Abstract
The arctiid moth Utetheisa ornatrix is protected against predation by pyrrolizidine alkaloids (PA) that it sequesters as a larva from its food plant. Earlier work had shown that males transmit PA to the female with the sperm package and that the female bestows part of this gift on the eggs, protecting these against predation as a result. We now show that the female herself derives protection from the gift. Females deficient in PA are vulnerable to predation from spiders (Lycosa ceratiola and Nephila clavipes). If mated with a PA-laden male, the females become unacceptable as prey. The effect takes hold promptly and endures; females are unacceptable to spiders virtually from the moment they uncouple from the male and remain unacceptable as they age. Chemical data showed that the female allocates the received PA quickly to all body parts. We predict that other instances will be found of female insects being rendered invulnerable by receipt of sexually transmitted chemicals.
Keywords: nuptial gift, sexual selection, pyrrolizidine alkaloids, Lepidoptera, Arctiidae
The moth Utetheisa ornatrix (Lepidoptera: Arctiidae; henceforth referred to as Utetheisa) depends on pyrrolizidine alkaloids (PA) for defense. It sequesters the PA as a larva from its food plants (Fabaceae; Crotalaria spp.) and retains the chemicals through pupation into adulthood. Females also transmit PA to their eggs (1). All developmental stages of the moth are protected as a result: the larvae and adults against spiders (2, 3) and the eggs against ants and ladybird beetles (4, 5). Notably, the PA in the eggs do not stem entirely from the mother moth. Mating in Utetheisa involves transfer from male to female of a sperm package (spermatophore) of considerable size, amounting on average to over 10% of male body mass (6). In addition to sperm and a quantity of nutrient that the female invests in egg production (7), the spermatophore contains PA. It has been shown experimentally that in endowing the eggs, the female resorts not only to her own PA but also to a portion of the PA received from the male (4).
The question of whether the female herself might benefit from receipt of the male’s alkaloidal gift remained unanswered. Might she derive a defensive advantage from mating? Here, we provide evidence that she does and that the advantage takes hold virtually from the moment the female uncouples from her partner.
Our experiments consisted of testing for the vulnerability of adult Utetheisa to predation by orb-weaving spiders (field tests with Nephila clavipes; Araneidae) and wolf spiders (laboratory tests with Lycosa ceratiola; Lycosidae). The Utetheisa offered to the spiders were of several categories, including, most importantly, females that had been raised to be PA-free but that had mated with males that were either PA-laden or PA-free. These categories permitted us to determine whether the alkaloidal gift from the male is itself sufficient to provide the females with protection. The tests were coupled with a quantitative assessment of the allocation of the male’s PA gift to different parts of the female’s body and with a determination of the deterrence of PA to one of the spiders (L. ceratiola).†
MATERIALS AND METHODS
Utetheisa.
Several categories of adult Utetheisa were offered in the tests with spiders.
Field-collected (wild) Utetheisa.
These were netted as adults in and around patches of Crotalaria mucronata, on the grounds of the Archbold Biological Station, near Lake Placid, Highlands County, FL. Such moths contain usaramine (1), the principal PA in C. mucronata (8). The moths were kept in individual vials with access to water (soaked cotton wad) and offered to N. clavipes within 2 days of capture.
Laboratory-reared (−) Utetheisa and (+) Utetheisa.
For years, we have raised Utetheisa in our Cornell laboratories on two types of larval diet: one with and one without PA. Details of the composition of these diets are given elsewhere (9). Briefly, the PA-free diet [(−) diet] is based on pinto beans, which are free of PA, whereas the PA-containing diet [(+) diet] includes a supplement of seeds of Crotalaria spectabilis, another major food plant of Utetheisa. Utetheisa raised on these diets are designated (−) Utetheisa and (+) Utetheisa, respectively. The (+) Utetheisa had been shown to contain monocrotaline (2), the principal PA in C. spectabilis (3, 10).
Once-mated laboratory-raised females [− ♀ (+ ♂) and − ♀ (− ♂)].
These females were raised on the (−) diet and were mated either with a (−) male and designated − ♀ (− ♂) or with a (+) male and designated − ♀ (+ ♂). Matings were effected by confining 2-day-old virgin males and females, in individual pairs, in small chambers (8-cm diameter and height) overnight. Pairs typically remain in copula for over 9 h (11). 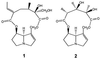
PA-injected virgin laboratory-raised females and their controls.
