Abstract
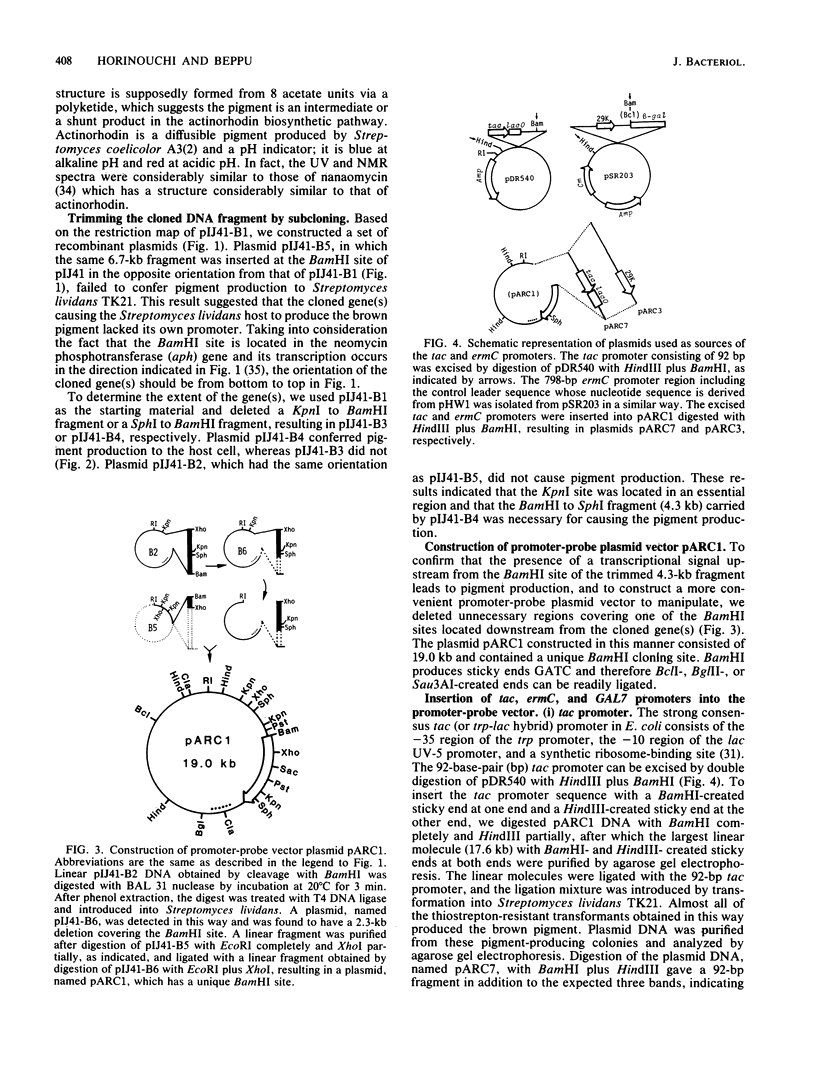
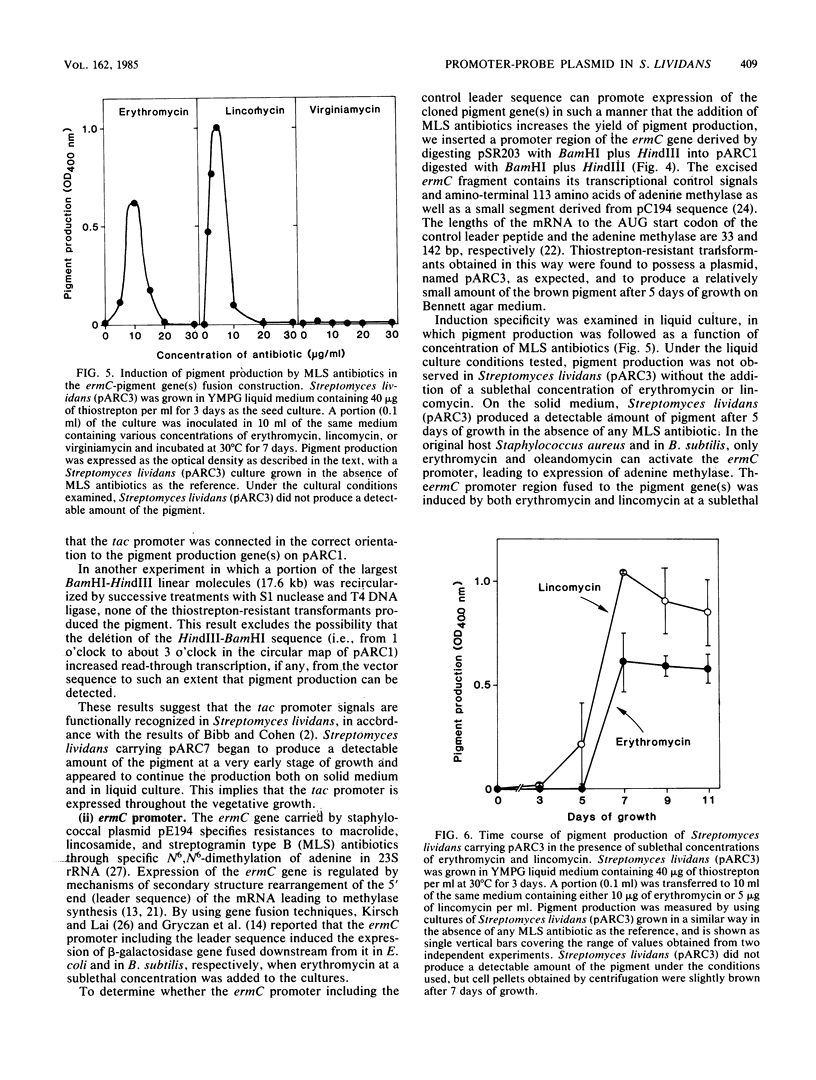
We cloned a Streptomyces coelicolor A3(2) DNA fragment which directed synthesis of a brown pigment, presumably a shunt product in the actinorhodin biosynthetic pathway, on the plasmid vector pIJ41 in Streptomyces lividans. The pigment production was observed only when the DNA fragment was inserted downstream from a functional promoter sequence. By subcloning the fragment together with in vitro manipulation, a promoter-probe plasmid vector (pARC1) with a unique BamHI cloning site was constructed that allows chromogenic identification of transcriptional control signals in Streptomyces lividans based on the expression of the cloned pigment gene(s). The Escherichia coli tac (trp-lac hybrid) promoter, consisting of 92 base pairs and a promoter region including the leader sequence of erythromycin resistance gene (ermC) on staphylococcal plasmid pE194, when ligated in the correct orientation in the BamHI site of pARC1, promoted expression of the cloned pigment gene(s) in Streptomyces lividans, whereas the Saccharomyces cerevisiae GAL7 promoter did not. In the case of the ermC, induction of the pigment production by the addition of either erythromycin or lincomycin, but not virginiamycin, was observed. The system was also shown to be useful and convenient in isolating transcriptional control signals of Streptomyces chromosomal DNA and estimating their activities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibb M. J., Cohen S. N. Gene expression in Streptomyces: construction and application of promoter-probe plasmid vectors in Streptomyces lividans. Mol Gen Genet. 1982;187(2):265–277. doi: 10.1007/BF00331128. [DOI] [PubMed] [Google Scholar]
- Bibb M., Schottel J. L., Cohen S. N. A DNA cloning system for interspecies gene transfer in antibiotic-producing Streptomyces. Nature. 1980 Apr 10;284(5756):526–531. doi: 10.1038/284526a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Bruton C. J. Mutational cloning in Streptomyces and the isolation of antibiotic production genes. Gene. 1983 Dec;26(1):67–78. doi: 10.1016/0378-1119(83)90037-9. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Chen C. W., Thomas C. A., Jr Recovery of DNA segments from agarose gels. Anal Biochem. 1980 Jan 15;101(2):339–341. doi: 10.1016/0003-2697(80)90197-9. [DOI] [PubMed] [Google Scholar]
