Abstract
The endocannabinoid, arachidonoylethanolamide (AEA), and the peroxisome proliferator-activated receptor (PPAR)-α ligand, oleylethanolamide (OEA) produce opposite effects on lipogenesis. The regulation of OEA and its anti-inflammatory congener, palmitoylethanolamide (PEA), in adipocytes and pancreatic β-cells has not been investigated. We report here the results of studies on acylethanolamide regulation in these cells during obesity and hyperglycaemia, and provide an overview of acylethanolamide role in metabolic control. We analysed by liquid chromatography-mass spectrometry OEA and PEA levels in: 1) mouse 3T3F442A adipocytes during insulin-induced differentiation, 2) rat insulinoma RIN m5F β-cells kept in ‘low' or ‘high' glucose, 3) adipose tissue and pancreas of mice with high fat diet-induced obesity (DIO), and 4) in visceral fat or blood of obese or type 2 diabetes (T2D) patients. In adipocytes, OEA levels remain unchanged during differentiation, whereas those of PEA decrease significantly, and are under the negative control of both leptin and PPAR-γ. PEA is significantly downregulated in subcutaneous adipose tissue of DIO mice. In RIN m5F insulinoma β-cells, OEA and PEA levels are inhibited by ‘very high' glucose, this effect being enhanced by insulin, whereas in cells kept for 24 h in ‘high' glucose, they are stimulated by both glucose and insulin. Elevated OEA and PEA levels are found in the blood of T2D patients. Reduced PEA levels in hypertrophic adipocytes might play a role in obesity-related pro-inflammatory states. In β-cells and human blood, OEA and PEA are down- or up-regulated under conditions of transient or chronic hyperglycaemia, respectively.
Keywords: anandamide, oleylethanolamide, palmitoylethanolamide, lipolysis, fat, pancreas
Introduction and review of the existing literature
Biosynthesis, molecular targets and degradation of acylethanolamides
Apart from classical hormones like leptin and insulin, other mediators that are receiving increasing attention in energy homeostasis are the fatty acid ethanolamides (or acylethanolamides (AEs); Figure 1; Lo Verme et al., 2005b; Matias and Di Marzo, 2007). In fact, emerging evidence indicates that both the cannabinoid receptor ligand arachidonoylethanolamide (anandamide, AEA), and oleoylethanolamide (OEA) regulate food intake and lipogenesis, although in opposing ways (Williams and Kirkham, 1999; Rodriguez de Fonseca et al., 2001; Fu et al., 2003; Oveisi et al., 2004; Di Marzo and Matias, 2005). Another well-known AE, palmitoylethanolamide (PEA), identified more than five decades ago (Long and Martin, 1956; Bachur et al., 1965), exhibits anti-inflammatory and cell-protective properties (Re et al., 2007 for a recent review).
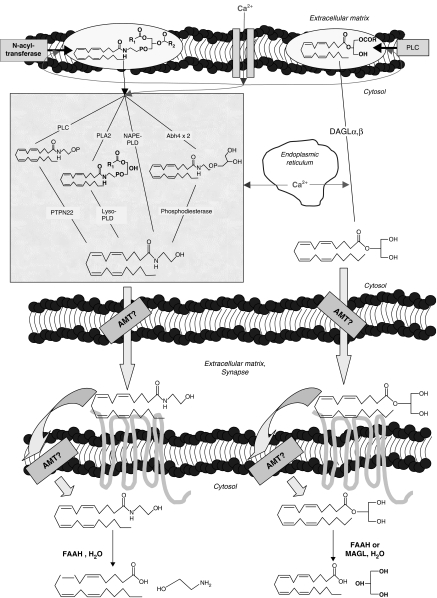
Figure 1.
Schematic representation of the anabolic and catabolic pathways so far proposed for the acylethanolamides and 2-AG. Adapted from Di Marzo and Petrosino (2007). Abbreviations used: Abh4, alpha/beta-hydrolase 4; 2-AG, 2-arachidonoylglycerol; DAGL, sn-1-selective diacylglycerol lipase; AMT, putative endocannabinoid membrane transporter; FAAH, fatty acid amide hydrolase; lyso-PLD, lysophospholipase D; MAGLs, monoacylglycerol lipases; NAPE-PLD, N-acylphosphatidyl-ethanolamine-selective phospholipase D; PLA2, phospholipase A2; PLC, phospholipase C; PTPN22, protein tyrosine phosphatase N22.
Interestingly, the orexigenic and lipogenic AEA, the anti-inflammatory PEA, and the anorectic and lipolytic OEA appear to share common biosynthetic and degradative pathways (Lambert and Di Marzo, 1999). The enzyme most likely responsible for the biosynthesis of AEs from their direct biosynthetic precursors, that is the corresponding N-acyl-phosphatidylethanolamines (NAPEs), is known as NAPE-selective phospholipase D (NAPE-PLD) and was cloned recently (Okamoto et al., 2004; Wang et al., 2006). N-Arachidonoyl-phosphatidylethanolamine (NArPE), N-oleoyl-phosphatidylethanolamine and N-palmitoyl-phosphatidylethanolamine are converted into AEA, OEA and PEA, respectively, by NAPE-PLD, although other possible pathways exist for the processing of NAPEs, as suggested also by the fact that NAPE-PLD null mice do not necessarily contain lower levels of AEA, OEA or PEA than wild-type mice (Figure 1; Leung et al., 2006). NAPEs can in fact also be hydrolysed by a secretory phospholipase 2 into N-acyl-lyso-phosphatidylethanolamines before being further hydrolysed to AEs by a lysophospholipase D (Sun et al., 2004). NAPEs are also substrates for α/β-hydrolase 4 acting as a lysophospholipase/phospholipase B for the formation of glycerol-phospho-AEA, glycerol-phospho-OEA and glycerol-phospho-PEA in the mouse brain (Simon and Cravatt, 2006). Finally, a PLC-dependent pathway for NArPE conversion into AEA via phospho-AEA has been recently suggested to occur in macrophages (Liu et al., 2006). Also the enzymes mostly involved in the degradation of these bioactive lipids were identified and cloned. An intracellular integral membrane protein of 597 amino acids belonging to the amidase family of enzymes, known as fatty acid amide hydrolase (FAAH), catalyses the hydrolysis of AEs (Cravatt et al., 1996; Giang and Cravatt, 1997). It is found not only in the brain (Tsou et al., 1998) but also in various peripheral tissues, for example, the vasculature (Deutsch et al., 1997), pancreas and adipose tissue (Juan-Pico et al., 2006; Matias et al., 2006). Additionally, an N-acylethanolamine-hydrolysing acid amidase (NAAA) was recently found to hydrolyse preferentially PEA and only to a low extent OEA and AEA (Ueda et al., 2001). NAAA has been recently cloned and appears to be expressed in macrophages and various rat tissues including lung and brain (Tsuboi et al., 2004, 2007).
Whereas it has been well-documented that, like AEA, PEA exhibits anti-inflammatory properties, OEA shows opposing effects on food intake as compared to the endocannabinoid. AEA activates the G-protein-coupled receptor targeted by Δ9-tetrahydrocannabinol (Matsuda et al., 1990), that is the cannabinoid receptor type 1 or CB1 (Devane et al., 1992), and much more less efficaciously the cannabinoid receptor type 2 or CB2, whereas OEA is inactive on these receptors and activates instead the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α) (Fu et al., 2003). Although initially described as a potential CB2 receptor agonist, PEA is also inactive at both cannabinoid receptor types, and was shown to bind to PPAR-α but with a lower potency than OEA. This receptor was suggested to mediate some of the anti-inflammatory effects of PEA (Lo Verme et al., 2005a).
