Abstract
Background and purpose:
We have investigated the mechanisms underlying the paradoxical ability of the antispasmodic, alverine, to enhance spontaneous activity in smooth muscles while suppressing evoked activity.
Experimental approach:
The effects of alverine on spontaneous and induced contractile activity were examined in preliminary experiments with various smooth muscles. More detailed effects were also investigated by recording membrane potential, intracellular Ca2+ concentration ([Ca2+]i) and tension from single-bundle detrusor smooth muscle (DSM) of the guinea-pig urinary bladder.
Key results:
Alverine (10 μM) increased the frequency and amplitude of spontaneous action potentials, transient increases in [Ca2+]i and associated contractions. Alverine also decreased action potential rate of decay, suggesting inhibition of L-type Ca channel inactivation. Charybdotoxin (50 nM) but neither cyclopiazonic acid (10 μM) nor Bay K 8644 (10 μM) attenuated alverine-induced enhancement of spontaneous contractions. Alverine suppressed contractions produced by high K (40 mM) or ACh (10 μM), without affecting electrical responses and with little suppression of increases in [Ca2+]i. This feature was very similar to that of the effects of the Rho kinase inhibitor Y-27632 (10 μM).
Conclusions and implications:
Alverine may increase Ca influx during action potentials due to inhibition of the inactivation of L-type Ca channels, but may also suppress evoked activity by inhibiting the sensitivity of contractile proteins to Ca2+. The proportional contribution of Ca-dependent and Ca-independent contractions in DSM may differ between spontaneous and evoked activity, necessitating further investigations into the interactions between these pathways for assessing the therapeutic potential of alverine to treat DSM dysfunction.
Keywords: alverine, smooth muscle, urinary bladder, L-type Ca channel, Ca sensitivity
Introduction
Alverine citrate (Spasmonal, Norgine, Harefield, Middlesex, UK) is thought of as a smooth muscle relaxant, and is currently used as an antispasmodic in irritable bowel syndrome and dysmenorrhoea. Alverine was shown to inhibit spontaneous electrical activity and nervous control of the proximal colon of the rabbit in vivo (Bouvier et al., 1992). It was also found to decrease the sensitivity of the intestinal mechanoreceptors in response to chemical stimulation in anaesthetized cats (Abysique et al., 1999), and reduce 5-hydroxytryptamine1A receptor-mediated rectal hypersensitivity in the rat (Coelho et al., 2001). However, the exact mechanisms of alverine's inhibitory action are still not clear, due to the lack of information of its effects on isolated smooth muscle in vitro.
Because of the Oxford group's interest in smooth muscles, in 1993 we were asked by Norgine, the manufacturers of alverine, to investigate the basic effects of alverine on smooth muscles, and since then several undergraduates and clinicians undertaking research projects with the group have studied the effects of the drug on contractile activity. The majority of this preliminary work was on smooth muscle dissected from the urinary bladder, but the pattern of effects was similar in all the smooth muscles we have examined. We found that alverine has paradoxical effects on contractile activity in smooth muscles, increasing the amplitude and frequency of spontaneous contractions, but simultaneously suppressing the amplitude of contractions evoked by applications of carbachol, high K solution or stimulation of the intrinsic nerves.
In the current paper we investigated this surprising finding in more detail in the detrusor smooth muscle (DSM) of guinea-pig bladder, since our present understanding of mechanisms underlying contractions in this smooth muscle suggests that spontaneous and depolarization contractions recruit the same mechanism, namely Ca influx thorough L-type Ca channels. Spontaneous contractions of DSM are initiated by Ca influx during individual action potentials, and are abolished by L-type Ca channel blockers (Hashitani and Brading, 2003). Similarly, high K-induced contractions of DSM may be initiated by the opening of L-type Ca channels subsequent to membrane depolarization, and are strongly attenuated by L-type Ca channel blockers (Maggi et al., 1988; Marti-Cabrera et al., 1994). This pathway should also contribute to a certain proportion of contractions induced by either muscarinic stimulation or neuromuscular transmission (Uchida et al., 1994; Hashitani et al., 2000).
We have examined whether alverine-induced enhancement of spontaneous contractions is associated with corresponding changes in the configuration of action potentials and spontaneous transient increases in [Ca2+]i (Ca transients). Next we have examined the correlation between Ca and contractile responses during high K- and acetylcholine (ACh)-induced excitation of DSM. The results indicate that in DSM of the guinea-pig urinary bladder, spontaneous contractions may recruit mainly Ca-dependent intracellular mechanisms, and evoked contractions may recruit mainly Ca-independent mechanisms.
Methods
The detailed procedures described have been approved by the animal experimentation ethics committee at the University of Oxford and Nagoya City University Medical School. Guinea pigs of either sex weighing 250–400 g were killed by a blow to the head followed by cervical dislocation. The urinary bladder was removed and its ventral wall was opened longitudinally from the bladder neck to the top of the dome. The mucosal layer, connective tissues and several smooth muscle layers were then removed leaving underlying single smooth muscle bundles attached to the serosal layer. A serosal sheet, which contained a single bundle of DSM, 2–3 mm long and 0.2–0.7 mm wide was then prepared, and thus experiments were carried out on the outer layer muscle bundles. In the preliminary experiments, strips of smooth muscle were also dissected from the taenia of the caecum, from the uterus and from the portal vein.
Intracellular recordings
Preparations were pinned out on a Sylgard plate (silicone elastomer, Dow Corning Corporation, Midland, MI, USA) at the bottom of the recording chamber (volume, approximately 1 ml), which was mounted on the stage of an inverted microscope. The preparations were superfused with warmed (36 °C) physiological salt solution (PSS) at a constant flow rate (2 ml min−1). Individual bladder smooth muscle cells in the muscle bundles were impaled with glass capillary microelectrodes, filled with 0.5 M KCl (tip resistance, 120–250 MΩ). Membrane potential changes were recorded using a high input impedance amplifier (Axoclamp-2B, Axon Instruments Inc., Foster City, CA, USA), and displayed on a cathode-ray oscilloscope (SS-5702, Iwatsu, Tokyo, Japan). After low-pass filtering (cutoff frequency, 1 kHz), membrane potential changes were digitized using either Digidata 1322 or Digidata 1200 interfaces (Axon Instruments Inc.) and stored on a personal computer for later analysis.
