Abstract
Background and purpose:
The TASK subfamily of two pore domain potassium channels (K2P) encodes for leak K currents, contributing to the resting membrane potential of many neurons and regulating their excitability. TASK1 and TASK3 channels are regulated by a number of pharmacological and physiological mediators including cannabinoids such as methanandamide. In this study, we investigate how methanandamide blocks these channels.
Experimental approach:
Currents through wild type and mutated TASK1 and TASK3 channels expressed in modified HEK-293 cells were measured using whole-cell electrophysiological recordings in the presence and absence of methanandamide.
Key results:
Methanandamide (3 μM) produced substantial block of hTASK1, hTASK3 and mTASK3 channels but was most potent at blocking hTASK3 channels. Block of these channels was irreversible unless cells were washed with buffer containing bovine serum albumin. Mutation of the distal six amino acids of TASK1 did not alter methanandamide inhibition, whilst C terminal truncation of TASK3 channels caused a small but significant reduction of inhibition. However, deletion of six amino acids (VLRFLT) at the interface between the final transmembrane domain and cytoplasmic C terminus of TASK3 channels gave functional currents that were no longer inhibited by methanandamide or by activation of GPCRs.
Conclusions and implications:
Methanandamide potently blocked TASK3 and TASK1 channels and both methanandamide and G protein-mediated inhibition converged on the same intracellular gating pathway. Physiologically, methanandamide block of TASK1 and TASK3 channels may underpin a number of CNS effects of cannabinoids that are not mediated through activation of CB1 or CB2 receptors.
Keywords: two-pore domain potassium channels, TASK1, TASK3, methanandamide, muscarinic receptor, PKA, PKC
Introduction
Background, or leak, potassium currents play an important role in the regulation of the resting membrane potential and excitability of mammalian neurons. The two-pore domain potassium (K2P) channel family are open across the physiological voltage range and are therefore believed to account for many of these leak currents (Goldstein et al., 2001; O'Connell et al., 2002; Lesage, 2003). There are currently 15 members of this family, which can be divided into six subfamilies on the basis of structural and functional properties (Goldstein et al., 2005; Kim, 2005; Alexander et al., 2006). Among these subfamilies is the TWIK-related acid-sensitive K (TASK) subfamily (TASK1 (K2P3.1), TASK3 (K2P9.1) and TASK5 (K2P15.1)). TASK1 and TASK3 K2P channels are regulated by a wide variety of chemical stimuli including activation of G protein-coupled receptor (GPCR) pathways (Mathie, 2007). TASK channels are responsible for leak K currents in many neurons including cerebellar granule neurons (see Millar et al., 2000; Talley et al., 2000; Mathie et al., 2003; Kang et al., 2004; Aller et al., 2005; Brickley et al., 2007).
The endocannabinoid, anandamide, produces a number of effects in the central nervous system (CNS) including hypothermia and analgesia (Di Marzo et al., 1998). While most actions of anandamide (and its non-hydrolysable analogue, methanandamide) in the CNS are mediated through CB1 receptors, some, such as effects on locomotion and ataxia (Chakrabarti et al., 1998; Chaperon and Thiebot, 1999) seem to occur independently of CB1 or CB2 receptors. Maingret et al. (2001) showed that anandamide could act directly to block TASK1 channels in recombinant systems and suggested that at least some of the receptor-independent effects of anandamide might be mediated in this way (see also Linden et al., 2006).
While K2P channels are not voltage sensitive in the classic sense and are seen as leak K currents, they nevertheless undergo gating. This can be observed both at a microscopic level where single-channel recordings reveal the opening and closing of individual channels (Kang et al., 2004) and also on a larger scale, where a variety of modulatory compounds are known to increase or decrease channel activity by changing the proportion of time the channels remain closed or open (Zilberberg et al., 2001). Relatively little is known, however, about the regions of mammalian K2P channels that underlie these gating processes. TASK K2P channels comprise four transmembrane domains, two ‘P' regions that contribute to the channel pore, a short intracellular N terminus and a large intracellular C terminus. Although the binding site for anandamide on TASK channels is not known, it is of interest that anandamide-mediated activation of TRPV1 channels occurs following direct binding of the compound to an identified region in the cytosolic face of the channel, accessible from the intracellular side of the membrane (Jordt and Julius, 2002). For TASK channels, both anaesthetic activation and transmitter inhibition of TASK1 and TASK3 require a region of six amino acids (VLRFLT in the case of TASK3) to be present at the interface between the final, fourth, transmembrane domain and the large cytoplasmic C terminus to be present, suggesting a shared molecular site of action (Talley and Bayliss, 2002).
