Abstract
Background and purpose:
Noradrenaline and ATP are sympathetic co-transmitters. In the rat perfused mesenteric bed cannabinoids have been shown to modify the overall response to sympathetic nerve stimulation. This study has assessed whether cannabinoid receptor activation modulates differentially the noradrenergic and purinergic components of sympathetic vasoconstriction.
Experimental approach:
Rat mesenteric beds were perfused with physiological salt solution and the effects of cannabinoids on responses to nerve stimulation, or exogenous noradrenaline or α,β-methylene ATP (α,β-meATP; P2X receptor agonist) were determined after raising tone with U46619. The effects of cannabinoids on the noradrenaline and ATP components of sympathetic neurotransmission were assessed using the α 1-adrenoceptor antagonist, prazosin, or after P2X receptor desensitization with α,β-meATP.
Key results:
Anandamide, WIN 55,212-2 and CP55,940 attenuated sympathetic neurogenic vasoconstrictor responses. The inhibitory actions of anandamide and WIN 55,212-2 were blocked by LY320135, a CB1 receptor antagonist, but not by SR144528, a CB2 receptor antagonist. The inhibitory actions of CP55,940 were unaffected by LY320135 and SR144528. WIN 55,212-3, the inactive S(−) enantiomer of WIN 55,212-2, had no effect on sympathetic neurogenic responses. None of the cannabinoids affected contractile responses to exogenous noradrenaline or α,β-meATP. Anandamide and WIN 55,212-2 inhibited both the noradrenaline and ATP components of the sympathetic neurogenic contractile responses, with effects on the ATP component being most marked.
Conclusions and implications:
These results indicate that prejunctional CB1-like receptors mediate the sympathoinhibitory action of anandamide and WIN 55,212-2, but not CP55,940, in the rat mesenteric bed. Cannabinoids inhibit both the noradrenergic and purinergic components of sympathetic neurotransmission.
Keywords: cannabinoids, sympathetic nerves, noradrenaline, ATP, P2X receptors, α1-adrenoceptors, perfused mesentery
Introduction
There is considerable evidence showing that noradrenaline and adenosine triphosphate (ATP) are co-stored and co-released from sympathetic nerves (Burnstock and Kennedy, 1985). The relative contribution of these sympathetic cotransmitters to vasoconstriction varies, depending on the species, tissue and stimulation parameters (Burnstock, 1990). The involvement of ATP as a mediator of sympathetic vasoconstriction has been demonstrated in the small mesenteric artery of rat (Angus et al., 1988; Sjoblom-Widfeldt et al., 1990; Gitterman and Evans, 2001; Luo et al., 2003). In the mesenteric vascular bed of rat under experimental conditions in which no tone has been induced, contractile responses to sympathetic nerve stimulation are mediated exclusively by the actions of noradrenaline at α1-adrenoceptors on postsynaptic sites (Eikenburg, 1984; Kong et al., 1994; Williams and Clarke, 1995). However, ATP can be shown to act as a functional cotransmitter via P2X receptors in this preparation under conditions of low temperatures (Yamamoto et al., 1992), or after raising tone with a vasoconstrictor, to more closely mimic conditions in vivo (Pakdeechote et al., 2007).
There are currently two main types of cannabinoid receptor, both G-protein-coupled: CB1 receptors are found mainly in the central nervous system and peripheral tissues, whereas CB2 receptors are found mainly in immune tissues (Pertwee, 1999; Pertwee and Ross, 2002; Demuth and Molleman, 2006). Non-CB1 and -CB2 actions of cannabinoids have also been described. Diverse effects of cannabinoids have been demonstrated in the cardiovascular system, including modulation of sympathetic nerve function (Ralevic, 2003; Randall et al., 2004). In anaesthetized rats and rabbits, natural (anandamide) and synthetic (WIN55,212-2 and CP55,940) cannabinoids cause hypotension involving a sympathoinhibitory action; the cannabinoids cause a decrease in noradrenaline release from peripheral sympathetic nerves, an effect involving CB1 receptors (Varga et al., 1996; Malinowska et al., 1997; Niederhoffer and Szabo, 1999; Niederhoffer et al., 2003). Furthermore, cannabinoids have been demonstrated to inhibit sympathetic neurotransmission in rabbit heart in vivo (Szabo et al., 2001) and isolated atria of rat (Ishac et al., 1996), also via CB1 receptors. Sympathoinhibitory effects of the synthetic cannabinoids HU210, CP55,940 and methanandamide have been reported in the isolated mesenteric vascular bed of rat at low tone (Ralevic and Kendall, 2002). HU210 inhibited both the neurogenic contractile response and the evoked release of endogenous noradrenaline, an effect involving CB1-like receptors located on the postganglionic sympathetic nerve fibres (Ralevic and Kendall, 2002).
While cannabinoids have been shown to inhibit the overall sympathetic response, there is little information about the effects of cannabinoids on sympathetic cotransmission. Therefore, the aim of the present study was to investigate whether synthetic and endogenous cannabinoids can modulate differentially the noradrenergic and purinergic components of sympathetic neurotransmission in the perfused mesenteric vascular bed of rat, under raised tone conditions.
