Abstract
Background and purpose:
Calcitonin gene-related peptide (CGRP) is a sensory neurotransmitter in the rat mesenteric arterial bed. Certain cannabinoids can inhibit, via CB1 receptors, vasorelaxant responses to electrical field stimulation (EFS) of sensory nerves in the rat mesentery, but the mechanism of the inhibitory effect of the cannabinoid Δ9-tetrahydrocannabinol (THC) is unclear. This study assessed directly the effect of THC on EFS-induced release of CGRP from sensory nerves in the rat mesenteric bed and investigated the possible involvement of cannabinoid receptors and transient receptor potential (TRP) ion channels.
Experimental approach:
Rat mesenteric beds were perfused with physiological salt solution. Sensory nerves were stimulated electrically and perfusate levels of CGRP measured by immunoassay. The effects of THC on EFS-induced CGRP release and vasorelaxant responses to sensory nerve stimulation were investigated in the absence and presence of cannabinoid antagonists and TRP channel blockers.
Key results:
EFS evoked a release of CGRP and vasodilatation of the mesenteric beds. THC inhibited the electrically-evoked release of CGRP and sensory neurogenic vasorelaxation. The effect of THC was unaffected by the CB1 antagonist AM251, the CB2 antagonist AM630 or the TRPV1 receptor antagonist capsazepine, but was blocked by the TRP channel blocker ruthenium red.
Conclusions and implications:
THC inhibits the EFS-induced release of CGRP (and subsequent vasorelaxation), from capsaicin-sensitive sensory nerves in the rat perfused mesentery. The effect of THC was not mediated by CB1, CB2 or TRPV1 receptors, but was sensitive to ruthenium red, suggesting a possible involvement of TRP ion channels.
Keywords: cannabinoids, sensory nerves, calcitonin gene-related peptide, perfused mesentery
Introduction
Capsaicin-sensitive sensory nerves are found throughout the cardiovascular system and have important roles in conveying information, such as disturbances to homeostasis, to the central nervous system (afferent function) and eliciting motor effects through the release of sensory neurotransmitters in the periphery (efferent function) (Maggi and Meli, 1988; Holzer, 1992). Calcitonin gene-related peptide (CGRP) is an important neurotransmitter in capsaicin-sensitive sensory nerves in the rat mesenteric arterial bed. Electrical field stimulation (EFS), to mimic the action potential, triggers a release of CGRP from capsaicin-sensitive sensory nerves leading to vasodilatation, an effect which can be blocked by the CGRP receptor antagonist CGRP(8-37) (Kawasaki et al., 1988). Cannabinoids have been shown to have diverse effects in the cardiovascular system (Randall et al., 2004), including modulation of sensory and autonomic nerve function (Ralevic, 2003). Certain cannabinoids (WIN55,212 and CP55,940) have been shown to inhibit EFS-induced capsaicin-sensitive sensory neurogenic relaxation in the rat isolated mesenteric arterial bed, and this effect could be reversed by the type-1 cannabinoid receptor (CB1) antagonists SR141716A and LY323501 (Duncan et al., 2004). Since the cannabinoids had no effect on relaxations to exogenously applied CGRP, their effects are likely to be prejunctional, via CB1 receptors on the peripheral ends of the sensory nerves (Duncan et al., 2004).
Not all cannabinoid-mediated responses in the cardiovascular system are mediated through the cannabinoid receptors CB1 and type-2 cannabinoid receptor (CB2). The archetypal cannabinoid, anandamide, was seminally shown to act at vanilloid transient receptor potential (TRP) channels, type 1 (TRPV1), to cause CGRP release and vasorelaxation (Zygmunt et al., 1999). In the rat isolated mesenteric arterial bed, Δ9-tetrahydrocannabinol (THC) and HU210, non-selective CB receptor agonists, inhibited the capsaicin-sensitive sensory neurogenic vasorelaxation induced by EFS, an effect which was likely to be through a prejunctional action, as THC and HU210 did not block relaxations to exogenously applied CGRP (Ralevic and Kendall, 2001; Duncan et al., 2003). However, the mechanism involved is unclear since CB1 and CB2 receptor antagonists failed to block the effects of THC and HU210 (Ralevic and Kendall, 2001; Duncan et al., 2003). It has been reported that THC acts independently of CB1 and CB2 receptors to evoke a release of CGRP from capsaicin-sensitive sensory nerves in the rat isolated mesenteric and hepatic arteries, resulting in a potent vasodilatation; relaxations to THC could be blocked by capsaicin, the CGRP antagonist CGRP(8-37), and ruthenium red (TRP ion channel blocker), but not by capsazepine (TRPV1 receptor antagonist), while CGRP release induced by THC in rat hepatic arteries was also blocked by ruthenium red (Zygmunt et al., 2002). Moreover, THC was still able to cause relaxation in mouse mesenteric arteries from TRPV1 knockout mice (Zygmunt et al., 2002). Furthermore, there is evidence that THC may activate the TRPA1 receptor channel, present in a subpopulation of capsaicin-sensitive sensory nerves (Jordt et al., 2004). On the other hand, it has been reported that capsaicin and ruthenium red have no effect on relaxation to THC in the rat isolated mesenteric arteries (O'Sullivan et al., 2005). The possible role of TRP channels in THC actions in the rat whole mesenteric arterial bed is especially unclear, since THC has been reported to cause vasoconstriction in this vascular bed in vitro (Wagner et al., 1999) and in vivo (O'Sullivan et al., 2007) or vasoconstriction followed by vasorelaxation in vitro (Duncan et al., 2004).
