Abstract
Background and purpose:
A putative novel cannabinoid receptor mediates vasorelaxation to anandamide and abnormal-cannabidiol and is blocked by O-1918 and by high concentrations of rimonabant. This study investigates VSN16, a novel water-soluble agonist, as a vasorelaxant potentially acting at non-CB1, non-CB2 cannabinoid receptors in the vasculature.
Experimental approach:
VSN16 and some analogues were synthesized and assayed for vasodilator activity in the rat third generation mesenteric artery using wire myography. Also carried out with VSN16 were haemodynamic studies in conscious rats and binding studies to CB1 receptors of rat cerebellum.
Key results:
VSN16 relaxed mesenteric arteries in an endothelium-dependent manner. The vasorelaxation was antagonized by high concentrations of the classical cannabinoid antagonists, rimonabant and AM 251, as well as by O-1918, an antagonist at the abnormal-cannabidiol receptor but not at CB1 or CB2 receptors. It did not affect [3H]CP55,940 binding to CB1 receptors in rat cerebellum. The vasorelaxation was not pertussis toxin-sensitive but was reduced by inhibition of nitric oxide synthesis, Ca2+-sensitive K+ channels (KCa) and TRPV1 receptors. In conscious rats VSN16 transiently increased blood pressure and caused a longer-lasting increase in mesenteric vascular conductance. Structure-activity studies on vasorelaxation showed a stringent interaction with the target receptor.
Conclusions and implications:
VSN16 is an agonist at a novel cannabinoid receptor of the vasculature. It acts on the endothelium to release nitric oxide and activate KCa and TRPV1. As it is water-soluble it might be useful in bringing about peripheral cannabinoid-like effects without accompanying central or severe cardiovascular responses.
Keywords: VSN16, cannabinoid receptors, endothelium, rat mesenteric artery, haemodynamics, O-1918, vasodilator, rimonabant, AM 251, GPR55
Introduction
There is growing evidence for a novel cannabinoid receptor in the vasculature. White and Hiley (1997a) showed that, in the isolated mesenteric arterial bed of the rat, anandamide, an endocannabinoid, causes vasorelaxation that is sensitive to the cannabinoid receptor antagonist rimonabant (formerly designated SR141716A). However, although the major active constituent of cannabis, Δ9-tetrahydrocannabinol causes relaxation, this is insensitive to pertussis toxin and the antagonist N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM 251) (O'Sullivan et al., 2005), which suggests that there is no CB1 receptor-mediated vasorelaxation in this vascular bed as the CB1 receptor is sensitive to both the antagonist and the toxin. Also rimonabant is a high-potency antagonist (Ki=21 nM) at CB1 receptors (Rinaldi-Carmona et al., 1995), but high concentrations of 1 μM or more are required to antagonize the vascular actions of anandamide (White and Hiley, 1997a; Chaytor et al., 1999; Járai et al., 1999). Mesenteric arterial vasodilatation in rat can also be induced by abnormal cannabidiol, a behaviourally inactive agent; this relaxation is also sensitive to rimonabant (Ho and Hiley, 2003b) but occurs in mice lacking both the CB1 and CB2 receptors (Járai et al., 1999). The vascular responses to abnormal cannabidiol can be antagonized in a competitive fashion by its analogue (−)-1,3-dimethoxy-2-(3-3,4-trans-p-menthadien-(1,8)-yl)-orcinol (O-1918) (Offertáler et al., 2003), which also antagonizes the vascular actions of anandamide (Offertáler et al., 2003) as well as vasorelaxation to other putative endocannabinoids such as virodhamine (Ho and Hiley, 2004) and oleamide (Hoi and Hiley, 2006).
The behavioural effects of most cannabinoids, such as sedation and the risk of the development of dependence, together with their long pharmacokinetic half-lives, limit their utility as therapeutic agents (Williamson and Evans, 2000; Pacher et al., 2006). Nevertheless, an extract of the cannabis plant, with the trade name Sativex, has gained a licence for use in pain relief in multiple sclerosis in Canada although it is argued that additional benefit in this preparation might be conferred by the presence at a carefully controlled ratio not only of Δ9-tetrahydrocannabinol but also of the behaviourally inactive cannabidiol (Smith, 2004). The desirability of separating the behavioural effects of cannabinoids from their therapeutic potential in diseases such as multiple sclerosis has led to a search for novel compounds. Here we report the vascular activity of one such compound, 3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide (VSN16). This agent is water soluble and we show that it causes vasorelaxation of a rat resistance artery in a manner that is sensitive to both O-1918 and rimonabant although it does not bind to rat CB1 cannabinoid receptors; VSN16 is therefore a candidate agonist at a novel vascular receptor for cannabinoids.
Methods
Chemistry: general synthetic procedures
Method A: general procedure for Sonogashira coupling reaction
Tetrakis(triphenyl-phosphine)palladium(0) (2 mol%) and copper(I) iodide (7 mol%) were added to pyrrolidine (15 ml) and stirred at room temperature under a nitrogen atmosphere, for 5 min. N-(2-hydroxy-1-methyl-ethyl)-3-iodobenzamide 1 (1 mmol) was added and stirred for an additional 15 min at room temperature. The alkyne (1 mmol) was added and the reaction mixture was stirred at 60°C for 3 h. The reaction mixture was concentrated under vacuum and the residue treated with DOWEX50 WX80 (10 × weight of the starting material), which had been washed with acetonitrile (3 × 20 ml) and then suspended in a mixture of acetonitrile/water (3:1, v v−1). The residue was dissolved in acetonitrile/water (1:1, 20 ml) before being added to the resin suspension and shaken for 20 min. The resin was filtered off, washed with acetonitrile/water (3:1) and the solvent removed from the filtrate under vacuum. The residue was purified by short flash column chromatography on silica gel (dichloromethane/MeOH/AcOH, 1–8% methanol gradient, with 1% AcOH) to yield the desired compound. As an alternative (method A′), the treatment of the crude material with DOWEX50 WX80 was performed in the presence of methanol instead of acetonitrile/water (3:1).
Method B: general procedure for amide couplings
Triethylamine (2 mmol) was added to a solution of the alkynoic acid (1 mmol) in dry tetrahydrofuran (6 ml) under an N2 atmosphere, and then cooled at −10°C. To the reaction mixture, ethyl chloroformate (1 mmol) was added and then stirred for further 15 min at −10°C. In the meantime, a solution of amine hydrochloride (3 mmol), water (0.88 ml), triethylamine (6 mmol) and tetrahydrofuran (1.76 ml) was prepared and added dropwise to the reaction mixture. The reaction was left warming up to 5°C in 1.5 h and then stirred at room temperature for a further 30 min. The mixture was poured into a 1:1 mixture of saturated brine and saturated NaHCO3 (50 ml) and then extracted with dichloromethane (DCM; 5 × 50 ml). The organic layer was evaporated under vacuum, the residue was purified by short column chromatography on silica gel (DCM/MeOH, 1–10% methanol gradient) to give the desired compound.
Method C: general method for Lindlar hydrogenation
Quinoline (25μl, 0.21 mmol), palladium on barium sulphate reduced (5%) (360 mg) and the alkyne (1 mmol) were combined in methanol (15 ml) and stirred under atmospheric pressure of hydrogen until the 1H NMR of the crude showed that the reduction was complete. The catalyst was removed by filtration through a pad of celite, which was washed several times with methanol. The filtrate was evaporated under vacuum and the product was purified by preparative HPLC.
Chemical synthesis of VSN16
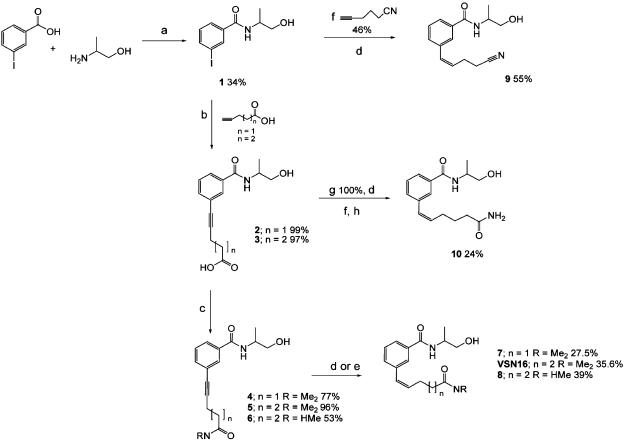
N-(2-hydroxy-1-methyl-ethyl)-3-iodobenzamide 1: At room temperature, 1-ethyl-3-(3′-dimethylaminopropyl)carbodiimide (EDCI: 40.3 mmol) was added to a solution of 3-iodobenzoic acid (40.3 mmol), in dry CH2Cl2 (180 ml) under an N2 atmosphere, followed by triethylamine (60.45 mmol). The mixture was stirred at room temperature for a further 5 min before adding DL-alaninol (40.3 mmol) and subsequently stirring the mixture for 16 h. The reaction mixture was washed with a mixture of saturated brine and saturated NaHCO3 (1:1; 2 × 150 ml) followed by saturated brine (100 ml). The organics were separated and dried over MgSO4 and the solvent evaporated under vacuum. The residue was purified by flash column chromatography on silica gel (DCM/MeOH, 1–8% methanol gradient) to afford compound 1 (13.6 mmol, 34% yield; Figure 1) as an off-white solid.