Materials and methods
Mesenteric vascular bed preparation
Male Wistar rats (225–250 g) (Charles River Laboratory; Kent, UK) were killed by CO2 overdose followed by exsanguination. The abdominal cavity was opened and the superior mesenteric artery was identified, cleaned of connective tissue and cannulated with a blunted hypodermic needle (no. 21). The superior mesenteric vein was cut and the preparation flushed with 0.5 ml Krebs' solution. The mesenteric vascular bed was separated from the gut by carefully cutting close to the intestinal wall. The preparation was then placed on a stainless steel grid (7 × 5 cm) in a humid chamber and perfused at a constant flow rate of 5 ml/min, using a peristaltic pump (model 7554-30; Cole-Parmer Instrument, Chicago, IL, USA). Krebs' solution was composed of the following (mM): NaCl 118, NaHCO3 25, KCl 4.8, KH2PO4 1.2, MgSO4·7H2O 1.2, CaCl2 1.25 and glucose 11.1. The solution was maintained at 37°C and continually gassed with a 95% O2/5% CO2 gas mixture.
Mesenteric vascular responses were detected as changes in perfusion pressure (mm Hg). This was monitored continuously using a pressure transducer (model P23XL; Viggo-Spectramed, Oxnard, CA, USA) and recorded using a PowerLab (ADInstruments Pty Ltd, Castle Hill, Australia). The preparation was allowed to equilibrate for 30 min before experimentation. Electrical field stimulation (EFS) was applied with a Grass SD9 stimulator.
Experimental protocols
Preparations were pretreated with capsaicin (1 μM) for 20 min, followed by a 30 min washout period, in order to cause a depletion of sensory neurotransmitters and desensitization of vanilloid receptors. Perfusion of the isolated mesenteric arterial bed of rat with 0.1 μM capsaicin for 20 min has been shown to abolish sensory neurogenic vasorelaxation induced by EFS, and perfusion with 0.3 μM capsaicin for 20 min has been shown to markedly diminish the intensity of calcitonin gene-related peptide (CGRP)-like immunoreactive nerves (Kawasaki et al., 1988). After 30 min washout, U46619 (0.1–0.5 μM) was added to the perfusate to raise tone (by 30–60 mm Hg above baseline). EFS (5–40 Hz, 90 V, 1 ms for 30 s at 5-min intervals) was applied. Contractile responses to EFS under raised tone conditions were examined in the absence (time control) and in the presence of the cannabinoid receptor agonists CP55,940 (1 μM), anandamide (1 μM) or WIN55,212-2 (1 μM). The effects of WIN55,212-3 (1 μM), an inactive enantiomer of WIN55,212-2, on responses to EFS were also tested. In separate experiments, either LY320135 (1 μM), a cannabinoid CB1 receptor antagonist, or SR144528 (1 μM), a cannabinoid CB2 receptor antagonist were added at the start of the experiment. To study the effects of the cannabinoids on the purinergic and noradrenergic components of sympathetic neurotransmission, either prazosin (0.1 μM; α1-adrenoceptor antagonist) or α,β-methyleneATP (α,β-meATP) (1 μM; P2X receptor agonist and desensitizing agent; Ralevic and Burnstock, 1988) was added to the perfusate, before the effects of anandamide or WIN55,212-2 on responses to EFS were evaluated.
In another set of experiments, dose-dependent contractile responses to exogenous noradrenaline or α,β-meATP were obtained, also under conditions of raised tone induced by U46619. Preparations were injected with bolus doses of noradrenaline (1 μM to 1 mM) or α,β-meATP (10 μM to 1 mM) through neoprene rubber tubing proximal to the tissue, under raised tone conditions, in the absence or presence of cannabinoid receptor agonists (anandamide, WIN55,212-2 or CP55,940).
Data analysis
Contractile responses (mm Hg) to EFS or drugs were expressed as mean±s.e.m. Data were compared by using two-way analysis of variance (ANOVA) with a Bonferroni post hoc test to assess differences between individual points on the curve. Other comparisons were made using a Student's paired or unpaired t-test, or by a one-way ANOVA followed by Dunn's multiple comparison test, as appropriate. A value of P<0.05 was taken to indicate statistical significance.
Drugs
Prazosin, α,β-meATP, 8-methyl-N-vanillyl-6-nonenamide (capsaicin) and U46619 were obtained from Sigma (Poole, Dorset, UK). Anandamide, WIN55,212-2, CP55,940, LY320125 and WIN55,212-3 were obtained from Tocris (Bristol, UK). SR144528 was supplied by Sanofi-Aventis (Montpellier, France). Prazosin and α,β-meATP were dissolved in water and stock solutions of all other drugs were dissolved in ethanol before dilution in distilled water.
