Abstract
Objective To systematically review evidence for the effectiveness of physical interventions to interrupt or reduce the spread of respiratory viruses.
Data extraction Search strategy of the Cochrane Library, Medline, OldMedline, Embase, and CINAHL, without language restriction, for any intervention to prevent transmission of respiratory viruses (isolation, quarantine, social distancing, barriers, personal protection, and hygiene). Study designs were randomised trials, cohort studies, case-control studies, and controlled before and after studies.
Data synthesis Of 2300 titles scanned 138 full papers were retrieved, including 49 papers of 51 studies. Study quality was poor for the three randomised controlled trials and most of the cluster randomised controlled trials; the observational studies were of mixed quality. Heterogeneity precluded meta-analysis of most data except that from six case-control studies. The highest quality cluster randomised trials suggest that the spread of respiratory viruses into the community can be prevented by intervening with hygienic measures aimed at younger children. Meta-analysis of six case-control studies suggests that physical measures are highly effective in preventing the spread of SARS: handwashing more than 10 times daily (odds ratio 0.45, 95% confidence interval 0.36 to 0.57; number needed to treat=4, 95% confidence interval 3.65 to 5.52); wearing masks (0.32, 0.25 to 0.40; NNT=6, 4.54 to 8.03); wearing N95 masks (0.09, 0.03 to 0.30; NNT=3, 2.37 to 4.06); wearing gloves (0.43, 0.29 to 0.65; NNT=5, 4.15 to 15.41); wearing gowns (0.23, 0.14 to 0.37; NNT=5, 3.37 to 7.12); and handwashing, masks, gloves, and gowns combined (0.09, 0.02 to 0.35; NNT=3, 2.66 to 4.97). The incremental effect of adding virucidals or antiseptics to normal handwashing to decrease the spread of respiratory disease remains uncertain. The lack of proper evaluation of global measures such as screening at entry ports and social distancing prevent firm conclusions being drawn.
Conclusion Routine long term implementation of some physical measures to interrupt or reduce the spread of respiratory viruses might be difficult but many simple and low cost interventions could be useful in reducing the spread.
Introduction
Although respiratory viruses usually cause minor disease, epidemics can occur. Mathematical models estimate that about 36 000 deaths and 226 000 admissions to hospital in the United States annually are attributable to influenza,1 and with incidence rates as high as 50% during major epidemics worldwide, respiratory viruses strain health services,2 are responsible for excess deaths,2 3 and result in massive indirect costs owing to absenteeism from work and school.4 Concern is now increasing about serious pandemic viral infections. In 2003 an epidemic of the previously unknown severe acute respiratory syndrome (SARS) caused by a coronavirus affected about 8000 people worldwide, with 780 deaths (disproportionately high numbers were in healthcare workers), and causing a social and economic crisis, especially in Asia.5 A new avian influenza pandemic caused by the H5N1 virus strain threatens greater catastrophe.6
High viral load and high viral infectiousness probably drive virus pandemics,7 hence the need for interventions to reduce viral load. Mounting evidence suggests, however, that single measures, particularly the use of vaccines or antivirals, will be insufficient to interrupt the spread of influenza. Agent specific drugs are also not available for other viruses.7,8,9,10
A recent trial found handwashing to be effective in lowering the incidence of pneumonia in the developing world.w1 Clear evidence has also shown a link between personal (and environmental) hygiene and infection.11 We systematically reviewed the evidence for the effectiveness of combined public health measures such as personal hygiene, distancing, and barriers to interrupt or reduce the spread of respiratory viruses.12 13 We did not include vaccines and antivirals because these have been reviewed.4 10 14,15,16,17,18
Methods
We considered trials (individual level, cluster randomised, or quasirandomised), observational studies (cohort and case-control), and any other comparative design in people of all ages provided some attempt had been made to control for confounding.
We included any intervention to prevent the transmission of respiratory viruses from animals to humans or from humans to humans (isolation, quarantine, social distancing, barriers, personal protection, and hygiene) compared with no intervention or with another intervention. We excluded vaccines and antivirals.
The outcome measures were deaths; numbers of cases of viral illness; severity of viral illness, or proxies for these; and other measures of burden, such as admissions to hospital.
Search strategy
We searched the Cochrane Central Register of Controlled Trials (Cochrane Library issue 4, 2006), Medline (1966 to November 2006), OldMedline (1950-65), Embase (1990 to November 2006), and CINAHL (1982 to November 2006). See bmj.com for details of our search terms for Medline and the Cochrane register (modified for OldMedline, Embase, and CINAHL). We applied no language restrictions. Study design filters included trials; cohort, case-control, and cross-over studies; and before and after and time series. We scanned the references of included studies to identify other potentially relevant studies.
We scanned the titles and abstracts of potentially relevant studies: when studies seemed to meet our eligibility criteria (or when information was insufficient to exclude them), we obtained the full text articles. We used a standardised form to assess the eligibility of each study, on the basis of the full article.
Quality assessment
We analysed randomised and non-randomised studies separately. Randomised studies were assessed according to the effectiveness of the randomisation method, the generation of the allocation sequence, allocation concealment, blinding, and follow-up. Non-randomised studies were assessed for the presence of potential confounders using the appropriate Newcastle-Ottawa Scales19 for case-control and cohort studies, and a three point checklist was used for controlled before and after studies.20
Using quality at the analysis stage as a means of interpretation of the results we assigned risk of bias categories on the basis of the number of items judged inadequate in each study: low risk of bias, up to one inadequate item; medium risk of bias, up to three inadequate items; and high risk of bias, more than three inadequate items.
Data extraction
Two authors (TJ, CDM) independently applied inclusion criteria to all identified and retrieved articles. Four authors (TJ, EF, BH, AP) extracted data from included studies and checked their accuracy on standard field forms used by Cochrane groups for vaccines, supervised and arbitrated by CDM.
