Abstract
The rabbit lacrimal gland undergoes an immunophysiological transformation during pregnancy, reminiscent of that of the mammary gland as it prepares to deliver secretory IgA into the nascent fluid product. The contents of TGF-β and prolactin (PRL) within ductal epithelial cells increase, and their primary localizations shift from the apical- to the basal cytoplasm, suggesting a transformation from exocrine- to paracrine secretion. Studies with ex vivo acinar cell models demonstrated that elevated PRL suppresses traffic of secretory proteins into the regulated exocrine apparatus and directs them into a novel, induced, regulated paracrine apparatus (Wang et al., Am. J. Physiol. 292, E1122-E1134, 2007). However, it was not clear whether PRL itself entered the induced paracrine apparatus. In the present study, confocal immunofluorescence microscopy revealed that natively-expressed PRL and over-expressed PRL co-localized with PRL receptors (PRLR); rab11, a marker for the recycling endosome; γ-adaptin, a marker for the Golgi complex and trans-Golgi network; and rab7, a marker for the autophagic - lysosomal apparatus. Natively expressed, over-expressed, and endocytosed PRL also co-localized with rab4 and rab5A, markers for the early endosome, and with rab3D, a marker for regulated exocrine secretory vesicles. Endocytosed PRL was stored in intact form and released in response to stimulation with carbachol. Subcellular fractionation analysis detected relative excesses of PRL over PRLR in fractions that contained fragments of the recycling endosome and fractions that contained both secretory vesicle fragments and pre-lysosomal- and autolysosomal fragments. EM-gold microscopy demonstrated PRL within small vesicles, consistent with endosomes or secondary lysosomes, and in large vesicles, consistent with regulated secretory vesicles. The secretory vesicles were preponderantly localized in the apical cytoplasm of control cells, and in the basal cytoplasm of PRL-over-expressing cells. These results indicate that when lacrimal epithelial cells synthesize PRL, and when they endocytose it from their ambient medium, they traffic it both into the endosomes that constitute the constitutive transcytotic - paracrine apparatus and also into regulated secretory vesicles, which are associated with the exocrine apparatus at low PRL levels and with the induced paracrine apparatus at high PRL levels.
INTRODUCTION
The lacrimal gland and tissues that comprise the ocular surface function in concert to maintain the constancy of the fluid film bathing the cornea and conjunctiva. This fluid represents a microenvironment, a physiologically-regulated milieu extérieur, which lubricates the lid-globe interface and buffers the superficial epithelia from the external environment (Mircheff et al., 2005, Mircheff et al., 2007). Failure to maintain homeostasis leads to sensations of dryness, itching, or burning; inflammation; vulnerability to abrasions and infections; and degradation of the visual image.
Like other tissues at the moist interfaces between the body and the external environment, i.e., the mucosae, the ocular surface system uses secretory IgA (sIgA) as an immunospecific barrier that prevents microbial infection (Kaetzel et al., 1991). IgA+ plasmacytes populating the lacrimal gland produce most of the dimeric IgA (dIgA) that appears in the ocular surface fluid (Franklin et al., 1973, Allansmith et al., 1976, Franklin et al., 1979). Epithelial cells in the mucosal immune system’s effector organs employ a common transcytotic apparatus, which uses the polymeric immunoglobulin receptor (pIgR) to internalize dIgA from the underlying tissue space and deliver sIgA into the nascent fluid secretion (Kuhn and Kraehenbuhl, 1979, Mostov and Deitcher, 1986). They also employ a paracrine apparatus to secrete paracrine signaling mediators and create specialized niches that recruit IgA-expressing plasmablasts (Tanneau, 1999, Hargreaves et al., 2001, Nakayama et al, 2003) and support their maturation and survival as dIgA-secreting plasmacytes (McGee et al., 1992, Palkowetz et al., 1994, Takahashi et al., 1997, Beagley et al., 1998). (A cellular model illustrating the various membrane traffic apparatus in lacrimal acinar cells is presented in Fig. 7).
