Abstract
The dopamine modulation of neuronal excitability in the prefrontal cortex (PFC) changes during critical late periods of postnatal development. In particular, D2 receptors activate fast-spiking interneurons after, and not before, adolescence. To test the functional impact of this change, we investigated the effects of dopamine agonists on PFC excitatory synaptic transmission with whole-cell recordings from deep-layer pyramidal neurons in brain slices obtained from prepubertal [postnatal day (PD) 28–35] and postpubertal (PD > 51) rats. Electrical stimulation of superficial layers elicited a fast AMPA/kainate excitatory postsynaptic potential (EPSP). In the adult PFC, the D2 agonist quinpirole decreased EPSP amplitude, an effect that lasted for at least 25 min after drug washout and was blocked by the D2 antagonist eticlopride. The late component of this effect was blocked by the GABA-A antagonist picrotoxin without affecting the early inhibition. Quinpirole also decreased EPSP amplitude in deep-layer pyramidal neurons from prepubertal rats, but this response was not affected by picrotoxin. A D1 agonist, on the other hand, did not affect the pyramidal neuron EPSP. These results indicate that D2, not D1, receptors attenuate local excitatory synaptic transmission in the adult PFC, and this effect of D2 involves a recruitment of local GABAergic activity.
Keywords: EPSP, interneurons, GABA-A, whole cell patch-clamp, electrophysiology, adolescence
INTRODUCTION
Dopamine (DA) modulates fast excitatory and inhibitory synaptic transmission in several brain regions. Early studies in the striatum revealed that DA, in particular via D1 receptors, depolarizes medium spiny neurons (Calabresi et al., 1987; Shen et al., 1992); this could be related to a D1 enhancement of NMDA currents, synaptic glutamatergic responses (Levine et al., 1996a,b) or L-type calcium channels (Cepeda et al., 1998; Hernández-López et al., 1997). In the prefrontal cortex (PFC), D1 receptors can also sustain plateau depolarizations (Lewis and O’Donnell, 2000; Tseng and O’Donnell, 2005), enhance NMDA currents (Seamans et al., 2001a) and pyramidal neuron excitability (Tseng and O’Donnell, 2004; Wang and O’Donnell, 2001), as well as activate interneurons (Gorelova et al., 2002; Tseng and O’Donnell, 2004, 2007). Not surprisingly then, PFC D1 receptors contribute to NMDA-dependent longterm potentiation (LTP) (Gurden et al., 1999, 2000) and improve memory retrieval and working memory performance (Floresco and Phillips, 2001; Seamans et al., 1998). Furthermore, D1-NMDA coactivation in the PFC is required for appetitive instrumental learning in adult rats (Baldwin et al., 2002). Thus, D1 receptors are critical for PFC cognitive functions, and may exert their influence by sustaining activity in PFC networks.
The role of D2 receptors in PFC physiology is less clear. There is evidence that D2 receptors are also critical for PFC-related cognitive functions (Arnsten et al., 1995; Druzin et al., 2000), but their mechanisms are controversial. For example, D2 agonists attenuate pyramidal cell excitability (Gulledge and Jaffe, 1998; Tseng and O’Donnell, 2004), the responses to NMDA and AMPA receptors (Tseng and O’Donnell, 2004), and activate fast-spiking interneurons (FSI) (Tseng and O’Donnell, 2004, 2007). Others, however, have shown D2 attenuation of inhibitory postsynaptic currents in PFC pyramidal neurons (Seamans et al., 2001b; Trantham-Davidson et al., 2004). Unfortunately, the few studies assessing DA modulation of PFC synaptic transmission in deep layer pyramidal neurons were carried out in developmentally immature animals; in vitro recordings from adult animals have only assessed DA modulation of responses to locally applied agents, not endogenous glutamate release. Thus, D2 receptors enhance FSI excitability in adult, not prepubertal slices (Tseng and O’Donnell, 2007). Here we investigated whether DA (specifically, D2) receptors modulate local excitatory synaptic transmission in adult PFC pyramidal neurons by conducting whole-cell patch clamp recordings in brain slices obtained from developmentally mature rats [postnatal day (PD) 51–80] and prepubertal animals (PD 28–35). We examined the effects of selective D2 and D1 agonists on synaptic responses evoked by electrical stimulation of superficial layers at a site ~1 mm lateral to the recorded neuron.
