Abstract
Alzheimer's disease is an age-related neurodegenerative condition associated with cognitive decline. The pathological hallmarks of the disease are the deposition of β-amyloid protein and hyperphosphorylation of tau, which evoke neuronal cell death and impair inter-neuronal communication. The disease is also associated with neuroinflammation, excitotoxicity and oxidative stress. In recent years the proclivity of cannabinoids to exert a neuroprotective influence has received substantial interest as a means to mitigate the symptoms of neurodegenerative conditions. In brains obtained from Alzheimer's patients alterations in components of the cannabinoid system have been reported, suggesting that the cannabinoid system either contributes to, or is altered by, the pathophysiology of the disease. Certain cannabinoids can protect neurons from the deleterious effects of β-amyloid and are capable of reducing tau phosphorylation. The propensity of cannabinoids to reduce β-amyloid-evoked oxidative stress and neurodegeneration, whilst stimulating neurotrophin expression neurogenesis, are interesting properties that may be beneficial in the treatment of Alzheimer's disease. Δ9-tetrahydrocannabinol can also inhibit acetylcholinesterase activity and limit amyloidogenesis which may improve cholinergic transmission and delay disease progression. Targeting cannabinoid receptors on microglia may reduce the neuroinflammation that is a feature of Alzheimer's disease, without causing psychoactive effects. Thus, cannabinoids offer a multi-faceted approach for the treatment of Alzheimer's disease by providing neuroprotection and reducing neuroinflammation, whilst simultaneously supporting the brain's intrinsic repair mechanisms by augmenting neurotrophin expression and enhancing neurogenesis. The evidence supporting a potential role for the cannabinoid system as a therapeutic target for the treatment of Alzheimer's disease will be reviewed herewith.
Keywords: Alzheimer's disease, cannabinoid, CB1 receptor, CB2 receptor, β-amyloid, neurodegeneration
Pathophysiology of Alzheimer's disease
Alzheimer's disease (AD) is a chronic debilitating neurodegenerative condition that is associated with progressive cognitive decline and profound neuronal loss, and estimated to affect 10% of people over the age of 65 years and 25% of people over the age of 80 years (Herbert et al., 2003). Western society is developing an increasingly aged population and this demographic shift is associated with a rise in the prevalence of age-related illnesses such as AD. The United Nations population projections estimate that 370 million people will be older than 80 years by 2050 and the associated increase in patients with AD will pose a substantial socio-economic burden. While a small proportion of AD cases have a genetic basis, the majority of cases are sporadic with unknown aetiology. A consistent feature of the AD brain is the presence of senile plaques composed of pathogenic extracellular deposits of β-amyloid (Aβ), a 1–42 amino acid peptide derived from aberrant processing of the transmembrane amyloid precursor protein (Walsh and Selkoe, 2007). Aβ fragments are proposed to play a central role in the genesis of the disease by evoking neuronal cell death (Boland and Campbell, 2003). The senile plaques are located within various brain regions but the hippocampus, cerebral cortex and amygdala are particularly vulnerable and plaques begin to form in these regions early in the disease process resulting in memory loss and behavioural changes (Ogomori et al., 1989). A second pathological hallmark of the disease is the hyperphosphorylation of the microtubule-associated protein, tau, resulting in formation of the intracellular neurofibrillary tangles that impair inter-neuronal communication (Mi and Johnson, 2006). AD is also associated with neuroinflammatory events and oxidative stress that are likely to exacerbate the disease process. Epidemiological studies support an involvement of inflammatory mechanisms in AD since patients using non-steroidal anti-inflammatory drugs for a 2-year period have a 60–80% reduction in the risk for the disease, while long-term non-steroidal anti-inflammatory drug treatment attenuates disease onset and reduces the severity of symptoms (Rich et al., 1995). Microglia are the Principal immune cells in the brain and in the AD brain they surround the senile plaques, possibly recruited to the plaque region in an attempt to clear the Aβ burden by phagocytosis (Wilkinson and Landreth, 2006). In AD, the Aβ deposition exceeds the phagocytic ability of the microglia and the persistent presence of activated microglia at the plaque results in a prolonged release of proinflammatory cytokines such as interleukin-1β (Bayer et al., 1999; Heneka and O'Banion, 2007) which mediate local inflammation and have the proclivity to increase the processing of amyloid precursor protein to generate more Aβ fragments (Heneka and O'Banion, 2007), as well as having a direct neurotoxic influence (Vereker et al., 2000). The association of activated microglia at the periphery of the senile plaque contributes to the generation of reactive oxygen species that mediate the oxidative damage found in the brains of patients with AD (Wilkinson and Landreth, 2006). Thus, inflammation and oxidative stress play a critical role in the disease process and anti-inflammatory and antioxidant strategies are likely to have enormous therapeutic potential for AD patients. Other factors that are thought to contribute to the pathophysiology of AD include dysregulation of intracellular calcium homeostasis and excitotoxicity (LaFerla, 2002). Cholinergic neurones are particularly vulnerable in AD and current therapeutics include acetylcholinesterase (AChE) inhibitors that aim to enhance acetylcholine (ACh) availability. However, such drugs are only suitable for the mild cognitive impairment that occurs early in the disease and no treatments are currently available to reverse the progression of the disease.
