Summary
Human menopause is remarkable in that reproductive senescence is markedly accelerated relative to somatic aging, leaving an extended post-reproductive period for a large proportion of women [1, 2]. Functional explanations for this are debated [4-11], in part because comparative data from closely-related species are inadequate. Existing studies of chimpanzees are based on very small samples and have not provided clear conclusions about the reproductive function of aging females [12-19]. These studies have not examined whether reproductive senescence in chimpanzees exceeds the pace of general aging, as in humans, or occurs in parallel with declines in overall health, as in many other animals [20, 21]. In order to remedy these problems, we examined fertility and mortality patterns in 6 free-living chimpanzee populations. Chimpanzee and human birth rates show similar patterns of decline beginning in the 4th decade, suggesting that the physiology of reproductive senescence was relatively conserved in human evolution. However, in contrast to humans, chimpanzee fertility declines are consistent with declines in survivorship, and healthy females maintain high birth rates late into life. Thus, in contrast to recent claims [16], we find no evidence that menopause is a typical characteristic of chimpanzee life histories.
Results and Discussion
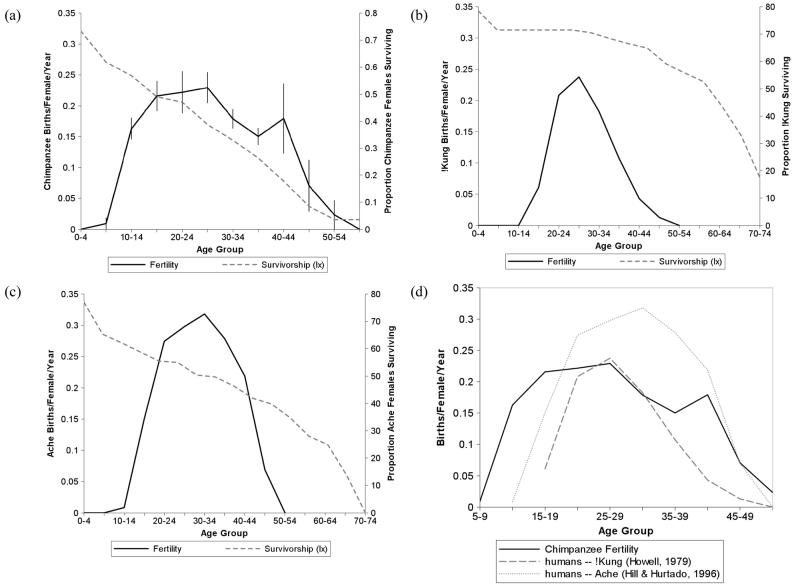
In this study we contrast age patterns of fertility in chimpanzees and humans. Our analysis focuses on two interrelated but differentiated processes: reproductive senescence, characterized by reduced reproductive performance with age; and menopause, characterized by species-typical patterns of reproductive senescence that significantly exceed the general aging trajectory and result in a post-reproductive life stage. We compared age-specific fertility patterns calculated from 534 chimpanzee births and 3416 female-risk-years with equivalent demographic data from two well-studied human foraging populations, the !Kung of Botswana [22] and the Ache of Paraguay [1]. In all datasets, age-specific fertility formed an inverted U-shape, characterized by lower birth rates at the beginning and end of the reproductive lifespan. Compared with humans, chimpanzees reproduced more broadly across the life cycle, experiencing an earlier onset of fertility (Fig. 1). Reproductive performance began to decline at a similar age group in chimpanzees and humans (25-35) and approached zero at approximately the same age (∼50). Peak fertility rates of chimpanzees were similar to the !Kung population, who have reduced fertility due to high incidence of secondary sterility [22], while Ache birth rates reached a markedly higher maximum. The slope of the age-related decline in chimpanzee fertility after age 25 was not significantly different from the hunter-gatherer populations (chimpanzees: −0.008; !Kung: −0.010, t = 1.280, p = 0.237; Ache: −0.013, t = 1.821, p = 0.106). These data support the hypothesis that the timing of human reproductive senescence has been largely conserved from our closest ancestors [2,7].
Fig 1.