Figure 1.
Scheme showing the synthetic pathway of VSN16 and some analogues from 3-iodobenzoic acid via N-(2-hydroxy-1-methyl-ethyl)-3-iodobenzamide (1) using the palladium-catalysed Sonogashira coupling reaction. Alkenes are shown in the Z configurations as these are the forms isolated in the synthesis. Reagents: (a) EDCI, DCM, Et3N, room temperature; (b) (i) tetrakis(triphenylphosphine)palladium(0), Cu(I)I, pyrrolidine, 60°C; (ii) DOWEX50 WX80 acetonitrile; (c) (i) EtOCOCl, Et3N, −10°C; (ii) Et3N, H2O, tetrahydrofuran, substituted amine HCL, 0°C to 5°C; (d) quinoline, palladium on barium sulphate (5%), MeOH, H2 atmospheric pressure; (e) borohydride polymer-supported, (CH3COO)2Ni·4H2O, MeOH, H2 atmospheric pressure; (f) bis(triphenylphosphine)palladium(II) chloride, Cu(I)I, Et3N, DMF, 60°C; (g) DOWEX50 WX80 MeOH; (h) concentrated NH3, room temperature.
δ(1H)(CDCl3); 1.41 (3H, d, J 6.8 Hz), 3.70 (1H, dd, J1 5.5, J2 10.9 Hz), 3.80 (1H, dd, J1 2.9, J2 10.9 Hz), 4.38 (1H, m), 6.46 (1H, m), 7.27 (2H, t, J 7.8 Hz), 7.93 (1H, d, J 7.88 Hz), 8.21 (1H, s). δ(13C) (CDCl3); 17.49 (CH3), 48.53 (C2), 67.19 (CH2), 94.59 (C), 126.79 (CH), 129.58 (CH), 130.62 (CH), 136.37 (CH), 136.83 (C), 166.71(C). Calculated C10H11NO2I·1/2H2O: C 38.23%, H 3.85%, N 4.46%; found: C 38.95%, H 3.80%, N 4.08%.
6-[3-(2-Hydroxy-1-methyl-ethylcarbamoyl)phenyl]-hex-5-ynoic acid 3: The iodobenzamide (1; Figure 1) (6.5 mmol) was coupled with 5-hexynoic acid using method A (Kadota et al., 1999) giving product 3 (6.42 mmol, 99% yield; Figure 1).
δ(1H)(CDCl3); 1.49 (3H, d, J 6.8 Hz), 2.14 (2H, t, J 7.2 Hz), 2.67-2.76 (4H, m), 3.83–3.90 (2H, m) 4.39–4.45 (1H, m) 7.64 (1H, t, J 7.7 Hz), 7.76 (1H, d, J 7.7 Hz), 7.99 (1H, d, J 7.8 Hz), 8.10 (1H, s). δ(13C) (CD3OD); 17.47 (CH2), 19.99 (CH3), 36.25 (CH2), 66.54 (CH2), 81.82 (C), 91.53 (C), 126.03 (C), 128.05 (CH), 129.99 (CH), 131.75 (CH), 135.69 (CH), 136.58 (C), 168.538 (C). MS (CI) m/z 290 (M+H).
3-(5-Dimethylcarbamoyl-pent-1-ynyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide 5: 6-[3-(2-hydroxy-1-methyl-ethylcarbamoyl)phenyl]-hex-5-ynoic acid 3 (Figure 1) (0.377 mmol) was reacted with dimethylamine hydrochloride using method B to obtain 5 (0.115 g, 0.363 mmol; 96% yield).
δ(1H)(CDCl3); 1.29 (3H, d, J 6.8 Hz), 1.81–1.94 (2 H, m), 2.37–2.47 (4H, m), 2.91 (3H, s), 3.00 (3H, s), 3.38–3.64 (2H, m) 4.19–4.43 (1H, m) 6.78 (1H, d, J 7.2 Hz), 7.29 (1H, t, J 7.7 Hz), 7.42 (1 H, d, J 7.7 Hz), 7.68 (1H, d, J 7.8 Hz), 7.75 (1H, s). δ (13C) (CDCl3); 17.42(CH3), 19.36 (CH2), 24.45 (CH2), 32.30 (CH2), 35.83(CH3), 37.67 (CH3), 48.51 (CH), 66.90 (CH2), 80.91 (C), 90.92 (C), 124.60 (C), 126.85 (CH), 128.85 (CH), 130.39 (CH), 134.58 (CH), 135.13 (C), 167.63 (C), 172.87(C). MS (ES) m/z 317 (M+H).
3-(5-Dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide: By Lindlar-catalysed reduction using method C, from alkyne 5 (0.10 g, 0.3 mmol) a mixture of VSN16 and the fully reduced compound 3-(5-dimethylcarbamoyl-pentyl)-N-(2-hydroxy-1-methyl-ethyl)-benzamide were obtained, which was separated by reverse-phase HPLC chromatography (20% acetonitrile/80% water 20 min isocratic program) (VSN16, 34 mg, yield 35%).
δ(1H)(CDCl3); 1.31 (3H, t, J 6.8 Hz), 1.81–1.91 (2H, m), 2.26–2.39 (4H, m), 2.90 (3H, s); 2.98 (3H, s); 3.65 (2H, dd, J1 5.5, J2 11.2 Hz), 3.83 (2H, dd, J1 3.2, J2 11.2 Hz), 4.27–4.30 (1H, m), 5.68–5.77 (1H, m), 6.46 (1H, d, J 11.6 Hz), 7.24–7.33 (1H, m), 7.38 (1H, d, J 7.6 Hz), 7.74–7.79 (2H, m). δ(13C)(CDCl3); 16.93 (CH3), 24.80 (CH2), 28.22 (CH2), 32.51 (CH2), 35.73 (CH), 37.45 (CH), 48.32 (CH2), 66.73 (CH2), 126.20 (CH), 126.35 (CH), 128.58 (CH), 129.12 (CH), 131.88 (CH), 132.63 (CH), 134.70 (C), 137.5 (C), 168.00(C), 173.11(C). Theoretical mass: (M+H) 318.19433. Measured mass: (M+H) 318.19507 MS (ES) m/z 319 (M+H).
Myograph studies
Male Wistar rats (300–400 g; Charles River UK Ltd, Kent, UK) were killed with an overdose of sodium pentobarbital (120 mg kg−1, i.p.; Sagatal, Rhône Mérieux, Harlow, Essex, UK); all animal care and use was in accordance with the UK Animal (Scientific Procedures) Act 1986. After rapid removal of the mesenteric arterial bed, it was placed into cold Krebs–Henseleit buffer solution of the following composition (mM): NaCl, 118; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25; CaCl2, 2.5; D-glucose, 5.5. The Krebs–Henseleit solution also contained 10 μM indomethacin and was bubbled with 95% O2/5% CO2 to give a pH of 7.4.
Third-generation (small) mesenteric arteries (internal diameter, 294±3 μm; basal tone after normalization, 3.9±0.1 mN, 240 vessels) were then dissected free and cleaned of adherent tissue. Two-millimetre-long segments were mounted in a wire myograph (Danish Myo Technology, Aarhus, Denmark) maintained at 37°C in Krebs–Henseleit solution, gassed with 95% O2/5% CO2, and normalized as described previously (White and Hiley, 1997a). Tension was measured and recorded on a PowerLab recording system (AD Instruments, Hastings, Sussex, UK) connected to an Apple Macintosh computer. The presence of a functional endothelium was tested by precontracting the vessels with methoxamine (10 μM), and then adding carbachol (10 μM) to cause relaxation; an intact endothelium was shown by relaxations >90% of the methoxamine-induced precontraction. When the endothelium was not required, vessels were denuded by rubbing the intimal surface with a human hair, and successful endothelial removal was confirmed by absence of a vasorelaxant response (<10% of the precontraction) to carbachol.