Isometric tension recordings
For the preliminary experiments, strips of approximately 10 × 1 × 1 mm were dissected from various smooth muscles following the direction of muscle bundles as viewed under a binocular microscope. Fine silk ligatures were tied to each end of the strips which were subsequently mounted in small (0.2 ml) organ baths (Brading and Sibley, 1983) between two platinum ring electrodes 1 cm apart and continuously superfused with PSS at 37 °C at a flow rate of approximately 1 ml min−1.
For the more detailed studies, an approximately 0.5–1 mm length from one end of the DSM preparation was pinned out on a Sylgard plate (silicone elastomer, Dow Corning Corporation) at the bottom of the recording chamber (volume, approximately 1 ml), which was mounted on a stage of an inverted microscope, and a thread was tied around the other end. The preparations were superfused with warmed (36 °C) PSS at a constant flow rate (2 ml min−1). The thread from the muscle strips was attached to an isometric force transducer, which was connected to a bridge amplifier (ADInstruments Ltd, Grove House, Hastings, UK). Isometric tension changes were digitized using Digidata 1200 interface (Axon Instruments Inc.) and stored on a personal computer for later analysis. A tension of approximately 1 mN was applied to preparations that were then left to equilibrate for 60–90 min until spontaneous phasic contractions, which were stable in both amplitude and frequency, were generated.
Intracellular calcium measurements
For measurements of changes in [Ca2+]i, DSM preparations were pinned out on the bottom of a recording chamber which was similar to that used for electrical and mechanical recordings. After 30 min incubation with warmed (36 °C) PSS, spontaneous contractions of the tissues were generated, and then the preparations were loaded with fluorescent dye, fura-PE3, by incubation in low Ca2+ physiological saline (Ca2+, 1 mM) containing 10 μM fura-PE3 AM for 1 h at room temperature. After being loaded, preparations were superfused with dye-free, warmed (36 °C) physiological saline at a constant flow (about 2 ml min−1) for 30 min. Preparations loaded with fura-PE3 were illuminated with ultraviolet light, wavelengths 340 and 380 nm, alternating at a frequency higher than 40 Hz. The ratio of the emission fluorescence (R340/380) in a desired size of rectangular window was measured through a barrier filter (peak transmission 510 nm; sampling time 100–250 ms), using a micro-photoluminescence measurement system (Aquacosmos, Hamamatsu Photonics, Hamamatsu, Shizuoka, Japan), and was taken as an index of [Ca2+]i.
Solutions
The composition of PSS was (in mM): Na+ 137.5, K+ 4.7, Ca2+ 2.5, Mg2+ 1.2, HCO3− 15.5, H2PO4− 1.2, Cl− 134 and glucose 15. The pH of PSS was 7.2 when bubbled with 95% O2 and 5% CO2 and 7.4 when bubbled with 97% O2 and 3% CO2. The measured pH of the recording bath was approximately 7.4.
Drugs used were fura-PE3 AM (Calbiochem-Novabiochem Ltd, San Diego, CA, USA), α,β-methylene ATP (Me-ATP), ACh chloride, atropine sulphate, Bay K 8644(1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)phenyl]-3-pyridinecarboxylic acid methyl ester), charybdotoxin (CTX), cyclopiazonic acid (CPA), nifedipine (Sigma, St Louis, MO, USA) and Y-26763 ((R)-(+)-trans-N-(4-Pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide, dihydrochloride; from Calbiochem). Drugs were dissolved in distilled water except nifedipine, which was dissolved in absolute ethanol and CPA, which was dissolved in dimethyl sulphoxide. The final concentration of these solvents in the physiological saline did not exceed 1:1000.
Calculations and statistics
Measured values are expressed as mean±s.d. Statistical significance was tested using paired t-test, and probabilities of less than 5% different from the control were considered significant. When drug effects were studied, the number of preparations refers to all the successful experiments carried out for each investigation.
The following action potentials parameters were measured: peak amplitude, measured as the value from the resting membrane potential to the action potential peak; maximum rate of rise (max dV/dtR), determined on the rising phase; half width, measured as the time between 50% peak amplitude on the rising and falling phases; and rate of decay (dV/dtD), measured as the slope between 20 and 80% of the peak amplitude of the events on the falling phase.
For isometric tension changes and Ca transients, the following parameters were measured: peak amplitude, measured as the value from the basal tension level to the peak of phasic contractions or Ca transients; frequency which was defined as an average of 5 min recordings.
Results
Preliminary studies
Effects of alverine on spontaneous activity
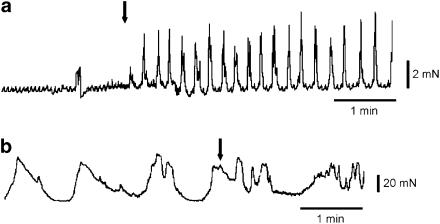
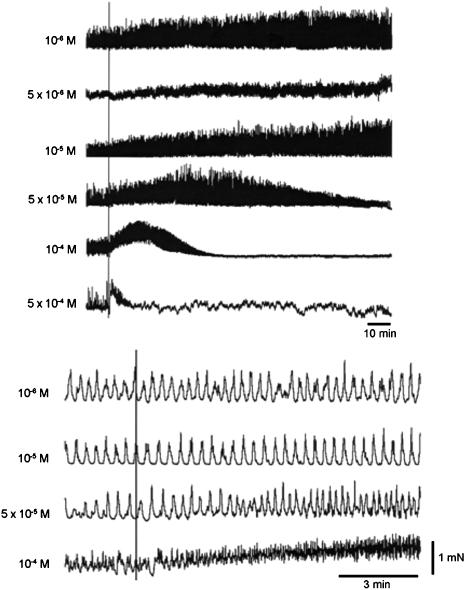
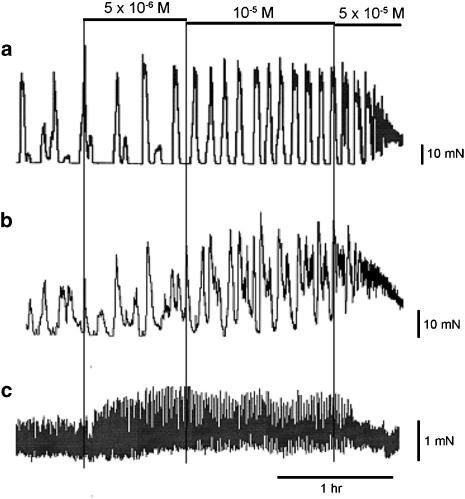
Because of its reputation as a spasmolytic, we were expecting alverine to inhibit spontaneous mechanical activity, and thus chose to work with tissues that often show such activity. We found, however, that alverine can induce spontaneous activity in some inactive preparations and enhance its frequency in those showing baseline activity. The effects are time and concentration dependent. Figure 1a shows induction of spontaneous mechanical activity in a quiescent bladder strip by exposure to 10 μM alverine. Figure 2 shows examples of spontaneous contractile activity in a set of strips dissected from the same bladder, after exposure to different concentrations of the drug. The lower figure is an expansion of the early part of the upper figure. Concentrations between 1 and 10 μM alverine increased the frequency and size of the spontaneous contractions, and the effects persisted for some hours. At higher concentrations the increased frequency resulted in a rise in the baseline tone of the strip and the effects become transient, with the activity suppressed in a concentration- and time-dependent manner. Similar effects were seen in the taenia of the caecum and the portal vein (Figure 3). Smooth muscles strips from the taenia or uterus can generate a pattern of very large but slow contractile events and in these cases, the effect of alverine was to break up the slow contractions into a pattern of more phasic and rapid contractile activity, usually reducing the size of the responses (Figure 1b).