Comparison of chimpanzee and human age-specific fertility and mortality patterns. (a) chimpanzee age-specific fertility (mean ± standard error of 6 populations) and female probability of surviving (lx) to end of each age class (5 wild populations only); (b) Dobe ! Kung hunter-gatherers, 1963-1973 [22]; (c) Ache hunter-gatherers, Forest Period [1]; (d) comparison plot of chimpanzee and human hunter-gatherer age-specific fertility. Age-specific fertility was calculated as the number of births as a fraction of risk years in each 5-year age interval. Intervals with ≤2 risk years in any population were excluded. All data are derived from true fertility and mortality rates rather than from model-fitted data.
Despite these similarities, the fertility patterns of chimpanzees and humans are markedly different when compared with each species' age-specific mortality patterns (Fig. 1). Fertility in chimpanzees declines at a similar pace to the decline in survival probability, while human reproduction nearly ceases at a time when mortality is still very low. This suggests that reproductive senescence in chimpanzees, unlike in humans, is consistent with the somatic aging process.
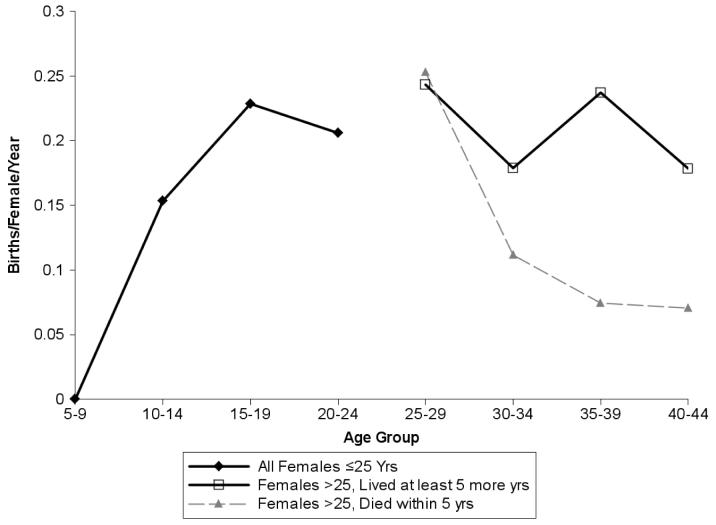
To further test this idea, we divided our age-specific fertility data for age classes over 25 into two subsets, with each birth and female-risk-year classified according to the subsequent survival of the female. The “unhealthy” subset contained risk years and births that occurred within 5 years of a female's death; the “healthy” subset consisted of data from females that lived an additional 5 or more years (an approximate chimpanzee birth interval). The cause of death is unknown in the majority of cases, thus this sample surely includes deaths from acute illness or violence [23], therefore underestimating the health-related differences in birth rate between the two groups. Even so, healthy individuals had higher fertility than unhealthy individuals (Wilcoxon signed-ranks test, Gombe: z=−1.924, N=19 years, p=0.054, Mahale: z=−2.172, N=18, p=0.030, Mean of two populations: z=−2.461, N=19, p=0.014). Healthy individuals did not have a significant age-specific decline in fertility (Fig. 2; R2=0.169, df=6 age groups ≥ 15, p=0.419), whereas unhealthy individuals reproduced less well at later ages (R2=0.760, df=6, p=0.023). This suggests that variance in somatic aging is systematically related to variance in reproductive senescence. Thus, these data are consistent with the hypothesis that the population-wide age decline in fertility reflects patterns of overall senescence, i.e., an increasing proportion of “unhealthy” individuals in older age groups.
Fig 2.
In females at or above the age of 25, healthy individuals had significantly higher fertility than females who died within five years of the birth or risk year considered. We used data for the two sufficiently-sampled long-term populations, Gombe and Mahale, and indicate the mean ± standard error of the two populations. Other populations have small samples but similar trends.