Experimental protocols in the myograph
After determination of the presence or absence of endothelium, vessels were left for 15–30 min and then precontracted submaximally with 10 μM methoxamine (yielding approximately 80% of the maximal response). When a stable level of tone was obtained (mean, 15.1±0.3 mN; n=170), concentration–response curves were generated by cumulative addition of the compound under investigation and each concentration was left for 10 min before addition of the next one.
Where the effect of VSN16 was investigated in the presence of antagonists (rimonabant, AM 251, O-1918 or capsazepine), apamin, charybdotoxin, NG-nitro-L-arginine methyl ester (L-NAME) or capsaicin, these agents were added to the organ bath 30 min before, and then were present during, the construction of the concentration–response curve. Note that in the case of pertussis toxin, preincubation was for 2 h. The concentrations of these inhibitors were based on those showing specific inhibition in previous experiments (White and Hiley, 1997a, 1998a, 1998b; Ho and Hiley, 2003b). None of these agents, except O-1918, had any effect on the tone induced by methoxamine; the mean tension in the test for endothelial function was 15.7±0.5 mN as compared with 15.6±0.6 mN (n=64) after preincubation with the antagonists or inhibitors. For O-1918 (30 μM), the mean tension developed by methoxamine in the endothelial integrity test was 14.6±0.7 mN and was 17.4±0.7 mN after application of the antagonist (n=15). A vehicle control with dimethyl sulfoxide (DMSO) was obtained for all compounds (except VSN16, which was dissolved in water) by adding an appropriate volume of vehicle alone to methoxamine-precontracted vessels. Since considerable variations were observed in the relaxant responses to the novel compounds, all the experiments were done in paired fashion with control and test experiments conducted in vessels from the same branch of artery from the same animal.
Data and statistical analysis for myograph experiments
Responses are expressed as the percentage relaxation of the precontraction induced by 10 μM methoxamine. Data are given as the mean±s.e.m. and n indicates the number of rats. When a defined maximum relaxation response was observed, the data were fitted to a logistic equation of the following form: where R is the reduction in tone, A the concentration of the agonist, Rmax the maximal reduction of established tone, nH the slope function and EC50 the agonist concentration giving half the maximal relaxation. The curve-fitting was carried out using KaleidaGraph (Synergy Software, Reading, PA, USA).
When concentration–relaxation curves did not reach a clearly defined maximal relaxation, thus rendering curve-fitting inappropriate, the mean EC50% was determined whenever possible. The EC50% was determined from the individual experimental concentration–response curves as the concentration of agonist required to produce 50% relaxation of the tone induced by methoxamine.
Statistical comparisons of concentration–response curves were made by two-way analysis of variance of the whole data set, followed by the Bonferroni post-hoc test for determining significant differences between treatment groups (StatView 4.5 for the Macintosh; Abacus Concepts Inc., Berkeley, CA, USA). P-values less than 0.05 were considered to be statistically significant.
Haemodynamic studies
Male Sprague–Dawley rats (n=8, 380–450 g, Charles River UK) were anaesthetized with fentanyl and medetomidine (300 μg kg−1 of each i.p.) and had miniaturized pulsed Doppler flow probes sutured around the left renal and superior mesenteric arteries, and around the distal abdominal aorta (to monitor hindquarters flow). After surgery, anaesthetic reversal and the provision of analgesia was achieved using atipamezole and buprenorphine (1 mg kg−1 and 0.02 mg kg−1, respectively, s.c.). At least 10 days after probe implantation, animals were re-anaesthetized (fentanyl and medetomidine, as above), and catheters were implanted in the distal abdominal aorta (via the ventral caudal artery) for monitoring mean arterial blood pressure and heart rate, and in the right jugular vein for the administration of substances. Anaesthesia was reversed and analgesia provided using atipamezole and buprenorphine as above. The procedures were approved by the University of Nottingham Ethical Review Committee and performed under a Home Office Project Licence; the fitness of the animals between surgical stages was certified by the Named Veterinary Surgeon.
Experimental protocol for haemodynamics
Experiments were run 48 h following catheterization when the animals were fully conscious and moving freely, with access to food and water ad libitum. After a period of baseline recording of at least 60 min, (R)-3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide (VSN16-R) was administered as i.v. bolus doses (0.1 ml) of 1, 3 and 15 mg kg−1. The order of dose administration was randomized with at least 3 h between doses.
Data acquisition and analysis in haemodynamic studies
Continuous recordings of cardiovascular variables were made using a customized, computer-based system (Haemodynamics Data Acquisition System; University of Limburg, Maastricht) connected to a transducer amplifier (Gould model 13-4615-50) and a Doppler flowmeter (Crystal Biotech VF-1 mainframe (pulse repetition frequency 125 kHz) fitted with high-velocity (HVPD-20) modules). Raw data were sampled by the haemodynamics data acquisition system every 2 ms, averaged every cardiac cycle, and stored to disc at 5-s intervals. Data were analysed offline using software (Datview, University of Limburg, Maastricht), which interfaced with the haemodynamics data acquisition system. Values were then exported into custom-designed software (Biomed, University of Nottingham) for statistical analysis using Friedman's test.
Binding experiments with [3H]CP 55940
Male Wistar rats (250–350 g; Charles River UK) were killed by CO2 asphyxiation (under Schedule 1 of the UK Animal (Scientific Procedures) Act 1986) and decapitated. Brains were removed quickly and the cerebella were dissected for preparation of a membrane homogenate from fresh or thawed tissue which had been frozen at −80°C until use. The cerebella were suspended in ice-cold buffer (50 mM Tris–HCl, 1 mM EDTA, 3 mM MgCl2, pH 7.4) and homogenized in a glass mortar using 15 strokes of a Teflon pestle. The homogenate was centrifuged at 1000 g at 4°C for 5 min, the pellet was discarded and the supernatant was centrifuged at 13 000 g for 30 min at 4°C. The resulting pellet was resuspended in 5 ml homogenization buffer and the protein concentration was determined by a BioRad (Hemel Hempstead, Hertfordshire, UK) protein assay.
Binding assays were carried out in triplicate in a final volume of 1 ml buffer (50 mM Tris–HCl, 1 mM EDTA, 3 mM MgCl2, 3 mg ml−1 bovine serum albumin, pH 7.4). Cerebellar membrane (50 μg protein) was incubated with 62 pM [3H]CP 55940 and non-specific binding (∼30–50% of the total) was determined as the residual binding in the presence of rimonabant or unlabelled (−)-cis-3-[2-hydroxy-4-(1,1dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol (CP 55940) (1 μM for both). Incubation was for 60 min at 30°C and the reaction was terminated by the addition of ice-cold wash buffer (10 mM Tris buffer, pH 7.4) and vacuum filtration using a 24-well Brandel cell harvester (SEMAT Technical (UK) Ltd, St Albans, Hertfordshire, UK) and GF/B filters (Whatman, Maidstone, Kent, UK) that had been presoaked in wash buffer at 4°C for 2 min. After drying the filters for 10 min, they were placed in 4 ml of Emulsifier-Safe (Perkin-Elmer, Waltham, MA, USA) for more than 3 h before measuring the radioactivity bound by liquid scintillation spectrometry.
Data analysis of radioligand binding
Results are expressed as the percentage inhibition of specific binding of the cannabinoid agonist, [3H]CP 55940. Data are given as the mean±s.e.m. and n indicates the number of membrane preparations from different groups of rats. When possible data were fitted to a logistic equation: where I is the percentage inhibition of [3H]CP 55940 binding to the rat membrane preparation, [L] the concentration of the competing ligand, Imax the maximal inhibition of radioligand binding, nH the Hill slope and IC50 the concentration of the competing ligand that reduced the specific binding by 50%. Curve-fitting was carried out as before and Ki values were determined from IC50 values using a Kd of 0.36 nM for [3H]CP 55940 determined by competition against unlabelled CP 55940 (see Results). Statistical comparison of concentration–inhibition curves was as for myograph-obtained relaxation curves.