Role and regulation of AEA and 2-AG in energy homeostasis
The endocannabinoid system, including the endocannabinoids and their metabolic enzymatic machinery, and the cannabinoid CB1 receptors, have been detected in all central and peripheral tissues involved in the control of energy intake, processing and storage, including the hypothalamus (Di Marzo et al., 2001), the nucleus accumbens (Berrendero et al., 1998), the vagus nerve and the nodose ganglion (Burdyga et al., 2004), myenteric neurons and epithelial cells of the large intestine (Coutts and Izzo, 2004), the liver and hepatocytes (Osei-Hyiaman et al., 2005), the white adipose tissue (Engeli et al., 2005) and the adipocytes (Bensaid et al., 2003; Cota et al., 2003; Matias et al., 2006; Roche et al., 2006), the skeletal muscle (Pagotto et al., 2006) and the pancreas (Juan-Pico et al., 2006; Matias et al., 2006). CB1 receptors and endocannabinoids control food intake via both central and peripheral mechanisms, and they also stimulate lipogenesis and fat accumulation (Cota et al., 2003). Data from our laboratory indicate that a peak of AEA precedes mouse 3T3F442A preadipocyte differentiation into mature adipocytes, and that stimulation of CB1 receptors during adipocyte differentiation accelerates the appearance of an early marker of differentiation, PPAR-γ, while inducing accumulation of lipid droplets (Matias et al., 2006). These findings reinforce the concept that endocannabinoids actively and directly participate in adipogenesis and fat accumulation. In the liver, the endocannabinoid system stimulates fatty acid synthesis. CB1 receptors are present in hepatocytes and are capable of stimulating the expression of the transcription factor SREBP-1c and of its targets acetyl-CoA-carboxylase-1 and fatty acid synthase. These effects are likely to underlie the stimulatory action on lipogenesis observed following CB1 receptor stimulation in these cells, and might be dramatically enhanced in obese animals, since the liver of mice with diet-induced obesity (DIO) exhibits higher levels of both AEA and CB1 receptors, as well as lower levels of FAAH (Osei-Hyiaman et al., 2005). We showed that the epidydimal fat of DIO mice and the visceral fat, but not the subcutaneous fat, of overweight and obese patients also exhibit higher levels of 2-arachidonoylglycerol (2-AG), but not AEA, than the respective lean controls (Matias et al., 2006). We also observed that the blood levels of AEA in obese women with binge-eating disorder (Monteleone et al., 2005) or in patients with strong hyperglycaemia caused by type 2 diabetes (T2D; Matias et al., 2006) are significantly higher than in age- and gender-matched control volunteers. In another study, circulating AEA levels were found to be increased also in postmenopausal women with uncomplicated obesity (Engeli et al., 2005).
In a model of pancreatic islet β-cells, the rat insulinoma RIN m5F cells, we reported that conditions mimicking hyperglycaemia, as well as hyperinsulinaemia, also lead to a dysregulation of endocannabinoid signalling. Under all conditions investigated, we found that a pulse of a ‘very high' concentration of glucose significantly elevates the levels of both anandamide and 2-AG in these cells. However, we also observed that, contrary to ‘low' glucose conditions, where insulin reduces the ‘very high' glucose-induced elevation of endocannabinoid levels and has no effect per se, in β-cells kept for 24 h in ‘high' glucose, endocannabinoid levels are no longer depressed by insulin, which instead elevates both AEA and 2-AG levels also in the absence of the ‘very high' glucose pulse. Accordingly, the pancreas of mice with DIO and sustained hyperglycaemia exhibits higher levels of AEA and 2-AG than that of lean mice (Matias et al., 2006).
Role and regulation of OEA in energy homeostasis
OEA, unlike anandamide, exerts anorexic actions (Rodriguez de Fonseca et al., 2001; Fu et al., 2003), and these effects appear to be mediated at least in part by PPAR-α (Fu et al., 2003). In fact, OEA fails to cause satiety and to reduce body weight in PPAR-α knockout mice (Fu et al., 2003). Whereas AEA induces food intake by activating CB1 receptors at both central and peripheral (small intestine) levels, as shown by the anorexic effect of the CB1 receptor blocker rimonabant administered intraperitoneally, OEA was suggested to act mostly at the peripheral level (Rodriguez de Fonseca et al., 2001). The effect of intestinal OEA results in central actions that could be erased after destruction of capsaicin-sensitive peripheral fibres, thus suggesting that this compound acts as a peripheral inhibitor of centrally controlled food ingestion behaviour. However, this latter finding is also in agreement with the proposal that another molecular target also participates in OEA anorexic effects, that is the transient receptor potential vanilloid type-1 (TRPV1) channel, which is activated and desensitized by capsaicin, and which OEA activates with moderate potency and good efficacy (Ahern, 2003; Movahed et al., 2005; Wang et al., 2005). OEA and AEA levels are differently regulated in the small intestine, where a sevenfold increase of AEA levels (Gomez et al., 2002) and a marked decrease of OEA were observed following food deprivation in comparison to ad lib fed control rats (Rodriguez de Fonseca et al., 2001; Fu et al., 2003). Recently, a 300-fold increase of OEA levels in the small intestine of fed compared with fasted Burmese pythons was also reported (Astarita et al., 2006). Two studies investigating the biochemical mechanisms underlying this phenomenon have been published, although with discrepant results. In one study, feeding was found to increase the small intestine levels of OEA and other unsaturated AEs without affecting those of saturated AEs, including PEA, nor the levels of the NAPE precursors for OEA and PEA, thus underscoring the selectivity of OEA in food intake (Fu et al., 2007). Increased OEA levels during feeding were accompanied by an increase of the expression and the activity of the NAPE-PLD and by a decrease of the expression and the activity of FAAH (Fu et al., 2007). Opposing results were obtained in a previous study by Petersen et al. (2006), who showed that the activity of biosynthetic and degrading enzymes did not change during food deprivation and refeeding, and that differential changes in AEA, OEA and PEA small intestine levels were likely due to differential changes in the levels of their NAPE precursors. The reasons why these studies produced discrepant outcomes are not known. Yet, the two studies emphasize how the levels of different AEs can be regulated in different and even opposing ways during food intake and food deprivation.
In addition to its anorexic properties, OEA also reduces visceral fat mass (Guzman et al., 2004; Fu et al., 2005) and produces several peripheral effects, in agreement with the concept that, like CB1, PPAR-α is also involved in lipid metabolism and adipocyte differentiation. Both PPAR-α and -β are in fact phosphorylated through an insulin-mediated pathway, thereby inhibiting the expression of genes favouring obesity and stimulating fatty acid β-oxidation (Christophe, 1997). During fasting conditions, fatty acids are mobilized from adipose tissue and taken up by the liver where they are β oxidized. The activation of PPAR-α has long since been known to increase hepatic fatty catabolism and reduce plasma triacylglycerols (Madsen et al., 2005). Accordingly, PPAR-α is highly expressed in hepatocytes, cardiomyocytes, the kidney cortex, skeletal muscles, adipocytes and enterocytes (Lefebvre et al., 2006). In adipocytes, OEA stimulates lipolysis as it increases the release of nonesterified fatty acids and glycerol in a dose-dependent manner, whereas it fails to do so in adipocytes from PPAR-α knockout mice (Guzman et al., 2004). Additionally, not only in adipocytes but also in jejunal enterocytes, OEA increases fatty acid uptake and the expression of the fatty acid translocase FAT/CD36, although in this case, the receptor through which these effects were caused was not investigated (Yang et al., 2007). Systemic administration of OEA produced a rapid elevation of the circulating levels of nonesterified fatty acids and glycerol and a decrease in triacylglycerols content in the epidydimal fat and in the liver but not in the skeletal muscle (Guzman et al., 2004). Since this latter effect is mediated by PPAR-α and this nuclear receptor stimulates fatty acid β-oxidation, it was not surprising to observe that OEA also stimulates fatty acid oxidation in rat soleus muscle and ketogenesis in rat hepatocytes (Guzman et al., 2004). In the liver, OEA regulates the expression of genes involved in lipid metabolism such as PPAR-α and some of its targets, the FAT/CD36, the liver fatty acid-binding protein (L-FABP) and the uncoupling protein-2 (UCP-2). Intraperitoneal administration of OEA increases the liver expression of FAT/CD36 and L-FABP without affecting acyl-CoA synthase, and decreases liver lipid droplets accumulation (Fu et al., 2005). Despite the several effects that exogenous OEA exerts on lipid metabolism, the regulation of its endogenous levels during adipocyte differentiation, or in the adipose depots of animals with disrupted energy balance (for example, DIO mice) or in obese humans has not been investigated to date. Furthermore, little is known on the possible role of OEA in the pancreas, despite the fact that another proposed molecular target for this compound, the ‘orphan' G-protein-coupled receptor GPR119, is most abundant in this organ (Overton et al., 2006).