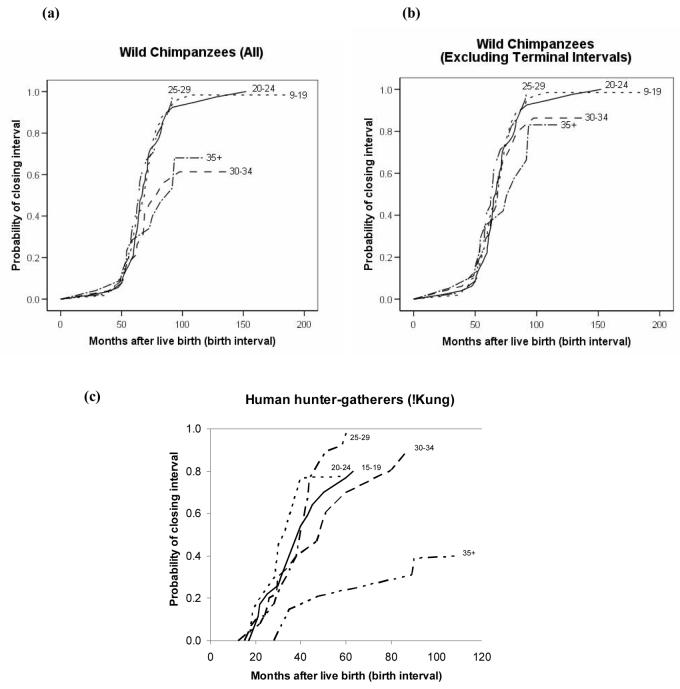
As a further test of age influences on reproduction, we investigated whether the duration of interbirth intervals increased with age by calculating a Cox proportional hazards model with an individual random effect to control for repeated intervals from the same individual. In this model, the length of interbirth intervals increased significantly with maternal age in chimpanzees (Fig. 3a; X2=28.2, df=l, p<0.0001). However, the effect size was very small (β = −0.05), particularly in relation to the age-related effect in humans (Fig. 3c). For chimpanzees, the individual effect of maternal identity was the strongest predictor of interbirth interval duration (X2=236.2, df=81, p<0.0001). As with the age-specific fertility analysis, we attempted to control for declining maternal health by eliminating birth intervals that culminated in maternal death. This substantially mitigated the effect, with healthy chimpanzees over 35 years of age experiencing a 20% decline in fertility, further supporting the hypothesis that fertility decline in chimpanzees is closely related to somatic senescence (Fig. 3b).
Fig 3.
Hazard for chimpanzee and human interbirth intervals according to the age of mother at the start of the interval. Lines reflect the probability of bearing a new infant at each time point when the previous infant has survived, (a) all wild chimpanzee birth intervals; (b) wild chimpanzee birth intervals, excluding intervals that terminated with the mother's death; (c) birth intervals of human hunter-gatherers in the Dobe !Kung population, adapted from Howell, 1979 [22].
Even with this combined dataset, the sample of females in the oldest age classes is low, given that only 7 percent of female chimpanzees born in the wild survive to age 40y. However, reproduction after the age of 40y occurred in all five wild chimpanzee populations. Thus, out of 34 mothers from the five sites that are estimated to have lived beyond the age of 40 (mean last age 45.3y), 47% subsequently produced at least one offspring, and 4 females (mean last age 51.3y) had two births after age 40y. Thus, we find little support in the wild data for the recent conclusion from captive studies that menopause occurs in chimpanzees at the age 35-40 years [16].
Our data on the reproductive patterns of wild chimpanzees help shed light on three aspects of the evolution of the human reproductive lifespan. First, age-specific fertility profiles of chimpanzees differ from those of humans. Chimpanzees had a wider, flatter profile than hunter-gatherer populations. This reflects in part the earlier maturity of chimpanzee females relative to humans [24]. In addition, the chimpanzee data do not indicate a strong peak in fertility, clear in the human datasets at approximately ages 25-35. This has several potential explanations. Ovarian hormone production in humans follows a very similar pattern, suggesting increased ability to conceive at these ages [25]. Available data suggest that estrogen levels do not change significantly with age in captive chimpanzees [16], but data for wild chimpanzees are not yet available. In addition, human mothers often care for more than one dependent offspring at a time, which may contribute to shortened birth intervals, particularly at ages when they are most likely to have living mothers or other relatives to assist with care [7, 10]; this is rare in chimpanzees [26]. Also, chimpanzees experience significant mortality in this age group, which may lead to substantial variance in reproductive function. However, even our data on ostensibly healthy chimpanzees does not suggest a significant peak in fertility within the reproductive lifespan.
