Abstract
Recent physiological, pharmacological and anatomical studies provide evidence that one of the main roles of the endocannabinoid system in the brain is the regulation of γ-aminobutyric acid (GABA) and glutamate release. This article aims to review this evidence in the context of its implications for pain. We first provide a brief overview of supraspinal regulation of nociception, followed by a review of the evidence that the brain's endocannabinoid system modulates nociception. We look in detail at regulation of supraspinal GABAergic and glutamatergic neurons by the endocannabinoid system and by exogenously administered cannabinoids. Finally, we review the evidence that cannabinoid-mediated modulation of pain involves modulation of GABAergic and glutamatergic neurotransmission in key brain regions.
Keywords: pain, brain, cannabinoids, GABA, glutamate, neurotransmission, nociception
Introduction
Pain is a complex sensory and psychological experience, and although many of the critical loci involved in pain have been identified, the precise mechanisms underlying the perception and modulation of pain are poorly understood. Acute pain is a protective facility, warning the organism of possible or actual damage. Peripheral noxious stimuli trigger a cascade of physiological events, which propagate to the brain and are integrated and processed by limbic and cortical structures to coordinate the appropriate behavioural response.
Chronic pain is more complicated and is a major health problem. Forty-eight million Americans experience chronic pain-related health problems with the cost of treatment estimated at $100 billion a year (Holden and Pizzi, 2003). Approximately four billion workdays are lost annually at a cost of $65 billion in lost productivity due to chronic pain (Gentry, 1999). In Europe, one in five people suffer from chronic pain of moderate-to-severe intensity (Holden and Pizzi, 2003; Breivik et al., 2006).
Cannabis has been used for pain relief for centuries. With the discovery and isolation of its main psychoactive constituent, Δ9-tetrahydrocannabinol (Mechoulam and Gaoni, 1967), and receptor targets, a better understanding of the antinociceptive properties of this drug and related cannabinoid compounds has been possible. However, the precise mechanisms underlying the modulation of pain by cannabinoids are as yet unclear. Extensive experimental and clinical evidence suggests a presynaptic location of cannabinoid receptors on GABAergic (GABA: γ-aminobutyric acid) and glutamatergic neurons in brain areas associated with pain modulation. Moreover, a large body of evidence implicates supraspinal GABA and glutamate in the regulation of pain, and functional studies have demonstrated that the release of these amino-acid neurotransmitters is controlled by the brain's endogenous (endo) cannabinoid system. This review examines the role of the brain's endocannabinoid system in modulation of pain with an emphasis on the regulation of GABA and glutamate in animal models of acute, inflammatory and neuropathic pain.
The pain pathways
The manifestation of pain, and its modulation, is mediated by ascending and descending pathways. Neurons in the ascending pain pathways receive input from peripheral primary afferent fibres and project from the dorsal horn of the spinal cord to a number of supraspinal sites. The two major ascending pain pathways in mammals are the spinothalamic and the spinoparabrachial tracts, which encode the sensory-discriminatory and affective aspects of pain respectively (for extensive reviews see Millan, 1999, 2002). The thalamus and parabrachial nucleus receive information from projection neurons in various laminae of the dorsal horn, and then relay this sensory information to cortical and amygdalar regions where the information is decoded as a ‘painful stimulus'. The descending pathways, in turn, modulate neuronal activity in ascending pathways, and can exert an inhibitory or facilitatory effect on the sensation of pain. Interestingly, the anatomical regions involved in facilitation and inhibition of nociception often overlap. Differences in the mechanisms underlying facilitation and inhibition of nociception lie primarily in the receptor subtypes coupled to differing intracellular mechanisms (Millan, 1999, 2002). Neurons of the descending inhibitory pain pathway originate in the amygdala and hypothalamus and project to the lower brainstem (including the A5, A6/A7 noradrenergic neurons) and spinal cord, via the periaqueductal gray (PAG) and rostral ventromedial medulla (RVM) (see below). There is an accumulating body of neurochemical, pharmacological, electrophysiological and behavioural evidence for the role of GABA receptors (GABAA and GABAB), and ionotropic (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid, N-methyl-D-aspartate and kainate) and metabotropic glutamate (mGlu1–8) receptors in modulating supraspinal pain pathways (for recent reviews see Bleakman et al., 2006; Enna and McCarson, 2006; Neto et al., 2006). Indeed, GABAergic and glutamatergic neurons at most, if not all, supraspinal components of the descending pain pathways mediate facilitatory and/or inhibitory effects on pain perception.
The endocannabinoid system
The endocannabinoid system is comprised of the cannabinoid1 (CB1) receptor, cannabinoid2 (CB2) receptor, endogenous cannabinoid ligands, their metabolizing enzymes and a putative anandamide uptake site (Figure 1). CB1 receptors are expressed presynaptically on neurons in both the peripheral and central nervous systems as well as on a wide range of peripheral tissues. CB2 receptors are expressed largely in non-neural tissues including immune cells, but now there is accumulating evidence that CB2 receptor protein and mRNA is also expressed in the brain (Van Sickle et al., 2005; Gong et al., 2006; Onaivi et al., 2006) and spinal cord (Zhang et al., 2003; Wotherspoon et al., 2005; Beltramo et al., 2006). Splice variants of the CB1 receptor have also been identified (Shire et al., 1995; Ryberg et al., 2005) and evidence suggests there may be additional, as yet undiscovered, cannabinoid receptor subtypes (Breivogel et al., 2001; Fride, 2002; Wenger et al., 2003; see review by Brown this issue). Within the central nervous system, the CB1 receptor is found in high density and its distribution is heterogenous. Both CB1 (Matsuda et al., 1990) and CB2 receptors (Munro et al., 1993) are Gi/o protein-coupled receptors that are negatively coupled to adenylyl cyclase (Howlett et al., 1999) and positively coupled to mitogen-activated protein kinase (Bouaboula et al., 1995). In addition, CB1 receptors are coupled to ion channels through Gi/o proteins, positively for A-type and inwardly rectifying potassium channels and negatively for N-type and P/Q-type calcium channels and D-type potassium channels (Pertwee, 1997, 1999; Mu et al., 1999). In this respect, CB1 receptor activation can affect the release of neurotransmitters by modulating calcium and potassium conductance.
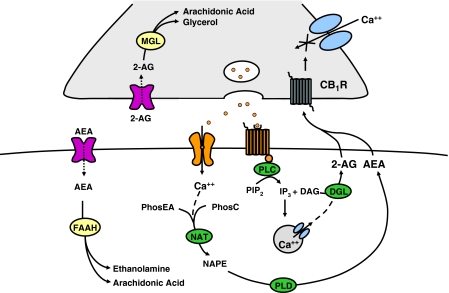
Figure 1.
Diagrammatical representation of an endocannabinoid synapse. Anandamide (AEA) and 2-arachidonylglycerol (2-AG) are synthesized following an increase in cytosolic calcium (Ca++) resulting from activation of postsynaptic ion channels or G protein-coupled receptors. The activation of Gq protein-coupled receptors results in the synthesis of inositol trisphosphate (IP3) and diacylglycerol (DAG) from phosphoinositol bisphosphate (PIP2). IP3 mobilizes calcium release from intracellular stores triggering the formation of 2-AG from DAG by the enzyme diacylglycerol lipase (DGL). The activation of Ca++ gating ion channels facilitates the influx of Ca++, which leads to the formation of N-arachidonoyl-phosphatidylethanolamine (NAPE) from phosphatidylethanolamine (PhosEA) and phosphatidylcholine (PhosC) via the enzyme N-acyltransferase (NAT). NAPE is then hydrolized to anandamide by a phospholipase D-type enzyme (NAPE-PLD). The cannabinoids are released from the postsynaptic neuron and travel retrogradely to the presynaptic membrane to activate cannabinoid receptors (e.g. cannabinoid1 receptor, CB1R). The activation of the CB1 receptor results in inhibition of Ca++ channels in the presynaptic membrane and a number of other signal transduction-mediated events, which generally result in suppression of neuronal activity and neurotransmitter release. 2-AG is catabolized to arachidonyl acid and glycerol by monoacylglycerol lipase (MGL), while fatty acid amide hydrolase (FAAH) breaks down AEA to arachidonic acid and ethanolamine.
The endogenous cannabinoid ligands, or endocannabinoids, are polyunsaturated fatty acids and include the compounds, arachidonyl ethanolamine (anandamide), 2-arachidonylglycerol (2-AG), noladin ether, palmitoylethanolamine, homo-g-linolenylethanolamide, 7,10,13,16-docosatetranylethanolamine, virodhamine and N-arachidonoyl-dopamine. Most endocannabinoids are derived from arachidonic acid, which is a known precursor for an array of other biochemical mediators. It is believed that endocannabinoids are biosynthesized as required and immediately released from cells to exert their physiological effects. In the case of anandamide and 2-AG, this biosynthesis is catalysed by calcium-sensitive enzymes and seems to occur with calcium influx following cell depolarization, or mobilization of intracellular calcium stores. The metabolism of the endocannabinoids occurs intracellularly; however, the precise mechanism by which these compounds are taken up into the cell is, as yet, unclear. It has been postulated that re-uptake may occur via more than one mechanism, including endocytosis and the interaction of endocannabinoids with transporter proteins to carry them across the membranes (Beltramo et al., 1997; Beltramo and Piomelli, 2000; Hillard and Jarrahian, 2003; McFarland and Barker, 2004).
Once inside the cell, endocannabinoids are metabolized by fatty acid amide hydrolase (FAAH), which demonstrates selectivity for anandamide (Cravatt et al., 1996), and by monoacylglycerol lipase, which selectively degrades 2-AG (Dinh et al., 2002). Immunohistochemistry has demonstrated that in many brain regions, FAAH (Egertova et al., 2003; Gulyas et al., 2004) and monoacylglycerol lipase (Dinh et al., 2002; Gulyas et al., 2004) are expressed in a pattern corresponding to that of the CB1 receptor (Egertova et al., 1998; Tsou et al., 1998; Ueda et al., 2000; Giuffrida et al., 2001). The neuroanatomy of the endocannabinoid system is, therefore, ideally organized to facilitate its role in retrograde signalling, the process by which endocannabinoids released postsynaptically modulate neurotransmission via an action at CB1 receptors located presynaptically.
