Abstract
Background and purpose:
Atypical cannabinoids are thought to cause vasodilatation through an as-yet unidentified ‘CBx' receptor. Recent reports suggest GPR55 is an atypical cannabinoid receptor, making it a candidate for the vasodilator ‘CBx' receptor. The purpose of the present study was to test the hypothesis that human recombinant GPR55 is activated by atypical cannabinoids and mediates vasodilator responses to these agents.
Experimental approach:
Human recombinant GPR55 was expressed in HEK293T cells and specific GTPγS activity was monitored as an index of receptor activation. In GPR55-deficient and wild-type littermate control mice, in vivo blood pressure measurement and isolated resistance artery myography were used to determine GPR55 dependence of atypical cannabinoid-induced haemodynamic and vasodilator responses.
Key results:
Atypical cannabinoids O-1602 and abnormal cannabidiol both stimulated GPR55-dependent GTPγS activity (EC50 approximately 2 nM), whereas the CB1 and CB2-selective agonist WIN 55,212-2 showed no effect in GPR55-expressing HEK293T cell membranes. Baseline mean arterial pressure and heart rate were not different between WT and GPR55 KO mice. The blood pressure-lowering response to abnormal cannabidiol was not different between WT and KO mice (WT 20±2%, KO 26±5% change from baseline), nor was the vasodilator response to abnormal cannabidiol in isolated mesenteric arteries (IC50 approximately 3 μ M for WT and KO). The abnormal cannabidiol vasodilator response was antagonized equivalently by O-1918 in both strains.
Conclusions:
These results demonstrate that while GPR55 is activated by atypical cannabinoids, it does not appear to mediate the vasodilator effects of these agents.
Keywords: GPR55, cannabinoid receptors, abnormal cannabidiol, CBx receptor
Introduction
Cannabinoids exert their effects on the central and peripheral nervous systems via two G-protein-coupled receptors, cannabinoid receptors type 1 and type 2 (CB1 and CB2, respectively) (Howlett et al., 2002). The CB1 receptor is expressed primarily in the central nervous system, mediating neurobehavioural responses to cannabinoids (Howlett et al., 2002), while CB2 is expressed peripherally in immune and haematopoietic cells (Munro et al., 1993). A third, as yet unidentified atypical cannabinoid receptor (CBx), has been proposed to mediate the effects of certain cannabinoids that show no binding affinity for CB1 or CB2 (Randall et al., 2004; Begg et al., 2005). These ‘atypical cannabinoids' have been shown to elicit endothelium-dependent vasodilator responses in isolated resistance arteries (Jarai et al., 1999; Wagner et al., 1999; Offertaler et al., 2003), and to lower blood pressure without any overt behavioural effects (Adams et al., 1977). While much information exists regarding the potential signaling mechanisms and biological effects of CBx, the molecular identity of this receptor has remained a mystery to date.
Recently, it was reported in patent literature (see Baker et al., 2006) that the orphan G-protein-coupled receptor GPR55 binds cannabinoid molecules with a profile, comparable to that expected of CBx. In the absence of selective agonists or antagonists for GPR55, evidence suggesting that GPR55 mediates cardiovascular effects of CBx agonists, or plays a role in any (patho)physiological function is lacking at present.
In the current study, mice deficient in GPR55 were utilized to evaluate the role of this purported atypical cannabinoid receptor in mediating the cardiovascular responses to atypical cannabinoids, which were characterized using human recombinant GPR55. The purpose of the present study was to test the hypothesis that GPR55 mediates vasodilatation to CBx agonists or atypical cannabinoids.
Methods
Animal procedures
All animal procedures were reviewed and approved by the GlaxoSmithKline Animal Care and Use Committee, and were performed in accredited facilities in accordance with institutional guidelines and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council).