Results
Effect of cannabinoid receptor agonists on contractile response to EFS in the perfused mesenteric vascular bed of rat with tone raised by U46619
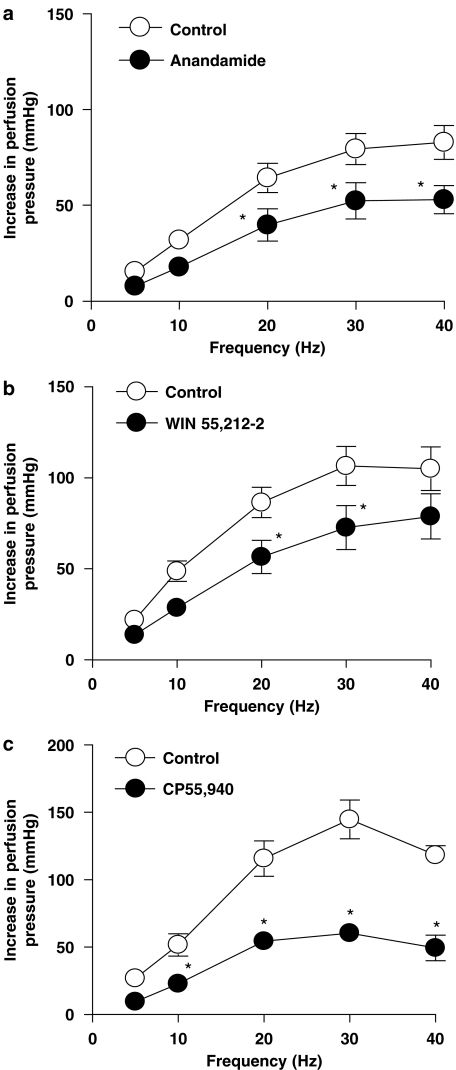
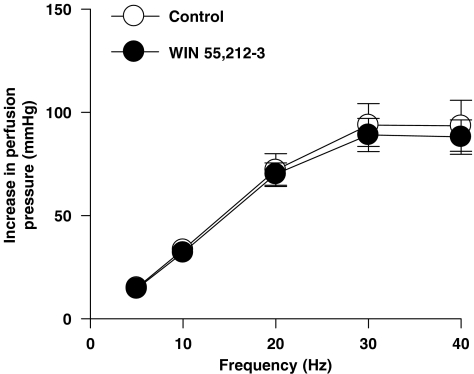
After exposure to U46619, perfusion pressure in the mesenteric vascular bed of rat significantly increased from 14.4±1.6 mm Hg in the absence of U46619 to 62.1±1.9 mm Hg in the presence of U46619 (P<0.05, n=5, Student's paired t-test). There was no significant difference in the level of tone produced by U46619 between any of the experimental conditions (ANOVA, P>0.05). In preparations in which tone had been raised with U46619, anandamide, WIN55,212-2 and CP55,940 (1 μM) caused a significant reduction of contractile responses to sympathetic nerve stimulation (ANOVA, P<0.05) (Figures 1a–c). In contrast, the contractile responses to EFS under U46619-induced raised tone conditions were not changed by the inactive enantiomer of WIN55212-2, WIN55,212-3 (Figure 2). The cannabinoid agonists had no significant effect on the tone of the preparations (Student's paired t-test, P<0.05).
Figure 1.
Effect of cannabinoid receptor agonists, anandamide (1 μM) (a), WIN55,212-2 (1 μM) (b) and CP55,940 (1 μM) (c) on contractile responses to EFS (5–40 Hz, 1 ms, 90 V, 30 s) in the mesenteric vascular bed of rat with tone raised by U46619 (n=5–7). Data are shown as mean±s.e.m. *P<0.05 vs control groups (ANOVA). ANOVA, analysis of variance; EFS, electrical field stimulation.
Figure 2.
Effect of WIN55,212-3 (1 μM), the inactive enantiomer of WIN55,212-2, on contractile responses to EFS (5–40 Hz, 1 ms, 90 V, 30 s) in the perfused mesenteric vascular bed of rat with tone raised by U46619. Data are shown as mean±s.e.m. (n=4) (ANOVA). ANOVA, analysis of variance; EFS, electrical field stimulation.
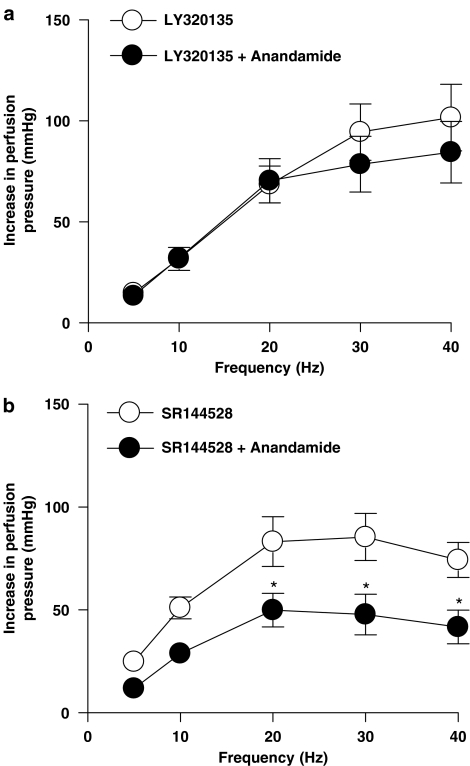
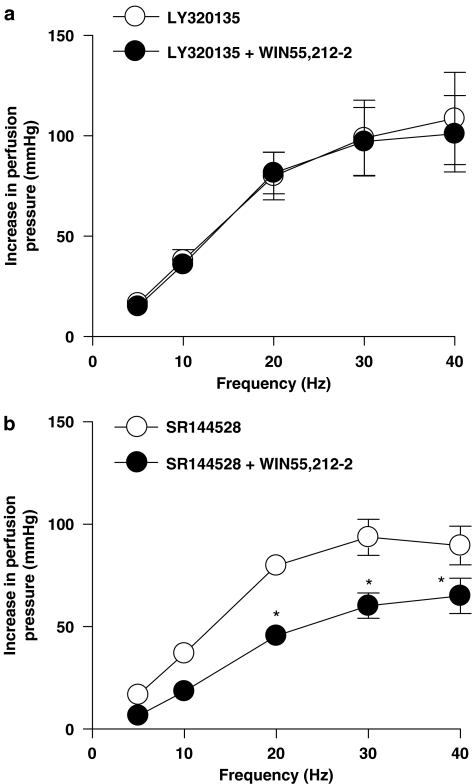
Effect of LY320135 and SR144528 on inhibitory effects of cannabinoid receptor agonists in the mesenteric vascular bed of rat with raised tone
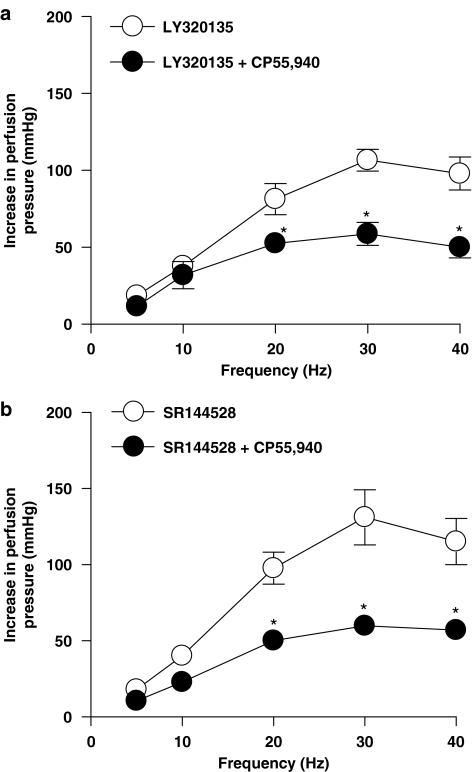