These females were raised on the (−) diet and injected either with a PA solution (150 μg of monocrotaline in 5 μl of saline) or with solvent only (5 μl saline). Injection was effected with a microsyringe to one side of the posterior half of the abdomen on day 4 after pupal emergence.
Orb-Weaving Spiders (N. clavipes).
Tests with this spider were carried out at Highlands Hammock State Park, Sebring, Highlands County, FL, a preserved site densely shaded by live oak and cabbage palm, where N. clavipes occur naturally. The feeding behavior of this spider is known (12). Typically, when an insect flies into the spider’s web, the spider darts toward it and bites it with the fangs to inject venom; the spider then envelopes the insect lightly in silk and carries it to the hub of the web to eat it. Consumption is by suctional uptake of fluid from the triturated, extraorally digested prey, a process that may take 1–3 h with insects the size of Utetheisa.
Presentation of Utetheisa to N. clavipes involved flipping individual moths from vials into webs and monitoring the course of events. We knew from previous studies that N. clavipes reject wild Utetheisa and that they are sensitive to PA; they tend to consume mealworms (larvae of Tenebrio molitor) only partially if these are treated by topical addition of crystalline monocrotaline (2). This spider was therefore well suited for our tests. Except where otherwise indicated, individual N. clavipes were given only a single Utetheisa and not reused in any test.
Wolf spiders (L. ceratiola).
This spider was abundant on the grounds of the Archbold Biological Station on sandy fire lanes, where we spotted them at night by using head lamps that reflected strongly off their eyes.
For laboratory testing, spiders were taken in vials and transferred singly into cylindrical plastic containers (17-cm diameter; 11-cm height; with a shallow bottom layer of sand), to which the spiders adapted quickly. Before testing, they received water only (daily misting from a spray bottle) or, in some cases, also a mealworm 2–3 weeks beforehand. We presented each test item by using forceps to seize an Utetheisa by a forewing and then dropping the Utetheisa from just above the spider to directly in front of its chelcers. Utetheisa that were accepted were pounced on by the spiders and killed by one or more bites; then, the spiders slowly triturated the Utetheisa and extracted their juices, a process that usually took over 2 h. Utetheisa that were rejected were judged to have survived the encounter if they were alive 24 h after the attack. Spiders that rejected an Utetheisa were given a mealworm within 20 min after the test and were tallied only if they ate this item (thus ruling out the possibility that the Utetheisa had been rejected because the spider was not hungry). We used only female L. ceratiola and tested each with only a single Utetheisa.
Oral Sensitivity of L. ceratiola to PA.
To obtain a measure of this parameter, individual female L. ceratiola were each given a live mealworm (presented in the same fashion as the Utetheisa), which they promptly attacked. Once they initiated feeding, they were stimulated by topical application of a droplet of PA solution (100 μg of monocrotaline in 5 μl of egg albumen), delivered with a micropipette on the mealworm as closely as possible to the spider’s chelicers. Control spiders were stimulated with 5 μl of albumen alone.
PA Analyses of Utetheisa Body Components.
Samples were weighed (wet weight) and extracted for 24 h with 500 μl of buffer solution (2.7 g of monopotassium phosphate/2 ml of triethylamine/0.4 ml of trifluoroacetic acid in 4 liters of water; pH adjusted to 3.0 with phosphoric acid). The samples then were centrifuged, and the residue was reextracted with additional buffer solution (200 μl). The two extracts were pooled, and the mixture was analyzed by HPLC with a Hewlett–Packard 1090 Series II instrument with a diode array detector (λ = 205; C-18 BDS Hypersil column; 250 × 4.6 mm; 5-μl particle size). The column was eluted (1 ml/min) with a buffer solution/acetonitrile mixture (92:8, vol/vol). The PA ridelline served as internal standard.
PA Analyses of Thoracic Froth.
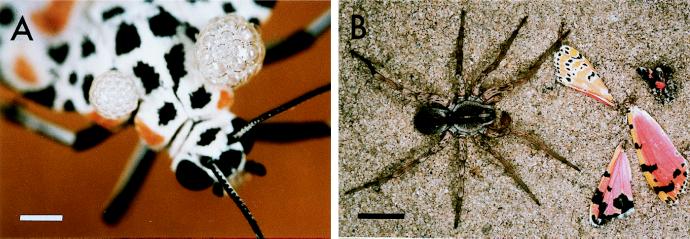
When adult Utetheisa are squeezed gently or sometimes even merely touched, they emit two bubbling masses of froth from the anterolateral margins of the thorax (Fig. 1A). They share this propensity, which is obviously defensive, with a number of other arctiid species (13). To obtain froth samples for PA analyses, individual female Utetheisa were squeezed gently with forceps, and the ensuing froth was picked up in microcapillary tubes, extracted with ethanol (100 μl), centrifuged, and then reextracted with buffer solution (100 μl). After evaporating the ethanol from the first extract (nitrogen stream), the two extracts were pooled and analyzed by HPLC as above.