Recombinant GPR55
Random basic local alignment search tool (BLAST)-based searches of the Genbank EST database identified a partial novel purinergic-like seven transmembrane receptor. The sequence for the missing regions was obtained by carrying out 5′ and 3′ rapid amplification of cDNA ends (using human fetal spleen, fetal liver and fetal lung cDNAs as a template. The full-length cDNA was cloned by PCR from a human placenta cDNA library (Invitrogen, Carlsbad, CA, USA) using primers at the predicted start codon (5′-AACATGAGTCAGCAAAACACCAGTGGG-3′) and the downstream stop codon (5′-GTGGGGACTGCCTGTTTGACG-3′). PCR was performed using Pfu Turbo polymerase (Stratagene, La Jolla, CA, USA) and a PCR9600 thermal cycler (Perkin Elmer, Waltham, MA, USA). The PCRs were carried out as follows: 94°C for 30 s, 52°C for 2 min and 72°C. The PCR product was run on a 1% agarose gel containing 0.1 μg ml−1 ethidium bromide. A single band of approximately 960 nucleotides was purified using the QIAquick Gel Extraction kit (Qiagen, Valencia, CA, USA) and cloned into the pCRII vector according to the manufacturer's protocol (Invitrogen). An independent clone was sequenced and found to contain an open reading frame of 319 amino acids, which was designated GPR55. This gene is identical to ‘GPR55A', as described from the patent literature in Baker et al. (2006).
HEK293T cells were maintained in T225 flasks in Dulbecco's minimum essential media/F12 media (Invitrogen) containing 10% (v/v) heat-inactivated fetal calf serum and 2 mM glutamine. At 90% confluency, cells were transiently transfected with human GPR55 DNA constructs (pcDNA3, 85 μg DNA per T225 in Optimem (Invitrogen)). Forty-eight hours later, plasma membranes were prepared from the transiently transfected cultured cells. All procedures were carried out at 4°C. Cell pellets were re-suspended in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) and 1 mM ethylenediaminetetraacetic acid (EDTA), pH 7.4, containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA) followed by homogenization for 45 s in a Waring blender. Cell lysates were centrifuged at 210 g for 10 min to pellet nuclei and unbroken cells and P2 particulate fractions were recovered by centrifugation 43 665 g for 30 min before being re-suspended in 50 mM HEPES and 1 mM EDTA, pH 7.4. Protein concentrations were determined using the bicinchoninic acid procedure (Pierce Biotechnology, Rockford, IL, USA) using bovine serum albumin (BSA) as a standard.
GTPγS activation
[35S]-labelled Guanosine 5′-(gamma thio) triphosphate (GTPγS) activation was measured as described by Breivogel (2006). Briefly, membranes (30 μg protein) from HEK293T cells transfected with human GPR55 were incubated with cannabinoid ligands (1 pM–10 μM) for 60 min at 30°C in assay buffer (50 mM Tris-HCl, pH 7.4; 3 mM MgCl2; 0.2 mM EGTA; 100 mM NaCl; 0.1% BSA) in the presence of 0.5 nM [35S]GTPγS and 50 μM guanosine diphosphate (GDP), in a final volume of 200 μl. The reaction was carried out in 96-well microtitration plates (Multiscreen FB Glass Fiber, Millipore Corp., Billerica, MA, USA). Non-specific binding was measured in the presence of 10 μM unlabelled GTPγS. The reaction was terminated by rapid filtration, the microfiltration plates were washed five times with ice-cold wash buffer (50 mM Tris-HCl, pH 7.4), and bound radioactivity was determined in TopCount Scintillation Counter (Perkin Elmer).