In the present study, we investigated for the first time, the direct prejunctional effect of THC on EFS-induced release of CGRP from capsaicin-sensitive sensory nerves in the rat isolated mesenteric arterial bed, by assaying perfusate levels of CGRP. The possible involvement of cannabinoid receptors and TRP channels in the mechanism(s) of action of THC was investigated using cannabinoid receptor antagonists and TRP channel blockers. Direct effects of THC alone on perfusate CGRP levels and tone of the mesenteric arterial beds, and the possible role of TRP channels and CB receptors, were also investigated. A preliminary report of our results on the effects of ruthenium red on THC- and HU210-evoked inhibition of sensory neurogenic vasodilatation (but not CGRP release) in the rat mesenteric arterial bed has been published in abstract form (Duncan et al., 2003).
Methods
All experiments were conducted in accordance with Home Office animal care regulations. Male Wistar rats (225–250 g; Charles River, Margate, Kent, UK) were killed by lethal exposure to CO2 in a rising concentration and subsequent cervical dislocation. For each experiment, the superior mesenteric artery was located and cannulated with a blunted stainless steel hypodermic needle, which also served as a contact for the positive electrode during EFS. Once secure, the superior mesenteric vein was cut and the arterial bed flushed with Krebs solution to remove residual blood and prevent formation of blood clots. The whole mesenteric arterial bed was isolated from the gut and immediately transferred to a humid chamber; the preparation was mounted on a stainless steel grid (70 × 50 mm) which, when connected to the negative electrode, completed the circuit for EFS. The preparation was perfused at a constant rate of 5 ml min−1 with Krebs solution (133 mM NaCl, 4.7 mM KCl, 1.35 mM NaH2PO4, 16.3 mM NaHCO3, 0.61 mM MgSO4, 2.52 mM CaCl2 and 7.8 mM glucose, gassed with 95% O2, 5% CO2) and kept at a constant temperature of 37 °C. Flow was maintained by a peristaltic pump (Model 7554-30; Cole Palmer Instruments Co., Illinois, USA) and temperature by a circulating water heater (Grant Instruments, Cambridge, UK).
Sympathetic neurotransmission was blocked by the addition of 5 μM guanethidine to the Krebs' solution (Kawasaki et al., 1988) and preparations allowed to stabilize for 30 min before adding methoxamine (α1-adrenoceptor agonist; 10–20 μM) to preconstrict the preparations by 30–50 mm Hg above baseline. EFS was applied via a Grass stimulator (S9D) at 8 Hz, 0.1 ms, 60 V and for 30 s (sub-maximal frequency; Duncan et al., 2003). Contractile and vasorelaxant responses were measured as changes in the perfusion pressure (mm Hg) with a pressure transducer mounted on a side arm adjacent to the cannula, and recorded using an A to D converter (Powerlab/400; ADInstruments, Sydney, Australia).
CGRP was isolated from the perfusate using solid phase extraction (Phenomenex, Macclesfield, UK), and the CGRP isolated was analyzed by CGRP enzyme immunoassay, with all reagents and methods, according to the manufacturer's instructions (SPIBio, Montigny, France).
Experimental protocol
Three consecutive EFSs, EFS1, EFS2 and EFS3 (each at 8 Hz, 0.1 ms, 60 V and for 30 s), were carried out on each preparation. In control experiments, EFS2 and EFS3 were found to be reproducible, both in terms of the amplitude of the vasorelaxant response and the evoked CGRP release. Thus, in subsequent experiments, EFS2 was used as the control response, after which THC was added to the perfusate and, after 30 min, EFS3 was applied. In some experiments, the following agents were used: CB1 receptor antagonist AM251 (100 nM) (Ki 8.1 nM; Lan et al., 1999); CB2 receptor antagonist AM630 (1 μM) (Ki 31.2 nM; Ross et al., 1999); TRP channel inhibitor ruthenium red (1 μM) (IC50 0.1–9 μM; Alexander et al., 2006; Bianchi et al., 2006); TRPV1 antagonist capsazepine (3 μM) (IC50 0.13 μM; Bianchi et al., 2006). These compounds were added to the perfusate at the beginning of the experiment, prior to preconstriction with methoxamine; none of these were found to have an effect on the response to methoxamine (data not shown). Perfusate was collected from each preparation prior to, during and after EFS and assayed for CGRP.