Cannabinoid system in the brain
The discovery of an endogenous cannabinoid (CB)-signalling system in the brain has prompted much research into understanding how this system regulates physiological and pathological events within the central nervous system. The endocannabinoid molecules, 2-arachidonoyl glycerol and anandamide, interact with the G-protein-coupled cannabinoid receptors, CB1 and CB2. These receptors are also activated by phytocannabinoids, such as Δ9-tetrahydrocannabinol (Δ9-THC), isolated from the Cannabis sativa plant. The action of endocannabinoids at their receptors is terminated by enzymatic degradation of the endocannabinoids, or by membrane transport (Piomelli, 2003). Early reports indicating a potential role for the cannabinoid system in the management of AD are based on the finding that Dronabinol, an oil-based solution of Δ9-THC, improves the disturbed behaviour and stimulates appetite in AD patients (Volicer et al., 1997), and alleviates nocturnal agitation in severely demented patients (Walther et al., 2006). More recently, an increasing body of evidence has accumulated to suggest antioxidant, anti-inflammatory and neuroprotective roles of the cannabinoid system (Jackson et al., 2005). Such properties may be harnessed to circumvent the neurodegenerative process and offer more effective approaches to treat AD (Pazos et al., 2004). In this review the recent experimental evidence that highlights the potential of the cannabinoid system to alleviate some of the pathology and cognitive decline associated with AD will be discussed.
The cannabinoid system in the AD brain
The CB1 receptor is abundant within the brain and associated with the cortex, hippocampus, cerebellum and basal ganglia (Herkenham et al., 1991). CB1 receptors in the hippocampus contribute to the effect of cannabinoids on learning and memory (Riedel and Davies, 2005); cognitive processes, which are disrupted early in the course of AD. CB2 receptors have a more limited expression in the central nervous system, being largely confined to neurones within the brainstem (Van Sickle et al., 2005), cerebellum (Ashton et al., 2006) and microglia (Nunez et al., 2004). Post-mortem studies of AD brains have detected increased expression of CB1 and CB2 receptors on microglia within the senile plaque, while CB1 expression is reduced in neurones more remote from the plaque (Ramirez et al., 2005). Also, cannabinoid receptors in the AD brain are nitrosylated, and this may contribute to the impaired coupling of these receptors to downstream effector signalling molecules (Ramirez et al., 2005). Other studies have failed to establish a link between changes in CB1 receptors in the AD brain and the specific pathological events that take place in this illness (Westlake et al. 1994), and report no changes in expression of CB1 receptors in the vicinity of the senile plaque (Benito et al., 2003). However, the endocannabinoid metabolizing enzyme, fatty acid amide hydrolase, is upregulated in the senile plaque (Benito et al., 2003), and may contribute to the increase in expression of anandamide metabolites, such as arachidonic acid, in the vicinity of the senile plaque. Such a pathway may be involved in increasing the production of prostaglandins and related pro-inflammatory molecules that are pertinent to the inflammatory process of AD. The association of fatty acid amide hydrolase with astrocytes within the senile plaque may participate in the astrocytic events that culminate in the reactive gliosis that is observed in regions rich in Aβ deposits (Wyss-Coray, 2006).
Cannabinoids mediate neuroprotection
Neuronal damage can increase the production of endocannabinoids (Stella et al., 1997; Marsicano et al., 2003), and cells lacking CB1 receptors are more vulnerable to damage (Marsicano et al., 2003). Those studies indicate that neural cannabinoid tone influences neuronal survival and suggest that augmentation of the cannabinoid system may offer protection against the deleterious consequences of pathogenic molecules such as Aβ. Recently, Aβ has been demonstrated to induce hippocampal degeneration, gliosis and cognitive decline, with a concomitant increase in the production of the endocannabinoid, 2-arachidonoyl glycerol, and this may reflect an attempt of the endocannabinoid system to provide neuroprotection from Aβ-induced damage (Van Der Stelt et al., 2006). Furthermore, in that study, when endocannabinoid uptake was inhibited by VDM-11, the Aβ-induced neurotoxicity and memory impairment were reversed, although this was dependent upon early administration of the reuptake inhibitor. Those findings suggest that robust and early pharmacological enhancement of brain endocannabinoid levels may protect against the deleterious consequences of Aβ. Other endocannabinoids, such as anandamide and noladin ether, have been found to reduce Aβ neurotoxicity in vitro via activation of the CB1 receptor and engagement the extracellular-regulated kinase pathway (Milton, 2002). Thus, endocannabinoids can reverse the negative consequences of exposure to Aβ, and such findings suggest that drugs designed to augment endocannabinoid tone, including inhibitors of membrane uptake and fatty acid amide hydrolase inhibitors, may have potential in the treatment of AD. However, the study by Van Der Stelt et al. (2006) cautions that the timing of endocannabinoid upregulation by pharmacological intervention in relation to the time-course of development of the disease pathology is crucial, since administration of VDM-11 later in the pathological cascade actually worsens memory retention in rodents. Also, the physiological role of the cannabinoid system in mnemonic processes should not be underestimated. In the hippocampus CB1 receptor activation is negatively associated with the performance of rodents in memory tasks (Castellano et al., 2003), possibly via a reduction in hippocampal ACh levels (Gifford et al., 2000), while the CB1 antagonist, SR141716A improves performance in memory tasks (Wolff and Leander, 2003). Furthermore, the impairment in memory evoked by Aβ in rodents is reversed by SR141716A (Mazzola et al., 2003), suggesting that CB1 receptor blockade may be beneficial in reversing the amnesia associated with AD. However, given the evidence for a neuroprotective role of the CB1 receptor (Marsicano et al., 2003; Alger, 2006), CB1 antagonists pose the risk of exacerbating the neurodegenerative component of the disease, which may negate the beneficial effects of such drugs on amnesia.