GPR55 KO mice
Gene targeting was performed in E14.1 mouse embryonic stem cells (129P2/OlaHsd). Two targeted clones were injected into C57Bl6/J-derived blastocysts. Male chimaeras were crossed with C57Bl6/J females to produce N1F0 offspring. The animals carrying the GPR55 mutant allele from one injected clone were repeatedly bred, or backcrossed, onto the C57Bl6/J genetic background for more than five generations. At each generation the C57Bl/6 genetic composition was assessed using a panel of single nucleotide polymorphism markers (Markel et al., 1997; Wakeland et al., 1997). The mice were considered fully backcrossed when they displayed greater than 98% coverage with a C57Bl/6 marker set. Backcrossed heterozygous animals were intermated to produce F1 mice homozygous for the GPR55 mutation (GPR55 KO) and wild-type littermate controls (WT). PCR genotyping was performed using primers designed to amplify PCR products specific to either the GPR55 wild-type locus (GPR55: DW1: 5′-TCTTCCCCCTGGAGATCTTT-3′; DW2: 5′-CTGGGAGAAAGGAGACCACA-3′; 30 cycles of 94°C (45 s), 58°C (45 s) and 72°C (45 s) were used to generate amplicons of 207 bp) or to the targeted GPR55 locus (N-5′, neomycin gene specific 5′-primer: 5′-CCGGCCGCTTGGGTGGAGAGG-3′ and N-3′, neomycin gene specific 3′-primer: 5′-TCGGCAGGAGCAAGGTGAGATGACA-3′; 30 cycles of 94°C (30 s), 68°C (30 s) and 72°C (30 s) were used to generate amplicons of 299 bp). Mice were housed in groups of 1–5 before use and maintained under a standard 12 h light–dark cycle with food and water available ad libitum. All testing began when the mice were approximately 10 weeks of age.
Blood pressure measurement
Male GPR55 KO mice or WT mice were anaesthetized with inhaled isoflurane (2% spontaneous respiration) and maintained at 37°C with a heating blanket. Blood pressure was recorded using a 1.4 F Millar mikro-tip catheter transducer (SPR-671) inserted into the right carotid artery. Blood pressure and heart rate data were collected by a Gould acquisition system (3P 6600). A jugular vein cannula (PE50) was used for intravenous bolus administration of abnormal cannabidiol (30 mg kg−1) or vehicle (ethanol:Alkamuls EL-620:phosphate-buffered saline, 1:1:8). In a pilot study utilizing WT mice, this dose of abnormal cannabidiol elicited reproducible hypotensive responses similar to published studies that used a dose of 10–20 mg kg−1 (Adams et al., 1977; Jarai et al., 1999; Offertaler et al., 2003).
Blood vessel reactivity in vitro
For myography experiments, mice were anaesthetized with isoflurane and killed by cervical dislocation. Small mesenteric arteries (∼100 μm internal diameter) were mounted in wire myograph organ baths (Danish Myotechnology, Aarhus, Denmark) containing 5 ml of Krebs buffer with the following components (mM): NaCl, 112.0; KCl, 4.7; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25.0; dextrose, 11.0. Krebs was maintained at 37°C and aerated with 95% O2/5% CO2 (pH 7.4). Vessel viability was verified with KCl (60 mM) and phenylephrine (1 μM) to which subsequent agonist-induced responses were normalized. Endothelial integrity was verified by responsiveness to carbachol (10 μM).
Cumulative concentration–response curves to phenylephrine (10 nM–30 μM) were obtained for each vessel by adding the spasmogen to the tissue bath at half-log increments. For relaxation studies, vessels were preconstricted with a predetermined EC80 concentration of phenylephrine and contractile tone was reversed by adding cumulative amounts of cannabinoid agonists (10 nM–30 μM). For some experiments, vessels were pretreated with the putative CBx antagonist O-1918 at a concentration previously shown to antagonize abnormal cannabidiol vasodilatation (30 μM; Offertaler et al., 2003) or dimethylsulphoxide vehicle for 30 min before concentration response determination with cannabinoid agents. The vasodilator activity of abnormal cannabidiol and its dependence on the CBx was verified first in phenylephrine-preconstricted mesenteric resistance arteries from C57Bl/6 mice unrelated to the GPR55 KO/WT strains. These vessels displayed concentration-dependent vasodilatation to abnormal cannabidiol (EC50 3.6±1.2 μM), which was inhibited by the classical ‘CBx' antagonist O-1918 (30 μM) with an antagonist affinity constant (Kb) of 4.8±1.2 μM.