In another series of experiments, the effects of THC (0.1–10 μM) on vascular tone were investigated in methoxamine-preconstricted preparations by adding single concentrations of THC to the perfusate after the preparations had stabilized (approximately 20 min after the addition of methoxamine). In these experiments, fractions of perfusate were collected before THC addition, and at fixed time intervals following THC addition and the CGRP levels assayed. EFS (8 Hz, 0.1 ms, 60 V, for 30 s) was applied at the end of each experiment. The effect of 1 μM THC on tone and perfusate CGRP levels was additionally investigated in the presence of ruthenium red (1 μM) and capsazepine (3 μM), which were added to the perfusate before preconstriction with methoxamine. The direct effects of THC (1 μM) on mesenteric vascular tone were also investigated after capsaicin pre-treatment (1 μM for 30 min before methoxamine preconstriction, followed by washout) and in the presence of the CB1 antagonist AM251 (100 nM, added before constriction with methoxamine); neither AM251 nor pre-treatment with capsaicin affected the contractile response to methoxamine.
Data analysis
Vasorelaxant responses of the mesenteric beds and the corresponding CGRP release (EFS3) are expressed as percentages of the control responses (EFS2). Data were compared by one-way analysis of variance with Newman–Keuls multiple comparisons post hoc test or two-way analysis of variance with Bonferroni post hoc test, as appropriate. A value of P<0.05 was taken to indicate a statistically significant difference between means. Basal release of CGRP in untreated and treated preparations was measured directly and reported as concentrations (pg ml−1) in the perfusate. Direct effects of THC on tone at 10 and 60 min are expressed as a percentage of the preconstricted tone 10 min prior to the addition of THC application. Analysis was performed using Prism4 software (GraphPad Software Inc., San Diego, CA, USA).
Drugs
Δ9-Tetrahydrocannabinol, methoxamine chloride, ruthenium red, capsazepine and capsaicin were all from Sigma-Aldrich (Poole, UK). Cannabinoid ligands AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) and AM630 (6-iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-[indol-3-yl](4-methoxyphenyl)methanone) were from Tocris Bioscience (Bristol, UK). Guanethidine monosulphate (Ismelin) was from Amdipharm Basildon (Essex, UK). Cannabinoids were dissolved in ethanol to a stock concentration (10−2 M) and diluted to a working concentration by addition to the perfusate reservoir.
Results
Effect of Δ9-tetrahydrocannabinol on electrical field stimulation-induced sensory neurogenic vasorelaxation and calcitonin gene-related peptide release in the rat mesenteric arterial bed
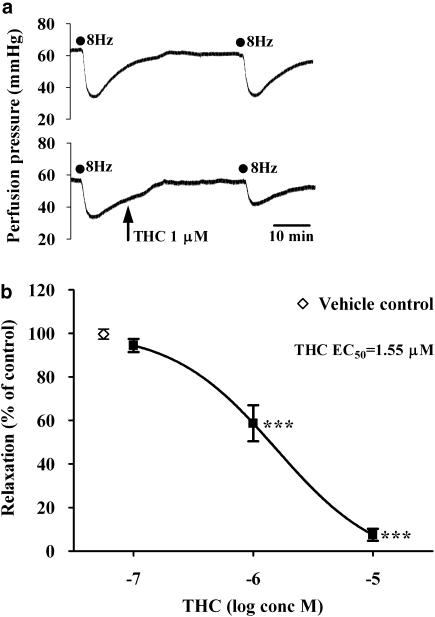
EFS (8 Hz, 0.1 ms, 60 V for 30 s) evoked reproducible vasorelaxations of the mesenteric arterial beds (due to activation of capsaicin-sensitive sensory nerves; Figure 1a). Vasorelaxation to EFS2 (62±4%; n=6) was not significantly different to vasorelaxation to EFS3 (58±5%; n=6; P>0.05). THC (0.1–10 μM) produced a concentration-dependent inhibition of the EFS-induced sensory neurogenic vasorelaxations, with responses being abolished in the presence of 10 μM THC (Figure 1b). A concentration of 1 μM THC was chosen for further investigation in the presence of cannabinoid receptor antagonists and TRP inhibitors.
Figure 1.
(a) Representative traces showing the inhibitory effect of the cannabinoid agonist Δ9-tetrahydrocannabinol (THC) (1 μM) on sensory neurogenic vasorelaxation in a methoxamine preconstricted rat isolated mesenteric arterial bed. Electrical field stimulation (EFS; 8 Hz, 0.1 ms, 60 V, 30 s) was used to evoke sensory neurogenic relaxations, and these were reproducible in the absence of THC (upper trace). (b) Effect of THC (0.1–10 μM) on sensory neurogenic vasorelaxation in the rat isolated mesenteric bed (n=4–9). EFS (8 Hz, 0.1 ms, 60 V, 30 s) was used to evoke sensory neurogenic relaxations. The vehicle control was ethanol at 0.1%. ***P<0.001, compared to neurogenic vasorelaxation in the presence of the vehicle control.