Cannabinoids and excitotoxicity
The dysregulation of intracellular Ca2+ homeostasis (Smith et al., 2005) and excessive activation of the N-methyl D-aspartate (NMDA) subtype of glutamate receptor, leading to excitotoxicity, are features of the AD brain (Sonkusare et al., 2005). All of the clinical mutations in the presenilin genes (PS1/PS2) that have been linked with the inherited form of AD disrupt calcium signalling (Smith et al., 2005), which may contribute to subsequent neurodegeneration and memory impairments (Rose and Konnerth, 2001). Also, Aβ can itself directly increase voltage-dependent Ca2+ channel activity (MacManus et al., 2000), as well as forming Ca2+-permeable pores in lipid bilayers (Arispe et al., 1993), to increase intracellular Ca2+ concentration as part of the pathogenic mechanism. Aβ also reduces glutamate uptake by astrocytes and increases the activation of glutamate receptors to evoke excitotoxicity (Sonkusare et al., 2005). Thus, strategies that reduce Ca2+ influx and limit excitotoxicity may confer neuroprotection in AD. The non-competitive NMDA receptor antagonist, memantine (Namenda, Ebixa) is used in the treatment of moderate to severe AD (Cosman et al., 2007), and its beneficial properties are based on an ability to inhibit pathological, but not physiological, functions of NMDA receptors, as well as antioxidant action and a propensity to increase production of brain-derived neurotrophic factor in the brain (Sonkusare et al., 2005). Manipulation of the cannabinoid system has several consequences that mirror those observed with memantine. Thus, the protective effects of some cannabinoids are related to the direct regulation of the NMDA receptor, since the non-psychotropic cannabinoid, HU-211, acts as a stereoselective inhibitor of the NMDA receptor and protects rat forebrain cultures (Nadler et al., 1993) and cortical neuronal cultures (Eshhar et al., 1993) from NMDA-induced neurotoxicity. Furthermore, activation of the CB1 receptor protects mouse spinal neurons (Abood et al., 2001) and cultured hippocampal neurones (Shen and Thayer, 1998) from excitotoxicity, possibly through inhibition of presynaptic Ca2+ entry (Mackie and Hille, 1992; Twitchell et al., 1997) and the subsequent suppression of excessive glutamatergic synaptic activity (Shen and Thayer, 1998; Takahashi and Castillo, 2006). CB1 receptor agonists also inhibit glutamate release, which may contribute to a reduction in excitotoxicity (Wang, 2003). The evidence for a Ca2+-dependent synthesis of anandamide and 2-arachidonoyl glycerol (Di Marzo et al., 1994; Stella et al., 1997) would suggest that endocannabinoids are generated in response to an intracellular Ca2+ load in an attempt to provide feedback inhibition of excitotoxicity. In this regard it is notable that endocannabinoid upregulation is a feature of a number of neurotoxic paradigms that are associated with elevated intracellular Ca2+ concentration (Hansen et al., 2001). Alternative mechanisms that are pivotal to cannabinoid-mediated protection include inhibition of [Ca2+]i by reducing calcium release from ryanodine-sensitive stores (Zhuang et al., 2005), inhibition of protein kinase A and reduced nitric oxide generation (Kim et al., 2006). Like memantine, cannabinoids are also capable of increasing brain-derived neurotrophic factor to confer protection against excitotoxicity (Khaspekov et al., 2004). In non-neuronal cells, the induction of nerve growth factor is also facilitated by cannabinoids, acting through the PI3K/PKB pathway (Sanchez et al., 2003), and activation of the CB1 receptor by the endocannabinoid, 2-arachidonoyl glycerol, can also couple to an axonal growth response, whereas CB1 receptor antagonists inhibit axonal growth (Williams et al., 2003). Thus, dampening excessive glutamatergic transmission and excitotoxicity, coupled with neurotrophic actions, may represent interesting actions of cannabinoids that could be exploited for the treatment of AD.