Statistical analysis
Competition-binding curves were analysed by nonlinear regression (GraphPad Software, Inc., San Diego, CA, USA) and concentration–response curves were fitted to a logistic equation as described previously (Douglas et al., 2005). Antagonist affinity determinations (for Kb) were carried out using the Schild equation (Jenkinson et al., 1998). All values are expressed as mean±s.e.mean. and n represents either the number of independent experiments carried out in triplicate or the number of total animals from which vessels were isolated. Statistical comparisons were made using a paired, two-tailed t-test or analysis of variance for repeated measures with Dunnett's post-test analysis. Differences were considered significant when P<0.05.
Materials
Abnormal cannabidiol, O-1602, O-1918, WIN 55212-2 were obtained from Cayman Chemical Co. (Ann Arbor, MI, USA), Alkamuls was obtained from Rhodia Inc. (Cranbury, NJ, USA). Unless otherwise noted, all other reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA) and were of the highest analytical grade possible.
Results
Atypical cannabinoids activate GPR55
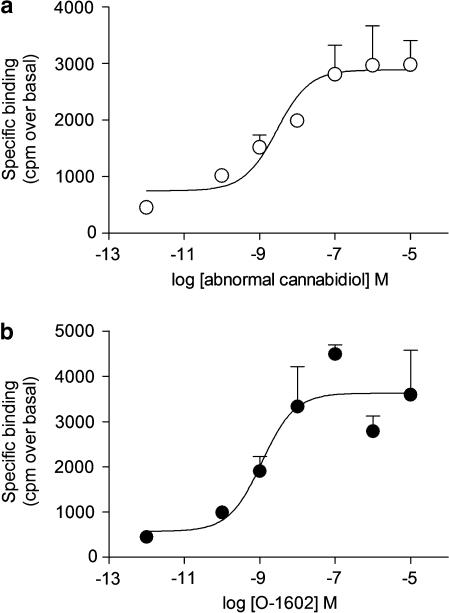
Membranes from HEK293 cells expressing empty plasmid showed no change in GTPγS activity when stimulated with the classical ‘CBx' agonists abnormal cannabidiol and its synthetic analogue O-1602 (Table 1). In membranes from HEK293 cells expressing human recombinant GPR55, both abnormal cannabidiol and O-1602 stimulated GTPγS activity with a EC50 of approximately 2 nM and Rmax values of approximately 3000 counts min−1 above basal for each compound (Table 1; Figure 1) WIN 55212-2 did not stimulate GTPγS activity in human recombinant GPR55-expressing cell membranes (Table 1).
Table 1.
Atypical cannabinoids stimulate GTPγS activation in membranes from human recombinant GPR55-expressing cells
| Compound | EC50 (nM) | Rmax (c.p.m. above basal) |
|---|---|---|
| GPR55-expressing cells | ||
| Abnormal cannabidiol | 2.5±1.4 | 2927±538 |
| O-1602 | 1.4±0.3 | 3637±578 |
| WIN 55,212-2 | >1000 | ND |
| Empty plasmid-transfected cells | ||
| Abnormal cannabidiol | >1000 | ND |
| O-1602 | >1000 | ND |
Abbreviation: GPR55, G-protein-coupled receptor 55.
Figure 1.
Atypical cannabinoids stimulate GPR55 GTPγS activity. Membranes from HEK293T cells expressing human recombinant GPR55 were utilized for GTPγS activity as described in Materials and methods. (a) Abnormal cannabidiol and (b) the synthetic abnormal cannabidiol analogue O-1602 stimulate GTPγS activity with similar potency. Data points represent means±s.e.mean of n=3 experiments.