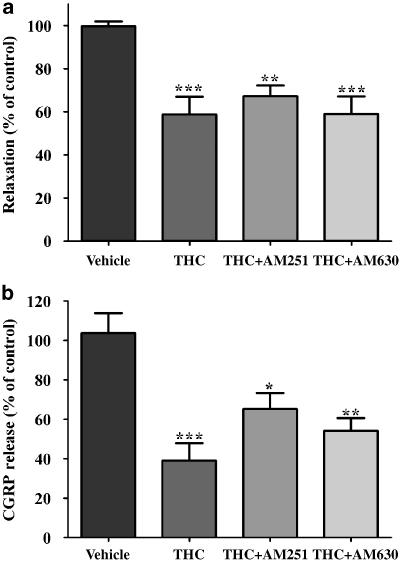
Effect of cannabinoid receptor antagonists on Δ9-tetrahydrocannabinol-induced inhibition of electrical field stimulation-induced sensory neurogenic vasorelaxation and calcitonin gene-related peptide release
THC (1 μM) significantly inhibited EFS-induced vasorelaxation (41±8%; n=7; Figure 1, Figure 2a). The inhibitory effect of THC on vasorelaxation was unaffected by the CB1 and CB2 receptor antagonists AM251 (100 nM) and AM630 (1 μM), respectively (Figure 2a). EFS (8 Hz, 0.1 ms, 60 V for 30 s) evoked a release of CGRP above baseline (5.3±2.9 pg ml−1; n=12). THC (1 μM) inhibited the electrically-evoked release of CGRP by 61±9% (n=9; Figure 2b). THC was still able to cause inhibition of CGRP release in the presence of the CB1 and CB2 receptor antagonists AM251 (100 nM) and AM630 (1 μM), respectively, although there was a trend for its effects to be reduced in the presence of AM251 (Figure 2b). The drug vehicle, ethanol (0.01%), had no significant effect on the vasorelaxant response or CGRP release (Figure 2).
Figure 2.
Effect of THC (1 μM) on electrical field stimulation (EFS)-induced vasorelaxation (a) and calcitonin gene-related peptide (CGRP) release (b) of the rat isolated mesenteric arterial bed in the absence and presence of the CB1 receptor antagonist AM251 (100 nM) and the CB2 receptor antagonist AM630 (1 μM). THC caused a significant inhibition of EFS-induced vasorelaxation in the absence and presence of AM251 and AM630, compared to vehicle controls. THC caused a significant inhibition of CGRP release in the absence and presence of AM251 and AM630, compared to vehicle controls. Relaxation responses are presented as % of control responses evoked in the same tissue. Analyzed by one-way ANOVA and Newman–Keuls multiple comparison; *P<0.05, **P<0.01 and ***P<0.001, significantly different from vehicle controls. Data are presented as means±s.e.mean (n=7–9). ANOVA, analysis of variance; THC, Δ9-tetrahydrocannabinol.
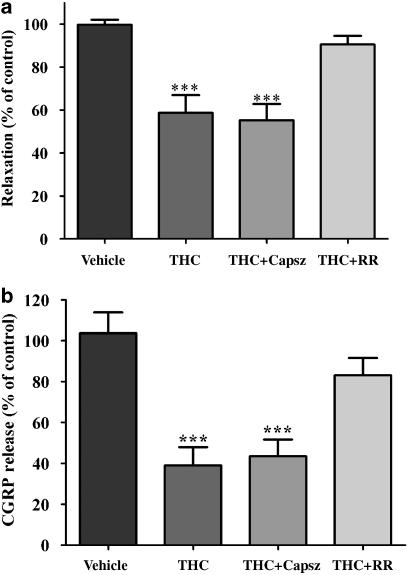
Effect of transient receptor potential inhibitors on Δ9-tetrahydrocannabinol-induced inhibition of electrical field stimulation-induced sensory neurogenic vasorelaxation and calcitonin gene-related peptide release
Ruthenium red (1 μM) blocked the inhibitory effect of THC (1 μM) on the vasorelaxant response to EFS (Figure 3a). Ruthenium red alone had no significant effect on the vasorelaxant response to EFS (n=9). In the presence of capsazepine (3 μM), THC (1 μM) was still able to cause a significant inhibition of vasorelaxant response to EFS (Figure 3a). Ruthenium red (1 μM) blocked the inhibitory effect of THC (1 μM) on the EFS-evoked CGRP release (Figure 3b). Ruthenium red alone had no significant effect on CGRP release (n=9). In the presence of capsazepine (3 μM), THC (1 μM) was still able to cause a significant inhibition of CGRP release (Figure 3b).
Figure 3.
Effect of THC (1 μM) on electrical field stimulation (EFS)-induced vasorelaxation (a) and calcitonin gene-related peptide (CGRP) release (b) of the rat isolated mesenteric arterial bed in the absence and presence of the vanilloid receptor antagonist, ruthenium red (RR, 1 μM) and the TRPV1 antagonist capsazepine (Capsz; 3 μM). THC caused a significant inhibition of EFS-induced vasorelaxation in the absence and presence of capsazepine, but had no significant effect on vasorelaxation in the presence of RR, compared to vehicle controls. THC caused a significant inhibition of EFS-induced CGRP release in the absence and presence of capsazepine, but had no significant effect on release in the presence of ruthenium red. Relaxation responses are presented as % of control responses evoked in the same tissue. Analyzed by one-way ANOVA and Newman–Keuls multiple comparison; ***P<0.001. Data are presented as means±s.e.mean (n=7–9). ANOVA, analysis of variance; THC, Δ9-tetrahydrocannabinol; TRPV1, vanilloid transient receptor potential type 1.
