Abstract
Background and purpose:
Rimonabant (AcompliaTM, SR141716A), a cannabinoid CB1 receptor inverse agonist, has recently been approved for the treatment of obesity. There are, however, concerns regarding its side effect profile. Developing a CB1 antagonist with a different pharmacological mechanism may lead to a safer alternative. To this end we have screened a proprietary small molecule library and have discovered a novel class of allosteric antagonist at CB1 receptors. Herein, we have characterized an optimized prototypical molecule, PSNCBAM-1, and its hypophagic effects in vivo.
Experimental approach:
A CB1 yeast reporter assay was used as a primary screen. PSNCBAM-1 was additionally characterized in [35S]-GTPγS, cAMP and radioligand binding assays. An acute rat feeding model was used to evaluate its effects on food intake and body weight in vivo.
Key results:
In CB1 receptor yeast reporter assays, PSNCBAM-1 blocked the effects induced by agonists such as CP55,940, WIN55212-2, anandamide (AEA) or 2-arachidonoyl glycerol (2-AG). The antagonist characteristics of PSNCBAM-1 were confirmed in [35S]-GTPγS binding and cAMP assays and was shown to be non-competitive by Schild analyses. PSNCBAM-1 did not affect CB2 receptors. In radioligand binding assays, PSNCBAM-1 increased the binding of [3H]CP55,940 despite its antagonist effects. In an acute rat feeding model, PSNCBAM-1 decreased food intake and body weight.
Conclusions and implications:
PSNCBAM-1 exerted its effects through selective allosteric modulation of the CB1 receptor. The acute effects on food intake and body weight induced in rats provide a first report of in vivo activity for an allosteric CB1 receptor antagonist.
Keywords: allosteric modulator, cannabinoid receptor, antagonist
Introduction
The expansion of research in cannabinoid pharmacology has arisen principally from the discovery that Δ9-tetrahydrocannabinol, the major active compound in herbal cannabis, exerts its effects through cannabinoid receptors in the brain (Devane et al., 1988). At least two cannabinoid receptor subtypes are known to exist, CB1 and CB2 (Matsuda et al., 1990; Munro et al., 1993), and there is also evidence for a CB3 receptor (Fride et al., 2003). These receptors belong to the G-protein-coupled receptor (GPCR) superfamily. CB2 receptors are expressed primarily in immune cells, whereas CB1 receptors exist predominantly in the brain (Matsuda et al., 1993) and to a lesser extent in peripheral tissues such as testis (Gerard et al., 1991) and adipose tissue (Bensaid et al., 2003). To date a number of endogenous cannabinoids and synthetic CB1 receptor ligands have been identified and many of these have therapeutic potential in a variety of disorders including obesity, nicotine and alcohol dependence, pain, multiple sclerosis, cancer, diarrhoea and cardiovascular diseases (Di Marzo et al., 2004).
In the past, the majority of GPCR-based drug discovery programmes have focussed on the development of molecules that compete with the endogenous ligands at the orthosteric binding site. In recent years, however, with the development of more sophisticated functional reporter-based assays it has been possible to identify active ligands that bind to other sites on the receptor, allosteric sites (Jensen and Spalding, 2004). Allosteric ligands mediate their effects by modifying receptor conformation leading to a change in the binding and/or functional properties of orthosteric ligands (May and Christopoulos, 2003). The formation of a ternary complex between two ligands and a protein is often necessary for such allosteric interactions to occur (Birdsall et al., 1996).
Various potential therapeutic advantages of allosteric modulation of GPCRs have been suggested. An allosteric modulator may only elicit its effects when the endogenous agonist is present, thereby resulting in a selective ‘tuning' of drug effects when and where they are required. This is in contrast to orthosteric ligands, which may also continuously affect receptor function for as long as they are present (Christopoulos and Kenakin, 2002). Furthermore, orthosteric sites of GPCR subtypes activated by the same ligand are often highly conserved; therefore, achieving subtype selectivity may be more challenging when targeting this site for drug design. Allosteric ligands act through less conserved sites and thus have a greater potential for receptor subtype specificity (Jensen and Spalding, 2004).
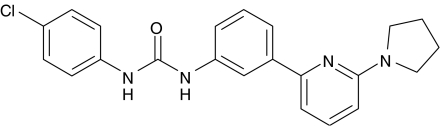
Currently, there is considerable interest in the therapeutic use of CB1 antagonists resulting from the recent approval in Europe of rimonabant (Acomplia), a CB1 inverse agonist, for the treatment of obesity. The anti-obesity potential of this compound was demonstrated in a Phase 3 trial in obese patients where significant reductions of body weight, waist circumference and triglyceride levels were observed in a 2-year study (Pi-Sunyer et al., 2006). While generally well tolerated, there was nonetheless a high dropout rate and an increased incidence of nausea and psychiatric disorders, highlighting the need to develop safer alternatives. Here, we provide evidence for allosteric antagonism of the CB1 receptor by a novel class of synthetic small molecules as exemplified by 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea (PSNCBAM-1; Figure 1). This compound has been optimized from a high-throughput screening lead, which was identified using a human cannabinoid receptor 1 (hCB1) receptor expressing yeast reporter-based assay. We also show that PSNCBAM-1 is active in vivo in an acute rat feeding model and believe this is the first report of a pharmacologically active CB1 allosteric modulator offering prospects as a novel treatment for obesity.
Figure 1.
Structure of PSNCBAM-1; 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea.