Role and regulation of PEA in energy homeostasis and inflammation
PEA binds to PPAR-α but is unable to exert on food intake and lipolysis effects similar to those of OEA, possibly because of its lower efficacy at this nuclear receptor, or perhaps because, as mentioned above, OEA might also exert some of these effects via other receptors. Interestingly, however, PEA activity at PPAR-α is efficacious enough to induce some anti-inflammatory actions (Lo Verme et al., 2005a). Nevertheless, despite the proposed inhibitory effects of PEA on the release of atherogenic cytokines (for example, tumour necrosis factor-α (TNF-α)) in vivo (Berdyshev et al., 1998) and in vitro (Berdyshev et al., 1997), and the previous observation that PEA inhibits leptin release from human adipocytes under inflammatory conditions (Hoareau et al., 2006), the regulation and role of this compound in atherogenic inflammation, a process that starts in the hypertrophic adipocyte with excessive release of inflammatory cytokines and decreased adiponectin production from white adipose tissues, has not yet been investigated. Furthermore, whereas skin PEA levels were shown to be upregulated in a rat model of type 1 diabetes (Darmani et al., 2005), no data exist on the regulation of PEA levels in β-cells and pancreas during the sustained hyperglycaemia, and the subsequent β-cell proinflammatory and proapoptotic state, which are typical of obesity and T2D.
Aim of the study
In view of this background and also of the fact that OEA and PEA have been recently detected in human adipocytes (Gonthier et al., 2007), but not yet reported in β-cells, we wanted to assess further the role of endogenous OEA and PEA in energy balance by investigating the regulation of their levels in (1) mouse 3T3F442A adipocytes, (2) a model of β-cells, the insulinoma RIN mF5 cells and (3) the adipose tissue and pancreas from lean and obese animals and humans. We decided to study the effect on OEA and PEA levels of exactly the same conditions previously shown to affect AEA and/or 2-AG levels in these cells and tissues (Matias et al., 2006). Thus, we investigated the regulation and dysregulation of OEA and PEA in isolated adipocytes and β-cells cultured under conditions mimicking either normal or unbalanced energy homeostasis, and in adipose tissues or pancreas or blood of DIO mice and obese or hyperglycaemic T2D patients.
Methods
Cell cultures
The mouse preadipocyte 3T3F442A cell line (fifth to tenth passage) was kindly provided by Dr Mohamed Bensaid from Sanofi-Aventis (Montpellier, France). 3T3F442A preadipocytes were grown according to the manufacturer's recommendations. After confluence, 3T3F442A adipose differentiation was obtained in the presence of the same culture medium supplemented with 0.9 μm insulin. Cells were placed in culture (day −6) and grown to confluence (day −2) before stimulation with insulin (day 0). Terminal differentiation occurred by day 12. For adipocyte stimulation, leptin (20 nM), WY14643 (a PPAR-α agonist, 20 μM) and ciglitazone (a PPAR-γ agonist, 20 μM) were added in dimethyl sulphoxide (final concentration 0.1%) to the culture medium.
RIN m5F rat insulonoma β-pancreatic cells were obtained from ATCC and grown according to the manufacturer's recommendations. For experiments with cells in low- and high-glucose conditions, cells were grown in the recommended culture medium containing respectively 2.4 g l−1 glucose (13 mM) and 4.5 g l−1 glucose (25 mM) for 24 h before experimentation. For RIN m5F cell stimulation, leptin (20 nM), insulin (100 nM), WY14643 (20 μM) and ciglitazone (20 μM) were added in dimethyl sulphoxide (final concentration 0.1%) to the culture medium depleted in fetal calf serum.
Animals
Male, 7-week-old C57Bl/6J mice were purchased from Harlan Italy (Corezzana, MI, USA). After 1 week acclimatization, animals were fed a diet containing 25.5% fat (49% of calories), 22% protein and 38.4% carbohydrate (TD97366, Harlan Italy) for up to 14 weeks. Control mice received standard diet. Mice (n=10 per group) were fed ad libitum, except for the 12-h period immediately preceding the killing at 14 weeks (Table 1). The pancreas and the subcutaneous and visceral fat tissues were removed and immediately frozen in liquid nitrogen until quantitative determination of endocannabinoids. Fasting plasma glucose levels were determined in 12-h-fasted animals using the glucose test kit with an automatic analyzer (AQccu-Chek Active; Roche, Basel, Switzerland) in blood samples obtained from tail vein. Guidelines for the use and care of laboratory animals of the authors' institutions were followed.
Table 1.
DIO vs lean mice
| Body weight (g) | Glucose (mM) | |
|---|---|---|
| Standard diet (9% fat) | 28.8±0.3 | 6.77±0.15 |
| HFD (25.5% fat) | 41.5±0.2*** | 9.07±0.18* |
Abbreviations: DIO, diet-induced obesity; HFD, high-fat diet.
Effect of a standard diet and HFD on body weight and blood glucose levels. Assays were performed after 14 weeks of dietary treatment. Blood samples were obtained following 12 h fasting. Data are means±s.e.mean of n=10. *P<0.05 and ***P<0.001 vs controls, respectively, as assessed by ANOVA followed by the Bonferroni's test.
Subjects and blood or fat sampling
Visceral/subcutaneous fat from normoweight and overweight/obese volunteers was collected during Roux-en-Y gastric bypass surgery for all 20 obese patients, and during cholecystectomy (n=4) and hiatal hernias removal (n=6) for the normoweight controls (Table 2). Samples were immediately frozen in liquid nitrogen until endocannabinoid quantification. Blood from patients with T2D and age-matched healthy volunteers were collected between 800 and 900 hours, the last treatment having been done not earlier than 12 h before blood sampling (Table 3). Regarding the determination of circulating preprandial and postprandial endocannabinoid levels, 12 healthy human subjects (eight men and four women) were recruited (age=32.3±3.9 years; body mass index (BMI)=21.7±2.9 kg m−2, means±s.d.; Table 4). After a 12-h-fasting period, volunteers received a high-fat meal providing 601.12 kcal and consisting of 16.60% protein, 39.25% carbohydrate and 44.15% fat. Preprandial and postprandial blood samples were collected 1 h before and after the test meal, respectively. All patients and volunteers were informed of the study procedures and signed an informed consent.
Table 2.
Obese vs normoweight patients
| Age (years) | BMI | Gender | Glucose (mM) | |
|---|---|---|---|---|
| Normoweight volunteers means (n=10)±s.e.mean | 56.7±5.9 | 21.3±0.3 | 4M/6F | 4.83±0.17 |
| Obese patients means (n=20)±s.e.mean | 45.9±2.1* | 45.1±1.8**** | 9M/11F | 5.67±0.11*** |
Abbreviations: BMI, body mass index, expressed in kg m−2; F, female; M, male.
None of the patients were under pharmacological treatment at the time of surgery. *,***,****P<0.05, 0.005, 0.0005, respectively vs normoweight volunteers, as assessed by the Kruskal–Wallis nonparametric test.
Table 3.
Data of hyperglycaemic type 2 diabetic vs healthy volunteers
| Age (years) | BMI | Gender | Glucose (mM) | |
|---|---|---|---|---|
| Healthy volunteers mean (n=8)±s.e.mean | 62.3±2.4 | 28.6±1.9 | 5M/3F | 5.72±0.22 |
| Diabetic patients mean (n=10)±s.e.mean | 69.0±4.0 | 33.5±3.0 | 4M/6F | 10.28±1.17*** |
Abbreviations: BMI, body mass index, expressed in kg m−2; F, female; M, male.
We used patients with type 2 diabetes with randomized pharmacological treatments. In fact, of the 10 patients, two were under treatment with metformin, one with metformin + insulin, one with glibenclamide, two with metformin+glibenclamide, one with acarbose+glibenclamide and three with insulin only. In each case, the last treatment was given not later than 12 h before blood sampling. As shown in the Table, this heterogeneity in treatment resulted in similar fasting glycaemia values. ***P<0.005 vs healthy volunteers, as assessed by the Kruskal–Wallis nonparametric test. BMI, body mass index, expressed in kg m−2.
Table 4.
Preprandial vs postprandial healthy volunteers
| Age (years) | BMI | Gender | Glucose (mM) | Insulin | C peptide | Leptin | Triglycerides | Total cholesterol | HDL | LDL | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Preprandial healthy volunteers means (n=12)±s.e.mean | 32.3±3.9 | 21.7±2.9 | 8M/4F | 4.61±0.05 | 4.02±0.63 | 417.83±46.92 | 7.92±1.21 | 0.71±0.12 | 1.76±0.06 | 0.57±0.05 | 1.05±0.05 |
| Postprandial healthy volunteers means (n=12)±s.e.mean | 5.06±0.22* | 25.2±6.47*** | 1587.25±216.05*** | 7.67±1.33 | 1.1±0.19**** | 1.76±0.05 | 0.56±0.04 | 1.02±0.05 |
Abbreviations: BMI, body mass index, expressed in kg m−2; HDL, high-density lipoprotein; LDL, low-density lipoprotein, OEA, oleoylethanolamide; PEA, palmitoylethanolamide.