The cannabinoid CB1 receptor antagonist, LY320135 (1 μM), and the cannabinoid CB2 receptor antagonist, SR144528 (1 μM), alone had no significant effect on contractile responses to EFS (n=6; ANOVA, P>0.05; data not shown). Anandamide (Figure 3a) and WIN55,212-2 (Figure 4a) had no effect on responses to sympathetic nerve stimulation in the presence of LY320135. SR144528 did not reverse the inhibitory effects of anandamide (Figure 3b) or WIN55,212-2 (Figure 4b). Neither LY320135 nor SR144528 blocked the inhibitory effects of CP55,940 (Figures 5a and b).
Figure 3.
Effect of anandamide (1 μM) in the presence of the CB1 cannabinoid receptor antagonist, LY320135 (a), and the CB2 receptor antagonist, SR144528 (b), on contractile responses to EFS (5–40 Hz, 1 ms, 90 V, 30 s) in the perfused mesenteric vascular bed of rat with tone raised by U46619 (n=6). Data are shown as mean±s.e.m. P<0.05 vs control groups (ANOVA). ANOVA, analysis of variance; EFS, electrical field stimulation.
Figure 4.
Effect of WIN55,212-2 (1 μM) in the presence of a CB1 cannabinoid receptor antagonist, LY320135 (a), and CB2 receptor antagonist, SR144528 (b), on contractile responses to EFS (5–40 Hz, 1 ms, 90 V, 30 s) in the perfused mesenteric vascular bed of rat with tone raised by U46619. Data are shown as mean±s.e.m. (n=4–6). *P<0.05 vs control groups (ANOVA). ANOVA, analysis of variance; EFS, electrical field stimulation.
Figure 5.
Effect of CP55,940 in the presence of the CB1 cannabinoid receptor antagonist, LY320135 (a), and CB2 receptor antagonist, SR144528 (b), on contractile responses to EFS (5–40 Hz, 1 ms, 90 V, 30 s) in the perfused mesenteric vascular bed of rat with tone raised by U46619 (n=5). Data are shown as mean±s.e.m. P<0.05 vs control groups (ANOVA). ANOVA, analysis of variance; EFS, electrical field stimulation.
Effects of cannabinoid agonists on responses to EFS in the presence of prazosin and α,β-meATP in the mesenteric vascular bed of rat with raised tone
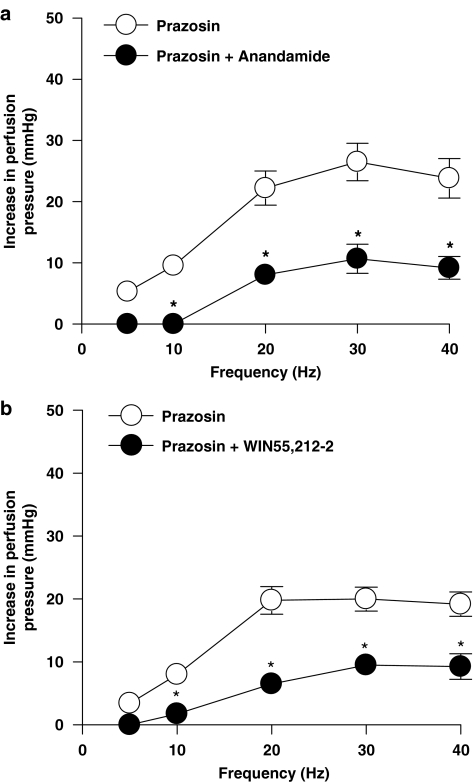
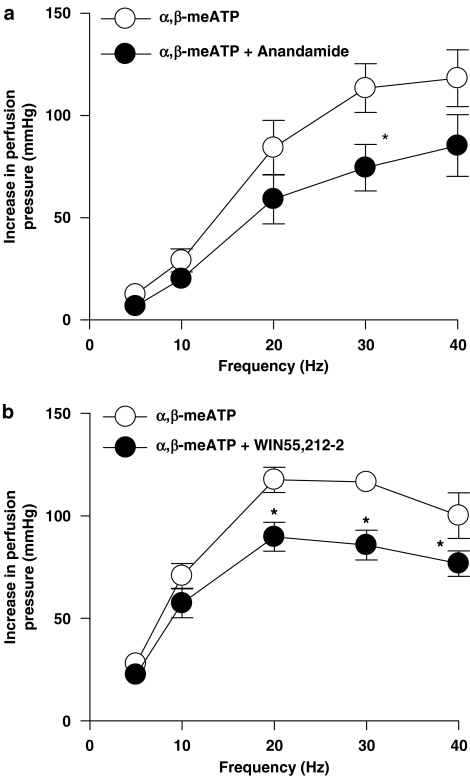
In the presence of prazosin (0.1 μM), anandamide (1 μM) (n=5) and WIN55,212-2 (1 μM) (n=6) significantly inhibited the remaining purinergic response to EFS (ANOVA, P<0.05) (Figures 6a and b). The inhibitory effects of anandamide and WIN55,212-2 were also apparent on the noradrenergic component, after desensitization of P2X receptors with α,β-meATP (1 μM) (ANOVA, P<0.05) (Figures 7a and b).