Aggregation of data depended on study design; types of comparisons; sensitivity; and homogeneity of definitions of exposure, populations, and outcomes used. We calculated the statistic I2 for each pooled estimate to assess the impact on heterogeneity.21 22
When possible we did a quantitative analysis and summarised effectiveness as an odds ratio with 95% confidence intervals, expressing absolute intervention effectiveness when significant as a percentage using the formula: intervention effectiveness=1−odds ratio. For studies that could not be pooled we used effect measures reported by the authors (such as relative risk or incidence rate ratio, with 95% confidence intervals or, when not available, relevant P values). We calculated numbers needed to treat (NNT) using the formula 1/absolute risk reduction whenever we thought the data were robust enough to allow it.
Results
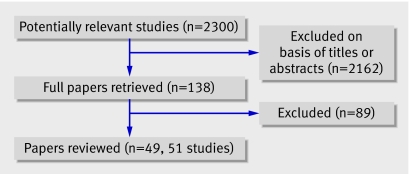
Overall, 2300 titles of reports of potentially relevant studies were identified and screened. In total, 2162 were excluded and 138 full papers retrieved, totalling 49 reports of 51 studies (fig 1).
Fig 1 Flow of papers through study
The quality of the methods of included studiesw1-w51 varied (tables 1-5). Considerable loss of information resulted from incomplete or no reporting of randomisation,w3 blinding,w5 numerators and denominators,w4 w6 interventions, outcomes,w39 attrition of participants,w34 confidence intervals,w33 and cluster coefficients in the relevant trials.w4 The impact of potential biases (such as cash incentives given to participantsw39) were not discussed. Some authors confused the cohort design with a before and after design, which provided conclusions unsupported by the data.w34 The quality of methods was sometimes eroded by the need to deliver behavioural interventions in the midst of service delivery.w37 Even when suboptimal designs were selected, authors rarely articulated potential confounders. A common confounder specific to this area is the huge variability in viral incidence over time, commonly ignored.w19 w41 Sometimes this was tackled in the study design,w30 even in controlled before and after studies (one attempted correlation between admissions for respiratory syncytial virus and respiratory syncytial virus circulating in the communityw21; another attempted linking exposure—measured as nasal excretion—and infection rate in the periods before and after interventionw14). Inadequate blinding or adjustment for confounders is a well known factor in exaggerating the effects of an intervention.23
Table 1.
Characteristics of included randomised trials
| Study | Participants | Interventions v comparisons | Outcomes | Risk of bias (notes) |
|---|---|---|---|---|
| Randomised controlled trials: | ||||
| Gwaltney 1980w2 | 15 and 20 participants in two experiments | Painting of hands with iodine v placebo (Ivory soap; Procter and Gamble, Cincinnati, OH), before experiment | Reduction in experimental rhinovirus infection (P=0.06) | High (poor description of randomisation process, concealment, and allocation) |
| Turner 2004w3 | 85 participants; 122 participants | Use of salicylic acid v salicylic acid and pyroglutamic acid, and v “placebo substance”; use of skin cleanser wipe containing 4% pyroglutamic acid formulated with 0.1% benzalkonium chloride v skin cleanser wipe containing ethanol | Reduction in experimental rhinovirus infection (P<0.05); reduction in experimental rhinovirus infection (not significant) | High (no description of randomisation process, concealment, and allocation); high (no description of randomisation process, concealment, and allocation) |
| Cluster randomised trials: | ||||
| Carabin 1999w4 | 1729 children aged 18-36 months | Training session (1 day) with washing hands, cleaning toys, opening windows, cleaning sandpits, and repeated requests to wash hands v standard practice | Reduced incidence of colds (incidence rate ratio 0.80, 95% CI 0.68 to 0.93) | High (no description of randomisation; partial reporting of outcomes, numerators, and denominators) |
| Farr 1988w5 | 186 families; 98 families | Use of virucidal tissues (Kimberly-Clark, Neenah, WI) over 26 weeks v placebo tissues, and v no tissues (no placebo); use of virucidal tissues (Kimberly-Clark) v placebo tissues | Acute respiratory infections. Total illness rate was lower in families using virucidal tissues than in either of other two groups, but only overall difference between active and placebo groups was significant (illnesses per person 3.4 v 3.9 for placebo group P=0.04, and 3.6 for no tissues control group P=0.2, and overall 14% to 5% reduction); acute respiratory infections reduced incidence per person per week in household by 5% (not significant) | High (failure of blinding); high (failure of blinding) |
| Kotch 1994w6 | 389 children aged ≤3 years in day care for at least 20 h/wk | Structured handwashing (disinfectant scrub Cal Stat donated by Calgon Vestal Laboratories, Merck) and disinfecting programme of environment (surfaces, sinks, toilets, and toys) with waterless liquid v standard practice | Acute respiratory infections (defined): No significant reduction (relative risk0.94, 95% CI −2.43 to 0.66) | High (poor reporting of randomisation, outcomes, numerators, and denominators) |
| Sandora 2005w7 | 292 families with children (6 months to 5 years) in child care (≥10 h/wk) | Alcohol based hand sanitiser (Purell Instant Hand Sanitizer; Gojo Industries, Akron, OH) with biweekly hand hygiene educational materials over five months v biweekly educational material on healthy diet | Acute respiratory infections. No significant reduction (relative risk 0.97, 95% CI 0.72 to 1.30) | Medium (relatively high attrition rate and confounder in respiratory droplet transmission route) |
| Ladegaard 1999w8 | 0-6 year olds | Educational programme: message on T shirts “Clean hands—yes, thank you”, performance of a fairytale “Princess who did not want to wash her hands,” exercises in handwashing; and importance of clean and fresh air | 34% decrease in “sickness” (probably mostly gastroenteritis) | Limited data only available |