This experiment was designed to assess whether or not a transient hyperglycaemia, corrected by well functioning insulin signalling, results in decreased serum OEA and PEA levels. Blood sampling was carried out 1 h before and after the meal, respectively. *,***,****P<0.05, 0.005, 0.0005, respectively vs paired volunteers, as assessed by the two-tailed paired Student's test. Insulin is expressed in mU ml−1. C peptide is expressed in pmol l−1. Leptin is expressed in ng ml−1. Triglycerides, total cholesterol, HDL and LDL are expressed in g l−1.
Oil Red-O staining
Light microscopy and Oil Red-O staining were used to monitor the characteristic cell rounding and lipid droplet accumulation in these cells during differentiation (Ramirez et al., 1992).
Real-time RT–PCR analyses
Real-time cDNA quantification was performed by a thermocycler iCycler iQ (Bio-Rad, Hercules, CA, USA). Fluorescence data were collected during elongation. Optimized PPAR-γ primers for SYBR Green analysis (and relative TaOpt) were designed by ‘Beacon Designer' software and synthesized by MWG-Biotech AG (Ebersberg, Germany). Assays were performed in triplicate (s.d. of threshold cycle mean <0.5) and a standard curve from consecutive fivefold dilutions (150–0.24 ng) of a cDNA pool representative of all samples was included for each determination. Relative expression analysis correct for PCR efficiency and normalized with respect to reference genes β-actin and hypoxanthine phosphoribosyltransferase was performed by ‘REST C' software for group-wise comparison and statistical analysis.
Purification and quantification of oleylethanolamide and palmitoylethanolamide
The extraction, purification and quantification of OEA and PEA from cells, tissues and blood were performed as described previously (Di Marzo et al., 2001; De Marchi et al., 2003). First, cells are dounce-homogenized and extracted with chloroform/methanol/Tris–HCl 50 mM pH 7.5 (2:1:1, v/v) containing internal standards. The lipid extract was prepurified by open-bed chromatography on silica columns eluted with increasing concentrations of methanol in chloroform. After extraction and purification, OEA and PEA fractions were subjected to isotope-dilution liquid chromatography–atmospheric pressure chemical ionization–mass spectrometric analysis (LC-APCI-MS) by using a Shimadzu HPLC apparatus (LC-10ADVP) coupled to a Shimadzu (LCMS-2010) quadrupole MS via a Shimadzu APCI interface as described previously (Di Marzo et al., 2001). The amounts of OEA and PEA are expressed as picomoles per milligram of lipids extracted or per milligram of weight tissue or per millitre of blood.
Results
Regulation of OEA and PEA in the adipose tissue
Regulation of OEA and PEA during adipocyte differentiation
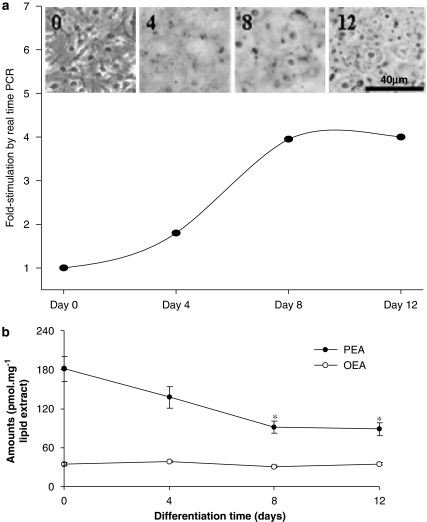
Mouse 3T3F442A preadipocytes were differentiated into adipocytes using conditions mimicking hyperinsulinaemia with a high concentration of insulin. Differentiation into mature adipocytes was monitored by measuring PPAR-γ expression by real-time reverse transcription (RT)–PCR (fold-enhancement over day 0) and Oil Red-O staining of lipid droplets, and occurred rapidly with insulin (Figure 2a). PPAR-γ expression was significantly different from day 0 on days 8 and 12 (P<0.01) under these conditions. Note the full differentiation occurring already at day 8 with insulin, and the increased formation of lipid droplets (red dots, black in the figure) between days 8 and 12. Adipocyte differentiation was accompanied by a decrease in the levels of the PEA (P<0.01 vs vehicle; n=3–6), which remained strongly decreased in mature (day 8) and hypertrophic (day 12) adipocytes (Figure 2b). OEA instead did not change during adipocyte differentiation (Figure 2b).
Figure 2.
Regulation of oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) levels during adipocyte differentiation. (a) Differentiation of mouse 3T3F442A pre-adipocytes into adipocytes induced by insulin (0.9 μM) was monitored by measuring peroxisome proliferator-activated receptor (PPAR)-γ expression by real-time reverse transcription (RT)–PCR (lower panel), or Oil Red-O staining under a microscope (upper panel; adapted from Matias et al., 2006). RNA expression is expressed as fold-enhancement over day 0. Error bars are not shown and they were always <10%. s.d. values for cycle threshold were always <1%. (b) Levels of OEA and PEA during adipocyte differentiation induced with insulin (0.9 μM). OEA and PEA levels were measured by isotope-dilution LC-MS (see Methods). Data are means±s.e.mean of n=3–6 separate experiments. *=P<0.01 vs day 0, respectively, as assessed by ANOVA followed by the Bonferroni's test.
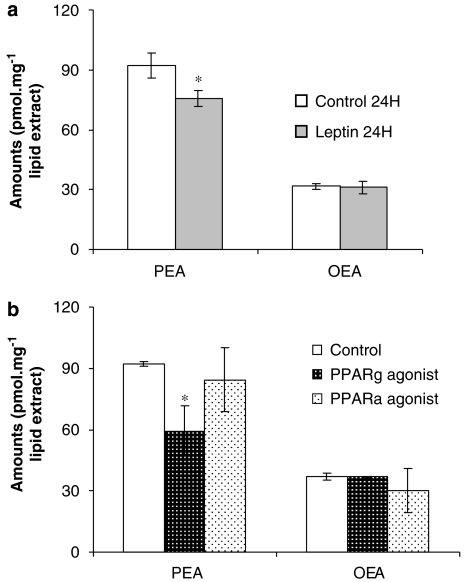
Regulation of endocannabinoid levels by leptin and PPAR-γ
In the rodent hypothalamus, uterus, lymphocytes and adipocytes (Di Marzo et al., 2001; Maccarrone et al., 2003, 2005; Matias et al., 2006), both AEA and 2-AG levels are regulated by leptin (Di Marzo et al., 2001). We observed here that in mature adipocytes (8 days), PEA but not OEA levels were decreased after prolonged (24 h) stimulation with leptin (20 nM) (Figure 3a). Ciglitazone (20 μM, 12 h), a selective agonist of PPAR-γ, but not WY14643 a selective agonist of PPAR-α, decreased PEA levels partially in mature adipocytes (8 days; n=4–6). In contrast, the levels of OEA were not affected by either ciglitazone or WY14643 (Figure 3b).
Figure 3.
Regulation of oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) levels in adipocytes. (a) Effect on OEA and PEA levels of a 24 h stimulation with leptin (20 nM) of differentiated adipocytes (8 days of treatment with 0.9 μM insulin). (b) Effect on OEA and PEA levels of prolonged 12 h stimulation with WY14643 (a peroxisome proliferator-activated receptor (PPAR)-α agonist, 20 μM) and ciglitazone (a PPAR-γ agonist, 20 μM) of differentiated adipocytes. Data are means±s.e.mean of n=4–6 separate experiments. *=P<0.05 vs vehicle, respectively, as assessed by ANOVA followed by Bonferroni's test.
Dysregulation of OEA and PEA levels in the adipose tissue of obese mice and patients
DIO mice were obtained as described in the Methods and exhibited significantly higher body weight and blood glucose levels than lean mice (Table 1). In these mice, we observed a significant decrease of both PEA and OEA in the subcutaneous but not visceral fat (Table 5).
Table 5.
Oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) levels in the visceral and subcutaneous fat and pancreas of mice fed a standard diet (STD) or a high-fat diet (HFD) for 14 weeks
| Organ |
OEA (pmol g−1 wet tissue) |
PEA (nmol g−1 wet tissue) |
||
|---|---|---|---|---|
| STD | DIO | STD | DIO | |
| Subcutaneous fat | 151.2±20.4 | 51.2±5.6*** | 4.3±1.7 | 0.5±0.06* |
| Visceral fat | 135.6±32.4 | 140.0±60.0 | 0.4±0.05 | 0.3±0.05 |
| Pancreas | 74.0±8.0 | 72.0±17.0 | 0.7±0.05 | 0.5±0.08 |
Abbreviation: DIO, diet-induced obesity.
Data are means±s.e.mean of n=4–10. Means were compared by ANOVA followed by Bonferroni's post hoc analysis. *P<0.05; ***P<0.005 vs the respective STD control.
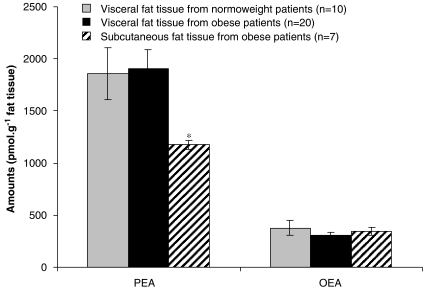
In obese humans as compared to normoweight controls, we observed no changes in both OEA and PEA levels in visceral adipose tissue (Figure 4, see Table 2 for patients' data). In obese patients, subcutaneous fat contained significantly lower levels of PEA, whereas no change in subcutaneous OEA levels were observed (Figure 4).
Figure 4.
Oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) levels in the visceral adipose tissue of normoweight and overweight/obese humans and in the subcutaneous fat of obese patients (Table 1). **=P<0.003 vs visceral fat from the corresponding obese patients, as assessed by paired Student's t-test.
Regulation of OEA and PEA in pancreas and blood
Regulation of OEA and PEA in rat RIN m5F pancreatic β-cells
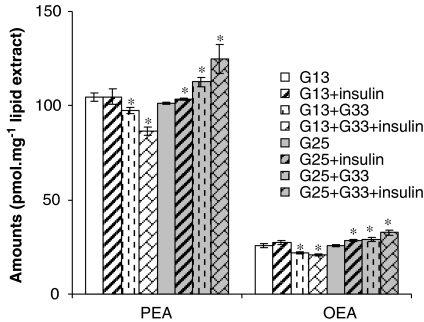
Rat insulinoma RIN m5F β-cells are a widely used model of pancreatic islet β-cells in as much as they release insulin in response to very high concentrations (for example 33 mM) of glucose, and also respond to this hormone (Hohmeier and Newgard, 2004). For optimal viability they need to be cultured in a relatively high concentration of glucose (25 mM), although they can survive for several hours also at a lower concentration (for example 13 mM). Near-confluent cells at a low number of passages were kept in ‘low' (13 mM) glucose for 24 h before stimulation with a 2-h ‘pulse' of 33 mM glucose, which caused a significant decrease of both OEA and PEA levels (Figure 5). In these conditions of relatively ‘low' glucose for these cells, costimulation with insulin enhanced 33 mM glucose-induced OEA and PEA level decrease (Figure 5). Conversely, in β-cells kept for 24 h on ‘high' glucose (25 mM) to mimic hyperglycaemic conditions, a 2-h ‘pulse' of 33 mM glucose and also insulin alone increased OEA and PEA levels, and insulin also enhanced the 33 mM glucose-induced OEA and PEA increase (Figure 5).
Figure 5.
Levels of oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) in RIN m5F β-cells kept on ‘low' (13 mM, G13) and ‘high' (25 mM, G25) glucose for 24 h before stimulation with either glucose (33 mM, 2 h, G33), insulin (100 nM, 2 h) or glucose+insulin (2 h). Data are means±s.e.mean of n=6 separate experiments. *=P<0.05 vs vehicle as assessed by ANOVA followed by the Bonferroni's test. Note that ‘low', ‘high' and ‘very high' glucose do not refer to, nor do they reflect, the fasting concentrations of glucose occurring in humans during normo- or hyperglycaemia, respectively. They refer instead to the optimal culturing conditions of RIN-m5F β-cells, which the manufacturer advises to grow in 25 mM glucose.
Regulation of OEA and PEA levels by leptin and PPARs
We observed no changes in OEA and PEA levels in β-cells kept on ‘low' glucose after prolonged (24 h) stimulation with leptin (20 nM), ciglitazone (20 μM, 12 h) and WY14643 (20 μM, 12 h; n=4–6, data not shown).
Dysregulation of OEA and PEA levels in the pancreas of obese mice
In these mice, we observed no changes in either OEA or PEA levels with respect to lean controls (Table 5).
Dysregulation of blood OEA and PEA levels in hyperglycaemia
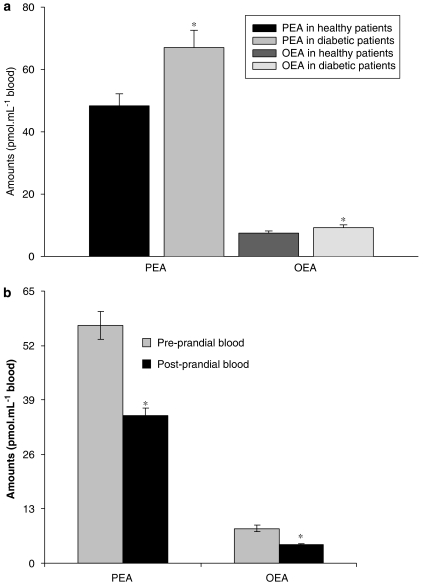
In the blood of nonobese T2D patients, as compared to age-, BMI- and gender-matched normoglycaemic volunteers (see Table 3 for patients' data), we observed an increase of PEA levels (Figure 6a). OEA levels were also increased (from 7.5±0.7 to 9.3±0.9 pmol ml−1). Conversely, following a meal in young normoglycaemic volunteers (see Table 4 for patients' data), both PEA and OEA blood levels were significantly decreased (Figure 6b).
Figure 6.
(a) Blood oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) levels in overweight type 2 diabetes (T2D) vs healthy volunteers. Data are means±s.e.mean of n=8 healthy volunteers and n=10 T2D patients. (b) Blood OEA and PEA levels in postprandial vs preprandial healthy normoweight volunteers. Blood sampling was carried out 1 h before and after the meal, respectively. Data are means±s.e.mean of n=12 subjects. *=P<0.05 vs controls, respectively, as assessed by the Kruskal–Wallis nonparametric test (a), or the two-tailed paired Student's t-test (b).
Discussion
With the present study, we wanted to (1) review the increasing evidence supporting the role of AEs, via cannabinoid and noncannabinoid receptors, in the regulation of metabolism and in inflammatory conditions accompanying metabolic disorders; and (2) investigate if the levels of two AEs, that is OEA and PEA, are regulated in a way similar to those of their chemically and metabolically related congener, and cannabinoid receptor ligand, AEA, in models of adipocytes and β-cells, and in the adipose tissue, pancreas and blood of DIO mice and obese or T2D patients (Matias et al., 2006).