- Feitelson J. S., Hopwood D. A. Cloning of a Streptomyces gene for an O-methyltransferase involved in antibiotic biosynthesis. Mol Gen Genet. 1983;190(3):394–398. doi: 10.1007/BF00331065. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y., Weisblum B. A family of r-determinants in Streptomyces spp. that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol. 1981 May;146(2):621–631. doi: 10.1128/jb.146.2.621-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J. A., Hopwood D. A. Cloning and expression of a p-aminobenzoic acid synthetase gene of the candicidin-producing Streptomyces griseus. Gene. 1983 Nov;25(1):119–132. doi: 10.1016/0378-1119(83)90174-9. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Grandi G., Hahn J., Grandi R., Dubnau D. Conformational alteration of mRNA structure and the posttranscriptional regulation of erythromycin-induced drug resistance. Nucleic Acids Res. 1980 Dec 20;8(24):6081–6097. doi: 10.1093/nar/8.24.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Israeli-Reches M., Dubnau D. Induction of macrolide-lincosamide-streptogramin B resistance requires ribosomes able to bind inducer. Mol Gen Genet. 1984;194(3):357–361. doi: 10.1007/BF00425544. [DOI] [PubMed] [Google Scholar]
- Hardy K., Haefeli C. Expression in Escherichia coli of a staphylococcal gene for resistance to macrolide, lincosamide, and streptogramin type B antibiotics. J Bacteriol. 1982 Oct;152(1):524–526. doi: 10.1128/jb.152.1.524-526.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Kikuchi Y., Nogi Y., Fukasawa T. Regulation of expression of the galactose gene cluster in Saccharomyces cerevisiae. Isolation and characterization of the regulatory gene GAL4. Mol Gen Genet. 1983;191(1):31–38. doi: 10.1007/BF00330886. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Hara O., Beppu T. Cloning of a pleiotropic gene that positively controls biosynthesis of A-factor, actinorhodin, and prodigiosin in Streptomyces coelicolor A3(2) and Streptomyces lividans. J Bacteriol. 1983 Sep;155(3):1238–1248. doi: 10.1128/jb.155.3.1238-1248.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Kumada Y., Beppu T. Unstable genetic determinant of A-factor biosynthesis in streptomycin-producing organisms: cloning and characterization. J Bacteriol. 1984 May;158(2):481–487. doi: 10.1128/jb.158.2.481-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pC194, a plasmid that specifies inducible chloramphenicol resistance. J Bacteriol. 