Abstract
Background and purpose:
In anaesthetized spontaneously hypertensive rats (SHR), there is evidence for up-regulation of cannabinoid (CB1) receptors: antagonism of CB1 receptors causes a rise in blood pressure, and administration of the endocannabinoid, anandamide, or inhibition of anandamide degradation causes hypotension. These findings have led to the suggestion that the endocannabinoid system may be a therapeutic target in hypertension. However, since the cardiovascular responses to cannabinoids are substantially influenced by anaesthesia, the purpose of this study was to assess regional haemodynamic responses to cannabinoid receptor stimulation and inhibition in conscious SHR.
Experimental approach:
Cardiovascular responses to i.v. administration of anandamide, the cannabinoid receptor agonist, WIN 55212-2, and the CB1 receptor antagonist, AM 251, were measured in male SHR, Wistar Kyoto rats and outbred Wistar rats, chronically instrumented for recording renal, mesenteric and hindquarters haemodynamics in the conscious, freely-moving state.
Key results:
Hypotensive responses to anandamide and WIN 55212-2 only occurred in SHR, but these were relatively modest and not associated with CB1 receptor-mediated vasodilatation. In SHR only, anandamide caused bradycardia, which was inhibited by AM 251. Furthermore, a pressor response to CB1 receptor antagonism occurred only in SHR, but was not associated with vasoconstriction. Moreover, there was some evidence for CB1 receptor-mediated vasoconstrictor actions of anandamide in SHR, which was not seen in the normotensive strains.
Conclusions and implications:
The results are consistent with activation of CB1 receptors in SHR by endogenous ligands exerting an antihypertensive effect, but the findings do not indicate enhanced CB1 receptor-mediated vasodilator mechanisms in SHR.
Keywords: anandamide, cannabinoids, hypertension, regional haemodynamics, spontaneously hypertensive rats
Introduction
The cardiovascular effects of cannabinoids are of interest, particularly since it has been suggested that targeting the endocannabinoid system may be a novel therapeutic strategy, either by inhibiting their action in hypotensive shock states, or by augmenting their effects in hypertensive conditions (for review, see Pacher et al., 2006). In the context of the latter, it has been shown that, in anaesthetized spontaneously hypertensive rats (SHR), there was an enhanced depressor response to administration of the endocannabinoid, anandamide and to an inhibitor of anandamide degradation (Bátkai et al., 2004). Furthermore, SHR, but not the normotensive controls, showed a pressor response to cannabinoid (CB1) receptor antagonists, and there was evidence for increased expression of CB1 receptors in the heart and aortic endothelium of the SHR (Bátkai et al., 2004). Collectively, these findings indicate an upregulation of the endocannabinoid system in SHR which was exerting a CB1 receptor-mediated antihypertensive effect.
There has been some debate about the underlying haemodynamic changes responsible for the effects of anandamide on blood pressure (for review see Randall et al., 2004), and although in vitro evidence indicates a vasodilator action of anandamide via multiple mechanisms (O'Sullivan et al., 2004), most in vivo studies have shown that, in the normotensive state, anandamide-induced hypotension is mainly, if not exclusively, due to a fall in cardiac output, rather than a peripheral vascular effect (Bátkai et al., 2004, Pacher et al., 2005). The very acute, short-lived (1–2 s), hypotensive response to anandamide appears to be due to vagally mediated bradycardia, which does not involve CB1 receptors (Lake et al., 1997a, 1997b; Gardiner et al., 2002a). In contrast, the slower onset and more sustained, hypotensive effect of anandamide seen in anaesthetized rats is accompanied by modest, CB1 receptor-mediated, bradycardia (Lake et al., 1997a, 1997b) and reduced cardiac contractility (Bátkai et al., 2004), consistent with in vitro evidence for a negative inotropic effect of anandamide, which may (Bonz et al., 2003), or may not (Ford et al., 2002), involve the CB1 receptor.
However, the study of Bátkai et al. (2004) showed that, in the anaesthetized SHR, the enhanced hypotensive effect of anandamide was also, in part, due to a fall in total peripheral resistance, that is attributable to a vasodilator action of the endocannabinoid.
Since the cardiovascular effects of anandamide are known to be influenced by anaesthesia (Lake et al., 1997b; Mendizábal and Adler-Graschinsky, 2007), the aim of the present study was to assess the haemodynamic effects of anandamide, and the effects of CB1 receptor antagonism, in conscious, freely moving, SHR. In addition to blood pressure and heart rate, measurements of regional vascular conductance changes were made to determine which vascular beds were contributing to the effects observed. Furthermore, cardiovascular responses to the cannabinoid agonist, WIN 55212-2, were evaluated to determine the extent to which any changes in responsiveness to anandamide were mirrored by changes in response to the synthetic agonist. Due to the long-standing controversy surrounding the appropriate control strain for the SHR (for example, Rapp, 1987; St Lezin et al., 1992), two normotensive strains were used for comparison, that is Wistar Kyoto rats (WKY) and outbred Wistar rats.