Supraspinal regulation of pain by cannabinoids
The development of potent, selective pharmacological agonists and antagonists for the CB1 and CB2 receptors (Little et al., 1988; Rinaldi-Carmona et al., 1994; Hillard et al., 1999), CB1 (Ledent et al., 1999; Zimmer et al., 1999; Marsicano et al., 2003; Domenici et al., 2006), CB2 (Buckley et al., 2000) and FAAH (Cravatt et al., 2001) knockout mice, and selective FAAH (Boger et al., 2000; Kathuria et al., 2003; Deutsch, 2005) and monoacylglycerol lipase inhibitors (Saario et al., 2004, 2006; Makara et al., 2005) has proven indispensable in the advancement of the field of cannabinoid research. There are now a large number of studies providing evidence of a role for the endocannabinoid system in nociception and these have been reviewed extensively elsewhere (Pertwee, 2001; Finn and Chapman, 2004; Hohmann and Suplita, 2006; Jhaveri et al., this issue). Moreover, the promise of this research may soon be realized in the clinical setting with the recent launch of the cannabis-based drug Sativex in Canada for the adjunctive relief of neuropathic pain in multiple sclerosis patients. Subsequent considerations in this review will focus on the supraspinal neural substrates and neurochemical mechanisms mediating cannabinoid-induced antinociception with an emphasis on the role of the amino-acid neurotransmitters GABA and glutamate.
Direct evidence for the involvement of supraspinal cannabinoid receptors in the modulation of pain has been obtained from a number of studies employing intracerebral microinjection of cannabinoids or endocannabinoid system modulators in animal models of acute, inflammatory or neuropathic pain (Table 1). Early work demonstrated that intracerebroventricular administration of antisense oligonucleotides directed against CB1 receptor mRNA inhibited the antinociceptive effect of the cannabinoid receptor agonist CP55,940 in mice, suggesting a role for supraspinal CB1 receptors in cannabinoid-mediated antinociception (Edsall et al., 1996). Further studies demonstrated that intracerebroventricular injection of non-selective cannabinoid receptor agonists suppressed nociception in the rat tail-flick test (Table 1), and these effects were reversed by the CB1 receptor antagonist, rimonabant (Lichtman et al., 1996; Lichtman and Martin, 1997; Martin et al., 1998; Welch et al., 1998). Martin et al. (1999) demonstrated that the cannabinoid receptor agonist WIN55,212-2 was antinociceptive in the tail-flick test when injected into a number of rat brain regions including subnuclei of the amygdala, thalamus, PAG and RVM (Table 1). Additional evidence supporting a role for the amygdala as an important site mediating cannabinoid-induced antinociception comes from work demonstrating that bilateral lesions to the amygdala abolish the antinociceptive effects of systemically administered WIN55,212-2 in the tail-flick test in rhesus monkeys (Manning et al., 2001).
Table 1.
The effects of supraspinal injection of cannabinoids in rat models of pain
| Cannabinoid | Injection location | Model | Effect | Reference |
|---|---|---|---|---|
| Cannabinoid receptor agonists | ||||
| WIN55,212-2 | ICV | TFT | Antinociceptive | Martin et al. (1993) |
| GiA | Monhemius et al. (2001) | |||
| dlPAG | de Novellis et al. (2005) | |||
| BLA | Hasanein et al. (2007) | |||
| RVM | Martin et al. (1998); Meng and Johansen (2004) | |||
| ICV, RVM, GiA, dPAG, BLA, CeA, thalamus, A5 NEergic group, DRN | Martin et al. (1999) | |||
| dlPAG | PWT | Antinociceptive | Palazzo et al. (2001) | |
| vlPAG | PWT | Antinociceptive/pronociceptive | Maione et al. (2006) | |
| GiA | FT | Antinociceptive | Monhemius et al. (2001) | |
| BLA | Hasanein et al. (2007) | |||
| Δ9-THC | ICV | TFT | Antinociceptive | Lichtman et al. (1996) |
| HU210 | RVM | TFT | Antinociceptive | Martin et al. (1998) |
| dPAG | FT | Antinociceptive | Finn et al. (2003) | |
| CP55,940 | ICV | TFT | Antinociceptive | Martin et al. (1993); Lichtman et al. (1996) |
| CB1 receptor antagonists | ||||
| Rimonabant | dlPAG | PWT | Pronociceptive | Palazzo et al. (2001) |
| BLA | SIA | Pronociceptive | Connell et al. (2006) | |
| RVM | Suplita et al. (2005) | |||
| dlPAG | Hohmann et al. (2005); Suplita et al. (2005) | |||
| dPAG | FT | No effect | Finn et al. (2003) | |
| Inhibitors of endocannabinoid degradation | ||||
| URB597 | vlPAG | PWT | Antinociceptive/pronociceptive | Maione et al. (2006) |
| dlPAG | SIA | Antinociceptive | Hohmann et al. (2005) | |
| BLA | No effect | Connell et al. (2006) | ||
| URB602 | dlPAG | SIA | Antinociceptive | Hohmann et al. (2005) |
| BLA | No effect | Connell et al. (2006) | ||
| MAFP | dlPAG | SIA | Antinociceptive | Hohmann et al. (2005) |
| AA-5-HT | RVM, dlPAG | SIA | Antinociceptive | Suplita et al. (2005) |
Abbreviations: BLA, basolateral amygdala; CeA, central nucleus of the amygdala; DRN, dorsal raphe nucleus; FT, formalin test; GiA, gigantocellularis pars-α; ICV, intracerebroventricular; PAG, periaqueductal gray; PWT, paw withdrawal test; RVM, rostral ventromedial medulla; SIA, stress-induced analgesia model; TFT, tail-flick test; Δ9-THC, Δ9-tetrahydrocannabinol.
This table reports the effects of cannabinoid compounds on nociception in a number of animal models including the TFT, PWT, FT and SIA. The TFT and PWT are models of acute thermal nociception measuring the latency to withdrawal of the animal's paw or tail from the heat source. The formalin test is a model of tonic persistent inflammatory pain, where formalin is injected into the plantar surface of the hind paw, and nociceptive behaviours are then observed and scored. The stress-induced analgesia model employs continuous footshocks and subsequent scoring of rat tail-flick responses with footshock stress increasing the latency to tail withdrawal.
In vivo electrophysiological studies have enabled the activity of ON and OFF cells in the RVM to be assessed in lightly anaesthetized rats during the tail-flick test. Microinjection of the cannabinoid receptor agonists WIN55,212-2 and HU210 into the RVM increased the rat tail-flick latency (Martin et al., 1998). WIN55,212-2 also decreased the firing of the ON cells while decreasing the duration of the OFF-cell pause and increasing ongoing OFF-cell activity (Meng and Johansen, 2004). Similarly, the local administration of WIN55,212-2 into the nucleus reticularis gigantocellularis pars-α, an area in the RVM, also increased latency to withdrawal in the rat tail-flick test and reduced nociceptive responses to subcutaneous formalin administration (Monhemius et al., 2001). Intra-RVM administration of rimonabant reversed the antinociceptive effects observed in all the above studies (Martin et al., 1998; Monhemius et al., 2001; Meng and Johansen, 2004) suggesting a modulatory role for RVM CB1 receptors in the descending pain pathway (Table 1).
In the rat thermal plantar test, the microinjection of WIN55,212-2 into the dorsolateral (Palazzo et al., 2001) and ventrolateral (Maione et al., 2006) PAG increased the latency of the nociceptive response; an effect which was reversed by rimonabant (Palazzo et al., 2001). The effects of microinjection of the FAAH inhibitor URB597 into the ventrolateral PAG were shown to depend on the dose administered. Low doses resulted in an immediate and prolonged hyperalgesic response to the rat thermal plantar test, while medium doses resulted in a bi-phasic analgesic/hyperalgesic response and high doses produced an immediate analgesic response (Maione et al., 2006). URB597 was shown to dose-dependently increase anandamide levels, while 2-AG levels were maximal with the lowest dose of URB597 administered. The antinociceptive responses coincided with changes in the activity of RVM ON- and OFF neurons. The differences between endocannabinoid concentrations and consequent nociceptive and electrophysiological responses were attributed to selective activation of CB1 and/or transient receptor potential vanilloid receptor type-1 receptors (Maione et al., 2006). These findings support the involvement of the endocannabinoid system in the descending pain pathway in animal models of acute pain (Table 1).
Evidence for a role of supraspinal cannabinoid receptors in the modulation of inflammatory pain comes from work demonstrating that microinjection of HU210 into the dorsal PAG decreased the second phase of formalin-evoked nociceptive behaviour in rats, an effect which was blocked by rimonabant and accompanied by an attenuation of formalin-evoked c-Fos expression in the caudal lateral PAG (Finn et al., 2003). Similarly, the intra-PAG microinjection of WIN55,212-2 delayed the response of formalin-treated rats to the tail-flick test, as well as the formalin-induced increase in activity of ON cells and decrease in OFF-cell pause in the rat RVM (de Novellis et al., 2005). Both these responses were blocked by rimonabant. A more recent study determined that intra-basolateral amygdala (BLA) microinjection of WIN55,212-2 dose-dependently increased the latency to withdrawal in the tail-flick test and decreased pain behaviours in both phases of the formalin test, effects reversed by the CB1 receptor antagonist AM251 (Hasanein et al., 2007). Further support for the involvement of the brain's endocannabinoid system in inflammatory pain was provided by the observation that electrical stimulation of the rat PAG, as well as formalin injection into the hindpaw, increased anandamide release in the PAG as determined by microdialysis coupled to liquid chromatography/mass spectrometry (Walker et al., 1999).
Additional evidence for an endogenous cannabinoid pain-suppressing system comes from work using an animal model of unconditioned stress-induced analgesia employing continuous footshocks with subsequent scoring of rat tail-flick responses (Table 1). It was demonstrated that intra-dorsolateral PAG, intra-RVM or intra-BLA microinjection of rimonabant suppressed stress-induced analgesia relative to control animals (Hohmann et al., 2005; Suplita et al., 2005; Connell et al., 2006). 2-AG levels in the dorsal midbrain were markedly increased 2 min post-footshock and returned to baseline after 15 min, while anandamide displayed an increased concentration which peaked at 7–15 min post-footshock (Hohmann et al., 2005). Further work demonstrated that intra-dorsal PAG, intra-RVM but not intra-BLA microinjection of inhibitors of endocannabinoid degradation enhanced stress-induced antinociception, while there was no effect on basal nociceptive thresholds in non-shocked rats (Hohmann et al., 2005; Suplita et al., 2005; Connell et al., 2006). The enhancement of stress-induced analgesia by these enzyme inhibitors was blocked by coadministration of rimonabant. Meanwhile, in a model of conditioned fear-induced analgesia which involves assessment of formalin-evoked nociceptive behaviour in an aversively conditioned context, Finn et al. (2004) demonstrated that this form of psychological stress-induced analgesia is attenuated by systemic administration of rimonabant. Despite good evidence for a role of the brain's endocannabinoid system in conditioned fear (Marsicano et al., 2002; Cannich et al., 2004), the neural substrates and neurochemical mechanisms involved in endocannabinoid-mediated fear-induced analgesia remain to be elucidated.