In this study, we show that methanandamide can potently inhibit both TASK1 and TASK3 channels. We show that deletion of the VLRFLT region (TASK3VLRFLT−DEL) not only abolishes inhibition of TASK3 channels by GPCR activation but also inhibition by methanandamide. A preliminary account of some of these results has been published (Veale et al., 2007b).
Methods
tsA-201 cell culture preparation
Modified HEK-293 cells (tsA-201, an HEK-293 subclone stably transfected with the SV40 large T antigen) were maintained in 5% CO2 in a humidified incubator at 37 °C in growth media (89% Dulbecco's modified Eagle's medium, 10% heat-inactivated fetal bovine serum, 1% penicillin (10 000 U ml−1) and streptomycin (10 mg ml–1). When the cells were 80% confluent, they were split and plated for transfection onto glass coverslips coated with poly-D-lysine (1 mg ml−1) to ensure good cell adhesion. The cells were transiently transfected using the calcium phosphate method. cDNA expression vector (0.3–1 μg) encoding a mouse or human TASK3 subunit was added to each 15 mm well, and 0.3–1 μg of a plasmid encoding the cDNA of green fluorescent protein was included to identify cells expressing K2P channels. Following an 18–24 h incubation period at 3% CO2 the cells were rinsed with saline and fresh growth medium was added to the wells. The cells were incubated at 37 °C with 5% CO2 for 24–60 h before electrophysiological measurements were made.
Mutations and truncations
Deletions and truncation mutants of TASK1 and TASK3 channel clones were generated by site-directed mutagenesis using the Quikchange kit (Stratagene, Amsterdam, The Netherlands). A pair of short (25–35 bases) complementary oligonucleotide primers, incorporating the intended mutation or deletion, were synthesized (MWG-Biotech, Ebersberg, Germany). Mutant DNA constructs were sequenced (MWG-Biotech) to confirm the introduction of the correct mutated bases.
Electrophysiological recordings from tsA-201 cells
Whole-cell voltage-clamp recordings were made from tsA-201 cells transiently transfected with hTASK3, hTASK1 or mTASK3 wild-type (WT) or mutated channels. The composition of the control extracellular solution was 145 mM NaCl, 2.5 mM KCl, 3 mM MgCl2, 1 mM CaCl2, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, titrated to pH 7.4 with NaOH or to pH 8.4 for experiments with hTASK1 channels). Glass microelectrodes were pulled from thick-walled borosilicate glass capillaries. Fire-polished pipettes were back-filled with 0.2 μm filtered intracellular solution (composition: 150 mM KCl, 3 mM MgCl2, 5 mM ethylene glycol tetraacetic acid, 10 mM HEPES; titrated to pH 7.4 with KOH). Cells were voltage clamped using an Axopatch 1D amplifier (Axon instruments, Sunnyvale, CA, USA) and low-pass filtered at 5 kHz before sampling (2–10 kHz) and online capture. Data acquisition was carried out using pClamp software (clampex 7, Axon Instruments). tsA-201 cells were usually held at −60 mV then subjected to a step to −80 mV for 100 ms followed by a 500 ms step to −40 mV. A 100 ms step to −110 mV was followed by a 500 ms ramp change in voltage to +20 mV. Finally, a 100 ms step to −80 mV preceded a step to the holding potential of −60 mV. This protocol was repeated once every 5 s. This protocol allows simultaneous measurement of the steady state current at particular voltages and the currents evoked by ramp changes in the voltage. Representative current traces evoked by this protocol can be seen in Figures 1a and b. Current amplitudes quoted in the results are the difference current between that seen at −40 mV and that at −80 mV, voltages where negligible endogenous currents are observed. All electrophysiological measurements were carried out at room temperature (21–23 °C).
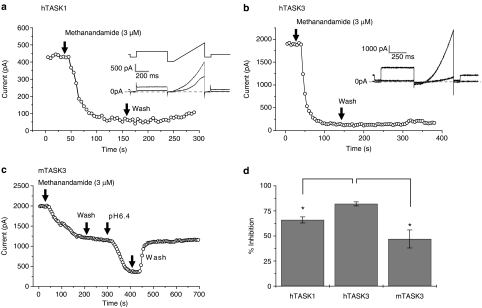
Figure 1.
Methanandamide is a potent blocker of both TASK1 and TASK3 channels. Time–course plots showing inhibition of (a) hTASK1 (b) hTASK3 and (c) mTASK3 channels by methanandamide (3 μM) (current measured as the difference current between that at −40 mV and that at −80 mV). Insets in (a) and (b) show representative current traces and voltage protocol (in a) before and after perfusion of methanandamide (see ‘Methods' for detailed description of voltage protocol). (d) Summary of inhibition of hTASK1 (n=6), hTASK3 (n=13) and mTASK3 (n=8) by methanandamide (3 μM). Note that hTASK3 is inhibited significantly more than the other two channels. *P<0.05; Student's t-test.
