Abstract
In regions with severe winters, global warming may be expected to cause earlier onset of breeding in most animals, yet no documentation of such a trend exists in North America. In a study of marked individuals of the Mexican jay (Aphelocoma ultramarina) in southeastern Arizona, from 1971 to 1998, the mean Julian date of first clutch in the population declined significantly by 10.1 days. The date of the first nest in the population also became earlier, by 10.8 days. These changes were associated with significant trends toward increased monthly minimum temperatures on the study area, traits that are associated with the onset of breeding in this population. Significant trends from 1971 to 1997 toward warmer minimum temperatures in the months before and during the initiation of breeding were observed. These trends parallel changes in minimum temperatures and community composition in a recent study of grassland ecology in the western United States. Together, they suggest that more attention should be given to the possible ecological importance of global change in minimum temperatures.
The global mean surface air temperature has risen 0.5°C in the 20th century (1). Global warming may be expected to affect plants and animals worldwide, especially in cooler parts of the world, but effects may vary regionally and with species. Evidence of such effects is still rare and circumstantial, but, using correlation evidence, ecologists have attempted to link to global climate change such effects as upward movement of alpine-nival floras (2), earlier breeding by amphibians (3), northward range changes in butterflies (4), increased photosynthesis (5), and changes in community composition (6, 7). In northern areas, global warming may be expected to cause trends toward earlier onset of breeding in most species of birds because birds in the south generally breed earlier than in the north. This regional difference is probably largely because the depressing effects of cold weather on food needed for reproduction attenuate earlier in the south. Analysis of a survey of nest records reported by amateurs in Europe revealed trends toward earlier breeding in a variety of avian species (8–10), but the hypothesis that global warming leads to earlier breeding by birds living in areas with cold winters needs testing in other regions of the world. Detailed examinations of well studied species are also needed. We report a trend toward early breeding in a New World animal, the Mexican jay (Aphelocoma ultramarina), and we consider the associated changes in relevant climate variables on the study area.
METHODS
We studied a natural population of individually recognizable, color-banded jays that occupied 7–9 territories centered at the Southwestern Research Station in the Chiricahua Mountains of Arizona, elevation 1,610–1,700 m. These territories were in pine-oak-juniper woodland, termed Madrean evergreen woodland (11, 12). Territory locations varied little or not at all from year to year. Descriptions of the vegetation of the study area, methods of trapping and banding jays, and the demography of the population are available elsewhere (13, 14). We did not monitor all potential influences on laying date, such as food, competing species, predators, or diseases. Although the Chiricahua Mountains are grazed and have been subject to fire control, they are relatively undisturbed by man, as shown by a healthy population of large mammalian predators and their prey. There were no fires or obvious changes in the vegetation on the study area during the period of observation or directly preceding it.
Weather data were recorded on the study area by employees of the Southwestern Research Station. We obtained these data from the monthly station summaries for Portal, AZ, published in Climatological Data, Arizona by the U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Environmental Data Service. Most of these data are available at the following website: http://www.wrcc.dri.edu/cgi-bin/cliMAIN.pl?azport.
Our analysis is based on 458 first clutches of color-banded females, 1971–1998; we ignore later clutches of the same individuals in the same year. The number of breeding females observed each year varied from 6 to 8 in 1971–1975 and from 11 to 27 from 1976–1998. We used three variables to test for trends in dates of first clutches. First, we examined first-clutch dates of all recognizable individuals in the population graphically. We then tested for trends by using the yearly means and medians of these distributions. We also used the earliest date among the members of the population (EARLIEST). EARLIEST was normally distributed without transformation (Shapiro-Wilk W test: W = 0.9550, P < 0.3672), as were the MEAN (Shapiro-Wilk W test: W = 9463, P < 0.2910) and MEDIAN (Shapiro-Wilk W test: W = 0.9683, P < 0.5770). We used linear regression to test for trends in EARLIEST and in climate variables. For examination of relationships of laying dates to climate variables, we used the mean laying date of individual first clutches each year [MEAN, as in Brown and Li (15)]. We examined the following climate variables: COLDEST, minimum temperature of preceding winter (December, January, February); MONSOON, summed precipitation in monsoon months, July and August, of previous year; LOMARCH, minimum temperature in March of same year; LOAPRIL, minimum temperature of April of same year. Because our climate variables were not normally distributed, being platykurtic, we used nonparametric statistics (Spearman rank correlations) to test for association between climate variables and year.
