Abstract
Background and purpose:
Studies in isolated preparations of vascular tissue (mainly resistance vessels) provide evidence that anandamide exerts vasorelaxation. The aim of the present work was to further characterize the mechanisms involved in the vascular response induced by anandamide in a conduit vessel, rat aorta.
Experimental approach:
Isometric tension changes in response to a cumulative concentration–response curve of anandamide (1 nM–100 μ M) were recorded in aortic rings from male Wistar rats. The involvement of a number of factors in this relaxation was investigated including endothelium-derived vasorelaxant products, cannabinoid and vanilloid receptors (transient potential vanilloid receptor-1 (TRPV1)), release of calcitonin gene-related peptide (CGRP), anandamide metabolism and the membrane transporter for anandamide.
Key results:
Anandamide caused a significant concentration-dependent vasorelaxation in rat aorta. This vasorelaxation was significantly inhibited by Pertussis toxin, by a non-CB1/non-CB2 cannabinoid receptor antagonist, by endothelial denudation, by inhibition of nitric oxide synthesis or inhibition of prostanoid synthesis via cyclooxygenase-2 (COX-2), by blockade of prostaglandin receptors EP4 and by a fatty acid amino hydrolase inhibitor. Antagonists for CB1, CB2, TRPV1 or CGRP receptors, an inhibitor of the release of endothelium-derived hyperpolarizing factor, and an inhibitor of anandamide transport did not modify the vascular response to anandamide.
Conclusions and implications:
Our results demonstrate, for the first time, the involvement of the non-CB1/non-CB2 cannabinoid receptor and an anandamide-arachidonic acid-COX-2 derived metabolite (which acts on EP4 receptors) in the endothelial vasorelaxation caused by anandamide in rat aorta.
Keywords: rat aorta, vasorelaxation, anandamide
Introduction
Studies in isolated preparations of vascular tissue and cells provide evidence that anandamide exerts vascular effects. The majority of these studies report that this endogenous cannabinoid elicits vasorelaxation in isolated arteries, although differences between species, as well as in the vascular beds used, should be noted (Ishioka and Bukoski, 1999; Zygmunt et al., 1999; Hillard, 2000; Grainger and Boachie-Ansah, 2001; O'Sullivan et al., 2004; Randall et al., 2004). The studies in resistance vessels show that anandamide induces clear vasodilator responses (Ellis et al., 1995; Randall and Kendall, 1997; Pratt et al., 1998; Jarai et al., 1999; Wagner et al., 1999; O'Sullivan et al., 2004), but in conduit vessels, different results have been reported. While some authors describe that in the rat hepatic and in guinea-pig basilar arteries (Zygmunt et al., 1999) and in rat aorta (López-Miranda et al., 2004) anandamide produces an important vasorelaxation, other researchers point out that in conduit vessels such as rat carotid and main mesenteric artery and aorta, this cannabinoid does not cause or causes only a slight vasorelaxation (Darker et al., 1998; Holland et al., 1999; O'Sullivan et al., 2004, 2005).
Several putative mechanisms have been put forward to explain the vasorelaxant effect of anandamide, but there is no clear consensus among researchers in this respect. Both extracellular and intracellular mechanisms have been described. With regard to the former, some studies have reported that anandamide acts through the stimulation of cannabinoid CB1 receptors and/or through a novel and putative endothelial ‘anandamide receptor' referred to as a ‘non-CB1/non-CB2 ‘cannabinoid receptor (White and Hiley, 1997; Jarai et al., 1999; Wagner et al., 1999; Offertáler et al., 2003), while other authors propose a new mechanism: the stimulation of vanilloid receptors (transient potential vanilloid receptor-1 (TRPV1)) on perivascular sensory nerves, with the subsequent release of the vasodilator neurotransmitter calcitonin gene-related peptide (CGRP) (Zygmunt et al., 1999; Ralevic et al., 2000, 2002; White et al., 2001; Ho and Hiley, 2003). With regard to the latter, the main mechanisms proposed include nitric oxide (NO) release, metabolism to vasoactive arachidonic metabolites or prostanoid analogues, or endothelium-derived hyperpolarizing factor (EDHF) release (Kunos et al., 2000; Randall et al., 2002). Most of these vasorelaxant mechanisms for anandamide have been established in resistance vessels, but the question of whether or not these mechanisms are same in all the vascular territory and, in particular, in conduit vessels has not been definitively answered.
Previous studies carried out in our laboratory showed that anandamide is able to produce a noteworthy vasorelaxation in rat aorta (López-Miranda et al., 2004). The aim of the present work was to further characterize the mechanisms involved in the vascular response induced by anandamide in this conduit vessel.
Methods
Tissue preparation
This study was carried out in accordance with the European Community Guidelines for the use of experimental animals. Male Wistar rats (250–300 g body weight) were anaesthetized with sodium pentobarbital (50 mg kg−1, intraperitoneal (i.p.)). The abdomen was opened by a midline incision, and the aorta was carefully excised and placed in ice-cold Krebs–Henseleit (K-H) solution with the following composition (mM): 118 NaCl; 4.75 KCl; 1.2 MgSO4; 1.19 KH2PO4; 2.54 CaCl2; 25 NaHCO3; 11 glucose (pH 7.4). All connective and perivascular adipose tissues were removed, with caution taken not to disrupt the endothelium. Transverse vascular rings 3–4 mm long were prepared. Some of the rings were deliberately denuded by rubbing and rolling them around stainless steel forceps before being mounted.
Afterwards, the rings were fixed vertically between two stainless steel hooks and suspended in a 5-ml jacketed glass organ bath, containing K-H solution at 37°C and continuously bubbled with 95% O2 and 5% CO2. The upper wire was connected to an isometric force transducer (Grass FT07), and tension measurements were recorded in a computer (PowerLab/4e program). The rings were mounted with a resting tension of 2 g. Tissues were equilibrated for 90 min, during which time the medium was replaced every 15 min.