Methods
Animals and surgical preparation
Animals
All procedures were approved by the University of Nottingham Ethical Review Committee, and were performed under UK Home Office Project Licence Authority. Male, spontaneously hypertensive rats (SHR), Wistar rats (Charles River UK) and Wistar Kyoto rats (WKY) (Harlan UK) were housed in a temperature-controlled environment (20–22°C) with a 12 h light/dark cycle (lights on at 0600 hours). Rats were allowed food and water ad libitum throughout, and were held within the Biomedical Services Unit in the University of Nottingham for at least a week before commencement of any procedures.
Surgical preparation
All surgery was carried out under general anaesthesia (fentanyl and medetomidine, 300 μg kg−1 of each, i.p.), which was reversed by nalbuphine and atipamezole (1 mg kg−1 of each, s.c.), with nalbuphine also providing analgesia. For some of the later experiments, buprenorphine (0.02 mg kg−1 s.c.) was used in place of nalbuphine, which was no longer available.
At the first surgical stage, miniaturized Doppler flow probes were sutured around the left renal and superior mesenteric arteries and the distal abdominal aorta (below the level of the ileocaecal artery) for measurement of hindquarters flow.
At least 10 days after probe implantation, and subject to veterinarian checks, rats were again anaesthetized. The Doppler flow probe wires were soldered into a plug (Microtech Inc., Boothwyn, PA, USA), which was mounted into a harness worn by the rat. Three separate catheters were inserted into the right jugular vein to allow drug administration, and a single catheter was inserted into the distal abdominal aorta via the caudal artery, enabling arterial blood pressure and heart rate measurement. Animals were left to recover for 24 h before experiments began.
At the time of experimentation, male Wistar rats weighed between 350 and 450 g, whereas SHR (∼20 weeks old) and WKY weighed approximately 300 g. In the SHR, protocols for the administration of anandamide and WIN 55212-2 were carried out in separate groups of animals. In WKY and Wistar rats, however, a combined anandamide and WIN 55212-2 protocol was used.
Experimental protocols
Spontaneously hypertensive rats
In one group of SHR (n=10), on the first experimental day, after a period of baseline recording, the vehicle for anandamide (Tocrisolve, 0.1 ml i.v.) was administered followed, at least 60 min later, by anandamide (3 mg kg−1 i.v.). On the second experimental day, AM 251 (3 mg kg−1 i.v. infused over 30 min at 2 ml h−1; Gardiner et al., 2002a, 2002b) was administered and 30 min after the end of the AM 251 infusion, animals were given anandamide (3 mg kg−1).
In a second group of SHR (n=8), on the first experimental day, after a period of baseline recording, the vehicle for WIN 55212-2 (saline containing 5% propylene glycol and 2% Tween-80) was administered (0.1 ml i.v.), followed by WIN 55212-2 (150 μg kg−1) at least 120 min later. On the second experimental day, AM 251 (3 mg kg−1 i.v. infused over 30 min at 2 ml h−1) was administered and 30 min after the end of the AM 251 infusion, animals were given WIN 55212-2 (150 μg kg−1).
Wistar Kyoto rats
One group of WKY (n=12) was used. On day 1, animals were given anandamide (3 mg kg−1) and the vehicle (0.1 ml) in random order, separated by at least 180 min. On day 2, the same animals were given the vehicle for WIN 55212-2 (see above) followed by WIN 55212-2 (150 μg kg−1) at least 120 min later. On day 3, in a subgroup of seven animals, AM 251 was administered (3 mg kg−1 as above) followed by WIN 55212-2 (150 μg kg−1) and anandamide (3 mg kg−1), given in random order, separated by at least 60 min.
Wistar rats
One group of Wistar rats was used (n=10). On day 1, after a period of baseline recording, the vehicle for WIN 55212-2 was administered (as above) followed by WIN 55212-2 (150 μg kg−1) at least 120 min later. After a further period of at least 120 min, when all the cardiovascular effects of WIN 55212-2 had waned, anandamide (3 mg kg−1) was given. On day 2, AM 251 (3 mg kg−1 as above) was administered, and 30 min after the end of infusion, anandamide (3 mg kg−1) was administered followed by WIN 55212-2 (150 μg kg−1) 60 min later. In other experiments (Gardiner et al., 2002b) we have shown that the dose of AM 251 used here is effective for at least 5 h.