Second, our data have implications for understanding the evolution of human menopause. Like recent data on follicular depletion in chimpanzees [17], age-specific fertility data show that reproductive senescence follows a similar time course in chimpanzees and humans. But while the decline in chimpanzee fertility mirrors declines in survival, humans experience an extended post-reproductive lifespan. Our findings thus support the hypothesis that the pattern of reproductive decline in both species has its origins in our last common ancestor, and that human evolution has resulted in an extended lifespan without complementary selection on extended reproduction [2, 4]. On the other hand, our data suggest that healthy chimpanzees maintain high fertility at ages when even healthy humans experience marked declines in reproductive function. This difference may provide some evidence that natural selection has slightly reduced the reproductive span of humans (or, less likely, extended that of chimpanzees). However, we have two caveats to this conclusion. Relatively few chimpanzees reach these oldest age classes, thus our sample size is still too small to draw strong conclusions about this difference. Additionally, later menopause has been linked with longevity in humans, suggesting that reproductive senescence might also be slower in relatively healthy humans [27-29]. Were there a similar relationship in chimpanzees, it could also mean that those chimpanzees left in the sample at late ages were those with particularly high fertility.
Finally, while some studies have emphasized an age-related decline in the fertility of captive chimpanzees as evidence of menopause, our examination of wild chimpanzees emphasizes the need to consider the general aging trajectory of the species in conjunction with reproductive senescence. In contrast to recent conclusions from captivity [16], we find no evidence to support the hypothesis that chimpanzees routinely experience menopause in the wild. On the contrary, our data on reproductive senescence conform to known differences in survivorship of individual animals. Somatic health has not been considered in previous studies on the fertility of captive chimpanzees, but may explain the very mixed results of these studies. Parallel contrasts are found between careful captive studies of aging gorillas, which support the occurrence of menopause [30], and wild studies, which do not [31].
Age declines in fertility are a common feature of mammalian life histories, and particularly among other primates, some older individuals cease reproducing years before death [20, 21, 32-37]. Likewise, some chimpanzees clearly do experience a short post-reproductive lifespan. However, we argue that this is not inconsistent with their generally slow reproductive schedule and the decline in general health that accompanies the aging process. While true menopause results when the supply of ovarian follicles is too depleted to sustain ovarian cycling [38, 39], other factors, such as inflammatory processes, likely also contribute to secondary infertility in some individuals [40]. These cannot be ruled out as causes of reproductive cessation in aging individuals. Additionally, evidence from other large-bodied mammals suggests that captive breeding schedules can artificially accelerate reproductive senescence; prolonged non-reproductive periods led to an increase in genital pathologies and accelerated follicular loss in captive elephants and rhinoceros [41-43]. Some studies have reported a correlation between contraceptive use or an increase in non-conceptive cycles and early menopause in humans [44-45], but this phenomenon has not yet been investigated in other primates.
The adaptive significance of human menopause, or post-reproductive lifespan, is still debated. This study provides greater evolutionary context to this debate by demonstrating that chimpanzees and humans experience a similar pace of reproductive senescence but that this pace does not exceed expectations from the overall somatic aging process in chimpanzees. These results, as well as recent data from wild gorillas [31] and orangutans [46] indicate that menopause is not a part of the life cycle of living apes and is a uniquely derived feature of humans.
Experimental Procedures
Study populations
Our subjects represent four wild populations of Pan troglodytes schweinjurthii in Gombe National Park (Tanzania), Mahale National Park (Tanzania), Kibale National Park (Uganda), and Budongo Forest (Uganda); one wild population of Pan troglodytes verus in Bossou, Guinea; and one free-ranging, provisioned population at the Chimpanzee Rehabilitation Project in the Gambia with reproductive parameters comparable to wild populations [47]. While these populations exhibit small differences in reproductive parameters (Table 1), variance in completed (birth to birth) interbirth intervals within each population was larger than the inter-population variance (F tests, all p<0.02). Thus, age-specific fertility patterns were comparable whether we weighed each geographic population equally or considered all females to be members of the same statistical population (Supplementary Online Material). In an additional analysis, we coded each female-risk year and birth according to whether the female (a) subsequently died within 5 years of the datapoint in question, or (b) lived an additional 5 years beyond that date, with indeterminate data (i.e., the most recent 5 years of living females) excluded. For ages over 25, we then calculated age-specific fertility rates separately and used a paired analysis to compare birth rates between “healthy” and “unhealthy” groups at each age.
