Abstract
Background and purpose:
2-arachidonoylglycerol (2-AG) is an endocannabinoid whose hydrolysis is predominantly catalysed by the enzyme monoacylglycerol lipase (MAGL). The development of MAGL inhibitors could offer an opportunity to investigate the anti-inflammatory and anti-nociceptive role of 2-AG, which have not yet been elucidated. On these bases, URB602, a MAGL inhibitor, was tested in a murine model of inflammation/inflammatory pain.
Experimental approach:
Acute inflammation was induced by intraplantar injection of λ-carrageenan into mice. The highest dose to be employed has been selected performing the tetrad assays for cannabimimetic activity in mice. URB602 anti-inflammatory and anti-nociceptive efficacy (assessed by plethysmometer and plantar test, respectively) was evaluated both in a preventive regimen (drug administered 30 min before carrageenan) and in a therapeutic regimen (URB602 administered 30 min after carrageenan). To elucidate the cannabinoid receptor involvement, rimonabant and SR144528, CB1 and CB2 selective antagonists, respectively, were given 15 min before URB602.
Key results:
Systemic administration of URB602 elicited a dose-dependent anti-oedemigen and anti-nociceptive effect that was reversed exclusively by the CB2 receptor antagonist. The efficacy of URB602 persisted also when the compound was administered in a therapeutic regimen, suggesting the ability of URB602 to improve established disease.
Conclusions and implications:
The present report highlighted the ability of the selective MAGL inhibitor, URB602, to prevent and treat an acute inflammatory disease without producing adverse psychoactive effects. The data presented herein also contributed to clarify the physiological role of 2-AG in respect to inflammatory reactions, suggesting its protective role in the body.
Keywords: 2-arachidonoylglycerol, carrageenan, cannabinoid receptor, endocannabinoid system, inflammation, monoacylglycerol lipase, mouse tetrad, pain, URB602
Introduction
2-Arachidonoylglycerol (2-AG), a monoglyceride of arachidonic acid esterified at the sn-2 position, which was isolated from rat brain (Sugiura et al., 1995) and from canine gut (Mechoulam et al., 1995), is currently recognized as the most abundant and efficacious endocannabinoid. 2-AG is present in rat brain in amounts 170–1000 greater than the other endogenous cannabinoid, anandamide (AEA) (Sugiura et al., 1995; Stella et al., 1997). 2-AG acts as a fully efficacious agonist at both cannabinoid receptor type 1 (CB1) and type 2 (CB2) (Stella et al., 1997; Sugiura et al., 1999, 2000; Gonsiorek et al., 2000; Savinainen et al., 2001). In contrast, AEA, is less potent and acts only as a partial agonist at the cannabinoid receptors (Sugiura et al., 1995, 2000; Savinainen et al., 2001). 2-AG, as anandamide, produced ‘on demand', acts near the site of its synthesis and is promptly metabolized to ensure rapid signal inactivation (Devane et al., 1992; Mechoulam et al., 1995; Sugiura et al., 1995). Although 2-AG might be metabolized by fatty acid amide hydrolase (FAAH) (Di Marzo et al., 1998; Bisogno et al., 2005), a distinct enzyme, monoacylglycerol lipase (MAGL) plays the predominant role in catalysing 2-AG hydrolysis (Dinh et al., 2004). This enzyme, which has been cloned and characterized in rat (Dinh et al., 2002) and human brain (Ho et al., 2002), hydrolyses preferentially 2-acylglycerols such as 2-AG and 2-oleolylglycerol (2-OG), but is unable to metabolize AEA and palmitoylethanolamide (PEA) (Dinh et al., 2002). The role of MAGL as a 2-AG hydrolysing enzyme in the cannabinoid system has only recently started to be elucidated and few MAGL inhibitors have been developed. Among these, URB602 ([1,1′-biphenyl]-3-yl-carbamic acid, cyclohexyl ester) can specifically and noncompetitively inhibit MAGL activity (Makara et al., 2005). In fact, URB602 increases 2-AG levels without altering levels of AEA both in vitro and in vivo (Hohmann et al., 2005), confirming the selectivity of this agent in inhibiting MAGL relative to FAAH activity. Moreover, URB602 did not affect the activities of lipid-metabolizing enzymes such as diacylglycerol lipase and cyclooxygenase-2 and did not significantly influence binding of [3H]WIN55212-2 to CB1 or CB2 receptors (IC50⩾5 μM) or [35S]GTP-γS to rat cerebellar membranes (EC50> 50 μM) (Hohmann et al., 2005). On these bases, URB602 could offer the opportunity to investigate the functions of 2-AG by blocking its deactivation and thus amplifying its intrinsic actions. To date, this approach led to the demonstration that URB602 magnifies endocannabinoid-dependent stress-induced analgesia (Hohmann et al., 2005; Suplita et al., 2006) and induces anti-nociception in formalin-evoked pain behaviour (Guindon et al., 2007). Notably, the enhanced stress-induced analgesia was CB1-mediated (Hohmann et al., 2005; Suplita et al., 2006), whereas the anti-nociceptive effect in formalin test knew CB1 and CB2 mediation (Guindon et al., 2007).