Figure 7. Cellular model for the PRL traffic in lacrimal acinar cells.
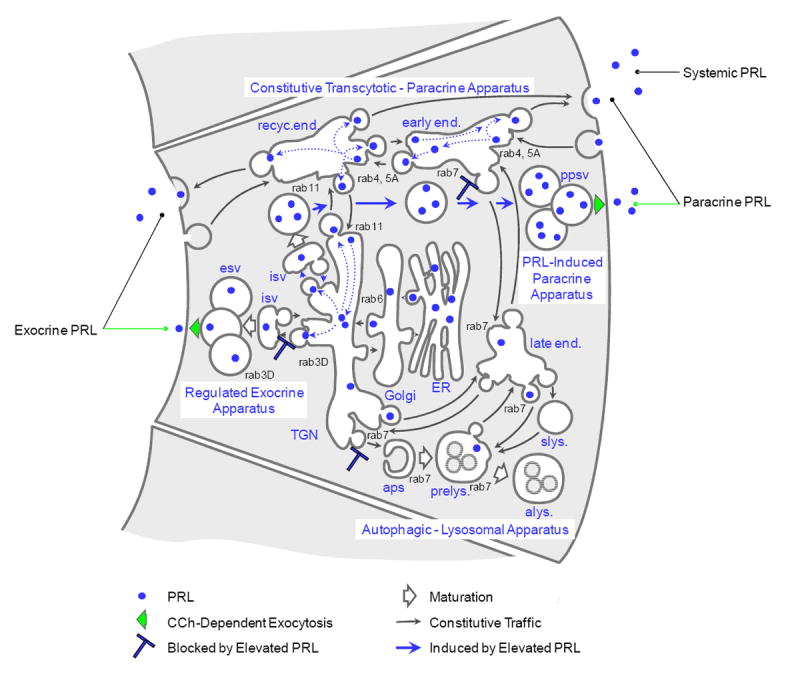
The major traffic apparatus diverge from, and reconverge with, the biosynthetic apparatus, i.e., the endoplasmic reticulum (ER) and Golgi complex, at the trans-Golgi network (TGN), the cell’s major sorting nexus. The constitutive transcytotic - paracrine apparatus consists of the recycling endosome (recyc.end.) and early endosome (early end.). The apparatus for regulated exocrine secretion of proteins consists of the immature secretory vesicle (isv) and mature secretory vesicles (esv). The apparatus for autophagy and degradation of proteins, lipids, and carbohydrates consists of the autophagosome (aps), pre-lysosome (prelys.), and autolysosome (alys), as well as the late endosome (late end.) and storage lysosome (slys.). Whether endocytosed from the ambient medium or emerging from the biosynthetic apparatus, PRL traffics through the TGN to the immature secretory vesicles as well as to the endosomes. Elevated levels of PRL suppress expression of the regulated exocrine apparatus and induce expression of the regulated paracrine apparatus, which consists of the immature secretory vesicle and paracrine secretory vesicles (ppsv). In reducing the cell’s content of rab7, elevated PRL also suppresses traffic into the autophagic - lysosomal apparatus.
As reviewed elsewhere (Mircheff et al., 2007), information is emerging that both acinar- and ductal epithelial cells in the lacrimal gland contribute to the local signaling milieu by secreting immunomodulatory cytokines and growth factors. Our working hypothesis has been that lacrimal epithelial cells’ constitutive paracrine secretory apparatus is to a large extent identical to their transcytotic apparatus. As newly-synthesized products emerge from the common biosynthetic apparatus of the endoplasmic reticulum, Golgi complex, and trans-Golgi network (TGN) (Farquhar, 1981), they are directed into a network of endomembrane compartments, i.e., the early endosome and apical recycling endosome, which are linked to each other and the plasma membranes by populations of transport vesicles, and which comprise a constitutive, transcytotic - paracrine apparatus. As transport vesicles that formed in the endosomes fuse with the basal-lateral plasma membrane to insert pIgR, they also exocytotically secrete cytokines and growth factors.