RESULTS
Trends in Laying Dates.
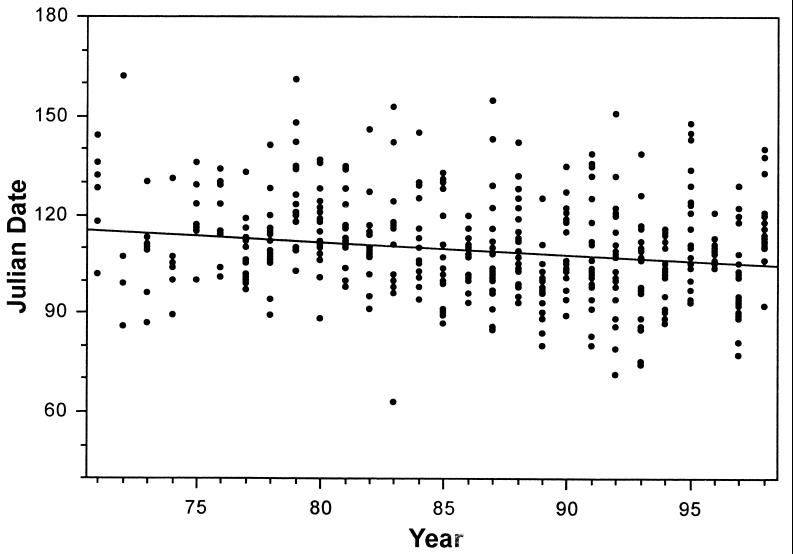
We observed a linear trend toward earlier laying of first clutches by known individuals from 1971 to 1998 (Fig. 1). Significant trends toward earlier laying were evident in both of our measures of central tendency of dates of first laying. The MEAN date of laying of individual first clutches became earlier by an average of 10.1 days (1971–1998, 28 years, MEAN = 750.1286–0.375802 YEAR, t = 2.068, P = 0.0487). Similar significant results were obtained by using the median of the distribution. A significant negative relationship with year also was observed for population first nests, with an advance of 10.8 days (1971–1998, n = 28, EARLIEST = 1007.5025–0.400617 YEAR, t = 2.229 P = 0.0346).
Figure 1.
Temporal changes in date of laying of first clutch of the spring by individually recognized female Mexican jays in the Chiricahua Mountains, AZ. Each point represents the first laying date of the year for one female (date = 141.7663 − 0.03712 year).
Climatic Correlates of Laying Dates.
To connect our observations of trends in laying dates to climate variables, we used an analysis of the climatic correlates of laying dates (15) and supplemented it with additional analyses. In our previous multiple regression analysis of the annual mean date of laying of first clutches, the only two significant correlates among 11 climate variables examined were the amount of rain in the previous monsoon (July–August, MONSOON) and the coldest temperature of the preceding winter (COLDEST). In that analysis, we also examined precipitation in four other periods (September–February, March–April, July–February, July–June), minimum temperatures in two other periods (March–April, September–November), maximum temperature in July–August, and two warmth sum variables (February–March, February–April). With one more year of data, we repeated this analysis of mean laying dates with similar results.