Drugs
Methoxamine hydrochloride, carbachol, charybdotoxin, L-NAME (Sigma Chemical Company, Poole, Dorset, UK) and apamin (Calbiochem, Nottingham, UK) were dissolved in deionized water as was the VSN16 synthesized as described above. Indomethacin (Sigma) was dissolved in 5% (w v−1) NaHCO3 solution. Capsaicin (Sigma), rimonabant (a generous gift from Sanofi-Synthélabo, Montpellier, France), AM 251 (Tocris Cookson, Bristol, UK), CP 55940 (Tocris Cookson) and O-1918 (a generous gift from Dr George Kunos, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, USA) were dissolved in 100% ethanol. For haemodynamic studies, fentanyl citrate was from Janssen-Cilag (High Wycombe, Buckinghamshire, UK); medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were from Pfizer (Sandwich, Kent, UK); buprenorphine (Vetergesic) was from Alstoe Animal Health (York). [side chain-2,3,4(N)-3 H]CP 55940 (specific activity 6.66 TBq mmol−1) was purchased from Perkin-Elmer.
Results
Chemistry
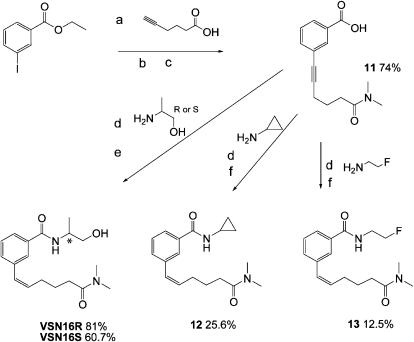
VSN16 is a water-soluble (>20 mg ml−1) benzamide derivative that was designed to be a cannabinoid-like compound that would not show central actions. The synthetic route for the synthesis of benzamide analogues utilizes simple, versatile chemistry, the key step is the palladium-catalysed Sonogashira coupling reaction, which is used to insert a variety of alkyl side chains into the N-(1-hydroxypropan-2-yl)-3-iodobenzamide 1 (Figure 1). 3-Iodobenzoic acid was reacted with DL-alaninol in the presence of a diimide (EDCI) to give amide 1 in good yield. Palladium-catalysed coupling of the amide with the alkyne acid in the presence of Cu(I)I and pyrrolidine proceeded smoothly to give alkynes 2 and 3. The acids were quantitatively transformed into 4, 5, 6 using ethylchloroformate and the appropriate substituted amine. Lindlar-catalysed reduction (Hopper et al., 1998) of alkyne 5 or 6 yielded the alkene 7, VSN16 or 8. Sonogashira coupling of hex-5-yne nitrile with 1 provided 9 after Lindlar reduction. When acid 2 was treated with DOWEX50 WX80 in methanol, the corresponding methyl ester was obtained and the unsubstituted amide 10 by aminolysis. We obtained several different analogues by reacting alkyl 11 with different amines as shown in Figure 2.
Figure 2.
Scheme showing the synthetic pathway to obtain the enantiomers of VSN16 (*the chiral centre) and some analogues from the ester of 3-iodobenzoic acid via the alkyl 11 using the appropriate amines for the coupling reaction. Alkenes are shown in the Z configurations as these are the forms isolated in the synthesis. Reagents: (a) (i) tetrakis(triphenylphosphine)palladium(0), Cu(I)I, pyrrolidine, 60°C; (ii) DOWEX50 WX80 acetonitrile; (b) (i) EtOCOCl, Et3N, −10°C; (ii) Et3N, H2O, tetrahydrofuran, substituted amine HCL, 0–5°C; (c) NaOH 1 M water; (d) EDCI, DCM, Et3N, room temperature; (e) quinoline, palladium on barium sulphate (5%), MeOH, H2 atmospheric pressure; (f) borohydride polymer-supported (CH3COO)2Ni·4H2O, MeOH, H2 atmospheric pressure.
Vasorelaxant effects of VSN16 and its enantiomers
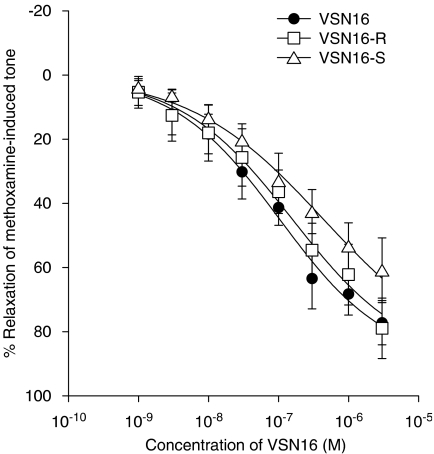
(R)-3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide (VSN16-R), (S)-3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide (VSN16-S) and VSN16 (the latter being used to designate the racemic mixture of the enantiomers) had similar potency at relaxing methoxamine-induced tone in the endothelium-intact small mesenteric artery of the rat (VSN16-R: EC50=110±69 nM, Rmax=91.6±9.6%; VSN16-S: EC50=140±17 nM, Rmax=71.0±1.8%; VSN16: EC50=88±3 nM, Rmax=86.7±6.4%; n=6 for all; Figure 3). Statistical analysis shows that VSN16-R is a little more potent than VSN16-S (P<0.05), although there was no significant difference in potency between VSN16-R and the racemic mixture.
Figure 3.
Concentration–response curves for relaxation of methoxamine-induced tone by VSN16 and its enantiomers in the isolated small mesenteric artery of the rat. Relaxation was determined in the presence of a functional endothelium (n=6 for all). Values are shown as means and vertical lines represent the s.e.m. from paired experiments. The curves drawn are those obtained from the curve-fitting procedure and values for Rmax and EC50 are given in the text.
Effects of cannabinoid receptor antagonists
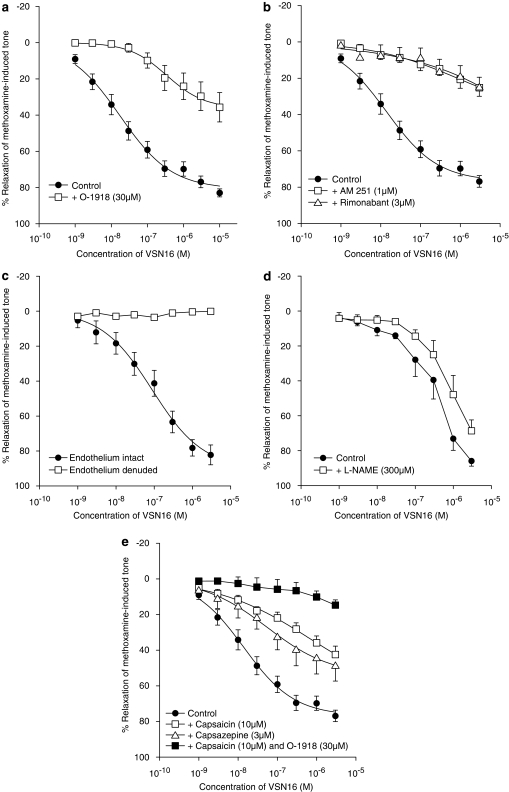
Figure 4a shows that O-1918 (30 μM), an antagonist for the novel cannabinoid receptor in the vasculature, markedly affected the vasorelaxation to VSN16. There was a 20-fold shift to the right of the concentration–response curve and the maximal relaxation was also reduced to about half the control value (control: EC50=17±3 nM, Rmax=80.9±2.6%; O-1918: EC50=341±91 nM, Rmax=36.2±2.5%; n=5; P<0.01). The cannabinoid receptor antagonist rimonabant (3 μM) also produced a prominent reduction of the relaxation leaving a residual relaxation of only 24.6±5.2% (n=4; P<0.01; Figure 4b). Similarly, the selective CB1 receptor antagonist, AM 251 (1 μM), blocked the VSN16-induced response with a residual relaxation of 25.2±4.8% at the highest concentration of VSN16 available (n=4; P<0.01; Figure 4b). A lower concentration of AM 251 (0.1 μM) had no significant effect on relaxations to VSN16 (control: EC50=35±17 nM; AM 251: EC50=52±26 nM; n=5).
Figure 4.