Figure 6.
Effect of the endogenous cannabinoid, anandamide (1 μM) (n=5) (a), and synthetic cannabinoid receptor agonist, WIN55,212-2 (1 μM) (n=6) (b), on contractile responses to EFS (5–40 Hz, 1 ms, 90 V, 30 s) in the presence of prazosin (0.1 μM) with tone raised by U46619. Data are shown as mean±s.e.m. *P<0.05 vs control groups (ANOVA). ANOVA, analysis of variance; EFS, electrical field stimulation.
Figure 7.
Effect of the endogenous cannabinoid, anandamide (1 μM) (n=7) (a), and synthetic cannabinoid receptor agonist, WIN55,212-2 (1 μM) (n=5) (b), on contractile responses to EFS (5–40 Hz, 1 ms, 90 V, 30 s) in the presence of α,β-meATP (1 μM) with tone raised by U46619. Data are shown as mean±s.e.m. *P<0.05 vs control groups (ANOVA). α,β-meATP, α, β-methylene ATP; ANOVA, analysis of variance; EFS, electrical field stimulation.
The cannabinoids had a quantitatively different effect on the noradrenaline and ATP components of sympathetic neurotransmission, with a greater inhibition observed on the purinergic component. For example, anandamide inhibited the purinergic component (in the presence of prazosin) by 100±0 and 59.4±8.1% (n=5) of the response at 10 and 30 Hz, respectively, compared with an inhibition of the noradrenergic component (in the presence of α,β-meATP) of 28.9±5.3 and 36±3.2% (n=7) at 10 and 30 Hz, respectively (one-way ANOVA followed by Dunn's multiple comparison test, P<0.05). It is possible that the greater inhibition produced by cannabinoids was a consequence of the purinergic responses being smaller than the noradrenergic component. However, anandamide caused a significantly greater inhibition of the purinergic response at 30 Hz; 26.5±3.1 mm Hg reduced by 59.4±8.1% (n=5), compared with its inhibition of the noradrenergic response at 10 Hz; 29.2±5.7 mm Hg reduced by 28.9±5.3% (n=7) (one-way ANOVA followed by Dunn's multiple comparison test, P<0.05), indicating that when responses are matched for amplitude, anandamide still produces a significantly greater inhibition of the purinergic component.
Effect of CP55,940, anandamide and WIN55,212-2 on contractile response to exogenous noradrenaline and α,β-meATP in the mesenteric vascular bed of rat with raised tone
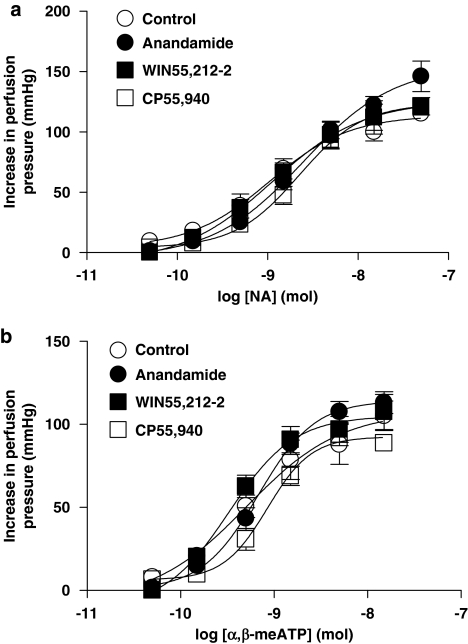
Dose-dependent pressor responses to noradrenaline (Figure 8a) and α,β-meATP (Figure 8b) were not affected by CP55,940 (1 μM) (n=4), anandamide (1 μM) (n=5) or WIN55,212-2 (1 μM) (n=6).
Figure 8.
Contractile response to exogenous NA (n=4–6) (a), and α,β-meATP (n=4–6) (b), in the absence (control) and in the presence of the cannabinoid receptor agonists, CP55,940, anandamide or WIN55,212-2 with tone raised by U46619. Data are shown as mean±s.e.m. There was no significant difference between the groups (ANOVA). α,β-meATP, α, β-methylene ATP; ANOVA, analysis of variance; NA, noradrenaline.
Discussion
The present findings show that anandamide, WIN55,212-2 and CP55,940 produce inhibitory actions on sympathetic neurotransmission in the perfused mesenteric vascular bed of rat with tone raised by U46619. The inhibitory actions of anandamide and WIN55,212-2 were blocked by LY320135, a cannabinoid CB1 receptor antagonist, but not by SR144528, a cannabinoid CB2 receptor antagonist. In addition, WIN55,212-3, the inactive S(−) enantiomer of WIN55,212-2 had no effect on sympathetic neurogenic responses under these conditions. None of the cannabinoids affected contractile responses to exogenous noradrenaline or α,β-meATP, or produced a direct vascular response. These results indicate that prejunctional CB1-like receptors mediate the sympathoinhibitory actions of anandamide and WIN55,212-2 in the mesenteric arterial bed of rat. Interestingly, anandamide and CP55,940, at the same concentrations used in the present study, had no effect on sympathetic neurotransmission in isolated small mesenteric arteries of rat (Lay et al., 2000), showing substantial differences between responses mediated by cannabinoids in the whole mesenteric bed and small mesenteric arteries of rat.