Cardiovascular measurements
Data acquisition
All data were recorded by a customized data capture system (Haemodynamics Data Acquisition System (HDAS), University of Limburg, Maastricht, Netherlands). Arterial catheters were attached via a fluid-filled (degassed water) pressure transducer (Gould, type 4-442) with a modified, low volume displacement dome, to a Gould transducer amplifier (model 13-4615-50) and into HDAS. The Doppler flow probe leads were then connected via a Doppler flowmeter (Crystal Biotech VF-1 Mainframe fitted with high velocity (HVPD-20) modules). These data were sampled by HDAS every 2ms, averaged every cardiac cycle and then stored to disc at 5-sec intervals thus enabling recording of heart rate, blood pressure and processed Doppler shift signals. The initial response to anandamide, which occurs within 1–2 cardiac cycles, is highly variable at the dose used (Gardiner et al., 2002a) and is generally agreed not to involve CB1 receptors (Lake et al., 1997b), and was not analysed in this study.
Statistical analysis
Offline analysis of averaged values from experimenter-selected time intervals was conducted using Datview software (University of Limburg, Maastricht, Netherlands). Values were then exported into custom-designed software (Biomed, University of Nottingham) for statistical analysis. The Friedman and Quade tests (non-parametric versions of ANOVA) were used for within-group analysis, Wilcoxon tests were conducted for comparing paired datasets, and Mann–Whitney tests were used for between-group comparisons, where P<0.05 was taken as significant. Multiple comparisons of integrated areas between strains and treatments were calculated using a Kruskal–Wallis test.
Drugs
Fentanyl citrate was from Janssen–Cilag (High-Wycombe, UK); medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were from Pfizer (Kent, UK); nalbuphine hydrochloride (Nubain) was from Bristol-Myers-Squibb (Hounslow, UK); buprenorphine (Vetergesic) was from Alstoe Animal Health (York, UK). Anandamide, Tocrisolve, WIN 55212-2, ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl) pyrrolo [1,2,3-de]-1,4-benzoxazin-6-yl]-1naphthalenylmethanone mesylate) and AM 251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrozole-3-carboxamide) were from Tocris (Bristol, UK), with anandamide supplied dissolved in Tocrisolve. AM 251 and WIN 55212-2 were dissolved in saline with 5% propylene glycol (Sigma UK) and 2% Tween-80 (BDH). AM 251 was infused (2 ml h−1) over 30 min, and all other drugs were administered as bolus intravenous injections given in a volume of approximately 0.1 ml.
Results
Cardiovascular responses to anandamide in the absence and presence of AM 251
Resting cardiovascular variables before administration of anandamide are shown in Table 1. In SHR, resting heart rates and blood pressures were significantly higher, and vascular conductances were generally lower (P<0.05 for renal and mesenteric vs WKY; P<0.05 for renal and hindquarters vs Wistar rats) than in either strain of control rat. Wistar rats had lower blood pressures and lower renal and mesenteric vascular conductances than WKY (Table 1).
Table 1.
Resting cardiovascular variables
|
Before anandamide |
Before WIN 55212-2 |
|||||
|---|---|---|---|---|---|---|
| Wistar (n=10) | WKY (n=12) | SHR (n=14) | Wistar (n=10) | WKY (n=11) | SHR (n=8) | |
| Heart rate (beats min−1) | 319±6 | 325±6 | 353±10*# | 327±6 | 309±4* | 330±11 |
| Mean arterial BP (mm Hg) | 111±2 | 120±3* | 176±5*# | 113±3 | 122±2* | 175±4*# |
| Renal VC (kHz mm Hg−1103) | 58±7 | 87±7* | 40±6*# | 59±9 | 80±5* | 41±4*# |
| Mesenteric VC (kHz mm Hg−1103) | 57±6 | 83±6* | 41±4# | 59±6 | 83±7* | 46±6# |
| Hindquarters VC (kHz mm Hg−1103) | 39±4 | 30±3 | 25±2* | 40±4 | 25±1* | 27±3* |
Abbreviations: SHR, spontaneously hypertensive rats; VC, vascular conductance; WKY, Wistar Kyoto rats.
Values are mean±s.e.means. VC is calculated as Doppler shift (kHz) divided by mean arterial blood pressure (mm Hg).
*P<0.05 vs Wistar, # P<0.05 vs WKY (Kruskal–Wallis).