| Longini 1988w9 | 143 households randomised to virucidal tissues during season of high circulating influenza H3N2 virus and rhinoviruses | Disposable three layered virucidal tissues containing sodium lauryl sulphate sandwiched between citric and malic acids (Kimberly-Clark) v placebo (succinic acid in tissue sandwich) | Acute respiratory infections reduced from 18.7% to 11.8% (NS) | High (inappropriate choice of placebo) |
| Luby 2005w1 | Householders living in slums in Karachi | Instruction programme and antibacterial bar soap containing 1.2% triclocarban (Safeguard Bar Soap; Procter and Gamble, Cincinnati, OH) v ordinary soap to be used throughout the day by householders v usual behaviour | Incidence of pneumonia: relative risk between soap and usual behaviour 0.50 (95% CI 0.65 to 0.34) in children aged <5 years | Low (cluster coefficients reported and analysis by unit of randomisation carried out) |
| Morton 2004w10 | 253 school children, (ages not reported) from kindergarten to third grade | Alcohol gel plus handwashing (AlcoSCRUB; Erie Scientific, Portsmouth, NH) v handwashing alone | Absenteeism from school reduced by 43% | High (no description of randomisation; partial reporting of outcomes, numerators, and denominators) |
| Roberts 2000w11 | Children aged ≤3 years | Handwashing programme (GloGerm, Moab, UT)including nursery rhymes and count to 10 seconds when handwashing or rinsing | Acute respiratory infections (defined) reduced in children aged ≤24 months (relative risk 0.90, 95% CI 0.83 to 0.97) but not in older children (0.95, 0.89 to 1.01) | Low (cluster coefficients reported and analysis by unit of randomisation carried out) |
| White 2001w12 | 769 5-12 year olds | Pump activated antiseptic hand rub with benzalkonium chloride (SAB formulation sanitiser; Woodward Laboratories) pump activated antiseptic hand rub plus water and soap handwash placebo | Acute respiratory infections (defined) relative risk for illness incidence 0.69, duration 0.71. Acute asthma. Gastrointestinal and other illnesses | High (no description of randomisation; partial reporting of outcomes, numerators, and denominators) |
Table 2.
Characteristics of included controlled before and after studies
| Study | Participants | Interventions | Outcomes | Risk of bias |
|---|---|---|---|---|
| Simon 2006w13 | Paediatric inpatients with diagnosis of respiratory syncytical virus admitted for at least 24 hours in Germany | Enhanced surveillance and feedback, rapid diagnosis, barriers and isolation, disinfection of surfaces | Nosocomial infection with respiratory syncytical virus decreased from 1.7 (year 1) to 0.2 per 1000 patient days (Year 3) | Low (reasonably reported study with incidence data presented by sex, age group, and birth weight to minimise bias) |
| Leclair 1987w14 | 695 children aged 5 days to 4 years and 11 months | Infection control intervention to increase use of gloves and gowns | Nosocomial infection with respiratory syncytical virus reduced by relative risk of 3 (95% CI 1.5 to 5.7) | Low (although prone to selection bias, study was better designed than some of its peers as attempt was made at adjusting for different levels of respiratory syncytical virus circulation by subanalysis of virus shedding days in infected participants) |
| Macartney 2000w15 | 1604 children in four seasons before and 2065 children after intervention seasons (aged about 1 year) with community acquired respiratory syncytical virus infection: inpatient children exposed to infected children, Philadelphia, USA | Education, high index of suspicion for case finding, barriers (not goggles or masks), and handwashing for patients and staff in contact with infected patients; two weeks’ isolation when possible: cohorting patients (assigning them to wards) and staff according to risk or symptoms, with enhanced surveillance and restriction of visits, and discouraging staff with acute respiratory infections from working unprotected | Infection with respiratory syncytical virus reduced (relative risk 0.61, 95% CI 0.53 to 0.69) | Medium (study well reported and conclusions reasonable, but no information given on background rate of infection and impact of intervention on morbidity in healthcare workers not analysed) |
| Gala 1986w16 | 74 children and 40 staff in before phase; 77 children and 41 staff in after phase | Use of disposable plastic eye-nose goggle and procedures for control of respiratory infections v procedures for control of respiratory infections alone (cohorting, isolation, and handwashing) | Infection with respiratory syncytical virus reduced from 42% (before) to 6% (after) | High (heavy play of confounders, missed opportunity for randomisation) |
| Hall 1981w17 | 31 volunteers caring for children with respiratory syncytical virus in hospital | Exposure to infants admitted with acute respiratory infection during community outbreak of respiratory syncytical virus | Rates of respiratory syncytical virus infection: 5/7 children cuddled, 4/10 children touched, and 0/14 kept away from their carers | Low (results are of low generalisability) |
| Hall 1981w18 | 162 inpatients with suspected respiratory syncytical virus infections from infants | Additional use of gowns and masks v standard infection control procedures (handwashing, isolation of affected cohorts) | Rates of respiratory syncytical virus infection increased from 32% to 41% | High (poor reporting) |
| Heymann 2004w19 | 186 094 children aged 6-12 years in Israel | Effect of school closure coinciding with “influenza” outbreak | Decreases in acute respiratory infections (42%), visits to doctor and emergency room (28%), and purchase of drugs (35%) | High (observed effect may result from school closure or possibly lower circulation of viruses) |
| Snydman 1988w20 | Healthcare workers and patients in special care baby unit | Active surveillance: gown, mask, and gloves used on contact; restricted visiting policy; and isolation of cohorts of cases, suspected cases, and staff | Rate of respiratory syncytical virus infection decreased from 8 (confirmed) cases to 0 cases per 1000 patient days | High (no denominators provided and exposure generically quantified by aggregate patient days of exposure. Unclear how circulation of respiratory syncytical virus outside related to claimed success of measures, as no information provided) |