The choice of concentration of cannabinoid receptor agonists used in this study, 1 μM, was influenced by our previous observations showing that CP55,940 and HU210 at this concentration caused submaximal inhibition (44 and 43% inhibition, respectively) of contractions mediated by sympathetic nerves in the mesenteric bed of rat (Ralevic and Kendall, 2002). In that study, 0.3 μM CP55,940 and HU210 had little or no effect, and at 3 μM, HU210 caused an 86% inhibition, indicating a steep concentration–response effect (Ralevic and Kendall, 2002). Micromolar concentrations of cannabinoid receptor agonists have also been used by other researchers investigating cannabinoid modulation of sympathetic neurotransmission in a variety of biological tissues (Ralevic, 2003). This is in line with negative log of the effector concentration for half-maximum response (pEC50) values of 6, 6.5 and 5.1 obtained for CP55,940, WIN55,212-2 and anandamide, respectively, for stimulation of basal cyclic adenosine monophosphate (cAMP) accumulation in Chinese hamster ovary (CHO) cells expressing human CB1 receptors (Bonhaus et al., 1998). However, CP55,940, WIN55,212-2 and anandamide are more potent at modulating forskolin-stimulated cAMP accumulation in CHO cells expressing human CB1 receptors (for example, pEC50 values of 8.9, 7.1 and 5.7, respectively, in causing inhibition of forskolin-stimulated cAMP accumulation) (Bonhaus et al., 1998). The cannabinoid receptor agonists we used have, in general, a relative potency and affinity order of CP55,940>WIN55,212-2>anandamide at the CB1 receptor (Bonhaus et al., 1998; Howlett et al., 2002), while at the CB2 receptor CP55,940 and WIN55,212-2 have similar affinities, and both have higher affinities than anandamide (a partial agonist at CB1 and CB2 receptors) (Howlett et al., 2002). In the present study, an apparently greater inhibitory effect of CP55,940 than of WIN55,212-2 and anandamide on sympathetic neurogenic contractions was observed.
LY320135 and SR144528 were used at 1 μM in the present study, the concentration at which they are selective antagonists at CB1 and CB2 receptors, respectively. LY3210135 has been shown to reverse anandamide-mediated stimulation of cAMP formation in CHO cells expressing human CB1 receptors with an IC50 (half-maximal inhibitory concentration) value of 734 nM, and in binding studies showed a 106-fold higher affinity for CB1 vs CB2 receptors expressed in CHO cells (Felder et al., 1998). SR144528 has an EC50 (effector concentration for half-maximum response) value of 10 nM in antagonizing the inhibitory effect of CP55,940 on forskolin-stimulated adenylyl cyclase activity in CHO cells expressing human CB2 receptors, but had no effect on CB1 receptors up to 10 μM (binding studies showed a 700-fold higher affinity for the CB2 vs the CB1 receptor) (Rinaldi-Carmona et al., 1998). In the present study, the effect of CP55,940 was not blocked by either LY320135 or SR144528, in agreement with our previous observations in the mesenteric arterial bed at low tone (Ralevic and Kendall, 2002), which indicates possible actions of CP55,940 at non-CB1/CB2 receptors (Lake et al., 1997; Jarai et al., 1999; Ford et al., 2002). Lay et al. (2000) have also reported that an inhibitory effect of CP55,940 on sympathetic neurogenic contractions, of the rat vas deferens, was not blocked by CB1 or CB2 antagonists. Non-CB1 and -CB2 receptor-mediated effects of cannabinoids on sympathetic neurotransmission have also been reported for WIN55,212, which inhibited the slow-onset second phase of contraction to EFS in the rabbit vas deferens in a manner that was unaffected by CB1 and CB2 receptor antagonists (Barun et al., 2005).
The cannabinoids used in the present study had no significant effect on tone of the isolated mesenteric arterial preparations of rat. Zygmunt et al. (1999) have shown that in the isolated mesenteric artery of rat, the endogenous cannabinoid anandamide is a potent vasodilator, acting by stimulation of vanilloid receptors on perivascular capsaicin-sensitive sensory nerves to release CGRP, a vasodilator neuropeptide. The analogue, methanandamide, is also a vasodilator in the mesenteric arterial bed of rat, also acting via vanilloid receptor-dependent mechanisms (Ralevic et al., 2000). The explanation for this difference is that, in the present study, mesenteric arterial beds of rat were pretreated with capsaicin in order to cause a depletion of sensory neurotransmitter and desensitization of sensory nerves; this removed a vasorelaxant effect of anandamide at vanilloid receptors on sensory nerves and also eliminated an opposing sensory neurogenic vasorelaxant component to our responses to EFS (Pakdeechote et al., 2007). Other cannabinoids, including WIN55,212-2 and CP55,940, have been shown to act as vasodilators in the mesenteric arteries of rat, via vanilloid receptor-independent mechanisms (White and Hiley, 1998), but had no significant direct vascular response in the present study. In the present study, none of the cannabinoids affected contractile responses to exogenous noradrenaline or α,β-meATP (acting via P2X receptors). Hence, these results are consistent with the fact that the CB1 receptors that mediate the sympathoinhibitory actions of anandamide and WIN55,212-2 in the mesenteric arterial bed of rat are located prejunctionally on sympathetic nerve varicosities.