Figure 1.
(A) Female Utetheisa emitting defensive froth. (Bar = 1 mm.) (B) Outcome of an encounter between a L. ceratiola spider and a PA-free Utetheisa from the − ♀ (− ♂) category. (Bar = 1 cm.)
Statistics.
All PA values are expressed as mean ± SEM.
PROCEDURES AND RESULTS
Tests with N. clavipes.
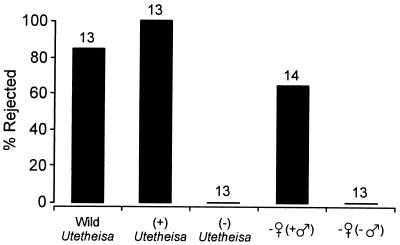
In tests, this spider was offered moths from five different categories. Results are shown next below and in Fig. 2.
Figure 2.
Fate of Utetheisa moths in N. clavipes webs. Numbers above bars indicate sample sizes.
Wild Utetheisa (six females and seven males).
With the exception of two individuals (one of each sex) that were eaten, wild Utetheisa were rejected. The rejection behavior was reminiscent of that noted with this spider presented with other inedible prey (e.g., blister beetles; ref. 14). The spider converged on the moth the moment it was dropped into the orb and, after briefly inspecting it with legs and palps, proceeded to cut the moth from the web. In cutting the moth free, the spider used palps and fangs in combination to sever each of the silken strands that were entangling the moth. Liberated, the moth fell to the ground or, more often, took to the wing in midfall and flew away.
Other categories of Utetheisa.
Utetheisa that were rejected in the following categories were similarly set free: of 7 female and 6 male (+) Utetheisa, all were rejected; of 7 female and 6 male (−) Utetheisa, all were eaten; of 14 − ♀ (+ ♂) females, 9 were rejected, and 5 were eaten; and of 13 − ♀ (− ♂) females, all were eaten.
It is clear from these data that not a single moth free of PA [i.e., from the categories (−) Utetheisa or − ♀ (− ♂)] was rejected by N. clavipes. The spider, however, showed at least some level of discrimination against all PA-containing Utetheisa, including the females from the category − ♀ (+ ♂) whose only PA was that received at mating. There is a caveat, however. The 14 data points in the − ♀ (+ ♂) category are not strictly independent, because they stem from tests done with only six spiders. We were forced to adopt this strategy because of a shortage of webs at the test site. However, none of the six spiders involved were tested more than once on the same day. A further point that held true for the encounters with all categories of Utetheisa is that the moths sometimes emitted froth in the course of being inspected by the spider. However, emission was not invariable, and most PA-containing moths that were rejected did not froth before rejection.
Tests with L. ceratiola.
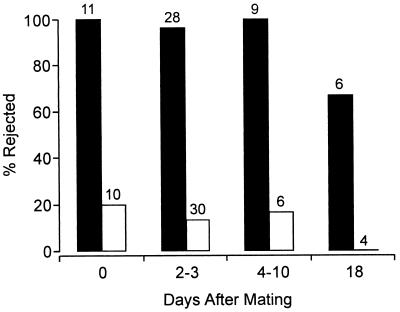
We knew from previous work (3) that this spider rejects wild Utetheisa (n = 20; all rejected) as well as (+) Utetheisa (n = 20; all rejected) but that it accepts (−) Utetheisa (n = 20; all eaten). It was not known whether a female Utetheisa endowed with no more PA than that received from a male was also unacceptable to the spider. To answer this question, we offered L. ceratiola a total of 54 − ♀ (+ ♂) female Utetheisa that had received PA at mating and 50 − ♀ (− ♂) females that had received no such gift. The Utetheisa were offered to the spiders at different times after mating [times ranging from day 0 (5 min to 4 h after termination of mating) to day 18]. The results (Fig. 3) show that the male’s alkaloidal gift provides the female with absolute or nearly absolute protection from virtually the moment mating is completed to day 10 after mating and even with substantial protection as late as day 18 after mating, when she is already senescent. The control − ♀ (− ♂) Utetheisa, as expected, proved largely acceptable to the spiders.