Some organs of the mammalian mucosal immune system are subject to pronounced gender-related dimorphisms; the prostate is unique to males; the vagina and uterus unique to females; the mammary gland well-developed in sexually mature females and undeveloped in males. The lacrimal gland exhibits somewhat more subtle dimorphisms, which pertain to size of acinar cells, capacity for secretion of electrolytes and fluid, size of the population of IgA+ plasmacytes, level of pIgR expression, and rates of secretion of sIgA and the pIgR secretory component (SC). In most species and strains that have been studied, females are more susceptible to age-related atrophy and to autoimmune processes that impair lacrimal fluid production. The glandular dimorphisms are thought to be maintained by the higher levels of androgens in males (Sullivan and Hann, 1989, Gao et al, 1995). However, it has been proposed that even the relatively lower androgen levels present in pre-menopausal females critically support maintenance of the glandular parenchyma (Schönthal, 2000) and expression of its mechanisms for secreting electrolytes and fluid (Azzarolo et al., 1993, Azzarolo et al., 1995, Azzarolo et al., 1997, Warren, 1994), as well as its ability to avoid autoimmune disease (Ariga et al, 1989, Sullivan and Sato, 1992, Mircheff et al., 1996). In contrast, estrogen replacement therapy is associated with increased occurrence and severity of dry eye symptoms in menopausal women; this finding has been interpreted as evidence that estradiol influences the lacrimal gland adversely (Schaumberg et al., 2001).
Prolactin (PRL), which plays a multiplicity of roles, primarily as a protein hormone produced by the pituitary, but also as a paracrine mediator produced by diverse tissues (including lacrimal epithelia), appears to be an additional factor influencing the lacrimal gland and ocular surface system. Serum PRL levels do not differ markedly between males and non-pregnant, non-lactating females (Kratz et al, 2004). However, in pregnant women they increase 10- to 15-fold over the course of gestation (Rigg et al., 1977), when PRL interacts with estradiol and progesterone to induce differentiated mammary epithelial functions in preparation for lactation, including synthesis of both pIgR and mediators that support IgA+ plasmacyte recruitment, differentiation, and survival (Weisz-Carrington et al., 1978, Rosato et al., 1995, Rincheval-Arnold et al., 2002). Physiological studies with rabbits indicate that the lacrimal gland’s transcytotic - paracrine apparatus, regulated apparatus for exocrine secretion of tear proteins, and regulated apparatus for secretion of electrolytes and fluid, undergo a constellation of changes during pregnancy (Schechter et al., 2000). In term pregnant rabbits, the basal rate of fluid production decreases by half; while the rate of fluid production under pilocarpine stimulation increases, and the concentration of protein in pilocarpine-induced fluid decreases (Ding et al, 2006). The contents of epidermal growth factor (EGF), transforming growth factor beta (TGF-β), and PRL increase within ductal epithelial cells. These mediators’ polarized subcellular distributions reverse. In the non-pregnant state, they are preferentially localized in the apical cytoplasm, consistent with exocrine secretion; during pregnancy, localization undergoes a shift toward the basal cytoplasm, consistent with a primarily paracrine mode of secretion. In accord with the hypothesis that the epithelium has undergone a functional transformation, the concentrations of mediators in pilocarpine-induced fluid decrease markedly. The cellular physiological changes are accompanied by a pronounced immunoarchitectural transformation, in which infiltrating lymphocytes redistribute away from periductal- and perivenular aggregates and accumulate in the interacinar space. Preliminary data suggest that the number of IgA+ cells and total content of IgA in the gland increase, while the number of IgG+ cells and total content of IgG decrease (Mircheff et al., 2006). The immunophysiological transformation in some ways mimics the immunophysiological transformation the mammary gland undergoes during pregnancy, and we infer that it is mediated by the cellular physiological transformation. Studies with ex vivo acinar cell models from rabbit lacrimal gland suggest that PRL is responsible for orchestrating certain aspects of the pregnancy-associated cellular physiological transformations. Elevated levels of recombinant rabbit PRL, either heterologously expressed and added to the culture medium, or over-expressed by transduction with an adenovirus vector (AdPRL), cause secretory vesicles to redistribute from the apical- to the basal cytoplasm and to exocytose their contents at the basal-lateral plasma membrane in response to stimulation with carbachol (Wang et al., 2007). This result suggests that elevated PRL, as a hormone, and also as an intracrine - autocrine mediator, suppresses expression of the regulated exocrine apparatus and induces expression of a novel, regulated paracrine apparatus.