These results add to a growing body of evidence that cannabinoids can inhibit autonomic neurotransmission in vitro and in vivo in a variety of tissues/organs and species by prejunctional mechanisms (see Ralevic, 2003 for review). However, little is known about whether the release of the sympathetic cotransmitters noradrenaline and ATP, is differently regulated by cannabinoids. In the present study, the tone of the mesenteric arterial bed of rat was raised with U46619, which, in addition to approximating more closely physiological conditions, uncovers a purinergic component of sympathetic neurotransmission (Pakdeechote et al., 2007). A part of our study, therefore, focussed on investigating cannabinoid effects on the noradrenergic and purinergic components of sympathetic neurotransmission (in the presence of α,β-meATP and prazosin, respectively).
In the presence of prazosin, to block α1-adrenoceptors, thereby revealing a purinergic component of sympathetic neurotransmission, anandamide and WIN55,212-2 decreased contractile responses to sympathetic nerve stimulation. Thus, cannabinoids can modulate the purinergic component of sympathetic neurotransmission in the perfused mesenteric vascular bed of rat with tone raised by U46619. Furthermore, we also observed an inhibitory action of anandamide and WIN55212-2 in the presence of α,β-meATP (to block responses at P2X receptors), indicating modulation of the noradrenergic component of sympathetic neurotransmission. Therefore, under raised tone conditions cannabinoids inhibited sympathetic neurogenic responses mediated by both of the cotransmitters, ATP and noradrenaline. In the vas deferens of rabbit, WIN55,212 and anandamide have been shown to inhibit both the first phase and the slow-onset second phase of the biphasic contraction to EFS, with the phases attributed to ATP acting at P2X receptors and noradrenaline acting at α1-adrenoceptors, respectively (Barun et al., 2005). In the urinary bladder of mouse, WIN55,212 attenuated both the muscarinic and purinergic components of neurotransmission (Martin et al., 2000).
Noradrenaline and ATP are co-stored in vesicles in the sympathetic nerve terminals, and there is some evidence that their release may be differentially modulated (Ellis and Burnstock, 1989; von Kügelgen and Starke, 1991; Driessen et al., 1994; Todorov et al., 1996; Dunn et al., 1999). In the present study, there appeared to be a quantitative difference in the effect of the cannabinoids on the noradrenaline and ATP components of sympathetic neurotransmission. Anandamide and WIN55,212-2 were more effective at reducing the purinergic component (in the presence of prazosin) than the noradrenergic component (in the presence of α,β-meATP) of responses to sympathetic nerve activation. It is possible that this was a consequence of the purinergic response being smaller than the noradrenergic response. However, when responses were matched for amplitude, anandamide still produced a greater inhibition of purinergic than of similarly sized noradrenergic contractile responses, indicating that the two components may be differentially regulated. Other researchers have also reported a greater prejunctional inhibition of the purinergic component compared to the noradrenergic component of sympathetic neurotransmission, for example, by A1 adenosine receptors (Driessen et al., 1994) and CGRP (Ellis and Burnstock, 1989) in the vas deferens of guinea-pig. In the vas deferens of rabbit, there is evidence of differential modulation by WIN55,212 of the noradrenergic and purinergic components of sympathetic neurotransmission, as only the effect of WIN55,212 on the first phase of the biphasic contractile response to EFS (purinergic component) was reversed by a CB1 receptor antagonist (Barun et al., 2005).
In conclusion, this study indicates that in the perfused mesenteric vascular bed of rat with tone raised by U46619, anandamide and WIN55,212-2 can activate prejunctional CB1 receptors to inhibit the release of both noradrenaline and ATP from sympathetic nerve terminals. CP55,940 also inhibits prejunctionally sympathetic neurotransmission, an effect that appears to be mediated by a non-CB1/CB2 receptor. Cannabinoids appear to have a greater inhibitory effect on the purinergic compared to the noradrenergic component of neurotransmission.
Acknowledgments
This work was supported by a grant from the British Heart Foundation (PG/03/116/16045). PP holds a scholarship from the Royal Thai Government.
Abbreviations
- α,β-meATP
α, β-methyleneATP
- CGRP
calcitonin gene-related peptide
- CHO
Chinese hamster ovary
- EFS
electrical field stimulation
Conflict of interest
The authors state no conflict of interest.