Experimental protocol
In the first series of experiments, the effect of anandamide on submaximal phenylephrine (1 μM)-induced tone in endothelium intact and denuded aorta rings were examined in the following manner. After the equilibration period, arteries were precontracted with phenylephrine and when a stable level of tone was established, cumulative concentration–response curves to anandamide were constructed (1 nM–100 μM). Control rings were similarly treated with phenylephrine, but corresponding vehicle additions (dimethylsulphoxide, DMSO 0.5%) (López-Miranda et al., 2004) were made. Only one experiment was carried out in each aorta ring. At the end of each cannabinoid concentration–response curve, carbachol (10 μM) was added to verify the existence of functional endothelium in the corresponding preparation. Arteries that relaxed to carbachol more than 70% were designated as endothelium-intact preparations and the preparations that relaxed to carbachol less than 10% were designated as endothelium-denuded preparations. Rings with intermediate responses were discarded. All subsequent series of experiments were carried out in intact arteries.
To identify the receptors involved in the vascular effects of anandamide, the following experiments were performed: (1) to evaluate the implication of Gi/0 protein-coupled receptors, aorta rings were pretreated with 300 ng ml−1 of Pertussis toxin for 3 h (Petitcolin et al., 2001); (2) to test the involvement of classical cannabinoid receptors (CB1 and CB2), aorta preparations were pretreated with (a) the selective CB1 receptor antagonist, N-(piperidin-1-yl) – 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (rimonabant) for 15 min at two different concentrations (1 and 3 μM) (Rinaldi-Carmona et al., 1994) and (b) the selective CB2 receptor antagonist, N-((1S)-endo-1,3,3-trimethyl bicycle (2.2.1) heptan-2yl)-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)pyrazole-3 carboxamide (SR144528) for 15 min at the same concentrations (1 and 3 μM) (Rinaldi-Carmona et al., 1998); (3) to test the involvement of the nonclassical and newly proposed non-CB1/non-CB2 endothelial cannabinoid receptor, aorta preparations were pretreated for 15 min with an antagonist of this receptor, O1918 (1,3-dimethoxy-5-methyl-2[(1R,6R)-3methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]benzene) (10 μM) (Offertáler et al., 2003); and (4) to investigate the involvement of TRPV1, two different protocols were performed: (a) arteries were pretreated with the TRPV1 blocker capsazepine 100 nM for 30 min (Jakab et al., 2005) and (b) arteries were pretreated with the inhibitor of CGRP receptors CGRP8−37 (3 μM) for 30 min (Ralevic et al., 2002). The vehicle used to dissolve the antagonists, ethanol, was also evaluated.
The contribution of the main vasorelaxant endothelial mediators was also investigated: (1) the involvement of NO and of EDHF in the vascular response elicited by anandamide was examined by pretreatment of aorta rings for 30 min with Nω-nitro-L-arginine methyl ester (L-NAME) 100 μM (Engler et al., 2000) or apamin plus charybdotoxin (10 nM each) (Perez-Vizcaino et al., 1999), respectively; (2) the involvement of vasorelaxant arachidonic acid-derived products was investigated by pretreatment of the preparations for 30 min with (a) 3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate (URB597) 1 μM, a fatty acid amide hydrolase (FAAH) inhibitor (Bátkai et al., 2004), which converts anandamide to arachidonic acid plus ethanolamine, (b) 17-octadecynoic acid (17-ODYA) 5 μM (cytochrome P450 epoxygenase and ω/ω-1-hydroxylase inhibitor) (Grainger and Boachie-Ansah, 2001), (c) indomethacin 10 μM (cyclooxygenase (COX) inhibitor) (Tep-areenan et al., 2003); (d) 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole (SC-560) 1 μM (a selective COX-1 inhibitor, Bolla et al., 2004) and (e) 5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2(5H)-furanone) (DFU) 0.1 μM (a selective COX-2 inhibitor, Riendeau et al., 1997). Prostaglandin E2 (PGE2) and analogues (PGE2 ethanolamide) have been identified as derived from anandamide metabolism pathways (Sugimura et al., 2002). These products act on PGE2 receptors (EP) to exert vascular effects. The involvement of the vasorelaxant EP4 has also been studied by pretreating rat aorta rings with GW627368X (N-(2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]-acetyl)benzenesulphonamide) (1 and 3 μM), a selective antagonist of EP4 receptors (Wilson et al., 2006).
In addition, the possible involvement of the anandamide transporter in its vascular effect on rat aorta was examined by pretreating the preparations with N-(4-hydroxy-phenyl)-5,8,11,14-eicosatetraenamine (AM404, anandamide transporter inhibitor) 10 μM for 30 min (Grainger and Boachie-Ansah, 2001).
Data analysis
Relaxation responses are expressed as the percentage of relaxation of the tone induced by phenylephrine (1 μM). Rmax is the maximal response obtained when the anandamide concentration–response curve is carried out.
pEC50 values refer to the negative logarithm of the concentration of anandamide that produced half (50%) of the maximal response obtained (EC50). EC50 values were obtained from individual concentration–response curves by fitting the data to a logistic equation (GraphPad PRISM V2.01).
Data are expressed as the mean±s.e.m. The number of animals in each group is expressed by n. Rmax values for anandamide in the different experimental groups were compared using analysis of variance (ANOVA), followed by Bonferroni/Dunn post hoc test, as appropriate. pEC50 values were compared by a Student's unpaired t-test. P-values <0.05 were considered significant.
Drugs
Phenylephrine carbachol, L-NAME, indomethacin, apamin, charybdotoxin, 17-ODYA and capsazepine were obtained from Sigma (Sigma Chemical Company, Poole, Dorset, UK), and URB597 was supplied from Cayman Chemical Company (Ann Arbor, MI, USA). Pertussis toxin was supplied by Research Biochemicals International (RBI, Natick, MA, USA). DMSO was supplied by Panreac Química S.A, Barcelona, Spain. Anandamide, CGRP8−37 (rat), AM 404 and O1918 were supplied by Tocris Cookson (Bristol, UK). SC-560 and DFU were obtained courtesy of Menarini Laboratorios (Barcelona, Spain) and Merck Sharp & Dohme (Madrid, Spain), respectively. Rimonabant and SR144528 were obtained from Sanofi Recherche (Montpellier, France). GW627368X was obtained courtesy of GlaxoSmithKline (Stevenage, UK).
Phenylephrine, carbachol, L-NAME, CGRP8−37 (rat), apamin and charybdotoxin were dissolved in distilled water. Indomethacin, capsazepine, rimonabant, SR144528, O1918, DFU, AM 404, URB597, SC-560, 17-ODYA and GW627368X were dissolved in ethanol (Panreac Química S.A.).