We infer that the physiological rationale for PRL’s actions in the lacrimal gland parallels the role PRL plays in the mammary gland as it enhances expression of the transcytotic - paracrine apparatus delivering sIgA into the milk. Since PRL is both a regulator of lacrimal cell physiology and potentially also a central factor determining the function of the immune cells that populate the gland, we wished to learn how it influences its own traffic through the constitutive transcytotic - paracrine apparatus, the regulated exocrine apparatus, and the regulated paracrine apparatus which it induces.
MATERIALS AND METHODS
Reagents
Carbamylcholine (carbachol, CCH), rhodamine-phalloidin, and goat anti-mouse secondary antibody conjugated to FITC were purchased from Sigma Chemical Co. (St. Louis, MO). Matrigel was from Collaborative Biochemicals (Bedford, MA). HepatoStim® Culture Medium (HSM) was obtained from Becton Dickinson (Bedford, MA). HyQ® HAM’S/F-12 culture medium was from HyClone (Logan, Utah). DMEM was from Mediatech, Inc. (Herndon, VA). Other cell culture reagents were from Life-Technologies. Matrisperse Cell Release Solution was purchased from Becton Dickinson (Franklin Lakes, NJ).
Guinea pig anti-rabbit-PRL antibody was purchased from Dr. Albert F. Parlow of the National Hormone and Peptide Program (NHPP, Torrance, CA). Normal guinea pig serum was from Antibodies, Inc. (Davis, CA). Mouse monoclonal anti-γ-adaptin antibody was from Transduction Laboratories (Lexington, KY). Polyclonal antibody to rab7 was from CytoSignal (Irvine, CA). Polyclonal antibody to rab11 was from Zymed Laboratories, Inc. (South San Francisco, CA). Polyclonal antibody to rab5a was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyclonal antibody to rab4 was from Stressgen Biotechnologies (Victoria, British Columbia, Canada). Polyclonal antibody to rab3D was generated in rabbits against recombinant rab3D produced in E. coli (Antibodies, Inc., Davis CA) and purified by chromatography over protein A/G Agarose. Mouse monoclonal antibody to the prolactin receptor was from Affinity BioReagent Inc (Golden, CO).
IRDye™800-conjugated secondary antibodies (goat anti-mouse, and goat anti-guinea pig) were purchased from Rockland Immunochemicals (Gilbertsville, PA). ProLong antifade mounting media and goat anti-rabbit secondary antibody conjugated to Alexa Fluor® 568 were from Molecular Probes (Eugene, OR). Donkey anti-guinea pig IgG conjugated with FITC was from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Paraformaldehyde was from Polysciences (Warrington, PA). All other chemicals were reagent grade and were obtained from standard suppliers.
Generation of vectors and heterologous expression of PRL
Previous publications describe the construction, as well as the production and cesium chloride purification of AdPRL [Wang et al. 2007], AdGFP, and AdLacZ [Wang et al., 2003].