Studies employing animal models of nerve injury have been carried out to determine the potential role of the brain's endocannabinoid system in modulation of neuropathic pain. An increase in CB1 receptor mRNA in the contralateral thalamus in rats with sciatic nerve ligation was reported (Siegling et al., 2001), suggesting that CB1 receptor upregulation may account for the increased analgesic efficacy of cannabinoids in chronic pain conditions. Microinjection of rimonabant into the nucleus reticularis gigantocellularis pars-α reversed the inhibitory effects of nerve ligation on formalin-evoked nociceptive behaviour (Monhemius et al., 2001), suggesting that increased endocannabinoid signalling through CB1 receptors in the nucleus reticularis gigantocellularis pars-α following nerve ligation acts to reduce nociception. A recent study evaluating changes in rat supraspinal endocannabinoid levels 3 or 7 days following chronic constriction injury of the sciatic nerve has yielded some interesting results (Petrosino et al., 2007). An increase in the levels of anandamide and 2-AG was reported in the PAG 3 days after chronic constriction injury, while after 7 days, anandamide levels were increased in the dorsal raphe nucleus, PAG and RVM, and levels of 2-AG were increased in the PAG and RVM. There were also decreases in palmitoylethanolamine in the dorsal raphe nucleus and RVM 7 days post-ligation. Similarly, Palazzo et al. (2006) demonstrated an increase in levels of anandamide, but not 2-AG, in the dorsal raphe nucleus 7 days after chronic constriction injury, effects accompanied by an increase in serotonergic firing and release. The effects of chronic constriction injury on serotonergic firing and release were reversed by either single or 7-day systemic administration of the anandamide reuptake inhibitor, AM404. The effects of AM404 were reversed by rimonabant. In further electrophysiological and microdialysis experiments, 7 days treatment with WIN55,212-2 also produced similar effects to AM404 (Palazzo et al., 2006). These results suggest that endocannabinoid-mediated modulation of central serotonergic function may facilitate antinociception, although further studies are necessary to confirm this hypothesis.
There is good evidence for localization of CB1 receptors on serotonergic (Haring et al., 2007), noradrenergic (Oropeza et al., 2007), dopaminergic (Rodriguez De Fonseca et al., 2001) and cholinergic (Nyiri et al., 2005b) neurons. In addition, cannabinoid compounds have been shown to impact on neuronal activity and/or neurotransmitter release from cholinergic (Table 2) and monoaminergic (Table 3) neurons. Despite this evidence, there are surprisingly few studies investigating the direct involvement of these neurotransmitters in supraspinally mediated cannabinoid-induced antinociception. In addition to the study by Palazzo et al. (2006) discussed above, it has been demonstrated that the antinociceptive effects of the cannabinoid receptor agonist, WIN55,212-2, in the rat tail-flick test are attenuated following lesion of the descending noradrenergic spinal pathways (Gutierrez et al., 2003). Thus, while the central serotonergic and noradrenergic systems may be involved in cannabinoid-induced antinociception, there is at present an insufficient body of data and a need for further research in this area. Cannabinoid-mediated modulation of central GABA and glutamate and its implications for pain is, however, better understood and is, therefore, the focus of the remainder of this review.
Table 2.
The effect of cannabinoid compounds on supraspinal acetylcholine release
| Cannabinoid | Effect | Brain area | Species | Reference |
|---|---|---|---|---|
| Cannabinoid receptor agonists | ||||
| WIN55,212-2 | ||||
| In vitro release | ↓[3H]ACh | Hippocampal neurons | Rat | Gifford et al. (2000) |
| Cortical neurons | ||||
| Hippocampal slices | Rat | Gifford and Ashby (1996); Kathmann et al. (2001b) | ||
| Mouse | Kathmann et al. (2001a, 2001b) | |||
| Cortical slices | Mouse | Kathmann et al. (2001b) | ||
| ↔[3H]ACh | Striatal slices | Mouse | Kathmann et al. (2001a, 2001b) | |
| Microdialysis | ↓ACh | Hippocampus | Rat | Gessa et al. (1997, 1998) |
| Prefrontal cortex | Rat | Gessa et al. (1998); Verrico et al. (2003b) | ||
| ↓↑ACh | Hippocampus | Rat | Tzavara et al. (2003a) | |
| ↑ACh | Hippocampus | Rat | Acquas et al. (2000) | |
| Prefrontal cortex | Acquas et al. (2001) | |||
| CP55,940 | ||||
| In vitro release | ↓[3H]ACh | Hippocampal slices | Rat | Gifford et al. (1997); Kathmann et al. (2001b) |
| Mouse | Kathmann et al. (2001b) | |||
| Microdialysis | ↓ACh | Hippocampus | Rat | Gessa et al. (1997) |
| Δ9-THC | ||||
| Microdialysis | ↓ACh | Hippocampus | Rat | Carta et al. (1998); Gessa et al. (1998); Nava et al. (2001) |
| ↑ACh | Hippocampus | Rat | Pisanu et al. (2006) | |
| Prefrontal cortex | Rat | Verrico et al. (2003b); Pisanu et al. (2006) | ||
| ↔ACh | Prefrontal cortex | Rat | Verrico et al. (2003b) | |
| HU210 | ||||
| Microdialysis | ↑ACh | Hippocampus | Rat | Acquas et al. (2000) |
| Prefrontal cortex | Acquas et al. (2001) | |||
| CB1 receptor antagonists | ||||
| Rimonabant | ||||
| In vitro release | ↑[3H]ACh | Hippocampal slices | Rat | Gifford et al. (1997, 2000); Gifford and Ashby (1996) |
| ↔[3H]ACh | Cortical, striatal slices | Rat | Gifford and Ashby (1996); Gifford et al. (2000) | |
| Striatal slices | Mouse | Kathmann et al. (2001a) | ||
| Microdialysis | ↑ACh | Prefrontal cortex | Rat | Gessa et al. (1998); Tzavara et al. (2003a) |
| Hippocampus | Rat | Gessa et al. (1997, 1998) | ||
| Mouse | Degroot et al. (2006) | |||
| ↔ACh | N.accumbens | Rat | Tzavara et al. (2003b) | |
| AM251 | ||||
| Microdialysis | ↑ACh | Hippocampus | Rat; mouse | Degroot et al. (2006) |
Abbreviation: Ach, acetylcholine; Δ9-THC, Δ9-tetrahydrocannabinol.
Upwards or downwards arrows indicate increases or decreases, respectively, in Ach release, whereas no change is indicated by a ‘↔'.
Table 3.
The effect of cannabinoid compounds on supraspinal monoaminergic neurotransmitter release and the firing of supraspinal monoaminergic neurons
| Cannabinoid | Effect | Brain area | Species | Reference |
|---|---|---|---|---|
| Cannabinoid receptor agonists | ||||
| WIN55,212-2 | ||||
| In vitro release | ↔[3H]DA | N. accumbens; C. striatum slices | Rat | Szabo et al. (1999) |
| ↓[3H]NE | HC slices | Human | Schlicker et al. (1997) | |
| HC, cerebellar, hypothalamic, cortical slices | G. pig | Schlicker et al. (1997) | ||
| ↔[3H]NE | HC slices | Rat; Mouse | Schlicker et al. (1997) | |
| ↓[3H]5-HT | Cortical membranes | Mouse | Nakazi et al. (2000) | |
| Tissue levels | ↔DA | N. accumbens, C. striatum | Rat | Verrico et al. (2003a) |
| ↓DA | Prefrontal cortex | |||
| Electrophysiology | ↑DA firing | Substantia nigra | Rat | French et al. (1997) |
| VTA | Rat | French et al. (1997); Diana et al. (1998); Gessa et al. (1998); Pistis et al. (2001) | ||
| ↓DA firing | VTA | Rat | Pillolla et al. (2007) | |
| ↔NE firing | L. coeruleus | Rat | Mendiguren and Pineda (2006) | |
| ↑NE firing | L. coeruleus | Rat | Mendiguren and Pineda (2006); Muntoni et al. (2006) | |
| Microdialysis | ↑DA | N. accumbens | Rat | Tanda et al. (1997); Lecca et al. (2006); Fadda et al. (2006) |
| ↑NE | Frontal cortex | Rat | Oropeza et al. (2005) | |
| CP55,940 | ||||
| In vitro release | ↔[3H]DA | N. accumbens, C. striatum slices | Rat | Szabo et al. (1999) |
| ↓[3H]5-HT | Cortical membranes | Mouse | Nakazi et al. (2000) | |
| Electrophysiology | ↔NE firing | L. coeruleus | Rat | Mendiguren and Pineda (2006) |
| ↑NE firing | L. coeruleus | Mendiguren and Pineda (2006) | ||
| Δ9-THC | ||||
| In vitro release | ↓↑[3H]DA; ↓↑[3H]NE | Hypothalamic, striatal neurons | Rat | Poddar and Dewey (1980) |
| Tissue levels | ↔DA | N. accumbens, C. striatum | Rat | Jentsch et al. (1998); Verrico et al. (2003a) |
| ↓DA | Prefrontal cortex | Jentsch et al. (1998); Verrico et al. (2003a) | ||
| Electrophysiology | ↑DA firing mesolimbic | VTA | Rat | French (1997); French et al. (1997); Diana et al. (1998); Gessa et al. (1998); Ng Cheong Ton et al. (1988); Malone and Taylor (1999); Melis et al. (2000); Wu and French (2000) |
| ↑DA firing | Substantia nigra | Rat | Tanda et al. (1997) | |
| Nigrostriatal | French et al. (1997); Wu and French (2000) | |||
| ↑NE firing | L. coeruleus | Rat | Melis et al. (2000); Muntoni et al. (2006) | |
| Microdialysis | ↑DA | N. accumbens | Rat | Tanda et al. (1999) |
| ↑DA | Prefrontal cortex | Rat | Chen et al. (1990) | |
| Anandamide | ||||
| Electrophysiology | ↑DA firing | N. accumbens | Rat | Solinas et al. (2006) |
| CB1 receptor antagonists | ||||
| Rimonabant | ||||
| In vitro release | ↔[3H]DA | N. accumbens; C. striatum slices | Rat | Szabo et al. (1999) |
| ↑[3H]NE | HC slices | Human | Schlicker et al. (1997) | |
| ↔[3H]NE | HC, cerebellar, hypothalamic, cortical slices | G. pig | Schlicker et al. (1997) | |
| ↔[3H]5-HT | Cortical membranes | Mouse | Nakazi et al. (2000) | |
| Electrophysiology | ↓DA firing Mesolimbic | VTA | Rat | Pistis et al. (2001); Pillolla et al. (2007) |
| ↓NE firing | L. coeruleus | Rat | Muntoni et al. (2006) | |
| Microdialysis | ↑DA; ↑NE | Prefrontal cortex | Rat | Tzavara et al. (2003b) |
| ↔DA; ↔NE | N. accumbens; | Rat | Tzavara et al. (2003b) | |
| ↑5-HT | Striatum prefrontal cortex, N. accumbens | Rat | Tzavara et al. (2003b) | |
| ↑DA;↔5-HT | Hypothalamus | Rat | Tzavara et al. (2001) | |
| Inhibitors of degradation | ||||
| URB597 | ||||
| Electrophysiology | ↑5-HT firing | Dorsal raphe | Rat | Gobbi et al. (2005) |
Abbreviations: C. striatum, corpus striatum; DA, dopamine; G. pig, guinea pig; HC, hippocampus; L. coeruleus, locus coeruleus; N. accumbens, nucleus accumbens; NE, noradrenaline; 5-HT, serotonin; Δ9-THC, Δ9-tetrahydrocannabinol; VTA, ventral tegmental area.