Methods
Yeast fluorescent reporter assay
Yeast reporter-based assays have previously been described in the literature (King et al., 1990; Campbell et al., 1999; Miret et al., 2002). Saccharomyces cerevisiae cells were engineered such that the endogenous yeast G-α protein (GPA1) was replaced with a Gαi/o-protein chimera. Additionally, the endogenous GPCR, Ste3, was deleted to allow for the heterologous expression of a mammalian GPCR of choice. In yeast, elements of the pheromone signalling transduction pathway drive the expression of Fus1. By placing the β-galactosidase (LacZ) gene under the control of the Fus1 promoter (Fus1p), receptor activation can by monitored by a fluorescent readout. Yeast cells were co-transformed with a CB1 cDNA expression plasmid carrying an LEU auxotrophic marker, and two Fus1p-LacZ reporter plasmids, with auxotrophic markers for URA and TRP. Transformants were grown on selective plates lacking leucine, uracil and tryptophan. For the fluorimetric functional assay, recombinant yeast strains were grown to mid-log phase in synthetic-defined medium without leucine, uracil and tryptophan, pH 6.8 (Qbiogene Inc., Carlsbad, CA, USA) and mixed with test compounds in opaque 96-well plates. Test compounds were added 30 min before addition of an EC90 concentration of agonist (100 nM CP55,940 (950 (−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl) cyclohexanol), 400 nM WIN55,212-2 (R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]-pyrrolo[1,2,3,-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate), 4 μM arachidonoyl ethanolamide (AEA) or 1.5 μM 2-arachidonoyl glycerol (2-AG)). Dimethyl sulphoxide was present at 1% final concentration and bovine serum albumin (BSA) at 0.1%. After 4 h incubation at 30°C, 4-methylumbelliferyl β-D-galactopyranoside substrate at a final concentration of 50 μM and Triton × 100 at a final concentration of 0.25% were added to each well. Incubation was continued for 45 min at 30°C and sodium carbonate then added to a final concentration of 140 μM to terminate the reaction and enhance the fluorescent signal. The plates were read in a fluorimeter at 365/445 nm.
For yeast assays used to observe inverse agonism at CB1, a transformant with a high level of constitutive receptor activity was selected. In this instance the assay was performed as described above but without the addition of agonist and the β-gal reporter activity measured after 6 h incubation at 30°C in the presence of a range of compound concentrations.
Cell culture and preparation of cell membranes
Human embryonic kidney (HEK)293 cells overexpressing hCB1 (HEK293-hCB1) were used for cAMP, binding and GTPγS experiments. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g l−1 glucose, L-glutamine and supplemented with 10% fetal bovine serum, 50 U ml−1 penicillin, 50 μg ml−1 streptomycin, 15 μg ml−1 blasticidin, 100 μg ml−1 hygromycin and 1 μg ml−1 tetracycline. To prepare cell membranes, cells were harvested by scraping, washed in phosphate-buffered saline containing 1 mM EDTA, and Dounce homogenized in buffer A (320 mM Sucrose, 10 mM HEPES, 1 mM EDTA at pH 7.4). Cell homogenates were then centrifuged at 1600 g for 10 min and the supernatant was collected. The pellet was resuspended in buffer A, homogenized and centrifuged as before and the supernatant was collected. Supernatants were pooled before undergoing further centrifugation at 50 000 g for 2 h. The supernatant was discarded and the pellet was resuspended in buffer B (50 mM HEPES, 0.5 mM EDTA, 10 mM MgCl2 at pH 7.4), aliquoted and stored at −80°C.
Preparation of rat cerebellar membranes
Isolated rat cerebellum (Pel-Freez Biologicals, Rogers, AR, USA) was Dounce homogenized in buffer A (as above). Tissue homogenates were then centrifuged at 1600 g for 10 min and the supernatant was collected. The pellet was resuspended in buffer A, homogenized and centrifuged as above and the supernatant was collected. Supernatants were pooled before undergoing further centrifugation at 40 000 g for 30 min. The supernatant was discarded and the pellet was resuspended in buffer B (as above) and stored at −80°C.
Radioligand binding experiments
Competition binding was performed by incubating 10 μg well−1 HEK293-hCB1 cell membranes with either 0.8. nM [3H]CP55,940 (Kd 0.39±0.13 nM) or 2 nM [3H]SR141716A (N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) (Kd 1.77±0.68 nM) in 50 mM Tris, 2.5 mM EDTA, 5 mM MgCl2 and 1 mg ml−1 BSA buffer (pH 7.4) at 30°C for 90 min in a final volume of 200 μl. A concentration range of test compound was added before the addition of radiolabelled compounds. Unlabelled CP55,940 (10 μM) and unlabelled SR141716A (10 μM) were used to define nonspecific binding for [3H]CP55,940 and [3H]SR141716, respectively. Following incubation, reactions were filtered onto GF/B filter mats pre-soaked in 0.05% polyethyleneimine. Filters were washed six times with ice-cold 50 mM Tris, 2.5 mM EDTA, 5 mM MgCl2 buffer (pH 7.4) then air-dried and the radioactivity counted in a Microbeta liquid scintillation counter.
[35S]GTPγS binding experiments
[35S]GTPγS binding was performed in 20 mM HEPES, 3 mM MgCl2, 100 mM NaCl, 1 mM dithiothreitol, 1 mg ml−1 BSA buffer (pH 7.4) with 20 μg cell membranes per reaction plus 10 μM GDP and 0.1 nM [35S]GTPγS in a final volume of 200 μl per reaction. Nonspecific binding was determined using 10 μM unlabelled GTPγS. When using rat cerebellar membranes, 20 nM 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, an adenosine A1 inverse agonist) was included to reduce basal stimulation of [35S]GTPγS. For competitive assays, approximate EC90 concentrations of either CP55,940 or AEA were used to stimulate [35S]GTPγS binding (for HEK293-hCB1 membranes 50 nM CP55,940 or 2.5 μM AEA; for rat cerebellar membranes 200 nM CP55,940 or 10 μM AEA; 10 nM CP55,940 for HEK293-human cannabinoid receptor 2 (hCB2) membranes). Test antagonist compounds were added 5 min before the addition of [35S]GTPγS and agonist compounds. Reactions were incubated at 30°C for 60 min, filtered onto GF/B filter mats pre-soaked in reaction buffer. Filters were then washed four times with ice-cold 20 mM HEPES, 3 mM MgCl2 buffer (pH 7.4) then air-dried and radioactivity counted in a Microbeta liquid scintillation counter.