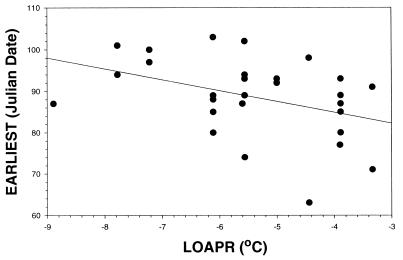
We supplement our previous report here with an analysis of the relationships of EARLIEST to climatic variables. In linear regression analyses, EARLIEST was not significantly related to the coldest temperature of the preceding winter, but EARLIEST was significantly related to minimum temperatures in the two months in which females began laying, namely March (1971–1997, n = 27, β= −0.407637, t = −2.232, P = 0.0348) and April (Fig. 2; β = −0.400358, t = −2.228, P = 0.0348). The amount of variance in EARLIEST explained by climatic variables (adjusted r2) increased moderately when LOMAR and LOAPR were used together (from r2 = 0.11634–0.19975) and again when MONSOON was added (r2 = 0.25096, F = 3.90370, P = 0.0217). MONSOON was not significant by itself (t = 1.064, P = 0.2973), but, when entered with LOMAR, it was close (t = 1.793, P = 0.0857). Thus, EARLIEST was not associated significantly with the specific climate variables that were associated with MEAN, but the two measures of the onset of breeding (the first clutches of all individuals and the first clutch of the first individual) were both associated with minimum temperatures in the months preceding breeding and at least weakly with precipitation in the previous monsoon.
Figure 2.
First clutches in the population (EARLIEST) are earlier when minimum temperatures in April are warmer (β= −0.400358, t = −2.228, P = 0.0348).
Trends in Annual Climate Indicators.
Because laying dates were related to both minimum temperatures and precipitation, we began our analysis of possible long-term climate trends by examining monthly precipitation and monthly minimum and maximum temperatures averaged over 12 months (prior moving average), but we did not test the significance of these trends because of nonindependence of the data points. Smoothed monthly precipitation and smoothed monthly minimum temperatures (lowest value of the month) showed gradual increases over the years. The average of daily minima for each month also showed a positive relationship with year (data not shown). In contrast, smoothed maximum temperatures of each month exhibited a lowering over the years, as did the monthly averages of daily maxima (data not shown).
Trends in Monthly Climate Variables.
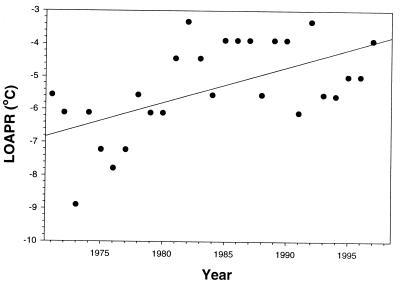
Because long-term trends in temperatures and precipitation were evident from the above analyses of all months together, we looked more specifically for trends in the particular precipitation variables already identified as being associated with laying dates. We also tested for warming trends in the minimum temperatures of the coldest winter months and the months preceding and during egg laying by using Spearman rank correlation. Significant positive relationships with year were observed for monthly minima in February (ρ = 0.5366, n = 29, P = 0.003), April (ρ = 0.5283, n = 29, P = 0.000), and May (ρ = 0.6516, n = 29, P = 0.000) but not for other months, although minima in January and March were weakly positively associated. Data for April, the month of most frequent first clutches, are shown in Fig. 3.
Figure 3.
Monthly minimum temperatures (Celsius) for April on the study area increased with year. (Linear regression: adjusted r2 = 0.35358, F = 15.76824, P = 0.0005, mintemp = −220.976355 + 0.108675 year)
Monthly values of precipitation were all weakly positively correlated with year except for July and October, which were negatively correlated, but none of these were significant (Spearman rank correlation, 27 or 28 years, depending on the month, P > 0.05). Neither the monsoon rains nor winter precipitation showed significant correlation with year. Precipitation in late winter (LWINTER = January and February) showed weak but positive association with year (ρ = 0.1904, n = 26, P = 0.351).