Upwards or downwards arrows indicate increases or decreases, respectively, in monoaminergic neurotransmitter release or firing of monoaminergic neurons as measured by electrophysiology, whereas no change is indicated by a ‘↔'.
Anatomical and functional evidence for modulation of supraspinal GABAergic and glutamatergic neurotransmission by the endocannabinoid system: implications for pain
Studies of CB1 receptor localization in the brain have been carried out using a number of techniques including retrograde/anterograde labelling, immunohistochemistry, in situ hybridization and autoradiography. Using the aforementioned techniques it has been determined that the expression of the CB1 receptor gene is restricted to specific cell types, which serve distinct functional roles in a variety of neurological processes (Freund and Hajos, 2003; Freund et al., 2003). There are a large number of studies demonstrating a role for supraspinal GABA and glutamate in animal models of pain (for review see Bleakman et al., 2006; Enna and McCarson, 2006; Neto et al., 2006). Here, we provide a summary of the distribution of CB1 receptor binding sites on GABAergic and glutamatergic neurons in brain regions known to play an important role in nociception, review the evidence for cannabinoid-mediated modulation of GABAergic and glutamatergic transmission (Table 4) and discuss its importance in the context of pain (Table 5).
Table 4.
Studies investigating the functional effects of cannabinoid compounds on supraspinal release of GABA and glutamate, and on the firing of supraspinal GABAergic and glutamatergic neurons
| Cannabinoid | Effect | Brain Area | Species | Reference |
|---|---|---|---|---|
| WIN55,212-2 | ||||
| In vitro electrophysiology | ↓IPSPs | HC neurons | Rat | Irving et al. (2000) |
| BLA | Rat | Katona et al. (2001) | ||
| Neocortex | Rat | Bodor et al. (2005) | ||
| PAG | Rat | Vaughan et al. (2000) | ||
| RVM | Rat | Vaughan et al. (1999) | ||
| HC slices | Rat | Hajos et al. (2000, 2001); Hoffman and Lupica (2000); Hajos and Freund (2002); Foldy et al. (2006) | ||
| Lateral amygdala | Mouse | Trettel and Levine (2002); Azad et al. (2003) | ||
| In vitro electrophysiology | ↓EPSPs | Prefrontal cortex | Rat | Auclair et al. (2000) |
| PAG | Rat | Vaughan et al. (2000) | ||
| HC slices | Rat | Hajos et al. (2001); Hajos and Freund (2002) | ||
| BLA, cortex | Mouse | Domenici et al. (2006) | ||
| Lateral amygdala | Mouse | Azad et al. (2003) | ||
| HC slices | Mouse | Misner and Sullivan (1999); Domenici et al. (2006) | ||
| In vitro release | ↓[3H]GABA | HC neurons | Rat | D'Amico et al. (2004) |
| HC slices | Rat | Katona et al. (1999) | ||
| Human | Katona et al. (2000) | |||
| ↑[3H]Glut | PFC neurons | Rat | Ferraro et al. (2001a) | |
| Microdialysis | ↓GABA | PFC | Rat | Ferraro et al. (2001a) |
| ↑Glutamate | PFC | Rat | Ferraro et al. (2001b) | |
| CP55,940 | ||||
| In vitro electrophysiology | ↓IPSPs | HC slices | Rat | Hajos et al. (2000) |
| BLA | Rat | Katona et al. (2001) | ||
| In vitro release | ↓[3H]GABA | HC neurons | Rat | D'Amico et al. (2004) |
| AM251 | ||||
| In vitro release | ↓[3H]GABA | HC slices | Rat | Neu et al. (2007) |
Abbreviations: BLA, basolateral amygdala; EPSPs, excitatory postsynaptic potentials; GABA, γ-aminobutyric acid; HC, hippocampus; IPSPs, inhibitory postsynaptic potentials; PAG, periaqueductal gray; PFC, prefrontal cortex; RVM, rostral ventromedial medulla.
Upwards or downwards arrows indicate increases or decreases, respectively, in the release of GABA and glutamate neurotransmitters, or in the firing of GABAergic and glutamatergic neurons, whereas no change is indicated by a ‘↔'. IPSPs and EPSPs are temporary changes in postsynaptic membrane potential caused by the flow of ions into or out of the cell. IPSPs are generally initiated by the activation of GABA receptors on the postsynaptic neuron and suppress the firing of the postsynaptic neuron, while glutamate receptor activation generally instigates EPSPs, which enhance the firing of the postsynaptic neuron.
Table 5.
The role of supraspinal GABA and glutamate in the antinociceptive effects of the cannabinoid receptor agonist, WIN55,212-2
| WIN55,212-2 injection location | Antinociception reversed by: | Model | Reference |
|---|---|---|---|
| dlPAG | Rimonabant -CB1 R antagonist | PWT | Palazzo et al. (2001) |
| MSOP-group III mGlu antagonist | |||
| MPEP-mGlu5 antagonist | |||
| EGlu-group II mGlu antagonist | |||
| APV-NMDA R antagonist | |||
| dlPAG | Rimonabant (CB1 R antagonist) MPEP (mGlu5 antagonist) | FT, EPhys | de Novellis et al. (2005) |
| I.v. | Intra-CeA muscimol (GABAA receptor agonist) | TFT, FT | Manning et al. (2003) |
| S.c. | Intra-RVM muscimol (GABAA receptor agonist) | TFT, EPhys | Meng et al. (1998) |
Abbreviations: CB1 R, cannabinoid1 receptor; CeA, central nucleus of the amygdala; Ephys, electrophysiology; GABA, γ-aminobutyric acid; FT, formalin test; i.v., intravenous; mGlu, metabotropic glutamate; PWT, paw withdrawal test; RVM, rostral ventromedial medulla; s.c., subcutaneous; TFT, tail-flick test.
This table reports the effects of GABAergic and glutamatergic compounds on the antinociceptive effects of the cannabinoid receptor agonist, WIN55,212-2, in a number of rat models including the TFT, PWT, FT and changes in the firing of various neurons as measured by EPhys. The TFT and PWT are models of acute thermal nociception measuring the latency to withdrawal of the animal's paw or tail from the heat source. The formalin test is a model of tonic persistent inflammatory pain, where formalin is injected into the plantar surface of the hind paw, and nociceptive behaviours are then observed and scored. The stress-induced analgesia model employs continuous footshocks with subsequent scoring of rat tail-flick responses with footshock stress increasing the latency to tail withdrawal.
Cortical and limbic areas
A number of cortical and limbic areas play an important role in the affective-motivational dimension of pain. Using in situ hybridization and immunohistochemistry, it has been shown that the CB1 receptor-positive cells in cortical areas represent a small percentage of the total cell population in rat brain and reside on heterogenous GABAergic interneurons (Mailleux and Vanderhaeghen, 1992; Matsuda et al., 1993; Tsou et al., 1998). Further double-labelling studies have shown that mice cortical cells expressing the CB1 receptor also co-express glutamic acid decarboxylase (GAD65), the GABA synthesizing enzyme that characterizes GABAergic cells (Marsicano and Lutz, 1999). These GABAergic interneurons can be further subdivided into separate groups based on the expression of cell type-specific neurochemical markers. Double immunostaining determined that the majority of CB1 receptor-positive GABAergic neurons also stained positive for cholecystokinin (CCK) in rat somatosensory cortex (Bodor et al., 2005), rat hippocampus (Katona et al., 1999; Nyiri et al., 2005a), rat septum (Nyiri et al., 2005b), rat BLA (Katona et al., 2001; McDonald and Mascagni, 2001) and mouse forebrain (Marsicano and Lutz, 1999). In addition to the large CCK-positive cells, a much smaller subset of CB1 receptor-positive GABAergic interneurons were reported to contain calcium-binding proteins in somatosensory cortex (Bodor et al., 2005), hippocampus (Katona et al., 1999; Marsicano and Lutz, 1999; Tsou et al., 1999) and BLA (Marsicano and Lutz, 1999; McDonald and Mascagni, 2001). It has been suggested that because CCK and calcium-binding proteins are expressed in separate populations of CB1 receptor-positive GABAergic interneurons, endocannabinoids could modulate population synchrony as well as individual neuronal plasticity (Bodor et al., 2005).