Figure 3.
Fate of Utetheisa females in laboratory tests with L. ceratiola. All females were raised to be PA-free and were mated either with PA-laden males [− ♀ (+ ♂); solid bars] or with PA-free males [− ♀ (− ♂); open bars]. Numbers above bars give sample sizes. The discrimination against the [− ♀ (+ ♂)] females is significant (G test on pooled data; P < 0.0001).
The three individuals from the − ♀ (+ ♂) category that were scored as having been accepted by the spiders were not in fact eaten. They were scored as accepted, because they died within 24 h of the attack. The one individual of the 2- to 3-day group so tallied was injured and died from the injury; however, this individual was consumed only partly (a portion of its abdomen was chewed away). The other two individuals (from the day 18 group) were not injured in the attack and may simply have died of “old age” (spontaneous mortality is high in 2- to 3-week-old females). The fate of control − ♀ (− ♂) females that were accepted was drastically different. These females were thoroughly macerated and typically reduced to a compacted amorphous mass (except for the wings, which tended to break away during the course of the meal; Fig. 1B).
As in the tests with N. clavipes, Utetheisa were sometimes noted to froth when attacked, but they did not do so consistently. In fact, the briefest physical contact with a PA-laden − ♀ (+ ♂) female at times seemed to suffice to discourage a spider; occasional rejections occurred after the spider had touched no more than the wings of the moth.
Fate of PA-Injected Utetheisa Females and Their Controls.
The eight females that received monocrotaline (150 μg) by injection on day 4 after pupal emergence were offered individually to L. ceratiola 5 min after injection, and all eight were rejected by the spiders. The controls (n = 10), with one exception, were eaten (Fisher exact test; P < 0.0005).
Oral Sensitivity of L. ceratiola to PA.
The results were clear cut. All spiders (n = 14) that were stimulated with the monocrotaline-containing fluid dropped the mealworm and then cleansed their mouth parts by dragging them in the sand. The response was not always immediate, because we sometimes failed to apply the droplet precisely between the spider’s chelicers. In cases of such misapplication, the response occurred later, when the spider repositioned its mouth parts and came into oral contact with the fluid. Spiders stimulated with albumen alone (n = 11) all failed to respond to application of the droplet (Fisher exact test; P < 0.0001).
PA Analyses.
Previous work (3) had established the PA content of two of the categories of Utetheisa moths used herein. Wild Utetheisa taken at the Archbold Biological Station in C. mucronata patches were shown to contain 540 ± 30 μg of usaramine per moth (n = 31; mixed lot of males and females). (+) Utetheisa raised on (+) diet in our Cornell laboratories contained 630 ± 50 μg monocrotaline per moth (n = 31; mixed lot of males and females).
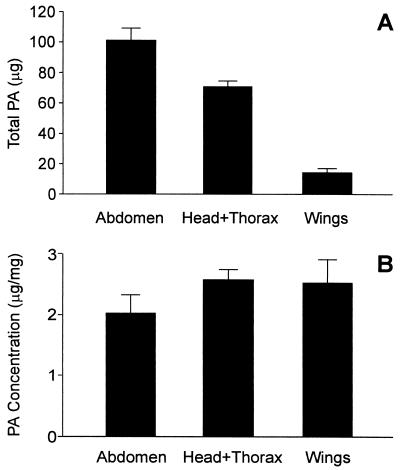
To obtain a measure of the amount of PA received by the female at mating and of the distribution of the PA in the body of the female, we analyzed three body components separately (abdomen, head + thorax, and wings) of 24 − ♀ (+ ♂) females that had uncoupled from their partners 0–2 h beforehand. The results (Fig. 4) show that, even at such an early time after mating, the received PA is already distributed throughout the body of the female and present in comparable concentrations in all body parts. Even the wings are already PA-laden by that time. From the content of the parts, we calculate the total PA content of − ♀ (+ ♂) females to be 186 ± 18 μg, a figure that provides a measure of the amount of PA transferred by the males at mating.
Figure 4.
Total PA content (A) and PA concentration (B) in three body components of 24 − ♀ (+ ♂) Utetheisa females whose only PA was that received at mating. The females were dissected within 0–2 h after mating. (B) The PA is shown to be equitably distributed in the three body components [single-factor ANOVA; P = 0.32].