| Krasinski 1990w21 | All in-hospital paediatric patients regarded as potentially infected with respiratory syncytical virus | Isolation of screening cohort for respiratory syncytical virus and service education programme v normal care | Respiratory syncytical virus infections to other children reduced from 5 to 3 infections per 1000 patient days | Medium (attempt at correlation between admissions with respiratory syncytical virus and circulation of virus in community) |
| Krilov 1996w22 | 33 children with Down’s syndrome (ages 6 weeks to 5 years) in special needs day care centre with staff-child ratio >5:1 | Training (reinforced by intensive monitoring of classroom behaviour), handwashing programme, and disinfectants on school buses, appliances, and toys | Decreased mean episodes per child per month: acute respiratory infection 0.7 to 0.4 (P<0.07), visits to doctor 0.5 to 0.3 (P<0.05), antibiotic courses 0.33 to 0.28 (P<0.05), days missed from school per study period from infection 0.8 to 0.4 (P<0.05) | High (disinfectants provided, and study sponsored, by manufacturer) |
| Pang 2003w23 | 2521 probable cases of SARS, mostly people admitted to hospital in Beijing, China | Management training and provision of gowns, gloves, and masks; and screening of port of entry | SARS public health measures (barriers, quarantine, screening, contact tracing); only 12 cases identified out of 13 000 000 screened | Low (efforts made to minimise impact of confounding) |
| Pelke 1994w24 | 230 infants, aged 22-42 weeks, of birth weight 464-6195 g | Additional use of gowns plus standard procedures (handwashing) v handwashing alone | No decrease in rates of respiratory syncytical virus infection, other infections, or death (1.2 v 1.4 deaths/100 patient days) | Medium (17% loss to follow-up) |
| Ryan 2001w25 | 136 225 naval recruits (mainly men, aged 19-20 years) undergoing training over three years compared with about 30 000 recruits for phase II of study | Structured ‘‘top-down’’, military ordered programme of handwashing (>4 times daily) v no programme of handwashing (that is, standard practice) | Three stratified samples of recruits: decreased self reported episodes of acute respiratory infections (4.7 v 3.2 per recruit, odds ratio 1.5, 95% CI 1.2 to 1.8) and fewer admissions to hospital (odds ratio 0.09, 95% CI 0.63 to 0.006) | Low (attempt at correlating effects in intervention cohort with viral circulation in non-intervention population on same military base) |
Table 3.
Characteristics of included prospective cohort studies
| Study | Participants | Interventions v comparisons | Outcomes | Risk of bias (notes) |
|---|---|---|---|---|
| Agah 1987w26 | 168 healthcare workers caring for children aged <5 years with differential diagnosis of respiratory syncytical virus infection | Mask and goggles (sometimes gowns) v normal care | Respiratory syncytial virus illness symptoms reduced from 61% (controls) to 5% (intervention) | Low (reasonably reported despite difficulties of carrying out study; standard procedures such as handwashing should not have acted as confounder given 100% coverage among healthcare workers) |
| Derrick 2005w27 | Six volunteers in experimental laboratory setting | Pleated rectangular three ply surgical masks worn singly up to five thick, subject to range of neck and head movements | Poor filtration of particles through masks | High (report too brief to allow assessment) |
| Dick 1986w28 | Eight men with laboratory induced cold using R16 virus (donors) and 12 antibody free men (recipients) | Use of virucidal paper handkerchiefs (Kleenex Mansize tissues; Kimberly-Clark, Neenah, WI), containing citric acid and other virucidal ingredients to stop spread of R16 virus v normal cotton handkerchiefs | 0% transmission of R16 virus in intervention groups compared with 42% of controls developing colds | Low (small, well designed and controlled study) |
| Dyer 2000w29 | 420 children aged 5-12 years in private school in California; cluster open label crossover cohort study over 10 weeks | Educational programme, surfactant, allontoin and benzal konium chloride spray hand sanitiser (CleanHands), and use of soap and water at will for handwashing v normal care | Absenteeism reduced by 41.9%; respiratory illnesses by 49.7% | Medium (authors described limitations of study as limited socioeconomic diversity in study population, limitation to single study site, and lack of blinding. Further washing using soap and water was not monitored. Generalisability of results questionable as participants underwent educational programme) |
| Falsey 1999w30 | Three adult day care centres with 97 staff and 204 elderly people | Addition of virucidal hand foam as supplement (Alcare Plus; Calgon Vestal Laboratories, St Louis, MO) v normal handwashing and educational programme | Rates of respiratory infection fell from 14.5 to 10.4 per 100 person months to 5.7 (P<0.001) in last four years, with accompanying decline in viral isolates (influenza, respiratory syncytial virus, coronavirus, parainfluenza virus, rhinovirus) | Low (one of few identified studies reporting circulating viruses in day care setting, in both staff and patients. Decline in flu-like illness episodes across four study years reflected in decline in viral isolates, suggesting that aspecific measures such as handwashing are effective against main respiratory viruses) |
| Kimel 1996w31 | 199 children of kindergarten and first grade (primary) schools | Handwashing and educational programme v no intervention | Absenteeism as a result of acute respiratory infections was about double that in control arm (P=0.01) | Medium (study did not control for health and hygiene practices at home or exposure to flu-like illness outside school. In addition student population was generally healthy, probably because families were able to provide adequate health and hygiene resources. Flu season was later than usual (February), therefore a confounder. Surveys of teachers indicated problems with handwashing facilities |
