Abstract
Cannabinoids have numerous physiological effects. In the years since the molecular identification of the G protein-coupled receptors CB1 and CB2, the ion channel TRPV1, and their corresponding endogenous ligand systems, many cannabinoid-evoked actions have been shown conclusively to be mediated by one of these specific receptor targets. However, there remain several examples where these classical cannabinoid receptors do not explain observed pharmacology. Studies using mice genetically deleted for the known receptors have confirmed the existence of additional targets, which have come to be known collectively as non-CB1/CB2 receptors. Despite intense research efforts, the molecular identity of these non-CB1/CB2 receptors remains for the most part unclear. Two orphan G protein-coupled receptors have recently been implicated as novel cannabinoid receptors; these are GPR119, which has been proposed as a receptor for oleoylethanolamide, and GPR55 which has been proposed as a receptor activated by multiple different cannabinoid ligands. In this review I will present an introduction to non-CB1/CB2 pharmacology, summarize information on GPR55 and GPR119 currently available, and consider their phylogenetic origin and what aspects of non-CB1/CB2 pharmacology, if any, they help explain.
Keywords: cannabinoid, CB1, CB2, non-CB1/CB2, GPR55, GPR119
Introduction
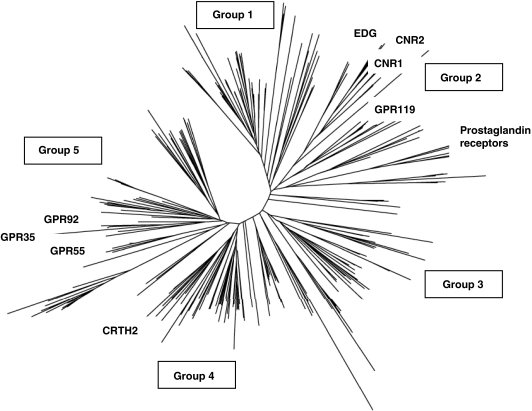
Cannabinoids, which include the bioactive constituents of the marijuana plant Cannabis sativa, as well as endogenous lipids (endocannabinoids) and synthetic compounds with cannabinoid-like activity, interact with specific receptors to cause their effects on target tissues (for review see Pacher et al., 2006). To date, three receptors have been identified by molecular cloning; these are the transient receptor potential vanilloid type 1 receptor (TRPV1) ion channel, and the G protein-coupled receptors (GPCRs) cannabinoid type 1 receptor (CB1) and cannabinoid type 2 receptor (CB2). At the phylogenetic level, CB1 and CB2 are most related to the family of lipid receptors, formerly EDG receptors, which are activated by the sphingolipids sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA; a phylogenetic tree of human family A GPCRs is shown in Figure 1). CB1 and CB2 are also lipid receptors, and recognize acylethanolamide analogues, typified by anandamide (arachidonoylethanolamide, AEA), and 2-arachidonoylglycerol (2-AG). TRPV1 is activated by various lipids including acylethanolamides such as AEA (Starowicz et al., 2007).
Figure 1.
Phylogenetic analysis of all non-sensory human family A G protein-coupled receptors (GPCRs; adapted from Foord, 2007). The neighbour-joining method was used, after forced alignment according to common amino-acid motifs. Each line represents a GPCR; known and putative cannabinoid receptors and other receptors described in this review are identified. Clusters have been assigned to groups (Foord, 2007): group 1, the monoamine-like receptors; group 2, a diverse group containing opsins and glycoprotein/leucine-rich repeat (LRG) type as well as cannabinoid, prostaglandin and lipid receptors; group 3, brain/gut peptide receptors; group 4, chemokine receptors; and group 5, metabolic receptors including purinergic, thrombin and free-fatty acid receptors. Cross-species comparisons show that groups 4 and 5 are not found in nematodes and insects. Abbreviations: CNR1 and CNR2 are the genes encoding cannabinoid type 1 receptor (CB1) and cannabinoid type 2 receptor (CB2), respectively. EDG indicates the sphingolipid receptor cluster, which comprises LPA1/EDG2, LPA2/EDG4 and LPA3/EDG7 and the sphingosine-1-phosphate (S1P) receptors (S1P1 to S1P5). LPA, lysophosphatidic acid.