Cell isolation and culture
Female New Zealand white rabbits weighing approximately 2 kg or 4 kg were obtained from Irish Farms (Norco, CA). They were used in accord with the Guiding Principles for the Use of Animals in Research, with a protocol approved by the Institutional Animal Care and Use Committee. Lacrimal gland acinar cells were isolated and cultured as described previously [Gierow et al., 1996; Hamm-Alvarez et al., 1997; Schechter et al., 2002]. For subcellular fractionation and functional studies, the cells from 4 kg rabbits were cultured in Matrigel® “rafts.” For immunofluorescence staining, cells from 2 kg rabbits were seeded onto 18 mm circular glass coverslips coated with Matrigel® and placed in 12 well plates. In co-culture experiments, non-transduced cells were placed on Matrigel®-coated coverslips in the wells of 12 well plates, and AdPRL -transduced cells were placed in Matrigel®-coated microporous culture inserts.
Cells were cultured for 3 or 4 days before analysis. For gene transduction, adenoviral constructs were added to 2-day cultures at a MOI of 6 pfu / cell and incubated 37°C for 1 to 2 hr. Virus was removed by washing three times with PBS. Cells were used 24 hr after infection, i.e., on day 3, for immunofluorescence staining and 48 hr after infection, i.e., on day 4, for secretion and subcellular fractionation studies. In co-culture experiments, non-transduced cells were grown in Matrigel® coated 12-well plates (2 × 106 per well), and AdPRL-transduced cells were placed in Matrigel®-coated microporous culture inserts for 18 hours. After removal of the inserts, cells in the wells were washed twice with PBS, then maintained in fresh PCM with or without 100 μM CCh for 0, 1, 3 and 5 hours. Collected supernatant culture media were concentrated ten times by centrifugation with 10 kDa cut-off Centricon centrifugal filter devices.
Western blotting
Aliquots of cell lysates, subcellular fractions, and concentrated supernatant culture media were analyzed by SDS polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes, blocked with Odyssey blocking buffer (LiCor Biosciences) at room temperature for 1 hr, then incubated with the appropriate primary antibody for 1- to 2 hr at room temperature or overnight at 4° C. After five 15 min washes with TBST, the blots were incubated with an appropriate secondary antibody conjugated to IRDye ™800 or Alexa Fluor® 680. After extensive washing with TBST, the blots were scanned with an Odyssey Scanning Infrared Fluorescence Imaging System (LiCor, Lincoln, Nebraska) and quantified using proprietary software supplied by the manufacturer.
Confocal fluorescence microscopy
For analysis of the distribution of PRL immunoreactivity in parallel with actin filaments or other intracellular markers, cells were rinsed with PBS, then either fixed and permeabilized with ethanol at −20°C for 10 min before rehydration in PBS as previously described [Wang et al., 2003] or fixed with 4% paraformaldehyde at room temperature for 15 min before permeabilization with 0.25% Triton X-100 at room temperature for 5- to10 min. Samples were blocked with 1% bovine serum albumin (BSA), then incubated with appropriate primary and FITC-conjugated or Alexa Fluor® 568-conjugated secondary antibodies and rhodamine-conjugated phalloidin. Images were acquired on a Nikon PCM Confocal System equipped with Argon ion (488 nm) and HeNe (543 nm) lasers attached to a Nikon TE300 Quantum inverted microscope. The immunofluorescence micrographic images were compiled in Adobe Photoshop 7.0 (Adobe Systems Inc, Mountain View, CA).
Electron Microscopy
Non-transduced- and AdPRL-transduced rafts were sedimented by centrifugation at 2,000 × g × 8 min. After removal of the supernatant, the rafts were fixed and processed as previously described (Schechter et al. 2002). The fixative was 2% formaldehyde, 2% glutaraldehyde, 0.02% calcium chloride, 0.1M cacodylate buffer, pH 7.4, with a small drop of methylene blue added to help in visualizing the pellet. After fixation for 1 hour, the pellet was rinsed in cacodylate buffer, and post-fixed in 1% osmium tetroxide, 0.02% calcium chloride, 0.1M cacodylate buffer, pH 7.4 for an additional hour. The pellets were then buffer rinsed and dehydrated in a graded series of ethanol rinses, and infiltrated and embedded in LR White. Thin sections were collected for electron microscopy on nickel grids and processed for immunogold localization with guinea pig anti-rabbit-PRL antibody and anti-guinea pig gold conjugate, 10 nm, (Aurion, Hatfield, PA). Electron microscopic sections were stained with uranyl acetate and lead citrate and viewed with a JEOL 1011 TEM.