Table 1.
Comparative data on reproduction in free-ranging chimpanzees
| N birth intervals |
N Females |
Median IBI (mos) ± S.E. after offspring death |
Median IBI (mos) ± S.E after surviving offspring |
Median IBI after surviving - Controlled by female |
Shortest completed interval |
Longest completed interval |
Youngest to give birth |
Oldest to give birth to surviving offspring |
|
|---|---|---|---|---|---|---|---|---|---|
| Gombe | 74/41 | 41 | 23.2 ± 2.5 | 67.4 ± 4.0 | 73.1 | 39.4 | 97.5 | 11.1 | 49.2* |
| Mahale | 116/58 | 62 | 26.6 ± 3.8 | 71.9 ± 2.0 | 69.0 | 44.1 | 132.0 | 12.0 | 44.0* |
| Kibale | 21/16 | 17 | 29.6 ± 8.1 | 79.1 ± 13.9 | 70.5 | 28.9 | 98.4 | 14.1 | 55.0* |
| Budongo | 13/17 | 17 | 37.1 ± 0.0 | 62.7 ± 3.0 | 63.8 | 57.5 | 83.7 | n/a | 40.6* |
| Bossou | 21/10 | 10 | 24.0 ± 0.4 | 63.9 ± 2.7 | 77.9 | 23.2 | 128.0 | 9.5 | 39.5* |
| Gambia | 43/25 | 25 | 29.6 ± 2.8 | 67.2 ± 5.8 | 73.4 | 21.7 | 110.7 | 12.6 | 32.7* |
| Composite | 288/173 | 165 | 26.6 ± 2.7 | 68.9 ± 1.2 | 70.6 | 21.7 | 132.0 | 9.5 | 55.0* |
|
Complete/ Censored |
Offspring died <4 years |
Offspring survived at least 4 years | Ages known | *Ages estimated | |||||
Female dispersal in chimpanzees precludes precise assignment of birth dates to most females in the study. The majority of females (N=300) were either first identified as juveniles or as young, nulliparous immigrants, thus we can be confident of their ages at least to within 5 years. Females who were first identified as mothers or older residents (N=55) were assigned ages based on their reproductive histories, including age and number of known or suspected offspring. Age estimates derived in this manner were typically conservative, though clues related to appearance are also used to rank the relative ages of individuals [48]. To include older females from all populations, we conducted analyses on the entire dataset for years of researcher presence. A more conservative analysis, excluding all females whose age estimates included a >5 year error, was also conducted with qualitatively similar results (Supplementary Online Material).
We compared chimpanzee data with the two available demographic datasets from human forager populations [1,22]. It should be noted that !Kung women were adversely impacted by infectious infertility; Howell [22] estimated that this may have lowered reproductive output by approximately 3% per year, though she concludes that this does not fully account for the relatively low fertility rates of the !Kung compared to other populations. This may or may not reflect a valid comparison with wild chimpanzees; therefore, curves of both available hunter-gatherer datasets are provided for comparison. Comparative data for Figure 3 are available only for the !Kung. However, because birth rates were lower throughout the life course for the !Kung relative to the Ache, data from the !Kung should provide accurate information on relative fertility in each age group. For Ache and chimpanzees, mortality data pertains to females specifically. Published data for !Kung mortality were available for the combined population only; this is a marginal overestimate because female mortality in this population was approximately 10-20% lower than male mortality depending on the age group [22].
Interbirth Intervals
Interbirth interval calculations were performed using Kaplan-Meier survival analyses. These analyses consider both completed birth intervals (i.e., those that conclude with a birth) and censored intervals (those that had not resulted in a birth by the date of study or the mother's death) in which the first infant survived to weaning. We considered only births with dates known to within one year (though more commonly within days or weeks). We also calculated a Cox proportional hazards model incorporating dependent variables of “infant survival to age 4” and “mother's age at start of interval”. To address non-independence of multiple intervals contributed by each female, we incorporated a gamma-frailty term [49] that specifies an individual-level multiplicative random effect on the fertility hazard with unit mean and variance estimated using a method of penalized likelihood [50]. The frailty term is essentially a latent trait which models additional variance in birth intervals not accounted for by the measured covariates. The model fit for 459 birth intervals was R2 = 0.606, Wald statistic = 206, df = 82.6, p < 0.0001.