Baseline haemodynamics and haemodynamic response to abnormal cannabidiol are not different between WT and GPR55 KO mice
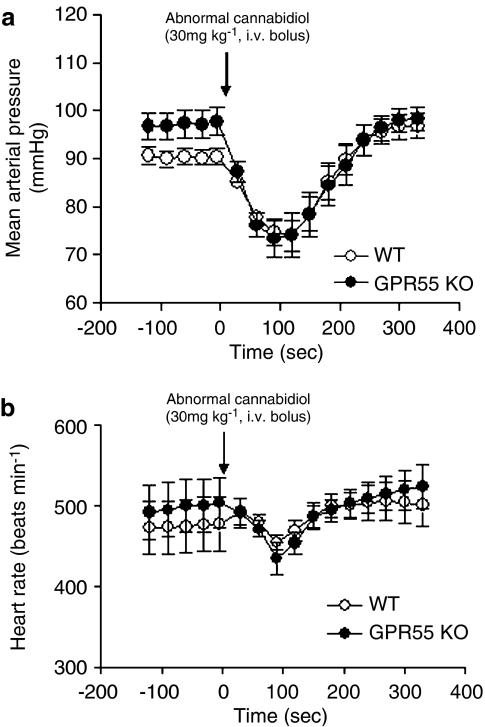
The baseline mean arterial blood pressure for WT mice (91±2 mm Hg) was not significantly different from GPR55 KO mice (96±4 mm Hg). Similarly, there was no significant difference in baseline heart rate between WT (449±23 beats min−1) and KO (426±12 beats min−1). As shown in Figure 2, administration of abnormal cannabidiol (30 mg kg−1, i.v. bolus) resulted in a rapid lowering of mean arterial pressure in both WT and GPR55 KO mice. The maximum change in mean arterial pressure was not significantly different between WT and GPR55 KO mice (WT −18±2 mm Hg, GPR55 KO −25±5 mm Hg (P=0.2), or 20±2 and 26±5% decrease from baseline (P=0.3), respectively). Abnormal cannabidiol administration was also associated with a minor decrease in heart rate immediately following injection (Figure 2), but the maximal response was not significantly different between strains (WT −19±42 beats min−1, GPR55 KO −34±34 beats min−1 (P=0.8), or 1±7 and 2±8% decrease from baseline (P=0.8), respectively). The changes in mean arterial pressure and heart rate followed a nearly identical time course (onset of response, time-to-maximum, time to return to baseline) between both strains (Figure 2).
Figure 2.
Abnormal cannabidiol administration (30 mg kg−1 i.v. bolus) results in similar blood pressure-lowering effect in GPR55 KO compared to WT. (a) Mean arterial pressure and (b) heart rate from baseline values are shown. Data are presented as mean±s.e.mean (n=8). GPR55 KO, mice homozygous for the GPR55 mutation; WT, wild-type littermate controls for GPR55 KO mice.
Vasodilatation to atypical cannabinoids
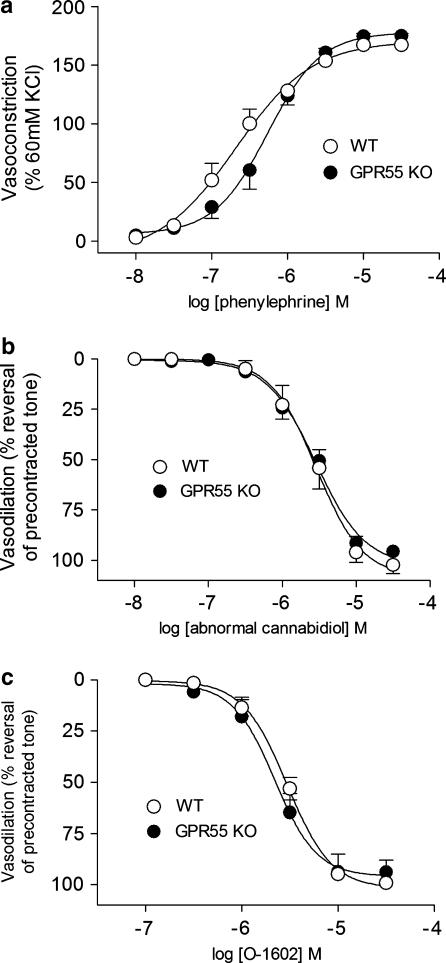
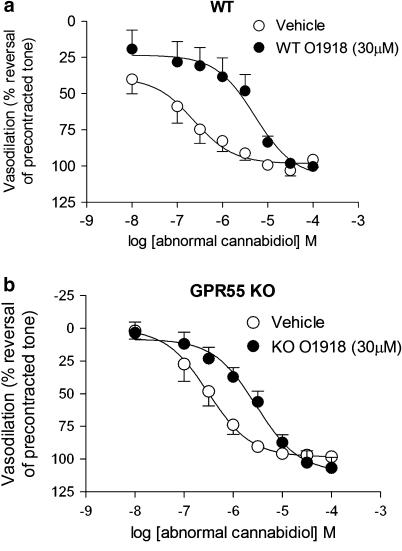