A cannabinoid stock solution (0.01 M) was prepared daily in DMSO 0.5% (v/v). Additional dilutions were made by mixing 1 volume of stock solution with up to 9 volumes of distilled water.
Results
Phenylephrine (1 μM) caused a submaximal increase in arterial tone that was similar in all the experimental groups (data not shown).
None of the antagonists or inhibitors, at the concentrations used, modified the vascular function in phenylephrine-precontracted intact arteries (data not shown). When ethanol was used as vehicle, the maximum volume administered in the organ bath was 5 μl to prevent any modification of the functionality of the preparation.
Vasorelaxation caused by anandamide in rat aorta rings
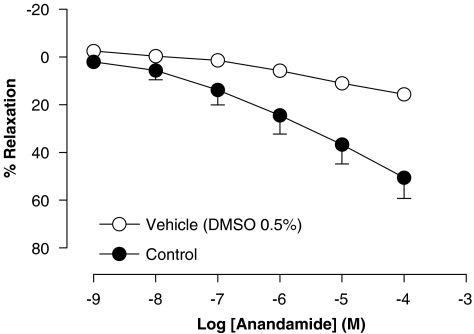
Anandamide caused a concentration-dependent vasorelaxation in intact rat aorta rings, resulting in an Rmax value of 51±9% and a pEC50 value of 5.92±0.04 (n=6) (Figure 1). This vasorelaxation was gradual in onset and took 7–10 min to reach plateau at each concentration step.
Figure 1.
Concentration–response curve for the relaxation of isolated rat aorta rings, precontracted by phenylephrine (1 μM), by anandamide. Values are expressed as mean±s.e. for 4–8 animals. The dimethylsulphoxide 0.5% curve represents the effect of the vehicle alone.
Involvement of Gi/0 protein-coupled receptors in vasorelaxation caused by anandamide in rat aorta rings
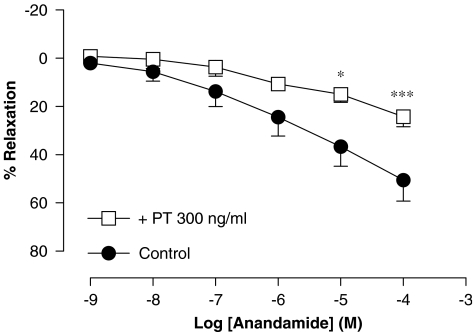
Incubation of the rat aorta ring preparations with pertussis toxin, 300 ng ml−1 for 3 h, significantly reduced the vasorelaxation caused by anandamide (Rmax of 24±4% and pEC50 value of 3.87±0.10 (n=4), P<0.001) (Figure 2).
Figure 2.
Effect of pretreatment (3 h) with Pertussis toxin (PT) on the relaxation produced by anandamide in intact rat aorta rings precontracted by phenylephrine (1 μM). Values are expressed as mean±s.e. for 4–8 animals. A two-way analysis of variance followed by Bonferroni/Dunn post hoc test was used for statistical analysis (*P<0.05, **P<0.01, ***P<0.001 vs control).
Involvement of CB1, CB2 and non-CB1/non-CB2 receptors in vasorelaxation caused by anandamide in rat aorta rings
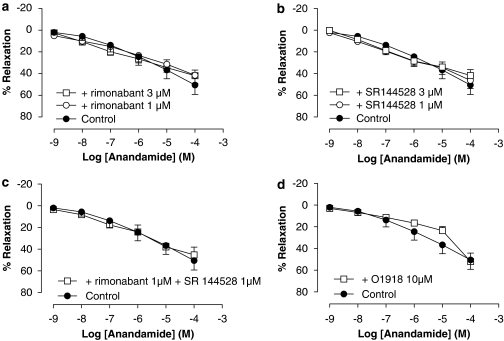
The vasorelaxation caused by anandamide in endothelium-intact aorta rings was not affected by either the pretreatment with the CB1 antagonist, rimonabant (rimonabant 1 μM: Rmax of 42±5% and pEC50 of 5.90±0.02 (n=6); rimonabant 3 μM: Rmax of 42±7% and pEC50 of 6.01±0.04 (n=8)) (Figure 3a) or the pretreatment with the CB2 antagonist, SR144528 (SR144528 1 μM: Rmax of 46±8% and pEC50 of 6.10±0.1 (n=5); SR144528 3 μM: Rmax of 42±5% and pEC50 of 5.98±0.07 (n=5)) (Figure 3b).
Figure 3.
Effect of rimonabant (a), SR144528 (b), rimonabant plus SR144528 (c) and O1918 (d) on the relaxation produced by anandamide in intact rat aorta rings precontracted by phenylephrine (1 μM). Values are expressed as mean±s.e. for 4–8 animals. A two-way analysis of variance followed by Bonferroni/Dunn post hoc test was used for statistical analysis. (*P<0.05, **P<0.01, ***P<0.001 vs control). O1918, 1,3-dimethoxy-5-methyl-2[(1R,6R)-3methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]benzene; SR144528, N-((1S)-endo-1,3,3-trimethyl bicycle (2.2.1) heptan-2yl)-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)pyrazole-3 carboxamide.
The influence of the combination of rimonabant and SR144528 on anandamide-induced vasorelaxation was also evaluated. Pretreatment with both antagonists simultaneously, at a concentration of 1 μM, did not cause any modification of the vascular effect of anandamide (Rmax of 45±7% and pEC50 of 6.01±0.04 (n=4)) (Figure 3c).
When aortic preparations were pretreated with O1918 at 10 μM, a significant right-ward shift in the concentration–response curve to anandamide without affecting the maximal response was observed (Rmax of 52±8%, P>0.05 and pEC50 of 5.3±0.12 (n=4), P<0.001) (Figure 3d).