A more recent study provides evidence for CB1 receptors on presynaptic glutamatergic terminals (Katona et al., 2006). It was shown that principal cell populations of the hippocampus contain high levels of diacylglycerol lipase-α (an enzyme involved in 2-AG formation) concentrated in heads of dendritic spines. Electron microscopy observations revealed that these specialized postsynaptic dendritic spine domains receive glutamatergic inputs. These dendritic spine domains release 2-AG by retrograde neurotransmission to activate CB1 receptors on presynaptic glutamatergic axon terminals. The colocalization of CB1 receptors with hippocampal vesicular glutamate transporter type 1 has also been demonstrated (Monory et al., 2006), suggesting that cannabinoids can impact on glutamate neurotransmission. We have recently demonstrated that CB1 receptors in the BLA colocalize with GAD67, a marker for GABAergic neurons, fibres and terminals (Figure 2).
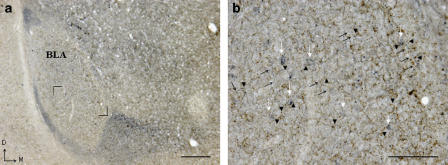
Figure 2.
CB1 receptor immunoreactivity on GABAergic neurones in the rat basolateral amygdala (BLA). A low magnification image of GABAergic neurons in the rat basolateral amygdala. (b) A section of panel (a) at higher resolution, as stated in the legend. Dual immunolabelling for GAD67 (blue) and CB1 receptor (brown) demonstrates that the CB1 receptor (arrow head) is expressed in close proximity to GAD67-immunoreactive cells (white arrows) and fibres (black arrows). (b) High magnification of boxed area in (a). Scale bar (a)=200 μm, (b)=100 μm. D=dorsal; M=medial.
In immunohistochemistry studies on primate brain slices, CB1 receptors were reported on putative glutamatergic pyramidal projection neurons as well as on GABAergic neurons in the cortex, hippocampus and amygdala (Lu et al., 1999; Ong and Mackie, 1999). However, in another study, CB1 immunoreactivity was found exclusively in GABAergic neurons and axon terminals in these regions (Eggan and Lewis, 2007). The authors suggest that the differences observed may be due to differential ability of antibodies to recognize different phosphorylated forms of the CB1 receptor.
Consistent with the anatomical localization studies, electrophysiological and neurotransmitter release studies have demonstrated a functional role of CB1 receptors in the modulation of GABA and glutamate release and firing (Table 4; for review see Doherty and Dingledine, 2003). In rat hippocampal brain slices, endocannabinoid and CB1 receptor agonist application decreased the amplitude of evoked inhibitory postsynaptic potentials of GABAergic neurons and this decrease was reversed by CB1 receptor antagonist application (Hajos et al., 2000, 2001; Hoffman and Lupica, 2000; Irving et al., 2000; Hajos and Freund, 2002). Furthermore, it was determined that cannabinoid-mediated inhibition of inhibitory postsynaptic potentials was dependent on the firing rates of the presynaptic interneurons, as an increase in the frequency of action potentials reversed WIN55,212-2-mediated inhibition of GABA release from hippocampal slices (Foldy et al., 2006). Further studies demonstrated that GABA release from CCK-positive CA1 hippocampal slices is under tonic inhibitory control by endocannabinoids, whose release can, in turn, be regulated by G protein-coupled receptors on the postsynaptic neuron (Neu et al., 2007). The inhibitory effects of cannabinoid receptor agonists on IPSPs were absent in CB1 receptor knockout mice and were reversed with the coapplication of rimonabant in wild-type mice, confirming that cannabinoid-mediated modulation of GABA action potentials is CB1 receptor-dependent (Hajos et al., 2000, 2001). Similarly, endocannabinoid-mediated suppression of GABA currents was shown both in slices from the rat amygdala (Katona et al., 2001) and mouse neocortex (Galarreta et al., 2004; Trettel et al., 2004; Bodor et al., 2005). Furthermore, the extracellular release of GABA from rat cerebral cortex (Ferraro et al., 2001a) and human and rat hippocampal brain slices (Katona et al., 2000; D'Amico et al., 2004) was decreased with the application of endocannabinoids and cannabinoid receptor agonists. Evidence for the direct involvement of the endocannabinoid system in GABA-mediated antinociception is provided by the observation that microinjection of the GABAA receptor agonist muscimol into the central nucleus of the amygdala, but not the BLA, prevented the antinociceptive effects of intravenous administration of WIN55,212-2 in the rat tail-flick and formalin tests (Manning et al., 2003) (Table 5).
Cannabinoid receptor agonists have also been shown to reduce the amplitude of glutamatergic excitatory postsynaptic potentials in slices from mouse hippocampus (Misner and Sullivan, 1999), rat prefrontal cortex (Auclair et al., 2000), mouse lateral amygdala (Azad et al., 2003) as well as other cortical and non-cortical areas such as the ventral tegmental area (Melis et al., 2004; Riegel and Lupica, 2004), substantia nigra (Szabo et al., 2000; Freiman and Szabo, 2005; Marinelli et al., 2007), nucleus accumbens (Robbe et al., 2001) and striatum (Huang et al., 2001; Kofalvi et al., 2005) (Table 4). However, the role of the CB1 receptor in cannabinoid-mediated release of glutamate is not yet clear, although the aforementioned studies would suggest that a reduction in firing would suppress glutamate release. In studies where rimonabant was administered, there was a reversal of these reductions in firing and presumably, glutamate release. The involvement of CB1 receptors in the regulation of glutamate release was complicated by the finding that in CB1 receptor knockout mice, WIN55,212-2 no longer reduced GABAergic transmission, but it still affected glutamate transmission (Hajos et al., 2001).
These findings, together with the limited evidence for CB1 receptor localization on glutamatergic neurons in various regions of the brain, led to the hypothesis that the effects of cannabinoids on glutamate transmission were mediated by a novel cannabinoid receptor, distinct from CB1, which has not yet been identified. However, a recent study using conditional mutant mice lacking CB1 receptors in all the principal forebrain neurons, but not in GABAergic interneurons, reported that WIN 55,212-2 did not reduce excitatory responses in glutamatergic neurons in the forebrain areas as it did in wild-type mice and mice lacking CB1 receptors exclusively in GABAergic neurons (Domenici et al., 2006). While these results do not preclude the existence of a novel CB1-like receptor, they provide strong evidence for the control of glutamatergic neurotransmission by CB1 receptors.
Thalamus
The thalamus, with its numerous subnuclei, plays a critical role in the sensory-discriminatory dimension of pain. In situ hybridization studies have reported low CB1 receptor mRNA expression in the thalamus (Mailleux et al., 1992; Mailleux and Vanderhaeghen, 1992) and subsequent studies have shown that there is CB1 receptor protein expression in certain nuclei within the thalamus (Cristino et al., 2006), for example the anterior dorsal thalamic nuclei, the habenular nucleus and the reticular thalamic nucleus (Tsou et al., 1998; Moldrich and Wenger, 2000). The precise identity of neurotransmitters involved in conveying nociceptive information to and from the thalamus remains unclear. A substantial proportion of thalamic neurons is GABAergic inhibitory interneurons (Ralston, 1991; Ulrich and Huguenard, 1997). Interestingly, the majority of neurons in the thalamus are output neurons and it is believed that they often target N-methyl-D-aspartate, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and mGlu receptors in target areas, suggesting a role for glutamatergic neurons originating in the thalamus. Yet, there is no direct anatomical evidence for expression of cannabinoid receptors on these neurons in the thalamus.
Hypothalamus
The hypothalamus is a brain area involved in the modulation of neuroendocrine function and is a component of the descending inhibitory pain pathway. It is also involved in coordinating the stress response and in mediating anxiety. Studies have shown that CB1 receptors in the hypothalamus are colocalized with calretinin, a marker for glutamatergic nuclei, but not with GAD65 or CCK (Marsicano and Lutz, 1999). This suggests that cannabinoid receptor activation in this area may alter the activity of glutamatergic neurons. Although there has been no direct evidence for the localization of CB1 receptors on GABAergic neurons in the hypothalamus, de Miguel et al. (1998) observed a parallel between hormone levels and GABA levels with cannabinoid receptor agonism and antagonism. It was also demonstrated that hypothalamic neuroendocrine cells can release endocannabinoids, which then suppresses glutamate release and postsynaptic spiking in the hypothalamus (Di et al., 2005). However, as with other regions of the brain including the midbrain and thalamus, there is still some uncertainty with respect to the precise identity and localization of CB1 receptor-containing neurons.
Periaqueductal gray and rostroventral medulla
The PAG is a longitudinally orientated tubular structure organized functionally into four columnar regions. Activation of the individual columns results in specific behavioural effects including confrontational defence, flight, quiescence, hypoactivity and analgesia. While GABAergic and glutamatergic neurons, as well as CB1 receptors, are known to exist in the PAG, only functional evidence exists to suggest the localization of CB1 receptors on the respective neuron types. Studies on rat brain PAG slices demonstrated that the amplitude of GABAergic and glutamatergic postsynaptic currents was reduced by the cannabinoid receptor agonists WIN55,212-2, anandamide and methanandamide, effects blocked by rimonabant (Vaughan et al., 2000).
In the rat thermal plantar test, the microinjection of WIN55,212-2 into the dorsolateral PAG increased the latency of the nociceptive response (Palazzo et al., 2001). These antinociceptive effects were prevented by intra-PAG administrations of rimonabant, as well as 2-methyl-6-(phenylethynyl)pyridine (MPEP), (2S)- α -ethylglutamic acid (EGlu), (RS)-α-methylserine-O-phosphate (MSOP) and 2-amino-5-phosphonopentanoic acid (APV) (mGlu5, group II mGlu, group III mGlu and N-methyl-D-aspartate receptor antagonists respectively), but not 7-(hydroxyimino)cyclopropa[b]chromen-la-carboxylate ethyl ester (CPCCOEt) (mGlu1 receptor antagonist) (Palazzo et al., 2001). In another study, intra-dorsolateral PAG microinjection of WIN55,212-2 resulted in a delayed tail-flick response in formalin-treated animals compared with controls (de Novellis et al., 2005). Intra-PAG WIN55,212-2 microinjection also prevented the formalin-induced increase in basal activity of ON cells and decreased the OFF-cell pause in the rat RVM. Interestingly, both the behavioural and electrophysiological responses were blocked by intra-PAG administrations of rimonabant, as well as MPEP but not CPCOOEt (de Novellis et al., 2005). Overall, these data suggest that endogenous glutamate acts via mGlu and N-methyl-D-aspartate receptors in the PAG to mediate cannabinoid-induced antinociception. However, the analgesic effect of intra-PAG CHPG (mGlu5 receptor agonist) as seen in the plantar test, was blocked by MPEP but not rimonabant (Palazzo et al., 2001), suggesting that while glutamate may mediate the antinociceptive effects of cannabinoids, the reverse (i.e. endocannabinoid mediation of glutamate-induced analgesia) does not appear to be the case.