MATERIALS AND METHODS
All experimental procedures were performed according to the USPHS Guide for Care and Use of Laboratory Animals and were approved by the Albany Medical College Institutional Animal Care and Use Committee. As previously reported (Tseng and O’Donnell, 2004), rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) before being decapitated. Brains were rapidly removed into ice-cold artificial cerebral spinal fluid (aCSF) containing (in mM): 125 NaCl, 25 NaHCO3, 10 glucose, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 3 MgCl2 (pH 7.45, 295 ± 5 mOsm). Coronal slices (350 μm thick) containing the medial PFC were cut in ice-cold aCSF with a Vibratome, and incubated in warm (~35°C) aCSF solution constantly oxygenated with 95% O2–5% CO2 for at least 60 min before recording. In the recording aCSF (delivered at 2 ml/min.), CaCl2 was increased to 2 mM and MgCl2 was decreased to 1 mM. Patch pipettes (6–9 MΩ) were pulled from 1.5 mm borosilicate glass capillaries (WPI) with a horizontal puller (Model P97, Sutter Instrument), and filled with a solution containing (in mM): 115 K-gluconate, 10 HEPES, 2 MgCl2, 20 KCl, 2 MgATP, 2 Na2-ATP, 0.3 GTP (pH 7.3, 280 ± 5 mOsm). All experiments were conducted at 33–35°C.
Pyramidal neurons in layers V and VI of the infralimbic and prelimbic regions were identified under visual guidance using infrared-differential interference contrast (IR-DIC) video microscopy with a 40× water-immersion objective (Olympus BX51-WI). The image was detected with an IR-sensitive CCD camera (DAGE-MTI) and displayed on a monitor. Whole-cell current-clamp recordings were performed with a computer- controlled amplifier (MultiClamp 700A; Axon Instruments), digitized (Digidata 1322 Axon Instruments), and acquired with Axoscope 8.1 (Axon Instrument) at a sampling rate of 10 KHz. The liquid junction potential was not corrected and electrode potentials were adjusted to zero before obtaining the whole-cell configuration. Electrical stimulation of layers I–II (0.4–0.9 mA square pulses of 0.3 ms duration) was delivered every 20 s with a bipolar electrode made from a pair of twisted Teflon-coated nichrome wires (tips separated by ~200 μm) and placed 0.8–1.2 mm lateral to the recorded neurons. Stimulation pulses were adjusted to half the intensity required to evoke an action potential. If synaptic responses exhibited more than 10% variation in amplitude during the initial 5 min of recording or the current intensity required was larger than 0.9 mA, the neuron was discarded. Input resistance (measured with hyperpolarizing square pulses), membrane potential, and evoked synaptic responses were analyzed before and after drug application. All drugs (quinpirole, eticlopride, SKF38393, SCH23390, APV, CNQX, and picrotoxin) were purchased from Sigma, and were mixed into oxygenated aCSF and applied in the recording solution in known concentrations. Control and drugcontaining aCSF were continuously oxygenated throughout the experiments. After 15 min. of baseline recordings, a solution containing drug mixtures was perfused for 5–7 min. followed by 20–30 min. of washout period. All measures are expressed as mean ± SD. Drug effects were compared using Student’s t-test or repeated measures ANOVA, and the differences between experimental conditions were considered statistically significant when P < 0.05. In some cases, a two-way ANOVA was performed to compare the interactions between different experimental conditions (drug treatments or age of animals) and the time course of synaptic changes obtained throughout the recording.
RESULTS
Whole-cell current clamp recordings were obtained from 86 medial PFC pyramidal neurons in brain slices from postpubertal rats (PD > 51) and 10 pyramidal neurons from prepubertal (PD 30–35) animals. The location and morphology of all neurons included in this study were confirmed by Neurobiotin staining (Fig. 1), revealing that they were all pyramidal cells in layers V and VI from the infralimbic and prelimbic regions of the medial PFC. PFC pyramidal neurons from PD51–80 rats were silent at rest, and exhibited a negative resting membrane potential (−70.7 ± 2.3 mV; mean ± SD). Their input resistance (150.6 ± 37.2 MΩ) was calculated from the linear negative portion of the current-voltage (I–V) curve (Fig. 1B). Action potentials could be elicited by depolarizing somatic current injections. Around 90% of the recorded pyramidal cells showed an initial spike doublet followed by spike frequency accommodation and a characteristic inward rectification to hyperpolarizing pulses (Figs. 1A and 1B). These properties are similar to what previously reported (Tseng and O’Donnell, 2004).
Fig. 1.

Whole-cell recordings of deep-layer PFC pyramidal neurons obtained from slices from a young adult (PD 56) rat. (A) Characteristic voltage responses (top) to depolarizing and hyperpolarizing somatic current pulses (bottom; 300 ms duration, −300 to +100 pA in amplitude) in a representative neuron. (B) Current-voltage (IV) plot obtained from the traces shown in A. Typically, currents larger than −100 pA yielded inward rectification in the hyperpolarizing direction (arrowhead). The oblique line highlights the regression slope for the linear part of the plot (−100 to +100 pA). (C) IR-DIC image of a deep-layer pyramidal neuron recorded from a PFC slice. Arrowheads indicate the shadow of the patch electrode. (D) Neurobiotin labeling of a representative pyramidal neuron recorded from the medial PFC (same cell from which traces shown in A were obtained). Small arrowheads point to the apical dendrite and the large arrowhead indicates the cell body.