References
- Angus JA, Broughton A, Mulvany MJ. Role of alpha-adrenoceptors in constrictor responses of rat, guinea-pig and rabbit small arteries to neural activation. J Physiol. 1988;403:495–510. doi: 10.1113/jphysiol.1988.sp017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barun S, Vural IM, Dileköz Ercan ZS, Sarioglu Y. Effects of cannabinoid receptor activation on rabbit vas deferens strips. Clin Exp Pharmacol Physiol. 2005;32:702–707. doi: 10.1111/j.1440-1681.2005.04261.x. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Chang LK, Martin GR. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther. 1998;287:884–888. [PubMed] [Google Scholar]
- Burnstock G. The fifth Heymans memorial lecture-Ghent, February 17, 1990. Co-transmission. Arch Int Pharmacodyn Ther. 1990;304:7–33. [PubMed] [Google Scholar]
- Burnstock G, Kennedy YC. Is there a basis for distinguishing two types of P2-purinoceptors. Gen Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signaling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Driessen B, von Kugelgen I, Starke K. P1-purinoceptor-mediated modulation of neural noradrenaline and ATP release in guinea-pig vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:42–48. doi: 10.1007/BF00180009. [DOI] [PubMed] [Google Scholar]
- Dunn WR, Brock JK, Hardy TA. Electrochemical and electrophysiological characterization of neurotransmitter release from sympathetic nerves supplying rat mesenteric arteries. Br J Pharmacol. 1999;128:174–180. doi: 10.1038/sj.bjp.0702760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenburg DC. Functional characterization of the pre- and postjunctional alpha-adrenoceptors in the in situ perfused rat mesenteric vascular bed. Eur J Pharmacol. 1984;105:161–165. doi: 10.1016/0014-2999(84)90661-7. [DOI] [PubMed] [Google Scholar]
- Ellis JL, Burnstock G. Modulation of neurotransmission in the guinea-pig vas deferens by capsaicin: involvement of calcitonin gene-related peptide and substance P. Br J Pharmacol. 1989;98:707–713. doi: 10.1111/j.1476-5381.1989.tb12646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Glass M, Mackie KP, Fahey KJ, et al. LY321035, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. J Pharmacol Exp Ther. 1998;284:291–297. [PubMed] [Google Scholar]
- Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitterman DP, Evans RJ. Nerve evoked P2X receptor contractions of rat mesenteric arteries; dependence on vessel size and lack of role of L-type calcium channels and calcium induced calcium release. Br J Pharmacol. 2001;132:1201–1208. doi: 10.1038/sj.bjp.0703925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;2396:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Kong JQ, Taylor DA, Fleming WW. Functional distribution and role of Alpha-1 adrenoceptor subtypes in the mesenteric vasculature of the rat. J Pharmacol Exp Ther. 1994;268:1153–1159. [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Lay L, Angus JA, Wright CE. Pharmacological characterisation of cannabinoid CB1 receptors in the rat and mouse. Eur J Pharmacol. 2000;391:151–161. doi: 10.1016/s0014-2999(00)00062-5. [DOI] [PubMed] [Google Scholar]
- Luo M, Hess MC, Fink GD, Olson LK, Rogers J, Kreulen DL, et al. Differential alterations in sympathetic neurotransmission in mesenteric arteries and veins in DOCA-salt hypertensive rats. Auton Neurosci. 2003;104:47–57. doi: 10.1016/S1566-0702(02)00287-4. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Godlewski G, Bucher B, Schlicker E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:197–202. doi: 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- Martin RS, Luong LA, Welsh NJ, Eglen RM, Martin GR, MacLennan SJ. Effects of cannabinoid receptor agonists on neuronally-evoked contractions of urinary bladder tissues isolated from rat, mouse, pig, dog, monkey and human. Br J Pharmacol. 2000;129:1707–1715. doi: 10.1038/sj.bjp.0703229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br J Pharmacol. 1999;126:457–466. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakdeechote P, Rummery NM, Ralevic V, Dunn WR. Raised tone reveals purinergic-mediated responses to sympathetic nerve stimulation in the rat perfused mesenteric vascular bed. Eur J Pharmacol. 2007;563:180–186. doi: 10.1016/j.ejphar.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- Ralevic V. Cannabinoid modulation of peripheral autonomic and sensory neurotransmission. Eur J Pharmacol. 2003;472:1–21. doi: 10.1016/s0014-2999(03)01813-2. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Actions mediated by P2-purinoceptor subtypes in the isolated perfused mesenteric bed of the rat. Br J Pharmacol. 1988;95:637–645. doi: 10.1111/j.1476-5381.1988.tb11686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA. Cannabinoids inhibit pre- and postjunctionally sympathetic neurotransmission in rat mesenteric arteries. Eur J Pharmacol. 2002;444:171–181. doi: 10.1016/s0014-2999(02)01597-2. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Högëstätt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed. Br J Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O' Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq J-M, Casellas P, Congy C, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Sjoblom-Widfeldt N, Gustafsson H, Nilsson H. Sympathetic transmission in small mesenteric arteries from the rat: influence of impulse pattern. Acta Physiol Scand. 1990;138:523–528. doi: 10.1111/j.1748-1716.1990.tb08880.x. [DOI] [PubMed] [Google Scholar]
- Szabo B, Nordheim U, Niederhoffer N. Effects of cannabinoids on sympathetic and parasympathetic neuroeffector transmission in the rabbit heart. J Pharmacol Exp Ther. 2001;297:819–826. [PubMed] [Google Scholar]
- Todorov LD, Mihaylova-Todorova S, Craviso GL, Bjur RA, Westfall DP. Evidence for the differential release of the cotransmitters ATP and noradrenaline from sympathetic nerves of the guinea-pig vas deferens. J Physiol. 1996;496:731–748. doi: 10.1113/jphysiol.1996.sp021723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Lake KD, Huangfu D, Guyenet PG. Mechanism of the hypotensive action of anandamide in anesthetized rats. Hypertension. 1996;28:682–686. doi: 10.1161/01.hyp.28.4.682. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Starke K. Release of noradrenaline and ATP by electrical stimulation and nicotine in guinea-pig vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:419–429. doi: 10.1007/BF00172581. [DOI] [PubMed] [Google Scholar]
- White R, Hiley CR. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Clarke DE. Characterization of alpha 1-adrenoceptors mediating vasoconstriction to noradrenaline and nerve stimulation in the isolated perfused mesentery of rat. Br J Pharmacol. 1995;114:531–536. doi: 10.1111/j.1476-5381.1995.tb13259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Takasaki K, Nickols GA. Purinergic vasoconstrictor component revealed by moderate cooling in the isolated mesenteric vasculature of Sprague–Dawley rats. J Pharmacol Exp Ther. 1992;262:1133–1138. [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]