There are controversial findings concerning the 2-AG role in inflammatory processes. In fact, Oka et al. (2005) found that 2-AG levels in mouse ear were markedly augmented following the application of 12-O-tetradecanoylphorbol-13-acetate. Moreover, topical application of 2-AG to mouse ear evoked swelling, which was abolished by treatment with the selective CB2 receptor antagonist N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide (SR144528). In contrast, several investigators reported that 2-AG and the cannabinoid CB2 receptors are involved in attenuation of inflammatory reactions and immune responses. Particularly, 2-AG has been shown to inhibit cytokine production, including tumour-necrosis factor-α released from both lipopolysaccharide-treated rat microglial cells (Facchinetti et al., 2003) and murine macrophages (Gallily et al., 2000) and interleukin-2 secretion in activated murine splenocytes (Ouyang et al., 1998). In contrast, more clear is the evidence concerning the analgesic property of 2-AG. Recently, Hohmann et al. (2005) demonstrated that the release of 2-AG in periacqueductal grey matter might mediate the stress-induced analgesia in rats and that URB602 local administration enhanced such anti-nociception, identifying MAGL as a potential therapeutic target for pain control.
Therefore, to clarify better the role of 2-AG in the inflammation, and to evaluate the potential pharmacological properties of MAGL inhibitors, we tested the preventive and therapeutic effect of URB602 in a murine model of inflammation/inflammatory pain such as that evoked by carrageenan. In addition, cannabinoid receptor antagonists were used to elucidate which receptor subtype is involved in the anti-inflammatory effect of 2-AG.
Methods
Animals
All experiments were performed in accordance with Italian State and European regulations governing the care and treatment of laboratory animals (permission no. 101/2004B) and conformed to the guidelines for the study of pain in awake animals established by the International Association for the Study of Pain (Zimmermann, 1983). Male C57BL/6J mice (9 weeks old, Harlan, Italy) were housed under controlled temperature (22±1°C), humidity (60±10%) and light (12 h/day) and allowed to acclimatize for at least 1 week before being used in the experiments. Standard food and water was available ad libitum. All the experiments were conducted in a randomized manner by the same experimenter blind to the pharmacological treatments.
Drugs
URB602 was purchased from the Cayman Chemical Co. (Ann Arbor, MI, USA); rimonabant and SR144528 were kindly supplied by Sanofi-Aventis (Montpellier, France).
Evaluation of psychoactive effects
A series of four consecutive observations were conducted on each mouse, according to the standard procedure employed to evaluate psychoactive cannabinoid-induced effects (Compton et al., 1993). Mice were consecutively tested for body temperature, nociceptive threshold, spontaneous locomotor activity and immobility on a ring, 30, 60 and 90 min after i.p. doses (10 and 20 mg kg−1) of URB602. Body temperature was measured with a rectal thermistor probe (Ellab Instruments, Roedrove, Denmark) inserted to a constant depth of 2 cm. To determine thermal nociception, mice were tested for responsiveness to radiant heat with a plantar test (Ugo Basile, Varese, Italy). Spontaneous locomotor activity was counted for 5 min by an activity cage (Ugo Basile, Varese, Italy), which recorded the number of horizontal animal movements through infrared sensors. Immobility time was measured by the ‘ring test' described by Pertwee, (1972). The apparatus consisted of a wire ring, 6 cm in diameter, fixed horizontally to a ring stand and 22 cm high. The experimenters placed the mouse across the ring, so that it was supported only by its front and rear paws. Its forepaws were placed on diametrically opposite points on the ring. The number of seconds the mouse remained motionless on the ring (except for breathing movements) was recorded. The test was conducted for 5 min and catalepsy was measured as immobility index, calculated as % of immobility=(time immobile in seconds/length of session in seconds)*100.
To verify the involvement of CB1 receptor in the URB602-induced psychoactive effects, mice were pre-treated (15 min) with the selective antagonist rimonabant (0.5 mg kg−1 i.p.) and submitted to behavioural test session, as described previously.
Induction of inflammation
Animals were anaesthetized with sodium pentobarbital (60 mg kg−1 i.p.). Acute inflammation was induced by intraplantar (i.pl.) injection of 20 μl of λ-carrageenan (2% w v−1 in saline), purchased from Sigma-Aldrich (Milano, Italy), into the right hind paw. Control animals received a corresponding i.p. injection of vehicle.