To determine whether GPR55 mediates vasodilatation to atypical cannabinoids, mesenteric resistance arteries from WT and KO mice were used. Vessels from WT and KO mice showed no significant difference in contractile responsiveness to phenylephrine (WT: EC50=255±88 nM, Rmax=165±11% of KCl response; KO: EC50=516±176 nM, Rmax=176±9% of KCl response) (Figure 3). When preconstricted with phenylephrine (EC80 concentration), vessels from both strains displayed similar vasodilator responses to atypical cannabinoids (EC50 in WT and KO tissues between 2 and 3 μM for both abnormal cannabidiol and O-1602) (Table 2, Figure 3). In all cases, the vascular tone induced by phenylephrine was completely reversed by each compound. O-1918 (30 μM) inhibited vasodilatation to abnormal cannabidiol in both WT and KO mouse vessels, with Kb values that were not significantly different (WT: 1.8±1.3 μM, KO: 2.8±2.0 μM, P=0.34 vs WT) (Figure 4).
Figure 3.
Vascular reactivity in unaltered in GPR55 KO mice compared to WT. (a) Contractile response to phenylephrine, normalized to contraction to 60 mM KCl challenge, (b) vasodilation to abnormal cannabidiol, (c) vasodilation to O-1602. Each data point represents mean±s.e.mean (n=5). GPR55 KO, mice homozygous for the GPR55 mutation; WT, wild-type littermate controls for GPR55 KO mice.
Table 2.
Mesenteric resistance artery vasodilation to atypical cannabinoids is not different between tissues from WT and GPR55 KO mice
| Compound | EC50 (μM) | Rmax (% reversal of PE pre-constriction) | EC50 (μM) | Rmax (% reversal of PE pre-constriction) |
|---|---|---|---|---|
| Abnormal cannabidiol | 3.0±0.6 | 107±5 | 2.6±0.5 | 99±4 |
| O-1602 | 2.8±0.3 | 101±2 | 2.2±0.4 | 95±7 |
Abbreviations: GPR55 KO, mice homozygous for the GPR55 mutation; WT, wild-type littermate controls for GPR55 KO mice.
Figure 4.
Antagonism of abnormal cannabidiol-induced vasodilatation by O-1918 is not different between GPR55 KO and WT mice. Preincubation of mesenteric resistance arteries with O-1918 (30 μM) caused similar shifts in the concentration–response curve to abnormal cannabidiol in (a) WT and (b) KO vessels. Data are presented as mean±s.e.mean (n=4). GPR55 KO, mice homozygous for the GPR55 mutation; WT, wild-type littermate controls for GPR55 KO mice.