Data analysis
Data were analyzed using Clampfit software (Clampfit 8, Axon Instruments), Excel (Microsoft Corporation, USA) and Origin (Microcal, USA). Statistical comparisons were carried out using Student's t-test and P-values <0.05 were regarded as significant (*). Results are given as mean±standard error of the mean with n as the number of experiments.
Drugs, chemicals and cDNA
Muscarine, methanandamide and bovine serum albumin (BSA) were obtained from Sigma, UK. Compounds were made up in either dimethyl sulfoxide or water and diluted in external or internal solution prior to experimentation. The human TASK3 and TASK1 K2P channel clones in the pcDNA 3.1 vector were from Dr Helen Meadows (GlaxoSmithKline, UK). Mouse TASK3 clones were from Professor Bill Wisden (University of Heidelberg, Germany). M3 muscarinic acetylcholine receptors and constitutively active Gα constructs were from the Guthrie cDNA Resource Center, USA.
Results
Methanandamide is a potent blocker of both TASK1 and TASK3 channels
Application of a maximally effective concentration of methanandamide (3 μM) to whole-cell voltage-clamped tsA-201 cells expressing TASK channels of interest resulted in a marked blockade of human (h) TASK1 and TASK3 channels (Figures 1a, b and d). Perhaps surprisingly (see Maingret et al., 2001), hTASK1 was significantly less inhibited (P<0.05) by methanandamide than hTASK3.
Methanandamide (3 μM) was also found to block the murine (m) form of TASK3 (Figures 1c and d). mTASK3 was significantly less inhibited (P<0.05) by methanandamide than hTASK3. A comparison of the h and mTASK3 channels showed their C termini to have only 44% similarity (using Blosum 62 similarity matrix), compared to 98% similarity for the remainder of the channel (see Veale et al., 2007a) suggesting that any differences in the effectiveness of pharmacological agents such as methanandamide may be due to differences in the respective C termini.
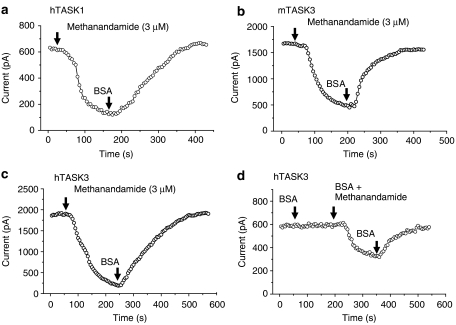
Block of TASK channels by methanandamide could only be reversed by BSA
Inhibition of all three channels by methanandamide could not be reversed by washing the preparation with normal, drug-free buffer (see Figure 1). Reversal of inhibition was only observed when the wash buffer contained lipid-free BSA (1 mg ml−1) (compare Figures 2a–c with Figures 1a–c). Pre-application of buffer with BSA, followed by application of methanandamide in BSA buffer, did not prevent block of TASK3 channels by methanandamide, but reduced its effectiveness (Figure 2d).
Figure 2.
Block of TASK channels by methanandamide could only be reversed by buffer containing bovine serum albumin (BSA). (a–c) Time–course plots showing inhibition of hTASK1, mTASK3 and hTASK3 channels by methanandamide (3 μM) and reversal of this block following perfusion of buffer with BSA (1 mg ml−1). (d) Reduced block of hTASK3 channels by methanandamide (3 μM) in the constant presence of BSA (1 mg ml−1). Note that BSA alone has no effect on hTASK3 current.
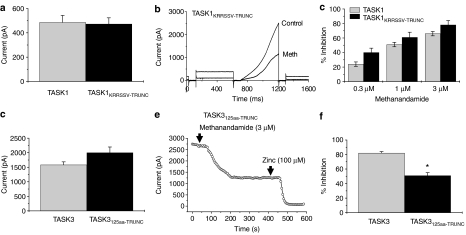
TASK1 inhibition is independent of its PKA phosphorylation sites
TASK1 channel inhibition by anandamide is a direct effect on the channel, independent of both CB1 and CB2 cannabinoid receptors (Maingret et al., 2001), but this effect of anandamide, while not directly mediated by phosphorylation, may be dependant on the phosphorylation state of the channel (Barbuti et al., 2002). Six amino acids (KRRSSV) at the end of the TASK1 C terminus encode for two putative protein kinase A (PKA) sites and a proposed binding site for two small adaptor proteins (p11 and 14-3-3), which regulate membrane expression levels (Girard et al., 2002; O'Kelly et al., 2002; Rajan et al., 2002). Removal of these amino acids (TASK1KRRSSV−TRUNC) had no significant effect on membrane expression of the channel as assessed by current amplitudes at pH 8.4 for TASK1 and TASK1KRRSSV−TRUNC (Figure 3a).