Figure 1.
Effects of 10 μM alverine on spontaneous activity. (a) Alverine-induced spontaneous activity in a previously inactive strip of detrusor, (b) alverine increased phasic activity in a strip of taenia caecum previously showing regular slow contractions. The drug was applied in the superfusing solution at the arrow. Scale bars (a) ordinate 2 mN, abscissa 1 min, (b) ordinate 20 mN, abscissa 1 min.
Figure 2.
Effects of alverine on the spontaneous contractile activity of strips dissected from the guinea-pig detrusor. The drug was applied continuously at the line at the concentration indicated. The lower traces are the same as the upper traces but on a faster time scale.
Figure 3.
Effects of alverine on the spontaneous contractile activity of smooth muscle strips dissected from the taenia of the guinea-pig caecum (a and b) and the portal vein (c). Scale bars are 10 mN for (a) and (b), 1 mN for (c). The whole trace lasts for 3.6 h.
Effects of alverine on the response of guinea-pig bladder to excitatory stimuli
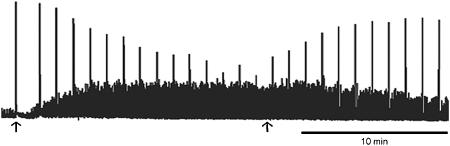
Spontaneous activity in the detrusor consists of contractions that are quite small in comparison to the maximum evoked contractions. Figure 4 demonstrates the paradoxical effect of alverine on these two types of contraction. Electrical field stimulation of the intrinsic nerves evoked large rapid contractions, which were superimposed on the spontaneous activity. Exposure to 10 μM alverine progressively increased spontaneous activity and suppressed evoked activity. Alverine was also able to suppress activity induced by K+ depolarization and activation of muscarinic receptors and P2X purinoceptors (not shown).
Figure 4.
Paradoxical effects of alverine on the guinea-pig detrusor. Electrical field stimulation was applied every 5 min (50 V, 20 Hz, 1 s, 0.05 ms pulse width). Alverine was added at the first arrow and washed out at the second arrow.
More detailed studies recorded from single-bundle DSM of the guinea pig
Effects of alverine on spontaneous contractions, action potentials and Ca transients
Detrusor smooth muscle generated either ‘single' contractions, which can be attributed to individual action potentials or ‘bursting' contractions, which result from bursts of action potentials (Hashitani et al., 2004). Since the amplitude of ‘bursting' contractions relies on the number of action potentials during each burst rather than the contractility of DSM associated with individual action potentials, we analysed spontaneous contractions focusing on ‘single' contractions where possible.
All single-bundle DSM preparations (n=18) generated spontaneous contractions. Alverine increased their frequency (10.8±4.4 min−1 in control, 15.3±5.4 min−1 in alverine, P<0.05) and amplitude (0.85±0.15 mN in control, 1.43±0.32 mN in alverine, P<0.05). This feature of alverine-induced enhancement of spontaneous contractions was similar to that studied using the larger, multi-bundle DSM preparations. To investigate the correlation between electrical and mechanical activity during the application of alverine, the membrane potential was recorded from single-bundle DSM preparations using an intracellular electrode either separately or simultaneously with mechanical activity.
All DSM preparations generated spontaneous action potentials. The resting membrane potential determined at the most stable negative potential between individual action potentials ranged between −41.3 and −49.5 mV (mean −44.7±3.9 mV, n=13).
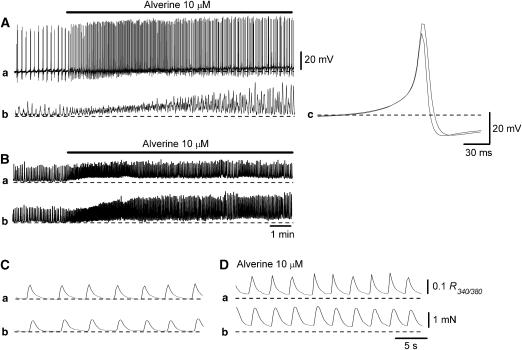
Alverine (10 μM) increased the frequency of spontaneous action potentials (11.2±4.3 min−1 in control, 18.7± 6.3 min−1 in alverine, n=13, P<0.05; Figure 5Aa) and associated contractions (Figure 5Ab). This was accompanied by a small depolarization (r.m.p.=−43.0±4.2 mV in alverine). Alverine also increased the amplitude of action potentials (49.8±3.8 mV in control, 53.5±4.6 mV in alverine, P<0.05), the half width (10.7±1.2 ms in control, 13.2±1.6 ms in alverine, P<0.05) and reduced dV/dtD (−12.5±1.8 mV ms−1 in control, −8.9±1.5 mV ms−1 in alverine, P<0.05; Figure 5Ac), but did not alter Max dV/dtR (5.3±0.9 mV ms−1 in control, 5.5±1.3 mV ms−1 in alverine, P>0.05).
Figure 5.