Supplementary Material
Acknowledgments
We are indebted to the work of the African field assistants who maintain continuous data collections on chimpanzees at our study sites. Long term research was supported by: DFID (Bd), DNRST République de Guinée (Bo), Getty Foundation (Bd), Golden Ark (Ga), JGI (Go), JSPS-HOPE Program (Bo), Government of the Gambia (Ga), IPPL (Ga), JICA (M), LSB Leakey Foundation (K, Bd), MUBFS (K), MEXT Japan (M, Bo), Harris Steel Group (Go), N.G.S. (K, Bd), US NIH (Go), Tanzanian COSTECH (Go, M), NORAD (Bd), TANAPA (Go, M), TAWIRI (Go, M), UNCST (K, Bd), Uganda Forest Dept. (Bd), UWA (K, Bd), NSF (K, Go), University of Minnesota (Go), Wenner-Gren Foundation (K), and WWF (Ga). We thank Peter Ellison, Craig Packer, Kristen Hawkes, Martin Muller, Michael Cant, and several anonymous reviewers for valuable suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hill K, Hurtado AM. Ache Life History: The Ecology and Demography of a Foraging People. Aldine de Gruyter; New York: 1996. [Google Scholar]
- 2.Pavelka MSM, Fedigan LM. Menopause: a comparative life history perspective. Yearb. Phys. Anthropol. 1991;34:13–38. [Google Scholar]
- 3.Williams G. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 4.Alexander R. The evolution of social behavior. Annu. Rev. Ecol. Syst. 1974;5:325–383. [Google Scholar]
- 5.Hawkes K, O'Connell J, Blurton Jones N. Hardworking Hadza grandmothers. In: Standen V, Foley R, editors. Comparative Socioecology: The Behavioural Ecology of Humans and Other Mammals. Blackwell Scientific Publications; Oxford: 1989. pp. 341–366. [Google Scholar]
- 6.Finn CA. Reproductive ageing and the menopause. Int. J. Dev. Biol. 2001;45:613–617. [PubMed] [Google Scholar]
- 7.Hawkes K, O'Connell J, Blurton-Jones NG, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. P Natl. Acad. Sci.USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan H. The evolution of the human life course. In: Wachter K, Finch C, editors. Between Zeus and the Salmon: The Biodemography of Longevity. National Academy of Sciences; Washington, D.C.: 1997. pp. 175–211. [PubMed] [Google Scholar]
- 9.Hill K, Kaplan H. Life history traits in humans: theory and empirical studies. Annu. Rev. Anthropol. 1999;28:397–430. doi: 10.1146/annurev.anthro.28.1.397. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan H, Hill KR, Lancaster JB, Hurtado AM. Atheory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 2000;9:156–184. [Google Scholar]
- 11.Marlowe F. The patriarch hypothesis: an alternative explanation of menopause. Hum. Nature. 2000;11:27–42. doi: 10.1007/s12110-000-1001-7. [DOI] [PubMed] [Google Scholar]
- 12.Graham CE. Reproductive function in aged female chimpanzees. Am. J. Phys. Anthropol. 1979;50:291–300. doi: 10.1002/ajpa.1330500302. [DOI] [PubMed] [Google Scholar]
- 13.Littleton J. Fifty years of chimpanzee demography at Taronga Park Zoo. Am. J.Primatol. 2005;67:281–298. doi: 10.1002/ajp.20185. [DOI] [PubMed] [Google Scholar]
- 14.Roof KA, Hopkins WD, Izard MK, Hook M, Schapiro SJ. Maternal age, parity, and reproductive outcome in captive chimpanzees (Pan troglodytes) Am. J. Primatol. 2005;67:199–207. doi: 10.1002/ajp.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould KG, Flint M, Graham CE. Chimpanzee reproductive senescence: A possible model for evolution of the menopause. Maturitas. 1981;3:157–166. doi: 10.1016/0378-5122(81)90007-4. [DOI] [PubMed] [Google Scholar]
- 16.Videan EN, Fritz J, Heward CB, Murphy J. The effects of aging on hormone and reproductive cycles in female chimpanzees (Pan troglodytes) Comparat. Med. 2006;56:291–299. [PubMed] [Google Scholar]