Local stimulation elicited synaptic responses in most PFC pyramidal neurons (n = 54 of 86 neurons). Electrical stimulation (0.4–0.8 mA; 0.5 ms) of layers I–II at 0.8–1.2 mm lateral to the axis defined by the apical dendrite of the recorded neuron (Fig. 2A) evoked a fast depolarizing postsynaptic potential that remained unchanged after 10 or more minutes of perfusing the slice with the GABA-A antagonist picrotoxin (10 μM, n = 6, Fig. 2B). In contrast, the AMPA/kainate antagonist CNQX (10 μM) completely eliminated the synaptic response in all cells tested (n = 8, Fig. 2C), whereas the NMDA antagonist APV (50 μM, n = 7) slightly reduced the duration without affecting the amplitude of the evoked response (Fig. 2D). These responses had short and constant onset latency, indicating their monosynaptic nature. These results indicate that superficial layer stimulation in the medial PFC induces primarily an AMPA-dependent excitatory postsynaptic potential (EPSP) in deep-layer pyramidal neurons, with NMDA contributing to the late component of this response and no obvious contribution of GABA receptors.
Fig. 2.
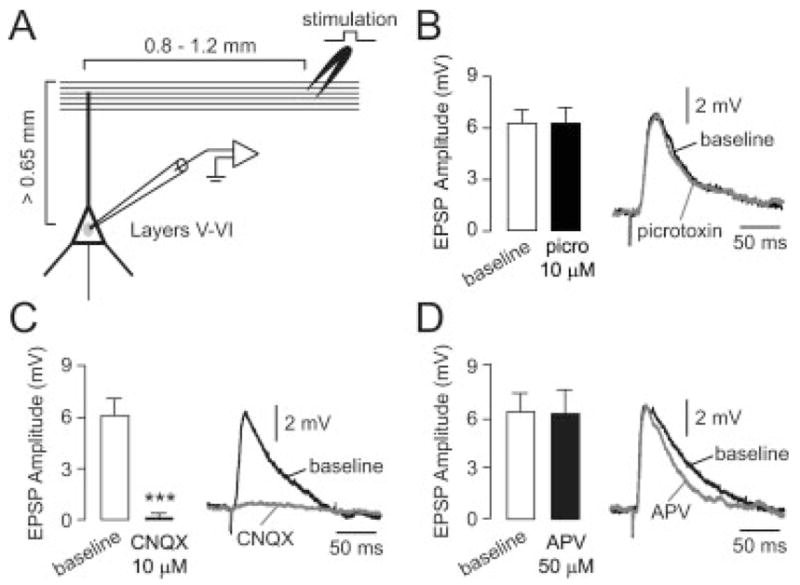
Electrical stimulation of superficial layers typically elicits a glutamatergic EPSP in deep-layer pyramidal neurons of the medial PFC. (A) Diagram illustrating the spatial arrangement of stimulating electrodes (layers I–II) and recording sites (layers V–VI). (B) Bath application of the GABA-A antagonist picrotoxin (10 μM) failed to change the amplitude of the evoked responses in all cells tested (n = 6). Left: bar graph summarizing EPSP amplitudes; right: example of the evoked response recorded before (black line) and after 5 min. of picrotoxin (gray line). Traces in this and subsequent figures are representative examples and not averages. (C) Bar graph (left) summarizing the effect of bath application of the AMPA/kainate antagonist CNQX (10 μM). The amplitude of the evoked response was gradually reduced and completely eliminated after 5 min. of CNQX in all cells tested (n = 8, ***P < 0.0001, paired t-test). Representative traces (right) showing the evoked EPSP before (baseline) and after 5 min. of CNQX. (D) Bath application of the NMDA antagonist APV (50 μM) failed to change EPSP amplitude in all cells tested (n = 7; left). Traces (right) recorded before (baseline) and after 5 min. of drug application illustrate the slight reduction of EPSP decay observed with APV.
D2 receptors affected intracortical synaptic responses. Bath application of the D2 agonist quinpirole, at a concentration that has been reported effective for attenuating pyramidal cell excitability (1 μM; Tseng and O’Donnell, 2004), reduced EPSP amplitude from 8.2 ± 1.3 mV to 6.3 ± 1.2 mV (n = 10; Fig. 3A; P < 0.0002, paired t-test). This effect was observed in every cell tested, despite the wide range of initial EPSP amplitudes (therefore, the large standard deviations and yet highly significant differences). The D2 antagonist eticlopride (20 μM) completely blocked this effect (n = 6, Figs. 3B and 3C), confirming that the quinpirole-induced synaptic depression was D2-mediated. Eticlopride had not effect when applied alone (n = 6, data not shown). Thus, D2 receptors attenuate intracortical synaptic responses in deep layer PFC pyramidal neurons from adult animals.