| Leung 2004w32 | 26 healthcare workers caring for probable or suspected people with SARS in Hong Kong | Triage and isolation for ultra high risk of SARS and strict infection control procedures v similar triage and isolation but less strict infection control procedures | No healthcare workers infected with SARS | Low (well done and clearly reported study in midst of major outbreak with previously unknown agent. Prince of Wales Hospital had previously experienced an outbreak in which index patient had infected 138 healthcare workers) |
| Madge 1992w33 | Four paediatric wards in one hospital; children had differential diagnosis of respiratory syncytial virus | Gowns, gloves, and isolated nursing of cohorts of suspected cases v normal care | Nosocomial infection with respiratory syncytial virus reduced (odds ratios reduced to between 0.76 and 0.013 of the baseline) | Low (possible “ward effect” not accounted for as confounder in study design. For practical reasons two wards continued with same policy over first two years of study. Possibility that another ward had been effective at implementing the assigned policy) |
| Makris 2000w34 | Eight private, freestanding, long term care facilities in USA | Infection control education programme reinforcing handwashing and other hygienic measures v normal care | Reported “reduced number of organisms present on hands and surfaces, and ARIs”; however, data showed incidence rate of 4.15 per 1000 patient days in test homes v 3.15 per 1000 patient days in control homes | High (internal inconsistencies) |
| Master 1997w35 | 305 healthy, predominantly upper middle class children aged 5-12 years | Handwashing programme v usual practice | Acute respiratory infections: no reduction of absenteeism (relative risk 0.79, P>0.75) | High (discrete population without socioeconomically diverse backgrounds, single institution, lack of blind assessment, low specificity of symptoms, lack of accurate symptom definition) |
| Murphy 1981w36 | 58 health workers caring for infants with respiratory infections | Handwashing, masks, and gowns (28 health workers) v handwashing only (n=30) | Viral infections (including respiratory syncytial virus) not reduced (5 in intervention arm v 4 in controls, P>0.20) | Medium (small study with potential confounders: heavy exposure of adults to respiratory viral illness in community; poor compliance with study protocol, modes of virus spread not able to be blocked by masks or gowns) |
| Niffenegger 1997w37 | Eight teachers and 26 children (aged 3-5 years) in test group | Three weekly cycles of teaching handwashing routine; encouragement for children, parents, and staff; and correct procedure for sneezing and coughing v unclear comparator | During first 11 weeks of study, test centre had double the incidence of colds compared with that of the control centre (19.4% v 12.7%, P<0.05) | High (wide range of infection incidence and unclear comparator) |
| Somogyi 2004w38 | One participant | Three masks; two without air filter and allowing external exhalation, one with manifold and air filter | Plumes of droplets observed and photographed: masks poor at preventing droplet spread | Low (small but simple, safe, and effective study) |
| White 2003w39 | 188 university students in communal residences | Education programme and alcohol gel hand sanitiser (Purell; Gojo Industries, Akron, OH) adjunct to handwashing in residence halls v standard hygiene | Acute respiratory infection reduced by 14.8% to 39.9%, and absenteeism from lectures reduced by 40% | Medium (unexplained attrition and unknown effect of cash incentives; relatively unclear definition of illness with hint of sensitivity analysis in footnote to table) |
Table 4.
Characteristics of included retrospective cohort studies
| Study | Participants | Interventions v comparisons | Outcomes | Risk of bias (notes) |
|---|---|---|---|---|
| Doherty 1998w40 | Children aged <2 years with differential diagnosis of respiratory syncytial virus infection | Diagnosis of respiratory syncytial virus infection and cohortingv normal care | “RSV infection reduced” (but data did not support conclusion) | High (poor descriptions) |
| Isaacs 1991w41 | Children aged <2 years with differential diagnosis of respiratory syncytial virus infection | Isolation and handwashing with alcohol based hand rubs (Amphisept 80; GoldsCHmidt) v normal care | Respiratory syncytial virus infection reduced by “up to 60%” | High (poor descriptions) |
| Ou 2003w42 w43 | 171 cases of SARS and 1210 people quarantined from selected districts in China | Quarantine at home or hospital for 14 days after exposure: comparisons between reductions of incidence (95% CIs) of SARS for carers 31% (20% to 44%), visitors 9% (3% to 22%), and cohabiting contacts 5% (2% to 9%) | SARS attack rates reduced for all groups except non-cohabitants living in same building; carers of cases during incubation period (quarantine therefore not necessary) | High (non-random basis for sample, selection bias of sample and responders, recall bias of responders, and absence of laboratory confirmed diagnosis may have affected conclusion. Overall, insufficient denominator data, or data on non-exposed people, precluded data extraction or calculation of odds ratios) |
| Yen 2006w44 | One intervention military hospital (459 healthcare workers) and 86 control hospitals in Taiwan | Integrated infection control strategy: triage and barrier traffic flow into hospital, zoning of risk, negative pressure areas of isolation, personal hygiene, and barrier interventions v normal isolation procedures | Only two healthcare workers infected with SARS, compared with 50 probable cases and 43 suspected cases in control hospitals | High (sketchily reported study with missing denominators and data on exposure to SARS. Not clear how intervention differed from high risk isolation procedures) |
Table 5.