Adipocytes and adipose tissue
Despite the previous observation that in adipocytes OEA stimulates lipolysis through a mechanism independent of cAMP and calcium (Guzman et al., 2004; Yang et al., 2007), and inhibits glucose uptake (Gonzalez-Yanes et al., 2005), in the present study, we could not observe any change in its levels in adipocytes under conditions previously demonstrated to modify AEA levels (Matias et al., 2006) and found to cause changes also in PEA levels. In fact, OEA concentrations did not change during differentiation from mouse 3T3F442A pre- to mature adipocytes and then hypertrophic adipocytes, induced by chronic treatment with a high concentration of insulin, whereas PEA levels were dramatically decreased in both mature and hypertrophic adipocytes (Figure 2b). Furthermore, unlike PEA, OEA was not downregulated by stimulation of either leptin or PPAR-γ receptors (Figure 3), possibly suggesting that the tonic stimulation of these receptors underlies the decrease of PEA in mature (by both leptin and PPAR-γ) and hypertrophic (by leptin only) adipocytes. The finding of lack of regulation over OEA levels by a key event like adipocyte differentiation, and by three major adipocyte-signalling systems (insulin, leptin and PPAR-γ) might suggest that this compound does not play a major autocrine role in adipocyte function and lipolysis. Thus, OEA originating from other cells might be responsible for the previously observed pharmacological effects of this compound on adipocytes. Conversely, AEA and PEA might play an autocrine function in adipocytes. The levels of the former compound peak before adipocyte maturation to then go back to preadipocyte levels (Matias et al., 2006). Therefore, AEA is likely to participate in adipocyte differentiation and lipogenesis by stimulating CB1 receptors, which, in turn, cause PPAR-γ overexpression (Matias et al., 2006), glucose uptake (Gasperi et al, 2007) and lipoprotein lipase activation (Cota et al., 2003). At higher concentrations, AEA might cause some of these effects by directly activating PPAR-γ (Bouaboula et al., 2005), but might also reduce lipogenesis by activating TRPV1 channels (Zhang et al., 2007), thus acting as a negative feedback signal to limit adipocyte size. On the other hand, PEA is known to counteract inflammatory cytokine production/release from mononuclear cells (Berdyshev et al., 1997, 1998), and might play a similar role also in adipocytes by activating PPAR-α. In fact, these cells and the adipose tissue produce a large number of proinflammatory cytokines like TNF-α and interleukin-6 (IL-6), thus causing macrophage infiltration into adipose tissue, an event that seems to be a crucial trigger for atherogenic inflammation during obesity (Wellen and Hotamisligil, 2003). In this perspective, the reduction of PEA levels in mature and hypertrophic adipocytes (Figure 2b) might be analogous to the downregulation of PPAR-γ (which is also an important anti-inflammatory signal) in these cells, and hence contribute to this inflammatory process. Indeed, some of us observed that PEA causes inhibition of lipopolysaccharide (LPS)-induced release of TNF-α from human subcutaneous adipocytes in culture (L Hoareau, M Buyse, P Ravanan, M-P Gonthier, I Matias, S Petrosino, H Caillens, C Lefebvre d'Hellencourt, M Cesari, V Di Marzo, F Festy and R Roche, in preparation). On the other hand, Hoareau et al. (2006) found that PEA does not affect LPS-induced IL-6 production in these adipocytes, and instead it enhances LPS-inhibition of leptin release. Therefore, it is also possible that PEA decrease in mouse hypertrophic adipocytes contributes to disinhibiting leptin release under inflammatory conditions. Conversely, leptin does not suppress the levels of OEA (perhaps in agreement with the fact that both compounds have anorectic properties), but does reduce PEA (Figure 3a) and AEA (Matias et al., 2006) levels, whereas PPAR-γ only affects PEA levels (Figure 3a and Matias et al., 2006). It must be emphasized that in human subcutaneous adipocytes cultured in vitro, leptin, but not PPAR-γ, inhibited the levels of PEA, but not those of OEA or AEA (Gonthier et al., 2007).
The downregulation of PEA levels in mature/hypertrophic adipocytes corresponds to what we have found here in vivo in the subcutaneous fat of DIO mice, where the levels of PEA were significantly reduced as compared to lean mice (Table 5), and where we recently observed a downregulation also of AEA levels (K Starowicz et al., submitted). Unlike isolated adipocytes, however, OEA levels were found to decrease in the subcutaneous fat of DIO mice also (although much less than PEA levels, that is, threefold vs ninefold, Table 5). PEA levels, but not OEA levels, were higher in subcutaneous than in visceral fat of lean mice, and none of the two compounds, nor AEA (K Starowicz et al., submitted), underwent any changes in this latter adipose depot following development of DIO (Table 5).
In human obese patients, unlike DIO mice, the amounts of PEA were found to be lower in the subcutaneous fat, whereas those of OEA (Figure 4) and AEA (Matias et al., 2006) were similar in the two adipose tissue compartments. Interestingly, PEA and OEA levels in the human were 2.5–5-fold higher than those in mouse visceral fat (Table 5 and Figure 4). Unfortunately, we could not analyse the subcutaneous fat of normoweight human volunteers, and hence we could not assess whether PEA levels in humans also decrease in subcutaneous fat following the development of obesity. In the visceral fat, however, the levels of neither PEA nor OEA changed as compared to the visceral fat of normoweight volunteers (Figure 4), similar to that previously observed for AEA, but nor for 2-AG (whose levels increase in this adipose compartment; Matias et al., 2006).
In summary, the regulation of PEA levels in the adipose tissue in vivo in obese mice seems to reflect to some extent the outcome of our experiments in mouse adipocytes only for what concerns the subcutaneous fat. In all cases that can be compared to one another, that is, when passing from just differentiated (4 days) to hypertrophic (12 days) isolated adipocytes, and from the adipose tissues of lean to those of obese mice, PEA levels change in exactly the same way as those of AEA, that is, they decrease in adipocytes and subcutaneous fat and seem to remain constant in visceral fat. In contrast, the levels of OEA change like those of the other two AEs only in in vivo experiments. From the biochemical point of view, this phenomenon might indicate that the levels of all three AEs are regulated in similar ways (that is by using the same biosynthetic and/or degrading enzymes or by similar changes in the levels of their NAPE ultimate precursors) in mouse adipose tissues in vivo, but not in mature mouse adipocytes, where only PEA and AEA levels responded to chronic insulin treatment in similar ways. However, it must be emphasized that, although these two compounds were downregulated in a similar way by leptin, PPAR-γ stimulation only reduced PEA levels (Figure 3b and Matias et al., 2006), thus indicating that this nuclear receptor might affect in a different way PEA and AEA biosynthesis in mouse 3T3F442A adipocytes. In human subcutaneous adipocytes, instead, it was leptin that inhibited only PEA levels without affecting AEA and OEA levels, whereas PPAR-γ has no effect on any of the three compounds (Gonthier et al., 2007). From a functional point of view, since PEA possesses anti-inflammatory effects but, unlike AEA, has no known direct effect on lipogenesis, the reduction of PEA levels can be reconciled with the proinflammatory profile typical of the adipose tissue of obese individuals, a profile that could be caused in part by a reduced tonic anti-inflammatory action by PEA. Furthermore, in view of its aforementioned enhancement of LPS-induced inhibition of leptin release from human subcutaneous adipocytes (Hoareau et al., 2006), it is also possible that PEA reduction in the subcutaneous fat is partly responsible for the higher leptin levels found in obese individuals.
Insulinoma β-cells and pancreas
We have reported here, for the first time, the presence of OEA and PEA in a model of rat pancreatic islet β-cells, the RIN mF5 cells. We found that leptin, ciglitazone or WY14643 do not regulate OEA and PEA levels in these cells, whereas leptin does inhibit AEA levels (Matias et al., 2006). In cells grown in 13 mM glucose, a 33 mM glucose pulse decreases significantly both PEA and OEA levels, whereas in cells kept for 24 h under conditions mimicking hyperglycaemia (25 mM glucose), we observed the opposite effect by 33 mM glucose (Figure 5). Under both conditions, insulin reinforced the glucose-induced effect on OEA and PEA levels, and insulin alone even increased both OEA and PEA levels in cells kept in 25 mM glucose (Figure 5). This suggests that a dysregulation of OEA and PEA levels may occur in β-cells under conditions mimicking hyperglycaemia, and that the capability of insulin to decrease OEA and PEA is lost under these conditions. The effect of glucose on OEA/PEA levels in RIN mF5 cells kept in ‘low' glucose is opposite to that previously observed with AEA and 2-AG in the same cells and under the same conditions, in which, however, insulin does inhibit 33 mM glucose-induced upregulation of the two endocannabinoids (Matias et al., 2006). On the other hand, in cells kept in 25 mM glucose, AEA and 2-AG are also upregulated by both 33 mM glucose and insulin, and to a larger extent than OEA and PEA (Matias et al., 2006). The finding of the reduction of OEA levels by glucose and glucose+insulin is in line with the anorexic properties of OEA and with the observation that OEA decreases in the small intestine of lean rats during feeding (Rodriguez de Fonseca et al., 2001). Furthermore, recently Gonzalez-Yanes et al. (2005) demonstrated that OEA impairs glucose tolerance and inhibits glucose uptake in adipocytes. If such a phenomenon also occurs in β-cells, or if the glucose-induced inhibition of OEA observed here in RIN mF5 cells occurs also in adipocytes, this latter effect might represent a negative feedback response aimed at reducing OEA inhibition of insulin-mediated glucose uptake by these two cell types. This effect would, however, be disrupted in cells under conditions of hyperglycaemia, where, on the contrary, elevation of OEA production might contribute to insulin resistance. The elevation of PEA levels by glucose in RIN mF5 cells kept in high glucose (Figure 5) might be seen, instead, as an adaptive response to the toxic and hence likely proinflammatory stimulus represented by hyperglycaemia. Interestingly, however, in the pancreas of DIO mice, we could not detect any increase of OEA and PEA levels, despite the fact that these mice were strongly hyperglycaemic (Table 5). It is possible that, out of the many cell types that comprise the pancreas, the dysregulation of these two lipid mediators only occurs in β-cells, and therefore cannot be seen when analysing the whole organ. Alternatively, species-dependent variations might also account for these differences, although it must be emphasized that AEA and 2-AG levels do increase in the pancreas of DIO mice, and that these two compounds were more strongly upregulated by glucose and insulin than OEA and PEA in RIN mF5 cells kept in 25 mM glucose (Matias et al., 2006). Furthermore, in agreement with our data, OEA was found to decrease in the pancreas of lean, normoglycaemic rats after free feeding (Fu et al., 2007).