Fig. 3.
Quinpirole depresses deep-layer pyramidal neuron EPSP amplitude in the PFC of postpubertal animals. (A) Graph summarizing the effect of the D2 agonist quinpirole on pyramidal neuron EPSP amplitude. Bath application of quinpirole (1 μM for 5 min.) significantly reduced EPSP amplitude in all cells tested (n = 10, ***P < 0.0001, paired t-test). (B) Graph illustrating the effect of quinpirole in presence of the D2 antagonist eticlopride (20 μM) in all cells tested. (C) Bar graph summarizing the effect of quinpirole and quinpirole + eticlopride as percentage changes relative to baseline. After 5 min. of quinpirole (1 μM), the average EPSP amplitude decreased by around 23%, an effect that was not evident in presence of eticlopride (***P < 0.0001, unpaired t-test). (D) Representative traces of evoked EPSP recorded in pyramidal neurons before and after bath application of 1 μM quinpirole alone (top) or in presence of 20 μM eticlopride (bottom).
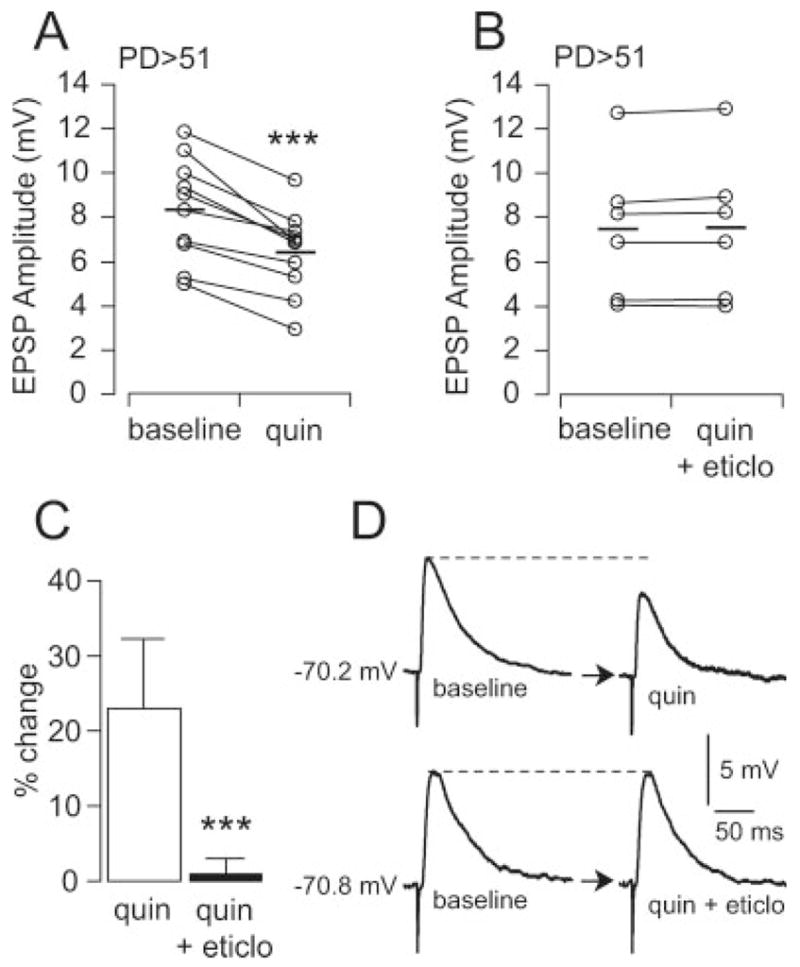
The EPSP remained attenuated for several minutes after quinpirole was removed. At least 25 min were necessary to see a trend towards recovery of EPSP amplitude to baseline (Fig. 4, solid squares). Eticlopride (20 μM) blocked the quinpirole effect in its entire duration (Fig. 4, open triangles). However, the GABAA antagonist picrotoxin (10 μM, n = 6) significantly shortened the quinpirole effect without affecting the initial inhibition (Fig. 4, open squares). A comparable effect was also observed with 10 μM bicuculline (n = 3, data not shown). These results indicate that a D2 attenuation of fast excitatory synaptic transmission in PFC pyramidal neurons could be prolonged by recruiting local GABAergic activity, also D2 mediated, in postpubertal animals.
Fig. 4.