Effects of endothelial removal, inhibitors and cannabinoid antagonists on the concentration–response curves for VSN16-induced relaxation of methoxamine-induced tone in the isolated small mesenteric artery of the rat. All vessels had an intact endothelium except where stated. (a) Antagonism by O-1918 (30 μM, n=5). (b) Antagonism by AM 251 (1 μM) or rimonabant (3 μM) (both n=4). (c) Removal of endothelium abolished the relaxant response to VSN16 (n=4). (d) Effect of L-NAME (300 μM; n=3). (e) Effects of pretreatment with capsaicin for 30 min (10 μM), or of the presence of capsazepine (3 μM) or combination of capsaicin pretreatment and O-1918 (30 μM) (n=3–4). All values are shown as means and vertical lines represent the s.e.m. from paired experiments; n shows the number of animals in each data set. The curves drawn are obtained from the curve-fitting procedure and values for Rmax and EC50 are given in the text.
Role of the endothelium and endothelial factors
VSN16-induced vasorelaxation was abolished by the removal of endothelium (n=4; Figure 4c) but the presence or absence of indomethacin (10 μM) did not have any significant effect (relaxation by VSN16 at 10 μM: control, 59.6±4%; indomethacin, 57.3±4%; n=6; P>0.05). In contrast, Figure 4d shows that inhibition of nitric oxide synthase by L-NAME caused a rightwards shift of the concentration–response curve (P<0.01); although solubility limitations prevented definition of a true maximum response, the shift at the level of 50% relaxation of tone was 3.1-fold (control: EC50%=0.35±0.05 μM; L-NAME: EC50%=1.1±0.1 μM; n=3).
Inhibition of the Ca2+-sensitive K+ channels (KCa) that are associated with the activity of endothelium-dependent hyperpolarizing factor, using a combination of apamin and charybdotoxin (both at 50 nM), significantly inhibited the relaxation induced by VSN16 (relaxation by 10 μM VSN16: control, 60.8±5%; apamin and charybdotoxin, 13.7±3%; n=8; P<0.01). Addition of L-NAME (300 μM) to the combination of apamin plus charybdotoxin further reduced the relaxant response by VSN16 (relaxation by 10 μM VSN16: control, 60.8±5%; apamin with charybdotoxin and L-NAME, 7.3±3%; n=4; P<0.01).
Involvement of TRPV1
Both functional desensitization of the vanilloid system by capsaicin (10 μM pretreatment for 30 min) and preincubation with capsazepine (an antagonist at the vanilloid TRPV1 receptor; 3 μM) significantly inhibited the relaxation induced by VSN16 (control: EC50=14±2 nM, Rmax=76.8±2.2%; capsaicin: EC50=643±45 nM, Rmax=66.5±8.0%; capsazepine: EC50=55±12 nM; Rmax=54.3±2.2%; both n=4; P<0.05; Figure 4e). In addition, while the capsaicin pretreatment or incubation with O-1918 alone did not completely abolish the relaxant response induced by VSN16, combination of the two had an additive effect, further reducing the relaxation and leaving a residual relaxation at 3 μM VSN16 of 14.7±2.9% (n=3; Figure 4e).
Effects of pertussis toxin on VSN16-induced relaxation
The relaxation produced by VSN16 was not inhibited by preincubation with pertussis toxin at a concentration of 400 ng ml−1 for 2 h (relaxation by VSN16 at 10 μM: control, 58.1±4.0%; pertussis toxin, 53.1±4.0%; n=4; P>0.05). This same treatment and batch of toxin did inhibit relaxation to oleamide in another study carried out during this work (Hoi and Hiley, 2006).
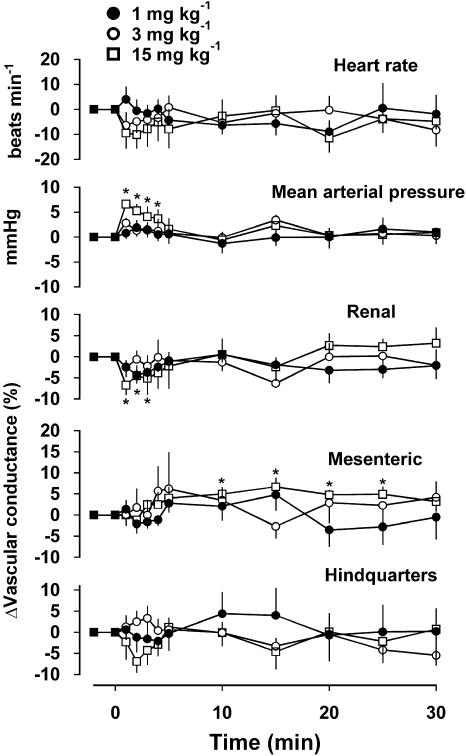
Haemodynamic studies with VSN16
Resting cardiovascular variables before administration of VSN16-R at 1, 3 and 15 mg kg−1 were: heart rate, 369±8, 364±10 and 380±13 beats min−1; mean arterial blood pressure, 108±3, 108±2 and 108±1 mmHg; renal vascular conductance, 100±9, 95±9 and 97±10 (kHz mmHg−1) 103; mesenteric vascular conductance, 61±5, 56±4 and 63±4 (kHz mmHg−1) 103; hindquarters vascular conductance, 49±3, 51±5 and 49±4 (kHz mmHg−1) 103.
At doses of 1 and 3 mg kg−1, there were no significant cardiovascular effects of VSN16-R (Figure 5). At a dose of 15 mg kg−1, there was a prompt and short-lived rise in blood pressure (P<0.05 between 1 and 4 min) and fall in renal vascular conductance (P<0.05 between 1 and 3 min), and a slower onset, longer-lasting rise in mesenteric vascular conductance (P<0.05 between 10 and 25 min); there were no changes in hindquarters vascular conductance or heart rate (Figure 5).
Figure 5.
Haemodynamic effects of VSN16-R, administered i.v., in conscious rats. Values are shown as means and vertical lines represent the s.e.m. (n=8). *Significantly different from the control value (P<0.05).
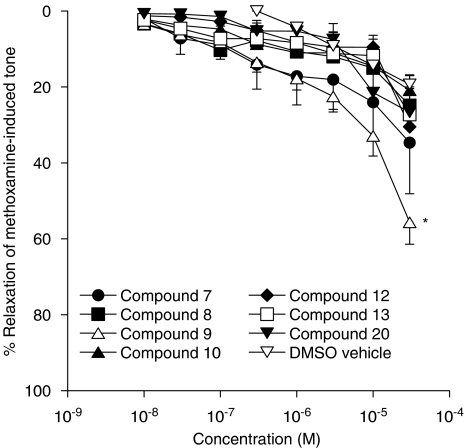
Relaxant effects of analogues of VSN16
A structure–activity study was undertaken with a series of analogues of VSN16. As shown in Figure 6, only compound 9 (with code number VSN15) showed a vasorelaxant effect that, at a concentration of 30 μM, was significantly greater than that evoked by the DMSO vehicle. Some vasorelaxation was seen with compound 7, but this did not reach significance relative to the DMSO control. For compound 9, the vasorelaxant effect was concentration-dependent but, as a maximal response was not found, an EC50 could not be determined. However, it is ∼80-fold less potent than the parent VSN16 as its EC50% of approximately 28 μM compares to 0.35 μM for VSN16 (cf Figure 4).
Figure 6.
Concentration–response curves for relaxation of methoxamine-induced tone by analogues of VSN16. Relaxation was determined in the presence of a functional endothelium (n=4–6). The effects of the appropriate amounts of vehicle (dimethyl sulfoxide; DMSO) are also shown; the highest amount of vehicle added gave 0.6% v v−1 in the bath. Values are shown as means and vertical lines represent the s.e.m. from paired experiments. *Significantly different from the relaxation obtained with the DMSO vehicle alone (P<0.05).
Effects on [3H]CP 55940 binding
Unlabelled CP 55940 inhibited the binding of [3H]CP 55940 according to a simple one-site competition model (Ki=0.36±0.05 nM, nH=0.76±0.05, n=3) and the maximal inhibition was 55±5% of the total bound [3H]CP 55940. The cannabinoid receptor antagonist rimonabant inhibited the specific binding of [3H]CP 55940 to rat cerebellar membranes in a concentration-dependent manner (Ki=9.0±1.3 nM, nH=0.81±0.07, n=5). The maximum inhibition by rimonabant at 1 μM was 56±4% of the total bound; since this is comparable to the effect of CP 55940, rimonabant (1 μM) was used to determine non-specific binding in further experiments. The putative antagonist at the vascular cannabinoid receptor, O-1918, showed limited inhibition of radioligand binding (15±1% of the specific binding at 30 μM, the highest concentration that could be used). VSN16 (300 μM) did not inhibit [3H]CP 55940 binding to rat cerebellar membranes (inhibition=−15±5%; n=4). Interestingly, compound 9, the compound in the series showing the greatest vasorelaxant activity after VSN16, significantly inhibited the specific binding of [3H]CP 55940 by 25±5% at a concentration of 100 μM (n=4) but compound 7, which showed some vasorelaxant activity, did not (inhibition at 100 μM=−4±15%; n=4).