Drug treatment and experimental design
URB602 at non-psychoactive doses (10, 5 and 1 mg kg−1) or an appropriate volume of its vehicle was administered i.p. 30 min before (preventive regimen) or 30 min after (therapeutic regimen) carrageenan injection. URB602 was first dissolved in 10% dimethylsulphoxide (DMSO), then a drop of Tween 80 was added for every 2–3 mg of compound to maintain its solubility when it is diluted with 90% saline. In the antagonism studies, rimonabant 0.5 mg kg−1 i.p. and SR144528 1 mg kg−1 i.p., selective antagonists for CB1 and CB2 receptors respectively, were given 15 min before 10 mg kg−1 URB602 administration. Rimonabant and SR144528 were dissolved in a mixture of Tween 80:DMSO:distilled water (1:2:7). The doses of SR144528 and rimonabant used here were based on previous works in rodents (Rinaldi-Carmona et al., 1998; Carta et al., 1999).
Evaluation of paw oedema
The volume of the injected paw as well as the contralateral paw was measured with a plethysmometer (Ugo Basile, Varese, Italy), 2 and 24 h after the carrageenan injection. Data are expressed as oedema (difference in volume between the right and left paws).
Evaluation of thermal hypersensitivity
Responses to thermal stimuli were measured on the same animals used to determine oedema, 5 and 24 h after carrageenan i.pl. injection. Since at the 2 h time point, some animals had not still recovered from anaesthesia, the test was performed when they had completely recovered, about 5 h after carrageenan. Heat hypersensitivity was tested according to the Hargreaves' procedure (Hargreaves et al., 1988) using the plantar test (Ugo Basile, Varese, Italy). Briefly, animals were placed in a clear plexiglass box and allowed to acclimatize. A constant intensity, radiant heat source was aimed at the midplantar area of the hind paw. The time, in seconds, from initial heat source activation until paw withdrawal was recorded.
Statistical analysis
All data were expressed as the mean±s.e.m. and analysed using analysis of variance followed by Tukey's post-hoc test for multiple comparison. Differences were considered significant at P<0.05. Linear regression analyses were done using GraphPAD Software (San Diego, CA, USA).
Results
Psychoactive effects of URB602
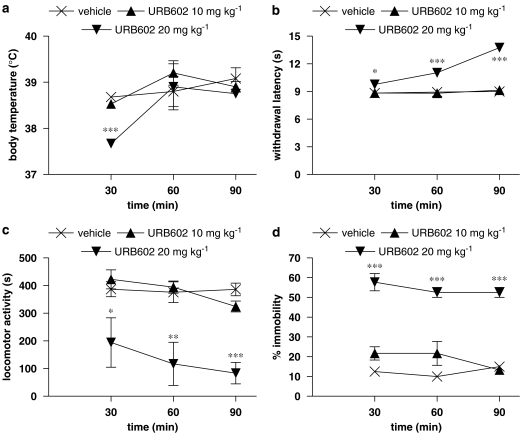
To select a dose of URB602 devoid of psychoactive effects that could be used to study its anti-inflammatory and anti-nociceptive activity, mice were tested in the tetrad of assays used to evaluate cannabinoid psychoactive effects (Compton et al., 1993). At 30, 60 and 90 min after a single i.p. administration of URB602 (10 and 20 mg kg−1), each mouse's body temperature, nociceptive threshold, locomotor activity and ring immobility were consecutively measured. The behavioural evaluations are given in Figure 1. Mice exerted the typical signs of cannabimimetic effects only at the dose of 20 mg kg−1, even if each effect showed a different time course. Particularly, body temperature decreased very early (at 30 min) of about 1°C (Figure 1a) and returned at basal levels already at 60 min. Nociceptive threshold (Figure 1b) increased in a time-dependent manner, reaching a maximum at 90 min time point, demonstrating a marked analgesia. Both hypomotility and catalepsy were always present at all time points; the first one was significantly reduced of about 80% (Figure 1c) and the second one showed an increase of 600% in immobility time (Figure 1d). On the contrary, the mouse tetrad showed that the dose of 10 mg kg−1 possessed no psychoactive effects, leading us to test its anti-inflammatory and anti-nociceptive properties in the mouse model of carrageenan. To ascertain whether the psychoactive effects induced by 20 mg kg−1 URB602 were mediated by cannabinoid receptor CB1, an antagonism study has been performed employing rimonabant (0.5 mg kg−1 i.p.). Based on the time course data obtained, the effect of rimonabant was evaluated at the peak time of every URB602-induced psychoactive effects (30 min for body temperature and 90 min for the others). Results, shown in Table 1, revealed that rimonabant blocked the hypothermic, cataleptic and analgesic effects of URB602 whereas it only attenuated hypoactivity. The employed dose of rimonabant did not modify the behavioural responses on its own (data not shown).