Time course of the effect of quinpirole on PFC pyramidal neuron EPSP amplitude, recorded in slices from postpubertal animals. Quinpirole (1 μM) significantly attenuated EPSP amplitude in pyramidal neurons after 5–7 min. of drug application (indicated with a gray shading), and this was blocked by 20 μM eticlopride (open triangles). In the absence of eticlopride, however, the EPSP attenuation remained even after quinpirole was removed from the bath (solid squares, n = 10). A period of at least 25 min. was required to partially washout the effect of quinpirole. The GABA-A antagonist picrotoxin (10 μM; open squares, n = 6) shortened the duration of this inhibition (*P < 0.01, **P < 0.001, ***P < 0.0001, Tukey posthoc test after significant 2-way ANOVA, interaction between drug and time P < 0.001). The initial D2-dependent EPSP attenuation (white and black arrowheads) was not affected by picrotoxin (open circle, n = 6).
We have recently reported that D2 receptors can increase interneuron excitability in PFC slices from adult, but not prepubertal, rats (Tseng and O’Donnell, 2007). Thus, it is possible that the D2-GABA-dependent modulation of local circuit EPSPs does not emerge until puberty. Therefore, we performed additional recordings in slices from prepubertal (PD < 35) animals. As observed in the mature PFC, bath application of 1 μM quinpirole decreased pyramidal neuron EPSP amplitude in slices from PD28-35 rats (Fig. 5A). EPSP amplitude decreased from 5.7 ± 0.6 mV to 4.9 ± 0.6 mV after 5 min of quinpirole (n = 5, P < 0.001, paired t-test). This effect was not affected by picrotoxin (Fig. 5B) and appears to be less robust than the D2-dependent synaptic attenuation observed in the mature PFC (14.2 ± 3.7% EPSP inhibition compared to 23.2 ± 9.1%; Fig. 5C). Unlike the adult response, the duration of the quinpirole effect was not reduced by picrotoxin (Fig. 5C). This suggests that the D2 recruitment of GABA activity is not present in the prepubertal PFC.
Fig. 5.

In slices from prepubertal animals the GABA component was not observed. (A) Plot summarizing the effect of quinpirole on EPSP amplitudes in pyramidal neurons recorded in the PFC of prepubertal animals. Bath application of quinpirole (1 μM) significantly reduced EPSP amplitude in all cells tested (n = 5, P < 0.001, paired t-test). (B) Plot summarizing the effect of quinpirole on EPSP amplitude in presence of the GABA-A antagonist picrotoxin (10 μM). In these conditions, quinpirole still reduced pyramidal neuron EPSP amplitude to a similar degree to that observed with quinpirole alone (n = 4, P < 0.005, paired t-test). (C) Line graph showing the time course of the quinpirole effect on EPSP amplitude in pyramidal neurons from prepubertal and postpubertal PFC slices. Bath application of quinpirole decreased EPSP amplitude by near 14% (solid squares/solid line) in prepubertal pyramidal neurons; a more pronounced effect was obtained in the PFC of postpubertal animals (23%, grey squares/dashed line;*P < 0.01, **P < 0.001, ***P < 0.0001, Tukey posthoc test after significant 2-way ANOVA, interaction between drug and time P < 0.001). In addition, the early postquinpirole inhibition observed in slices from prepubertal animals (open circles/solid line) was not affected by picrotoxin as it was in the adult PFC (arrowheads).
We also investigated the impact of D1 receptors on local excitatory synaptic transmission in the PFC of PD51-80 rats. Bath application of the D1 agonist SKF38393 enhances pyramidal neuron excitability in adult animals (Tseng and O’Donnell, 2004, 2005). However, no apparent changes in EPSP amplitude were observed with SKF38393 (8 μM, a concentration effective in affecting pyramidal cell excitability; Fig. 6). EPSP amplitudes were 5.4 ± 2.0 mV before and 5.5 ± 1.7 mV after 5–7 min. of SKF38393 (n = 5; Fig. 6A, open circles). Picrotoxin (10 μM) failed to affect the (lack of) SKF38393 response (n = 4; Fig. 6A, open triangles). These results suggest a minor effect of D1 receptors in the modulation of intracortical AMPA-mediated synaptic responses in deep-layer pyramidal neurons.
Fig. 6.