Figure 3.
TASK1 inhibition by methanandamide is independent of its PKA phosphorylation sites but C-terminal truncation of hTASK3 reduces the effectiveness of methanandamide. (a) Mean current through hTASK1 channels (measured as the difference current between that at −40 mV and that at −80 mV) for WT channels (n=14) and for channels with the six distal amino acids in the C terminus deleted (TASK1KRRSSV−TRUNC; n=12). (b) Representative TASK1KRRSSV−TRUNC current traces before (Control) and after perfusion of methanandamide (Meth (3 μM); see ‘Methods' for detailed description of voltage protocol). (c) Summary of inhibition of TASK1 and TASK1KRRSSV−TRUNC channels by methanandamide (0.3, 1 and 3 μM; n=3–5 at each concentration). (d) Mean currents through hTASK3 channels (measured as the difference current between that at −40 mV and that at −80 mV) for WT channels (n=37) and for channels with C terminus deleted (TASK3125aa−TRUNC; n=12). (e) Time–course plot showing inhibition of TASK3125aa−TRUNC channels by methanandamide (3 μM). Note that zinc (100 μM) inhibits the remaining TASK3125aa−TRUNC current. (f) Summary of inhibition of TASK3 (n=13) and TASK3125aa−TRUNC (n=4) channels by methanandamide (3 μM). *P<0.05; Student's t-test.
Methanandamide block of this mutated TASK1 channel was also unaffected by the lack of its two PKA phosphorylation sites, over a range of concentrations (0.3, 1 and 3 μM) (see Figures 3b and c).
C-terminal truncation of hTASK3 reduces the effectiveness of methanandamide
The C terminus of TASK3 contains all of its putative protein kinase C (PKC) and PKA phosphorylation sites. Truncation of the C terminus (the distal 125 amino acids) of TASK3 (TASK3125aa−TRUNC) had no significant effect on membrane expression of the protein, in terms of the current amplitudes for WT or TASK3125aa−TRUNC channels (Figure 3d, also Talley and Bayliss, 2002; Veale et al., 2007a). These currents were blocked as normal by zinc (see Figure 3e).
The inhibitory effect of methanandamide (3 μM) on the TASK3125aa−TRUNC channel however was significantly less than on the WT channel (P<0.05). In other words, TASK3125aa−TRUNC channels were still blocked by methanandamide, although block was significantly reduced (Figures 3e and f), despite the fact that all three putative PKC phosphorylation sites on the TASK3 channel had been removed by this truncation (see ‘Discussion', also Barbuti et al., 2002; Veale et al., 2007a).
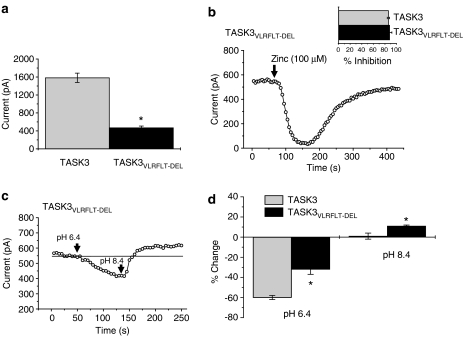
The VLRFLT region of the TASK3 channel is critical for inhibition mediated by muscarinic receptors, Gαq and methanandamide
A six-residue sequence at the interface of the final transmembrane domain and the C terminus of TASK1 and TASK3 has previously been found to be important for both halothane activation and thyrotropin-releasing hormone (TRH) inhibition of these channels (Talley and Bayliss, 2002). We constructed a TASK3 mutant (TASK3VLRFLT−DEL), where these six amino acids (residues 243–248) had been deleted. TASK3 channel current density was significantly decreased (P<0.05) by the deletion of these VLRFLT residues (Figure 4a).
Figure 4.
Mutation of the VLRFLT region of TASK3 channels gives functional currents regulated by zinc and pH. (a) Mean currents through TASK3 channels (measured as the difference current between that at −40 mV and that at −80 mV) for WT channels (n=37) and for TASK3VLRFLT−DEL channels (n=41). (b) Time–course plot showing inhibition of TASK3VLRFLT−DEL channels by zinc (100 μM). Inset shows a summary of inhibition of TASK3 (n=14) and TASK3VLRFLT−DEL (n=4) channels by zinc (100 μM). (c) Time–course plot showing alteration in TASK3VLRFLT−DEL channels by changes in extracellular pH (6.4 then 8.4 from a control pH of 7.4). (d) Summary of changes in TASK3 and TASK3VLRFLT−DEL channel currents by alterations in extracellular pH; at pH 6.4, TASK3, n=20; TASK3VLRFLT−DEL, n=8: at pH 8.4, TASK3, n=9; TASK3VLRFLT−DEL, n=8. *P<0.05; Student's t-test.