Discussion
The purpose of the present study was to determine whether the orphan G-protein-coupled receptor GPR55 is the cannabinoid-binding receptor responsible for vasodilatation to ‘atypical' agonists such as abnormal cannabidiol and O-1602. The vasodilator effects of abnormal cannabidiol are well reported (Jarai et al., 1999; Wagner et al., 1999; Offertaler et al., 2003; Begg et al., 2005) and are thought to be mediated by a cannabinoid-like receptor distinct from the classical CB1 and CB2 subtypes (Jarai et al., 1999). The putative receptor, termed ‘CBx', is thought to be endothelial in origin and a GPR55 (Jarai et al., 1999; Offertaler et al., 2003). Recent patent literature suggests that GPR55 might interact with endocannabinoids and synthetic cannabinoids, compounds that are reported to have activity at CBx (Baker et al., 2006).
Data from the current study demonstrates that human recombinant GPR55 is stimulated by abnormal cannabidiol and O-1602, compounds reported to be putative CBx agonists (Jarai et al., 1999; Offertaler et al., 2003), but not by WIN 55212-2, an agonist at CB1 and CB2 (Felder et al., 1995). Little information regarding cannabinoid activation of human recombinant GPR55 is available in the public domain, with the exception of data described in patent literature (see Baker et al., 2006) and in abstract form (Sjogren et al., personal communication, XV Symposium on the Cannabinoids, International Cannabinoid Research Society, Clearwater, FL, USA, 2005). In these descriptions, the potency of atypical cannabinoids at recombinant GPR55 is well within the range as that reported in the current study (nM EC50 in GTPγS) with no activity reported at CB1 and CB2. Taken together, these data supported the hypothesis that human GPR55 is an atypical (non-CB1, CB2) cannabinoid receptor, and validated the use of abnormal cannabidiol and O-1602 as reagents to probe the involvement of GPR55 in vivo and in vitro.
Generation of a GPR55-deficient mouse enabled the testing of the hypothesis that GPR55 is the vasodilatory CBx in the mouse. The observation that baseline blood pressure and heart rate were not different between the GPR55 KO mouse and WT cohorts suggests that GPR55 is not involved in the baseline regulation of mean arterial pressure under normal physiological conditions. However, this would not necessarily exclude a role for GPR55 in mediating blood pressure effects of atypical cannabinoids administered exogenously. Previous studies have reported blood pressure lowering effects of abnormal cannabidiol when infused into anesthetized animals, including the dog (Adams et al., 1977) and mouse (Jarai et al., 1999; Offertaler et al., 2003). Furthermore, these effects were attributed to CBx, evidenced by inhibition of the abnormal cannabidiol-induced hypotensive response by O-1918 (Offertaler et al., 2003). In the current study, the lack of a significant difference in the blood pressure-lowering effect of abnormal cannabidiol at a similar dose used in studies reporting a similar effect supports the notion that murine GPR55 is not involved in the blood pressure response to this CBx agonist. Despite the in vitro observation that abnormal cannabidiol-induced vasodilatation was not different between WT and KO, demonstration of a direct link between vasodilatation and hypotension requires more complete haemodynamic evaluation (for example, regional blood flow, total peripheral resistance and cardiac effects of abnormal cannabidiol).
The lack of a difference in blood pressure response to abnormal cannabidiol is further substantiated by the lack of any difference in vasodilator response to either abnormal cannabidiol or O-1602 in isolated mesenteric resistance vessels from each strain. The potencies of vasodilatation for both agonists are similar to the single-digit micromolar potencies reported in the literature (Jarai et al., 1999; Offertaler et al., 2003). This suggests that while these compounds are vasodilators, their effects on vascular tone do not require GPR55. Furthermore, these data indicate that GPR55 deficiency does not result in any apparent change in the function of the CBx system that mediates abnormal cannabidiol/O-1602 vasodilatation. The observation that O-1918 inhibits vasodilatation to abnormal cannabidiol equally in WT and KO mouse arteries further suggests that the CBx responsible for vasodilatation to atypical cannabinoids such as abnormal cannabidiol is a target other than GPR55. The degree of antagonism of abnormal cannabidiol-induced vasodilatation by O-1918 in WT and KO mice (Kb=2 and 3 μM, respectively) was similar to that reported previously (Offertaler et al., 2003). Despite this observation, which supports the notion that GPR55 is not the receptor mediating atypical cannabinoid vasodilatation, a lack of antagonism of abnormal cannabidiol-induced GTPγS by O-1918 in GPR55-expressing cell membranes would provide even further differentiation. The lack of these data is a limitation of the current study, but remains an important focus for more complete investigation of the GPR55 and CBx systems.