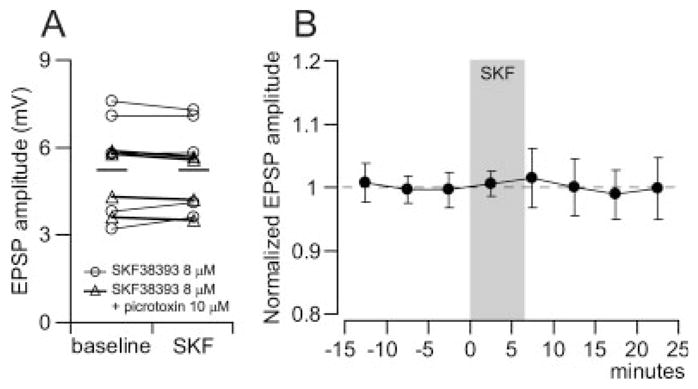
Bath application of the D1 agonist SKF38393 failed to elicit significant changes on medial PFC pyramidal neuron EPSP amplitude. (A) Plot illustrating the effect of 8 μM SKF38393 on EPSP amplitude recorded in deep-layer pyramidal neurons. No consistent changes were observed after 5–7 min. of SKF38393 alone (n = 5; open circles) or in presence of picrotoxin (10 μM, n = 4; open triangles). (B) Time course analysis of normalized EPSP amplitude revealing that SKF38393 does not affect significantly evoked synaptic responses.
DISCUSSION
D2 DA receptors attenuated AMPA-mediated synaptic transmission in PFC pyramidal neurons in slices from both pre- and postpubertal animals. The magnitude and duration of this D2 modulation were more pronounced in the PFC of PD51–80 rats; at least 25 min were required to partially washout the D2 effect. The GABA-A antagonist picrotoxin shortened the duration of the synaptic depression in slices obtained from older animals to values similar to what was observed in slices from prepubertal animals. On the other hand, D1 stimulation failed to elicit significant changes on evoked EPSPs. Thus, DA attenuation of local circuit excitatory synaptic transmission may involve both a direct D2 action on pyramidal neurons and a D2-mediated upregulation of local GABAergic activity that can sustain the inhibition, but only in the adult PFC.
Our data shows that D2 receptors can attenuate the amplitude of EPSPs evoked by superficial layer stimulation in slices from PD51–80 rats. The EPSPs were primarily glutamatergic, as evidenced by their almost complete blockade by CNQX and the lack of effect of picrotoxin. As the distance between stimulating and recording electrode was around 1 mm, it is unlikely that stimulating current spread to activate local GABA projections. All evoked responses seemed to be driven by activation of cortico-cortical fibers in superficial layers. The D2 modulation of these responses; however, may include a fast GABA-independent component and a more protracted one that can be blocked by a GABA-A antagonist. Even though GABA-A blockade with picrotoxin did not affect EPSP amplitude directly, D2 activation attenuated the responses with a late, GABA-A—dependent component, suggesting that D2 receptors may induce long-term changes that affect GABA neurons. Thus, D2 activation can engage several mechanisms, including a direct inhibition of pyramidal neurons and recruitment of local GABA interneurons. In fact, D2 agonists reduce neuronal excitability and attenuate glutamatergic responses in several cortical and subcortical brain regions (Cepeda et al., 1993, 1998; Gulledge and Jaffe, 1998; Hernandez-Echeagaray et al., 2004; Hernandez-Lopez et al., 2000; Kotecha et al., 2002; O’Donnell and Grace, 1994; Perez et al., 2006; Tseng and O’Donnell, 2004). In the PFC, a direct postsynaptic activation of phospholipase lipase C-IP3 and inhibition of protein kinase A (PKA) by D2 receptors can decrease pyramidal cell excitability (Tseng and O’Donnell, 2004). D2 receptors can also enhance local GABA release (Grobin and Deutch, 1998) and GABA interneuron excitability in the adult PFC (Tseng and O’Donnell, 2004, 2007). Indeed, FSI can become spontaneously active in presence of a D2 agonist (Tseng and O’Donnell, 2004). However, other studies have shown that D2 receptors can attenuate GABA currents in pyramidal neurons from prepubertal animals (Seamans et al., 2001b). It is possible that the periadolescent maturation of the D2 modulation of GABA interneuron excitability (Tseng and O’Donnell, 2007) is responsible for this difference. Thus, a D2-dependent, GABA-A-mediated EPSP depression that outlasts the direct effect of the D2 agonist on pyramidal neurons could emerge in PFC circuits during adolescence.
Several studies have reported changes in DA and its actions in the PFC during adolescence. In primates, the DA innervation of the PFC changes dramatically during this critical period (Benes et al., 2000; Rosenberg and Lewis, 1994). In rodents, immediate early gene expression induced by amphetamine changes during adolescence in the PFC and nucleus accumbens (Andersen et al., 2001). Furthermore, neurophysiological studies reveal that event-related potentials are still maturing during adolescence in humans (Segalowitz and Davies, 2004). We have recently shown that the excitatory effect of D2 receptors on PFC FSI excitability emerge during adolescence (Tseng and O’Donnell, 2007). This late maturation of D2 actions may explain the findings reported here. As interneurons acquire the ability to be driven by D2 receptors during adolescence, these receptors may, in addition to any direct action on pyramidal neurons, recruit interneurons that would in turn modulate pyramidal synaptic responses.