Inhibition by the selective TASK3 inhibitor, zinc (Clarke et al., 2004) was unaffected in TASK3VLRFLT−DEL channels with zinc (100 μM) giving about 80% block of TASK3VLRFLT−DEL or WT TASK3 channels (Figure 4b). Another fingerprint for TASK K2P channels is their sensitivity to changes in pH. The sensitivity of the TASK3VLRFLT−DEL channels to changes in extracellular pH was altered compared to WT TASK3 channels. In an acidified extracellular solution (pH 6.4) the TASK3VLRFLT−DEL channel was significantly less inhibited (P<0.05) than its WT isoform. However, in an alkaline extracellular solution (pH 8.4) the TASK3VLRFLT−DEL channel current was significantly enhanced (P<0.05), compared to WT TASK3 channels (Figures 4c and d).
As previously shown, the VLRFLT region of TASK3 is critical for GPCR-mediated inhibition of the channel (Talley and Bayliss, 2002). In our experiments, these TASK3VLRFLT−DEL channels were no longer inhibited by the activation of muscarinic receptors, with 0.1 μM muscarine inducing no change in TASK3VLRFLT−DEL channel current, unlike the WT TASK3 channels, which were markedly inhibited (Figures 5a, b and d).
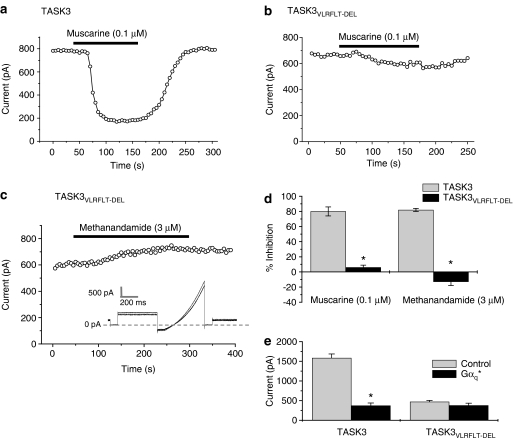
Figure 5.
The VLRFLT region of the TASK3 channel is critical for muscarinic receptor, Gαq and methanandamide-mediated inhibition. (a) Time–course plot showing inhibition of TASK3 channels by muscarine (0.1 μM) acting on co-transfected M3 receptors (current measured as the difference current between that at −40 mV and that at −80 mV). (b and c) Time–course plots showing inhibition of TASK3VLRFLT−DEL channels by (b) muscarine (0.1 μM) and (c) methanandamide. Inset in (c) shows representative TASK3VLRFLT−DEL channel current traces before and after perfusion of methanandamide (see ‘Methods' for detailed description of voltage protocol). (d) Summary of inhibition of TASK3 (n=9) and TASK3VLRFLT−DEL (n=3) channel currents by muscarine (0.1 μM) and methanandamide (3 μM). (e) Mean currents through TASK3 channels (n=15) showing inhibition following co-transfection with Gαq* (n=9). In contrast, mean current through TASK3VLRFLT−DEL channels (n=37) was unaltered following co-transfection with Gαq* (n=13). *P<0.05; Student's t-test.
Furthermore, these TASK3VLRFLT−DEL channels were no longer inhibited by the co-transfection of cells with the constitutively active G-protein subunit, Gαq* (Chen et al., 2006; Veale et al., 2007a) (Figure 5e).
Interestingly methanandamide (3 μM) no longer inhibited and, in fact, now slightly enhanced current through this TASK3VLRFLT−DEL channel suggesting that in addition to GPCR-mediated inhibition, methanandamide inhibition converges on this intracellular gating pathway (Figures 5c and d).
Discussion
In this study, we have shown that methanandamide is a non-selective blocker of both TASK1 and TASK3 channels, with hTASK3 channels being more sensitive to block, than hTASK1 and, indeed, mTASK3 channels (see also Maingret et al., 2001; Berg et al., 2004). Block by methanandamide was irreversible, unless cells were washed with buffer containing lipid-free BSA. This suggests that methanandamide enters the cell membrane to produce its blocking effect and that its site of action is located either intracellularly or in the membrane-spanning region of the protein.