Effects of alverine on spontaneous action potentials, contractions and Ca transients recorded from DSMs of the guinea-pig bladder. Alverine (10 μM) facilitated spontaneous action potentials (Aa) and corresponding contractions (Ab) in a single-bundle DSM. It also increased the amplitude and reduced the rate of decay (Ac). Overlaid action potentials (solid line in control, dotted line in alverine) are averaged traces of 30 action potentials each. In another DSM preparation, alverine (10 μM) enhanced spontaneous Ca transients (Ba) and corresponding contractions (Bb). It also increased the basal Ca level (Ba) and caused a sustained contraction (Bb). With a fast time scale (C and D), alverine (10 μM) increased the amplitude of individual Ca transients (Ca) and contractions (Cb). Scale bars on the right in (Da) and (Db) refer to all Ca and tension traces, respectively.
In preparations that had been treated with nifedipine (10 μM), a blocker for L-type Ca channel, the generation of spontaneous contractions was abolished (n=4). Subsequent applications of alverine (10 μM) failed to cause any detectable contractions. Conversely, in preparations that had been treated with alverine (10 μM), subsequent application of nifedipine (10 μM) abolished spontaneous contractions (n=4), indicating that alverine-induced enhancement of spontaneous contractions resulted from increased Ca influx through L-type Ca channels.
In a separate series of experiments, the correlation between Ca and mechanical responses during the application of alverine (10 μM) was also examined. Alverine (10 μM) increased the frequency of spontaneous Ca transients (11.3±3.6 min−1 in control, 18.1±3.3 min−1 in alverine, n=8, P<0.05; Figure 5Ba) and contractions (Figure 5Bb). Alverine also increased the amplitude of Ca transients (0.13±0.04 R340/380 in control, 0.16±0.04 R340/380 in alverine, n=8, P<0.05; Figures 5C and D), and increased basal Ca level by about 0.025 R340/380. These results indicate that the excitatory action of alverine on spontaneous contractions is associated with increases in Ca transients, which may be causally related to the altered configuration of spontaneous action potentials.
Effects of pharmacological manipulation of L-type Ca channels and intracellular Ca stores on alverine-induced reinforcements of spontaneous contractions
Since alverine reduced the rate of decay of action potentials and prolonged their half width, it is likely to inhibit the inactivation of L-type Ca channels and increase the Ca influx during individual action potentials. The inactivation of L-type Ca channels has been shown to result from both Ca-dependent inhibition of L-type Ca channels (Nakayama and Brading, 1993) and Ca store-dependent negative feedback regulation through the activation of BK channels (Herrera et al., 2000; Herrera and Nelson, 2002; Hashitani and Brading, 2003). Therefore, we investigated the effects of CTX, a blocker for BK channels, CPA, a sarco-endoplasmic reticulum Ca ATPase inhibitor, and Bay K 4688, an L-type Ca channel agonist, on the excitatory action of alverine.
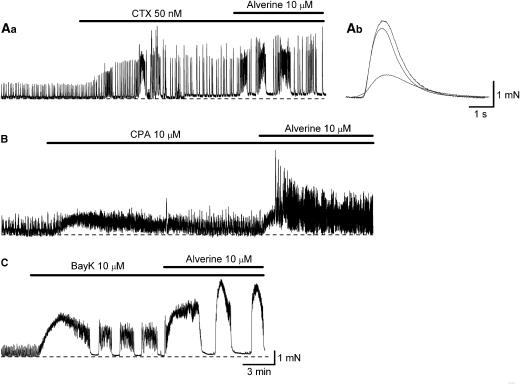
Charybdotoxin (50 nM) increased the frequency and amplitude of spontaneous contractions, and often converted ‘single' contractions into ‘bursting' contractions. Since the magnitude of bursting contractions reflects the number of ‘single' contractions rather than the amplitude of individual ‘single' contractions, we have used only ‘single' contractions for analysis. ‘Single' contractions could be readily identified by their smooth rising and falling phase, and their simple exponential decay. In preparations that had been treated with CTX for 20 min, the amplitude of spontaneous contractions was about three times larger than that in control conditions (Figure 6a). Subsequent application of alverine (10 μM) increased the amplitude of spontaneous contractions by about 20% (116.3±6.9% of in CTX alone, n=7; Figure 6a). However, alverine often increased the amplitude of bursting contractions by about 50–100% presumably by increasing the number of ‘single' contractions during each burst.
Figure 6.
Effects of cyclopiazonic acid (CPA), charybdotoxin (CTX) and Bay K 8644 on alverine-induced facilitations of spontaneous contractions recorded from DSMs of the guinea-pig bladder. In a single-bundle DSM, CTX (50 nM) caused a threefold increase in the amplitude of spontaneous contractions (A). Subsequent addition of alverine (10 μM) increased the amplitude of ‘single' contractions by only 20%. The overlaid traces (Ab) showed averaged ‘single' contractions in control (dash), in CTX (solid) and in CTX plus alverine (dotted) preparations. CPA (10 μM) increased the frequency and amplitude of spontaneous contractions (B). Subsequent application of alverine (10 μM) increased the amplitude of spontaneous contractions by about 100%. Bay K (10 μM) initiated bursting contractions (C), and subsequent application of alverine (10 μM) caused a further twofold increase in their amplitude. Scale bar in (C) also refers to (Aa) and (B).
Cyclopiazonic acid (10 μM) had complex effects on spontaneous contractions in single DSM bundles. In three preparations, CPA converted individual contractions into bursting contractions and thus apparently caused 2- to 3-fold increases in their amplitude. Subsequent application of alverine (10 μM) caused a further 2- to 3-fold increase in their amplitude. In the remaining four preparations, CPA increased the frequency of spontaneous contractions but did not increase their amplitude (Figure 6b). In preparations that had been treated with CPA for 30 min, subsequent application of alverine (10 μM) increased the amplitude of contractions by about 60% (162.5±36.7% of in CPA alone, n=4; Figure 6b). In addition, alverine often prolonged and enlarged bursting contractions and increased their amplitude by about 50–200%.
Bay K 4688 (1 μM) invariably converted individual contractions into bursting contractions, thus we were unable to analyse ‘single' contractions (Figure 6c). Subsequent application of alverine (10 μM) further increased the amplitude of bursting contractions by about 50–200% (Figure 6c).