Characteristics of included case-control studies
| Study | Participants | Interventions v comparisons | Outcomes | Risk of bias (notes) | |
|---|---|---|---|---|---|
| Lau 2004w45 | 330 probable cases of SARS reported to Department of Health, Hong Kong | Natural exposure to SARS during serious epidemic | Community transmission of SARS reduced (odds ratio 0.30, 95% CI 0.23 to 0.39) | Medium (inconsistencies in text: controls not described) | |
| Nishiura 2005w46 | 29 survivors of laboratory confirmed SARS; cases admitted to hospital and retained or transferred | Handwashing before contact with patient infected with SARS; handwashing after contact with infected patient; masks; gloves; gowns; all measures combined | Masks (odds ratio 0.3, 95% CI 0.1 to 0.7) and gowns (0.2, 0.0 to 0.8) were significantly associated with protection from SARS during phase 1 trials but in phase 2 trials masks (0.1, 0.0 to 0.3) and all measures (0.1, 0.0 to 0.3) were associated with protection probably because of increased awareness of danger of outbreak and increased use of measures | Low (well written and reported study) | |
| Seto 2003w47 | 13 healthcare workers infected with confirmed SARS within 2-7 days of exposure, with no community exposure | Handwashing, masks, gloves, and gowns | Handwashing, masks, and gowns (odds ratio 5, 95% CI 1 to 19) were effective, but only masks (13, 3 to 60) were significant using logistic regression, possibly through lack of power | Medium (inconsistencies in text: lack of description of controls) | |
| Teleman 2004w48 | 36 healthcare workers caring for patients with probable or suspected SARS | Distance from source of infection <1 m, duration of exposure ≥60 minutes, wearing N95 mask, wearing gloves, wearing gown, touched patients, touched patients’ personal belongings, contact with respiratory secretions, did venepuncture, carried out or assisted in intubation, carried out suction of body fluids, gave oxygen; washed hands after contact with each patient | Three factors were associated with significant risks or protection against SARS: wearing N95 mask (odds ratio 0.1, 95% CI 0.02 to 0.86), contact with respiratory secretions (21.8, 1.7 to 274.8), and | handwashing after contact with each patient (0.07, 0.008 to 0.66) | Low (well written and reported study) |
| Wu 2004w49 | 94 patients with probable or suspected SARS admitted to hospital | Always wearing a mask, intermittently wearing a mask, washing hands after returning home, owning a pet, visited farmers’ market, visited clinics, eaten out, or used taxis | Always wearing a mask was strongly protective (70% reduction in risk, odds ratio 0.3, 95% CI 0.2 to 0.7) and wearing one intermittently (0.5, 0.2 to 0.9) or always washing hands after returning home (0.3, 0.2 to 0.7) showed smaller significant reductions in risk. Of great interest was role of fever clinics in spreading the disease, probably because of poorly implemented isolation and triage procedures (13.4, 3.8 to 46.7), having eaten out (2.3, 1.2 to 4.5), or used taxis more than once a week (3.2, 1.3 to 8.0) | Medium (inconsistencies in text: controls not described) | |
| Yin 2004w50 | 77 healthcare workers caring for patients with probable or suspected SARS | Mouth masks, thick mouth masks (>12 layers of cloths), one-off paper mouth mask, wearing eye mask when indicated, protection for mucosa of nose and eyes, shoes, gloves, barrier gown, gloves, rinsing out mouth, bathing, fresh clothes before going home, checking mouth mask, taking oseltamivir orally, avoiding eating or smoking in ward, hand washing and disinfection, nose clamps, taking herbal Banlangen (Indigowoad Root) orally | Single measures such as wearing masks (odds ratio 0.78, 95% CI 0.60 to 0.99), goggles (0.20, 0.10 to 0.41), and footwear (0.58, 0.39 to 0.86) were effective against SARS | Medium (inconsistencies in text: controls not described) |
Inappropriate interventions for comparison caused problems with study designs: in some studies these probably carried sufficient effect to dilute the intervention outcomew9; in two studies blinding may have failed because placebo handkerchiefs were impregnated with a dummy compound that stung the users’ nostrils.w5
Some interventions were tested under impractical and unrealistic situations: participants allocated to the intervention hand cleaner (organic acids) were not allowed to use their hands between cleaning and challenge with virus, so the effect of normal use of the hands on the intervention remains unknown,w3 and 2% aqueous iodine is a successful antiviral intervention when painted on the hands but it stains and is impractical for all but the highest risk of epidemic contagion.w51
Compliance with interventions—especially educational programmes—was problematic for several studies, despite the importance of many such low cost interventions.
The most impressive effects came from high quality cluster randomised trials in preventing the spread of respiratory virus into the community using hygienic measures aimed at younger children. One study reported a significant decrease in respiratory illness in children up to age 24 months (relative risk 0.90, 95% confidence interval 0.83 to 0.97), although the decrease was not significant in older children (0.95, 0.89 to 1.01).w11 Another study reported a 50% (95% confidence interval 65% to 34%) lower incidence of pneumonia in children aged less than 5 years in a developing country.w1 Additional benefit from reduced transmission to other household members is broadly supported by the results of other study designs although the potential for confounding is greater.