Involvement of TRPV-1 and CGRP in vasorelaxation caused by anandamide in rat aorta rings
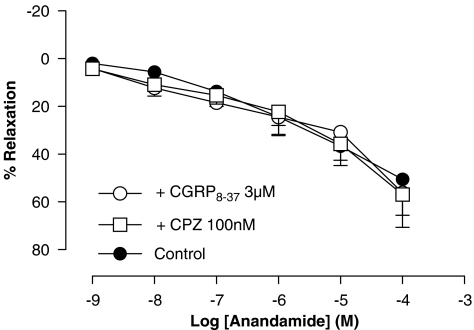
Neither pretreatment with capsazepine (100 nM) nor with CGRP8−37 (3 μM) modified the vasorelaxation caused by anandamide in intact rat aorta ring preparations (Rmax of 57±9% and pEC50 of 5.99±0.13 (n=4) and Rmax of 56±15% and pEC50 of 6.09±0.16 (n=4), respectively) (Figure 4).
Figure 4.
Effect of capsazepine (CPZ) and CGRP8−37 on the relaxation produced by anandamide in intact isolated rat aorta rings precontracted by phenylephrine (1 μM). Values are expressed as mean±s.e. for 4–8 animals. A two-way analysis of variance followed by Bonferroni/Dunn post hoc test was used for statistical analysis (*P<0.05, **P<0.01, ***P<0.001 vs control). CGRP, calcitonin gene-related peptide.
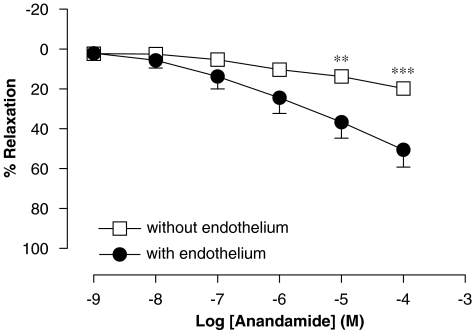
Involvement of endothelium in the vasorelaxation caused by anandamide in rat aorta rings
Endothelial denudation resulted in a complete inhibition of the vasorelaxation caused by anandamide, obtaining a Rmax value of 20±1% (n=5), similar to that obtained in the vehicle (DMSO 0.5%) group (Rmax of 16±2% (n=5)) (Figure 5).
Figure 5.
Concentration–response curve for the relaxation of isolated rat aorta rings, precontracted by phenylephrine (1 μM), by anandamide in the presence or absence of functional endothelium. Values are expressed as mean±s.e. for 4–8 animals. A two-way analysis of variance followed by Bonferroni/Dunn post hoc test was used for statistical analysis (*P<0.05, **P<0.01, ***P<0.001 vs control).
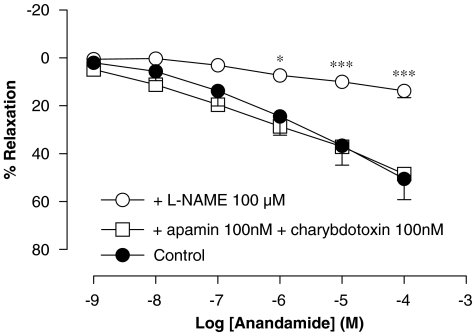
Involvement of NO and EDHF in vasorelaxation caused by anandamide in rat aorta rings
Pretreatment with L-NAME (100 μM) for 30 min significantly inhibited the vasorelaxation produced by anandamide in rat aorta rings (Rmax of 14±3% and pEC50 of 2.56±0.23 (n=5), P<0.001) (Figure 6). Pretreatment with apamin plus charybdotoxin (10 nM each) did not modify the endothelium-dependent vasorelaxation elicited by anandamide in isolated rat aorta rings (Rmax of 49±6% and pEC50 of 6.2±0.10 (n=4)) (Figure 6).
Figure 6.
Effect of Nω-nitro-L-arginine methyl ester (L-NAME) and apamin plus charybdotoxin on the relaxation produced by anandamide in intact isolated rat aorta rings precontracted by phenylephrine (1 μM). Values are expressed as mean±s.e. for 4–8 animals. A two-way analysis of variance followed by Bonferroni/Dunn post hoc test was used for statistical analysis (*P<0.05, **P<0.01, ***P<0.001 vs control).
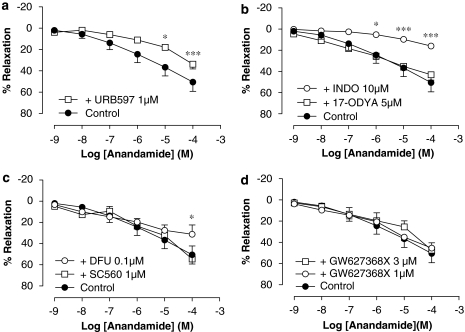
Involvement of metabolism-derived products in vasorelaxation caused by anandamide in rat aorta rings
Inhibition of FAAH by pretreatment with URB597 (1 μM) caused a significant reduction of the vasorelaxant effect of anandamide in rat aorta (Rmax of 34±5% and pEC50 of 4.50±0.04, P<0.001 (n=4)) (Figure 7a).
Figure 7.
Effect of the FAAH inhibitor, URB597 (a), the cytochrome P450 epoxygenase and ω/ω-1-hydroxylase inhibitor, 17-ODYA and the COX inhibitor indomethacin (INDO) (b), the selective COX-1 inhibitor, SC560, and the selective COX-2 inhibitor, DFU (c) and the selective EP4 receptor antagonist GW627368X (d) on the relaxation produced by anandamide in intact rat aorta rings precontracted by phenylephrine (1 μM). Values are expressed as mean±s.e. for 4–8 animals. A two-way ANOVA followed by Bonferroni/Dunn post hoc test was used for statistical analysis (*P<0.05, **P<0.01, ***P<0.001 vs control). FAAH, fatty acid amino hydrolase; URB597, 3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate.
Pretreatment with the cytochrome P450 epoxygenase and ω/ω-1-hydroxylase inhibitor, 17-ODYA (5 μM), did not modify the vasorelaxation induced by anandamide in rat aorta rings (Rmax of 43±5% and pEC50 of 6.01±0.03 (n=9), P>0.05) (Figure 7b), while pretreatment with indomethacin 10 μM for 30 min significantly reduced the vasorelaxation produced by anandamide in rat aorta rings (Rmax of 16±6% (n=8) and pEC50 of 3.07±0.09 (n=5), P<0.001) (Figure 7b).
When the selective COX-1 inhibitor, SC-560 (1 μM), was added, the vasorelaxation caused by anandamide was not affected (Rmax of 55±12% and pEC50 of 5.81±0.11 (n=5)). However, when the selective COX-2 inhibitor, DFU (0.1 μM) was used, a significant inhibition of the vasorelaxant response provoked by anandamide in rat aorta was observed (Rmax of 31±9%, P<0.05, and pEC50 of 4.97±0.07, P<0.001 (n=4)) (Figure 7c).