We have also reported here for the first time that in human blood, OEA and PEA levels are decreased following a meal in normoglycaemic volunteers (Figure 6b) and are instead permanently increased under conditions of chronic hyperglycaemia, that is in patients with T2D under randomized pharmacological treatment (Figure 6a), exactly as shown previously for both AEA and 2-AG (Matias et al., 2006). Both phenomena might be related to our findings in β-cells, although we have provided no evidence here that the switch from inhibition to stimulation of the levels of OEA and PEA by glucose and insulin observed in RIN mF5 cells when passing from ‘low' to ‘high' glucose occurs also in those peripheral tissues that might release the two compounds into the blood. Indeed, we do not know the cellular source(s) responsible for the presence of these two compounds in the blood, nor whether their blood concentrations (ranging from ∼35 to ∼65 nM for PEA and from ∼5 to ∼10 nM for OEA) are sufficient to produce any pharmacological effects. Since these concentrations are much lower than those normally found in several tissues, we can hypothesize that OEA and PEA found in the blood are due to ‘spillover' from peripheral organs, in which these compounds are biosynthesized and exert their effects. Nevertheless, our findings in the blood of normoglycaemic patients are in full agreement with a recent study carried out in rats (Fu et al., 2007), in which OEA was found to decrease after food intake in the white adipose tissue, pancreas, spleen, liver and blood. In this same study, it was found that PEA levels did not change following food consumption in the jejunum, whereas we also found that PEA levels decrease in the blood of normoglycaemic patients following a meal, and that they are permanently increased in T2D patients. Since postprandial hyperglycaemia increases the magnitude and duration of the systemic inflammatory responses, particularly during T2D (Kempf et al., 2006), it is possible that the changes in blood PEA levels in postprandial vs preprandial human blood and in T2D patients are somehow related to glucose-induced inflammation.
In conclusion, whereas in insulinoma β-cells, OEA and PEA are regulated by glucose differently from AEA (although all three compounds are upregulated by glucose under culturing conditions rich in glucose), the levels of all three AEs seem to be regulated exactly in the same way in human blood following transient (postprandial) or permanent (T2D) hyperglycaemia. This is in agreement with the fact that the biosynthetic and degradative mechanisms are similar for the three compounds, at least to some extent.
Conclusions
In this study, we reviewed the current knowledge of the potential role of three bioactive AEs in the control of energy metabolism (in the case of AEA and OEA) and inflammation associated with obesity and T2D (in the case of PEA). In doing so, we laid particular emphasis on adipocytes and β-cells, and on the organs that contain these two cell types. Our overview reveals that (1) despite the fact that OEA exerts strong pharmacological effects on adipocytes, little is known on the regulation of its levels in adipocytes during differentiation or in the adipose tissue during obesity; (2) despite the fact that OEA inhibits glucose tolerance, no study have been carried out on the regulation of its levels in β-cells and blood; (3) whereas the anti-inflammatory effects of PEA at the level of blood cells and adipocytes have been investigated, no information is available on its regulation in hypertrophic (and hence proinflammatory) adipocytes and in β-cells grown under hyperglycaemic (and hence proinflammatory and proapoptotic) conditions; and (4) no data exist on the levels of OEA and PEA in the adipose tissue of obese patients or in the blood of T2D patients. We have, therefore, attempted here to fill in part these gaps, by using the same experimental approach that previously led to the understanding of AEA regulation during the above-mentioned conditions of unbalanced energy homeostasis (Matias et al., 2006; K Starowicz et al., submitted). Our data can be summarized as follows (Table 6): (1) In mouse adipocytes, PEA, but not OEA, is regulated during differentiation, and inhibited by both leptin and PPAR-γ; (2) PEA and OEA are present in a model of rat β-cells and are either down- or upregulated by glucose and insulin during pseudonormoglycaemic and -hyperglycaemic conditions, respectively; (3) PEA levels decrease in the subcutaneous fat, but not in the visceral fat and pancreas of DIO mice, and do not change in the visceral fat of obese patients; and (4) circulating PEA and OEA levels are lower after a meal in normoglycaemic humans, and are higher in T2D patients. Clearly, further studies will be required to understand the role of OEA in β-cells and insulin release, and of PEA in the inflammation accompanying obesity and T2D.
Table 6.
Summary of the metabolic regulation of acylethanolamide levels in various organs and cells
| 3T3F442A mouse adipocytes | RIN mF5 rat insulinoma β-cells | Adipose tissue | Pancreas | Small intestine | Hypothalamus | Blood | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| During differentiation with insulin | Leptin | PPAR-α | PPAR-γ | Pulse of 33 mM glucose in cells kept in 13 mM glucose | Pulse of 33 mM glucose in cells kept in 25 mM glucose | Leptin | PPAR-α, PPAR-γ | Obesity | Obesity | Food consumption | Food consumption | Food consumption | T2D | |
| AEA | Peaks at day 4 and then decreases | Decreases | Does not change | Does not change | Increases and insulin blocks this effect | Increases and insulin does not block this effect | Decreases | No effect | Increases in epidydimal fat of DIO mice and visceral fat of obese patients, does not change in the visceral fat of DIO mice and decreases in subcutaneous fat of DIO mice | Increases in DIO mice | Decreases | Decreases | Decreases in human blood | Increases |
| OEA | Does not change | Does not change | Does not change | Does not change | Decreases and insulin enhances this effect | Increases and insulin enhances this effect | No effect | No effect | Does not change in visceral fat of DIO mice and obese patients and decreases in subcutaneous fat of DIO mice | Does not change in DIO mice | Increases | Does not change | Decreases in both human and rat blood | Increases |
| PEA | Decreases starting from day 4 | Decreases | Does not change | Decreases | Decreases and insulin enhances this effect | Increases and insulin enhances this effect | No effect | No effect | Does not change in visceral fat of DIO mice and obese patients and decreases in subcutaneous fat of DIO mice | Does not change in DIO mice | Does not change | ND | Decreases in human blood | Increases |
Abbreviations: AEA, arachidonoylethanolamide; DIO, high-fat diet-induced obesity; ND, not determined; OEA, oleoylethanolamide; PEA, palmitoylethanolamide; PPAR, peroxisome proliferator-activated receptor; T2D, type 2 diabetes.
Based on the data reported here and in Matias et al. (2006); Fu et al. (2007); and K Starowicz et al. (submitted).
Acknowledgments
We thank Mr Marco Allarà, Endocannabinoid Research Group, CNR, Italy for technical assistance. This study was partly supported by a grant from Sanofi-Aventis (to Vincenzo Di Marzo). Isabel Matias, Stefania Petrosino and Vincenzo Di Marzo are the recipients of a research grant from Sanofi-Aventis.
Abbreviations
- AE
acylethanolamide
- AEA
arachidonoylethanolamide
- 2-AG
2-arachidonoylglycerol
- DIO
diet-induced obesity
- FAAH
fatty acid amide hydrolase
- LPS
lipopolysaccharide
- NAAA
N-acylethanolamine-hydrolysing acid amidase
- NAPE
N-acyl-phosphatidylethanolamine
- NAPE-PLD
NAPE-selective phospholipase D
- NArPE
N-arachidonoyl-phosphatidylethanolamine
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamide
- PPAR
peroxisome proliferator-activated receptor
- T2D
type 2 diabetes
- TRPV1
transient receptor potential vanilloid type-1
Conflict of interest
The authors state no conflict of interest.