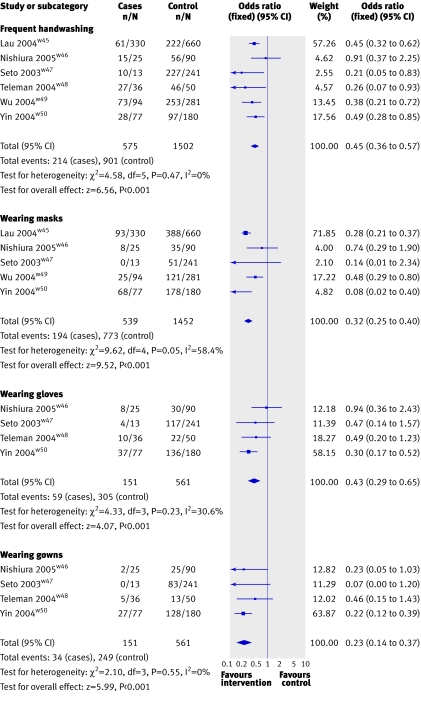
Six case-control studies assessed the impact of public health measures to curb the spread of the SARS epidemic in China, Singapore, and Vietnam in 2003. Homogeneity of case definition, agent, settings, and outcomes made meta-analysis possible, using a fixed effects model because no comparisons showed significant heterogeneity (fig 2 and table 6). Only binary data were pooled despite the availability of continuous data because the variables differed or were measured in different units, and standard deviations were usually missing. The data suggest that implementing barriers to transmission, isolation, and hygienic measures are effective and relatively cheap interventions to contain epidemics of respiratory viruses, such as SARS, with estimates of effect ranging from 55% to 91%: washing hands more than 10 times daily (odds ratio 0.45, 95% confidence interval 0.36 to 0.57, NNT=4, 95% confidence interval 3.65 to 5.52); wearing masks (0.32, 0.25 to 0.40, NNT=6, 4.54 to 8.03); wearing N95 masks (0.09, 0.03 to 0.30, NNT=3, 2.37 to 4.06); wearing gloves (0.43, 0.29 to 0.65, NNT=5, 4.15 to 15.41); wearing gowns (0.23, 0.14 to 0.37, NNT=5, 3.37 to 7.12); and handwashing, masks, gloves, and gowns combined (0.09, 0.02 to 0.35, NNT=3, 2.66 to 4.97). All studies selected hospital cases, except onew45 in which the cases were people with probable SARS reported to the Department of Health in the territory of Hong Kong up to 16 May 2003. Evidence was limited for the superior effectiveness of barrier devices to droplets such as the N95 masks (respirators with 95% filtration capability against non-oily particulate aerosolsw48) over simple surgical masks. An incremental effect was found for decreased burden of respiratory disease by adding virucidals or antiseptics to normal handwashing in atypical settings, but the extra benefit may have been, at least partly, from confounding additional routines.
Fig 2 Evidence from case-control studies on effect of frequent handwashing or wearing of masks, gloves, or gowns on prevention of severe respiratory syndrome (SARS)
Table 6.
Pooled estimates of effect of public health interventions to interrupt transmission of SARS from case-control studies
| Intervention | No of studies (references) | Odds ratio (95% CI) | Intervention effectiveness* (%) | Number needed to treat (95% CI)† |
|---|---|---|---|---|
| Frequent handwashing (>10 times daily) | 6 (w48, w45-w47, w49, w50) | 0.45 (0.36 to 0.57) | 55 | 4.00 (3.65 to 5.52) |
| Wearing mask | 5 (w45-w47, w49, w50) | 0.32 (0.25 to 0.40) | 68 | 6.00 (4.54 to 8.03) |
| Wearing N95 mask | 2 (w45, w47) | 0.09 (0.03 to 0.30) | 91 | 3.00 (2.37 to 4.06) |
| Wearing gloves | 4 (w46, w47 w45, w50) | 0.43 (0.29 to 0.65) | 57 | 7.00 (4.15 to 15.41) |
| Wearing gown | 4 (w45, w46, w47, w50) | 0.23 (0.14 to 0.37) | 77 | 5.00 (3.37 to 7.12) |
| Handwashing, mask, gloves, and gown combined | 2 (w46, w47) | 0.09 (0.02 to 0.35) | 91 | 3.00 (2.66 to 4.97) |
*Odds ratio−1.
†Number needed to treat to prevent one case.
Studies on interventions to prevent the transmission of respiratory syncytical virus and similar viruses in more typical settings suggested good effectiveness, although doubt was cast on the findings because of method quality inherent in controlled before and after studies, especially different virus infection rates.
Few studies reported on resource consumption for the physical intervention evaluated. One case-control studyw45 concluded that handwashing needs to be carried out more than 10 times daily to be effective. One study,w25 in a military training setting, reported a need to wash hands more than four times daily. During one month of the respiratory syncytical virus “season” on a ward containing 22 cribs, one study reported that 5350 gowns and 4850 masks were used.w18
Proper evaluation of global and highly resource intensive measures such as screening at entry ports and social distancing was lacking. The handful of studies (mostly done during the SARS epidemic) did not allow firm conclusions to be drawn.
Discussion
In this systematic review we found that physical barriers such as handwashing, wearing a mask, and isolation of potentially infected patients were effective in preventing the spread of respiratory virus infections. It is not surprising that methods of the included studies were at risk of bias as these types of interventions are difficult to blind, are often set up hurriedly in emergency situations, and funding is less secure than for profit making interventions. Hasty design of interventions to minimise public health emergencies, particularly the six included case-control studies, is understandable but not when no randomisation (not even of clusters) was done in the several unhurried cohort and before and after studies, despite randomisation leading to minimal disruption to service delivery. Inadequate reporting often made interpretation of before and after studies difficult.
The settings of the studies, carried out over four decades, were heterogeneous, ranging from suburban schoolsw4 w37 w29 to military barracks,w25 intensive care units, paediatric wardsw14 w16 in industrialised countries, slums in developing countries,w1 and day care centres for children with special needs.w22 Few attempts were made to obtain socioeconomic diversity by, for example, involving several schools in the evaluations of one programme.w29 We identified few studies from developing countries where the most burden lies and where cheap interventions are needed. Even in Israel, the decrease in acute respiratory tract infections subsequent to school closure may have been related to atypical features: the high proportion of children in the population (34%) and limited access to over the counter drugs, which together with the national universal comprehensive health insurance means that symptomatic treatment is generally prescribed by doctors.w19
Compliance with interventions—especially educational programmes—was a problem for several studies, despite the importance of such low cost interventions. Routine long term implementation of some would be problematic—particularly maintaining strict hygiene and barrier routines for long periods, probably only feasible in highly motivated environments such as hospitals without the threat of an epidemic.