Cannabinoid type 1 receptor is highly expressed in brain and mediates many of the neurobehavioural and psychotropic effects of Δ9-tetrahydrocannabinol (THC), the primary bioactive cannabinoid of C. sativa. CB1 is also present at lower levels in testis, heart, vascular tissue, and in immune cells. The fundamental importance of the CB1–endocannabinoid axis is reflected in the ongoing development of high-affinity CB1 antagonists and inverse agonists as therapeutic drugs for diabetes, metabolic syndrome and drug dependence. The first of these, SR141716A (rimonabant, Accomplia), is now marketed for treatment of obesity. CB2 plays a role in inflammatory reactions and the immune response and is expressed predominantly by immune and haematopoietic cells and is also present in the CNS during neuroinflammatory states (Elmes et al., 2004; Klein, 2005). CB2−/− mice lack the helper T-cell-activating response to THC observed in wild type and are deficient in particular subsets of B and T cells (Buckley et al., 2000; Ziring et al., 2006). Activation of both CB1 and CB2 contribute to the antinociceptive and immunomodulatory effects of cannabinoid ligands, and both CB2-selective and non-selective agonists have entered clinical development for pain. However, studies performed with CB1−/− and CB2−/− mice have indicated that certain effects of cannabinoids on tissues are mediated by neither CB1 nor CB2 (for review see Begg et al., 2005; Mackie and Stella, 2007). Some effects appeared still to involve GPCRs rather than other potential targets such as ion channels or intracellular lipid receptors such as the PPAR (peroxisome proliferator-activated receptor) family, as deduced from the stimulation of GTP binding or sensitivity to pertussis toxin (PTX). Indeed, only a subset of cannabinoids has affinity for CB1 or CB2. Two naturally occurring acylethanolamides: oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), and two phytocannabinoids: cannabinol (CBN) and cannabidiol (CBD), all lack affinity at CB1/CB2 but evoke pharmacological effects. Recently, two orphan GPCRs have emerged as candidate non-CB1/CB2 receptors. These are GPR119, which is reportedly a receptor for OEA (Overton et al., 2006), and GPR55, which is reportedly activated by various cannabinoids. Initially, little information on GPR55 was available in the public domain, and its identification as a cannabinoid receptor came solely from patent and meeting abstracts (that is, non-peer-reviewed sources; Baker et al., 2006). In this issue, Johns et al. (2007) have described cardiovascular phenotyping of GPR55−/− mice as well as preliminary studies of the pharmacology of recombinantly expressed GPR55. Also, Staton et al. (2006) have explored the phenotype of the same GPR55−/− line in models of inflammatory and neuropathic pain. This review will evaluate whether, based on current data, GPR119 and GPR55 can be considered as novel cannabinoid receptors and, if so, whether they might explain any observed non-CB1/CB2 effects.
Non-CB1/CB2 sites in the vasculature
The most extensively studied non-CB1/CB2 site occurs in resistance arteries of the mesenteric vasculature, where AEA and analogues, but not synthetic cannabinoid agonists, cause vasodilatation (Begg et al., 2005). This effect is abolished by endothelial denudation and is sensitive to blockade by SR141716A, but not by the chemically similar CB1 antagonist AM251, and only at concentrations higher than required to inhibit CB1 (Begg et al., 2005). Abnormal cannabidiol (abn-cbd), which is a synthetic structural analogue of cannabidiol, is a selective agonist at this endothelial site, inducing vasodilatation, but having negligible activity at CB1 or CB2 (Jarai et al., 1999). Cannabidiol itself and the analogue O-1918 are selective antagonists, blocking vasodilatation induced by AEA or abn-cbd but having negligible affinity or activity at CB1 or CB2 (Offertaler et al., 2003). Several but not all studies report the mesenteric vasodilator effect of abn-cbd to be PTX-sensitive (Begg et al., 2005; Hiley and Kaup, 2007). A similar ligand profile is detected in human umbilical vein endothelial cells, where abn-cbd activates p42/44 MAP kinase and potentiates voltage-gated K+ currents via BKCa channels, and O-1918 or PTX block these effects (Begg et al., 2003). The endothelial abn-cbd-sensitive site may be related to another non-CB1/CB2 site in heart, which mediates the hypotensive response to systemic bacterial endotoxin that is thought to result from release of AEA by activated macrophages. Endotoxin-evoked hypotension is sensitive to blockade by SR141716A but not AM251, and is comparable in wild-type, CB1−/− or CB1−/−/CB2−/− mice (Begg et al., 2005). There may also be a non-CB1/CB2 site on smooth muscle cells, as THC causes PTX-sensitive vasodilatation that is endothelium-independent (O'Sullivan et al., 2005).
Non-CB1/CB2 sites in the CNS
Breivogel et al. (2001) showed that AEA and the aminoalkylindole WIN55212-2, but not HU210 or THC, can stimulate [35S]-GTPγS binding in brain slices and in membrane preparations from CB1−/− mice. In separate studies, WIN55212-2 and also CP55940 inhibited glutaminergic excitatory postsynaptic currents (EPSCs) on hippocampal CA1 pyramidal cells from CB1−/− mice, allowing electrophysiological characterization of this site (Hajos et al., 2001). These two putative non-CB1/CB2 sites in the CNS share with the vascular endothelial site sensitivity to blockade by PTX, or by high concentrations of SR141716A and not by AM251. However, they appear pharmacologically distinct from the endothelial site since the synthetic cannabinoids WIN55212-2 and/or CP55940 were efficacious at the CNS site(s) whereas neither stimulated the endothelial site. Conversely abn-cbd had no effect on glutaminergic EPSCs but activated the endothelial site. Hints about the role of hippocampal non-CB1/CB2 receptors have come from the demonstration that SR141716A but not AM251 has anxiolytic effects in CB1−/− mice, hence the non-CB1/CB2 site might mediate anxiogenesis, opposing the anxiolytic effects of CB1 (Haller et al., 2004).
Non-CB1/CB2 sites on immune cells
As in the CNS and cardiovascular systems, many of the effects of cannabinoids on the immune system are induced by binding to CB1 and/or CB2 receptors, particularly CB2 which is abundantly expressed by macrophages, dendritic cells, and B cells (Massi et al., 2006). However studies using CB1−/− and CB2−/− mice again support the existence of additional receptor targets. Resting T-cells exhibit robust elevations of intracellular calcium ([Ca2+]i) in response to THC, CBN and HU-210 but not CP55940, an effect which is similar in splenocytes isolated from either wild-type or CB1−/−/CB2−/− mice (Rao and Kaminski, 2006). Both SR141716A and SR144528 blocked these effects, though only at concentrations (1–5 μM) higher than their reported affinities for CB1 and CB2. This observation serves to highlight the challenges inherent in using these agents, which are well established to be selective (for CB1 over CB2, or vice versa), but whose specificity over other, novel cannabinoid receptors, is less well defined. Non-CB1/CB2 effects have also been characterized on activated T cells, where 2-AG suppresses production of interleukin-2, though this seems to occur via intracellular PPARγ (Rockwell et al., 2006). On neutrophils, high-micromolar concentrations of CP55940 but not AEA nor the non-hydrolysable analogue methanandamide reduced superoxide production from chemoattractant-stimulated cells (Kraft et al., 2004).