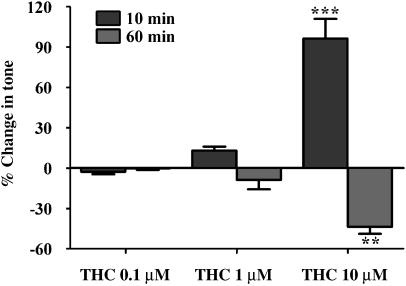
Effect of Δ9-tetrahydrocannabinol on tone and on calcitonin gene-related peptide levels in perfusate from rat mesenteric arterial beds
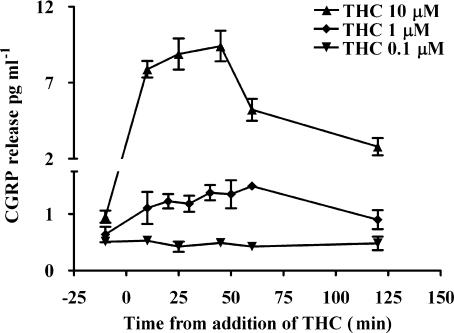
THC (0.1–10 μM) evoked a concentration-dependent biphasic effect on tone of the mesenteric arterial beds (Figure 4). THC at 1 and 10 μM evoked an initial slow contraction, 8±2 (n=10) and 34±5 mm Hg (n=5), respectively, which peaked at approximately 10 min. This was followed by a slow vasorelaxation, 3±2 and 16±2 mm Hg below methoxamine-constricted baseline at 1 and 10 μM THC, respectively; these responses are shown as a percentage of tone in Figure 4. The CB1 antagonist, AM251 (100 nM), had no significant effect on the contraction evoked by THC (1 μM; 6±1.5 mm Hg, n=4). THC (0.1–10 μM) evoked a concentration dependent increase in CGRP levels in perfusate collected from the mesenteric arterial beds (Figure 5). The vehicle used at the highest concentration of THC (0.1% ethanol) had no significant effect on tone or CGRP levels. A concentration of 1 μM THC was chosen for further investigation in the presence of cannabinoid receptor antagonists and TRP inhibitors.
Figure 4.
Effect of THC (0.1–10 μM) on tone of the rat isolated perfused mesenteric arterial beds. THC evoked a biphasic response, initial slow contraction followed by a prolonged relaxation, and these responses were measured at 10 and 60 min (n=4). Data are presented as means±s.e.mean. Analyzed by one-way ANOVA and Newman–Keuls multiple comparison; **P<0.01 and ***P<0.001, compared to THC at both 0.1 and 1 μM. ANOVA, analysis of variance; THC, Δ9-tetrahydrocannabinol.
Figure 5.
Effect of THC (0.1–10 μM) on perfusate calcitonin gene-related peptide (CGRP) levels of the perfusate collected from the rat isolated perfused mesenteric arterial beds (n=4). Data are presented as means±s.e.mean.
Effect of transient receptor potential channel inhibitors on Δ9-tetrahydrocannabinol-induced effects on tone and perfusate calcitonin gene-related peptide levels
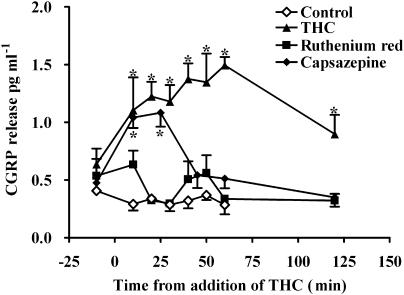
THC (1 μM) caused a significant increase in the basal release of CGRP that was maintained for 60 min (Figure 6). This was blocked by ruthenium red (1 μM; Figure 6). Capsazepine (3 μM) had no significant effect on the early release of CGRP but blocked the sustained release (after 25 min; Figure 3a). Pretreatment with capsaicin caused a large increase in CGRP release that did not return to normal levels even after 120 min, and under these circumstances, no effect of THC could be observed.
Figure 6.
Effect of THC (1 μM) on calcitonin gene-related peptide (CGRP) release from the rat isolated mesenteric arterial bed in the absence and presence of the vanilloid receptor antagonist, ruthenium red (RR, 1 μM) and the TRPV1 antagonist capsazepine (3 μM). THC (n=6) evoked significant release of CGRP (*P<0.001) into the perfusate, but this was blocked in the presence of RR (n=5). Capsazepine partially blocked the effect of THC (n=5). The vehicle control (0.1% ethanol) had no effect on CGRP levels. Analyzed by two-way ANOVA and Bonferroni post test, data are presented as means±s.e.mean. ANOVA, analysis of variance; TRPV1, vanilloid transient receptor potential type 1.