1982 May;150(2):815–825. doi: 10.1128/jb.150.2.815-825.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. The control region for erythromycin resistance: free energy changes related to induction and mutation to constitutive expression. Mol Gen Genet. 1981;182(2):341–348. doi: 10.1007/BF00269681. [DOI] [PubMed] [Google Scholar]
- Jaurin B., Cohen S. N. Streptomyces lividans RNA polymerase recognizes and uses Escherichia coli transcriptional signals. Gene. 1984 Apr;28(1):83–91. doi: 10.1016/0378-1119(84)90090-8. [DOI] [PubMed] [Google Scholar]
- Kirsch D. R., Lai M. H. Regulation of a macrolide resistance-beta-galactosidase (ermC-lacZ) gene fusion in Escherichia coli. J Bacteriol. 1984 Jul;159(1):381–384. doi: 10.1128/jb.159.1.381-384.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Nogi Y., Fukasawa T. Nucleotide sequence of the transcriptional initiation region of the yeast GAL7 gene. Nucleic Acids Res. 1983 Dec 20;11(24):8555–8568. doi: 10.1093/nar/11.24.8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Schupp T., Toupet C., Stålhammar-Carlemalm M., Meyer J. Expression of a neomycin phosphotransferase gene from Streptomyces fradiae in Escherichia coli after interplasmidic recombination. Mol Gen Genet. 1983;189(1):27–33. doi: 10.1007/BF00326051. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Koyama Y., Nagai T., Marumo H., Omura S. Nanomycins, new antibiotics produced by a strain of Streptomyces. II. Structure and biosynthesis. J Antibiot (Tokyo) 1975 Nov;28(11):868–875. doi: 10.7164/antibiotics.28.868. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Gray G. S. Nucleotide sequence of a streptomycete aminoglycoside phosphotransferase gene and its relationship to phosphotransferases encoded by resistance plasmids. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5190–5194. doi: 10.1073/pnas.80.17.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J., Kieser T., Ward J. M., Hopwood D. A. Physical analysis of antibiotic-resistance genes from Streptomyces and their use in vector construction. Gene. 1982 Nov;20(1):51–62. doi: 10.1016/0378-1119(82)90086-5. [DOI] [PubMed] [Google Scholar]
- Thompson C. J., Ward J. M., Hopwood D. A. DNA cloning in Streptomyces: resistance genes from antibiotic-producing species. Nature. 1980 Jul 31;286(5772):525–527. doi: 10.1038/286525a0. [DOI] [PubMed] [Google Scholar]