Orphan endocannabinoids: PEA and OEA
Palmitoylethanolamide reduces the pain behaviour resulting from immune challenge, an effect blocked by SR144528 (Calignano et al., 1998; Jaggar et al., 1998). Since PEA lacks affinity for CB2, its effects have been postulated to involve indirect activation of CB2, either through inhibition of AEA hydrolysis, which is catalysed by fatty-acid amide hydrolase (FAAH), or by coupling of a putative PEA receptor to PLC and diacylglycerol lipase, resulting in increased 2-AG production. However, PEA has anti-inflammatory effects not blocked by SR144528 (Lo Verme et al., 2005b). In tissues, production of PEA can occur independently of production of AEA and 2-AG, and a PEA-specific hydrolytic enzyme has been isolated, named N-acylethanolamine-hydrolysing acid amidase (NAAA), distinct from FAAH. NAAA is ∼30-fold selective for PEA over AEA, differs in its optimal pH for hydrolytic activity, and has unique tissue distribution and subcellular location. Hence, PEA and its receptor target have the characteristics of a parallel endocannabinoid-signalling pathway, distinct from CB1, CB2, AEA and 2-AG (Mackie and Stella, 2007). OEA also has characteristics of a paracrine- or endocrine-signalling mediator. OEA has been linked to satiety and mechanisms controlling food intake. In the mucosal layer of the small intestine, levels of OEA as well as OEA-synthesizing and OEA-degrading activities change in proportion to food intake (Nielsen et al., 2004). OEA is detected in other tissues but at levels unaffected by feeding (Fu et al., 2007). Dosing OEA to rodents prolongs the time between feeding activity and decreases overall food intake (Rodriguez de Fonseca et al., 2001). Mechanistically, the effects of both OEA and PEA are believed to involve PPARα. OEA binds with high affinity to purified ligand-binding domain of PPARα, and both ligands activate PPARα in cell-based assays with EC50 values 120 nM and 3.1 μM for OEA and PEA, respectively (Fu et al., 2003; Lo Verme et al., 2005a, 2005b). PPARα is a familiar regulator of energy balance and lipid metabolism and is expressed in the small intestine (Bocher et al., 2002). Synthetic PPARα agonists have similar behavioural effects on feeding as OEA, and dosing either synthetic PPARα agonists or OEA to PPARα−/− mice fails to induce hypophagic effects (Fu et al., 2003). Likewise, activation of PPARα reduces inflammation (Chinetti et al., 2000), and PEA lacks its anti-inflammatory activity in PPARα−/− mice (Lo Verme et al., 2005a). Hence, the anti-nociceptive effects of PEA may be due to synergistic activity on both cannabinoid and PPARα systems (Russo et al., 2007).
GPR55
GPR55 was identified as an orphan GPCR in the purinergic subfamily, most closely related to two other orphans, GPR35 and GPR23, and the purinoceptor P2Y5 (Figure 1; Sawzdargo et al., 1997). The first association between GPR55 and cannabinoids appeared in a patent from GlaxoSmithKline describing expression of human GPR55 in yeast host strains that coexpressed yeast/human chimeric G proteins (Brown and Wise, 2001). These cells are engineered to grow only under conditions of receptor activation, allowing sensitive and specific detection of either receptor constitutive activity, or receptor activation by agonist ligands (Brown et al., 2000). Owing to the lack of endogenous GPCRs in the yeast cells, this approach is a useful method to identify endogenous or other ligands for orphan GPCRs (Dowell and Brown, 2002). GPR55-expressing yeast were activated by AM251 and SR141716A (see Table 1; Brown and Wise, 2001). A subsequent patent from AstraZeneca (London, UK) corroborated the link between GPR55 and cannabinoids, showing that membranes from HEK293 cells transiently transfected with GPR55 bound [3H]CP55940 and [3H]SR141716A, but not [3H]WIN55212-2 (Drmota et al., 2004). [35S]GTPγS binding in response to a panel of chemically diverse cannabinoids was also determined in these membranes. Several cannabinoids including AEA, PEA, 2-AG, THC, virodhamine and CP55940 behaved as potent agonists. SR141716A, AM251, SR144528 and OEA also activated GPR55 (see Table 1). Virodhamine, an endogenously occurring isomer of anandamide in which arachidonic acid is linked to ethanolamide via an ester moiety, appeared to have the greatest intrinsic activity (Drmota et al., 2004). Johns et al. (2007) describe similar findings for several of these ligands. [35S]GTPγS binding was insensitive to PTX or cholera toxins, implicating a G protein distinct from Gi or Gs (Drmota et al., 2004). Consistent with this, GPR55-constitutive activity in yeast was only detected in the presence of a chimeric G protein α-subunit incorporating the C terminus of Gα13 (Brown and Wise, 2001). Further abstracts presented by the AstraZeneca group using G protein-specific peptides or antibodies to inhibit [35S]GTPγS binding also implicate G13 as the coupling partner of GPR55 (Baker et al., 2006). The association with G13 tends to indicate that GPR55 is not responsible for any of the known PTX-sensitive non-CB1/CB2 effects. However, all other GPCRs known to activate G13 also activate other G proteins (Riobo and Manning, 2005), so further G protein-signalling pathways (potentially, PTX-sensitive) for GPR55 may remain to be discovered.