Since the initial effects of alverine on spontaneous activity were marked, the release of excitatory transmitters may be involved in its action, although alverine suppressed evoked activity induced by bath-applied ACh. Hence, we examined the effects of desensitization of P2X purinoceptors with Me-ATP (10 μM) and atropine (1 μM) on the excitatory effects on spontaneous contractions induce by alverine.
In preparations that had been treated with atropine and Me-ATP for about 20 min, a time sufficient to block muscarinic receptors and to desensitize P2X purinoceptors, alverine still increased the frequency of spontaneous contractions and also increased their amplitude.
Effects of alverine on high K- and ACh-induced activity
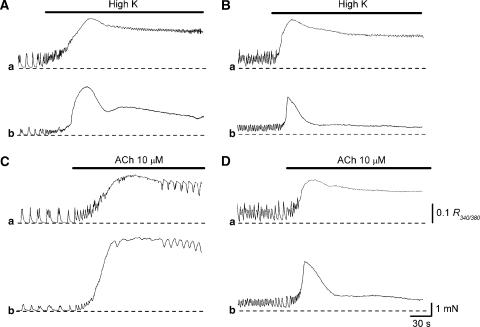
In DSM preparations, increasing [K+]o from 5.9 to 40 mM (high K solution) caused an increase in [Ca2+]i, which was composed of an initial phasic component (0.26±0.08 R340/380, n=8) and a following sustained component (0.19±0.065 R340/380; Figure 7Aa). This increase in [Ca2+]i was associated with a biphasic contraction (Figure 7Ab). In preparations that had been treated with alverine (10 μM), high K solution evoked an increase in [Ca2+]i (initial 0.24±0.07 R340/380, sustained 0.17±0.057 R340/380, n=8), which was similar to that in control conditions (Figure 7Ba). However, high K-induced contractions were much smaller than those in control conditions (Figure 7Bb). Thus, the amplitude of the initial component of high K-induced contractions was about 60% of the control values (6.9±1.5 mN in control, 4.1±1.3 mN in alverine), and that of sustained component was only about 25% of the control values (3.3±1.1 mN in control, 0.72±0.35 mN in alverine). These results are summarized in Table 1a.
Figure 7.
Effects of alverine on changes in Ca and contractile responses induced by high K solution and acetylcholine (Ach) recorded from DSMs of the guinea-pig bladder. In a DSM bundle, increasing [K+]o caused a phasic increase in [Ca2+]i, which was followed by a sustained increase in [Ca2+]i (Aa) and an associated contraction (Ab). In the same preparation that had been treated with alverine (10 μM), a subsequent increase in [K+]o caused a similar increase in [Ca2+]i (Ba) but caused a much smaller contraction (Bb). In another DSM, ACh (10 μM) caused a biphasic increase in [Ca2+]i (Ca), and an associated contraction (Cb). In the same preparation, alverine (10 μM) suppressed ACh-induced contractions (Db) but had little effect on the ACh-induced increase in [Ca2+]i (Da). Scale bars in (Da) and (Db) refer to all Ca and tension traces, respectively.
Table 1.
Comparison between the effects of alverine and Y-27632 on evoked contractions and changes in [Ca2+]i
|
(a) Effects of alverine (10 μM>on [Ca2+]i and contractile responses produced by high K+-containing solution and ACh | ||||
|---|---|---|---|---|
| Tension (initial) (%) | Calcium (initial) (%) | Tension (sustained) (%) | Calcium (sustained) (%) | |
| High K+ solution ([K+]o=40 mM) | 59.5±13.7 | 94.0±7.3 | 24.6±14.8 | 90.1±9.1 |
| ACh (10 μM) | 50.8±17.5 | 93.3±4.2 | 25.0±16.9 | 90.4±4.3 |
|
(b) Effects of Y-27632 (10 μM) on [Ca2+]i and contractile responses produced by high K+-containing solution and ACh | ||||
| High K+ solution (K+]o=40 mM) | 73.6±11.3 | 94.1±5.2 | 14.4±9.8 | 91.6±6.3 |
| ACh (10 μM) | 83.9±8.9 | 97.1±7.5 | 36.3±5.8 | 91.3±4.9 |
Abbreviation: Ach, acetylcholine; initial, initial component; sustained, sustained components.
All values=(peak amplitude in drugs)/(peak amplitude in control).
Acetylcholine (10 μM) caused an increase in [Ca2+]i, which was composed of an initial component (0.29±0.072 R340/380, n=8) and a sustained component (0.21±0.065 R340/380; Figure 7Ca). This increase in [Ca2+]i was associated with a biphasic contraction (Figure 7Cb). In preparations that had been treated with alverine (10 μM), ACh evoked an increase in [Ca2+]i (initial 0.27±0.064 R340/380, sustained 0.19±0.06 R340/380, n=8), which was similar to that in control conditions (Figure 7Da), but induced much smaller contractions (Figure 7Db). Thus, the amplitude of the initial component of ACh-induced contractions was about 50% of the control values (8.1±2.2 mN in control, 3.9±0.9 mN in alverine), and that of sustained component was only about 25% of the control values (5.6±2.1 mN in control, 1.3±0.9 mN in alverine). These results are also summarized in Table 1a.
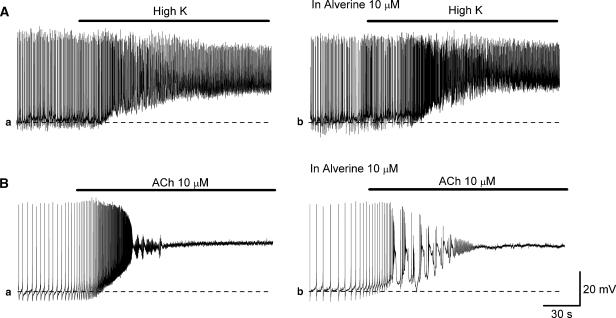
In a separate series of experiments, the effects of alverine on high K- or ACh-induced depolarizations were examined. High K solution depolarized the membrane from −44.4±2.4 to −23.5±1.8 mV (20.9±2.3 mV, n=5) and increased the frequency of spontaneous action potentials (Figure 8Aa). In preparations that had been treated with alverine (10 μM), high K solution depolarized the membrane from −43.0±1.4 to −22.2±1.7 mV (20.8±2.5 mV, n=5) and increased action potential frequency (Figure 8Ab).