Global and highly resource intensive measures such as screening at entry ports and social distancing lacked proper evaluation. The handful of studies (mostly done during the SARS epidemic) did not allow us to reach any firm conclusions, although a recent analysis of historical and archival data from the 1918-9 influenza pandemic in the United States suggests an effect of social distancing measures such as school closures and bans on public gatherings.24
Nevertheless our systematic review of available research does provide some important insights. Perhaps the impressive effect of the hygienic measures aimed at younger children derives from their poor capability with personal hygiene.w1 w11
Simple public health measures seem to be highly effective at reducing the transmission of respiratory viruses, especially when they are part of a structured programme including instruction and education and when they are delivered together. Further large pragmatic trials are needed to evaluate the best combinations. In the meantime we recommend implementing the following interventions combined to reduce the transmission of respiratory viruses: frequent handwashing (with or without antiseptics), barrier measures (gloves, gowns, and masks), and isolation of people with suspected respiratory tract infections.
What is already known on this topic
People are increasingly concerned about pandemics of virus infections such as avian influenza and SARS
Preparation against pandemics includes developing vaccines and stockpiling antiviral agents—interventions that are virus specific and of unknown effectiveness in epidemic disease
What this study adds
Several physical barriers, especially handwashing, masks, and isolation of potentially infected people, were effective in preventing the spread of respiratory virus infections
Such interventions should be better evaluated and given higher priority in preparation for pandemics
Supplementary Material
We thank Peter Doshi, Anne Lyddiatt, Stephanie Kondos, Tom Sandora, Kathryn Glass, Max Bulsara, and Allen Cheng for commenting on the draft protocol; Jørgen Lous for translating a Danish paper and extracting data; Taixiang Wu for translating Chinese text; and Ryuki Kassai for translating Japanese text.
Contributors: RF and AR constructed the search strategy. TJ, CDM, and LD drafted the protocol. LD, CDM, and RF incorporated the referees’ comments. TJ, FE, BH, and AP extracted study data. SN carried out the analyses. TJ and CDM wrote the final report and are the guarantors for the paper. All authors contributed to the final paper.
Funding: Cochrane Collaboration Steering Group, UK, and each author’s institution.
Competing interests: None declared.
Ethical approval: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.United States Department of Health and Human Services. HHS pandemic influenza plan. Appendix B: pandemic influenza background. 2005. www.hhs.gov/pandemicflu/plan/appendixb.html (accessed 20 Jun 2006).
- 2.Fleming DM. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun Dis Public Health 2000;3:32-8. [PubMed] [Google Scholar]
- 3.Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NH, Schonberger LB. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health 1997;87:1944-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith S, Demicheli V, Di Pietrantonj C, Harnden AR, Jefferson T, Matheson NJ, et al. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2006:CD004879. [DOI] [PubMed]
- 5.Shute N. SARS hit home. US News World Rep 2003;134:38-42, 44. [PubMed] [Google Scholar]
- 6.WHO. WHO interim guidelines on clinical management of humans infected by influenza A(H5N1). 2004. www.who.int/csr/disease/avian_influenza/guidelines/clinicalmanage/en/index.html (accessed 15 Jun 2007).
- 7.Jefferson T, Demicheli V, Rivetti D, Jones M, Di Pietrantonj C, Rivetti A. Antivirals for influenza in healthy adults: systematic review. Lancet 2006;367:303-13. [DOI] [PubMed] [Google Scholar]
- 8.Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A, Di Pietrantonj C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: systematic review. Lancet 2005;365:773-80. [DOI] [PubMed] [Google Scholar]
- 9.Jefferson T, Smith S, Demicheli V, Harnden A, Rivetti A. Safety of influenza vaccines in children. Lancet 2005;366:803-4. [DOI] [PubMed] [Google Scholar]
- 10.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005;366:1165-74. [DOI] [PubMed] [Google Scholar]
- 11.Aiello AE, Larson EL. What is the evidence for a causal link between hygiene and infections? Lancet Infect Dis 2002;2:103-10. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2004-2005 interim guidance for the use of masks to control influenza transmission. 2005. www.cdc.gov/flu/professionals/infectioncontrol/maskguidance.htm (accessed 15 Jun 2007).
- 13.Centers for Disease Control and Prevention. Infection control guidance for the prevention and control of influenza in acute-care facilities. 2007. www.cdc.gov/flu/professionals/infectioncontrol/healthcarefacilities.htm (accessed 15 Jun 2007).
- 14.Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2007;(2):CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Jefferson T, Demicheli V, Deeks J, Rivetti D. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev 2000:CD001265. [DOI] [PubMed]
- 16.Jefferson T, Demicheli V, Di Pietrantonj C, Rivetti D. Amantadine and rimantadine for influenza A in adults. Cochrane Database Syst Rev 2006; (2):CD001169. doi: 10.1002/14651858.CD001169.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matheson NJ, Harnden AR, Perera R, Sheikh A, Symmonds-Abrahams M. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database Syst Rev 2007:CD002744. [DOI] [PubMed]
- 18.Swingler G, Fransman D, Hussey G. Conjugate vaccines for preventing Haemophilus influenzae type b infections. Cochrane Database Syst Rev 2003:CD001729. [DOI] [PubMed]
- 19.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.htm, 2005.
- 20.Khan SK, ter Riet G, Popay J, Nixon J, Kleijnen J. Stage II Conducting the review: phase 5: study quality assessment. In: Khan SK, ter Riet G, Glanville J, Sowden AJ, Kleijnen J, eds. Undertaking systematic reviews of research on effectiveness. CRD’s guidance for carrying out or commissioning reviews CRD Report No 4. 2nd ed. York: University of York, 2000
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M, Juni P, Bartlett C, Sterne J. Importance of different sources of bias in systematic reviews of controlled trials: systematic review of empirical studies. Cochrane Methodology Register (CMR) 2007:issue 3.
- 24.Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918-1919 influenza pandemic. JAMA 2007;298:644-54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.