Discussion
VSN16 was designed to be a cannabinoid-like compound which would not show central actions and which might be beneficial in multiple sclerosis. It is water-soluble, unlike the analogues also tested here, and, in addition to neuronal effects to be reported elsewhere, we have now found it to induce vasorelaxation in the isolated third-generation mesenteric artery of the rat. Its actions show only slight stereoselectivity, are strictly endothelium-dependent, apparently involve the vanilloid receptor system and are sensitive to cannabinoid antagonists including one (O-1918) active at a currently unidentified cannabinoid receptor in the vasculature (Offertáler et al., 2003).
The role of cannabinoid receptors
The endothelium-dependent vasorelaxation induced by VSN16 was antagonized by two CB1 receptor antagonists, rimonabant (Showalter et al., 1996) and AM 251 (Lan et al., 1999). A similar reduction in the relaxation at the highest agonist concentration available brought about by both antagonists could indicate involvement of the cannabinoid CB1 receptor. However, this is unlikely because, firstly, a lower concentration of AM 251 (100 nM), which might be expected to give a shift of over 100-fold in the concentration–response curve based on its Kd<1 nM (Gatley et al., 1997), did not antagonize the responses to VSN16. Secondly, CB1 receptors, even if they are present in some parts of the vasculature (Darker et al., 1998; Ho and Hiley, 2004), have not been reported to contribute to any vasodilator action in the rat mesenteric vasculature (White and Hiley, 1998a; Wagner et al., 1999; O'Sullivan et al., 2005).
The interpretation of the effects of the antagonists is not simple as they did not produce a parallel shift in the concentration–response curve but rather depressed the maximum response. However, the lack of documented CB1 receptor-mediated responses in the third-generation mesenteric artery of the rat as well as the observation that the responses to VSN16 are insensitive to pertussis toxin (while CB1 receptor-mediated responses are sensitive to the toxin; Matsuda et al., 1990) indicate that its actions are not likely to be due to CB1 receptor activation.
The absence of involvement of the CB1 receptor in the responses to VSN16 is further substantiated by the results with [3H]CP 55940 binding to rat cerebellar membranes. Rat cerebellum has been shown to be a tissue rich in CB1 receptors whereas the CB2 receptor is very sparse (Griffin et al., 1999) and the affinity observed for rimonabant in rat cerebellum in this study is similar to those reported in the literature for its binding to CB1 receptors. Here, the Ki value for rimonabant was 9.0 nM, which compares with those reported at stably expressed human CB1 receptors (5.6–12.3 nM; see Howlett et al., 2002 for review), though a slightly higher affinity at rat brain CB1 receptors (Ki=1.98 nM) was reported by Rinaldi-Carmona et al. (1994). The absence of any inhibitory effect of VSN16 on specific [3H]CP 55940 binding shows that it does not bind to the CB1 receptor in rat tissues.
Interestingly, the vasorelaxation induced by VSN16 was also inhibited by O-1918, which has been proposed by Offertáler et al. (2003) to be a selective antagonist for the putative endothelial ‘abnormal-cannabidiol receptor' (Járai et al., 1999) or ‘anandamide receptor' (Wagner et al., 1999); O-1918 blocks the action of both abnormal cannabidiol and anandamide in rat blood vessels (Offertáler et al., 2003) and, as shown here, has a very low binding potency at rat cerebellar CB1 receptors. Furthermore, the concentrations of rimonabant and AM 251 used in this study to show antagonism are much higher than those needed to block CB1 receptors, and sensitivity to high concentrations of rimonabant is a feature of responses mediated by the postulated vascular cannabinoid receptor (Ho and Hiley, 2003b).
The relaxant response to VSN16 was entirely dependent on a functional endothelium. It therefore resembles those to anandamide and abnormal cannabidiol in that the vasorelaxation they induce shows some endothelium dependence and sensitivity to rimonabant (Offertáler et al., 2003). Therefore, VSN16 could be an agonist for a novel endothelial cannabinoid receptor, or receptors, activated by anandamide or abnormal cannabidiol. Taken together, there is strong evidence for the involvement of receptors for cannabinoid-like compounds in the production of vasorelaxation by VSN16. However, the nature of these receptors cannot be clearly identified, as the relaxant effect of VSN16 is not consistent with action at CB1 receptors. Nor does its pharmacology agree totally with the profile of the putative endothelial abnormal cannabidiol receptor in the mesentery as the latter has been reported to be insensitive to AM 251 (Ho and Hiley, 2003b). Clearly, further detailed investigation is necessary for characterizing the cannabinoid receptor, or receptors, involved in these responses. Thus far no other receptor has been definitively identified as a cannabinoid receptor although the orphan receptor GPR55 has been put forward as a candidate (Baker et al., 2006). It has been contended that GPR55 is unlikely to be a cannabinoid receptor on the basis of the ‘functional fingerprint' of amino acids involved in ligand binding at CB1 and CB2 receptors (Petitet et al., 2006), but the value of this particular analysis is questionable as cannabinoid ligands are known to bind to GPR55 (Brown and Wise, 2001; Drmota et al., 2004). It would be interesting to determine the actions of VSN16 at this receptor, especially as its mRNA has been identified in whole rat mesenteric artery (Hoi, 2007). Indeed, we have some preliminary data that show that, at a concentration of 10 μM, it increases intracellular Ca2+ in HEK293 cells stably expressing GPR55 in a manner that is sensitive to rimonabant (3 μM) but not pretreatment with pertussis toxin. (Values as fold increase over baseline±s.e.m. Control: 1.70±0.06, n=30; with rimonabant: 1.06±0.02, n=30, P<0.001, Student's t-test. Control: 1.76±0.10, n=38; after pretreatment for 20 h with toxin: 1.85±0.02, n=41, not significant.)
Processes involved in vasorelaxation to VSN16
The relaxant response to VSN16 was sensitive to the inhibition of nitric oxide synthase by L-NAME, though there also was a role for KCa as the responses were sensitive to the combination of apamin with charybdotoxin; this combination has been extensively used to show a role for endothelium-dependent hyperpolarization in a vasorelaxant effect (Chataigneau et al., 1998). As the responses were abolished by removal of the endothelium, VSN16 is the first cannabinoid-like agonist that displays an entirely endothelium-dependent vasorelaxation. Other cannabinoids that have been investigated until now induce relaxation that is wholly or partly endothelium-independent (for example, CP 55940, HU 210, palmitoylethanolamide and WIN 55212-2; White and Hiley, 1997a, 1997b, 1998a: anandamide; Ho and Hiley, 2003a: abnormal cannabidiol; Offertáler et al., 2003; Ho and Hiley, 2003b: virodhamine, Ho and Hiley, 2004). These cannabinoids and endocannabinoids therefore show a wide range of mechanisms to bring about their vascular actions that are sometimes very markedly different. For example, unlike the vasorelaxation caused by VSN16, responses induced by abnormal cannabidiol are not affected by L-NAME but are similar in that they are sensitive to apamin plus charybdotoxin (Offertáler et al., 2003; Ho and Hiley, 2003b). In the absence of a certain identification of the receptor, or receptors, mediating the endothelium-dependent responses to the cannabinoids, it is difficult to untangle these diverse mechanisms to see how they are activated by the receptors concerned. The fact that VSN16 is entirely endothelium-dependent in its actions might make it a more useful agent in resolving the pathways involved than those compounds, which show both endothelium-mediated and -independent mechanisms.