The PKA phosphorylation sites in the distal C terminus of TASK1 did not seem to be important for methanandamide action since their removal had no influence on the compound's effectiveness. Perhaps more surprisingly, the trafficking of this mutated channel to the membrane of the cell also appeared to be unaltered. A number of studies have shown that in addition to two PKA phosphorylation sites, the distal C terminus sequence (RRSSV) interacts with two types of small adaptor protein (p11 and 14-3-3) which have both been suggested to be essential for trafficking of TASK1 channels to the membrane (Girard et al., 2002; O'Kelly et al., 2002; Rajan et al., 2002). Instead our results are in agreement with a more recent study (Renigunta et al., 2006) that suggests that although p11 is a powerful factor for the retention in the endoplasmic reticulum (ER) of TASK1 channels, its binding site is located more proximally in the C terminus. Indeed, the small size of TASK1 currents compared to TASK3 currents in our recordings (see ‘Results') suggests that much of the WT TASK1 channel protein was retained in the ER (see also Renigunta et al., 2006). Therefore, we observed functional TASK1 currents in the absence of the proposed 14-3-3 binding site (see also Girard et al., 2002), despite the suggestion that binding of this protein is essential for membrane trafficking of TASK1 (O'Kelly et al., 2002).
We cannot, however, discount the importance of the phosphorylation state of the channel and the significance of the C terminus on the cannabinoid effect. Indeed, previous work has shown that PKC may have a role in transducing the cannabinoid effect. For example, Barbuti et al. (2002) have shown that pre-application of the PKC inhibitor bisindolylmaleimide 1 hydrochloride, caused a significant decrease in the methanandamide sensitivity of the TASK1 current present in guinea pig ventricular myocytes. In support of these data, Maingret et al. (2001) had previously shown a reduced sensitivity to anandamide for a C terminus-truncated TASK1 channel, with all its putative PKC and PKA sites removed, with anandamide (10 μM) producing around 80% inhibition of the truncated channel (Figure 3 of Maingret et al., 2001) compared to >90% inhibition by a lower concentration of anandamide (3 μM) for WT TASK1 channels (Figure 1 of Maingret et al., 2001).
Similarly, our data show that the removal of all phosphorylation sites from TASK3 by a C-terminal truncation (TASK3125aa−TRUNC; see Veale et al., 2007a) significantly reduced the effectiveness of the compound on the TASK3 channel. A similar reduced effectiveness for GPCR-mediated modulation of these truncated channels has been seen (Talley and Bayliss, 2002). However, as substantial modulation of the truncated channel remained, methanandamide block was not solely dependant upon the phosphorylation state of the channel.
Previously, Talley and Bayliss (2002) had shown that TRH-mediated modulation of TASK3 channels and anaesthetic activation of these channels converged on a single pathway, dependant on a six-residue sequence (VLRFLT) at the interface of the last transmembrane domain and the beginning of the C terminus of TASK3. In this study we have confirmed the importance of this region for G-protein regulation and we show for the first time that this region is critical for inhibition of TASK3 channels by methanandamide. Additionally, deletion of this region altered the pH sensitivity of TASK3 channels. This suggests that alterations of the gating of the channel through an intracellular pathway can influence modulators that act extracellularly. A similar effect has been seen for Drosophila KCNK0 channels where phorbol ester-mediated regulation of gating through an intracellular pathway altered extracellular modulation by zinc (Zilberberg et al., 2001). However, since inhibition of TASK3VLRFLT−DEL channels by zinc was no different from that of WT TASK3 channels, this region does not underpin all regulatory pathways that act on these channels.
Furthermore, it is not clear whether this region in TASK channels is involved in binding of these modulatory molecules or in the transduction of the signal to the gate following binding. The diverse structures of these modulatory molecules (and/or the mediators they activate) would suggest, perhaps, that the latter is more likely. A recent study by Andres-Enguix et al. (2007) has shown that the Lymnaea ortholog of hTASK1 (LyTASK) is highly sensitive to general anaesthetic activation and, in contrast to hTASK1, this activation shows stereoselectivity. When the same six-residue region of LyTASK was mutated to that of hTASK1, the sensitivity of the channel to anaesthetics was reduced to that of hTASK1, but the stereoselectivity remained. Thus, these amino acids determine sensitivity to general anaesthetics but are unlikely to form the binding site for these agents.
Other ion channels that possess similar regions at the interface between the final transmembrane domain and their C termini appear to transduce modulatory signals. For example, the related TREK1 (K2P2.1) K2P channels are highly regulated by a wide range of physiological and pharmacological mediators including mechanical stretch, intracellular acidification, polyunsaturated fatty acids, volatile anaesthetics and temperature, all of which increase their activity, and certain GPCRs and pharmacological agents which inhibit their activity (see Honore, 2007). Like the VLRFLT region in TASK3 channels, the cytosolic C terminus of the TREK channel immediately following the fourth transmembrane domain plays a key structural role in these regulatory mechanisms. The actions of many of these regulatory compounds converge on a single intracellular glutamic acid residue, E306 (Honore et al., 2002; Chemin et al., 2005; Kennard et al., 2005), which is critical in the gating pathway. In addition, a cluster of positively charged residues around E306 interact with membrane lipids and bind to the A-kinase anchoring protein AKAP150, which integrates TREK1 channels into a postsynaptic scaffold with other regulatory proteins (Sandoz et al., 2006). Similarly, a region analogous to VLRFLT in cyclic nucleotide-gated channels (in this case close to the sixth transmembrane domain) is important for transmission of intracellular gating signals in these channels (Flynn et al., 2001).