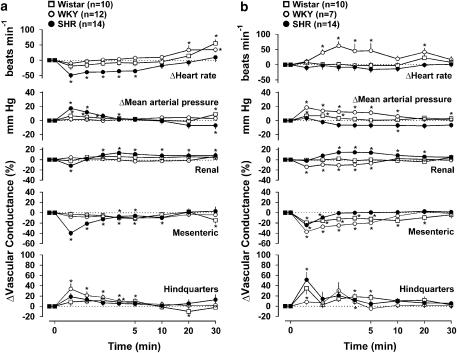
As described above (see Methods), the initial, rapid and very transient response to anandamide, which tends to be highly variable in conscious animals at the dose used (Gardiner et al., 2002a), was not analysed in this study. In WKY and in Wistar rats, the cardiovascular effects of anandamide were modest, the only significant (P<0.05 Friedman's test) changes directly associated with anandamide being a small rise in blood pressure in Wistars, and hindquarters vasodilatation in WKY (Figure 1). In contrast, in SHR, anandamide caused pronounced, sustained bradycardia, together with a rise followed by a fall in blood pressure and renal and mesenteric vasoconstriction, followed by some tendency for vasodilatation in all three vascular beds, albeit only significant in the renal vascular bed (Figure 1). The integrated (0–30 min) bradycardic response to anandamide in SHR (−700±225 beats) was significantly (P<0.05) greater than in WKY (−86±31 beats) and Wistar rats (−205±80 beats). Moreover, although the integrated pressor responses were not different between the three strains, the integrated depressor response was greater (P<0.05) in SHR (−222±67 mm Hg min) than in WKY (−36±14 mm Hg min) and Wistar rats (−27±10 mm Hg min). However, there were no significant between-strain differences in the integrated changes in vascular conductance in response to anandamide (either increases or decreases). Administration of the vehicle for anandamide (Tocrisolve) to WKY caused no significant changes (Friedman's test) in any measured cardiovascular variable (data not shown). In SHR, following administration of Tocrisolve, there was a small reduction in heart rate which was only significant 1 min following the vehicle administration (−15±4 beats min−1, P<0.05, Friedman's test), and the integrated (0–30 min) bradycardic response to anandamide in SHR (−700±225 beats) was significantly greater than the integrated change following the vehicle in that strain (−239±115 beats). There were no significant changes in any other measured cardiovascular variable following vehicle administration to SHR (data not shown).
Figure 1.
Regional haemodynamic responses to anandamide (3 mg kg−1 i.v.) in conscious Wistar rats, WKY and spontaneously hypertensive rats, in the absence (a) and presence (b) of AM 251 (3 mg kg−1 i.v.). Values are mean and vertical bars show s.e.m. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in the text.
Administration of AM 251 had no consistent cardiovascular effects in either Wistar rats or in WKY, but in SHR, there was a significant increase in blood pressure between 20 and 60 min after the onset of AM 251 administration (+12±3 mm Hg at 20 min, +14±3 mm Hg at 40 min), but this pressor effect was not accompanied by significant changes in heart rate or vascular conductance in any monitored vascular bed (at 20 min: heart rate +13±9 beats min−1, renal vascular conductance −5±3%, mesenteric vascular conductance −8±3%, hindquarters vascular conductance +4±4%; at 40 min: heart rate +19±10 beats min−1, renal vascular conductance −1±3%, mesenteric vascular conductance −9±4%, hindquarters vascular conductance −4±3%).
In the presence of AM 251, the cardiovascular effects of anandamide in Wistar rats were still modest, although there was some mesenteric vasoconstriction and hindquarters vasodilatation which were not seen in the absence of AM 251 (Figure 1b). Paradoxically, the cardiovascular effects of anandamide in WKY in the presence of AM 251 were more substantial than in the absence of AM 251, comprising an early rise in blood pressure and renal and mesenteric vasoconstriction and a slower onset tachycardia, none of which occurred in the absence of AM 251 (Figure 1b). In contrast, in SHR, AM 251 abolished the bradycardic, pressor and renal vasoconstrictor responses to anandamide and attenuated the mesenteric vasoconstriction. Under those conditions, anandamide still caused some fall in blood pressure and there was renal and hindquarters vasodilatation (Figure 1b).
Cardiovascular responses to WIN 55212-2 in the absence and presence of AM 251
In SHR, before administration of WIN 55212-2, resting blood pressures were significantly higher than in either control strain of rat, and vascular conductances were lower (P<0.05 for renal and hindquarters vs Wistar rats; P<0.05 for renal and mesenteric vs WKY) (Table 1). In Wistar rats, blood pressures and renal and mesenteric vascular conductances were lower, and heart rate and hindquarters vascular conductance were higher, than in WKY (Table 1).