Figure 8.
Effects of alverine on high K solution and ACh-induced depolarizations recorded from DSMs of the guinea-pig urinary bladder. In a DSM preparation, increasing [K+]o depolarized the membrane by about 20 mV and increased the frequency of action potentials (Aa). In the same preparation that had been treated with alverine (10 μM), an increase in [K+]o caused a similar depolarization (Ab). In another DSM, ACh (10 μM) depolarized the membrane by about 25 mV, and also initially increased the frequency of action potentials and then their generation ceased (Ba). In the same preparation, alverine (10 μM) did not prevent either ACh-induced depolarization or its effects on action potentials (Bb). Scale bars in (Bb) refer to all traces.
Acetylcholine (10 μM) depolarized the membrane from −47.0±4.9 to −20.4 mV (26.6±2.8 mV, n=4). ACh initially increased action potential frequency, and subsequently either induced oscillatory membrane potential changes or inhibited their generation (Figure 8Ba). In preparations that had been treated with alverine (10 μM), ACh depolarized the membrane from −46.9 to −19.6 mV (27.3±4.9 mV, n=4) (Figure 8Ba). These results show that the effects of high K and ACh on electrical activity were not altered by alverine.
Effects of Y-27632 on evoked activity
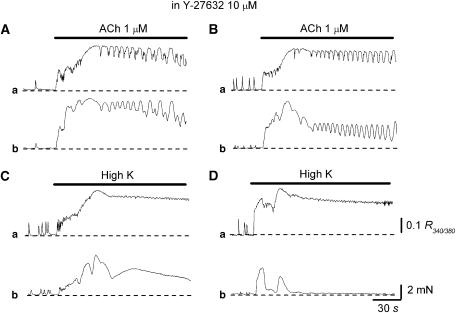
Since alverine suppressed the evoked contractions with little change in the evoked increases in [Ca2+]i, the effects of inhibition of Rho kinase with Y-26763, which is known to suppress carbachol-induced contractions without reducing associated Ca response in human DSM (Takahashi et al., 2004), were examined. The results are shown in Figure 9 and are summarized in Table 1b.
Figure 9.
Effects of Y-26763 on changes in Ca and contractile responses induced by high K solution and acetylcholine (Ach) recorded from DSMs of the guinea-pig bladder. In a DSM bundle, increasing [K+]o caused a phasic increase in [Ca2+]i, which was followed by a sustained increase in [Ca2+]i (Aa) and an associated contraction (Ab). In the same preparation that had been treated with Y-26763 (10 μM), a subsequent increase in [K+]o caused a similar increase in [Ca2+]i (Ba) but caused a much smaller contraction (Bb). In another DSM, ACh (10 μM) caused a biphasic increase in [Ca2+]i (Ca), and an associated contraction (Cb). In the same preparation, Y-26763 (10 μM) suppressed ACh-induced contractions (Db), while having little effect on the ACh-induced increase in [Ca2+]i (Da). Scale bars in (Da) and (Db) refer to all Ca and tension traces, respectively.
In control conditions, high K solution evoked an increase in [Ca2+]i, which was composed of an initial phasic component (0.22±0.03 R340/380, n=4) and a sustained component (0.18±0.02 R340/380; Figure 9Aa). This increase in [Ca2+]i was associated with a biphasic contraction (Figure 9Ab). In preparations that had been treated with Y-26763 (10 μM), high K solution evoked an increase in [Ca2+]i, which was similar to that in control conditions (initial 0.21±0.04 R340/380, sustained 0.17±0.02 R340/380, n=4; Figure 9Ba). However, high K-induced contractions were much smaller than those in control conditions (Figure 9Bb). Thus, the amplitude of the initial component of high K-induced contractions was about 75% of the control values, and that of the sustained component was only about 15% of the control values. Note that spontaneous Ca transients generated much smaller contractions in preparations that had been treated with Y-26763.
Acetylcholine (10 μM) caused an increase in [Ca2+]i, which was composed of an initial component (0.21±0.03 R340/380, n=4) and a sustained component (0.19±0.03 R340/380; Figure 9Ca). This increase in [Ca2+]i was associated with a biphasic contraction (Figure 9Cb). In preparations that had been treated with Y-26763 (10 μM), ACh (10 μM) evoked an increase in [Ca2+]i that was similar to that in control conditions (Figure 9Da), but induced much smaller contractions (Figure 9Db). Thus, the amplitude of the initial component of ACh-induced contractions was about 85% of the control values, and that of the sustained component was only about 40% of the control values.
Discussion
Preliminary studies
The paradoxical ability of alverine citrate at concentrations between 1 and 10 μM to enhance the frequency of spontaneous contractions in endogenously active smooth muscles while suppressing the size of evoked contractions is a robust observation and well established. Although higher concentrations will suppress spontaneous and evoked activity, at clinically used doses it is very unlikely that the concentration would be high enough to do this. Alverine should not be thought of as a drug that relaxes smooth muscles. The most likely explanation of the clinical effects of the drug is its ability to reduce the duration of spontaneous contractions in the gut and uterus. It is probable that the long contractions lead to local ischaemia in the smooth muscles, and activation of intrinsic nociceptive sensory nerves. Shortening the contractions and preventing tetanic build-up can alleviate this. Thus, the drug is truly spasmolytic as it relieves spasms.
Detailed studies on guinea-pig DSM
Alverine-induced enhancement of spontaneous contractions was associated with altered configuration of the action potentials. It also reinforced spontaneous Ca transients, and thus its action on spontaneous contractions occurred in a Ca-dependent manner. In contrast, alverine suppressed high K- or ACh-induced contractions with apparently no change in electrical responses and much less suppression of the Ca responses, suggesting that its action on evoked activity may result from Ca-independent mechanisms.