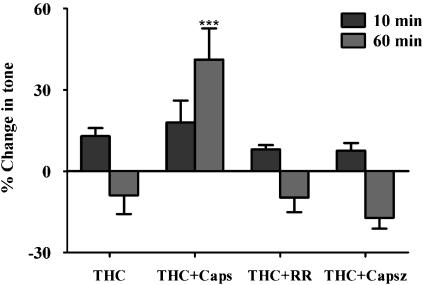
The contraction to THC (1 μM) was unaffected by ruthenium red (1 μM) or capsazepine (3 μM; Figure 7). Capsaicin (1 μM) pretreatment also had no significant effect on the contraction at 10 min, but significantly blocked the relaxation resulting in a contraction at 60 min (Figure 7). In control experiments, where no agents were added to the methoxamine preconstricted mesenteric arterial preparations, there was no significant change in tone up to 60 min after capsaicin pretreatment (results not shown).
Figure 7.
Effect of THC (1 μM) on tone of the rat isolated perfused mesenteric arterial beds in the absence (n=9) and presence of the vanilloid receptor antagonist, ruthenium red (RR, 1 μM) (n=7) and the TRPV1 antagonist capsazepine (Capsz; 3 μM) (n=6), and after pre-treatment with capsaicin (1 μM for 30 min) (n=8). THC evoked a biphasic response, initial slow contraction, measured at 10 min, followed by a prolonged relaxation, measured at 60 min. ***P<0.001, significance compared to THC, analyzed by one-way ANOVA and Newman–Keuls multiple comparison. Data are presented as means±s.e.mean. ANOVA, analysis of variance; TRPV1, vanilloid transient receptor potential type 1.
Discussion
The present findings show clearly, by direct measurement, that THC inhibits the EFS-induced release of CGRP from perivascular capsaicin-sensitive sensory nerves in the rat mesenteric arterial bed, which is likely to account for the attenuation of sensory neurogenic vasorelaxation observed in the presence of THC. This confirms and extends our previous conclusions of a prejunctional inhibitory action of THC on capsaicin-sensitive sensory neurotransmission, based on indirect evidence showing that THC inhibits sensory neurogenic vasorelaxation, but has no effect on vasorelaxation to exogenously applied CGRP, in the rat mesenteric arterial bed (Duncan et al., 2004).
The effects of THC on both EFS-induced CGRP release and sensory neurogenic vasorelaxation were not significantly altered by the CB1 and CB2 antagonists AM251 and AM630, respectively (although there was a trend for the effects of THC to be reduced in the presence of the CB1 antagonist), indicating the predominant involvement of a non-CB1/CB2 mechanism. This is supported by our previous results showing that THC-induced inhibition of capsaicin-sensitive sensory neurogenic vasodilatation is unaffected by the CB1 and CB2 receptor antagonists SR141716A and SR144528, respectively (Duncan et al., 2004). It is also in line with evidence for a lack of effect of CB1 and CB2 antagonists on the vasorelaxant effects of THC in the rat isolated small mesenteric arteries (Zygmunt et al., 2002; O'Sullivan et al., 2005). In the report by Duncan et al. (2004), we tentatively suggested that THC was mediating its effects via a novel non-CB1/CB2 cannabinoid receptor. However, the results of the present study make this less likely as they identify the involvement of an alternative mechanism in the prejunctional inhibitory effect of THC.
The effects of THC on both EFS-induced CGRP release and sensory neurogenic vasorelaxation were blocked by ruthenium red, but not by capsazepine. These results are consistent with evidence that THC acts at ruthenium red-sensitive, capsazepine-insensitive, sites on capsaicin-sensitive sensory nerves in rat small mesenteric arteries, causing CGRP release and vasodilatation (Zygmunt et al., 2002). The effect of ruthenium red indicates a possible involvement of TRP ion channels, while the lack of effect of capsazepine indicates a lack of involvement of TRPV1. Thus, the prejunctional actions of THC are in contrast to those of certain other synthetic cannabinoids, namely WIN55,212 and CP55,940, which act at CB1 receptors to inhibit EFS-induced capsaicin-sensitive sensory neurogenic vasorelaxation of the rat mesenteric bed (their effects were reversed by the CB1 receptor antagonists SR141716A and LY323501, but not by the CB2-selective antagonist SR144528) (Duncan et al., 2004). This indicates that there are at least two different mechanisms through which cannabinoids can inhibit, prejunctionally, perivascular capsaicin-sensitive sensory neurotransmission, namely CB1 and TRP ion channels.
Insight into the possible mechanism of action of THC came from investigations of the direct effects of THC on perfusate levels of CGRP. THC evoked a release of CGRP into the perfusate of the mesenteric arterial beds and this was blocked by ruthenium red, further indicating a possible involvement of TRP channels. This suggests that the prejunctional inhibitory effect of THC on capsaicin-sensitive sensory neurotransmission (CGRP release and vasorelaxation) is likely to occur secondary to its excitatory effects on sensory nerves, leading to depletion of CGRP, desensitization of the sensory nerves and/or desensitization of postjunctional CGRP receptors.
A part of the THC-induced CGRP release, that which occurred after approximately 25 min perfusion with THC, was additionally blocked in the presence of capsazepine, indicating a possible involvement of TRPV1. However, THC is inactive at the rat and human TRPV1 receptor (Lam et al., 2005). Moreover, Zygmunt et al. (2002) found that the vasorelaxant effect of THC in mouse mesenteric arteries was still observed in knockout mice lacking the TRPV1 receptor. It is likely, therefore, that the inhibitory effect of capsazepine is due to a block of ion channels other than TRPV1, as suggested by others (Rousseau et al., 2005).