Cannabidiol prevents Aβ-mediated neurotoxicity
Cannabidiol (CBD) is the principal non-psychoactive component of Cannabis sativa, with potent antioxidant properties that offer neuroprotection against glutamate toxicity (Hampson et al., 1998). In differentiated PC12 cells exposed to Aβ, CBD reduces the induction of inducible nitric oxide synthase (iNOS), nitric oxide production and activation of the stress-activated protein kinase p38 and the inflammatory transcription factor, nuclear factor-κB (Esposito et al., 2006a), providing evidence for a CBD-mediated downregulation of the inflammatory signalling events associated with exposure to Aβ. As well, CBD reduces Aβ-induced neuronal cell death by virtue of its ability to scavenge reactive oxygen species and reduce lipid peroxidation; antioxidant properties that occur independently of the CB1 receptor (Iuvone et al., 2004). CBD also reverses tau hyperphosphorylation, a key hallmark of AD, by reducing phosphorylation of glycogen synthase kinase-3β, a tau protein kinase responsible for the tau hyperphosphorylation in AD (Esposito et al., 2006b). Moreover, since glycogen synthase kinase-3β also evokes amyloid precursor protein processing to increase Aβ production (Phiel et al., 2003), the CBD-mediated inhibition of glycogen synthase kinase-3β is likely to be effective in reducing the amyloid burden. Thus, from such in vitro studies one can speculate that CBD may be therapeutically beneficial in AD, since it can prevent the deleterious effects of Aβ and ameliorate several features of AD pathology, including tau hyperphosphorylation, oxidative stress, neuroinflammation and apoptosis. Whether such actions of CBD are retained in the AD brain remains to be established, and experiments to test the effect of CBD in the various transgenic animal models of AD are eagerly awaited. In the meantime, reports that CBD is effective as an antioxidant and neuroprotectant in an animal model of Parkinson's disease (Lastres-Becker et al., 2005), and orally effective in a rat model of chronic inflammation (Costa et al., 2007), lend support to its potential therapeutic value in AD. There are a number of advantages of CBD as a therapeutic agent for AD; it is devoid of psychoactive activity and since CB receptors are nitrosylated in the AD brain, a feature that may hinder CB receptors coupling to their downstream effectors (Ramirez et al., 2005), a therapy that does not depend on signalling through CB receptors may have a distinct advantage. Sativex is a cannabinoid-based oromucosal spray, containing CBD and THC, that is devoid of tolerance or withdrawal symptoms (Perez, 2006). This therapy is already available for the treatment of neuropathic pain and multiple sclerosis and may be exploited in the future for the treatment of AD.
CB2 receptors and neuroinflammation
The CB2 receptor is largely confined to glial cells in the brain (Nunez et al., 2004), although some studies have reported CB2 receptors in neuronal populations within the brainstem and cerebellum (van Sickle et al., 2005; Ashton et al., 2006). CB2 receptors have been implicated in the control of neural survival (Fernandez-Ruiz et al., 2007) and mediate neuroprotection through their anti-inflammatory actions (Ehrhart et al., 2005). CB2 receptors are upregulated in activated microglia and astrocytes, and this upregulation is proposed to control the local production of proinflammatory mediators such as interleukin-1β, reactive oxygen species and prostaglandins. In the AD brain and in animal models of AD-like pathology, CB2 receptors are upregulated within the active microglia present in those brain regions where senile plaques are abundant (Benito et al., 2003; Ramirez et al., 2005). The upregulation of CB2 in such pathological situations may be an attempt to reduce neuroinflammation since CB2 receptor activation in vitro reduces the microglial production of pro-inflammatory molecules (Facchinetti et al., 2003). Such control in the production of inflammatory mediators may be due to a direct impact on activity of transcription factors, such as nuclear factor κB (Panikashvili et al., 2005; Esposito et al., 2006a). Thus, the neuroprotective mechanisms of cannabinoids are likely to include a downregulation in activity of the transcription factors that are pertinent to induction of the pro-inflammatory cytokines that serve as key players in neurodegenerative disease, while also stimulating the production of anti-inflammatory species such as IL-1ra (Molina-Holgado et al., 2003). The manipulation of such inflammatory pathways may be exploited for the treatment of AD. In support of this contention, Ramirez et al. (2005) have demonstrated that in rats treated with Aβ, the induction of AD-like pathology and cognitive impairment, is reversed by the CB1/CB2 agonist, WIN,55212–22 and the CB2-selective agonist, JWH-133. Since the CB2 receptor was only associated with activated microglia located within the plaque, those authors have suggested that the CB2 receptor may be a promising target for AD by virtue of its ability to serve as a brake for the neuroinflammatory cascade that is a feature of AD. CB2 agonists offer the advantage of being devoid of psychoactivity, although it is important to recognize that they may have other side effects such as immune suppression (Pertwee, 2005), which would be undesirable in an elderly population.
Cannabinoids and neurogenesis in the adult brain
Another exciting mechanism that could account for the ability of cannabinoids to confer neuroprotection may be related to their regulation of neurogenesis. Adult neurogenesis can occur in the dentate gyrus of the hippocampus and the subventricular zone (Grote and Hannan, 2007), resulting in the presence of newly generated neurones. In several mouse models of AD neurogenesis is reduced (Dong et al., 2004), although it should be noted that in the post-mortem AD brain, neurogenesis is increased (Jin et al., 2004). Factors that enhance neurogenesis, such as dietary restriction and upregulation of brain-derived neurotrophic factor, enhance neurogenesis and improve the memory performance in animal models of AD (Lee et al., 2000). Thus, targeting adult neurogenesis is receiving interest as a means to mitigate the symptoms of AD. In this regard it is notable that the cannabinoid system also regulates neurogenesis (Galve-Roperh et al., 2007). Adult neurogenesis is defective in mice lacking CB1 receptors (Jin et al., 2004), and the synthetic cannabinoid, WIN55212-2, stimulates adult neurogenesis by opposing the antineurogenic effect of nitric oxide (Kim et al., 2006). Also, the CB1 agonist HU-210 has anxiolytic and antidepressant effects, which may be a functional consequence of enhanced neurogenesis (Jiang et al., 2005). CB2 receptor activation also stimulates neural progenitor proliferation in vitro and in vivo (Palazuelos et al., 2006), and targeting neurogenesis via the CB2 receptor would avoid undesired psychoactive side effects. Thus, the neuroprotective effects of cannabinoids may involve short-term adaptation to neuronal stress, such as limiting excitotoxicity, as well as longer-term adaptations, such as enhancing neurogenesis. It remains to be established whether or not the beneficial effects of cannabinoids on memory, neuroinflammation and neurodegeneration in animal models of AD are due to a functional consequence of an enhancement in neurogenesis.