Table 1.
Activity of cannabinoid ligands at GPR55
| Ligand | GPR55 EC50 (nM) GTPγS binding | GPR55 EC50 (nM) yeast reporter | GPR119 fold induction of yeast reporter |
|---|---|---|---|
| Endocannabinoids | |||
| AEA | 18a | Inactiveb | 2c |
| Noladin ether | 11a | ||
| PEA | 3a | 20c | |
| Virodhamine | 10a | Inactiveb | |
| 2-AG | 3a | Inactiveb | Inactivec |
| OEA | 420a | Inactiveb | 60c |
| Phytocannabinoids | |||
| THC | 8a | ||
| CBD | 350a (IC50) | ||
| CBN | >30 000a | ||
| Synthetic cannabinoids | |||
| Methanandamide | Inactiveb | Inactivec | |
| CP55940 | 7a | 20b (pA2) | Inactivec |
| WIN55212-2 | >30 000,a >1000c | Inactiveb | Inactivec |
| HU210 | 33a | Inactiveb | |
| JWH015 | 4a | ||
| JWH133 | >30 000a | Inactivec | |
| abn-cbd | 2780,a 2.5c | ||
| SR144528 | ≈1000a | ||
| SR141716A | ≈600a | 3000b | |
| AM251 | 39e | 3000b | |
| AM630 | ≈1500a | Inactiveb | |
| O-1602 | 13,e 1.4c | ||
Abbreviations: abn-cbd=abnormal cannabidiol; AEA=arachidonoylethanolamide; 2-AG=2-arachidonoylglycerol; CBD=cannabidiol; CBN=cannabinol; OEA=oleoylethanolamide; PEA=palmitoylethanolamide; THC=Δ9-tetrahydrocannabinol.
The table shows reported ligand activities at the putative cannabinoid receptors, GPR55 and GPR119. For GPR55, data show agonist EC50 (nM) or IC50/pA2 (in bold), where the compound acts as an antagonist. For SR141716A, SR144528 and AM251, EC50 values in GTPγS binding have been estimated from graphical data (Drmota et al., 2004). For GPR119, data show estimated fold induction of a yeast reporter gene in the presence of 30-μM compound.
GlaxoSmithKline abstract (see Baker et al., 2006).
AstraZeneca abstract (see Baker et al., 2006).
The function of GPR55 remains an open question. Similarities between GPR55 and the endothelial vasodilator site (AEA, abn-cbd and O-1602 as agonists; cannabidiol as antagonist; Drmota et al., 2004) led Johns et al. (2007) to evaluate the cardiovascular phenotype of GPR55−/− mice. Blood pressure and heart rate were not significantly different between GPR55−/− and wild-type littermates, and both genotypes showed similar decreases in blood pressure following systemic dosing with O-1602. Isolated mesenteric resistance arteries from GPR55−/− mice had normal contractile responses to carbachol, and pre-contracted arteries from GPR55−/− and wild-type mice were relaxed by O-1602 with identical potencies. This did not disprove the hypothesis that GPR55 may be present and functional in the vasculature, but did indicate that GPR55 was unlikely to be the vasodilator site-of-action of abn-cbd (Johns et al., 2007). This conclusion was consistent with the efficacy of CP55940 at recombinant GPR55 but not at the endothelial site (Begg et al., 2005). Also, the endothelial site appears to signal through Gi whereas G13-linked receptors are more commonly associated with vasoconstrictor effects (Lee et al., 2004; Begg et al., 2005). Currently, there are no published data measuring GPR55 expression in vascular tissue, though abstracts have suggested the presence of GPR55 in vascular smooth muscle (Baker et al., 2006).
Human GPR55 mRNA is expressed in brain, most abundantly in the caudate nucleus and putamen, with lesser levels in the hippocampus, thalamus, pons, cerebellum and frontal cortex (Sawzdargo et al. (1997) and data not shown). In rat brain, GPR55 mRNA was detected by in situ hybridization in hippocampus, thalamic nuclei and regions of the mid-brain. GPR55 mRNA is also present in spleen in both human and rodent (Sawzdargo et al., 1997). Robust data on the location of GPR55 protein await the generation of antibodies reactive with mouse GPR55 and their validation on tissues from GPR55−/− and wild-type littermates. However, the overall pattern of expression in CNS and immune cells leads to the proposition of GPR55 as a putative non-CB1/CB2 site in these tissues. WIN55212-2 activates the non-CB1/CB2 site in CNS (Breivogel et al., 2001), whereas WIN55212-2 reportedly lacks activity at GPR55 (Drmota et al., 2004), suggesting GPR55 does not mediate the non-CB1/CB2 effects of this ligand. In an abstract, Staton et al. (2006) described a study of GPR55−/− mice in models of inflammatory and neuropathic pain. The hypersensitivity to mechanical nociception observed in wild-type mice following either injection of Freund's complete adjuvant (FCA) into the hind paw, or surgery to partially constrict the sciatic nerve, was completely absent in GPR55−/− animals at all time points observed (1–28 days). Increased plasma cytokine levels resulting from the FCA challenge were not significantly different between GPR55−/− and wild-type mice on day 1, though at day 14, a subset of cytokines including interferon-γ remained significantly elevated in GPR55−/− relative to wild-type mice. These results suggest a role for GPR55 in pain signalling, though whether the site-of-action is neuronal, immune cell, or other, remains to be determined.