Physiologically, methanandamide block of TASK1 and TASK3 channels may underpin a number of effects that cannot be explained through activation of CB1 or CB2 receptors (Maingret et al., 2001; Di Marzo et al., 2002). In the cerebellum, for example, TASK1 and TASK3 channels have important roles in regulating neuronal excitability (e.g. Aller et al., 2005; Brickley et al., 2007). Block of these channels by anandamide and other cannabinoids may contribute to the ataxia and movement disorders that can be observed following treatment with these agents.
TASK1 and TASK3 channels have been shown to be expressed and have functional importance in many regions of the CNS. In addition to the cerebellum, they have been shown to be particularly important in the brain stem and thalamus (see Maingret et al., 2001; Meuth et al., 2006). The enzyme directly responsible for the formation of anandamide in the brain, N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD), is expressed in many regions of the CNS but particularly high levels of NAPE-PLD mRNA are found in the thalamus (Morishita et al., 2005). While high levels of CB1 receptors are seen in the cerebellum, only moderate levels are observed in brain stem and low levels in thalamus (Morishita et al., 2005). Thus, anandamide generated in the brain stem and, particularly, the thalamus may have primary targets that are not CB1 receptors, such as TASK channels. Since the analgesic, sedative and hypothermic effects of the cannabinoid receptor agonist WIN55212-2 (R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate) were reduced in TASK-1 knockout mice compared to WT littermates (Linden et al., 2006), this might suggest a physiologically important role for TASK channels and their regulation by cannabinoids in supra-spinal pain pathways in the thalamus (see also Meuth et al., 2006).
Acknowledgments
This work was supported by the MRC and ARC. CEC is an Australian Research Council Postdoctoral Fellow. We thank Bill Wisden and Helen Meadows for the kind gifts of cDNA for TASK channels.
Abbreviations
- BSA
bovine serum albumin
- K2P
two-pore domain potassium
- NAPE-PLD
N-acylphosphatidylethanolamine-hydrolyzing phospholipase D
- PKA
protein kinase A
- PKC
protein kinase C
- WT
wild type
- WIN55212-2
(R-(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphtalenyl)methanone mesylate)
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels 2nd edition. Br J Pharmacol. 2006;147 Suppl 3:S1–S168. doi: 10.1038/sj.bjp.0706651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, et al. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Enguix I, Caley A, Yustos R, Schumacher MA, Spanu PD, Dickinson R, et al. Determinants of the anesthetic sensitivity of TASK channels: molecular cloning of an anesthetic-activated potassium channel from Lymnaea stagnalis. J Biol Chem. 2007;282:20977–20990. doi: 10.1074/jbc.M610692200. [DOI] [PubMed] [Google Scholar]
- Barbuti A, Ishii S, Shimuzi T, Robinson RB, Feinmark SJ. Block of the background K channel TASK-1 contributes to arrhythmogenic effects of platelet activating factor. Am J Physiol Heart Circ Physiol. 2002;282:2024–2030. doi: 10.1152/ajpheart.00956.2001. [DOI] [PubMed] [Google Scholar]
- Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Aller MI, Sandu C, Veale EL, Alder FG, Sambi H, et al. TASK-3 two-pore domain potassium channels enable sustained high-frequency firing in cerebellar granule neurons J Neurosci 200727doi:10.1523/JNEUROSCI.1427-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Ekuta JE, Onaivi ES. Neurobehavioral effects of anandamide and cannabinoid receptor gene. Brain Res Bull. 1998;45:67–74. doi: 10.1016/s0361-9230(97)00291-8. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, et al. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci USA. 2006;103:3422–3427. doi: 10.1073/pnas.0507710103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CE, Veale EL, Green PJ, Meadows HJ, Mathie A. Selective block of the human 2-P domain potassium channel, TASK-3, and the native leak potassium current, IKSO, by zinc. J Physiol. 2004;560:51–62. doi: 10.1113/jphysiol.2004.070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. Anandamide receptors. Prostaglandins Leukot Essen Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Flynn GE, Johnson JP, Zagotta WN. Cyclic nucleotide-gated channels: shedding light on the opening of a channel pore. Nature Rev Neurosci. 2001;2:643–652. doi: 10.1038/35090015. [DOI] [PubMed] [Google Scholar]