Cyclic AMP accumulation assay
HEK293-hCB1 cells were cultured for 24 h in 96-well microtitre plates seeded at 2.5 × 104 cells well−1 in DMEM (as above). After 24 h, the medium was removed and cells were incubated with test compounds in the presence of an EC90 concentration of agonist (10 nM CP55,940 or 1 μM AEA) and 5 μM Forskolin (added simultaneously) at 21°C for 30 min in 50 μl per reaction in stimulation buffer (Hank's-buffered salt solution containing 5 mM HEPES, 0.5 mM IBMX, 1 mg ml−1 BSA at pH 7.4). Lysis buffer (75 μl; 50 mM HEPES, 10 mM CaCl2, 0.35% Triton X-100 at pH 7.4) was then added and the plates were incubated at 21°C for a further 10 min to terminate the reaction. cAMP was then measured using a LANCE cAMP detection kit.
Feeding experiments
Acute feeding experiments were carried out using singly housed male Sprague–Dawley (SD) rats (Charles River Laboratories, Kent, UK). Rats were maintained on a reverse-phase light–dark cycle and allowed free access to a standard powdered rat diet and tap water. Animals were accustomed to these conditions for at least 2 weeks before experimentation. On the day of the experiment, the animals were allocated to weight-matched treatment groups containing six rats. Compounds were administered by intraperitoneal (i.p.) injection 30 min before the start of the dark phase in a vehicle consisting 5% propylene glycol/5% Tween 80 and 90% saline at a dose volume of 5 ml kg−1. Food intake was monitored by weighing the feeding jars at 1, 2, 4, 6 and 24 h. The animals were weighed at the 24 h reading.
Data and statistical analysis
For yeast receptor assays, competition radioligand binding and [35S]GTPγS binding, EC50 and IC50 values were obtained by fitting data to a four parameter, one-site dose–response equation. Ki values were calculated using the Cheng and Prusoff (1973) equation. Ligand-induced changes in [35S]GTPγS binding were expressed as a percentage of basal binding. Schild analysis was performed using the method described by Arunlakshana and Schild (1959). Excel Fit 4 (IDBS) curve fitting software was used for all in vitro data analysis.
Results from the feeding experiments (body weights at 0 and 24 h; change in body weight over 24 h; food intake at 1, 2, 4, 6 and 24 h) were expressed as mean values±s.e.m. Food intakes were expressed in gram per kilogram to account for variations in body weight between the different animals. Statistical comparisons between the food intake of different groups of rats were made by analysis of variance followed by Dunnett's multiple comparisons test. P<0.05 was considered to be statistically significant.
Materials
CP55,940, WIN55,212-2 and AM630 ((6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl)(4-methoxyphenyl) methanone) were purchased from Tocris (Bristol, UK). AEA and 2-AG were purchased from Cayman (Tyne & Wear, UK). SR141716A was prepared according to a published procedure (Casellas et al., 1997). PSNCBAM-1 was synthesized as described by Bloxham et al. (2006).
[3H]CP55,940 and [3H]SR141716A were purchased from Perkin Elmer (Buckinghamshire, UK) and [35S]GTPγS from Amersham (Buckinghamshire, UK). LANCE cAMP kits and GF/B filter mats were purchased from Perkin Elmer.
Results
CB1 receptor yeast assay experiments
The budding yeast, Saccharomyces cerevisiae, is an attractive host for the study of heterologously expressed GPCRs since the signalling pathways are similar to mammalian cells. Moreover, it contains only two endogenous GPCRs, either of which can be replaced by a mammalian GPCR of interest thereby minimizing interference by the multitude of other GPCRs, which would otherwise be present if using a mammalian host. Two such recombinants were constructed with a lacZ reporter: one expressing a constitutively active hCB1 receptor and the other where the same receptor is activated only upon the addition of specific agonist ligands. The latter was used to screen a proprietary small molecule compound library for antagonists and from this emerged a distinct chemical series that underwent optimization leading to the identification of PSNCBAM-1 (Figure 1).
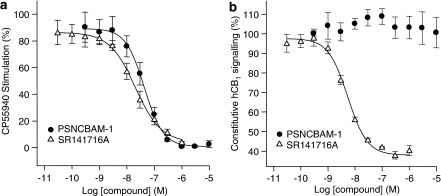
In the hCB1 yeast reporter assay, PSNCBAM-1 dose-dependently antagonized the stimulation of hCB1 receptor signalling elicited by CP55,940 (Figure 2a). Other CB1 agonists, WIN55,212-2, AEA and 2-AG (each used at a concentration that produced 90% maximal stimulation) were also antagonized with IC50 values from about 40 nm to over 200 nM (Table 1). This wide range of IC50 values implied that functional antagonism was agonist ligand dependent. On the other hand, the potent selective CB1 inverse agonist, SR141716A, in the same experimental system produced IC50 values, which were more consistent (Table 1) suggesting a ligand-independent effect.
Figure 2.
Effect of compounds on receptor signalling in Saccharomyces cerevisiae stably expressing recombinant human CB1 receptors. Antagonism of 100 nM CP55,940-induced signalling by SR141716A and PSNCBAM-1 (a). Effect of SR141716A and PSNCBAM-1 on constitutive hCB1 signalling (b). Yeast cells were incubated with compounds at 30°C and reporter gene expression was measured by a fluorescent readout. Agonist concentrations used were determined by their EC90 values in the assay. Data points are mean±s.e.m. from three experiments and curves are fitted to a four parameter, one-site, dose–response equation. PSNCBAM-1, 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea; SR141716A, N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Table 1.