The vasomotor response to THC in the rat mesenteric arterial bed was biphasic, with a concentration-dependent vasocontraction being followed by vasorelaxation. This is consistent with our previous observations of biphasic responses to THC in the rat mesenteric arterial bed (Duncan et al., 2004). In contrast, it has been reported that THC causes vasoconstriction only, without any evidence for vasodilatation, in the rat whole mesenteric arterial bed in vitro (Wagner et al., 1999) and in vivo (O'Sullivan et al., 2007). A reason for the differences between these studies and the present report may be due to the different times of exposure to THC; the present study used a continuous perfusion of a steady-state concentration of THC, while Wagner et al. (1999) used a bolus injection and O'Sullivan et al. (2007) infused THC for 20 min intravenously. In the present study, vasoconstriction to THC was unchanged in the presence of capsaicin, ruthenium red and capsazepine, indicating that vasoconstriction is not functionally antagonised by vasorelaxation resulting from THC-evoked CGRP release. The CB1 receptor antagonist AM251 also had no effect on THC-induced vasoconstriction of the mesenteric arterial bed. Indeed, an involvement of CB1 seems unlikely since the CB1/CB2 agonists CP55,940 and WIN55,212, both at 1 μM, had no effect on tone of the rat isolated mesenteric arterial beds (Duncan et al., 2004), and they have higher affinities for CB1 and CB2 receptors than THC (vasoconstriction observed at 1 μM; Pertwee, 2006). CB1 receptors were also not involved in the small contractions to CP55,940 and anandamide observed in endothelium-denuded rat isolated mesenteric arteries, as these were unaffected by the CB1 antagonist SR141716A (White and Hiley, 1998). In contrast, in the rat isolated superior mesenteric artery, the contractile effect of THC was blocked in the presence of the CB1 receptor antagonist SR141716A and by endothelium removal (O'Sullivan et al., 2005). Similarly, mesenteric vasoconstriction to THC in rats in vivo was abolished in the presence of AM 251 (O'Sullivan et al., 2007). It is unclear why such diverse conclusions have been produced by these studies of vasocontractile effects of cannabinoids as, in some cases, there are only relatively subtle differences in methodology employed; there seems to be an extraordinary sensitivity of cannabinoid pharmacology in this regard.
It is clear that cannabinoids can produce substantially different effects depending on species, blood vessel and even on the size of vessel within a vascular bed (Randall et al., 2004). For example, THC has diverse vasomotor effects in the rat isolated small mesenteric arteries, which vary depending on the size of the vessel studied (Zygmunt et al., 2002; O'Sullivan et al., 2005). THC produced vasoconstriction in the superior mesenteric artery, and vasorelaxation or no effect in smaller vessels (Zygmunt et al., 2002; O'Sullivan et al., 2005). Together with the results of the present study, this indicates that responses of the rat whole mesenteric arterial bed are a composite of responses mediated by both the superior mesenteric artery and smaller mesenteric arteries, as both vasoconstriction and vasorelaxation to THC were observed.
It might be expected that the release of CGRP evoked by THC in the rat mesenteric arterial bed would cause vasorelaxation, as has been shown to accompany THC-evoked CGRP release in the rat small mesenteric arteries (Zygmunt et al., 2002). However, ruthenium red had no effect on vasorelaxation mediated by THC (1 μM), although it abolished THC-evoked CGRP release. This means that there is a dissociation between the effects of THC on CGRP release and vasorelaxation in the rat whole mesenteric arterial bed, that is, THC-evoked CGRP release does not account for the relaxation observed to THC. It is possible that the levels of CGRP released by THC at 1 μM are too low to evoke vasorelaxation. O'Sullivan et al. (2005) have also observed a lack of effect of ruthenium red and capsaicin on THC-induced relaxations in rat small mesenteric arteries (O'Sullivan et al., 2005). In that study, the vasorelaxant actions of THC were attributed to activation of smooth muscle K+ channels and inhibition of Ca2+ channels (O'Sullivan et al., 2005). In the present study, capsaicin pre-treatment reversed the THC-induced vasorelaxation of the mesenteric arterial bed and in its place vasoconstriction was revealed. The mechanism involved is unclear. Functionally antagonistic motor effects of sensory nerves mediated through CGRP do not appear to be involved because ruthenium red had no effect on THC-induced vasocontraction or vasorelaxation, but did block CGRP release. Moreover, non-specific augmentation of contractile responses by capsaicin pre-treatment seems unlikely since contractions to methoxamine were not different with and without capsaicin pre-treatment.