Froth samples were taken from three batches of − ♀ (+ ♂) females at three different times after termination of mating: 0 days (1–12 h after mating; n = 20), 3 days (n = 23), and 18 days (n = 11). The samples ranged in mass from 0.5 mg to 3.2 mg (the oldest females emitted the least froth). The monocrotaline content for the three samples was, at day 0, 4.0 ± 0.6 μg per female (concentration = 2.8 ± 0.4 μg/mg); at day 3, 4.1 ± 0.7 μg per female (concentration = 2.9 ± 0.4 μg/mg); and at day 18, 2.7 ± 0.6 μg per female (concentration = 2.7 ± 0.7 μg/mg). The froth is evidently laden with PA shortly after mating, at a concentration that does not attenuate over time (single-factor ANOVA; P = 0.95).
DISCUSSION
The notion of transmission of defensive chemicals by seminal infusion from a male to female insect is not new. We ourselves had shown such chemical transfer to occur in a danaid butterfly, Danaus gilippus, where the male, as in Utetheisa, transmits PA that it sequesters from plants to the female (15), and in a pyrochroid beetle, Neopyrochroa flabellata, where the terpenoid toxin cantharidin is bestowed on the female (16, 17). In all these cases, including Utetheisa, the assumption had been that the beneficiaries of the transfer are the offspring, and evidence did indeed indicate that the eggs of Utetheisa and N. flabellata derive protection from the PA or cantharidin they receive from their respective fathers (5, 17). What we have established now with Utetheisa is that the female herself profits from receipt of the male’s chemical gift.
The female Utetheisa is protected by receipt of the male’s PA, and the protection takes effect quickly. Within the first 2 h after mating, the male’s PA was already found to be evenly distributed throughout the female’s body, and froth taken from females on the day of mating already contained PA at maximal levels. All females that we offered to L. ceratiola on the day of mating, even those that were tested within minutes after the end of mating, were rejected by the spiders. We conclude that the male’s PA is distributed systemically throughout the female during mating itself, over the course of the several hours that the pair remains coupled. The fact that female Utetheisa experimentally injected with PA were rejected by L. ceratiola 5 min after injection, attests to the speed with which received PA can be deployed defensively by the moth.
A single mating with a PA-laden male is evidently sufficient to convey lasting protection on the Utetheisa female. Even senescent females offered to L. ceratiola on day 18 after receipt of their alkaloidal gift proved unacceptable to the spiders. Froth from such females contained undiminished concentrations of PA. Over time after mating, as the female bestows PA on the eggs (4), she evidently does not exhaust her supply of the chemicals. She retains PA in sufficient quantity for her own protection, thereby exercising a strategy that appears intended to protect the egg carrier as well as the eggs.
In a strict sense, our experiments with PA-free females were not realistic, inasmuch as such females are not likely to occur in nature. Female Utetheisa themselves sequester PA as larvae so that at adult emergence they already contain a self-acquired quantity of the chemicals. However, that quantity is variable, and it can be low, as is the case when the larvae have access to the leaves of Crotalaria only, rather than to the PA-rich seeds as well (18). The male’s PA gift is therefore to be viewed as an important supplement, which, during times of PA-shortage in the female, could be the bonus that “makes the difference.”
Also unrealistic is the fact that our experimentally mated females were but once-mated. Utetheisa females mate on average with 4–5 males over their life span and may take as many as 10 or more partners (11). Over time, therefore, females are likely to receive periodic infusions of PA from males and accrue far larger quantities of PA than they can obtain from a single mate.
An interesting feature of the mating strategy of Utetheisa is that the female does not accept males at random but shows preference for those of high PA content, which are able to bestow large alkaloidal gifts (19, 20). She is capable of discerning such males, because they produce higher titers of a courtship pheromone. That pheromone, hydroxydanaidal, is derived chemically from PA and therefore is produced in larger quantities by PA-rich males (20). In exercising mate choice, therefore, the female accesses those males best able to meet her defensive needs (1).
Orb-weavers and lycosids are not likely to be the only spider enemies of adult Utetheisa. Crab spiders (Thomisidae) and jumping spiders (Salticidae) could be natural predators as well and could also be deterred by PA. The PA could also protect Utetheisa against entirely different enemies, including possibly vertebrates, but evidence to this effect is lacking.