Targeting acetylcholinesterase with cannabinoids
Currently there are four approved drugs (tacrine, Cognex; donepezil, Aricept; rivastigmine, Exelon; galantamine, Reminyl) that are used to alleviate the symptoms of early stage AD by inhibiting the active site of AChE, thus increasing the levels of ACh at the synaptic cleft and enhancing cholinergic transmission. In addition, AChE accelerates that assembly of Aβ peptides into fibrillar species by forming complexes with Aβ via the peripheral anionic site on AChE (Inestrosa et al., 1996), an interaction that increases the neurotoxicity of the Aβ fibrils (Alvarez et al., 1998). Thus, AChE inhibitors offer a two-pronged attack for the treatment of AD by virtue of their ability to enhance ACh availability, as well as reduce amyloidogenesis and subsequent neurotoxicity. A recent study has demonstrated that Δ9-THC competitively inhibits AChE and prevents the AChE-induced aggregation of Aβ by virtue of Δ9-THC binding to the peripheral anionic site on AChE (Eubanks et al., 2006). Compared with tacrine and donepezil, Δ9-THC was found to be more robust inhibitor of Aβ aggregation, suggesting that Δ9-THC and its analogues warrant further investigation as AChE inhibitors for use in the treatment of AD.
Do cannabinoids have a role for the treatment of other neurodegenerative conditions?
It is also worth considering how the aforementioned properties of cannabinoids may be beneficial in ameliorating the symptoms of other diseases in which neuroinflammation, oxidative stress and neurodegeneration are key features, such as multiple sclerosis and Parkinson's disease. Benito et al. (2007) have reported that components of the cannabinoid system are upregulated in multiple sclerosis (MS) plaques, suggesting that endocannabinoids either have a role in the pathogenesis of MS or may be upregulated as a consequence of the pathology. MS is associated with excitotoxicity (Pitt et al., 2000; Smith et al., 2000) and neuroinflammation (Ziemssen, 2005), and these represent features of the disease that cannabinoids may be able to circumvent. In encephalomyelitis virus-induced demyelinating disease, an animal model of MS, the mixed cannabinoid agonist HU210 reduces axonal damage and improves motor function as a consequence of a concomitant activation of the CB1 receptor in neurones and CB2 in astrocytes (Docagne et al., 2007). Other studies in animal models of MS have demonstrated a role for the CB2 receptor in enhancing T-cell apoptosis (Sanchez et al., 2006) and suppressing microglial activation (Ehrhart et al., 2005), while the CB1 receptor is associated with neuroprotection (Pryce and Baker, 2007). Such neuroprotective and antioxidant properties of cannabinoids also underlie their ability to reverse the motor deficits in animal models of Parkinson's disease (Lastres-Becker et al., 2005; Garcia-Arencibia et al., 2007), and lend support of a potential role for cannabinoid-based therapies to mitigate the symptoms of a range of neurodegenerative conditions.
Conclusion
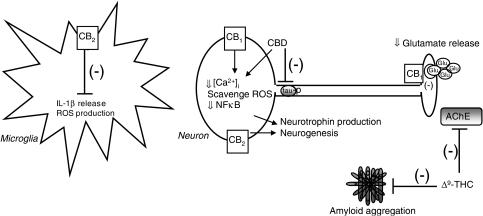
Alzheimer's disease is a devastating illness for which there is no cure. Current AD drugs, which serve as AChE inhibitors, have a number of unpleasant side effects such as hepatotoxicity and gastrointestinal disturbances. While the NMDA receptor antagonist, memantine, can modify the disease, it cannot reverse the process of neurodegeneration. Manipulation of the cannabinoid pathway offers a novel pharmacological approach for the treatment of AD that may be more efficacious than current treatment regimes. Cannabinoids can reduce the oxidative stress, neuroinflammation and apoptosis that is evoked by Aβ, while promoting the brain's intrinsic repair mechanisms. Certain cannabinoids, such as Δ9-THC, may also increase ACh availability and reduce amyloidogenesis, although potential psychoactive side effects may hinder its clinical usefulness. Cannabinoids clearly offer a multifaceted approach for the treatment of AD and future studies should focus on examining the efficacy of cannabinoids in the array of animal models that exhibit AD-like pathology and cognitive decline. Targeting the CB2 receptor to reduce neuroinflammation while stimulating neurogenesis is likely to be of particular interest, given the reduced risk of psychoactive activity and the close association of the CB2 receptor with the senile plaque, thus limiting drug effects to the region of pathology and sparing the potential for widespread effects on normal neurophysiological processes. In conclusion, manipulation of the cannabinoid system offers the potential to upregulate neuroprotective mechanisms while dampening neuroinflammation. Whether these properties will be beneficial in the treatment of AD in the future is an exciting topic that undoubtedly warrants further investigation (Figure 1).