As with some endocannabinoids, relaxation to VSN16 was sensitive to capsazepine, a competitive antagonist of TRPV1, and to functional desensitization of the vanilloid receptor by capsaicin pretreatment; both drugs significantly reduced the maximum response and, moreover, to similar levels. VSN16 therefore shares with anandamide (Zygmunt et al., 1999; Ho and Hiley, 2003a) and its non-hydrolysable derivative methanandamide (Ralevic et al., 2000), the property of acting through the TRPV1 receptor system. This ability to act through vanilloid receptors is shared by a few compounds structurally related to each other by having an amide bond and an aliphatic side chain. These include olvanil (N-vanillyloleamide: Szallasi and Blumberg, 1999) and the putative endocannabinoid N-arachidonoyl dopamine (Huang et al., 2002; O'Sullivan et al., 2004) and they are clearly quite different structurally from VSN16. In contrast, other endocannabinoids including 2-arachidonoyl glycerol (Zygmunt et al., 1999) and virodhamine (Ho and Hiley, 2004), as well as abnormal cannabidiol and synthetic cannabinoids, including HU 210, WIN 55212-2 and CP 55940, do not behave in this way (Zygmunt et al., 1999; Offertáler et al., 2003; Ho and Hiley, 2003a, 2003b). It is clear therefore that quite different structure–activity properties are associated with vasorelaxation mediated by TRPV1 and the as-yet-undefined cannabinoid receptor(s) of the rat mesenteric artery but it must be borne in mind that it is not possible to determine at this stage if VSN16 directly or indirectly stimulates the vanilloid receptor although preliminary data suggest that it is not a direct stimulant (V Di Marzo, personal communication).
In spite of this, the relaxation of the mesenteric artery in response to VSN16 is unlikely to occur via sensory nerves, since the effect is entirely endothelium-dependent. Thus, VSN16 might activate a TRPV1 receptor in the endothelium. Poblete et al. (2005) reported that they could detect the mRNA for the rat TRPV1 receptor in the endothelial cell layer in the mesenteric arterial bed, in addition to its expression in sensory nerves. They also showed that anandamide (at a sub-vasorelaxant concentration) and capsaicin elicited nitric oxide release, and that this was reduced by the TRPV1 receptor antagonists 5′-iodoresiniferatoxin, SB 366791 and capsazepine, as well as by removal of the endothelium or nitric oxide synthase inhibition.
Moreover, the sensitivity of vasorelaxation to capsaicin treatment does not always imply the involvement of TRPV1 receptors; for example, Δ9-tetrahydrocannabinol stimulates calcitonin gene-related peptide release from perivascular sensory nerves in TRPV1 receptor-knockout mice, and it was speculated that a novel receptor or ion channel on the sensory nerves might be involved (Zygmunt et al., 2002). Indeed, the relaxant effects of anandamide in blood vessels are not only sensitive to capsaicin pretreatment, but also to the calcitonin gene-related peptide antagonist, CGRP(8–37), which suggests that some of the vasorelaxant action of the endocannabinoid is due to activation of TRPV1 leading to the release of the vasodilator calcitonin gene-related peptide from sensory nerves (Zygmunt et al., 1999). In this respect, functional studies on sensory neurogenic vasorelaxation to electrical field stimulation in the rat-isolated mesenteric arterial bed have provided evidence that non-CB1, non-CB2 cannabinoid receptors might exist on capsaicin-sensitive sensory nerves in the rat mesenteric bed (Ralevic and Kendall, 2001; Duncan et al., 2004). However, since the responses to VSN16 were completely abolished by removal of the endothelium, it seems unlikely that the cannabinoid receptors of the sensory nerves are involved in the vasodilator response that it induces in the rat mesenteric artery.
The situation concerning the inhibition of VSN16 function with other cannabinoid and vanilloid receptor-related antagonists currently appears complex and will remain so until the identification of the target and its signalling cascade. While CB1- and TRPV1-binding agents modified VSN16 function, currently VSN16 cannot be shown to bind to either of these receptors (see above for results on the CB1 receptor). This suggests either that VSN16 may bind to allosteric sites or there is a complex interaction of the different receptor systems within a cascade that controls vasodilatation or that the current reagents have off-target specificities. It is already known that rimonabant has actions at other than CB1 receptors (White and Hiley, 1998b) and can inhibit the actions of abnormal cannabidiol at the ‘abnormal-cannabidiol receptor' (Járai et al., 1999; Ho and Hiley, 2003b), whereas we have shown that the abnormal cannabidiol receptor is relatively insensitive to the actions of AM 251 (Ho and Hiley, 2003b). Likewise, O-1918 inhibits the abnormal-cannabidiol responses at its vascular receptor. The finding that VSN16 is sensitive to inhibition by O-1918, and AM 251, but is pertussis toxin-insensitive suggests that the vascular target for VSN16 may be distinct from the receptor for abnormal cannabidiol.
Recently it has been reported that abnormal cannabidiol and O-1918, AM 251 and rimonabant bind to the orphan receptor GPR55 and signal via pertussis toxin-insensitive mechanisms (Sjögren et al., 2005; Johns et al., 2007). However, although GPR55 does not appear to mediate the vasodilator effects of abnormal cannabidiol (Johns et al., 2007), the receptor has been implicated in control of blood pressure in a pathological system (Sjögren et al., 2005). These points underline further that work is needed to clarify the interactions of VSN16 with GPR55.
Systemic cardiovascular effects
In view of the actions on the isolated third-generation artery, which is considered to make some contribution to mesenteric vascular resistance, a haemodynamic study was performed in conscious rats. At doses of 1 and 3 mg kg−1, VSN16 caused no significant changes in heart rate, blood pressure or regional vascular conductances. However, at the highest dose used, 15 mg kg−1, there was a transient pressor response that was accompanied by a decrease in renal vascular conductance. Interestingly, at this dose, there was a significant increase in mesenteric conductance, which would be consistent with the mesenteric vasodilatation observed in isolated vessels. These results may reflect a sparse distribution of receptors for VSN16 in the vasculature since a dose of 5 mg kg−1 VSN16-R gave a peak concentration of 7 μg ml−1 (22 μM) at 5 min after i.v. injection (data not shown); this concentration is ∼20 times that of its EC50 in the mesenteric artery. The relatively small in vivo effect on blood pressure of VSN16 could suggest that in vitro vasorelaxation of third-order mesenteric arteries has poor predictive value in generalized blood pressure effects. Alternatively, there is a possibility that there might be a strain difference in the distribution of receptors for VSN16 as the myograph studies used Wistar rats while the systemic haemodynamics were carried out in Sprague–Dawley rats.
The modest haemodynamic effects of VSN16-R contrast with the complex picture presented by anandamide, which some of us have previously found to evoke both a pressor response, at all doses studied between 0.075 and 3 mg kg−1, as well as vasoconstriction not only in the mesenteric but also in the renal and hindquarters vascular beds (Gardiner et al., 2002). At higher doses (2.5 and 3 mg kg−1), anandamide both slowed the heart and decreased the blood pressure while the hindquarters vasoconstriction was followed by an increase in vascular conductance. None of these effects was sensitive to AM 251. This pattern shows limited similarity to those responses observed with VSN16-R: both induce systemic pressor responses and increase renal vascular resistance. However, anandamide had no effect on mesenteric vascular resistance while VSN16-R did not affect hindquarters conductance which was increased by higher doses of anandamide. This suggests that the two compounds do not share sites of action that can be readily identified at the systemic level even though the mesenteric artery effects of both anandamide and VSN16-R observed in the myograph share the property of sensitivity to the novel antagonist O-1918.
Nevertheless, although VSN16-R had a modest effect in normotensive rats, it remains to be determined if it may bring about hypotension under pathological conditions. However, the lack of cardiovascular effect would be beneficial in a drug developed for use in non-cardiovascular conditions such as multiple sclerosis. Indeed, other work with VSN16 have shown that it has therapeutic potential in this condition as it reduces spasticity in vivo in the autoimmune encephalomyelitis mouse model of the disease (Okuyama et al., 2005; D Baker, unpublished results).
Chirality and structure–activity relationships
VSN16 showed little stereospecificity in its activity as a vasodilator. This probably indicates either that the part of the molecule that includes the chiral centre does not interact with the receptor or that the element of the binding surface that interacts with this region does not have a structure which is sensitive to the orientation of the side chain. Until the target and the binding moieties of VSN16 are better defined, the role of the chiral elements in relation to function will remain unclear. Based on the in vitro experiments presented here, VSN16-R is slightly more active than VSN16-S and this is seen also in relation to the in vivo therapeutic activity in models of multiple sclerosis (D Baker, unpublished results).
A limited number of analogues is presented here to illustrate the structure–activity relationships; in general, very few structural alterations are tolerated. Thus changes to the ethanolamide group, for example, to 2-fluoroethylamide, as in compound 13, or to cyclopropylamide (compound 12) caused loss of activity, though these groups are tolerated in simple anandamide analogues (Ryan et al., 1997). The interactions of the terminal carboxamide group with the receptor seem to be similarly precise with apparently conservative changes to the monomethyl amide or the unsubstituted amide not tolerated. In contrast, a cyano group was allowed in this position (compound 9, which is also termed VSN15). These data indicate that H-bond donor groups at this position are unfavourable whereas H-bond acceptor groups are apparently allowed. Reducing the length of the alkyl side chain as in compound 7 again reduced the activity markedly.