Antagonism of agonist stimulated yeast expressing hCB1 receptors
| Compound | 100 nM CP55,940 IC50 (nM) | 400 nM WIN55212-2 IC50 (nM) | 4 μM AEA IC50 (nM) | 1.5 μM 2-AG IC50 (nM) |
|---|---|---|---|---|
| PSNCBAM-1 | 45.2±7.5 | 208.7±27.9 | 37±5.3 | 230.3±12.2 |
| SR141716A | 22.5±7.3 | 6.5±1.4 | 16±3.2 | 11.4±2.9 |
Abbreviations: AEA, arachidonoyl ethanolamide; PSNCBAM-1, 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea; SR141716A, N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Potency of PSNCBAM-1 and SR141716A against a range of agonist-stimulated receptor signalling in Saccharomyces cerevisiae stably expressing recombinant human CB1 receptors. Yeast cells were incubated with compounds at 30°C and reporter gene expression was measured by a fluorescent read-out. Agonist concentrations used were determined by their EC90 values in the assay. Data are mean IC50±s.e.m. from three experiments.
In constitutive hCB1 yeast reporter assays, SR141716A dose-dependently reduced CB1 activity with an IC50 of 4.8±0.4 nM, confirming its reported inverse agonist properties. In contrast, PSNCBAM-1 had no effect on constitutive activity at concentrations up to 10 μM (Figure 2b), indicating that this compound did not behave as an inverse agonist.
[35S]GTPγS binding experiments
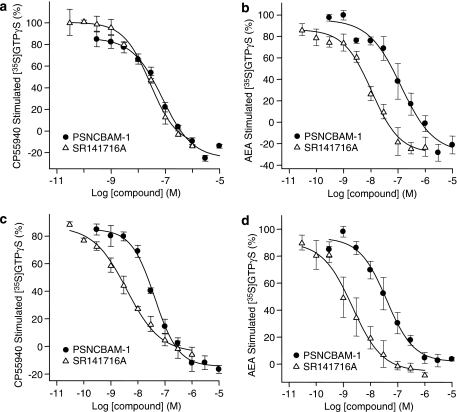
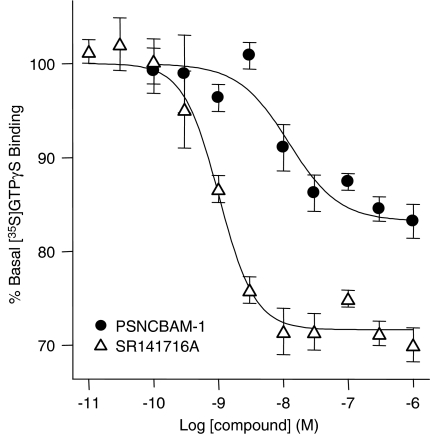
To confirm the yeast data, PSNCBAM-1 was further investigated in a human cell line (HEK293) overexpressing hCB1 receptors and on endogenously expressed CB1 receptors in rat cerebellum. In recombinant HEK293-hCB1 membranes, PSNCBAM-1 displayed a concentration-dependent reversal of [35S]GTPγS binding stimulated by either 50 nM CP55,940 or 1 μM AEA (Figures 3a and b, respectively). PSNCBAM-1 also reversed [35S]GTPγS binding stimulated by either 200 nM CP55,940 or 10 μM AEA in rat cerebellar membranes (Figures 3c and d respectively). The potencies of PSNCBAM-1 at inhibiting CP55,940 and AEA-induced responses in these assays were similar (Table 2). Furthermore the potency of PSNCBAM-1 in both HEK293-hCB1 and rat cerebellar membranes was comparable, indicating no significant species difference and no great disparity between recombinant and endogenous expression levels of receptor (Table 2). However, the difference between PSNCBAM-1 and SR141716A potency was greater in rat cerebellar membranes than in HEK293-hCB1 membranes. It was also noted in these experiments that PSNCBAM-1, like SR141716A, reduced [35S]GTPγS binding below the baseline suggesting that it may indeed act as an inverse agonist contrary to what was observed in the yeast assay. Consequently its effects on basal [35S]GTPγS binding were investigated. The results (Figure 4) confirmed SR141716A to be a potent inverse agonist reducing [35S]GTPγS binding by 29.25±0.68% with an IC50 of 0.84±0.02 nM, while PSNCBAM-1 produced a partial reduction of 16.3±0.83% with an IC50 of 7.02±1.25 nM.
Figure 3.
Effect of PSNCBAM-1 and SR141716A on [35S]GTPγS binding in HEK293-hCB1 cell membranes stimulated by either 50 nM CP55,940 (a) or 2.5 μM AEA (b); and rat cerebellar membranes stimulated by either 200 nM CP55,940 (c) or 10 μM AEA (d). Agonist concentrations used were determined by their EC90 values for each membrane type. [35S]GTPγS and compounds were mixed with membranes and incubated at 30°C for 60 min. Plates were then filtered and the radioactivity counted. Data points are mean±s.e.m. from three experiments and curves are fitted to a four parameter, one-site, dose–response equation. AEA, arachidonoyl ethanolamide; HEK, human embryonic kidney; PSNCBAM-1, 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea; SR141716A, N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Table 2.