As discussed earlier, the RVM is a critical component of the descending inhibitory pain pathway. Evidence for localization of CB1 receptors in the RVM has been provided by autoradiography (Herkenham et al., 1991) and in situ hybridization (Matsuda et al., 1993), although the expression of CB1 receptors on GABAergic or glutamatergic neurons in the RVM is yet to be confirmed anatomically. Application of submicromolar concentrations of WIN55,212-2, anandamide and methanandamide reduced the amplitude of postsynaptic GABAergic currents in the rat brain slices, an effect which was blocked by rimonabant (Vaughan et al., 1999). The antinociceptive effect of systemic CB1 receptor activation was prevented by preinjection of muscimol into the RVM (Meng et al., 1998), suggesting a role for RVM GABAergic receptors in the mediation of cannabinoid-induced antinociception.
Spinal cord
The spinal cord is a projection target for neurons descending as part of the inhibitory pain pathway. An interaction between cannabinoid and mGlu receptors at the spinal level has been demonstrated with evidence that the antihyperalgesic effect of WIN55,212-2, administered intrathecally to rats with loose ligation of the sciatic nerve, was reversed by intrathecal administration of the mGlu5 receptor antagonist, MPEP (Hama and Urban, 2004). In the rat formalin test, intrathecal pretreatment with rimonabant attenuated the antinociceptive effect of the GABAB receptor agonist baclofen administered intrathecally suggesting a role for endocannabinoids in mediating the antinociceptive effects of GABA agonists at the spinal level (Naderi et al., 2005).
Summary and general discussion
It is now clear from work in animal models that activation of supraspinal cannabinoid receptors or elevation of brain endocannabinoid levels is sufficient to induce antinociception. Moreover, anatomical and functional evidence points towards an involvement of supraspinal GABA and glutamate in mediating the antinociceptive effects of cannabinoids (Figure 3). However, further studies are needed to fully elucidate the mechanisms involved and their potential clinical importance. An integrative approach employing powerful techniques such as in vivo electrophysiological recording from GABAergic and glutamatergic neurons and microdialysis to assess GABA and glutamate release in discrete brain regions may afford the best opportunity to study the mechanisms underlying cannabinoid-induced antinocieption in clinically relevant animal models of pain. In this respect, there is a paucity of these studies in models of inflammatory and neuropathic pain. Small animal functional and/or pharmacological magnetic resonance imaging also provide an opportunity to explore the effects of modulators of the endocannabinoid, glutamatergic and GABAergic systems, and their interactions, in discrete brain regions in the presence or absence of nociceptive tone.
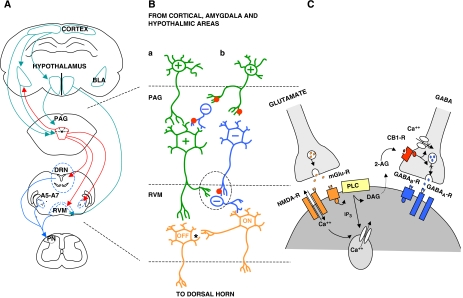
Figure 3.
Possible mechanism for endocannabinoid-mediated control of nociception. (A) Diagrammatical representation of some of the interactions between various brain regions of the descending pain pathway. The PAG receives critical input from various cortical areas as well as from the hypothalamus and amygdala. The net input of afferent neurons to the PAG determines the firing of the various PAG cell types. (B) Two possible outcomes of this net input. In resting conditions (no pain) the sum effect on the input of ON and OFF cells to the dorsal horn is neutral. Painful stimuli are proposed to selectively activate pathway (b), where these excitatory neurons from pathways upstream of the PAG project onto inhibitory projection neurons (possibly GABAergic) as well as inhibitory GABAergic interneurons. This activation of inhibitory interneurons in the PAG prevents firing of excitatory projection neurons (possibly glutamate) and negatively impacts on OFF cells in the RVM. Simultaneously, GABAergic projection neurons from the PAG synapse on GABAergic interneurons in the RVM and disinhibit their suppression of firing of ON neurons to result in nociception. The mediation of antinociception is achieved through pathway (a), when excitatory neurons from pathways upstream of the PAG activate excitatory neurons in the PAG. These excitatory neurons in turn activate the firing of OFF cells, and inhibit the firing of ON cells through GABAergic interneurons. It is also proposed that the activity of OFF cells negatively impacts on the firing of ON cells through an inhibitory mechanism and possibly impacts on OFF-cell duration (represented by an asterix). (C) The circled section of (B), illustrates the possible mechanism behind cannabinoid-mediated antinociception. The activation of various receptor subtypes leads to an increase in intracellular calcium by various pathways. This increase in calcium concentration initiates endocannabinoid synthesis and release. The released endocannabinoids can then prevent the presynaptic release of neurotransmitters possibly by inhibiting calcium influx or vesicular release of neurotransmitters. See abbreviations list.
Work to date has focused largely on the role of supraspinal CB1 receptors. However, accumulating evidence for the presence of the CB2 receptor in the brain (Van Sickle et al., 2005; Gong et al., 2006; Onaivi et al., 2006) now justifies the need for studies to address the gap in knowledge regarding the potential role of supraspinal CB2 receptors in nociception and modulation of neurotransmission. Our understanding of the endocannabinoid system and its complexity is expanding rapidly. The implications of the recent discovery that many cannabinoids also target and mediate biological effects through an action at peroxisome proliferator-activated receptors for the pain field remain unknown (Burstein, 2005; LoVerme et al., 2005; Sun et al., 2006). Studies are required to examine the extent to which these nuclear receptors may mediate the antinocieptive effects of cannabinoids.
The goal of much of this work is the development of therapies relevant to the clinical setting. To this end, clinical trials, which combine pain outcome measures with functional magnetic resonance imaging and/or positron emission tomography, would be very informative with respect to identifying supraspinal sites of action of novel cannabinoid-based analgesics. Targeted, site-specific intracerebral delivery of cannabinoids or coadministration of cannabinoids with drugs modulating GABAergic and glutamatergic activity in pain pathways may one day be used as a therapeutic strategy to treat some types of intractable pain.
Acknowledgments
CB1 receptor antibody was a kind gift from Professor Maurice Elphick, School of Biological and Chemical Sciences, Queen Mary, University of London. Financial support from Science Foundation Ireland, and the Irish Health Research Board is gratefully acknowledged.
Abbreviations
- ↑
Increase
- ↓
Decrease
- ↔
no change
- 2-AG
2 arachidonylglycerol
- BLA
basolateral amygdala
- CCK
cholecystokinin
- FAAH
fatty acid amide hydrolase
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- mGlu
metabotropic glutamate
- PAG
periaqueductal gray
- RVM
rostral ventromedial medulla
Conflict of interest
The authors state no conflict of interest.
References
- Acquas E, Pisanu A, Marrocu P, Di Chiara G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur J Pharmacol. 2000;401:179–185. doi: 10.1016/s0014-2999(00)00403-9. [DOI] [PubMed] [Google Scholar]
- Acquas E, Pisanu A, Marrocu P, Goldberg SR, Di Chiara G. Delta9-tetrahydrocannabinol enhances cortical and hippocampal acetylcholine release in vivo: a microdialysis study. Eur J Pharmacol. 2001;419:155–161. doi: 10.1016/s0014-2999(01)00967-0. [DOI] [PubMed] [Google Scholar]
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Azad SC, Eder M, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learn Mem. 2003;10:116–128. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, et al. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Piomelli D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. NeuroReport. 2000;11:1231–1235. doi: 10.1097/00001756-200004270-00018. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger DL, Sato H, Lerner AE, Hedrick MP, Fecik RA, Miyauchi H, et al. Exceptionally potent inhibitors of fatty acid amide hydrolase: the enzyme responsible for degradation of endogenous oleamide and anandamide. Proc Natl Acad Sci USA. 2000;97:5044–5049. doi: 10.1073/pnas.97.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Burstein S. PPAR-gamma: a nuclear receptor with affinity for cannabinoids. Life Sci. 2005;77:1674–1684. doi: 10.1016/j.lfs.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem. 2004;11:625–632. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta G, Nava F, Gessa GL. Inhibition of hippocampal acetylcholine release after acute and repeated Delta9-tetrahydrocannabinol in rats. Brain Res. 1998;809:1–4. doi: 10.1016/s0006-8993(98)00738-0. [DOI] [PubMed] [Google Scholar]
- Chen J, Paredes W, Lowinson JH, Gardner EL. Delta 9-tetrahydrocannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. Eur J Pharmacol. 1990;190:259–262. doi: 10.1016/0014-2999(90)94136-l. [DOI] [PubMed] [Google Scholar]
- Connell K, Bolton N, Olsen D, Piomelli D, Hohmann AG. Role of the basolateral nucleus of the amygdala in endocannabinoid-mediated stress-induced analgesia. Neurosci Lett. 2006;397:180–184. doi: 10.1016/j.neulet.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- D'Amico M, Cannizzaro C, Preziosi P, Martire M. Inhibition by anandamide and synthetic cannabimimetics of the release of [3H]D-aspartate and [3H]GABA from synaptosomes isolated from the rat hippocampus. Neurochem Res. 2004;29:1553–1561. doi: 10.1023/b:nere.0000029569.20266.3f. [DOI] [PubMed] [Google Scholar]
- de Miguel R, Romero J, Munoz RM, Garcia-Gil L, Gonzalez S, Villanua MA, et al. Effects of cannabinoids on prolactin and gonadotrophin secretion: involvement of changes in hypothalamic gamma-aminobutyric acid (GABA) inputs. Biochem Pharmacol. 1998;56:1331–1338. doi: 10.1016/s0006-2952(98)00185-3. [DOI] [PubMed] [Google Scholar]
- de Novellis V, Mariani L, Palazzo E, Vita D, Marabese I, Scafuro M, et al. Periaqueductal grey CB1 cannabinoid and metabotropic glutamate subtype 5 receptors modulate changes in rostral ventromedial medulla neuronal activities induced by subcutaneous formalin in the rat. Neuroscience. 2005;134:269–281. doi: 10.1016/j.neuroscience.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Degroot A, Kofalvi A, Wade MR, Davis RJ, Rodrigues RJ, Rebola N, et al. CB1 receptor antagonism increases hippocampal acetylcholine release: site and mechanism of action. Mol Pharmacol. 2006;70:1236–1245. doi: 10.1124/mol.106.024661. [DOI] [PubMed] [Google Scholar]