Overall, we have shown that THC, perfused into the lumen, produces time- and concentration-dependent effects in the rat mesenteric arterial bed. THC produces an initial vasoconstriction that, at 1 μM, is independent of CB1 receptors and does not involve sensory nerves. This is followed by a slow vasorelaxation which, at 1 μM, is independent of CGRP release and TRP channels, including TRPV1, although intriguingly it is blocked by capsaicin pre-treatment. It is possible that THC causes vasorelaxation through the release of sensory neurotransmitter through a TRP-independent mechanism, for example, activation of voltage-operated calcium channels (although there is evidence against an involvement of voltage-operated calcium channels in the rat small mesenteric arteries; Zygmunt et al., 2002). However, if this is the case, it is difficult to reconcile with the lack of involvement of CGRP, since this is the principal vasorelaxant sensory neurotransmitter in rat mesenteric arteries. THC is able to permeate the blood vessel wall to reach sensory nerves in the adventitia and cause a release of CGRP through TRP channels distinct from TRPV1, and inhibition of sensory neurogenic vasodilatation. THC appears to be more potent at inhibiting prejunctionally sensory neurotransmission, than at evoking vasomotor effects (vasoconstriction and vasorelaxation), which suggests that, at least at 1 μM and below, its major role may be to inhibit, rather than to evoke, blood vessel relaxation.
In conclusion, we have shown, by direct measurement, that THC inhibits the EFS-induced release of CGRP from capsaicin-sensitive sensory nerves in the rat mesenteric arterial bed. The effect of THC on sensory neurogenic responses (CGRP and vasorelaxation) was inhibited by ruthenium red, but not by capsazepine or CB1/CB2 antagonists, indicating a possible involvement of TRP ion channels, but not TRPV1. The mechanism may involve depletion/desensitization of sensory neurotransmitter, since THC elicited a ruthenium red-sensitive release of CGRP. However, this released CGRP is not involved in vasomotor responses to THC since vasocontraction and vasorelaxation was unaffected by ruthenium red. In summary, we hypothesized that TRP channels were involved in THC responses in the rat isolated perfused mesenteric arterial bed. We are able to accept this hypothesis with regard to an involvement of TRP channels in THC inhibition of EFS-induced capsaicin-sensitive neurotransmission (CGRP release and vasorelaxation).
Acknowledgments
This study was supported by the British Heart Foundation (Project Grant No. PG/03/158).
Abbreviations
- CGRP
calcitonin gene-related peptide
- EFS
electrical field stimulation
- THC
Δ9-tetrahydrocannabinol
- TRP
transient receptor potential
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels. Br J Pharmacol. 2006;147 Suppl 3:S120–S125. doi: 10.1038/sj.bjp.0706651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi BR, Lee C-H, Jarvis MF, Kouhen RE, Morelland RB, Faltynek CR, et al. Modulation of human TRPV1 receptor activity by extracellular protons and host cell expression system. Eur J Pharmacol. 2006;537:20–30. doi: 10.1016/j.ejphar.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Duncan M, Kendall DA, Ralevic V.The effects of ruthenium red on the inhibitory actions of noladin ether, THC and HU210 on sensory neurotransmission in the rat isolated mesenteric arterial bed 2003. 1st European Workshop on Cannabinoid Research
- Duncan M, Kendall DA, Ralevic V. Characterisation of cannabinoid modulation of sensory neurotransmission in the rat isolated mesenteric arterial bed. J Pharmacol Exp Ther. 2004;311:411–419. doi: 10.1124/jpet.104.067587. [DOI] [PubMed] [Google Scholar]
- Holzer P. Peptidergic sensory neurons in the control of vascular functions: mechanisms and significance in the cutaneous and splanchnic vascular beds. Rev Physiol Biochem Pharmacol. 1992;121:49–146. doi: 10.1007/BFb0033194. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Lam PMW, McDonald J, Lambert DG. Characterization and comparison of recombinant human and rat TRPV1 receptors: effects of exo- and endocannabinoids. Br J Anaesthesia. 2005;94:649–656. doi: 10.1093/bja/aei098. [DOI] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Frenando SR, McCallion D, et al. Structure–activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive neurons. Gen Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. The effects of Δ9-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br J Pharmacol. 2005;145:514–526. doi: 10.1038/sj.bjp.0706218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Randall MD, Gardiner SM. The in vitro and in vivo cardiovascular effects of Δ9-tetrahydrocannabinol in rats made hypertensive by chronic inhibition of nitric oxide synthase. J Pharmacol Exp Ther. 2007;321:663–672. doi: 10.1124/jpet.106.116566. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obesity. 2006;30:S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- Ralevic V. Cannabinoid modulation of peripheral autonomic and sensory neurotransmission. Eur J Pharmacol. 2003;472:1–21. doi: 10.1016/s0014-2999(03)01813-2. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA. Cannabinoid inhibition of capsaicin-sensitive sensory neurotransmission in the rat mesenteric arterial bed. Eur J Pharmacol. 2001;418:117–125. doi: 10.1016/s0014-2999(01)00940-2. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O' Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterisation at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E, Cloutier M, Morin C, Proteau S. Capsazepine, a vanilloid antagonist, abolished tonic responses induced by 20-HETE on guinea pig airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288:L460–L470. doi: 10.1152/ajplung.00252.2004. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Járai Z, Kunos G. Mesenteric vasodilatation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- White R, Hiley R. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Andersson DA, Högestätt ED. Δ9-Tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J Neurosci. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Dimarzo V, Julius D, Högestätt ED. Vacilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]