Figure 1.
Effect of URB602 (20 and 10 mg kg−1 i.p.) 30, 60 and 90 min after the administration on (a) body temperature, (b) nociceptive threshold, (c) spontaneous locomotor activity, (d) immobility on a ring. Data represent mean±s.e.m. of 5–6 mice. ***P<0.001, **P<0.01, *P<0.05 versus vehicle. URB602, [1,1′-biphenyl]-3-yl-carbamic acid, cyclohexyl ester.
Table 1.
Effect of rimonabant administration (0.5 mg kg−1) on URB602 (20 mg kg−1)-induced psychoactive effects
| Vehicle | URB602 | Rimonabant/URB602 | |
|---|---|---|---|
| Body temperature (°C) | 38.7±0.12 | 37.66±0.05* | 38.9±0.06° |
| Nociceptive threshold (s) | 8.83±0.14 | 13.73±0.15* | 8.30±0.10° |
| Spontaneous locomotor activity (s) | 361.2±15.85 | 83.67±22.2* | 130.0±18.00* |
| Immobility on a ring (%) | 15.0±2.04 | 53.33±1.67* | 12.0±4.00° |
Values shown in the table are the mean±s.e.m. *P<0.001 versus vehicle; °P<0.001 versus URB602.
Abbreviation: URB602, [1,1′-biphenyl]-3-yl-carbamic acid, cyclohexyl ester.
Anti-inflammatory and anti-nociceptive preventive dose-related effect of URB602
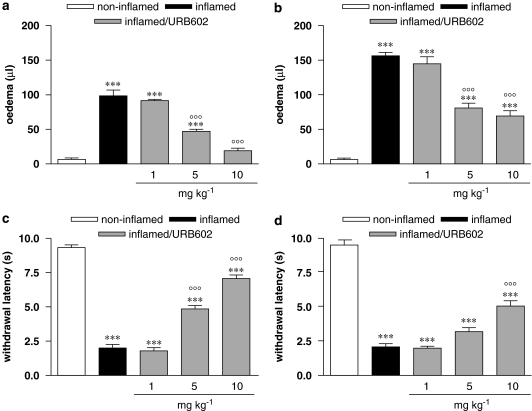
I.pl. injection of carrageenan resulted in an increase in ipsilateral hindpaw volume, expressed as oedema, that is the difference between the ipsilateral injected paw and the contralateral non-injected paw. Paw oedema was present both at 2 and 24 h after carrageenan (Figures 2a and b), even if at 24 h it was further increased. At 2 h, oedema was abolished by the highest dose (10 mg kg−1) of URB602 administered 30 min before the inflammatory stimulus, it was diminished by the intermediate dose of 5 mg kg−1, while it was not counteracted by the lowest dose (1 mg kg−1). Figure 2a shows this dose-correlated effect (r2=0.9592, P<0.0001, F=164.6). At 24 h after carrageenan injection, the animals treated with the highest doses (10 and 5 mg kg−1) were significantly different from both non-inflamed and inflamed mice, suggesting that this effect was still present (Figure 2b). Carrageenan injection also caused a marked thermal hypersensitivity, as shown in Figures 2b and c, that remained unaltered at 24 h. All doses of URB602, except the lowest one (1 mg kg−1), diminished pain hypersensitivity to thermal stimulus, in a dose-correlated manner (r2=0.9484, P<0.0001, F=128.8), as shown in Figure 2c. At the 24 h time point, only the dose of 10 mg kg−1 elicited a partial relief of pain hypersensitivity, even if this effect was diminished versus the 5 h time point. Contralateral hindpaw volume did not change significantly (data not shown) and no differences in paw-withdrawal latencies were found in the contralateral hindpaw (data not shown) at any time point.
Figure 2.
Dose-related effect of URB602 (10, 5 and 1 mg kg−1 i.p.), administered in a preventive regimen, on oedema, evaluated (a) 2 h, and (b) 24 h after carrageenan, and on thermal hypersensitivity, evaluated (c) 5 h and (d) 24 h after carrageenan. Data represent mean±s.e.m. of 8–10 mice. ***P<0.001 versus non-inflamed; °°°P<0.001 versus inflamed. URB602, [1,1′-biphenyl]-3-yl-carbamic acid, cyclohexyl ester.