Antagonism of agonist stimulated [35S]-GTPγS binding
|
HEK293-hCB1 membranes |
Rat cerebellar membranes |
HEK293-hCB2 membranes |
|||
|---|---|---|---|---|---|
| Compound | CP55,940 IC50 (nM) | AEA IC50 (nM) | CP55,940 IC50 (nM) | AEA IC50 (nM) | CP55,940 IC50 (μM) |
| PSNCBAM-1 | 74.3±12.7 | 131±66 | 42.1±8.3 | 29.7±10.2 | >10* |
| SR141716A | 28±1.3 | 20±9.4 | 4.7±2.1 | 2.4±1 | 3.6* |
Abbreviations: AEA, arachidonoyl ethanolamide; HEK, human embryonic kidney; PSNCBAM-1, 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea; SR141716A, N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Antagonism of agonist-stimulated [35S]-GTPγS binding in HEK293-hCB1 and rat cerebellar membranes by PSNCBAM-1 and SR141716A. [35S]-GTPγS and compounds were mixed with membranes and incubated at 30°C for 60 min. Plates were then filtered and the radioactivity counted. Mean IC50±s.e.m. from three experiments. AM630, a CB2 control antagonist, produced an IC50 value of 0.65 μM.
Data from a single experiment.
Figure 4.
Effect of PSNCBAM-1 and SR141716A on [35S]GTPγS binding in unstimulated HEK293-hCB1 cell membranes. [35S]GTPγS and compounds were mixed with membranes and incubated at 30°C for 60 min. Plates were then filtered and the radioactivity counted. Data points are mean±s.e.m. from four experiments and curves are fitted to a four parameter, one-site, dose–response equation. HEK, human embryonic kidney; PSNCBAM-1, 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea; SR141716A, N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
In recombinant HEK293-hCB2 membranes, PSNCBAM-1 displayed no significant reversal of 10 nM CP55,940-stimulated [35S]GTPγS binding up to a concentration of 10 μM (Table 2), indicating a high degree of receptor subtype selectivity with regards to functional antagonism. In this experiment the CB2 antagonist, AM630, produced dose-dependent antagonism of CP55,940-stimulated membranes (Table 2).
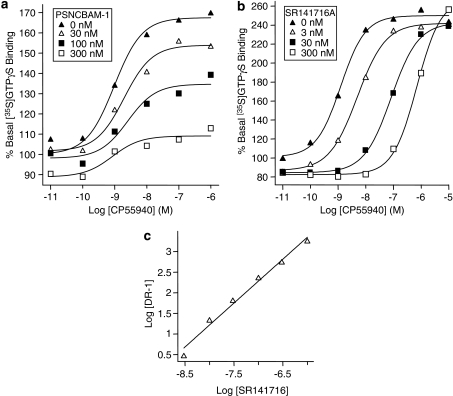
To gain further insight into the mode of antagonism of PSNCBAM-1, a Schild analysis experiment was performed using the [35S]GTPγS assay. Unlike a competitive antagonist, PSNCBAM-1 did not significantly affect the EC50 value of CP55,940. However, the efficacy (Emax) of CP55,940 was reduced in a concentration-dependent manner from 167.5% of basal [35S]GTPγS with no PSNCBAM-1 to 109.1% of basal [35S]GTPγS at 300 nM PSNCBAM-1 (Figure 5a). These data provided further evidence that PSNCBAM-1 was a non-competitive antagonist. In the same experimental system, the presence of the competitive inverse agonist SR141716A caused an increase in the EC50 of CP55,940 (Figure 5b). The Schild plot for SR141716A provided a pA2 value of 8.84±0.08 (consistent with previously calculated Kd values) and a gradient of 1.18±0.1 indicating classical competitive reversible antagonism (Figure 5c). Due to the non-competitive action of PSNCBAM-1, a Schild plot was not generated for this compound.
Figure 5.
CP55,940-stimulated [35S]GTPγS binding in HEK293-hCB1 membranes in the presence of a range of concentrations of PSNCBAM-1 (a) and SR141716A (b). Data for SR141716A was used to generate a Schild plot (c). [35S]GTPγS and compounds were mixed with membranes and incubated at 30°C for 60 min. Plates were then filtered and the radioactivity counted. Curves are fitted to a four parameter, one-site, dose–response equation. HEK, human embryonic kidney; PSNCBAM-1, 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea; SR141716A, N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Cyclic AMP accumulation assay
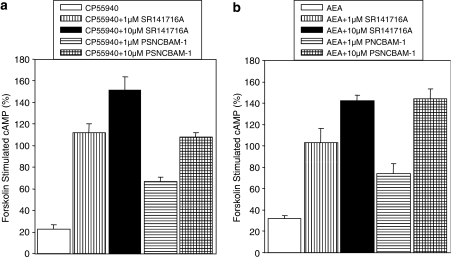
Compounds were tested in whole-cell cAMP assays to assess whether the observed inhibition of [35S]GTPγS accumulation would translate to antagonism of agonist-induced inhibition of adenylate cyclase. In HEK293-hCB1 cells, 10 μM PSNCBAM-1 and 1 μM SR141716A were able to completely reverse either 10 nM CP55,940 or 1 μM AEA-induced inhibition of forskolin-stimulated cyclic AMP accumulation (Figures 6a and b). As was the case for the [35S]GTPγS assay, the ability of PSNCBAM-1 to antagonize CP55,940- and AEA-induced responses was similar although the inhibition was only partial at 1 μM. As observed in the rat [35S]GTPγS assays, SR141716A was more potent than PSNCBAM-1 in the cAMP assay. In summary, the cAMP assay data confirm the data obtained in the yeast reporter and [35S]GTPγS assays and demonstrates that PSNCBAM-1 acts as a CB1 antagonist of second messenger signalling in mammalian cells. Thus we have established that PSNCBAM-1 inhibits CB1 receptor signalling at both the G-protein and second messenger levels of signalling.
Figure 6.