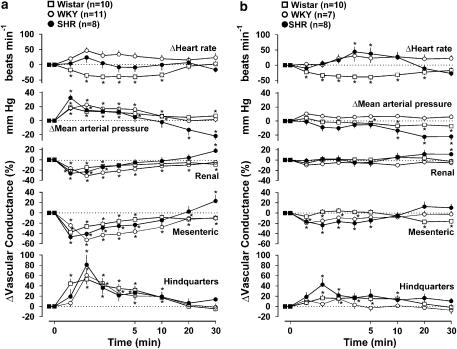
Administration of WIN 55212-2 (150 μg kg−1) caused rises in blood pressure, falls in renal and mesenteric vascular conductances and rises in hindquarters vascular conductance in all three strains of rat (Figure 2a), and the integrated (0–10 min) pressor and vascular conductance changes were not significantly different between the strains. In SHR and WKY there was no significant change in heart rate, but in Wistar rats there was bradycardia lasting up to 10 min after WIN 55212-2 administration (Figure 2a). In SHR only, the rise in blood pressure was followed by a fall (−22±5 mm Hg at 30 min), accompanied by vasodilatation (significant in the renal and mesenteric vascular beds) (Figure 2a). The vehicle for WIN 55212-2 caused no changes in heart rate in either SHR or WKY, and, although it caused a small fall in heart rate in Wistar rats (P<0.05 at 4 and 5 min, −17±7 and −18±7 beats min−1, respectively), the integrated change in heart rate caused by WIN-55212-2 in that strain was significantly greater than the effect of vehicle. Blood pressure was not significantly affected by the vehicle in either WKY or Wistar rats, and in SHR, although there was a small significant increase in blood pressure following the vehicle (P<0.05 between 0 and 5 min), the magnitude of change (+7±3 mm Hg at 1 min) was markedly less than the corresponding change following WIN 55212-2 (+33±5 mm Hg), and there was no subsequent hypotensive response to the vehicle (+5±4 mm Hg at 30 min). Renal vascular conductance was not affected by vehicle administration, mesenteric vascular conductance fell transiently in SHR and WKY (maximum change −14±5 and −8±2% at 1 min respectively), and hindquarters vascular conductance rose transiently in WKY (+17±7% at 1 min), but the magnitude of change in all variables was substantially and significantly smaller than the effect of WIN 55212-2.
Figure 2.
Regional haemodynamic responses to WIN 55212-2 (150 μg kg−1 i.v.) in conscious Wistar rats, WKY and spontaneously hypertensive rats, in the absence (a) and presence (b) of AM 251 (3 mg kg−1 i.v.). Values are mean and vertical bars show s.e.m. *P<0.05 vs baseline (Friedman's test). Statistical comparisons of integrated responses are given in the text.
In the presence of AM 251, the pressor and renal vasoconstrictor responses to WIN 55212-2 were abolished in all three strains of rat. The integrated (0–10 min) fall in mesenteric vascular conductance in response to WIN 55212-2 was significantly reduced by AM 251 in WKY (before=−339±28% min, after=−102±14% min) and Wistar rats (before=−178±36% min, after=−66±22% min), and, although the response was reduced in SHR (before=−264±68% min, after=−155±47% min) the difference was not significant. Similarly, the integrated (0–10 min) hindquarters vasodilator response to WIN 55212-2 was reduced by AM 251 in all three strains (WKY before=+288±56% min, after=+81±26% min; Wistar rats before=+322±54% min, after=+158±49% min; SHR before=+324±54% min, after=+233±55% min), but the effect was not significant in SHR. The delayed fall in blood pressure, seen in SHR in response to WIN 55212-2 in the absence of AM 251, was still present following AM 251 administration (−22±5 mm Hg at 30 min); indeed a small fall in blood pressure also occurred in Wistar rats under these conditions, but the only significant vasodilatation was in the renal vascular bed in SHR (Figure 2b).
Discussion and conclusions
Against the background of evidence for increased involvement of endocannabinoids in cardiovascular regulation in anaesthetized SHR (Bátkai et al., 2004), the specific aim of the present study was to determine the regional haemodynamic effects of the endocannabinoid, anandamide, the CB1 receptor antagonist, AM 251 and the synthetic cannabinoid agonist, WIN 55212-2, in conscious SHR, and compare them with the responses in two normotensive Wistar strains, that is WKY and outbred Wistar rats.
