated protein kinase signaling cascades. Anesthesiology. 1997;87:1118–1126. doi: 10.1097/00000542-199711000-00016. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim KA, Yu YJ, von Zastrow M. Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Allouche S, Sichel F, Stanasila L, Massotte D, Landemore G, et al. Internalization and recycling of delta-opioid receptor are dependent on a phosphorylation–dephosphorylation mechanism. J Pharmacol Exp Ther. 2000;293:237–247. [PubMed] [Google Scholar]
- Hasbi A, Polastron J, Allouche S, Stanasila L, Massotte D, Jauzac P. Desensitization of the delta-opioid receptor correlates with its phosphorylation in SK-N-BE cells: involvement of a G protein-coupled receptor kinase. J Neurochem. 1998;70:2129–2138. doi: 10.1046/j.1471-4159.1998.70052129.x. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM. The antidepressant-like effects of delta-opioid receptor agonists. Mol Interv. 2006;6:162–169. doi: 10.1124/mi.6.3.7. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Woods JH, Winsauer PJ. Effects of the delta-opioid receptor agonist SNC80 on learning relative to its antidepressant-like effects in rats. Behav Pharmacol. 2003;14:509–516. doi: 10.1097/00008877-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, et al. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Lecoq I, Marie N, Jauzac P, Allouche S. Different regulation of human delta-opioid receptors by SNC-80 [(+)-4-[(alphaR)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide] and endogenous enkephalins. J Pharmacol Exp Ther. 2004;310:666–677. doi: 10.1124/jpet.103.063958. [DOI] [PubMed] [Google Scholar]
- Marie N, Aguila B, Allouche S. Tracking the opioid receptors on the way of desensitization. Cell Signal. 2006;18:1815–1833. doi: 10.1016/j.cellsig.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Marie N, Landemore G, Debout C, Jauzac P, Allouche S. Pharmacological characterization of AR-M1000390 at human delta opioid receptors. Life Sci. 2003a;73:1691–1704. doi: 10.1016/s0024-3205(03)00489-2. [DOI] [PubMed] [Google Scholar]
- Marie N, Lecoq I, Jauzac P, Allouche S. Differential sorting of human delta-opioid receptors after internalization by peptide and alkaloid agonists. J Biol Chem. 2003b;278:22795–22804. doi: 10.1074/jbc.M300084200. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Smadja C, Valverde O, Vonesch JL, Foutz AS, Boudinot E, et al. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor. J Neurosci. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namir N, Polastron J, Allouche S, Hasbi A, Jauzac P. The delta-opioid receptor in SK-N-BE human neuroblastoma cell line undergoes heterologous desensitization. J Neurochem. 1997;68:1764–1772. doi: 10.1046/j.1471-4159.1997.68041764.x. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Tesmer JJ, Freeman JL, Capel WD, Stone WC, Lefkowitz RJ. Feedback inhibition of G protein-coupled receptor kinase 2 (GRK2) activity by extracellular signal-regulated kinases. J Biol Chem. 1999;274:34531–34534. doi: 10.1074/jbc.274.49.34531. [DOI] [PubMed] [Google Scholar]
- Polastron J, Mur M, Mazarguil H, Puget A, Meunier JC, Jauzac P. SK-N-BE: a human neuroblastoma cell line containing two subtypes of delta-opioid receptors. J Neurochem. 1994;62:898–906. doi: 10.1046/j.1471-4159.1994.62030898.x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Shahabi NA, McAllen K, Sharp BM. Phosphorylation of activating transcription factor in murine splenocytes through delta opioid receptors. Cell Immunol. 2003;221:122–127. doi: 10.1016/s0008-8749(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Szatmari I, Toth G, Kertesz I, Schiller PW, Borsodi A. Synthesis and binding characteristics of [3H] H-Tyr-Ticpsi[CH2-NH] Cha-Phe-OH, a highly specific and stable delta-opioid antagonist. Peptides. 1999;20:1079–1083. doi: 10.1016/s0196-9781(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Trapaidze N, Gomes I, Cvejic S, Bansinath M, Devi LA. Opioid receptor endocytosis and activation of MAP kinase pathway. Brain Res Mol Brain Res. 2000;76:220–228. doi: 10.1016/s0169-328x(00)00002-4. [DOI] [PubMed] [Google Scholar]
- Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, et al. Anxiolytic- and antidepressant-like activities of H-Dmt-Tic-NHCH(CH2-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist Peptides 2007. in press [DOI] [PubMed]
- Vergura R, Valenti E, Hebbes CP, Gavioli EC, Spagnolo B, McDonald J, et al. Dmt-Tic-NH-CH2-Bid (UFP-502), a potent DOP receptor agonist: in vitro and in vivo studies. Peptides. 2006;27:3322–3330. doi: 10.1016/j.peptides.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson S, Law P, Barak L, Caron M. Agonist-specific regulation of delta-opioid receptor trafficking by G protein-coupled receptor kinase and beta-arrestin. J Recept Signal Transduct Res. 1999a;19:301–313. doi: 10.3109/10799899909036653. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xin SM, Wu GX, Zhang WB, Ma L, Pei G. Endogenous delta-opioid and ORL1 receptors couple to phosphorylation and activation of p38 MAPK in NG108-15 cells and this is regulated by protein kinase A and protein kinase C. J Neurochem. 1999b;73:1502–1509. doi: 10.1046/j.1471-4159.1999.0731502.x. [DOI] [PubMed] [Google Scholar]