GW627368X, the selective inhibitor of EP4 receptors, did not cause a modification in the maximal vasorelaxation caused by anandamide at the lower concentration (1 μM) tested (Rmax of 46±1% (n=4) and pEC50 of 5.77±0.08 (n=4)). However, this antagonist at the higher concentration (3 μM) caused a significant rightward shift in the concentration–response curve to anandamide without affecting the maximal response (Rmax of 47±7%, P>0.05 and a pEC50 of 5.43±0.10, P<0.001 (n=4)) (Figure 7d).
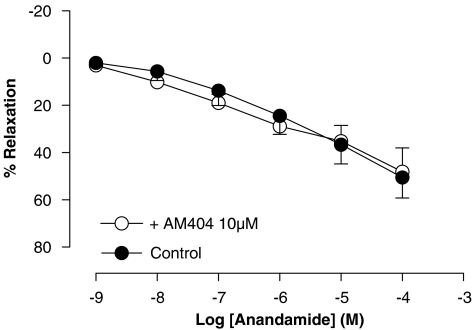
Involvement of the anandamide transporter in vasorelaxation caused by anandamide in rat aorta rings
Blockade of the anandamide membrane transporter by pretreatment with AM404 (10 μM) did not modify the vascular response of anandamide in rat aorta rings (Rmax of 48±10% and pEC50 of 6.1±0.10 (n=6) (Figure 8).
Figure 8.
Effect of the anandamide transporter inhibitor AM404 on the relaxation produced by anandamide in intact rat aorta rings precontracted by phenylephrine (1 μM). Values are expressed as mean±s.e. for 4–8 animals. A two-way analysis of variance followed by Bonferroni/Dunn post hoc test was used for statistical analysis (*P<0.05, **P<0.01, ***P<0.001 vs control). AM404, N-(4-hydroxy-phenyl)-5,8,11,14-eicosatetraenamine.
Discussion
The aim of the present study was to further characterize the vasorelaxant mechanisms of anandamide in rat aorta. Currently, there are only two studies that have evaluated the vasorelaxant effect of anandamide in conduit vessels. One describes a clear vasorelaxant effect of anandamide, around 60%, in rabbit aorta (Mukhopadhyay et al., 2002) and the other refers to no more than a slight vasorelaxation (around 20%) in rat aorta (O'Sullivan et al., 2005). The present study shows a slowly developing concentration-dependent relaxation induced by anandamide in intact isolated rat aorta rings, reaching a maximal value of around 35% (versus vehicle). Although these results are not in agreement with those obtained by O'Sullivan et al. (2005) in spite of using the same animal species, it is possible that methodological differences could explain these differing findings: O'Sullivan's group used ethanol as anandamide solvent, while in the present study, the vehicle is DMSO 0.5%. We have previously demonstrated that the vascular responses of anandamide can be lost depending on the solvent/vehicle used (López-Miranda et al., 2004).
Anandamide can exert its vascular actions through three different Gi/0-regulatory protein-coupled cannabinoid receptors: CB1, CB2 and the non-CB1/non-CB2 cannabinoid receptors (Bouaboula et al., 1999; Holland et al., 1999; Jarai et al., 1999; Randall et al., 2002; Begg et al., 2003; Offertáler et al., 2003). In our experimental conditions, the involvement of Gi/0-protein-coupled receptors in the vasorelaxation caused by anandamide has been confirmed because of the significant reduction of anandamide's effects in the presence of Pertussis toxin. But, is a cannabinoid receptor responsible for the vascular effect? If this were the case, which is/are the subtype/s involved?
To answer these questions, the vasorelaxation of anandamide in the presence of CB1, CB2 and non-CB1/non-CB2 receptor antagonists, rimonabant, SR144528 and O1918, respectively, was evaluated. The rimonabant and SR144528 concentrations used in the present study (1 and 3 μM) were chosen taking into account the results obtained by White and Hiley (1998). They concluded that concentrations of rimonabant higher than 3 μM are not appropriate for the investigation of cannabinoid receptor-dependent processes in vascular tissue. Due to the lack of similar studies with SR144528, we decided to use the same range of concentrations as for the CB1 antagonist. In the present study, anandamide vasorelaxation was insensitive to CB1 and CB2 receptor antagonists at the different concentrations tested, either when both antagonists were administered separately or simultaneously. In studies in which anandamide exerts only a modest vasorelaxation in rat aorta, other authors have also suggested CB1-and CB2-independent vasorelaxant mechanisms for anandamide (O'Sullivan et al., 2004). We used O1918 at 10 μM, a concentration which does not bind to cloned CB1 and CB2 receptors and which has been shown to cause a 10-fold rightward shift of the concentration–relaxation curve for abnormal cannabidiol in rat mesenteric arteries (Offertáler et al., 2003). In our experiments with O1918, a significant rightward shift of the concentration–relaxation curve of anandamide was obtained, suggesting that this non-CB1/non-CB2 receptor may be involved in the vascular effect of anandamide in rat aorta. Other researchers have also described similar results in rat mesenteric and coronary vessels (Jarai et al., 1999; Ford et al., 2002; Offertáler et al., 2003; O'Sullivan et al., 2004), and some of them have even suggested that this receptor is specific for anandamide (Offertáler et al., 2003).
Anandamide binds to TRPV-1 with micromolar affinity, and this interaction was shown to result in the release of the potent vasodilator peptide CGRP from sensory nerve endings, provoking vasorelaxation (Jarai et al., 1999; Zygmunt et al., 1999; Mukhopadhyay et al., 2002). In the present study, neither the inhibition of the TRPV-1 by capsazepine nor the inhibition of CGRP receptors by CGRP8−37 modified the vasorelaxant effect of anandamide, which rules out the involvement of this mechanism in the vascular effect of anandamide in rat aorta. Although it has not been discussed in the present study, the vasorelaxation caused by anandamide in rat aorta is endothelium-dependent. There are studies which have demonstrated that only the endothelium-independent component in the vasorelaxation induced by anandamide is blocked by capsazepine or by CGRP inhibitors (Jarai et al., 1999; Zygmunt et al., 1999; Mukhopadhyay et al., 2002), while the endothelium-dependent vasodilator effect of anandamide is unaffected by these antagonists (Jarai et al., 1999; Mukhopadhyay et al., 2002; Ho and Hiley, 2003; Offertáler et al., 2003). This may explain the absence of TRPV-1 and CGRP involvement in our preparation.