Effect of PSNCBAM-1 and SR141716A on either (a) 10 nM CP55,940 or (b) 1 μM AEA-stimulated inhibition of cAMP accumulation induced by 5 μM forskolin, in HEK293-hCB1 cells. Agonist concentrations used were determined by their EC90 values. Cells were incubated with compounds for 30 min at room temperature. Intracellular cAMP levels were subsequently measured using the AlphaScreen kits from Perkin Elmer Inc. Data are means±s.e.m. from three experiments. The direct effects of PSNCBAM-1 and SR141716A on forskolin-induced cAMP responses were investigated as part of the validation of this assay and although some effects were noted, in particular with SR141716A, they did not alter the data interpretation when the agonist CP55,940 was present. AEA, arachidonoyl ethanolamide; HEK, human embryonic kidney; PSNCBAM-1, 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea; SR141716A, N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Radioligand binding experiments
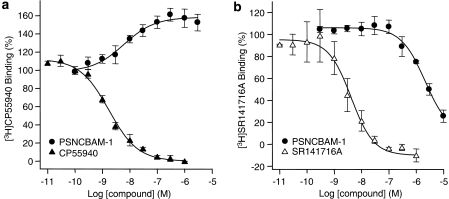
To further elucidate the mode of interaction of PSNCBAM-1 at the CB1 receptor, competitive radioligand experiments were conducted. When the orthosteric agonist [3H]CP55,940 was used in such experiments, unlabelled CP55,940 displaced the radioligand as expected with a Ki of 0.52±0.05 nM. By contrast, PSNCBAM-1 dose dependently increased [3H]CP55,940 binding by 58±9% with an EC50 of 14.4±6.6 nM (Figure 7a).
Figure 7.
Effect of compounds on the binding of 0.8 nM [3H]CP55,940 (a) and 2 nM [3H]SR141716A (b) to HEK293-hCB1 cell membranes. Compounds were mixed with membranes and incubated at 30°C for 90 min. Plates were then filtered and the radioactivity counted. Data points are mean±s.e.m. from three experiments and curves are fitted to a four parameter, one-site, dose–response equation. HEK, human embryonic kidney; SR141716A, N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Substituting the radioligand with the orthosteric inverse agonist, [3H]SR141716A resulted in the competitive displacement of the radioligand by unlabelled SR141716A with a Ki of 1.77±0.56 nM. In contrast to its effect on [3H]CP55,940 binding, PSNCBAM-1 dose dependently reduced [3H]SR141716A binding with an IC50 of 2.29±0.37 μM (Figure 7b). However, this reduction in radioligand binding was incomplete at 10 μM (74±6% reduction). It was not possible to test concentrations above 10 μM due to compound insolubility. Thus, even though PSNCBAM-1 displayed antagonism in several functional assays it did not compete with, but enhanced, the binding of the agonist CP55,940.
Feeding studies
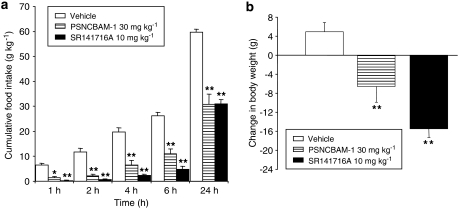
In acute food intake studies in male SD rats, PSNCBAM-1 (30 mg kg−1 i.p.) and SR141716A (10 mg kg−1 i.p.) significantly reduced food intake by 83±6% (P<0.01) and 94±2% (P<0.01) after 2 h, respectively, when compared to the vehicle-treated group. PSNCBAM-1 also significantly reduced food intake by 48±7% over the 24-h period (P<0.01) compared with 48±3% for SR141716A (P<0.01; Figure 8a). PSNCBAM-1 significantly decreased body weight when compared to the vehicle-treated group that gained weight over the same period (P<0.01). As expected, SR141716A also reduced body weight (P<0.01 vs vehicle; Figure 8b). No adverse effects on animal behaviour or obvious signs of toxicity were observed at any point in the experiment for any of the groups tested.
Figure 8.
Effect of PSNCBAM-1 and SR141716A on acute feeding (a) and body weight (b) in male SD rats. Compounds were administered by i.p. injection before the start of the dark phase. Food intake was monitored by weighing the feeding jars at 1, 2, 4, 6 and 24 h and animals were weighed at 24 h. Results are means±s.e.m. for groups of six rats. *P<0.05 and **P<0.01, analysis of variance followed by Dunnett's multiple comparison test. i.p., intraperitoneal; PSNCBAM-1, 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea; SD, Sprague–Dawley; SR141716A, N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Discussion
There are now numerous reports describing allosteric modulation of family A GPCRs including muscarinic M1–M5 receptors (Ellis et al., 1991), dopamine receptors (Hoare et al., 2000) and adenosine A3 receptors (Gao et al., 2002). However, to our knowledge, there is only one recent report describing allosteric modulation of cannabinoid receptors (Price et al., 2005). We have described in the present study that a novel small molecule, PSNCBAM-1, is also an allosteric modulator at the CB1 receptor. This allosterism is principally characterized by differential effects on the binding of orthosteric CB1 ligands depending on whether they are agonists or antagonists, and non-competitive antagonism of CB1 signalling.
In hCB1 yeast assays, PSNCBAM-1 behaved as an antagonist of agonist-induced responses. Such effects were also seen in both [35S]GTPγS binding and cAMP assays. Paradoxically, in competition binding experiments, PSNCBAM-1 caused a dose-dependent increase in [3H]CP55,940 binding to CB1 membranes, indicating positive modulation of agonist binding. Consequently the antagonist effects of PSNCBAM-1 cannot be attributed to competitive displacement of agonist and are likely to arise through the formation of a ternary complex that disrupts function. This non-competitive mode of action was confirmed by [35S]GTPγS binding and Schild analysis. In contrast, PSNCBAM-1 produced a significant, but incomplete, decrease in binding of the inverse agonist, [3H]SR141716A, indicating a moderate negative effect on antagonist binding. A possible explanation for the opposing effects on agonist and antagonist binding could be that the allosteric site is in close proximity to the orthosteric site and acts cooperatively with the agonist CP55,940 but is overlapped and occupied upon binding of the antagonist SR141716A. It is also possible that SR141716A has both a high- and a low-affinity binding site and it is at the latter site that PSNCBAM-1 competes, resulting in the observed partial incomplete inhibition. Thus even though CP55,940 and SR141716A are both orthosteric ligands, PSNCBAM-1 appears to modulate their binding in different ways.