References
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- Astarita G, Rourke BC, Andersen JB, Fu J, Kim JH, Bennett AF, et al. Postprandial increase of oleoylethanolamide mobilization in small intestine of the Burmese python (Python molurus) Am J Physiol Regul Integr Comp Physiol. 2006;290:R1407–R1412. doi: 10.1152/ajpregu.00664.2005. [DOI] [PubMed] [Google Scholar]
- Bachur NR, Masek K, Melmon KL, Udenfriend S. Fatty acid amides of ethanolamine in mammalian tissues. J Biol Chem. 1965;240:1019–1024. [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Berdyshev E, Boichot E, Corbel M, Germain N, Lagente V. Effects of cannabinoid receptor ligands on LPS-induced pulmonary inflammation in mice. Life Sci. 1998;63:PL125–PL129. doi: 10.1016/s0024-3205(98)00324-5. [DOI] [PubMed] [Google Scholar]
- Berdyshev EV, Boichot E, Germain N, Allain N, Anger JP, Lagente V. Influence of fatty acid ethanolamides and delta9-tetrahydrocannabinol on cytokine and arachidonate release by mononuclear cells. Eur J Pharmacol. 1997;330:231–240. doi: 10.1016/s0014-2999(97)01007-8. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Romero J, Garcia-Gil L, Suarez I, De la Cruz P, Ramos JA, et al. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim Biophys Acta. 1998;1407:205–214. doi: 10.1016/s0925-4439(98)00042-8. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–181. doi: 10.1016/j.ejphar.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe J. [Molecular endocrinology of hereditary obesity] Bull Mem Acad R Med Belg. 1997;152:189–194. [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids: an update. Curr Opin Pharmacol. 2004;4:572–579. doi: 10.1016/j.coph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Izzo AA, Degenhardt B, Valenti M, Scaglione G, Capasso R, et al. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available pre-clinical data, and first human studies. Neuropharmacology. 2005;48:1154–1163. doi: 10.1016/j.neuropharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- De Marchi N, De Petrocellis L, Orlando P, Daniele F, Fezza F, Di Marzo V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:1–9. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Petrosino S.Endocannabinoids and the regulation of their levels in health and disease Curr Opin Lipidol 200718129–140.Review [DOI] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, et al. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem. 2007;282:1518–1528. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 2005;48:1147–1153. doi: 10.1016/j.neuropharm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Gasperi V, Fezza F, Pasquariello N, Bari M, Oddi S, Agro AF, et al. Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cell Mol Life Sci. 2007;64:219–229. doi: 10.1007/s00018-006-6445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giang DK, Cravatt BF. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc Natl Acad Sci USA. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonthier MP, Hoareau L, Festy F, Matias I, Valenti M, Bes-Houtmann S, et al. Identification of endocannabinoids and related compounds in human fat cells. Obesity (Silver Spring) 2007;15:837–845. doi: 10.1038/oby.2007.581. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Yanes C, Serrano A, Bermudez-Silva FJ, Hernandez-Dominguez M, Paez-Ochoa MA, Rodriguez de Fonseca F, et al. Oleylethanolamide impairs glucose tolerance and inhibits insulin-stimulated glucose uptake in rat adipocytes through p38 and JNK MAPK pathways. Am J Physiol Endocrinol Metab. 2005;289:E923–E929. doi: 10.1152/ajpendo.00555.2004. [DOI] [PubMed] [Google Scholar]
- Guzman M, Lo Verme J, Fu J, Oveisi F, Blazquez C, Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha) J Biol Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- Hoareau L, Ravanan P, Gonthier MP, Delarue P, Goncalves J, Cesari M, et al. Effect of PEA on LPS inflammatory action in human adipocytes. Cytokine. 2006;34:291–296. doi: 10.1016/j.cyto.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol. 2004;228:121–128. doi: 10.1016/j.mce.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Juan-Pico P, Fuentes E, Bermudez-Silva FJ, Javier Diaz-Molina F, Ripoll C, Rodriguez de Fonseca F, et al. Cannabinoid receptors regulate Ca(2+) signals and insulin secretion in pancreatic beta-cell. Cell Calcium. 2006;39:155–162. doi: 10.1016/j.ceca.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Kempf K, Rose B, Herder C, Kleophas U, Martin S, Kolb H. Inflammation in metabolic syndrome and type 2 diabetes: impact of dietary glucose. Ann NY Acad Sci. 2006;1084:30–48. doi: 10.1196/annals.1372.012. [DOI] [PubMed] [Google Scholar]
- Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic. Curr Med Chem. 1999;6:757–773. [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005a;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D. Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci. 2005b;62:708–716. doi: 10.1007/s00018-004-4494-0. [DOI] [PubMed] [Google Scholar]
- Long DA, Martin AJ. Factor in arachis oil depressing sensitivity to tuberculin in BCG-infected guineapigs. Lancet. 1956;270:464–466. doi: 10.1016/s0140-6736(56)90529-3. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agro A, Rossi A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J Biol Chem. 2003;278:32726–32732. doi: 10.1074/jbc.M302123200. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Fride E, Bisogno T, Bari M, Cascio MG, Battista N, et al. Up-regulation of the endocannabinoid system in the uterus of leptin knockout (ob/ob) mice and implications for fertility. Mol Hum Reprod. 2005;11:21–28. doi: 10.1093/molehr/gah130. [DOI] [PubMed] [Google Scholar]
- Madsen L, Petersen RK, Kristiansen K. Regulation of adipocyte differentiation and function by polyunsaturated fatty acids. Biochim Biophys Acta. 2005;1740:266–286. doi: 10.1016/j.bbadis.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Matias I, Martiadis V, De Petrocellis L, Maj M, Di Marzo V. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology. 2005;30:1216–1221. doi: 10.1038/sj.npp.1300695. [DOI] [PubMed] [Google Scholar]
- Movahed P, Jonsson BA, Birnir B, Wingstrand JA, Jorgensen TD, Ermund A, et al. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280:38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oveisi F, Gaetani S, Eng KT, Piomelli D. Oleoylethanolamide inhibits food intake in free-feeding rats after oral administration. Pharmacol Res. 2004;49:461–466. doi: 10.1016/j.phrs.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- Petersen G, Sorensen C, Schmid PC, Artmann A, Tang-Christensen M, Hansen SH, et al. Intestinal levels of anandamide and oleoylethanolamide in food-deprived rats are regulated through their precursors. Biochim Biophys Acta. 2006;1761:143–150. doi: 10.1016/j.bbalip.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Ramirez ZJL, Castro MF, Kuri HW. Quantitation of adipose conversion and triglycerides by staining intra-cytoplasmic lipids with Oil Red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- Re G, Barbero R, Miolo A, Di Marzo V. Palmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: potential use in companion animals. Vet J. 2007;173:21–30. doi: 10.1016/j.tvjl.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Roche R, Hoareau L, Bes-Houtmann S, Gonthier MP, Laborde C, Baron JF, et al. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem Cell Biol. 2006;126:177–187. doi: 10.1007/s00418-005-0127-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, et al. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J. 2004;380:749–756. doi: 10.1042/BJ20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Nogueron MI, Muthian S, Sanudo-Pena MC, Hillard CJ, Deutsch DG, et al. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci Lett. 1998;254:137–140. doi: 10.1016/s0304-3940(98)00700-9. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem. 2005;280:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Zhao LY, Okamoto Y, Araki N, Ueno M, Sakamoto H, et al. Predominant expression of lysosomal N-acylethanolamine-hydrolyzing acid amidase in macrophages revealed by immunochemical studies. Biochim Biophys Acta. 2007;1771:623–632. doi: 10.1016/j.bbalip.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Ueda N, Yamanaka K, Yamamoto S. Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J Biol Chem. 2001;276:35552–35557. doi: 10.1074/jbc.M106261200. [DOI] [PubMed] [Google Scholar]
- Wang J, Okamoto Y, Morishita J, Tsuboi K, Miyatake A, Ueda N. Functional analysis of the purified anandamide-generating phospholipase D as a member of the metallo-beta-lactamase family. J Biol Chem. 2006;281:12325–12335. doi: 10.1074/jbc.M512359200. [DOI] [PubMed] [Google Scholar]
- Wang X, Miyares RL, Ahern GP. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J Physiol. 2005;564:541–547. doi: 10.1113/jphysiol.2004.081844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen M, Georgeson KE, Harmon CM. Mechanism of oleoylethanolamide on fatty acid uptake in small intestine after food intake and body weight reduction. Am J Physiol Regul Integr Comp Physiol. 2007;292:R235–R241. doi: 10.1152/ajpregu.00270.2006. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]