- Girard C, Tinel N, Terrenoire C, Romey G, Lazdunski M, Borsotto M. P11, an anexin ii subunit, an auxiliary protein associated with the background K+ channel, TASK-1. EMBO J. 2002;21:4439–4448. doi: 10.1093/emboj/cdf469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- Goldstein SAN, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International union of pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nature Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K(+) channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species–species sensitivity to ‘hot' chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br J Pharmacol. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Linden AM, Aller MI, Leppa E, Vekovischeva O, Aitta-aho T, Veale EL, et al. The in vivo contributions of TASK-1-containing channels to the actions of inhalation anesthetics, the α2 adrenergic sedative dexmedetomidine and cannabinoid agonists. J Pharmacol Exp Ther. 2006;317:615–626. doi: 10.1124/jpet.105.098525. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K+ channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A. Neuronal two pore domain potassium channels and their regulation by G protein coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie A, Clarke CE, Ranatunga KM, Veale EL. What are the roles of the many different types of potassium channel expressed in cerebellar granule cells. Cerebellum. 2003;2:11–25. doi: 10.1080/14734220310015593. [DOI] [PubMed] [Google Scholar]
- Meuth SV, Aller MI, Munsch T, Schuhmacher T, Seidenbecher T, Meuth P, et al. The contribution of TWIK-related acid-sensitive K+-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol Pharmacol. 2006;69:1468–1476. doi: 10.1124/mol.105.020594. [DOI] [PubMed] [Google Scholar]
- Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, et al. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci USA. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita J, Okamoto Y, Tsuboi K, Ueno M, Sakamoto H, Maekawa N, et al. Regional distribution and age-dependent expression of N-acylphosphatidylethanolamine-hydrolyzing phospholipase D in rat brain. J Neurochem. 2005;94:753–762. doi: 10.1111/j.1471-4159.2005.03234.x. [DOI] [PubMed] [Google Scholar]
- O'Kelly I, Butler MH, Zilberberg N, Goldstein SA. Forward transport: 14-3-3 binding overcomes retention in endoplasmic reticulum by dibasic signals. Cell. 2002;111:577–588. doi: 10.1016/s0092-8674(02)01040-1. [DOI] [PubMed] [Google Scholar]
- O'Connell AD, Morton MJ, Hunter M. Two-pore domain K+ channels-molecular sensors. Biochim Biophys Acta. 2002;1566:152–161. doi: 10.1016/s0005-2736(02)00597-7. [DOI] [PubMed] [Google Scholar]
- Rajan S, Presig-Muller R, Wischmeyer E, Nehring R, Hanley PJ, Renigunta V, et al. Interaction with 14-3-3 proteins promotes functional expression of the potassium channels TASK-1 and TASK-3. J Physiol. 2002;545:13–26. doi: 10.1113/jphysiol.2002.027052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renigunta V, Yuan H, Zuzarte M, Rinne S, Koch A, Wischmeyer E, et al. The retention factor p11 confers an endoplasmic reticulum-localization signal to the potassium channel TASK-1. Traffic. 2006;7:168–181. doi: 10.1111/j.1600-0854.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- Sandoz G, Thummler S, Duprat F, Feliciangeli S, Vinh J, Escoubas P, et al. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K+ channels into open leak channels. EMBO J. 2006;25:5864–5872. doi: 10.1038/sj.emboj.7601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Bayliss DA. Modulation of TASK-1 (Kcnk3) and TASK-3 (Kcnk9) potassium channels: volatile anesthetics and neurotransmitters share a molecular site of action. J Biol Chem. 2002;277:17733–17742. doi: 10.1074/jbc.M200502200. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Veale EL, Clarke CE, Mathie A.Identification of a region of TASK3 two pore domain K channels critical for their modulation by methanandamide 2007bPC187Life Science Conference (Glasgow) [DOI] [PMC free article] [PubMed]
- Veale EL, Kennard LE, Sutton GL, MacKenzie G, Sandu C, Mathie A. Gαq mediated regulation of TASK3 two pore domain potassium channels: the role of protein kinase C. Mol Pharmacol. 2007a;71:1666–1675. doi: 10.1124/mol.106.033241. [DOI] [PubMed] [Google Scholar]
- Zilberberg N, Ilan N, Goldstein SAN. KCNKO: opening and closing the 2-P-domain potassium leak channel entails ‘C-type' gating of the outer pore. Neuron. 2001;32:635–648. doi: 10.1016/s0896-6273(01)00503-7. [DOI] [PubMed] [Google Scholar]