Allosteric enhancement of both agonist binding and function has previously been described for a number of GPCRs including adenosine A3 receptors (Gao et al., 2002) and GABAB (γ-aminobutyric acid B) receptors (Urwyler et al., 2001), but small molecules that allosterically enhance agonist binding while reducing efficacy are less widely reported. The recent description by Price et al. (2005) of molecules acting allosterically at CB1 has noted very similar opposing effects on agonist binding and receptor function to those observed here with PSNCBAM-1. Allosteric modulation by the Organon (Org) compounds described in their paper was unambiguously demonstrated by dissociation kinetics using [3H]CP55,940. Although such studies have not been conducted with PSNCBAM-1, it is expected that a similar effect will be noted since the Org and Prosidion (PSN) compounds share some common structural features which suggest that they would target the same binding.
The cAMP and [35S]GTPγS functional assays also show that the non-competitive antagonism displayed by PSNCBAM-1 is not restricted to stimulation by the synthetic agonist CP55,940 as stimulation by the endogenous agonist AEA is also inhibited. Antagonism of the AEA-induced responses in these assays also demonstrates the potential of PSNCBAM-1 to elicit CB1 antagonist-like effects in vivo.
A number of CB1 antagonists, such as SR141716A, have been reported to produce inverse agonist effects on CB1 signalling in a variety of in vitro bioassays including [35S]GTPγS binding to CHO-hCB1 membranes (Bouaboula et al., 1997) and we have shown such effects of SR141716A in both a constitutively active CB1 yeast assay and [35S]GTPγS binding. In the constitutive yeast assay, PSNCBAM-1 displayed no intrinsic negative effects on the constitutive activity of CB1 and therefore does not appear to behave as an inverse agonist. However, this observation was not supported in the [35S]GTPγS assay where PSNCBAM-1 caused a partial decrease in basal activity equivalent to approximately 50% of the effect seen with SR141716A. One possible explanation for this discrepancy could be the presence of a low level of endocannabinoid in the membrane preparation contributing to the basal activity that PSNCBAM-1 is able to antagonize, hence the partial effect. Whether PSNCBAM-1 acts functionally as a pure antagonist or may have some inverse agonist properties remains unresolved at present. Inverse agonism by allosteric ligands has been observed in several family C GPCRs such as mGluR receptors (Kew, 2004), GABAB receptors (Urwyler et al., 2001) and also some receptors from family A (of which cannabinoid receptors are a member), such as M2 muscarinic receptors (Hilf and Jakobs, 1992; Zahn et al., 2002).
The effects of PSNCBAM-1 are highly selective for CB1 over CB2 as shown by a lack of reversal of CP55,940-induced [35S]GTPγS binding in HEK293-CB2 membranes. The lack of effects at CB2 and several other GPCRs (data not shown) suggest that the mechanism by which PSNCBAM-1 inhibits receptor signalling is likely to be CB1 receptor specific rather than through nonspecific interactions with G-proteins or other signalling molecules. This high level of subtype selectivity also supports the theory that allosteric compounds have greater potential for selectivity (Jensen and Spalding, 2004). As CB1 receptors are highly expressed in the central nervous system (CNS), a high degree of specificity is desired to avoid CNS-related side effects. The use of allosteric inhibitors of CB1 receptors could therefore allow greater receptor selectivity to be achieved.
Results of acute food-intake experiments in male SD rats displayed the hypophagic properties of PSNCBAM-1 when administered i.p. The hypophagic effects of the CB1 inverse agonist SR141716A have previously been demonstrated in a range of animal species including non-obese Wistar rats (Colombo et al., 1998) and lean and obese (fa/fa) Zucker rats (Vickers et al., 2003), but we are not aware of any descriptions of decreased food consumption induced by allosteric inhibitors of CB1. Hence, data presented herein provides the first in vivo indication that allosteric antagonism of CB1 might induce similar effects to the well-characterized anti-obesity agent SR141716A. Further studies comparing the effects of PSNCBAM-1 in CB1+/+ and CB1−/− animals may be required to fully elucidate whether its hypophagic effects are mediated via CB1.
The exact mechanism by which GPCR allosterism occurs is not yet fully understood. Nonetheless, it is generally accepted that binding of allosteric modulators results in conformational changes to the receptor that in turn influence function (Soudijn et al., 2004). In the case of PSNCBAM-1 it appears that such conformational change induced by the allosteric compound results in an increase in agonist binding to the receptor, yet signalling is inhibited. In most cases of allosterism it can be useful to apply a model of interaction between ligands and receptor. A version of the ternary complex model is often applied to the activity of such compounds to obtain a larger picture of allosteric interactions (Christopoulos and Kenakin, 2002). In this model ligand A binds to the orthosteric site, whereas ligand B, the allosteric modulator, binds to the allosteric site. A cooperativity factor-α is applied to the effect of B on A. Where α>1, there is positive cooperativity, and where α <1, there is negative cooperativity. It appears likely that PSNCBAM-1 would possess a positive effect (α>1) on the agonist, CP55,940, but a negative effect (α <1) on the inverse agonist, SR141716A. However, even if α-values can be used to quantify the effects of allosteric compounds on orthosteric ligands, it still remains difficult to relate these interactions to their outcome on receptor signalling.
In conclusion, this report presents evidence for a novel CB1 receptor-specific allosteric antagonist in PSNCBAM-1. This molecule is also shown to produce acute hypophagia and weight loss in male SD rats, and therefore may represent an alternative approach to the current strategies for the treatment of obesity, based on inverse agonists for the CB1 receptor.