Other important mechanisms proposed for the vasorelaxant effect of anandamide are endothelial intracellular pathways (Kunos et al., 2000; Randall et al., 2002). Our results show that the vasorelaxation provoked by anandamide in rat aorta is endothelium-dependent. For this reason, a more detailed investigation of the implication of the different endothelial vasorelaxant pathway/s was carried out. The production of NO, the release of EDHF and the production of prostanoids have been investigated.
The results of the present study show that while the production of NO is a pivotal step in the vasorelaxation caused by anandamide in rat aorta, the contribution of a hyperpolarizing mechanism (via EDHF) is not involved. These data are in agreement with those obtained by other authors (Mukhopadhyay et al., 2002; O'Sullivan et al., 2004).
With regard to prostanoids, it has been known that anandamide can be converted to arachidonic acid plus ethanolamine through its hydrolysis via FAAH (Deutsch et al., 1997). Besides, there is evidence from some resistance vessels that anandamide vasorelaxation is dependent on arachidonic acid metabolism via COX and/or epoxygenase pathways (Pratt et al., 1998; Fleming et al., 1999; Grainger and Boachie-Ansah, 2001). To date, there is no study that has assessed the involvement of arachidonic acid metabolites in the anandamide vasorelaxant effect in conduit vessels, but, since it is a possible mechanism, we investigated this option.
Pretreatment of aorta preparations with the selective and potent FAAH inhibitor, URB597, caused a significant reduction of the anandamide vasorelaxation, suggesting an involvement of anandamide metabolism in its vascular effect. Some experiments were carried out to further investigate this possibility. As cyclooxygenase and/or epoxygenase pathways have been implicated in vasorelaxation of anandamide in resistance vessels, these metabolism routes were tested in the present study. Our data showed that, while the epoxygenase pathway was not involved in the anandamide vasorelaxant response in rat aorta, the COX metabolism pathway was important in the vasodilatory response in this blood vessel, as there was no inhibition of this vascular effect after pretreatment with 17-ODYA and but inhibition after indomethacin. In addition, the selective inhibition of COX-1 and COX-2 separately showed that the vasorelaxation induced by anandamide was only affected by the presence of the selective COX-2 inhibitor (DFU). So, we can propose that, at least, a vasodilator metabolite of arachidonic acid derived via COX-2 is involved in the vasorelaxation caused by anandamide in rat aorta. Recently, other authors have described that vasorelaxation caused by anandamide in rat mesenteric vessels is limited by its catabolism by FAAH and COX-2 metabolites (Ho and Randall, 2007).
The most important arachidonic acid metabolite related to anandamide pharmacology via COX-2 metabolism is prostaglandin E2 (Kozak and Marnett, 2002). PGE2 exerts its vascular actions through EPs: EP1, EP2, EP3 and EP4. The EP2 and EP4 receptors mediate vasorelaxant responses (Davis et al., 2004; Wilson and Giles, 2005) and, while EP2 receptors are the least abundant among the EP receptors, EP4 receptors are widely distributed throughout the body (Narumiya et al., 1999). For this reason, in the present study, the involvement of EP4 receptors in anandamide vasorelaxation was evaluated. In the presence of GW627368X (an EP4 selective blocker), a rather small but statistically significant inhibitory effect was observed. This indicates that this receptor may be involved in the vasorelaxant effect of anandamide in rat aorta.
At this time, it is important to mention that anandamide can also be metabolized directly by COX-2 leading to a major product, the prostamide, PGE2-ethanolamide (Yu et al., 1997; Burstein et al., 2000) and PGE2-ethanolamide acts directly on EP4 receptors and causes vasorelaxant responses in blood vessels (Ross et al., 2002). So, it could be possible that PGE2-ethanolamide can act on EP4 receptors, causing vasorelaxation in rat aorta. However, if data obtained with URB597 and DFU are compared, it can be observed that the magnitude of the anandamide vasorelaxation inhibition is very similar to both inhibitors (Rmax of 34±5% (n=4) and Rmax of 31±12% (n=5), respectively). This leads us to suggest that the ‘PGE2 like' product proposed should be PGE2, because if PGE2-ethanolamide were the compound responsible for the anandamide vasorelaxation, the inhibition with URB597 would not have been obtained.
Anandamide is readily taken up into cells by a process of facilitated diffusion (Hillard and Jarrahian, 2000; Fowler and Jacobsson, 2002). Some studies have demonstrated that vascular responses to anandamide are affected by an inhibitor of carrier-mediated anandamide uptake, AM404 (Calignaro et al., 1997; Chaytor et al., 1999). The results obtained in the present study show that the vasorelaxation caused by anandamide was not affected by the presence of AM404. This finding suggests that anandamide does not need a specific transporter protein to access the cytosol to exert its vasorelaxant effect, which would agree with data described by Glaser et al. (2003) in neuronal cells. Alternatively, the anandamide transporter may not exist or operate in this particular conduit vessel. When this transporter protein is definitively identified, these questions can be clearly answered. It is important to note that, although AM404 can exert other actions (agonist at TRPV-1 (Zygmunt et al., 2000; Ralevic and Kendall, 2001) and inhibitor of FAAH in neuronal cells (Glaser et al., 2003)), in the present study, the AM404 concentration used (10 μM) did not modify vascular function or interfere with results obtained with the FAAH inhibitor, discounting the possibility of these other effects.
In summary, the present study demonstrates, for the first time, a clear and important endothelium-mediated vasorelaxant effect of anandamide in rat aorta mainly mediated by two mechanisms: (a) anandamide may act on the endothelial non-CB1/non-CB2 cannabinoid receptor promoting NO synthesis and the subsequent vasorelaxation; (b) anandamide could be metabolized by FAAH resulting in arachidonic acid formation, which is a substrate of COX-2 leading to the formation of a ‘PGE2 like' product, which binds to the EP4 receptor in vascular smooth muscle and causes vasorelaxation.