The D1 agonist failed to affect intra-PFC EPSPs in deep-layer pyramidal neurons. It is well known that D1 receptors increase AMPA-evoked striatal cell firing (Cepeda et al., 1993) but produce little net change on excitatory synaptic responses within corticostriatal synapses (Levine et al., 1996b; O’Donnell and Grace, 1994; West and Grace, 2002). In the PFC, the D1 modulation of glutamate-mediated responses is complex, with evidence supporting both positive and negative interactions depending on the glutamatergic receptor subtypes involved (Tseng and O’Donnell, 2004). D1 agonists enhance NMDA responses in deep-layer pyramidal neurons in the PFC of pre- (Wang and O’Donnell, 2001) and postpubertal (Tseng and O’Donnell, 2005) animals. Furthermore, D1-NMDA co-activation elicited recurrent plateau depolarizations resembling in vivo up states, but only in the adult PFC (Tseng and O’Donnell, 2005). These depolarizations require a combination of intrinsic and synaptic mechanisms, including intracellular Ca2+, L-type Ca2+ channels, and PKA, as well as upregulation of voltage-gated Na+ channels. At a synaptic level, D1 activation also facilitates AMPA postsynaptic currents in superficial-layer pyramidal neurons and attenuates recurrent excitation presynaptically (Gao et al., 2001). Here, we did not observe a consistent D1 effect on deep-layer pyramidal neuron excitatory synaptic response to superficial layer stimulation. Although this negative finding is consistent with our previous report showing an absence of D1 modulation of AMPA-mediated excitation in the PFC (Tseng and O’Donnell, 2004), it remains to be determined whether D1 receptors may play a role in modulating excitatory synaptic responses in discrete ensembles of pyramidal neurons, particularly those enabled by a D1-NMDA coactivation (Tseng and O’Donnell, 2005). In fact, it has been recently shown that D1 receptor stimulation increases extrasynaptic GluR1 expression and subsequent NMDA activation is required to translocate AMPA receptors into synapses (Sun et al., 2005). Therefore, a D1 enhancement of AMPA synaptic events may only occur when NMDA function is enabled such as during periods of sustained depolarization or up states (Tseng and O’Donnell, 2005; Tseng et al., 2007). In any event, PFC circuits are normally affected by endogenous DA, which activates both D1 and D2 receptors. Thus, in addition to specific D1 or D2 effects the possibility exists for D1–D2 interactions that can shape the responses to DA. The regulation of PFC persistent activity by mesocortical DA has been associated with working memory and other executive functions (Goldman-Rakic et al., 2000; Horvitz, 2000; Jay, 2003; O’Donnell, 2003). It has been proposed that mesocortical DA increases the impact of behaviorally relevant information by attenuating irrelevant inputs to the PFC (O’Donnell, 2003). For example, mesocortical stimulation with trains of pulses mimicking DA cell burst firing typically elicits sustained membrane potential depolarization along with suppression of action potential firing in PFC pyramidal neurons (Lewis and O’Donnell, 2000). This characteristic inhibition in pyramidal cell activity is usually obtained in the absence of coincident excitatory inputs to the PFC and matches the temporal course of FSI excitation (Tseng et al., 2006), suggesting that part of the response to mesocortical stimulation could be mediated by local GABAergic circuitry. Both D1 and D2 receptors can exert a powerful excitatory effect on PFC GABAergic interneurons (Gorelova et al., 2002; Tseng et al., 2006; Tseng and O’Donnell, 2007). This modulation could influence the timing and spatial selectivity of ensembles of output neurons, probably by the D2- and GABA-mediated attenuation of excitatory inputs to deep-layer pyramidal neurons described here. In addition to the postpubertal emergence of a D1 enhancement of NMDA function (Tseng and O’Donnell, 2005), an increased impact of D2-dependent inhibition with the postpubertal acquisition of DA modulation of local GABAergic interneurons (Tseng and O’Donnell, 2004, 2007) may represent another important functional characteristic of the adult mesocortical system. These postpubertal changes may ultimately enhance the detection of relevant and salient signals through two concurrent events. A mature D2-GABA interaction may provide a more efficient mechanism to limit neuronal firing originated from asynchronous inputs, which in turn will facilitate relevant signals to drive specific ensembles of PFC pyramidal neurons into up states by virtue of a D1 enhancement of NMDA function (Lewis and O’Donnell, 2000; Tseng and O’Donnell, 2005). If these events were coincident with the arrival of strong excitatory inputs (e.g., from the hippocampus), the representation encoded in the ensemble of activated PFC neurons would be reinforced and action potential firing during up states would be enabled. Thus, the periadolescent maturation of the control of excitatory and inhibitory neuro-transmission by DA could be critical to fine-tuning PFC activity responsible for mature cognitive processes. Disruption of these complex modulations could lead to inappropriate PFC function, and this would become evident during or after late adolescence, as is the case for several deficits observed in schizophrenia (Carter et al., 1998; Lewis et al., 2004).