DISCUSSION
We have shown a significant trend toward earlier laying of first clutches of known individuals in the Mexican Jay graphically (Fig. 1) and have tested the trend by using the yearly means and medians. We also analyzed the date of the earliest single clutch in the population each year (rather than the mean or median) because this approach was used in previous papers on the onset of breeding by others (3, 8). A significant trend to earlier laying again was observed. We consider these observations in relation to climate change on our study area and then consider some alternative hypotheses.
The Climate Change Hypothesis.
The rise in global mean surface air temperature during the 20th century (1) has resulted in part from the daily minimum temperature increasing at a faster rate than the daily maximum, resulting in a decrease in the diurnal temperature range (16). The observed pattern of change in temperature varies in different regions of the earth. Easterling et al. (16) reported that maximum temperatures increased over most areas, with exceptions that include the southern United States. Minimum temperatures, however, increased almost everywhere, including southern United States, resulting in a decreased diurnal temperature range in southern United States, as observed on our study area and in Colorado (7). Thus, observations of climate change on our study area (Fig. 3 and above) agree well with the regional pattern as described in the study of global warming by Easterling et al. (16).
In desert Arizona, El Niño is associated with winter rains (October–April), whose increase has been suggested to have changed the composition of a desert community that is within 20 km of our very different woodland community (6, 17). In contrast to the desert study, we did not find a significant increase in precipitation on our study area in any specific months, though the trends in most months were positive. Several reasons for this discrepancy may be suggested. First, we considered only our study area whereas the analysis of the desert community used data from a collection of five localities ranging from Tucson in the west to Jornada, NM in the east (not actually including their study area). Second, our study area was at a higher elevation and was located in pine-oak juniper woodland, in contrast to the low-elevation desert community. Third, we considered only the years 1971–1998 whereas the desert-community study compared precipitation to a mean based on data as far back as 1867 at one station, thus using larger sample sizes and different time scales.
Taking a more global view, our results on a single, intensively studied avian species in North America complement those from a European national nest-records scheme (8, 9) and work on British titmice (10) by providing data from a more natural environment in a completely different climate and geographical region. Additional assurance in our study comes from recognition of individuals on a specific study area.
Our two indicators of laying date, the mean of individuals and the first individual, gave somewhat different though related results. The mean implicated previous monsoon rains and the coldest temperature of the preceding winter. The timing of laying of the first individual was not as closely associated with the monsoon rains as was the mean, but the first individual was more strongly related to minimum temperatures in the months of initiation of laying. Our analysis of yearly trends on a monthly basis provided little evidence of a trend toward more precipitation, but it strongly indicated a trend toward increasing minimum temperatures in the months immediately preceding laying. The coupling of a trend toward warmer minimum temperatures and the sensitivity of the initiation of laying to minimum temperatures is circumstantial evidence for a relationship between these variables.
Physiological and Ecological Mechanisms.
Because the onset of breeding in some northern hemisphere birds is well known to be cued by photoperiod and because the photoperiod has presumably not changed during the years of our observations, either Mexican Jays have some flexibility with regard to photoperiod or their sensitivity to photoperiod has changed. The pioneer experiments of Rowan showed that another corvid species, the American crow (Corvus brachyrhynchos), is photoresponsive (18). However, a closer relative of the Mexican jay, the pinyon jay (Gymnorhinus cyanocephalus, 19), is known to breed in response to local food abundance rather than being strictly limited by photoperiod (20, 21). Therefore, some flexibility in this respect is plausible for the Mexican jay. In the Mexican jay, the blood levels of reproductive hormones, such as testosterone (22) and prolactin (23), are known to peak at about the time of egg laying, but the physiological, evolutionary, and ecological causation of these patterns have not been determined. Considerable research on the onset of laying has, however, been done on other corvids. In the rook (Corvus frugilegus), breeding appears to be timed so that young are reared when their principal food, earthworms, is abundant (24); the number of rooks in the population is statistically related to the abundance of earthworms in the preceding breeding season (25). Onset of laying in the black-billed magpie (Pica pica), like the Mexican jay, is correlated with temperatures in early spring (26).