Figure 1.
Potential sites of action of the cannabinoid system for the treatment of AD. Activation of the CB2 receptor reduces the formation of reactive oxygen species (ROS) and the release of interleukin-1β from microglia, thus exerting an anti-inflammatory effect. In neurones, activation of the CB1 receptor reduces intracellular Ca2+ concentration ([Ca2+]i), protects against oxidative stress and reduces inflammatory signalling by inhibition of nuclear factor κB. CB1 activation also inhibits glutamate release to reduce excitotoxicity, and enhances neurotrophin expression and neurogenesis. CBD is neuroprotective and anti-inflammatory in a CB receptor-independent manner, and also reduces tau phosphorylation. Δ9-THC inhibits AChE, resulting in enhanced cholinergic transmission and reduced amyloidogenesis. AD, Alzheimer' disease; AChE, acetylcholinesterase; CB, cannabinoid; CBD, cannabinoid; Δ9-THC, Δ9-tetrahydrocannabinol.
Abbreviations
- Aβ
β-amyloid
- AD
Alzheimer's disease
- CB
cannabinoid
- CBD
cannabidiol
- NMDA
N-methyl D-aspartate
- Δ9-THC
Δ9-tetrahydrocannabinol
Conflict of interest
The authors state no conflict of interest.
References
- Abood ME, Rizvi G, Sallapudi N, McAllister S. Activation of the CB1 cannabinoid receptor protects cultured mouse spinal neurons against excitotoxicity. Neuroscience Lett. 2001;309:197–201. doi: 10.1016/s0304-3940(01)02065-1. [DOI] [PubMed] [Google Scholar]
- Alger BE. Not too excited? Thank your endocannabinoids. Neuron. 2006;51:393–395. doi: 10.1016/j.neuron.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Alvarez A, Alarcon A, Opazo C, Campos EO, Munoz F, Calderon FH, et al. Stable complexes involving acetylcholinesterase and amyloid-β-fibrils change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer's fibrils. J Neurosci. 1998;18:407–416. doi: 10.1523/JNEUROSCI.18-09-03213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Pollard HB, Rojas E. Giant multilevel cation channels formed by Alzheimer's disease Aβ1-40 in bilayer membranes. Proc Natl Acad Sci USA. 1993;90:10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci Lett. 2006;396:113–116. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett. 1999;271:126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J Neurosci. 2003;23:11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, Romero JP, Tolon RM, Clemente D, Docagne F, Hillard CJ, et al. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J Neurosci. 2007;27:2396–2402. doi: 10.1523/JNEUROSCI.4814-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland B, Campbell V. Amyloid-induced apoptosis of cultured cortical neurones involves calpain-mediated cleavage of poly-ADP ribose polymerase. Neurobiol Aging. 2003;24:179–186. doi: 10.1016/s0197-4580(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. Cannabinoids and memory: animal studies. Curr Drug Targets CNS Neurol Disord. 2003;2:389–402. doi: 10.2174/1568007033482670. [DOI] [PubMed] [Google Scholar]
- Cosman KM, Boyle LL, Porsteinsso AP. Memantine in the treatment of mild-to-moderate Alzheimer's disease. Expert Opin Pharmacother. 2007;8:203–214. doi: 10.1517/14656566.8.2.203. [DOI] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol. 2007;556:75–83. doi: 10.1016/j.ejphar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinell S, Clmlno G, Schwartz J, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurones. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Docagne F, Muñetón V, Clemente D, Ali C, Loría F, Correa F, et al. Excitotoxicity in a chronic model of multiple sclerosis: neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol Cell Neurosci. 2007;34:551–561. doi: 10.1016/j.mcn.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Ehrhart J, Obergon D, Mri T, Hou H, Sun N, Bai Y, et al. Stimulation of CB2 suppresses microglial activation. J Neuroinflamm. 2005;12:22–29. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar N, Streim S, Biegon A. HU-211, a non-psychotropic cannabinoid, rescues cortical neurones from excitatory amino acid toxicity in culture. Neuroreport. 1993;5:237–240. doi: 10.1097/00001756-199312000-00013. [DOI] [PubMed] [Google Scholar]
- Esposito G, De Filippis D, Carnuccio R, Izzo AG, Iuvone T. The marijuana component, cannabidiol, inhibits β-amyloid-induced tau protein hyperphosphorylation through Wnt/β-catenin pathway rescue in PC12 cells. J Mol Med. 2006b;84:253–258. doi: 10.1007/s00109-005-0025-1. [DOI] [PubMed] [Google Scholar]
- Esposito G, De Filippis D, Maiuri MC, De Stefano D, Carnuccio R, Iuvone T. Cannabidiol inhibits inducible NOS expression and NO production in β-amyloid-stimulated PC12 neurons through p38 MAP kinase and NFκB involvement. Neurosci Lett. 2006a;399:91–95. doi: 10.1016/j.neulet.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Eubanks LM, Rogers CJ, Beuscher AE, Koob GF, Olson AJ, Dickerson TJ, et al. A molecular link between the active component of marijuana and Alzheimer's disease pathology. Mol Pharm. 2006;3:773–777. doi: 10.1021/mp060066m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchinetti F, Del Giudice E, Furegato S, Passarotto M, Leon A. Cannabinoids ablate release of TNFα in rat microglial cells stimulated with lipopolysaccharide. Glia. 2003;41:161–168. doi: 10.1002/glia.10177. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival. Trends Pharm Sci. 2007;28:39–45. doi: 10.1016/j.tips.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Aguada T, Palazuelos J, Guzman M. The endocannabinoid system and neurogenesis in health and disease. Neuroscientist. 2007;13:109–114. doi: 10.1177/1073858406296407. [DOI] [PubMed] [Google Scholar]