In normotensive rats, the cardiovascular actions of anandamide are complex and influenced by the presence of anaesthesia (for reviews see Randall et al., 2004; Mendizábal and Adler-Graschinsky, 2007). In conscious Sprague–Dawley rats, within 1–2 min after administration of anandamide (that is, after the very rapid, short-lived changes), the cardiovascular effects are modest, comprising a small rise in blood pressure and a bradycardia (Stein et al., 1996; Lake et al., 1997a, 1997b; Gardiner et al., 2002a; Wheal et al., 2007a), with some renal and mesenteric vasoconstriction and hindquarters vasodilatation (Gardiner et al., 2002a; Wheal et al., 2007a). In the normotensive rat strains used in the present study, the cardiovascular effects of anandamide were qualitatively similar to those described previously (excluding the rapid initial effects not measured here), but smaller than observed in response to the same dose in Sprague–Dawley rats under the same conditions (Gardiner et al., 2002a; Wheal et al., 2007a).
Lake et al. (1997b) showed that the pressor response to anandamide in conscious Sprague–Dawley rats was enhanced by the CB1 receptor antagonist, SR 141716A, and, although we previously found no effect of AM 251 on responses to anandamide in Sprague–Dawley rats (Gardiner et al., 2002a), in the present study we observed some AM 251-induced enhancement of pressor and vasoconstrictor responses to anandamide in the Wistar rats and, particularly, in WKY. These findings are consistent with there being a covert CB1 receptor-mediated vasodilator effect of anandamide in normotensive rats that is not normally seen, because the pressor and vasoconstrictor effects of anandamide predominate. The mechanisms underlying the latter are complex, but are generally agreed not to involve CB1 receptors (Lake et al., 1997a, 1997b; Kwolek et al., 2005). Kwolek et al. (2005) proposed a central mechanism for the anandamide-induced pressor response involving β-adrenoceptors and NMDA receptors located in the medulla oblongata. Although the evidence for an involvement of sympathetic activation is not strong (Kwolek et al., 2005), our finding of a tachycardic effect of anandamide in WKY in the presence of AM 251, would be consistent with it causing sympathetic activation, either by peripheral or central mechanisms. Alternatively, it has been shown in isolated atria, that anandamide caused an increase in cardiac contractility in the presence of CB1 receptor antagonism and a decrease in contractility in the presence of CB2 receptor antagonism, suggesting the presence of functional myocardial CB1 and CB2 receptors causing cardio-depression and cardio-excitation, respectively (Sterin-Borda et al., 2005). The tachycardic effect of anandamide in WKY in the presence of AM 251 may, therefore, be a manifestation of CB2 receptor-mediated cardioexcitation.
To our knowledge, only three studies have reported cardiovascular effects of anandamide in SHR previously (Lake et al., 1997b; Li et al., 2003; Bátkai et al., 2004), and only one of those was in conscious animals (Lake et al., 1997b). In that study, after the initial, rapid hypotensive and bradycardic effects of anandamide, there was a short-lived (1–2 min) rise in blood pressure. The present findings corroborate and extend that observation, showing that the transient pressor response to anandamide in SHR, which was more marked than in the control strains, was accompanied by renal and mesenteric vasoconstriction. Furthermore, and in contrast to the findings in the normotensive animals, we found these effects were attenuated by AM 251, indicating CB1 receptor-mediated vasoconstrictor effects in SHR.
The vast majority of evidence indicates that anandamide is a vasorelaxant in isolated arterial vessels from normotensive rats (see Randall et al., 2004) and, in our experience, SHR (Wheal et al., 2007b). To our knowledge, there is only one report of anandamide causing vasoconstriction, and that was in rat mesenteric arteries in calcium-free buffer (White and Hiley, 1998). Therefore, it is most likely that the pressor and vasoconstrictor effects are indirect. As indicated above, studies by Kwolek et al. (2005) have gone some way to identifying the mechanisms involved in the pressor response to anandamide in anaesthetized normotensive rats, but it seems that the mechanisms may differ in SHR, since the effect of AM 251 was the opposite in normotensive rats (that is, augmentation) than in SHR (that is, attenuation). Our finding that the pressor response was reduced by AM 251 in SHR is in contrast to the reports of Lake et al. (1997b), who showed that the pressor response was unchanged by SR 141716A in that strain, although they saw an enhancement by SR 141716A in their normotensive animals.