- 17.Jones K, Walker L, Anderson D, Lacreuse A, Robson SL, Hawkes K. Depletion of ovarian follicles with age in chimpanzees: similarities to humans. Biol. Reprod. 2007;77:247–251. doi: 10.1095/biolreprod.106.059634. [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama Y. Demographic parameters and life history of chimpanzees at Bossou, Guinea. Am. J. Phys. Anthropol. 2004;124:154–165. doi: 10.1002/ajpa.10345. [DOI] [PubMed] [Google Scholar]
- 19.Nishida T, Corp N, Hamai M, Hasegawa T, Hiraiwa-Hasegawa M, Hosaka K, Hunt KD, Itoh N, Kawanaka K, Matsumoto-Oda A, et al. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. Am. J. Primatol. 2003;59:99–121. doi: 10.1002/ajp.10068. [DOI] [PubMed] [Google Scholar]
- 20.Packer C, Tatar M, Collins A. Reproductive cessation in female mammals. Nature. 1998;392:807–811. doi: 10.1038/33910. [DOI] [PubMed] [Google Scholar]
- 21.Moss CJ. The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya. Journal of Zoology, London. 2001;255:145–156. [Google Scholar]
- 22.Howell N. Demography of the Dobe! Kung. Aldine de Gruyter; New York: 1979. [Google Scholar]
- 23.Wrangham RW, Wilson ML, Muller MN. Comparative rates of violence in chimpanzees and humans. Primates. 2006;47:14–26. doi: 10.1007/s10329-005-0140-1. [DOI] [PubMed] [Google Scholar]
- 24.Hill K. Life history theory and evolutionary anthropology. Evolutionary Anthropology. 1993;2:78–88. [Google Scholar]
- 25.Ellison PT, Panter-Brick C, Lipson SF, O'Rourke MT. The ecological context of human ovarian function. Hum. Reprod. 1993;8:2248–2258. doi: 10.1093/oxfordjournals.humrep.a138015. [DOI] [PubMed] [Google Scholar]; Hill K. Life history theory and evolutionary anthropology. Evol. Anthropol. 1993;2:78–88. [Google Scholar]
- 26.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Belknap Press; Cambridge: 1986. [Google Scholar]
- 27.Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16:556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- 28.Amagai Y, Ishikawa S, Gotoh T, Kayaba K, Nakamur Y, Kajii E. Age at menopause and mortality in Japan: the Jichi Medical School cohort study. J. Epidemiol. 2006;16:161–166. doi: 10.2188/jea.16.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong JS, Yi S, Kang HC, Kang HG, Bayasgalan G, Ohrr H. Age at menopause and cause-specific mortality in South Korean women: Kangwha cohort study. Maturitas. 2006;56:411–419. doi: 10.1016/j.maturitas.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Atsalis S, Marguiles SW. Sexual and hormonal cycles in geriatric Gorilla gorilla gorilla. International Journal of Primatology. 2006;27:1664–1685. [Google Scholar]
- 31.Robbins AM, Robbins MM, Gerald-Steklis N, Steklis HD. Age-related patterns of reproductive success among female mountain gorillas. American Journal of Physical Anthropology. 2006;131:511–521. doi: 10.1002/ajpa.20474. [DOI] [PubMed] [Google Scholar]
- 32.Berube CH, Festa-Bianchet M, Jorgenson JT. Individual differences, longevity, and reproductive senescence in bighorn ewes. Ecology. 1999;80:2555–2565. [Google Scholar]
- 33.Schwartz CC, Keating KA, Reynolds Harry V., III, Barnes Victor G., Jr., Sellers RA, Swenson JE, Miller SD, McLellan BN, Keay J, McCann R, et al. Reproductive maturation and senescence in the female brown bear. Ursus. 2003;14:109–119. [Google Scholar]
- 34.Henriksen HB, Anderson R, Hewison A, Gaillard J, Bronndal M, Jonsson S, Linnell JD, Odden J. Reproductive biology of captive female Eurasian lynx, Lynx lynx. Eur. J. Wildlife Res. 2005;51:151–156. [Google Scholar]