GPR55: outstanding questions
A focus of current research is to understand which tissues express functional GPR55 protein. This arises from the apparent paradox between the strong binding of [3H]CP55940 to GPR55 in vitro (Drmota et al., 2004), and autoradiographic studies that show a lack of binding of this ligand to mouse brain after genetic deletion of CB1 (Zimmer et al., 1999). This might be explained by a lower affinity of GPR55 for CP55940, compared with CB1. Repetition of these studies at higher concentrations of radioligand, and comparison of CB1−/− with CB1−/−/GPR55−/− mice, may in future reveal a GPR55-mediated component. Also, Buckley et al. (2000) were unable to detect specific binding of [3H]CP55940 to spleen membranes derived from CB2−/− mice even at high radioligand concentrations (30 nM). Further investigation is also required to understand whether G13 is important for GPR55 signalling in vivo, or whether other G protein-mediated or G protein-independent mechanisms are involved, and to explain the discrepancies in pharmacology between GPR55 expressed recombinantly in yeast or mammalian hosts (see Table 1).
The endogenous ligand of GPR55 is unclear. The association of both GPR55 and PEA with inflammatory pain signalling reinforces speculation that PEA, in addition to its action at PPARα described above, might also activate GPR55 in vivo (Mackie and Stella, 2007). The activity of CBD at GPR55 is also noteworthy, since CBD is reported to have anticonvulsive, anxiolytic, antipsychotic, antiemetic and antiarthritic properties. CBD does not bind to CB1 or CB2, and its effects have not been conclusively attributed to any receptor or other target, though its ability to inhibit AEA transport and degradation or its antioxidant capacity have been suggested to mediate in vivo activity (Mechoulam et al., 2002).
For any orphan receptor, it is usual to consider whether homologues offer any clues to pharmacology. The closest relative of GPR55 in phylogenetic clustering is GPR35 (Figure 1), which shares low (30%) amino-acid identity. In human and rodent, GPR35 mRNA is detected in immune tissues and isolated immune cells, gastrointestinal tract, and in the case of rat, in neural tissue from the dorsal root ganglia (Wang et al., 2006; Taniguchi et al., 2006). This profile is somewhat similar to that of GPR55, except that little GPR35 was detected in brain. However, ligand specificity of GPR35 appears divergent from GPR55. GPR35 is activated by kynurenic acid, a metabolite of tryptophan (Wang et al., 2006), and by zaprinast, a synthetic PDE inhibitor (Taniguchi et al., 2006). Chemically, zaprinast is a xanthine analogue of cGMP, and was a lead molecule in the development of sildenafil (Viagra). Both of these ligand pairings have been corroborated by expression in yeast, where human GPR35 was activated by kynurenic acid and zaprinast with potencies comparable to literature reports (SJ Dowell, personal communication). Neither structure bears obvious chemical similarity to proposed ligands of GPR55 nor are cannabinoids reported to activate GPR35, suggesting little similarity between GPR55 and GPR35 at the level of pharmacophore.
If GPR55 is a receptor for AEA, its phylogenetic divergence from CB1 and CB2 suggests that it may have arisen by convergent evolution towards the pre-existing signalling molecule. Is there precedence for such convergent evolution in the GPCR superfamily? The answer is affirmative—two examples are worthy of note. First, GPR23 and GPR92 were originally orphans but recently have been shown to respond to LPA and designated LPA4 and LPA5, respectively (Noguchi et al., 2003; Lee et al., 2006). Phylogenetically, they are only distally related to the other known LPA receptors (LPA1/EDG2, LPA2/EDG4 and LPA3/EDG7; Figure 1). GPR23/LPA4 and GPR92/LPA5 cluster proximal to GPR55 in the purinergic subfamily of evolutionarily recent origin, whereas LPA1–3 cluster proximal to S1P receptors (S1P1–5) and CB1 and CB2 in a much more ancient branch of family A GPCRs. Hence, LPA4 and LPA5 appear to have diverged not from a pre-existing LPA receptor but from an unrelated receptor. McPartland et al. (2006) noted these relationships, and using data from point-mutation studies on LPA receptors (Fujiwara et al., 2005), extrapolated by alignment of GPR55, GPR23, CB1 and LPA1 sequences to propose four candidate residues or motifs potentially involved in the GPR55 ligand-binding site. These will form a starting point for point-mutational analysis of GPR55, once robust mammalian cell-based assays for this target are available. The second example of putative convergent evolution is among prostaglandin receptors. The eight originally identified prostaglandin receptors (EP1–4, TP, FP, DP1 and IP; Figure 1) are evolutionarily ancient and have related primary sequences. The ninth and most recently described receptor, originally named CRTH2 (for chemoattractant receptor on TH2 cells) but now renamed DP2, is activated by prostaglandin D2 (Hirai et al., 2001) but bears minimal sequence similarity to DP1 beyond motifs common to all family A GPCRs. Residues involved in ligand-binding in DP1 are not conserved in DP2 (Hata et al., 2005), and DP2 clusters with chemoattractant receptors (FPR, FPRL-1) in the purinergic subfamily.