In the European great tit (Parus major), mean laying date was strongly correlated with the appearance of the caterpillars of the winter moth (Operophtera brumata) (27, 28). Thus, breeding is timed so as to have young in the nest when the principal food of the nestlings is at its peak (29). Several European studies have found that warm temperatures early in spring are associated with earlier laying in resident titmice (28–34). The positive results of experimental provision of supplemental food early in the spring reinforce the view that food conditions influence laying date (reviewed in refs. 35 and 36).
A second mechanism by which minimum temperatures in early spring could influence laying dates is through their effects on the energy budgets of females that are about to produce eggs. Experimental insulation of nest boxes caused great tits to lay earlier in the spring than controls (37), suggesting that cold temperatures at night critically affect laying dates. Passerine birds commonly lose nearly 10% of their body mass over cold nights (38–40), and we have confirmed this for free-ranging Mexican jays using electronic, self-weighing scales (J.L.B., unpublished work). Thus, two plausible environmental mechanisms exist for a trend to warmer minimum temperatures in early spring to cause earlier breeding in birds, namely by leading to earlier emergence of insect food for the female and her nestlings and by energy savings for females on cold nights.
Another possibility is that natural selection has favored earlier breeding genetically. We have no evidence for this hypothesis, but genetic adaptation has been suggested to explain a trend in recent years toward decreased body mass in a nearby population of woodrats (Neotoma sp.) (41).
Other Hypotheses.
We cannot “prove” with correlation data that climate changes caused the observed changes in laying dates, nor can we reject this hypothesis; but other hypotheses are not convincing, and suitable controlled experiments with proper replication are impossible. A full exploration of other hypotheses at present seems premature in the absence of support for them. However, the following information is relevant to some of them. Our observations were made systematically on a small, year-round resident population at a single elevation. We had direct knowledge of the individual female birds involved, and demographic details of the population are available (13, 14, 42). The population density fluctuated around a stable mean (J.L.B., unpublished data). The impact of man on the study area during the study appeared to be slight and stable. Grazing occurs on part of the study area, but its intensity has changed little during the period of study. There is no human habitation in seven of our nine flock territories. On the other two, there has been little change since the start of the study. Finally, our results with respect to the importance of minimum temperatures are in good agreement with a recent study of climatic correlates of changes in community composition in Colorado (7).
As discussed by Crick et al. (8), the possibility exists that their results are attributable to changes in the behavior of the amateur observers in U.K. Perhaps amateur observers are influenced themselves by the temperature, leading them to discover nests earlier. There also may have been changes in effort and effectiveness of observers because of recently increased interest in birdwatching in UK. Crick et al. (8) presented plausible arguments against these interpretations, but they need to be considered in other studies too. Our procedures did not allow for such possibilities because our schedule of field work was geared to the birds, not to the temperatures, and our observations did not depend on amateur bird watchers who go out when they feel like it.
North American Birds.
We have reported data for only one species, but it may be useful to consider the possibility that other animals may be breeding earlier or later in response to climate changes in North America. We caution that not all species are expected to breed earlier. In the southern United States, only species that are sensitive mainly to minimum temperatures are expected to breed earlier whereas species that are sensitive to maximum temperatures would actually be expected to breed later, according to the global weather patterns summarized regionally (16). In northern states and Canada, earlier laying should be more apparent, according to regional maps of temperature change (16) and photosynthesis (5).
Global Perspective.