Effect of CB1 and CB2 receptor antagonists on URB602-induced anti-oedemigen and anti-nociceptive effect
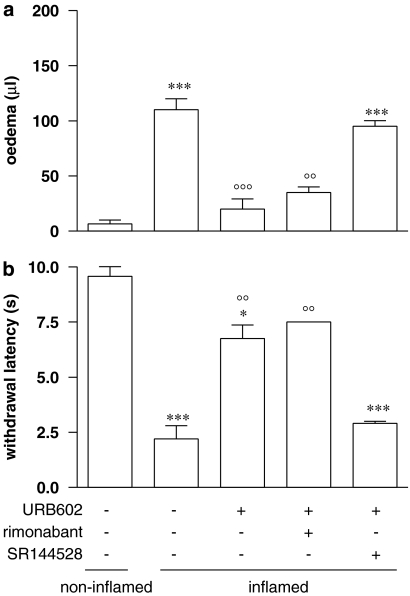
The ability of specific CB1 and CB2 antagonists to reverse the effect elicited by URB602 treatment was tested both on oedema and thermal hypersensitivity. Antagonists were administered 15 min before URB602 10 mg kg−1 and oedema evaluations were performed 2 h after drug administration while thermal hypersensitivity was measured 5 h later as described previously. Results in Figure 3 show that only SR144528 reversed both URB602-induced anti-oedemigen (Figure 3a) and anti-nociceptive (Figure 3b). Rimonabant and SR144528, given alone, did not affect the oedema paw and the nociceptive response of animals (data not shown).
Figure 3.
Effect of rimonabant (0.5 mg kg−1 i.p.) and SR144528 (1 mg kg−1 i.p.) on (a) anti-oedemigen and (b) anti-nociceptive effect evoked by URB602 (10 mg kg−1 i.p.). Antagonists were administered 15 min before the drug and behavioural evaluations were assessed 2 h (oedema) and 5 h (thermal hypersensitivity) after carrageenan. Data represent mean±s.e.m. of 8–10 mice. ***P<0.001, *P<0.05 versus non-inflamed, °°°P<0.001, °°P<0.01 versus inflamed. URB602, [1,1′-biphenyl]-3-yl-carbamic acid, cyclohexyl ester.
Anti-inflammatory and anti-nociceptive therapeutic effect of URB602
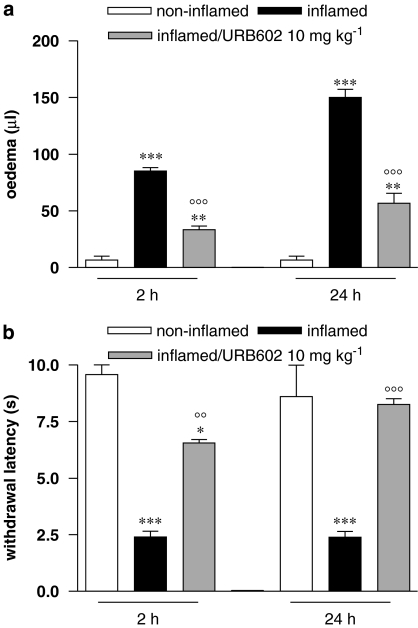
To test the ability of URB602 to counteract an established inflammatory process, the maximal dose of the drug (10 mg kg−1) was administered in a therapeutic regimen, that is 30 min after carrageenan injection. Figure 4a shows that URB602 partially counteracted oedema (about 60%) evoked by carrageenan injection, 2 h after carrageenan injection. The effect remained unaltered after 24 h. URB602 also showed an anti-nociceptive activity, increasing inflamed paw thermal withdrawal latency of about 170% (Figure 4b), assessed 5 h after carrageenan; 24 h later, thermal hypersensitivity was completely abolished.
Figure 4.
Effect of URB602 (10 mg kg−1 i.p.), administered in a therapeutic regimen, on (a) oedema and (b) thermal hypersensitivity, evaluated 2 h (oedema) or 5 h (thermal hypersensitivity) and 24 h after carrageenan. Data represent mean±s.e.m. of 8–10 mice. ***P<0.001, **P<0.01, *P<0.05 versus non-inflamed; °°°P<0.001, °°P<0.01 versus inflamed. URB602, [1,1′-biphenyl]-3-yl-carbamic acid, cyclohexyl ester.