GPR119
GPR119 is an orphan receptor originally identified in genome-sequencing efforts and expressed predominantly in the pancreas and gastrointestinal tract (Fredriksson et al., 2003). Immunohistochemical staining colocalized GPR119 to cells expressing pancreatic polypeptide (PPY) within mouse pancreatic islets; no immunoreactivity was detected in α- or β-cells (Sakamoto et al., 2006). This striking expression pattern prompted the initiation of drug screening, which yielded selective synthetic small-molecule agonists of GPR119, including AR-231453 (EC50=25 nM; Arena Pharmaceuticals patent WO 2004065380), and PSN632408 (EC50=5 μM; Overton et al., 2006). GPR119 behaves as a Gs-coupled receptor: transfection of GPR119 into mammalian cells resulted in elevation of intracellular cAMP levels and sensitization to forskolin (Sakamoto et al., 2006), and small-molecule agonists evoke concentration-dependent increases in cAMP in GPR119-transfected cells (Overton et al., 2006). When dosed systemically into rodent, both small-molecule agonists have effects on metabolic status. In acute studies, PSN632408 reduced cumulative food intake in free-feeding rats for 24-h post-dosing. Longer term oral dosing of PSN632408 to fat-fed male rats reduced both cumulative food intake and body weight gain (Overton et al., 2006). AR-231453 elevated plasma insulin in a glucose-dependent fashion in normal mice and improved glucose tolerance in both normal and diabetic mouse models, but not in mice genetically deleted for GPR119 (Jones, 2006). These studies strongly suggest a role for GPR119 in the pancreas, regulating energy balance. It is still unclear whether this regulation is secondary to effects on PPY, a peptide known to inhibit pancreatic exocrine secretion and attenuate the elevation of post-prandial glucose, since Jones (2006) described increased cAMP levels in isolated pancreatic β-cells treated directly with AR-231453.
The identification of GPR119 as a putative cannabinoid receptor comes from reports of activation of GPR119 by OEA. Overton et al. (2006) described activation of yeast expressing either human or mouse GPR119 by OEA. Significant induction of the yeast reporter gene was achieved only at >10 μM OEA; PEA was more weakly active, and other cannabinoids tested including AEA were inactive (see Table 1). Host cells lacking GPR119 failed to respond to OEA, though the control, of OEA tested on yeast expressing unrelated receptors, was not described. HEK293 cells stably expressing tetracycline-inducible human GPR119 (Overton et al., 2006), or HEK293 or COS-7 cells transiently transfected with mouse GPR119 (Sakamoto et al., 2006) were also reported to respond to >1 μM OEA with increases in intracellular cAMP. The hypothesis that OEA is the endogenous ligand of GPR119 is initially compelling, given the hyperphagic effects of OEA (Rodriguez de Fonseca et al., 2001). However, it is unclear whether the high concentrations of OEA required to activate recombinant GPR119 occur (patho)physiologically, or whether another as-yet unidentified ligand with greater potency might be the endogenous ligand. Not all groups have observed specific agonism of GPR119 by OEA, using mammalian cell-based assays in which small-molecule agonists exhibit high potencies (H Sauls, personal communication). Given the evidence that OEA acts via PPARα, it will be crucial to test whether the characteristic effects of OEA on feeding remain after genetic ablation of GPR119. Soga et al. (2005) also report activation of GPR119 by oleoyl- and palmitoyl-lysophosphatidylcholine (18:1-LPC and 16:0-LPC) in the micromolar range (EC50=1.5 and 1.6 μM, respectively), whereas Overton et al. (2006) detected activation by 18:1-LPC only at higher concentrations (EC50>30 μM). LPC has a history of mis-assignation as a ligand of orphan GPCRs possibly due to pleiotropic effects on host cells; LPC was described as an agonist at the orphan G2A but these reports were later retracted (Kabarowski et al., 2001; Witte et al., 2005), and it now appears that direct activators of G2A are oxidized free-fatty acids including 9-hydroxyoctadecadienoic acid (9-HODE; Obinata et al. (2005) and SJ Dowell and AJ Brown, unpublished data).
Conclusion
It is now established that phytocannabinoids, endocannabinoids, and synthetic cannabinoids have multiple in vivo sites-of-action additional to CB1, CB2 and TRPV1. Some of the observed non-CB1/CB2 effects may be explained by cross-activity at PPARs or other known receptors, but novel targets, particularly GPCRs, likely also play a part. On the basis of the variety of observed pharmacological profiles, these have been estimated to number three or more (Mackie and Stella, 2007). Two orphan GPCRs, GPR55 and GPR119, have so far been reported as novel cannabinoid receptors. GPR55 has been demonstrated to interact with chemically unrelated cannabinoid ligands, in both mammalian and non-mammalian recombinant expression systems, and by independent research groups. Clearly, there is some relationship between the ligand-binding sites of GPR55 and CB1/CB2; however, the endogenous agonist and physiological relevance of GPR55 are not yet clear. On the basis of the reasonable assumption that GPR55 might mediate the unexplained vasodilatatory effects of AEA and abn-cbd, Johns et al. (2007) evaluated cardiovascular phenotypes of GPR55−/− mice, but found no differences to wild-type mice. A more speculative pain study showed a clear phenotype in GPR55−/− animals, of reduced mechanical nociception following inflammatory or neuropathic challenge (Staton et al., 2006). By analogy, CB2 was readily linked to inflammatory pain signalling in whole-animal studies using selective ligands (Clayton et al., 2002; Ibrahim et al., 2003; Giblin et al., 2007). However, defining the effects of CB2 activation at the cellular level and how they relate to mechanisms of inflammation has been a far greater challenge. Different hypotheses hold that the antinociceptive site-of-action of CB2 may be directly on immune cells to reduce local release of inflammatory mediators, or neuronal, or on other cells such as keratinocytes, acting through release of β-endorphins (Cheng and Hitchcock, 2007). For GPR55, the generation of selective pharmacological tools suitable for in vivo studies will confirm whether this GPCR is associated with pain signalling in wild-type animals. If this holds to be the case, detailed studies will be required to define mechanisms involved, with the initial focus being on immune regulation. The second reported novel cannabinoid receptor, GPR119, has been the focus of intensive screening efforts in several pharmaceutical laboratories, and is strongly implicated in the regulation of energy balance and body weight. However, further corroborating data of the activity of acylethanolamides at GPR119 will be required before it can be regarded unequivocally as a cannabinoid receptor. Overall, it appears none of the known non-CB1/CB2 effects is associated with these putative novel cannabinoid receptors. Ironically, even though the original precept was that ligand fishing was expected to identify novel cannabinoid GPCRs, such as that mediating abn-cbd-evoked vasodilatation, this approach has instead yielded novel cannabinoid receptors involved in diverse other aspects of physiology. The implication is that further novel cannabinoid receptors mediating non-CB1/CB2 effects remain to be identified.