The ecological details of laying dates and climate change in Arizona differ from those in Europe, but recognition of similar trends on both continents in very different environments is consistent with the interpretation that some avian populations are already responding to climate changes in the last 29 years or so. The causes of these regional climate changes, especially the possible contribution of greenhouse gases, and the possibility of changes on other time scales before our study began are beyond the scope of this paper. Finally, if natural communities are already responding to a global warming of 0.5°C, what can we expect from the rise of ≈2.8°C by the middle of next century that is simulated by climate models (43)? If the breeding season of one species is changing, perhaps other North American animals also are changing their breeding seasons. Some species may benefit from such changes whereas others may be harmed. More information is needed to assess these possibilities.
Acknowledgments
Our long-term study would have been impossible without the cooperation of the Southwestern Research Station of the American Museum of Natural History. The help of J. Rozen, W. Sherbrooke, E. Sherbrooke, R. Morse, P. Limberger, C. Swartz, and other employees of these institutions was invaluable. For permission to work on neighboring properties we thank O. Bruhlman, the M. Cazier family, R. Mendez, and officials of the Coronado National Forest. We are extremely grateful to the dedicated field workers who have made many of the observations reported here. These range from undergraduates to post-docs. In chronological order they include L. G. Herlin, D. Slaymaker, H. Alvarez, E. Wilkins, K. Zemanek, A. Lewis, S. Corbett, P. Trail, D. Smith, S. Strahl, G. Lipford, C. Barkan, L. Barkan, J. Craig, A. Stewart, R. K. Johnson, L. Elliot, D. Siemens, E. Horvath, S. Stoleson, J. Harding, P. Kleeman, T. Kennedy, J. Adams, J. Kaiser, P. Jablonski, L. Herbeck, K. Short, J. McCormack, and E. Brown. For advice on statistical analyses, we thank C. Bingham, J. Sedransk, and J. Chen. We thank E. Brown, G. Robinson, W.-C. Wang, and the reviewers for comments on the manuscript. We are grateful to the U.S. National Institute of Mental Health and the U.S. National Science Foundation for nearly continuous financial support during this project. We thank the State University of New York for support in the 1998 field season.
References
- 1.Houghton J T, Meria Filho L G, Callender B, Harris N, editors. The Science of Climate Change. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 2.Grabherr G, Gottfried M, Pauli H. Nature (London) 1994;369:448. doi: 10.1038/369448a0. [DOI] [PubMed] [Google Scholar]
- 3.Beebee T J C. Nature (London) 1995;374:219–220. [Google Scholar]
- 4.Parmesan C. Nature (London) 1996;382:765–766. [Google Scholar]
- 5.Myneni R B, Keeling C D, Tucker C J, Asrar G, Nemani R R. Nature (London) 1997;386:698–702. [Google Scholar]
- 6.Brown J H, Valone T J, Curtin C G. Proc Natl Acad Sci USA. 1997;94:9729–9733. doi: 10.1073/pnas.94.18.9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alward R D, Detling J K, Milchunas D G. Science. 1999;283:229–231. doi: 10.1126/science.283.5399.229. [DOI] [PubMed] [Google Scholar]
- 8.Crick H P Q, Dudley C, Glue D E, Thomson D L. Nature (London) 1997;388:526. [Google Scholar]
- 9.Forchhammer M C, Post E, Stenseth N C. Nature (London) 1998;391:29–30. [Google Scholar]