We propose that when anandamide acts on non-CB1/non-CB2 cannabinoid receptors, it promotes the release of NO in rat aorta. Our study does not demonstrate this proposal, but it is in agreement with the signalling cascade of endothelial non-CB1/non-CB2 cannabinoid receptor proposed by Begg et al. (2003) in human umbilical vein endothelial cells. Besides, recently, McCollum et al. (2007) have demonstrated that the synthetic analogue of anandamide, methanandamide, promotes NO production acting through the novel non-CB1/non-CB2 anandamide receptor in rabbit aortic endothelial cells (McCollum et al., 2007). A detailed investigation when this receptor is cloned should be performed to definitively test this hypothesis.
We would also like to point out that, although either NO (via non-CB1/non-CB2 cannabinoid receptor) or prostaglandin (via a ‘PGE2 like' product which binds to the EP4 receptor) is involved in the vasorelaxant effect of anandamide in rat aorta, these endothelial vasorelaxant pathways need not be mutually exclusive. Indeed, there is much evidence for ‘crosstalk' between endothelial NO and COX pathways. NO regulates COX activity in normal and inflamed tissues, and it has been demonstrated that NO can modulate the synthesis of PGE2 via activation of the COX enzymes. On the other hand, there is also much evidence for the action of prostaglandins on the L-arginine:NO pathway (Di Rosa et al., 1996.). Such interactions would explain the similar inhibition of anandamide's vasorelaxant effects when L-NAME or indomethacin were used in our study.
Acknowledgments
This study was supported by grants from Ministerio de Educación y Ciencia (SAF2003-08003-C2-01 and SAF2006-13391-C03-01). We thank Sanofi Recherche (Montpellier, France), Merck Sharp & Dohme (Madrid, Spain), Menarini (Barcelona, Spain) and GlaxoSmithkline (Stevenage, United Kingdom) for generous supply of Rimonabant and SR144528, DFU, SC560 and GW627368X respectively.
Abbreviations
- AM404
N-(4-hydroxy-phenyl)-5,8,11,14-eicosatetraenamine
- DFU
5,5-dimethyl-3-(3-fluorophenyl)-4-(4-methylsulphonyl)phenyl-2(5H)-furanone)
- DMSO
dimethylsulphoxide
- EDHF
endothelium-derived hyperpolarizing factor
- EP
PGE2 receptors
- FAAH
fatty acid amide hydrolase
- GW627368X
N-(2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]-acetyl)benzenesulphonamide
- L-NAME
Nω-nitro-L-arginine methyl ester
- O1918
1,3-dimethoxy-5-methyl-2[(1R,6R)-3methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]benzene
- 17-ODYA
17-octadecynoic acid
- PGE2
prostaglandin E2
- rimonabant
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- SC-560
5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole
- SR144528
N-((1S)-endo-1,3,3-trimethyl bicycle (2.2.1) heptan-2yl)-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)pyrazole-3 carboxamide
- URB597
3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate
Conflict of interest
The authors state no conflict of interest.
References
- Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertáler L, Bátkai S, Pacher P, Razdan RK, et al. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Bolla M, You D, Loufrani L, Levy BI, Levy-Toledano S, Habib A, et al. Cyclooxygenase involvement in thromboxane-dependent contraction in rat mesenteric resistance arteries. Hypertension. 2004;43:1264–1269. doi: 10.1161/01.HYP.0000127438.39744.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Mol Pharmacol. 1999;55:473–480. [PubMed] [Google Scholar]
- Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 2000;61:29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Calignaro A, La Rana G, Beltramo M, Makriyannis A, Piomelli D. Potentiation of anandamide hypotension by the transport inhibitor AM404. Eur J Pharmacol. 1997;337:R1–R2. doi: 10.1016/s0014-2999(97)01297-1. [DOI] [PubMed] [Google Scholar]
- Chaytor AT, Martin PE, Evans WH, Randall MD, Griffith TM. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J Physiol. 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darker IT, Millns PJ, Selbie L, Randall MD, S-Baxter G, Kendall DA. Cannabinoid (CB1) receptor expression is associated with mesenteric resistance vessels but not thoratic aorta in the rat. Br J Pharmacol. 1998;125:95P. [Google Scholar]
- Davis RJ, Murdoch CE, Ali M, Purbrick S, Ravid R, Baxter GS, et al. EP4 prostanoid receptor-mediated vasodilatation of human middle cerebral arteries. Br J Pharmacol. 2004;141:580–585. doi: 10.1038/sj.bjp.0705645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, Goligorsky MS, Schmid PG, Krebsbach RJ, Schmid RJ, Das SK, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M, Ialenti A, Ianaro A, Sautebin L. Interaction between nitric oxide and cyclooxygenase pathways. Prostaglandins Leukot Essent Fatty Acids. 1996;54:229–238. doi: 10.1016/s0952-3278(96)90053-8. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Moore SF, Willoughby KA. Anandamide and THC dilation of cerebral arterioles is blocked by indomethacin. Am J Physiol. 1995;38:H1859–H1864. doi: 10.1152/ajpheart.1995.269.6.H1859. [DOI] [PubMed] [Google Scholar]
- Engler MB, Engler MM, Browne A, Sun YP, Sievers R. Mechanisms of vasorelaxation induced by eicosapentaenoic acid (20:5n-3) in WKY rat aorta. Br J Pharmacol. 2000;131:1793–1799. doi: 10.1038/sj.bjp.0703754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Schermer B, Popp R, Busse R. Inhibition of the production of endothelium-derived hyperpolarizing factor by cannabinoid receptor agonists. Br J Pharmacol. 1999;126:949–960. doi: 10.1038/sj.bjp.0702381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Jacobsson SO. Cellular transport of anandamide, 2-arachidonoylglycerol and palmitoylethanolamide--targets for drug development. Prostaglandins Leukot Essent Fatty Acids. 2002;66:193–200. doi: 10.1054/plef.2001.0357. [DOI] [PubMed] [Google Scholar]
- Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative initropic and coronary vasodilator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci USA. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J, Boachie-Ansah G. Anandamide-induced relaxation of sheep coronary arteries: the role of the vascular endothelium, arachidonic acid metabolites and potassium channels. Br J Pharmacol. 2001;134:1003–1012. doi: 10.1038/sj.bjp.0704340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ. Endocannabinoids and vascular function. J Pharmacol Exp Ther. 2000;294:27–32. [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A. The movement of N-arachidonoylethanolamine (anandamide) across cellular membranes. Chem Phys Lipids. 2000;108:123–134. doi: 10.1016/s0009-3084(00)00191-2. [DOI] [PubMed] [Google Scholar]