Acknowledgments
Simon Dowell and Jenny Stables are thanked for critical reading of the manuscript, and Jo Holbrook, Alan Lewis and Steve Foord for providing Figure 1.
Abbreviations
- abn-cbd
abnormal cannabidiol
- AEA
arachidonoylethanolamide
- 2-AG
2-arachidonoylglycerol
- CBD
cannabidiol
- CBN
cannabinol
- EPSC
excitatory postsynaptic current
- GPCR
G protein-coupled receptor
- LPA
lysophosphatidic acid
- LPC
lysophosphatidylcholine
- NAAA
N-acylethanolamine-hydrolysing acid amidase
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamide
- PPAR
peroxisome proliferator-activated receptor
- PTX
pertussis toxin
- S1P
sphingosine-1-phosphate
- THC
Δ9-tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid type 1 receptor
Conflict of interest
The author is an employee of GlaxoSmithKline.
References
- Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertaler L, Batkai S, Pacher P, Razdan RK, et al. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bocher V, Pineda-Torra I, Fruchart JC, Staels B. PPARs: transcription factors controlling lipid and lipoprotein metabolism. Ann NY Acad Sci. 2002;967:7–18. doi: 10.1111/j.1749-6632.2002.tb04258.x. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Brown AJ, Dyos SL, Whiteway MS, White JHM, Watson M-A, Marzioch M, et al. Functional coupling of mammalian receptors to the yeast mating pathway using novel yeast/mammalian G protein α-subunit chimeras. Yeast. 2000;16:11–22. doi: 10.1002/(SICI)1097-0061(20000115)16:1<11::AID-YEA502>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Wise A.Identification of modulators of GPR55 activity 2001. Assignee: GlaxoSmithKline. Patent WO00186305
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Hitchcock SA. Targeting cannabinoid agonists for inflammatory and neuropathic pain. Expert Opin Investig Drugs. 2007;16:951–965. doi: 10.1517/13543784.16.7.951. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Clayton N, Marshall FH, Bountra C, O'Shaughnessy CT. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253–260. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Dowell SJ, Brown AJ. Yeast assays for G-protein-coupled receptors. Receptors Channels. 2002;8:343–352. [PubMed] [Google Scholar]
- Drmota E, Greasley P, Groblewski T.Screening assays for cannabinoid-ligand type modulators 2004. AssigneeAstraZeneca. Patent WO2004074844
- Elmes SJR, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- Foord SM.G protein-coupled receptors and comparative genomics Comparative Genomics: Basic and Applied Research 2007Taylor & Francis: Boca Raton; 283–300.In: Brown JA (ed) [Google Scholar]
- Fredriksson R, Hoglund PJ, Gloriam DEI, Lagerstrom MC, Schioth HB. Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett. 2003;554:381–388. doi: 10.1016/s0014-5793(03)01196-7. [DOI] [PubMed] [Google Scholar]
- Fu J, Astarita G, Gaetani S, Kim J, Cravatt BF, Mackie K, et al. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J Biol Chem. 2007;282:1518–1528. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez de Fonseca F, et al. Oleoylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-[alpha] Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Sardar V, Tokumura A, Baker D, Murakami-Murofushi K, Parrill A, et al. Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J Biol Chem. 2005;280:35038–35050. doi: 10.1074/jbc.M504351200. [DOI] [PubMed] [Google Scholar]
- Giblin GMP, O'Shaughnessy CT, Naylor A, Mitchell WL, Eatherton AJ, Slingsby BP, et al. Discovery of 2-[(2,4-dichlorophenyl)amino]-N-[(tetrahydro-2H-pyran-4-yl)methyl]-4-(trifluoromethyl)-5-pyrimidinecarboxamide, a selective CB2 receptor agonist for the treatment of inflammatory pain. J Med Chem. 2007;50:2597–2600. doi: 10.1021/jm061195+. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Hata AN, Lybrand TP, Breyer RM. Identification of determinants of ligand binding affinity and selectivity in the prostaglandin D2 receptor CRTH2. J Biol Chem. 2005;280:32442–32451. doi: 10.1074/jbc.M502563200. [DOI] [PubMed] [Google Scholar]
- Hiley CR, Kaup SS.GPR55 and the vascular receptor for cannabinoids Br J Pharmacol; 2007 10.1038/sj.bjp.0707421[e-pub ahead of print: 20 August 2007doi:] [DOI] [PMC free article] [PubMed]
- Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–262. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Deng HF, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker D, Ao Z, Parsons M, Daniels D, et al. The novel endocannabinoid receptor GPR55 binds atypical cannabinoids but does not mediate their vasodilatory effects Br J Pharmacol 2007 10.1038/sj.bjp.0707419[e-pub ahead of print: 20 August 2007doi:] [DOI] [PMC free article] [PubMed]
- Jones R.Discovery of agonists of the glucose dependent insulinotropic receptor, GPR119, a pancreatic beta-cell oGPCR, for the treatment of NIDDM Drugs Future 200631Suppl AAbstract L48 [Google Scholar]