Spontaneous contractions in DSM are initiated by the opening of L-type Ca channels during action potentials, which lead to spontaneous Ca transients, and thus Ca influx through the channels plays a fundamental role in their generation. Since alverine had no effect on DSM contractility in the presence of nifedipine, it may act primarily on L-type Ca channels rather than either intracellular Ca stores or the Ca sensitivity of contractile proteins. As single-bundle DSM acts as an electrical syncytium, electrical activity invariably resulted in corresponding contractions, irrespective of the pattern of action potential generation, that is individual or bursting (Hashitani et al., 2004). In contrast, overall contractility of multi-bundle DSM depends not only on the contractility of individual bundles but also the synchronicity between bundles, which varies between preparations (Hashitani et al., 2000). Therefore, alverine-induced enhancement of spontaneous contractions in larger, multi-bundle DMS may also result from its action on Ca influx through L-type Ca channels in individual DSM muscle bundles. In addition, alverine might increase the synchronicity between bundles by altering the property of interstitial cells that may play a role in integrating signals within the bladder wall (Hashitani, 2006). This would also be consistent with the lack of excitatory effect of alverine on the responses evoked in individual DSM cells uniformly stimulated by either high K or ACh.
Action potentials are generated by the opening of L-type Ca channels, and are repolarized by both the inactivation of L-type Ca channels (Nakayama and Brading, 1993) and activation of a negative feedback mechanism, mediated by store-released intracellular Ca acting on BK channels (Herrera et al., 2000; Herrera and Nelson, 2002; Hashitani and Brading, 2003). Alverine increased the amplitude and half width of each action potential, and reduced the rate of decay, suggesting that it may suppress either the inactivation of L-type Ca channels or the negative feedback mechanism. However, we were not able to examine the role of Ca-dependent inactivation of L-type Ca channels in alverine's action because Bay K 4688 invariably converted ‘single' contractions into ‘bursting' contractions.
Charybdotoxin, a blocker for large (BK) and intermediate (IK) conductance Ca-activated potassium channels, has been shown to increase the amplitude and half width of action potentials and reduce the rate of decay, by suppressing the negative feedback mechanism on L-type Ca channels and enhanced associated contractions (Hashitani and Brading, 2003). Iberiotoxin, a specific blocker for BK channels has a similar effect on action potential configuration, suggesting that BK channels play a central role in the negative feedback mechanism. It might be expected, therefore, that CTX would prevent alverine-induced enhancement of spontaneous contractions. In preparations that had been treated with CTX, subsequent application of alverine did increase the amplitude of ‘single' contractions that resulted from individual action potentials, but by only 20%, suggesting that alverine and CTX may share a common mechanism for reinforcing spontaneous contractions. However, CTX often initiated bursting contractions, and subsequent application of alverine further increased their amplitude by about 50–100%. This effect of alverine may result from it increasing the frequency of action potentials, which would increase the number of action potentials during a bursting contraction. Therefore, alverine may increase the frequency of action potentials by additional mechanisms to its action on BK channel-mediated negative feedback on L-type Ca channels, presumably by inhibiting other potassium channels.
Cyclopiazonic acid has also been shown to diminish Ca store- BK channel-mediated negative feedback mechanism to augment action potentials and associated contractions (Hashitani and Brading, 2003). However, in preparations that had been treated with CPA, subsequent application of alverine still increased the amplitude of ‘single' contractions by 60%, suggesting that alverine-induced enhancement of spontaneous contractions does not rely on intracellular Ca stores. CPA also often induced bursting contractions, and subsequent application of alverine increased their amplitude by about 50–100%. It was noteworthy that CPA itself increased the amplitude of spontaneous contractions in three out of seven preparations but did not alter their amplitude in the remaining four preparations. This heterogeneity may result from the known but controversial effects of CPA on contractility of DSM. Thus, CPA can enhance spontaneous contractions by suppressing the negative feedback mechanisms (Hashitani and Brading, 2003), but at the same time it can reduce the contractility of DSM by disrupting Ca-induced Ca release (CICR). In our previous study, CPA did not increase the amplitude of spontaneous Ca transients (Hashitani et al., 2000), but enhanced spontaneous contractions by increasing the number of action potential during a burst (Hashitani et al., 2004).
Alverine suppressed both high K- and ACh-induced contractions without suppressing the associated electrical responses. In addition, alverine had much less effect on changes in [Ca2+]i induced by high K or ACh, suggesting that alverine-induced suppression of evoked contractions may result from the reduced sensitivity of contractile proteins to Ca2+. It is also noteworthy that alverine preferentially suppressed the sustained phase of evoked contractions rather than their initial, phasic components. ACh contracts DSM by increasing [Ca2+]i through both the Ca release from intracellular stores and the Ca influx through L-type Ca channels (Wu et al., 1999; Rivera and Brading, 2006). ACh also increased the sensitivity of the contractile proteins to Ca by activating both Rho kinase and PKC, and thus generates larger contraction than does high K solution at similar increases in Ca (Takahashi et al., 2004; Durlu-Kandilci and Brading, 2006). Therefore, it was not surprising that alverine suppressed ACh-induced contractions by disrupting these Ca-independent mechanisms. High K solution generates smaller contractions in DSM than ACh at the same level of [Ca2+]i, and is considered to cause contractions mainly by Ca influx though L-type Ca channels (Maggi et al., 1988; Marti-Cabrera et al., 1994). However, high K solution is also known to be capable of increasing Ca sensitivity by Ca-induced activation of the Rho kinase pathway in vascular smooth muscles (Sakurada et al., 2003). Therefore, high K solution may initiate contractions of DSM by depolarization of the membrane to activate Ca influx through L-type Ca channels, and subsequently activate Rho kinase to maintain the contractions, and alverine may inhibit this pathway. Indeed, alverine selectively suppressed the sustained phase of high K-induced contractions with much less effect on the initial phase, which may result from Ca influx through L-type Ca channels and Ca release from intracellular stores via CICR.
Regardless of alverine-induced suppression of high K- and ACh-induced contractions, one might expect alverine to reinforce the evoked increases that are assumed to result from Ca influx through L-type Ca channels, in particular those of the sustained components. Since the inhibitory action of alverine on BK channels is relatively modest in comparison with venomous BK channel blockers, which reduce dV/dtD to less than one-tenth and cause 5- to 10-fold increase in half width, the concentrations of alverine used may not have been high enough to block BK channels that have been fully activated by sustained depolarizations and massive increases in [Ca2+]i.