Discussion and conclusions
There is evidence supporting the role of the endocannabinoid system in a wide variety of physiological and pathological processes. In fact, endocannabinoid levels appear to be transiently elevated as an adaptive reaction to re-establish normal homeostasis when this is perturbed. In some chronic states, however, changes in endocannabinoid levels also contribute to the progress or symptoms of diseases. In this latter case, the development of drugs blocking/stimulating the endocannabinoid action could be very useful to restore the physiological endocannabinoid levels thus blocking the progression of the disease. Inhibitors of endocannabinoid hydrolases could offer a rational therapeutic approach in treating certain inflammatory diseases where higher endocannabinoid activity would be beneficial. The development of FAAH inhibitors, together with the generation of mice lacking the FAAH gene, has deepened considerably the understanding of the pathophysiological role of AEA in the central nervous system and in the periphery. By contrast, the functional role of 2-AG is only beginning to be explored. Inhibition of endocannabinoid metabolism is considered a promising therapeutic target on its own with respect to the use of exogenously administered cannabinoid ligands, since it presumably increases the endocannabinoid level mainly at the location where their synthesis has been stimulated, thus minimizing the adverse side effects produced by the direct activation of cannabinoid receptors in the brain. Consistent with the efficacy of this strategy, we have shown previously that the systemic administration of FAAH inhibitor URB597 reduced the oedema response to carrageenan in the mouse via CB2 receptors (Holt et al., 2005). A very recent report by Guindon et al. (2007) demonstrated that exogenous 2-AG induced peripheral anti-nociceptive effects in the formalin test and that the MAGL inhibitor URB602, administered locally to the paw, enhanced the anti-nociceptive effect of the exogenously applied 2-AG. On these bases, the work reported here was designed to investigate the efficacy of the MAGL inhibitor URB602 in a model of inflammation/inflammatory pain such as that evoked by the i.pl. injection of carrageenan in mice, after systemic administration and, importantly, under both prophylactic and therapeutic regimen.
Since 2-AG acts as potent endogenous ligand for both cannabinoid receptor subtypes, the systemic administration of URB602 at too high doses might enhance the 2-AG level in brain areas, thereby producing adverse psychoactive effects that could limit the therapeutic employment of such a compound. To select a dose of URB602 devoid of psychoactivity which could be used to study its anti-inflammatory and anti-nociceptive efficacy, we first tested mice in the tetrad of assays employed to evaluate cannabinoid central effects (Compton et al., 1993). At 30, 60 and 90 min after i.p. doses of URB602 (10 and 20 mg kg−1) each mouse's body temperature, locomotor activity, nociceptive threshold and ring immobility were consecutively measured. The data clearly indicated that only mice given 10 mg kg−1 URB602 did not show any cannabimimetic activity. Consequently, this dose has been selected as the highest to be administered to mice in the following experimental sessions. However, the finding that 20 mg kg−1 URB602 evoked a marked cannabimimetic activity adds an important insight about the efficacy of this MAGL inhibitor, demonstrating that it is able to enhance the endocannabinoid tone also in vivo. In addition this result suggests that, although URB602 showed a relative low potency in inhibiting MAGL activity in vitro (Hohmann et al., 2005), its systemic administration at high dose led to a significant blockade of 2-AG hydrolysis in vivo. Besides the high dose employed and the route of administration (i.p.) causing reasonable bioavailability, the species and strain employed in our experiments could account for the systemic effect observed. To ascertain whether CB1 receptor mediated the psychoactive effects following URB602 administration, the selective CB1 receptor antagonist rimonabant was pre-administered to mice. Rimonabant (0.5 mg kg−1 i.p.) completely blocked the hypothermic, cataleptic and analgesic effects of URB602 whereas it only attenuated hypoactivity, highlighting the contribution of cannabinoid CB1 receptor in the cannabimimetic activity of URB602. The limited antagonism of URB602-induced hypoactivity by rimonabant has been reported previously (Jarbe et al., 2002; McMahon et al., 2005; McMahon and Koek, 2007) suggesting that locomotor activity seems to be differently sensitive to cannabinoid CB1 receptor activity. The data strongly suggest that 2-AG, whose level increased following URB602 administration, bound to CB1 receptors inducing cannabimimetic effects; however, in the light of the high dose employed (20 mg kg−1) and of the affinity of URB602 for CB1 and CB2 receptors (IC50⩾5 μM) (Hohmann et al., 2005), we cannot exclude a direct action of URB602 upon CB1 receptors.