- Garcia-Arencibia M, Gonzalez S, deLago E, Ramos JA, Mechoulam R, Fernandez-Ruiz J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson's disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007;1134:162–170. doi: 10.1016/j.brainres.2006.11.063. [DOI] [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Volkow ND. Cannabinoid receptor-mediated inhibition of acetylcholine release from hippocampal and cortical synaptosomes. Br J Pharmacol. 2000;131:645–650. doi: 10.1038/sj.bjp.0703599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote HE, Hannan AJ. Regulators of adult neurogenesis in the healthy and diseased brain. Clin Exp Pharm Phys. 2007;34:533–545. doi: 10.1111/j.1440-1681.2007.04610.x. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci USA. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HH, Ikonomidou C, Bittigau P, Hansen SH, Hansen HS. Accumulation of the anandamide precursor and other N-acylethanolamine phospholipids in infant rat models of in vivo necrotic and apoptotic neuronal death. J Neurochem. 2001;76:39–46. doi: 10.1046/j.1471-4159.2001.00006.x. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Herbert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer's disease in the US population: prevalence estimates using the 2000 census. Archiv Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Alvarez A, Pecez CA, Moreno RD, Vicente M, Linker C, et al. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer's fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16:881–891. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on β-amyloid-induced toxicity in PC12 cells. J Neurochem. 2004;89:134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- Jackson SJ, Diemel LT, Pryce G, Baker D. Cannabinoids and neuroprotection in CNS inflammatory disease. J Neurol Sci. 2005;233:21–25. doi: 10.1016/j.jns.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji S, Bai G. Cannabinoids promote embryonic and adult hippocampal neurogenesis and produce anxiolytic and anti-depressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XL, Xie L, Cottrell B, Henshall DC, et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaspekov LG, Brenz Verca MS, Frumkina LE, Hermann H, Marsicano G, Lutz B. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur J Neurosci. 2004;19:1691–1698. doi: 10.1111/j.1460-9568.2004.03285.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao A, Ledent C, Jin K, Greenberg DA. Role for neuronal nitric-oxide synthase in cannabinoid-induced neurogenesis. J Pharmacol Exp Ther. 2006;319:150–154. doi: 10.1124/jpet.106.107698. [DOI] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signaling in Alzheimer's disease. Nat Rev Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernandez-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol Dis. 2005;19:96–107. doi: 10.1016/j.nbd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram GK, Mattson MP. Dietary restriction increases the number of newly generated neural cells and induced BDNF expression in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma–glioma cell lines. Proc Natl Acad Sci USA. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacManus A, Ramsden M, Murray M, Pearson HA, Campbell V. Enhancement of 45Ca2+ influx and voltage-dependent Ca2+ channel activity by β-amyloid(1–40) in rat cortical synaptosomes and cultured cortical neurones: modulation by the proinflammatory cytokine interleukin-1β. J Biol Chem. 2000;275:4713–4718. doi: 10.1074/jbc.275.7.4713. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Mazzola C, Micale V, Drago F. Amnesia induced by β-amyloid fragments in counteracted by cannabinoid CB1 receptor blockade. Eur J Pharmacol. 2003;477:219–225. doi: 10.1016/j.ejphar.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Mi K, Johnson GV. The role of tau phosphorylation in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2006;3:449–463. doi: 10.2174/156720506779025279. [DOI] [PubMed] [Google Scholar]
- Milton NGN. Anandamide and noladin ether prevent excitotoxicity of the human amyloid-β-peptide. Neurosci Lett. 2002;332:127–130. doi: 10.1016/s0304-3940(02)00936-9. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Pinteaux E, Moore JD, Molina-Holgado E, Guaza C, Gibson RM, et al. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J Neurosci. 2003;12:6470–6474. doi: 10.1523/JNEUROSCI.23-16-06470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler V, Mechoulam R, Sokolovsky M. The non-psychotropic cannabinoid (+)-3S,4S)-7-hydroxy-Δ6-tetrahydrocannabinol 1,1-dimethylheptyl (HU-211) attenuates N-methyl-D-aspartate receptor-mediated neurotoxicity in primary cultures of rat forebrain. Neurosci Lett. 1993;162:43–45. doi: 10.1016/0304-3940(93)90555-y. [DOI] [PubMed] [Google Scholar]
- Nunez E, Benito C, Pazos MR, Barbachano A, Fajardo O, Gonzalez S, et al. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 2004;53:208–213. doi: 10.1002/syn.20050. [DOI] [PubMed] [Google Scholar]
- Ogomori K, Kitamoto T, Tateishi J, Sato Y, Suetsugu M, Abe M. Protein amyloid is widely distributed in the central nervous system of patients with Alzheimer's disease. Am J Pathol. 1989;134:243–251. [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzman M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Mechoulam R, Beni SM, Alexandrovich A, Shohami E. CB1 cannabinoid receptors are involved in neuroprotection via NFκB. Blood Flow Metab. 2005;25:477–484. doi: 10.1038/sj.jcbfm.9600047. [DOI] [PubMed] [Google Scholar]