Y-26763, an inhibitor of Rho kinase suppressed both ACh- and high K-induced contractions with little reduction in [Ca2+]i, and preferentially suppressed the sustained components. These properties of Y-26763 resemble the action of alverine, suggesting that alverine may inhibit Rho kinase activation subsequent to either muscarinic stimulation or increasing Ca2+. However, unlike alverine which enhanced spontaneous contractions, Y-26763 suppressed spontaneous contractions without inhibiting either electrical or Ca responses (Hashitani et al., 2004). This discrepancy may arise from different contributions of Ca-dependent and Ca-independent contractions of DSM between spontaneous and evoked contractions. In obstructed bladder, a causal relationship between increased DSM contractility and Rho kinase activity has been observed (Bing et al., 2003). Alverine, which may inhibit Rho kinase activity without suppressing Ca-dependent DSM contractions, might therefore be useful for the pharmacological treatment of some particular types of overactive bladder associated with increased Rho kinase activity.
In conclusion, the paradoxical effects of alverine on DSM contractility may result from the complexity of its action, involving inhibition on both BK channel-mediated negative feedback to L-type Ca channels and Rho kinase-mediated Ca sensitization. This unique drug revealed a different contribution of Ca-dependent, that is Ca influx through L-type Ca channels and subsequent CICR, and Ca-independent mechanisms to DSM contractility between spontaneous and evoked contractions. This should encourage further investigation of changes in DSM contractility under different situations.
Acknowledgments
The preliminary studies were begun by IW as an undergraduate and later as a graduate. The following undergraduates also carried out projects using alverine, Tom Cominos, Alison Brown, Siew Ling Harrison and Jonathan O'Keeffe, and the following trainee urologists also worked part time on the effects of alverine: Mo Belal, James Thorpe and Katherine Kennedy. The excellent work of these people underlies the preliminary work. This more detailed work was supported by Grant-in-Aid from JSPS to HH (no. 19390418).
Abbreviations
- BK channel
large conductance Ca2+-activated K+ channel
- [Ca2+]i
intracellular concentration of free calcium ions
- CICR
calcium-induced calcium release
- CPA
cyclopiazonic acid
- CTX
charybdotoxin
- DSM
detrusor smooth muscle
- dV/dtD
rate of decay
- dV/dtR
rate of rise
Conflict of interest
The authors state no conflict of interest.
References
- Abysique A, Lucchini S, Orsoni P, Mei N, Bouvier M. Effects of alverine citrate on cat intestinal mechanoreceptor responses to chemical and mechanical stimuli. Aliment Pharmacol Ther. 1999;13:561–566. doi: 10.1046/j.1365-2036.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- Bing W, Chang S, Hypolite JA, DiSanto ME, Zderic SA, Rolf L, et al. Obstruction-induced changes in urinary bladder smooth muscle contractility: a role for Rho kinase. Am J Physiol Renal Physiol. 2003;285:F990–F997. doi: 10.1152/ajprenal.00378.2002. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Grimaud JC, Abysique A, Chiarelli P. Effects of alverine on the spontaneous electrical activity and nervous control of the proximal colon of the rabbit. Gastroenterol Clin Biol. 1992;16:334–338. [PubMed] [Google Scholar]
- Brading AF, Sibley GNA. A superfusion apparatus to study field stimulation of smooth muscle from mammalian urinary bladder. J Physiol (London) 1983;334:11P–12P. [Google Scholar]
- Coelho AM, Jacob L, Fioramonti J, Bueno L. Rectal antinociceptive properties of alverine citrate are linked to antagonism at the 5-HT1A receptor subtype. J Pharm Pharmacol. 2001;53:1419–1426. doi: 10.1211/0022357011777783. [DOI] [PubMed] [Google Scholar]
- Durlu-Kandilci NT, Brading AF. Involvement of Rho kinase and protein kinase C in carbachol-induced calcium sensitization in beta-escin skinned rat and guinea-pig bladders. Br J Pharmacol. 2006;148:376–384. doi: 10.1038/sj.bjp.0706723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol. 2003;140:159–169. doi: 10.1038/sj.bjp.0705320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H. Interaction between interstitial cells and smooth muscles in the lower urinary tract and penis. J Physiol. 2006;576:707–714. doi: 10.1113/jphysiol.2006.116632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol. 2004;141:183–193. doi: 10.1038/sj.bjp.0705602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Bramich NJ, Hirst GDS. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol. 2000;524:565–579. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol. 2000;279:R60–R68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Manzini S, Parlani M, Conte B, Giuliani S, Meli A. The effect of nifedipine on spontaneous, drug-induced and reflexly-activated contractions of the rat urinary bladder: evidence for the participation of an intracellular calcium store to micturition contraction. Gen Pharmacol. 1988;19:73–81. doi: 10.1016/0306-3623(88)90008-0. [DOI] [PubMed] [Google Scholar]
- Marti-Cabrera M, Llopis P, Abengochea A, Ortiz JL, Climent VJ, Cortijo J, et al. Effects of Ca2+ channel antagonists and benzodiazepine receptor ligands in normal and skinned rat urinary bladder. Eur J Pharmacol. 1994;255:157–165. doi: 10.1016/0014-2999(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Brading AF. Inactivation of the voltage-dependent Ca2+ channel current in smooth muscle cells isolated from the guinea-pig detrusor. J Physiol. 1993;471:107–127. doi: 10.1113/jphysiol.1993.sp019893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera L, Brading AF. The role of Ca2+ influx and intracellular Ca2+ release in the muscarinic-mediated contraction of mammalian urinary bladder smooth muscle. BJU Int. 2006;98:868–875. doi: 10.1111/j.1464-410X.2006.06431.x. [DOI] [PubMed] [Google Scholar]
- Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, et al. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res. 2003;93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Nishimura J, Hirano K, Seki N, Naito S, Kanaide H. Ca2+ sensitization in contraction of human bladder smooth muscle. J Urol. 2004;172:748–752. doi: 10.1097/01.ju.0000130419.32165.6b. [DOI] [PubMed] [Google Scholar]
- Uchida W, Masuda N, Shirai Y, Shibasaki K, Satoh N, Takenada T. The role of extracellular Ca2+ in carbachol-induced tonic contraction of the pig detrusor smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:398–402. doi: 10.1007/BF00178958. [DOI] [PubMed] [Google Scholar]
- Wu C, Bayliss M, Newgreen D, Mundy AR, Fry CH. A comparison of the mode of action of ATP and carbachol on isolated human detrusor smooth muscle. J Urol. 1999;162:1840–1847. [PubMed] [Google Scholar]