Lake et al. (1997b) described, in addition, a delayed hypotensive response to anandamide (significant between 10 and 45 min) in conscious SHR, and other studies have reported exaggerated hypotensive responses in anaesthetized SHR compared to normotensive rats (Lake et al., 1997b; Li et al., 2003; Bátkai et al., 2004). Li et al. (2003) found enhanced hypotensive responses to methanandamide (the stable analogue of anandamide) in SHR relative to WKY and provided evidence for an involvement of vanilloid receptors in addition to CB1 receptors, whereas Bátkai et al. (2004) found no effect of the vanilloid receptor antagonist, capsazepine, on the enhanced hypotensive response to anandamide in SHR and Lake et al. (1997b) showed that the CB1 receptor antagonist, SR 141716A, abolished the depressor response. In the present study we observed a small (about 7 mm Hg), delayed hypotensive response to anandamide in conscious SHR, which was accompanied by some evidence for vasodilatation, albeit only significant in the renal vascular bed. The magnitude of effect was considerably less than reported by Lake et al. (1997b), who showed a fall of about 25 mm Hg at 20 min after a similar dose of anandamide (4 mg kg−1), but their data are not inconsistent with the findings of Bátkai et al. (2004), who showed a more marked (about 50 mm Hg) hypotensive response to a higher dose of anandamide (10 mg kg−1) in anaesthetized SHR which was, in part, due to vasodilatation. In the present study, neither the fall in blood pressure nor the renal vasodilatation were inhibited by AM 251, indicating that they may not involve CB1 receptors. However, the bradycardic response to anandamide which was marked in the SHR, was inhibited by AM 251, suggesting a CB1 receptor-mediated cardiac effect in that strain of rats, consistent with the results of Lake et al. (1997a, 1997b), and consistent with evidence for increased expression of CB1 receptors in the myocardium of SHR (Bátkai et al., 2004). It is feasible that the CB1 receptor-mediated bradycardic effect of anandamide was due to a direct (Bonz et al., 2003; Sterin-Borda et al., 2005) and/or indirect action, such as inhibition of noradrenaline release (Molderings et al., 1999). Additionally, our results showed a modest pressor response to AM 251 in SHR, which was not accompanied by vasoconstriction or a change in heart rate suggesting an underlying change in cardiac function. These data with AM 251 support the findings of Bátkai et al. (2004), although the magnitude of change in our conscious rats (about 14 mm Hg) was substantially less than the effect reported by Bátkai et al. (2004) with SR 141716A in anaesthetized SHR (about 30 mm Hg) and with AM 251 in anaesthetized rats made hypertensive by infusion of angiotensin II (about 50 mm Hg).
If some of the present findings with anandamide and AM 251 are interpreted as indicating upregulation of CB1 receptor-mediated cardiovascular effects in SHR, then exaggerated responses to a synthetic CB1 receptor agonist would have been expected. However, we have shown previously that although the pressor and vascular responses to WIN 55212-2 in conscious rats involve CB1 receptors, the latter are involved indirectly via activation of sympathoadrenal mechanisms (Gardiner et al., 2001, 2002b). Thus, the present findings indicate those processes are not altered in SHR. However, following the pressor response to WIN 55212-2 in SHR, there was a delayed hypotension, accompanied by vasodilatation in renal and mesenteric vascular beds, and although the hypotension and renal vasodilatation were not inhibited by AM 251, the mesenteric vasodilatation was abolished.
An unexpected effect of WIN 55212-2 was a bradycardia in Wistar rats, which was not sensitive to AM 251, and not attributable to a baroreceptor reflex, since it was still present when the pressor effect was abolished. This may reflect a non-cannabinoid action of WIN 55212-2, for example, direct inhibition of calcium channels (Shen and Thayer, 1998; Ho and Hiley, 2003), but it is not clear why it is not seen in other normotensive strains either in this study (WKY) or previous studies in Sprague–Dawley rats (Gardiner et al., 2002b; Wheal et al., 2007a).
Collectively, the results show some differences in the responses to anandamide and WIN 55212-2 in SHR compared to normotensive rats, but the differences are not convincingly explained by upregulation of CB1 receptor-mediated vasodilator mechanisms. We have shown a small hypotensive response to anandamide, and a more marked hypotensive response to WIN 55212-2 in SHR, neither of which are susceptible to inhibition by AM 251, and neither of which are likely to be wholly attributable to vasodilatation, suggesting a negative effect on cardiac function (Ford et al., 2002), rather than a vasodilator effect of vanilloid receptor stimulation (Li et al., 2003). Interestingly, we have recently shown that in rats made hypertensive by nitric oxide synthase inhibition, there is no evidence for a delayed hypotensive effect of anandamide and/or enhanced vasodilator actions, and no evidence for a pressor response to CB1 receptor antagonism (Wheal et al., 2007a), indicating that the phenomenon is not common to all hypertensive states.
Thus, while there is persuasive evidence for vasodilator effects of cannabinoids in vitro, and some evidence for enhanced vasodilator effects of cannabinoids in hypertensive states under anaesthesia, the outcome of the present study in conscious rats makes it unlikely that CB1 receptor-mediated vasodilator effects of cannabinoids could be exploited as a potential target for antihypertensive therapy.