- Deutsch DG. Design of on-target FAAH inhibitors. Chem Biol. 2005;12:1157–1158. doi: 10.1016/j.chembiol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Gessa GL. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci. 1998;10:2825–2830. doi: 10.1111/j.1460-9568.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Doherty J, Dingledine R. Functional interactions between cannabinoid and metabotropic glutamate receptors in the central nervous system. Curr Opin Pharmacol. 2003;3:46–53. doi: 10.1016/s1471-4892(02)00014-0. [DOI] [PubMed] [Google Scholar]
- Domenici MR, Azad SC, Marsicano G, Schierloh A, Wotjak CT, Dodt HU, et al. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J Neurosci. 2006;26:5794–5799. doi: 10.1523/JNEUROSCI.0372-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall SA, Knapp RJ, Vanderah TW, Roeske WR, Consroe P, Yamamura HI. Antisense oligodeoxynucleotide treatment to the brain cannabinoid receptor inhibits antinociception. NeuroReport. 1996;7:593–596. doi: 10.1097/00001756-199601310-00052. [DOI] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Egertova M, Giang DK, Cravatt BF, Elphick MR. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc R Soc Lond B Biol Sci. 1998;265:2081–2085. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L, et al. Cannabinoid self-administration increases dopamine release in the nucleus accumbens. NeuroReport. 2006;17:1629–1632. doi: 10.1097/01.wnr.0000236853.40221.8e. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Cassano T, Bebe BW, Siniscalchi A, O'Connor WT, et al. Cannabinoid receptor agonist WIN 55,212-2 inhibits rat cortical dialysate gamma-aminobutyric acid levels. J Neurosci Res. 2001a;66:298–302. doi: 10.1002/jnr.1224. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, Antonelli T. The cannabinoid receptor agonist WIN 55,212-2 regulates glutamate transmission in rat cerebral cortex: an in vivo and in vitro study. Cereb Cortex. 2001b;11:728–733. doi: 10.1093/cercor/11.8.728. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Richardson D, Kendall DA, Marsden CA, Chapman V. Evidence for differential modulation of conditioned aversion and fear-conditioned analgesia by CB1 receptors. Eur J Neurosci. 2004;20:848–852. doi: 10.1111/j.1460-9568.2004.03509.x. [DOI] [PubMed] [Google Scholar]
- Finn DP, Chapman V. Cannabinoids as analgesic agents: evidence from in vivo studies. Curr Neuropharmacol. 2004;2:75–89. [Google Scholar]
- Finn DP, Jhaveri MD, Beckett SR, Roe CH, Kendall DA, Marsden CA, et al. Effects of direct periaqueductal grey administration of a cannabinoid receptor agonist on nociceptive and aversive responses in rats. Neuropharmacology. 2003;45:594–604. doi: 10.1016/s0028-3908(03)00235-1. [DOI] [PubMed] [Google Scholar]
- Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman I, Szabo B. Cannabinoids depress excitatory neurotransmission between the subthalamic nucleus and the globus pallidus. Neuroscience. 2005;133:305–313. doi: 10.1016/j.neuroscience.2005.01.058. [DOI] [PubMed] [Google Scholar]
- French ED. Delta(9)-tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. NeuroReport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- Freund TF, Hajos N. Excitement reduces inhibition via endocannabinoids. Neuron. 2003;38:362–365. doi: 10.1016/s0896-6273(03)00262-9. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fride E. Endocannabinoids in the central nervous system—an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:221–233. doi: 10.1054/plef.2001.0360. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Erdelyi F, Szabo G, Hestrin S. Electrical coupling among irregular-spiking GABAergic interneurons expressing cannabinoid receptors. J Neurosci. 2004;24:9770–9778. doi: 10.1523/JNEUROSCI.3027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry C.Where does it hurt Wall Street J 1999R6published on October 18, 1999
- Gessa GL, Casu MA, Carta G, Mascia MS. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. Eur J Pharmacol. 1998;355:119–124. doi: 10.1016/s0014-2999(98)00486-5. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Mascia MS, Casu MA, Carta G. Inhibition of hippocampal acetylcholine release by cannabinoids: reversal by SR 141716A. Eur J Pharmacol. 1997;327:R1–R2. doi: 10.1016/s0014-2999(97)89683-5. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Ashby CR., Jr Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J Pharmacol Exp Ther. 1996;277:1431–1436. [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Volkow ND. Cannabinoid receptor-mediated inhibition of acetylcholine release from hippocampal and cortical synaptosomes. Br J Pharmacol. 2000;131:645–650. doi: 10.1038/sj.bjp.0703599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford AN, Samiian L, Gatley SJ, Ashby CR., Jr Examination of the effect of the cannabinoid receptor agonist, CP 55,940, on electrically evoked transmitter release from rat brain slices. Eur J Pharmacol. 1997;324:187–192. doi: 10.1016/s0014-2999(97)00082-4. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez T, Nackley AG, Neely MH, Freeman KG, Edwards GL, Hohmann AG. Effects of neurotoxic destruction of descending noradrenergic pathways on cannabinoid antinociception in models of acute and tonic nociception. Brain Res. 2003;987:176–185. doi: 10.1016/s0006-8993(03)03324-9. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hajos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, et al. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Hama AT, Urban MO. Antihyperalgesic effect of the cannabinoid agonist WIN55, 212-2 is mediated through an interaction with spinal metabotropic glutamate-5 receptors in rats. Neurosci Lett. 2004;358:21–24. doi: 10.1016/j.neulet.2003.12.111. [DOI] [PubMed] [Google Scholar]
- Haring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Hasanein P, Parviz M, Keshavarz M, Javanmardi K. CB1 receptor activation in the basolateral amygdala produces antinociception in animal models of acute and tonic nociception. Clin Exp Pharmacol Physiol. 2007;34:439–449. doi: 10.1111/j.1440-1681.2007.04592.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A. Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol. 2003;140:802–808. doi: 10.1038/sj.bjp.0705468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, DiCamelli R, Ross RA, Stevenson LA, et al. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) J Pharmacol Exp Ther. 1999;289:1427–1433. [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL., II Endocannabinoid mechanisms of pain modulation. AAPS J. 2006;8:E693–E708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Holden JE, Pizzi JA. The challenge of chronic pain. Adv Drug Deliv Rev. 2003;55:935–948. doi: 10.1016/s0169-409x(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Mukhopadhyay S, Shim JY, Welsh WJ. Signal transduction of eicosanoid CB1 receptor ligands. Life Sci. 1999;65:617–625. doi: 10.1016/s0024-3205(99)00284-2. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving AJ, Coutts AA, Harvey J, Rae MG, Mackie K, Bewick GS, et al. Functional expression of cell surface cannabinoid CB(1) receptors on presynaptic inhibitory terminals in cultured rat hippocampal neurons. Neuroscience. 2000;98:253–262. doi: 10.1016/s0306-4522(00)00120-2. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Verrico CD, Le D, Roth RH. Repeated exposure to delta 9-tetrahydrocannabinol reduces prefrontal cortical dopamine metabolism in the rat. Neurosci Lett. 1998;246:169–172. doi: 10.1016/s0304-3940(98)00254-7. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Weber B, Schlicker E. Cannabinoid CB1 receptor-mediated inhibition of acetylcholine release in the brain of NMRI, CD-1 and C57BL/6J mice. Naunyn Schmiedebergs Arch Pharmacol. 2001a;363:50–56. doi: 10.1007/s002100000304. [DOI] [PubMed] [Google Scholar]
- Kathmann M, Weber B, Zimmer A, Schlicker E. Enhanced acetylcholine release in the hippocampus of cannabinoid CB(1) receptor-deficient mice. Br J Pharmacol. 2001b;132:1169–1173. doi: 10.1038/sj.bjp.0703987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, et al. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona IN, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, et al. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Cacciapaglia F, Valentini V, Di Chiara G. Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212-2 self-administration. Psychopharmacology (Berl) 2006;188:63–74. doi: 10.1007/s00213-006-0475-3. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. The selective cannabinoid antagonist SR 141716A blocks cannabinoid-induced antinociception in rats. Pharmacol Biochem Behav. 1997;57:7–12. doi: 10.1016/s0091-3057(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247:1046–1051. [PubMed] [Google Scholar]
- LoVerme J, La Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005;77:1685–1698. doi: 10.1016/j.lfs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Lu XR, Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in monkey basal forebrain. J Neurocytol. 1999;28:1045–1051. doi: 10.1023/a:1007052507911. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Parmentier M, Vanderhaeghen JJ. Distribution of cannabinoid receptor messenger RNA in the human brain: an in situ hybridization histochemistry with oligonucleotides. Neurosci Lett. 1992;143:200–204. doi: 10.1016/0304-3940(92)90265-9. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, et al. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Makara JK, Mor M, Fegley D, Szabo SI, Kathuria S, Astarita G, et al. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141. doi: 10.1038/nn1521. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Modulation by fluoxetine of striatal dopamine release following delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br J Pharmacol. 1999;128:21–26. doi: 10.1038/sj.bjp.0702753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH, Martin WJ, Meng ID. The rodent amygdala contributes to the production of cannabinoid-induced antinociception. Neuroscience. 2003;120:1157–1170. doi: 10.1016/s0306-4522(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Manning BH, Merin NM, Meng ID, Amaral DG. Reduction in opioid- and cannabinoid-induced antinociception in rhesus monkeys after bilateral lesions of the amygdaloid complex. J Neurosci. 2001;21:8238–8246. doi: 10.1523/JNEUROSCI.21-20-08238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Florenzano F, Fezza F, Viscomi MT, van der Stelt M, et al. N-arachidonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology. 2007;32:298–308. doi: 10.1038/sj.npp.1301118. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Coffin PO, Attias E, Balinsky M, Tsou K, Walker JM. Anatomical basis for cannabinoid-induced antinociception as revealed by intracerebral microinjections. Brain Res. 1999;822:237–242. doi: 10.1016/s0006-8993(98)01368-7. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Lai NK, Patrick SL, Tsou K, Walker JM. Antinociceptive actions of cannabinoids following intraventricular administration in rats. Brain Res. 1993;629:300–304. doi: 10.1016/0006-8993(93)91334-o. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Tsou K, Walker JM. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjection into the rostral ventromedial medulla. Neurosci Lett. 1998;242:33–36. doi: 10.1016/s0304-3940(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001;107:641–652. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- McFarland MJ, Barker EL. Anandamide transport. Pharmacol Ther. 2004;104:117–135. doi: 10.1016/j.pharmthera.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1967;86:1646–1647. [Google Scholar]