- Kabarowski JHS, Zhu K, Le LQ, Witte ON, Xu Y. Lysophosphatidylcholine as a ligand for the immunoregulatory receptor G2A. Science. 2001;293:702–705. doi: 10.1126/science.1061781. [DOI] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Kraft B, Wintersberger W, Kress HG. Cannabinoid receptor-independent suppression of the superoxide generation of human neutrophils (PMN) by CP55940, but not by anandamide. Life Sci. 2004;75:969–977. doi: 10.1016/j.lfs.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- Lee DL, Webb RC, Jin LM. Hypertension and RhoA/Rho-kinase signaling in the vasculature—highlights from the recent literature. Hypertension. 2004;44:796–799. doi: 10.1161/01.HYP.0000148303.98066.ab. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005a;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, La Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005b;77:1685–1698. doi: 10.1016/j.lfs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J. 2007;8:E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Parolaro D. Cannabinoids, immune system and cytokine network. Curr Pharm Des. 2006;12:3135–3146. doi: 10.2174/138161206777947425. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Matias I, Di Marzo V, Glass M. Evolutionary origins of the endocannabinoid system. Gene. 2006;370:64–74. doi: 10.1016/j.gene.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- Nielsen MJ, Petersen G, Astrup A, Hansen HS. Food intake is inhibited by oral oleoylethanolamide. J Lipid Res. 2004;45:1027–1029. doi: 10.1194/jlr.C300008-JLR200. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the EDG family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. The effects of Delta(9)-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br J Pharmacol. 2005;145:514–526. doi: 10.1038/sj.bjp.0706218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obinata H, Hattori T, Nakane S, Tatei K, Izumi T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem. 2005;280:40676–40683. doi: 10.1074/jbc.M507787200. [DOI] [PubMed] [Google Scholar]
- Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MCT, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GK, Kaminski NE. Cannabinoid-mediated elevation of intracellular calcium: a structure–activity relationship. J Pharmacol Exp Ther. 2006;317:820–829. doi: 10.1124/jpet.105.100503. [DOI] [PubMed] [Google Scholar]
- Riobo NA, Manning DR. Receptors coupled to heterotrimeric G proteins of the G12 family. Trends Pharmacol Sci. 2005;26:146–154. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- Russo R, LoVerme J, La Rana G, D'Agostino G, Sasso O, Calignano A, et al. Synergistic antinociception by the cannabinoid receptor agonist anandamide and the PPAR-alpha receptor agonist GW7647. Eur J Pharmacol. 2007;566:117–119. doi: 10.1016/j.ejphar.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y, Inoue H, Kawakami S, Miyawaki K, Miyamoto T, Mizuta K, et al. Expression and distribution of Gpr119 in the pancreatic islets of mice and rats: predominant localization in pancreatic polypeptide-secreting PP-cells. Biochem Biophys Res Commun. 2006;351:474–480. doi: 10.1016/j.bbrc.2006.10.076. [DOI] [PubMed] [Google Scholar]
- Sawzdargo M, George SR, Nguyen T, Xu SJ, Kolakowski LF. A cluster of four novel human g protein-coupled receptor genes occurring in close proximity to cd22 gene on chromosome 19q13.1. Biochem Biophys Res Commun. 1997;239:543–547. doi: 10.1006/bbrc.1997.7513. [DOI] [PubMed] [Google Scholar]
- Soga T, Ohishi T, Matsui T, Saito T, Matsumoto M, Takasaki J, et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun. 2005;326:744–751. doi: 10.1016/j.bbrc.2004.11.120. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Nigam S, Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol Ther. 2007;114:13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Staton PC, Hatcher JP, Morrison AD, Walker DJ, Parsons M, Brown AJ, et al. Determining the role of a third cannabinoid receptor, GPR55, in inflammatory and neuropathic pain 16th ICRS Annual Symposium on the Cannabinoids; 24–28 June 2006; Hungary 2006ICRS: Hungary; Abstract 149 [Google Scholar]
- Taniguchi Y, Tonai-Kachi H, Shinjo K. Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Lett. 2006;580:5003–5008. doi: 10.1016/j.febslet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- Witte ON, Kabarowski JH, Xu Y, Le LQ, Zhu K. Retraction. Science. 2005;307:206b. doi: 10.1126/science.307.5707.206b. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziring D, Wei B, Velazquez P, Schrage M, Buckley NE, Braun J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics. 2006;58:714–725. doi: 10.1007/s00251-006-0138-x. [DOI] [PubMed] [Google Scholar]