- 10.McCleery R H, Perrins C M. Nature (London) 1998;391:30–31. [Google Scholar]
- 11.Brown D E. In: Biotic Communities of the American Southwest-United States and Mexico (Special issue of Desert Plants) Brown D E, editor. Vol. 4. Superior, Arizona: Boyce Thompson Southwest Arboretum; 1982. pp. 59–65. [Google Scholar]
- 12.Brown D E, Reichenbacher F, Franson S E. A Classification of North American Biotic Communities. Salt Lake City: Univ. of Utah Press; 1998. [Google Scholar]
- 13.Brown J L. In: The Birds of North America. Poole A, Stettenheim P, Gill F, editors. Philadelphia: Academy of Natural Sciences of Philadelphia; 1994. pp. 1–16. [Google Scholar]
- 14.Brown J L, Brown E R. In: Cooperative Breeding in Birds: Long-Term Studies of Ecology and Behavior. Stacey P B, Koenig W D, editors. Cambridge, U.K.: Cambridge Univ. Press; 1990. pp. 268–288. [Google Scholar]
- 15.Brown J L, Li Shou-H. Condor. 1996;98:879–884. [Google Scholar]
- 16.Easterling D R, Horton B, Jones P D, Peterson T C, Karl T R, Parker D E, Salinger M J, Razuvayev V, Plummer N, Jamason P, et al. Science. 1997;277:364–367. [Google Scholar]
- 17.Valone T J, Brown J H. In: Long-Term Studies of Vertebrate Communities. Cody M L, Smallwood J A, editors. San Diego: Academic; 1996. pp. 555–583. [Google Scholar]
- 18.Rowan W. Proc Natl Acad Sci USA. 1932;18:639–654. doi: 10.1073/pnas.18.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Los Monteros A E, Cracraft J. Condor. 1997;99:490–502. [Google Scholar]
- 20.Ligon J D. Condor. 1971;73:147–153. [Google Scholar]
- 21.Marzluff J M, Balda R P. The Pinyon Jay: Behavioral Ecology of a Colonial and Cooperative Corvid. London: Poyser; 1992. [Google Scholar]
- 22.Vleck C, Brown J, Brown E, Adams J. VI International Symposium on Avian Endocrinology. Edmonton, Canada: Univ. of Alberta Press; 1996. (abstr.). [Google Scholar]
- 23.Brown J L, Vleck C M. Behav Ecol. 1998;9:541–545. [Google Scholar]
- 24.Murton R K, Westwood N J. Avian Breeding Cycles. Oxford: Clarendon; 1977. [Google Scholar]
- 25.Murton R K, Westwood N J. Avian Breeding Cycles. Oxford: Clarendon; 1977. [Google Scholar]
- 26.Birkhead T. The Magpies. London: Poyser; 1991. [Google Scholar]
- 27.Perrins C M. J Anim Ecol. 1965;34:601–647. [Google Scholar]
- 28.Perrins C M, McCleery R H. Wilson Bull. 1989;101:236–253. [Google Scholar]
- 29.Perrins C M. Ibis. 1995;133, Suppl.1:49–54. [Google Scholar]
- 30.Kluijver H N. Ardea. 1951;39:1–135. [Google Scholar]
- 31.Kluyver H N. Ardea. 1952;40:123–141. [Google Scholar]
- 32.Lack D. Ardea. 1958;46:93–123. [Google Scholar]
- 33.Perrins C M. Ibis. 1970;112:242–255. [Google Scholar]
- 34.VanBalen J H. Ardea. 1973;61:1–93. [Google Scholar]
- 35.Martin T E. Annu Rev Ecol Syst. 1987;18:453–487. [Google Scholar]
- 36.Nilsson J, Svensson E. Ecology. 1993;74:244–251. [Google Scholar]
- 37.O’Connor R J. Ibis. 1978;120:534–537. [Google Scholar]
- 38.Ekman J B, Hake M K. Behav Ecol. 1990;1:62–67. [Google Scholar]
- 39.Ekman J B, Lilliendahl K. Behav Ecol. 1993;4:232–238. [Google Scholar]
- 40.Lilliendahl K E, Carlson A, Welander J, Ekman J B. Can J Zool. 1996;74:1612–1616. [Google Scholar]
- 41.Smith F A, Browning H, Shepherd U L. Ecography. 1998;21:140–148. [Google Scholar]
- 42.Brown J L, Brown E R, Sedransk J, Ritter S. Auk. 1997;114:279–286. [Google Scholar]
- 43.Wang W-C, Dudek M P, Liang X-Z. In: Future Climates of the World. Henderson-Sellers A, editor. New York: Elsevier; 1995. pp. 317–346. [Google Scholar]