While these data might rule out the involvement of GPR55 in regulation of blood pressure under normal conditions, potential non-specific (non-GPR55) effects are possible and require further investigation. Endocannabinoids such as anandamide have been shown to stimulate other non-putative cannabinoid (non-CB1, CB2 or CBx) targets such as the transient receptor potential vallinoid type 1 (TRPV1) channel resulting in CGRP-dependent vasodilatation (Zygmunt et al., 1999). However, the vasodilator effects of abnormal cannabidiol are reported to be insensitive to TRP antagonists (Jarai et al., 1999; Offertaler et al., 2003; Ho and Hiley, 2004), which adds credence to a lack of involvement of this particular pathway. Clearly, the development of selective agonists and antagonists for GPR55 would greatly assist the interrogation of the relevance of this receptor in cardiovascular physiology.
In conclusion, the current study demonstrates that GPR55 is, indeed, activated by atypical cannabinoids; however, it does not appear to mediate vasodilatation induced by these compounds. Therefore, the molecular identity of the elusive CBx remains to be determined.
Abbreviations
- BLAST
basic local alignment search tool
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- CBx
atypical cannabinoid receptor
- GDP
guanosine diphosphate
- GPR55
G-protein-coupled receptor 55
- GPR55 KO
mice homozygous for the GPR55 mutation
- GTPγS
guanosine 5′-(gamma thio) triphospate
- Kb
antagonist affinity constant
- WT
wild-type littermate controls for GPR55 KO mice
Conflict of interest
All authors are employees of GlaxoSmithKline.
References
- Adams MD, Earnhardt JT, Martin BR, Harris LS, Dewey WL, Razdan RK. A cannabinoid with cardiovascular activity but no overt behavioral effects. Experientia. 1977;33:1204–1205. doi: 10.1007/BF01922330. [DOI] [PubMed] [Google Scholar]
- Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Breivogel C. Cannabinoid receptor binding to membrane homogenates and cannabinoid-stimulated [35S]GTPgammaS binding to membrane homogenates or intact cultured cells. Methods Mol Med. 2006;123:149–162. doi: 10.1385/1-59259-999-0:149. [DOI] [PubMed] [Google Scholar]
- Douglas SA, Behm DJ, Aiyar NV, Naselsky D, Disa J, Brooks DP, et al. Non-peptidic urotensin-II receptor (UT) antagonists I: in vitro pharmacological characterisation of SB-706375. Br J Pharmacol. 2005;145:620–635. doi: 10.1038/sj.bjp.0706229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- Ho WS-V, Hiley CR. Vasorelaxant activities of the putative endocannabinoid virodhamine in rat isolated small mesenteric artery. Br J Pharmacol. 2004;138:1320–1332. doi: 10.1211/0022357023682. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Bonner TI, Cabral GA, Casellas P, Devane WA, Felder CC, et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson DH, Barnard EA, Hoyer D, Humphrey PPA, Leff P, Shankley NP.Terms and symbols in quantitative pharmacology The IUPHAR Compendium of Receptor Characterization and Classification 1998IUPHAR Media: London; 6–20.In: Girdlestone D (ed) [Google Scholar]
- Markel P, Shu P, Ebeling C, Carlson GA, Nagle DL, Smutko JS, et al. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet. 1997;17:280–284. doi: 10.1038/ng1197-280. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Offertaler L, Mo F-M, Batkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharm. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O'Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Jarai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- Wakeland E, Morel L, Achey K, Yui M, Longmate J. Speed congenics: a classic technique in the fast lane (relatively speaking) Immunol Today. 1997;18:472–477. doi: 10.1016/s0167-5699(97)01126-2. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]