- Pazos MR, Nunez E, Benito C, Tolon RM, Romero J. Role of the endocannabinoid system in Alzheimer's disease: new perspectives. Life Sci. 2004;75:1907–1915. doi: 10.1016/j.lfs.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Perez J. Combined cannabinoid therapy via oromucosal spray. Drugs Today. 2006;42:495–503. doi: 10.1358/dot.2006.42.8.1021517. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK3 regulates production of Alzheimer's disease β-amyloid peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol. 2007;150:519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Caballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich JB, Rasmusson DX, Folstein MF, Carson KA, Kawas C, Brandt J. Nonsteroidal anti-inflammatory drugs in Alzheimer's disease. Neurology. 1995;45:51–55. doi: 10.1212/wnl.45.1.51. [DOI] [PubMed] [Google Scholar]
- Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. Handb Exp Pharmacol. 2005;168:445–477. doi: 10.1007/3-540-26573-2_15. [DOI] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. Stores not just for storage, intracellular calcium release and synaptic plasticity. Neuron. 2001;31:519–522. doi: 10.1016/s0896-6273(01)00402-0. [DOI] [PubMed] [Google Scholar]
- Sanchez AJ, Gonzalez-Perez P, Galve-Roperh I, Garcia-Merino A. R-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)-pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphtalenylmethanone (WIN-2) ameliorates experimental autoimmune encephalomyelitis and induces encephalitogenic T cell apoptosis: partial involvement of the CB(2) receptor. Biochem Pharmacol. 2006;72:1697–1706. doi: 10.1016/j.bcp.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Sanchez MG, Ruiz-Llorente L, Sanchez AM, Diaz-Laviada I. Activation of phosphoinositol 3-kinase/PKB pathway by CB1 and CB2 cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell Signal. 2003;15:851–859. doi: 10.1016/s0898-6568(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Shen M, Thayer SA. Cannabinoid receptor agonists protect cultured mouse hippocampal neurons from excitotoxicity. Brain Res. 1998;783:77–84. doi: 10.1124/mol.54.3.459. [DOI] [PubMed] [Google Scholar]
- Smith IF, Green KN, LaFerla FM. Calcium dysregulation in Alzheimer's disease: recent advances gained from genetically modified animals. Cell Calcium. 2005;38:427–437. doi: 10.1016/j.ceca.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat Med. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- Sonkusare SK, Kaul CL, Ramarao P. Dementia of Alzheimer's disease and other neurodegenerative disorders—memantine, a new hope. Pharmacol Res. 2005;51:1–17. doi: 10.1016/j.phrs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Castillo PE. CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience. 2006;139:795–802. doi: 10.1016/j.neuroscience.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Van Der Stelt M, Mazzola C, Espositp G, Mathias I, Petrosino S, De Filippis D, et al. Endocannabinoids and β-amyloid-induced neurotoxicity in vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci. 2006;63:1410–1424. doi: 10.1007/s00018-006-6037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vereker E, Campbell V, Roche E, McEntee E, Lynch MA. Lipopolysaccharide inhibits long-term potentiation in the rat dentate gyrus by activation of caspase-1. J Biol Chem. 2000;275:26252–26258. doi: 10.1074/jbc.M002226200. [DOI] [PubMed] [Google Scholar]
- Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of Dronabinol on anorexia and disturbed behaviour in patients with Alzheimer's disease. Int J Geriatr Psych. 1997;12:913–919. [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Aβ oligomers—a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Walther S, Mahlberg R, Eichmann U, Kunz D. Δ9-Tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology. 2006;185:524–528. doi: 10.1007/s00213-006-0343-1. [DOI] [PubMed] [Google Scholar]
- Wang SJ. Cannabinoid CB1 receptor-mediated inhibition of glutamate release from rat hippocampal synaptosomes. Eur J Pharmacol. 2003;469:47–55. doi: 10.1016/s0014-2999(03)01734-5. [DOI] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and mRNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry of normal aged and Alzheimer's brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J Neuroinflamm. 2006;3:30–42. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EJ, Walsh FS, Doherty P. The FGF receptor uses the endocannabinoid signalling system to couple to an axonal growth response. J Cell Biol. 2003;160:448–481. doi: 10.1083/jcb.200210164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur J Pharmacol. 2003;477:213–217. doi: 10.1016/j.ejphar.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer's disease: driving force, bystander or beneficial response. Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Zhuang S-Y, Bridges D, Grigoenko E, McCloud S, Boon A, Hampson RE, et al. Cannabinoids produce neuroprotection by reducing intracellular calcium release from ryanodine-sensitive stores. Neuropharmacology. 2005;28:1086–1096. doi: 10.1016/j.neuropharm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Ziemssen T. Modulating processes within the central nervous system is central to therapeutic control of multiple sclerosis. J Neurol. 2005;252:38–45. doi: 10.1007/s00415-005-5007-2. [DOI] [PubMed] [Google Scholar]