Acknowledgments
Contract grant sponsors: USPHS, MH57683, and NARSAD Independent Investigator Award (P.O’D).
References
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41:345–350. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: The effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Kelley AE. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci. 2002;22:1063–1071. doi: 10.1523/JNEUROSCI.22-03-01063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: Implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri N, Stanzione P, Stefani A, Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: Evidence for D1 receptor involvement. Neuroscience. 1987;20:757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Colwell CS, Itri JN, Chandler SH, Levine MS. Dopaminergic modulation of NMDA-induced whole cell currents in neostriatal neurons in slices: Contribution of calcium conductances. J Neurophysiol. 1998;79:82–94. doi: 10.1152/jn.1998.79.1.82. [DOI] [PubMed] [Google Scholar]
- Druzin MY, Kurzina NP, Malinina EP, Kozlov AP. The effects of local application of D2 selective dopaminergic drugs into the medial prefrontal cortex of rats in a delayed spatial choice task. Behav Brain Res. 2000;109:99–111. doi: 10.1016/s0166-4328(99)00166-7. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, Williams GV. D1 receptors in prefrontal cells and circuits. Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Deutch AY. Dopaminergic regulation of extracellular γ-aminobutyric acid levels in the prefrontal cortex of the rat. J Pharmacol Exp Ther. 1998;285:350–357. [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 1998;18:9139–9151. doi: 10.1523/JNEUROSCI.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Tassin J-P, Jay T. Integrity of the mesocortical dopaminergic system is necesary for complete expression of in vivo hippocampal-prefrontal cortex long-term potentiation. Neuroscience. 1999;94:1019–1027. doi: 10.1016/s0306-4522(99)00395-4. [DOI] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Echeagaray E, Starling AJ, Cepeda C, Levine MS. Modulation of AMPA currents by D2 dopamine receptors in striatal medium-sized spiny neurons: Are dendrites necessary? Eur J Neurosci. 2004;19:2455–2463. doi: 10.1111/j.0953-816X.2004.03344.x. [DOI] [PubMed] [Google Scholar]
- Hernández-López S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[β]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Jay TM. Dopamine: A potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Oak JN, Jackson MF, Perez Y, Orser BA, Van Tol HH, MacDonald JF. A D2 class dopamine receptor transactivates a receptor tyrosine kinase to inhibit NMDA receptor transmission. Neuron. 2002;35:1111–1122. doi: 10.1016/s0896-6273(02)00859-0. [DOI] [PubMed] [Google Scholar]
- Levine MS, Altemus KL, Cepeda C, Cromwell HC, Crawford C, Ariano MA, Drago J, Sibley DR, Westphal H. Modulatory actions of dopamine on NMDA receptor-mediated responses are reduced in D1A-deficient mutant mice. J Neurosci. 1996a;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MS, Li Z, Cepeda C, Cromwell HC, Altemus KL. Neuromodulatory actions of dopamine on synaptically-evoked neostriatal responses in slices. Synapse. 1996b;24:65–78. doi: 10.1002/syn.890240102. [DOI] [PubMed] [Google Scholar]
- Lewis BL, O’Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential ‘up’ states in pyramidal neurons via D1 dopamine receptors. Cerebral Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann NY Acad Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Res. 1994;634:105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Perez MF, White FJ, Hu XT. Dopamine D2 receptor modulation of K+ channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. J Neurophysiol. 2006;96:2217–2228. doi: 10.1152/jn.00254.2006. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: A tyrosine hydroxylase immunohistochemical study. Biol Psychiat. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA. 2001a;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001b;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: An electrophysiological strategy. Brain Cogn. 2004;55:116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Shen R-Y, Asdourian D, Chiodo LA. Microiontophoretic studies of the effects of D-1 and D-2 receptor agonists on type I caudate nucleus neurons: Lack of synergistic interaction. Synapse. 1992;11:319–329. doi: 10.1002/syn.890110407. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–10659. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA coactivation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O’Donnell P. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–417. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Snyder-Keller A, O’Donnell P. Dopaminergic modulation of striatal plateau depolarizations in corticostriatal organotypic cocultures. Psychopharmacology (Berlin) 2007;191:627–640. doi: 10.1007/s00213-006-0439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, O’Donnell P. D1 dopamine receptors potentiate NMDA-mediated excitability increase in rat prefrontal cortical pyramidal neurons. Cerebral Cortex. 2001;11:452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: Studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