Acknowledgments
We thank the following for their contributions to this work: J Bloxham, JG Mc Cormack, M Procter and PS Widdowson (Prosidion Limited). We also thank Sharon Cheetham and colleagues at RenaSci Consultancy Limited, Nottingham, UK, who performed the in vivo experiments on a fee for service basis. We also acknowledge Professor RG Pertwee and Dr RA Ross, University of Aberdeen, Institute of Medical Sciences, Forresterhill, Aberdeen, AB25 2ZD, Scotland, UK, for helpful discussion.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- AEA
arachidonoyl ethanolamide
- AM630
(6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl)(4-methoxyphenyl) methanone
- CP55
950 (−)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl) cyclohexanol
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- hCB1
human cannabinoid receptor 1
- hCB2
human cannabinoid receptor 2
- HEK
human embryonic kidney
- IBMX
3-isobytyl-1-methylxanthine
- PSNCBAM-1
1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea
- SR141716A
N-(piperidin-1-yl)-5-(4-cholrophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- WIN55,212-2
R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]-pyrrolo[1,2,3,-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate
Conflict of interest
The authors are employees of Prosidion Ltd.
References
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- Birdsall NJ, Lazareno S, Matsui H. Allosteric regulation of muscarinic receptors. Prog Brain Res. 1996;109:147–151. doi: 10.1016/s0079-6123(08)62096-8. [DOI] [PubMed] [Google Scholar]
- Bloxham J, Fyfe MCT, Horswill J, Jeevaratnam RP, Keily J, Procter MJ, et al. Arylurea derivatives International Patent Application Publication 2006. WO 2006/018662
- Bouaboula M, Perrachon S, Milligan l, Canat X, Rinaldi-Carmona M, Portier M, et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interaction. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Campbell RM, Cartwright C, Chen W, Chen Y, Duzic E, Fu JM, et al. Selective A1-adenosine receptor antagonists identified using yeast Saccharomyces cerevisiae functional assays. Bioorg Med Chem Lett. 1999;9:2413–2418. doi: 10.1016/s0960-894x(99)00398-4. [DOI] [PubMed] [Google Scholar]
- Casellas P, Congy C, Martinez S, Rinaldi M, Anne-Archard G.Substituted N-piperidino 3-pyrazolecarboxamide European patent 1997. EP 0656354
- Cheng YC, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR141716. Life Sci. 1998;63:113–117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;36:605–613. [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Dis. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Ellis J, Huyler J, Brann MR. Allosteric regulation of cloned m1–m5 muscarinic receptor subtypes. Biochem Pharmacol. 1991;42:1927–1932. doi: 10.1016/0006-2952(91)90591-r. [DOI] [PubMed] [Google Scholar]
- Fride E, Foox A, Rosenberg E, Faigenboim M, Cohen V, Barda L, et al. Milk intake and survival in newborn cannabinoid CB1 receptor knockout mice: evidence for a ‘CB3' receptor. Eur J Pharmacol. 2003;7:27–34. doi: 10.1016/s0014-2999(03)01295-0. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Kim SG, Soltysiak KA, Melman N, Ijzerman AP, Jacobson KA. Selective allosteric enhancement of agonist binding and function at human A3 adenosine receptors by a series of imidazoquinoline derivatives. Mol Pharmacol. 2002;62:81–89. doi: 10.1124/mol.62.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf G, Jakobs KH. Activation of solubilized G-proteins by muscarinic acetylcholine receptors. Cell Signal. 1992;4:787–794. doi: 10.1016/0898-6568(92)90059-h. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Coldwell MC, Armstrong D, Strange PG. Regulation of human D(1), D(2(long)), D(2(short)), D(3) and D(4) dopamine receptors by amiloride and amiloride analogues. Br J Pharmacol. 2000;130:1045–1059. doi: 10.1038/sj.bjp.0703370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Spalding TA. Allosteric modulation of g-protein coupled receptors. Eur J Pharm Sci. 2004;21:407–420. doi: 10.1016/j.ejps.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kew JN. Positive and negative allosteric modulation of metabotropic glutamate receptors: emerging therapeutic potential. Pharmacol Ther. 2004;104:233–244. doi: 10.1016/j.pharmthera.2004.08.010. [DOI] [PubMed] [Google Scholar]
- King K, Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ. Control of yeast mating signal transduction by a mammalian beta 2-adrenergic receptor and Gs alpha subunit. Science. 1990;250:121–123. doi: 10.1126/science.2171146. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;22:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- May LT, Christopoulos A. Allosteric modulators of G-protein-coupled receptors. Curr Opin Pharmacol. 2003;3:551–556. doi: 10.1016/s1471-4892(03)00107-3. [DOI] [PubMed] [Google Scholar]
- Miret JJ, Rakhilina l, Silverman L, Oehlen B. Functional expression of heteromeric calcitonin gene-related peptide and adrenomedullin receptors in yeast. J Biol Chem. 2002;277:6881–6887. doi: 10.1074/jbc.M107384200. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of Rimonabamt, a cannabinoid-1 receptor blocker, on weight and cardiometric risk factors in overweight or obese patients. RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- Soudijn W, Van Wijngaarden J, Ijzerman AP. Allosteric modulation of G protein-coupled receptors: perspectives and recent developments. Drug Discov Today. 2004;9:752–758. doi: 10.1016/S1359-6446(04)03220-9. [DOI] [PubMed] [Google Scholar]
- Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, et al. Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(b) receptors by 2, 6-di-tert-butyl-4-(3-hydroxy-2, 2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol. 2001;60:963–971. [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology. 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Zahn K, Ekstein N, Trankle C, Sadee W, Mohr K. Allosteric modulation of muscarinic receptor signaling: alcuronium-induced conversion of pilocarpine from an agonist into an antagonist. J Pharmacol Exp Ther. 2002;301:720–728. doi: 10.1124/jpet.301.2.720. [DOI] [PubMed] [Google Scholar]