Compound 7, which showed some vasorelaxant activity, is like VSN16 in that it did not inhibit [3H]CP 55940 binding at CB1 receptors in rat cerebellar membranes. Thus, it might be suggested that chemicals of this series do not have any ability to bind to classical cannabinoid receptors. However, compound 9, which was the most effective vasodilator after VSN16, brought about modest inhibition of the binding of [3H]CP 55940 to the CB1 receptor; thus although the interaction is weak, at least one of the series has the ability to bind to the CB1 receptor even if, for the reasons discussed above, this receptor is not responsible for the vasorelaxant activity of the series.
Conclusions
VSN16 represents a novel water-soluble vasorelaxant that produces a strictly endothelium-dependent response through several mechanisms, possibly involving novel cannabinoid receptor-dependent and -independent pathways. Thus, results reported here support the proposal that an additional cannabinoid receptor (or receptors) different from either the CB1 or the CB2 receptor is responsible for the actions of VSN16. The sensitivity to O-1918 suggests that the proposed endothelial cannabinoid receptor, the ‘abnormal-cannabidiol' or ‘anandamide' receptor might contribute to some of its effects, although it is possible that another unknown but similar entity also exists. One candidate target is GPR55. Structure–activity studies of analogues in the VSN series suggest a very stringent interaction between the compound and the binding pocket of the potential receptor in the vasculature.
Acknowledgments
This work was supported by a Wellcome Trust University Translation Award (076001/Z/04/Z), the Brain Research Trust and the Bloomsbury Bioseed Fund.
Abbreviations
- DCM
dichloromethane
- DMSO
dimethyl sulfoxide
- EDCI
1-ethyl-3-(3′-dimethylaminopropyl)carbodiimide
- KCa
Ca2+-sensitive K+ channels
- L-NAME
NG-nitro-L-arginine methyl ester
- nH
Hill slope
- O-1918
(−)-1,3-dimethoxy-2-(3-3,4-trans-p-menthadien-(1,8)-yl)-orcinol
- TRPV1
transient receptor potential vanilloid 1
- VSN16
(±)-3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide
- VSN16-R
(R)-3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide
- VSN16-S
(S)-3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide
Conflict of interest
VSN16 is subject to patent WO2005/080316 granted to M Okuyama, D Selwood, C Visintin, D Baker, G Pryce and University College, London.
References
- Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Wise A.GlaxoSmithKline Identification of modulators of GPR55 activity 2001. Patent WO01/86305
- Chataigneau T, Félétou M, Thollon C, Villeneuve N, Vilaine JP, Duhault J, et al. Cannabinoid CB1 receptor and endothelium-dependent hyperpolarization in guinea-pig carotid, rat mesenteric and porcine coronary arteries. Br J Pharmacol. 1998;123:968–974. doi: 10.1038/sj.bjp.0701690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Martin PE, Evans WH, Randall MD, Griffith TM. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J Physiol. 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darker IT, Millns PJ, Selbie L, Randall MD, S-Baxter G, Kendall DA. Cannabinoid (CB1) receptor expression is associated with mesenteric resistance vessels but not thoracic aorta in the rat. Br J Pharmacol. 1998;125:95P. [Google Scholar]
- Drmota T, Greasley P, Groblewski T.AstraZeneca Screening assays for cannabinoid-ligand type modulators of GPR55 2004. Patent WO2004/074844
- Duncan M, Kendall DA, Ralevic V. Characterization of cannabinoid modulation of sensory neurotransmission in the rat isolated mesenteric arterial bed. J Pharmacol Exp Ther. 2004;311:411–419. doi: 10.1124/jpet.104.067587. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Complex regional haemodynamic effects of anandamide in conscious rats. Br J Pharmacol. 2002;135:1889–1996. doi: 10.1038/sj.bjp.0704649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sci. 1997;61:PL 191–PL 197. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- Griffin G, Wray EJ, Martin BR, Abood ME. Cannabinoid agonists and antagonists discriminated by receptor binding in rat cerebellum. Br J Pharmacol. 1999;128:684–688. doi: 10.1038/sj.bjp.0702806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx. Br J Pharmacol. 2003a;139:585–597. doi: 10.1038/sj.bjp.0705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003b;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasorelaxant activities of the putative endocannabinoid virodhamine in rat isolated small mesenteric artery. J Pharm Pharmacol. 2004;56:869–875. doi: 10.1211/0022357023682. [DOI] [PubMed] [Google Scholar]
- Hoi PM. Cannabinoid receptor pharmacology in the rat small mesenteric artery 2007University of Cambridge, Cambridge, UK; PhD thesis; [Google Scholar]
- Hoi PM, Hiley CR. Vasorelaxant effects of oleamide in rat small mesenteric artery indicate action at a novel cannabinoid receptor. Br J Pharmacol. 2006;147:560–568. doi: 10.1038/sj.bjp.0706643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper AT, Witiak DT, Ziemniak J. Design, synthesis, and biological evaluation of conformationally constrained aci-reductone mimics of arachidonic acid. J Med Chem. 1998;41:420–427. doi: 10.1021/jm970034q. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker D, Ao Z, Shapland EM, Daniels DA, et al. The novel endocannabinoid receptor GPR55 binds atypical cannabinoids but does not mediate their vasodilator effects Br J Pharmacol; 2007 10.1038/sj.bjp.0707419e-pub ahead of print, 20 August 2007doi [DOI] [PMC free article] [PubMed]
- Kadota I, Shibuya L, Lutete LM, Yamamtoto Y. Palladium/benzoic acid catalyzed hydroamination of alkynes. J Org Chem. 1999;64:4570–4571. doi: 10.1021/jo990498r. [DOI] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, et al. Structure–activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Okuyama M, Selwood D, Visintin C, Baker D, Pryce G.University College London. Modulators of cannabinoid receptors 2005. Patent WO2005/080316
- O'Sullivan SE, Kendall DA, Randall MD. Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA) Br J Pharmacol. 2004;141:803–812. doi: 10.1038/sj.bjp.0705643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. The effects of Δ9-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br J Pharmacol. 2005;145:514–526. doi: 10.1038/sj.bjp.0706218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offertáler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitet F, Donlan M, Michel A. GPR55 as a new cannabinoid receptor: still a long way to prove it. Chem Biol Drug Des. 2006;67:252–253. doi: 10.1111/j.1747-0285.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- Poblete IM, Orliac ML, Briones R, Adler-Graschinsky E, Huidobro-Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol. 2005;568:539–551. doi: 10.1113/jphysiol.2005.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA. Cannabinoid inhibition of capsaicin-sensitive sensory neurotransmission in the rat mesenteric arterial bed. Eur J Pharmacol. 2001;418:117–125. doi: 10.1016/s0014-2999(01)00940-2. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Hogestatt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br J Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Alonso R, Shire D, Congy C, et al. Biochemical and pharmacological characterisation of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Ryan WJ, Banner WK, Wiley JL, Martin BR, Razdan RK. Potent anandamide analogs: the effect of changing the length and branching of the end pentyl chain. J Med Chem. 1997;40:3617–3625. doi: 10.1021/jm970212f. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Sjögren S, Ryberg E, Lindblom A, Larsson N, Hermansson N-O, Åstrand A, et al. A new receptor for cannabinoid ligands. International Cannabinoid Research Society XVth Symposium on the Cannabinoids: 106 2005()
- Smith PF. Medicinal cannabis extracts for the treatment of multiple sclerosis. Curr Opin Investig Drugs. 2004;5:727–730. [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Wagner JA, Varga K, Járai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptor. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol. 1997a;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. Endothelium and cannabinoid receptor involvement in levcromakalim vasorelaxation. Eur J Pharmacol. 1997b;339:157–160. doi: 10.1016/s0014-2999(97)01397-6. [DOI] [PubMed] [Google Scholar]
- White R, Hiley CR. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br J Pharmacol. 1998a;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br J Pharmacol. 1998b;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson EM, Evans FJ. Cannabinoids in clinical practice. Drugs. 2000;60:1303–1314. doi: 10.2165/00003495-200060060-00005. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Andersson DA, Hogestatt ED. Δ9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J Neurosci. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]