We have shown here for the first time that the systemic administration of MAGL inhibitor URB602 prevented the development of carrageenan-induced oedema. A clear dose–response relationship was seen in the present study for the anti-oedema effect of URB602, where a dose of 10 mg kg−1 decreased the oedema development to a level undistinguishable from the non-inflamed animals. The anti-oedema effect was still present at 24 h, suggesting a lasting efficacy of this inhibitor. Of course, the finding that this MAGL inhibitor produces effects in the carrageenan model does not conclusively prove that the key of the efficacy is its inhibition upon MAGL. However, the demonstration reported by Hohmann et al. (2005) that in vivo microinjection of URB602 into the periacqueductal grey of rats increased 2-AG concentration without affecting AEA levels, strongly suggests that the URB602 anti-inflammatory property could be ascribed to a selective enhancement of 2-AG. The ability of URB602 to prevent the inflammatory process also highlights the significance of 2-AG as an endogenous protective substance against inflammation, pointing to the possibility that 2-AG shares with AEA and PEA the capability to modulate inflammation. With respect to 2-AG physiological role in inflammation, controversial findings have been reported so far. Some studies suggested that CB2 receptors and 2-AG are involved in the stimulation of different types of inflammatory and immune responses (Smith et al., 2001; Oka et al., 2005, 2006), whereas other investigators reported that 2-AG is involved in the attenuation of both inflammatory and immune reactions (Ouyang et al., 1998; Gallily et al., 2000; Facchinetti et al., 2003), even if these findings have been obtained in vitro. The data presented herein strongly support the in vitro results, since the URB602-induced inhibition of 2-AG degradation in vivo offers the opportunity to investigate the functions of 2-AG by amplifying its intrinsic actions that show anti-inflammatory effects. We demonstrated here that URB602 has dose-dependent action in reducing not only oedema but also thermal hypersensitivity associated with inflammation, extending the recently described ability of this compound to counteract formalin-induced pain behaviour (Guindon et al., 2007) to acute inflammatory pain. Similar to the anti-inflammatory effect, the anti-nociceptive property of URB602 persisted also at 24 h after the administration. In addition, the same treatment did not affect the nociceptive thresholds of the paw contralateral to the carrageenan injection, indicating that the doses employed are not directly analgesic and confirming the results obtained by us in the tetrad assay with URB602 10 mg kg−1. This finding prompted us to suggest that the enhancement of 2-AG in tissue (that is, spinal cord, skin) in which there is an ongoing production of the endocannabinoid, accounts for the effect observed validating the hypothesis of the beneficial use of the so-called ‘indirect agonists'. To ascertain the relative contribution of CB1 and CB2 receptors in the anti-oedema and anti-nociceptive effect of URB602, we employed selective antagonists for these receptors. Both the URB602-induced effects in inflamed mice can be prevented by SR144528 but not by rimonabant (at the same dose able to reverse the effect of URB602 on the tetrad assay) clearly indicating that exclusively CB2 receptor mediated the anti-inflammatory and anti-nociceptive properties of URB602. With respect to inflammation, there is good evidence in the literature that the activation of CB2 receptors expressed by mast cells and macrophages is involved in downregulation of the inflammatory response. In the light of the potent agonist activity of 2-AG at the CB2 receptor, its involvement in URB602-induced anti-inflammation was strongly predictable. Conversely, the fact that also the relief of thermal hypersensitivity evoked by the MAGL inhibitor was mediated solely by CB2 receptors was unexpected but welcomed. We can speculate that the anti-nociceptive effect of URB602 might be mediated by a direct action of 2-AG on CB2 receptors located in activated microglia within the spinal cord or in the mouse paw tissue. The activation of CB2 receptors could contribute to the relief of pain by decreasing the release of sensitizing substances from mast and immune cells. In addition, the lack of CB1 involvement in the URB602-induced anti-nociception confirms that URB602 can be systemically administered without producing central effects. In addition, it has been recently identified a novel MAGL, sensitive to URB602 inhibition, that is mainly expressed in microglia cells (Muccioli et al., 2007). Interestingly, this suggests that the cloned MAGL which is thought to be responsible for the majority of the 2-AG hydrolysis in the brain, does not play a major role in microglia. Consequently, we cannot exclude that the anti-nociceptive property of URB602 in unhealthy animals could be ascribed to a selective inhibition of this novel MAGL.
Finally, our findings demonstrated that the anti-inflammatory and anti-nociceptive efficacy of URB602 persists also when the compound was administered in a therapeutic regimen, suggesting that the ability of URB602 to improve the established disease may have potential therapeutic application.
In conclusion, the present report highlighted the ability of the selective MAGL inhibitor, URB602, to prevent and treat an acute inflammatory disease without producing adverse psychoactive effects. The data presented herein also contributed to clarify the physiological role of 2-AG with respect to inflammatory reactions, suggesting its protective role in the body. More work is necessary to demonstrate that MAGL may be considered a novel molecular target to be exploited for the treatment of inflammation and pain and the development of new more potent and selective inhibitors as well as the generation of mice lacking the MAGL gene could help us to understand better the potential of this pharmacological tool.
Acknowledgments
We are grateful to Sanofi-Aventis for kindly providing rimonabant and SR144528.
Abbreviations
- AEA
arachidonoylethanolamide (anandamide)
- 2-AG
2-arachidonoylglycerol
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- DMSO
dimethylsulphoxide
- FAAH
fatty acid amide hydrolase
- MAGL
monoacylglycerol lipase
- PEA
palmitoylethanolamide
- rimonabant (SR141716)
N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide
- SR144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
- URB602
[1,1′-biphenyl]-3-yl-carbamic acid, cyclohexyl ester
Conflict of interest
The authors state no conflict of interest.