- Ho VWS, Hiley RC. Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca++ influx. Br J Pharmacol. 2003;139:585–597. doi: 10.1038/sj.bjp.0705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WSV, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cycloxygenases limits vasorelaxation to anandamide and 2-arachidonoilglycerol. Br J Pharmacol. 2007;150:641–651. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M, Challis RA, Standen NB, Boyle JP. Cannabinoid CB1 receptors fail to cause relaxation, but couple via Gi/Go to inhibition of adenylyl cyclase in carotid artery smooth muscle. Br J Pharmacol. 1999;128:597–604. doi: 10.1038/sj.bjp.0702842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishioka N, Bukoski RD. A role for N-arachidonylethanolamida (anadamide) as a mediator of sensory nerve-dependent Ca++-induced relaxation. J Pharmacol Exp Ther. 1999;289:245–250. [PubMed] [Google Scholar]
- Jakab B, Helyes Z, Varga A, Bolcskei K, Szabo A, Sandor K, et al. Pharmacological characterization of the TRPV1 receptor antagonist JYL1421 (SC0030) in vitro and in vivo in the rat. Eur J Pharmacol. 2005;517:35–44. doi: 10.1016/j.ejphar.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BM, et al. Cannabinoid-induced mesenteric vasodilation through an endotelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Marnett LJ. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot Essent Fatty Acids. 2002;66:211–220. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- Kunos G, Jarai Z, Batkai S, Goparaju SK, Ishac EJN, Liu J, et al. Endocannabinoids as cardiovascular modulators. Chem Phys Lipids. 2000;108:159–168. doi: 10.1016/s0009-3084(00)00194-8. [DOI] [PubMed] [Google Scholar]
- López-Miranda V, Herradón E, Dannert MT, Alsasua A, Martín MI. Anandamide vehicles: a comparative study. Eur J Pharmacol. 2004;505:151–161. doi: 10.1016/j.ejphar.2004.10.017. [DOI] [PubMed] [Google Scholar]
- McCollum L, Howlet AC, Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor-indeoendent nitric oxide production in rabbit aortic endothelial cells. J Exp Pharm Ther. 2007;321:930–937. doi: 10.1124/jpet.106.117549. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Chapnick BM, Howlett AC. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol Heart Circ Phisiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Offertáler L, Mo FM, Bátkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G Protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistance and conduit rat mesenteric arteries. Br J Pharmacol. 2004;142:435–442. doi: 10.1038/sj.bjp.0705810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Vascular effects of Delta9-tetrahydrocannabinol (THC), anandamide and N-arachidonoyldopamine (NADA) in the rat isolated aorta. Eur J Pharmacol. 2005;507:211–221. doi: 10.1016/j.ejphar.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Perez-Vizcaino F, Cogolludo AL, Zaragoza-Arnaez F, Fajardo S, Ibarra M, Lopez-Lopez JG, et al. Vasodilator effects of sodium nitroprusside, levcromakalim and their combination in isolated rat aorta. Br J Pharmacol. 1999;128:1419–1426. doi: 10.1038/sj.bjp.0702924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitcolin MA, Vandeputte C, Spitzbarth-Regrigny E, Bueb JL, Capdeville-Atkinson C, Tschirhart E. Lack of involvement of pertussis toxin-sensitive G-proteins in norepinephrine-induced vasoconstriction of rat aorta smooth muscle. Biochem Pharmacol. 2001;61:485–491. doi: 10.1016/s0006-2952(00)00547-5. [DOI] [PubMed] [Google Scholar]
- Pratt PF, Hillard CJ, Edgemond WS, Campbell WB. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Physiol Heart Circ Phisiol. 1998;43:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Smart D. Cannabinoid modulation of sensory neurotransmission via cannabinoid and vanilloid receptors: roles in regulation of cardiovascular function. Life Sci. 2002;71:2577–2594. doi: 10.1016/s0024-3205(02)02086-6. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA. Cannabinoid inhibition of capsaicin-sensitive sensory neurotransmission in rat mesenteric arterial bed. Eur J Pharmacol. 2001;418:117–125. doi: 10.1016/s0014-2999(01)00940-2. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Zygmunt PM, Movahed P, Högestätt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed. Br J Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall MD, Harris D, Kendall DA, Ralevic V. Cardiovascular effects of cannabinoids. Pharmacol Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA. The involvement of an endogenous cannabinoid in EDHF-mediated vasorelaxation in the rat coronary vasculature. Eur J Pharmacol. 1997;335:205–209. doi: 10.1016/s0014-2999(97)01237-5. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O'Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riendeau D, Percival MD, Boyce S, Brideau C, Charleson S, Cromlish W, et al. Biochemical and pharmacological profile of a tetra substituted furanone as a highly selective COX-2 inhibitor. Br J Pharmacol. 1997;121:105–117. doi: 10.1038/sj.bjp.0701076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Craib SJ, Stevenson LA, Pertwee RG, Henderson A, Toole J, et al. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J Pharmacol Exp Ther. 2002;301:900–907. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Sugimura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:123–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Tep-areenan P, Kendall DA, Randall MD. Mechanisms of vasorelaxation to testosterone in the rat aorta. Eur J Pharmacol. 2003;465:125–132. doi: 10.1016/s0014-2999(03)01453-5. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol. 1997;121:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Giblin GM, Roomans S, Rhodes AA, Cartwright KA, Shield VJ, et al. GW627368X ((N-(2-[4-(4, 9-diethoxy-1-oxo-1, 3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]-acetyl) benzenesulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006;148:326–339. doi: 10.1038/sj.bjp.0706726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RJ, Giles H. Piglet saphenous vein contains multiple relaxatory prostanoid receptors: evidence for EP4, EP2, DP and IP receptor subtypes. Br J Pharmacol. 2005;144:405–415. doi: 10.1038/sj.bjp.0706088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide fromanandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol. 2000;396:39–42. doi: 10.1016/s0014-2999(00)00207-7. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediated the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]