- Melis M, Gessa GL, Diana M. Different mechanisms for dopaminergic excitation induced by opiates and cannabinoids in the rat midbrain. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:993–1006. doi: 10.1016/s0278-5846(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur J Pharmacol. 2006;534:83–88. doi: 10.1016/j.ejphar.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Meng ID, Johansen JP. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience. 2004;124:685–693. doi: 10.1016/j.neuroscience.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. J Neurosci. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Monhemius R, Azami J, Green DL, Roberts MH. CB1 receptor mediated analgesia from the nucleus reticularis gigantocellularis pars alpha is activated in an animal model of neuropathic pain. Brain Res. 2001;908:67–74. doi: 10.1016/s0006-8993(01)02605-1. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, Zhuang SY, Kirby MT, Hampson RE, Deadwyler SA. Cannabinoid receptors differentially modulate potassium A and D currents in hippocampal neurons in culture. J Pharmacol Exp Ther. 1999;291:893–902. [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23:2385–2394. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Naderi N, Shafaghi B, Khodayar MJ, Zarindast MR. Interaction between gamma-aminobutyric acid GABAB and cannabinoid CB1 receptors in spinal pain pathways in the rat. Eur J Pharmacol. 2005;414:159–164. doi: 10.1016/j.ejphar.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:19–24. doi: 10.1007/s002109900147. [DOI] [PubMed] [Google Scholar]
- Nava F, Carta G, Colombo G, Gessa GL. Effects of chronic Delta(9)-tetrahydrocannabinol treatment on hippocampal extracellular acetylcholine concentration and alternation performance in the T-maze. Neuropharmacology. 2001;41:392–399. doi: 10.1016/s0028-3908(01)00075-2. [DOI] [PubMed] [Google Scholar]
- Neto FL, Ferreira-Gomes J, Castro-Lopes JM. Distribution of GABA receptors in the thalamus and their involvement in nociception. Adv Pharmacol. 2006;54:29–51. doi: 10.1016/s1054-3589(06)54002-5. [DOI] [PubMed] [Google Scholar]
- Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Cheong Ton JM, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, et al. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005a;136:811–822. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Szabadits E, Cserep C, Mackie K, Shigemoto R, Freund TF. GABAB and CB1 cannabinoid receptor expression identifies two types of septal cholinergic neurons. Eur J Neurosci. 2005b;21:3034–3042. doi: 10.1111/j.1460-9568.2005.04146.x. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann NY Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience. 1999;92:1177–1191. doi: 10.1016/s0306-4522(99)00025-1. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Palazzo E, de Novellis V, Petrosino S, Marabese I, Vita D, Giordano C, et al. Neuropathic pain and the endocannabinoid system in the dorsal raphe: pharmacological treatment and interactions with the serotonergic system. Eur J Neurosci. 2006;24:2011–2020. doi: 10.1111/j.1460-9568.2006.05086.x. [DOI] [PubMed] [Google Scholar]
- Palazzo E, Marabese I, de Novellis V, Oliva P, Rossi F, Berrino L, et al. Metabotropic and NMDA glutamate receptors participate in the cannabinoid-induced antinociception. Neuropharmacology. 2001;40:319–326. doi: 10.1016/s0028-3908(00)00160-x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Palazzo E, de Novellis V, Bisogno T, Rossi F, Maione S, et al. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology. 2007;52:415–422. doi: 10.1016/j.neuropharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Pillolla G, Melis M, Perra S, Muntoni AL, Gessa GL, Pistis M. Medial forebrain bundle stimulation evokes endocannabinoid-mediated modulation of ventral tegmental area dopamine neuron firing in vivo. Psychopharmacology (Berl) 2007;191:843–853. doi: 10.1007/s00213-007-0733-z. [DOI] [PubMed] [Google Scholar]
- Pisanu A, Acquas E, Fenu S, Di Chiara G. Modulation of delta(9)-THC-induced increase of cortical and hippocampal acetylcholine release by micro opioid and D(1) dopamine receptors. Neuropharmacology. 2006;50:661–670. doi: 10.1016/j.neuropharm.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Pistis M, Porcu G, Melis M, Diana M, Gessa GL. Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur J Neurosci. 2001;14:96–102. doi: 10.1046/j.0953-816x.2001.01612.x. [DOI] [PubMed] [Google Scholar]
- Poddar MK, Dewey WL. Effects of cannabinoids on catecholamine uptake and release in hypothalamic and striatal synaptosomes. J Pharmacol Exp Ther. 1980;214:63–67. [PubMed] [Google Scholar]
- Ralston HJ., III Local circuitry of the somatosensory thalamus in the processing of sensory information. Prog Brain Res. 1991;87:13–28. doi: 10.1016/s0079-6123(08)63045-9. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez De Fonseca F, Gorriti MA, Bilbao A, Escuredo L, Garcia-Segura LM, Piomelli D, et al. Role of the endogenous cannabinoid system as a modulator of dopamine transmission: implications for Parkinson's disease and schizophrenia. Neurotox Res. 2001;3:23–35. doi: 10.1007/BF03033228. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Vu HK, Larsson N, Groblewski T, Hjorth S, Elebring T, et al. Identification and characterisation of a novel splice variant of the human CB1 receptor. FEBS Lett. 2005;579:259–264. doi: 10.1016/j.febslet.2004.11.085. [DOI] [PubMed] [Google Scholar]
- Saario SM, Poso A, Juvonen RO, Jarvinen T, Salo-Ahen OM. Fatty acid amide hydrolase inhibitors from virtual screening of the endocannabinoid system. J Med Chem. 2006;49:4650–4656. doi: 10.1021/jm060394q. [DOI] [PubMed] [Google Scholar]
- Saario SM, Savinainen JR, Laitinen JT, Jarvinen T, Niemi R. Monoglyceride lipase-like enzymatic activity is responsible for hydrolysis of 2-arachidonoylglycerol in rat cerebellar membranes. Biochem Pharmacol. 2004;67:1381–1387. doi: 10.1016/j.bcp.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Timm J, Zentner J, Gothert M. Cannabinoid CB1 receptor-mediated inhibition of noradrenaline release in the human and guinea-pig hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:583–589. doi: 10.1007/pl00005093. [DOI] [PubMed] [Google Scholar]
- Shire D, Carillon C, Kaghad M, Calandra B, Rinaldi-Carmona M, Le Fur G, et al. An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J Biol Chem. 1995;270:3726–3731. doi: 10.1074/jbc.270.8.3726. [DOI] [PubMed] [Google Scholar]
- Siegling A, Hofmann HA, Denzer D, Mauler F, De Vry J. Cannabinoid CB1 receptor upregulation in a rat model of chronic neuropathic pain. Eur J Pharmacol. 2001;415:R5–R7. doi: 10.1016/s0014-2999(01)00798-1. [DOI] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Kendall DA, Bennett AJ. Cannabinoids and PPARalpha signalling. Biochem Soc Trans. 2006;34:1095–1097. doi: 10.1042/BST0341095. [DOI] [PubMed] [Google Scholar]
- Suplita RL, II, Farthing JN, Gutierrez T, Hohmann AG. Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharmacology. 2005;49:1201–1209. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Szabo B, Muller T, Koch H. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J Neurochem. 1999;73:1084–1089. doi: 10.1046/j.1471-4159.1999.0731084.x. [DOI] [PubMed] [Google Scholar]
- Szabo B, Wallmichrath I, Mathonia P, Pfreundtner C. Cannabinoids inhibit excitatory neurotransmission in the substantia nigra pars reticulata. Neuroscience. 2000;97:89–97. doi: 10.1016/s0306-4522(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Tanda G, Loddo P, Di Chiara G. Dependence of mesolimbic dopamine transmission on delta9-tetrahydrocannabinol. Eur J Pharmacol. 1999;376:23–26. doi: 10.1016/s0014-2999(99)00384-2. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Trettel J, Fortin DA, Levine ES. Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurones in juvenile mouse neocortex. J Physiol. 2004;556:95–107. doi: 10.1113/jphysiol.2003.058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Levine ES. Cannabinoids depress inhibitory synaptic inputs received by layer 2/3 pyramidal neurons of the neocortex. J Neurophysiol. 2002;88:534–539. doi: 10.1152/jn.2002.88.1.534. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Perry KW, Rodriguez DE, Bymaster FP, Nomikos GG. The cannabinoid CB(1) receptor antagonist SR141716A increases norepinephrine outflow in the rat anterior hypothalamus. Eur J Pharmacol. 2001;426:R3–R4. doi: 10.1016/s0014-2999(01)01228-6. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Wade M, Nomikos GG. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: site and mechanism of action. J Neurosci. 2003a;23:9374–9384. doi: 10.1523/JNEUROSCI.23-28-09374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, et al. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003b;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N, Puffenbarger RA, Yamamoto S, Deutsch DG. The fatty acid amide hydrolase (FAAH) Chem Phys Lipids. 2000;108:107–121. doi: 10.1016/s0009-3084(00)00190-0. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Huguenard JR. GABA(A)-receptor-mediated rebound burst firing and burst shunting in thalamus. J Neurophysiol. 1997;78:1748–1751. doi: 10.1152/jn.1997.78.3.1748. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127:935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Jentsch JD, Roth RH. Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse. 2003a;49:61–66. doi: 10.1002/syn.10215. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Jentsch JD, Dazzi L, Roth RH. Systemic, but not local, administration of cannabinoid CB1 receptor agonists modulate prefrontal cortical acetylcholine efflux in the rat. Synapse. 2003b;48:178–183. doi: 10.1002/syn.10202. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch SP, Huffman JW, Lowe J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. J Pharmacol Exp Ther. 1998;286:1301–1308. [PubMed] [Google Scholar]
- Wenger T, Moldrich G, Furst S. Neuromorphological background of cannabis addiction. Brain Res Bull. 2003;61:125–128. doi: 10.1016/s0361-9230(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wu X, French ED. Effects of chronic delta9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment. Neuropharmacology. 2000;39:391–398. doi: 10.1016/s0028-3908(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O'Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]