- 35.Harley D. Aging and reproductive performance in langur monkeys (Presbytis entellus) Am. J. Phys. Anthropol. 1990;83:253–261. doi: 10.1002/ajpa.1330830213. [DOI] [PubMed] [Google Scholar]
- 36.Strum SC, Western JD. Variations in fecundity with age and environment in olive baboons (Papio anubis) Am. J. Primatol. 1982;3:61–76. doi: 10.1002/ajp.1350030106. [DOI] [PubMed] [Google Scholar]
- 37.Fedigan L. Life span and reproduction in Japanese macaque females. In: Fedigan LM, Asquith PJ, editors. The Monkeys of Arashiyama: Thirty-Five Years of Research in Japan and the West. State University of New York Press; New York: 1991. pp. 140–154. [Google Scholar]
- 38.Faddy M, Gosden R, Gougeon A, Richardson S, Nelson J. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum. Reprod. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 39.Faddy M, Gosden R. A model conforming the decline in follicle numbers to the age of menopause in women. Hum. Reprod. 1996;11:1484–1486. doi: 10.1093/oxfordjournals.humrep.a019422. [DOI] [PubMed] [Google Scholar]
- 40.Wood JW. Dynamics of Human Reproduction: Biology, Biometry, Demography. Aldine de Gruyter; New York: 1994. [Google Scholar]
- 41.Hildebrandt T, Goritz F, Pratt NC, Brown JL, Montali RJ, Schmitt DL, Fritsch G, Hermes R. Ultrasonagraphy of the urogenital tract in elephants (Loxodonta africana and Elephas maximus): an important tool for assessing female reproductive function. Zoo Biol. 2000;19:321–333. [Google Scholar]
- 42.Hermes R, Hildebrandt T, Goritz F. Reproductive problems directly attributable to long-term captivity -- asymmetric reproductive aging. Anim. Reprod. Sci. 2004;82-83:49–60. doi: 10.1016/j.anireprosci.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Hermes R, Hildebrandt TB, Walzer C, Goritz F, Patton ML, Silinski S, Anderson MJ, Reid CE, Wibbelt G, Tomasova K, et al. The effect of long non-reproductive periods on the genital health in captive female white rhinoceroses (Ceratotherium simum simum, C.s. cottoni) Theriogenology. 2006;65:1492–1515. doi: 10.1016/j.theriogenology.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Cramer DW, Xu H, Harlow BL. Gynecology: does “incessant” ovulation increase risk for early menopause? American Journal of Obstetrics and Gynecology. 1995;172:568–573. doi: 10.1016/0002-9378(95)90574-x. [DOI] [PubMed] [Google Scholar]
- 45.Sowers MR, La Pietra MT. Menopause: its epidemiology and potential association with chronic disease. Epidemiologic Reviews. 1995;17:287–302. doi: 10.1093/oxfordjournals.epirev.a036194. [DOI] [PubMed] [Google Scholar]
- 46.Wich SA, Utami-Atmoko S, Mitra Setia T, Rijksen H, Schumann C, van Hooff JA, van Schaik CP. Life history of wild Sumatran orangutans (Pongo abelii) J. Hum. Evol. 2004;47:385–398. doi: 10.1016/j.jhevol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Brewer-Marsden S, Marsden D, Emery Thompson M. Demographic and female life history parameters of free-ranging chimpanzees at the Chimpanzee Rehabilitation Project, River Gambia National Park. Int. J. Primatol. 2006;27:391–410. [Google Scholar]
- 48.Hill K, Boesch C, Goodall J, Pusey AE, Williams J, Wrangham RW. Mortality rates among wild chimpanzees. J. Hum. Evol. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- 49.R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2004. [Google Scholar]
- 50.Therneau T, Grambash P. Modeling survival data: extending the Cox model. Springer; New York: 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.