References
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signalling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Carta G, Gessa GL, Nava F. Dopamine D2 receptor antagonists prevent Δ9-tetrahydrocannabinol-induced antinociception in rats. Eur J Pharmacol. 1999;384:153–156. doi: 10.1016/s0014-2999(99)00696-2. [DOI] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, et al. Cannabinoid structure–activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Sugiura T, Melck D, De Petrocellis L. The novel endogenous cannabinoid 2-arachidonoylglycerol is inactivated by neuronal and basophil-like cells: connections with anandamide. Biochem J. 1998;331:15–19. doi: 10.1042/bj3310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–1264. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Del Giudice E, Furegato S, Passarotto M, Leon A. Cannabinoids ablate release of TNFα in rat microglial cells stimulated with lipopolysaccharide. Glia. 2003;41:161–168. doi: 10.1002/glia.10177. [DOI] [PubMed] [Google Scholar]
- Gallily R, Breuer A, Mechoulam R. 2-Arachidonoylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-α production in murine macrophages, and in mice. Eur J Pharmacol. 2000;406:R5–R7. doi: 10.1016/s0014-2999(00)00653-1. [DOI] [PubMed] [Google Scholar]
- Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. 2000;57:1045–1050. [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injection of 2-Arachidonoylglycerol are mediated by cannabinoid CB2 receptors. Br J Pharmacol. 2007;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves KM, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Ho SY, Delgado L, Storch J. Monoacylglycerol metabolism in human intestinal Caco-2 cells: evidence for metabolic compartmentation and hydrolysis. J Biol Chem. 2002;277:1816–1823. doi: 10.1074/jbc.M108027200. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Manieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Holt S, Comelli F, Costa B, Fowler C. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br J Pharmacol. 2005;146:467–476. doi: 10.1038/sj.bjp.0706348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TUC, Andrzejewski ME, DiPatrizio NV. Interactions between the CB1 receptor agonist Δ9-THC and the CB1 receptor antagonist SR-141716 in rats: open-field revisited. Pharmacol Biochem Behav. 2002;73:911–919. doi: 10.1016/s0091-3057(02)00938-3. [DOI] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, Szabo SI, Kathuria S, Astarita G, et al. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Koek W. Differences in the relative potency of SR 141716A and AM 251 as antagonists of various in vivo effects of cannabinoid agonists in C57BL/6J mice. Eur J Pharmacol. 2007. pp. 1–7. [DOI] [PMC free article] [PubMed]
- McMahon LR, Amin MR, France CP. SR 141716A differentially attenuates the behavioral effects of Δ9-THC in rhesus monkeys. Behav Pharmacol. 2005;16:363–372. doi: 10.1097/00008877-200509000-00008. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Muccioli GG, Xu C, Odah E, Cudaback E, Cisneros JA, Lambert DM, et al. Identification of a novel endocannabinoid-hydrolyzing enzyme expressed by microglial cells. J Neurosci. 2007;27:2883–2889. doi: 10.1523/JNEUROSCI.4830-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka S, Wakui J, Gokoh M, Kishimoto S, Sugiura T. Suppression by WIN55212-2, a cannabinoid receptor agonist, of inflammatory reactions in mouse ear: Interference with the actions of an endogenous ligand, 2-arachidonoylglycerol. Eur J Pharmacol. 2006;538:154–162. doi: 10.1016/j.ejphar.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Oka S, Yanagimoto S, Ikeda S, Gokoh M, Kishimoto S, Waku K, et al. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J Biol Chem. 2005;280:18488–18497. doi: 10.1074/jbc.M413260200. [DOI] [PubMed] [Google Scholar]
- Ouyang Y, Hwang SG, Han SH, Kaminski NE. Suppression of interleukin-2 by the putative endogenous cannabinoid 2 arachidonoyl-glycerol is mediated through down-regulation of the nuclear factor of activated T cells. Mol Pharmacol. 1998;53:676–683. doi: 10.1124/mol.53.4.676. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The ring test: a quantitative method for assessing the ‘cataleptic' effect of cannabis in mice. Br J Pharmacol. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Savinainen JR, Jarvinen T, Laine K, Laitinen JT. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB(1) receptor-dependent G-protein activation in rat cerebellar membranes. Br J Pharmacol. 2001;134:664–672. doi: 10.1038/sj.bjp.0704297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Denhardt G, Terminelli C. The anti-inflammatory activities of cannabinoid receptor ligands in mouse peritonitis models. Eur J Pharmacol. 2001;432:107–119. doi: 10.1016/s0014-2999(01)01477-7. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, et al. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure–activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. 1999;274:2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Kishimoto S, Miyashita T, Nakane S, Kodaka T, et al. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J Biol Chem. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Suplita RL, Gutierrez T, Fegley D, Piomelli D, Hohmann AG. Endocannabinoids at the spinal level regulate, but do not mediate, nonopioid stress-induced analgesia. Neuropharmacology. 2006;50:372–379. doi: 10.1016/j.neuropharm.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]