The defensive strategy exemplified by Utetheisa, in which the males help to protect not only the eggs but also the female partner, seems to be without parallel in nature. We predict, however, that the strategy will be shown to have counterparts in insects, where males so often transmit sizeable spermatophores to females (21). One can certainly imagine the strategy being at play in such insects as D. gilipus and N. flabellata, where seminal transfer of defensive chemicals has been established (15–17).
Acknowledgments
We thank Ken Amerman for excellent laboratory help, the staff of the Archbold Biological Station for numerous favors, and Jerrold Meinwald and W. Mitchell Masters for comments on the manuscript. This study was supported in part by National Institutes of Health Grant AI02908 (to T.E.) and by Johnson & Johnson Fellowships in Chemical Ecology (to C.R. and A.G.).
ABBREVIATION
- PA
pyrrolizidine alkaloid(s)
Footnotes
This paper is no. 159 in the series “Defense Mechanisms of Arthropods.” Paper no. 158 is ref. 22.
References
- 1.Eisner T, Meinwald J. Proc Natl Acad Sci USA. 1995;92:50–55. doi: 10.1073/pnas.92.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisner T, Meinwald J. In: Pheromone Biochemistry. Prestwich G D, Blomquist G J, editors. Orlando, FL: Academic; 1987. pp. 251–269. [Google Scholar]
- 3.Eisner T, Eisner M. Psyche. 1991;98:111–118. [Google Scholar]
- 4.Dussourd D E, Ubik K, Harvis C, Resch J, Meinwald J, Eisner T. Proc Natl Acad Sci USA. 1988;85:5992–5996. doi: 10.1073/pnas.85.16.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hare J F, Eisner T. Oecologia. 1993;96:9–18. doi: 10.1007/BF00318024. [DOI] [PubMed] [Google Scholar]
- 6.LaMunyon C W, Eisner T. Proc Natl Acad Sci USA. 1994;91:7081–7084. doi: 10.1073/pnas.91.15.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaMunyon C W. Ecol Entomol. 1997;22:69–73. [Google Scholar]
- 8.Sauthon I W, Buckingham J. Dictionary of Alkaloids. London: Chapman & Hall; 1989. [Google Scholar]
- 9.Bogner F, Eisner T. J Chem Ecol. 1991;17:2063–2075. doi: 10.1007/BF00987992. [DOI] [PubMed] [Google Scholar]
- 10.Johnson A E, Molyneux R J, Merrill G B. J Agric Food Chem. 1985;33:50–55. [Google Scholar]
- 11.LaMunyon C W, Eisner T. Proc Natl Acad Sci USA. 1993;90:4689–4692. doi: 10.1073/pnas.90.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson M H, Mirick H. Psyche. 1971;78:123–139. [Google Scholar]
- 13.Dethier V G. J N Y Entomol Soc. 1939;47:131–144. [Google Scholar]
- 14.Smedley S R, Blankespoor C L, Yang Y, Carrel J E, Eisner T. Zoology. 1996;99:211–217. [Google Scholar]
- 15.Dussourd D E, Harvis C A, Meinwald J, Eisner T. Experientia. 1989;45:896–898. doi: 10.1007/BF01954068. [DOI] [PubMed] [Google Scholar]
- 16.Eisner T, Smedley S R, Young D K, Eisner M, Roach B, Meinwald J. Proc Natl Acad Sci USA. 1996;93:6494–6498. doi: 10.1073/pnas.93.13.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisner T, Smedley S R, Young D K, Eisner M, Roach B, Meinwald J. Proc Natl Acad Sci USA. 1996;93:6499–6503. doi: 10.1073/pnas.93.13.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conner W E, Roach B, Benedict E, Meinwald J, Eisner T. J Chem Ecol. 1990;16:543–552. doi: 10.1007/BF01021785. [DOI] [PubMed] [Google Scholar]
- 19.Conner W E, Eisner T, Vander Meer R K, Guerrero A, Ghiringelli D, Meinwald J. Behav Ecol Sociobiol. 1980;7:55–63. [Google Scholar]
- 20.Dussourd D E, Harvis C A, Meinwald J, Eisner T. Proc Natl Acad Sci USA. 1991;88:9224–9227. doi: 10.1073/pnas.88.20.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mann T. Spermatophores. Berlin: Springer; 1984. [Google Scholar]
- 22.Knight, M., Glor, R., Smedley, S. R., González, A., Adler, K. & Eisner, T. (1999) J. Chem. Ecol., in press.