Acknowledgments
We are grateful to the British Heart Foundation for financial support, and thank Philip Kemp and Julie March for technical assistance.
Abbreviations
- AM 251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- CB
cannabinoid
- HDAS
haemodynamics data acquisition system
- SHR
spontaneously hypertensive rats
- WIN 55212-2
(R)-(+)-(2,3-dihydro-5-methyl-3-[(morphonolinyl)methyl] pyrrolo[1,2,3-de]-1,4-benzoxazin-yl)(1-napthalenyl)methanone mesylate
- WKY
Wistar Kyoto rats.
Conflict of interest
The authors state no conflict of interest.
References
- Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Lui J, Harvey-White J, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonz A, Laser M, Kullmer S, Kniesch S, Babin-Ebell J, Popp V, et al. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Regional haemodynamic responses to the cannabinoid agonist, WIN 55212-2, in conscious, normotensive rats, and in hypertensive, transgenic rats. Br J Pharmacol. 2001;133:445–453. doi: 10.1038/sj.bjp.0704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Complex regional haemodynamic effects of anandamide in conscious rats. Br J Pharmacol. 2002a;135:1889–1896. doi: 10.1038/sj.bjp.0704649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Influence of the CB1 receptor antagonist, AM 251, on the regional haemodynamic effects of WIN 55212-2 or HU 210 in conscious rats. Br J Pharmacol. 2002b;136:581–587. doi: 10.1038/sj.bjp.0704750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WSV, Hiey CR. Endothelium-independent relaxation to cannabinoids in rat isolated mesenteric artery and role of Ca2+ influx. Br J Pharmacol. 2003;139:585–597. doi: 10.1038/sj.bjp.0705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwolek G, Zakrzeska A, Schlicker E, Göthert M, Godlewski G, Malinowska B. Central and peripheral components of the pressor effect of anandamide in urethane-anaesthetized rats. Br J Pharmacol. 2005;145:567–575. doi: 10.1038/sj.bjp.0706195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake KD, Compton DR, Varga K, Martin BR, Kunos G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J Pharmacol Exp Ther. 1997a;281:1030–1037. [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997b;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats. Role of the vanilloid receptor. Hypertension. 2003;41:757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- Mendizábal VE, Adler-Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: a tale of passions and illusions. Br J Pharmacol. 2007;151:427–440. doi: 10.1038/sj.bjp.0707261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molderings GJ, Likungu J, Gothert M. Presynaptic cannabinoid and imidazoline receptors in the human heart and their potential relationship. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:157–164. doi: 10.1007/s002109900043. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistance and conduit rat mesenteric arteries. Br J Pharmacol. 2004;142:435–442. doi: 10.1038/sj.bjp.0705810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Osie-Hyiaman D, Offertáler L, Liu J, Harvey-White J, et al. Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. Am J Physiol. 2005;289:H533–H541. doi: 10.1152/ajpheart.00107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O'Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp JP. Use and misuse of control strains for genetically hypertensive rats. Hypertension. 1987;10:7–10. doi: 10.1161/01.hyp.10.1.7. [DOI] [PubMed] [Google Scholar]
- Shen M, Thayer SA. The cannabinoid agonist WIN 55,212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- St Lezin E, Simonet L, Pravenec M, Kurtz TW. Hypertensive strains and normotensive ‘control' strains. How closely are they related. Hypertension. 1992;19:419–424. doi: 10.1161/01.hyp.19.5.419. [DOI] [PubMed] [Google Scholar]
- Stein EA, Fuller SA, Edgemond WS, Campbell WB. Physiological and behavioural effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide) in the rat. Br J Pharmacol. 1996;119:107–114. doi: 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterin-Borda L, Del Zar CF, Borda E. Differential CB1 and CB2 cannabinoid receptor-inotropic response of rat isolated atria: endogenous signal transduction pathways. Biochem Pharmacol. 2005;69:1705–1713. doi: 10.1016/j.bcp.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Wheal AJ, Bennett T, Randall MD, Gardiner SM. Effects of chronic nitric oxide synthase inhibition on the cardiovascular responses to cannabinoids in vivo and in vitro. Br J Pharmacol. 2007a;150:662–671. doi: 10.1038/sj.bjp.0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheal AJ, Bennett T, Gardiner SM, Randall MD.Vascular activity of anandamide in spontaneously hypertensive rats Proc Br Pharmacol Soc 2007b. In press [DOI] [PMC free article] [PubMed]
- White R, Hiley CR. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]