Subcellular analysis
Acinar cells were released from Matrigel rafts by incubation with Matrisperse™ Cell Release Solution for 1 hr on ice. After washing with PBS, cells were resuspended in 2 ml of ice-cold 5% sorbitol cell lysis buffer with water-soluble protease inhibitors, then lysed by 10 passages through a 20-gauge syringe needle followed by 60 passages through a Balch cell press (H & Y Enterprises, Redwood City, CA, USA). Lysates were analyzed by differential sedimentation and isopycnic density gradient centrifugation as described previously [Gierow et al. 1996, Hamm-Alvarez et al., 1997, Xie et al. 2004, Rose et al. 2005). As in the previous studies, Western blot signals and biochemical marker contents were expressed as percentages of the totals recovered in the 13 density gradient fractions, and, to account for possible differences in total marker and protein contents between reference and experimental gradients, the total marker contents were normalized to the total protein contents according to the following equation:
RESULTS
The confocal immunofluorescence micrographs in Fig. 1 reveal different levels of immunopositivity but similar punctate- and somewhat reticular distributions of PRL in non-transduced cells, AdPRL-transduced cells, and non-transduced cells that had been incubated in media containing recombinant rPRL. Some accumulations of PRL, indicated by arrows, can be detected in the cells that had been exposed to rPRL.
Figure 1. Localization of PRL in non-transduced cells, AdPRL-transduced cells, and non-transduced cells maintained in the presence of recombinant rabbit PRL.
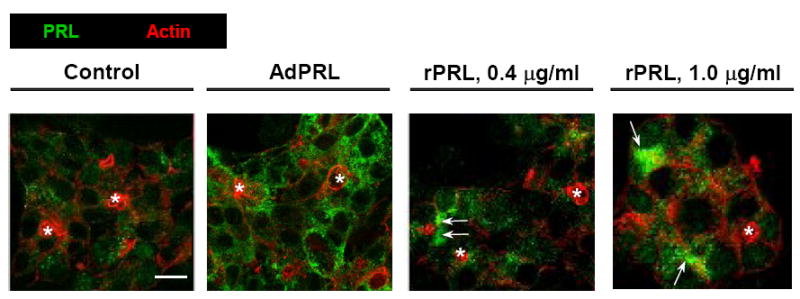
Control and AdPRL-transduced lacrimal acini grown on Matrigel® coated coverslips were fixed with 4% paraformaldehyde, then permeabilized with 0.25% Triton X-100 . They were stained for PRL with guinea pig anti-prolactin antibody followed by a donkey anti-guinea pig IgG secondary antibody conjugated to FITC (green). Acini were stained in parallel with rhodamine phalloidin (red) to mark the actin filaments underlying the apical- and basal-lateral membranes. (*, acinar lumena; bar, 10 μm.)
Confocal immunofluorescence microscopy was then used to examine the co-localizations of PRL with γ-adaptin, a marker for the Golgi complex and trans-Golgi network (TGN); rab11, a marker for recycling endosomes; and rab7, an effector of traffic from the TGN through the late endosome and pre-lysosome to the autolysosome. Fig. 2 shows that PRL was co-localized with all three markers in non-transduced- and AdPRL-transduced cells. These co-localizations were more intense in the AdPRL-transduced cells (arrows), but both over-expressed PRL and natively-expressed PRL were present in the biosynthetic compartments, the recycling endosome, and compartments of the autophagic - lysosomal apparatus.
Figure 2. Co-localizations of PRL with rab11, rab7, and γ-adaptin in control and AdPRL transduced cells.
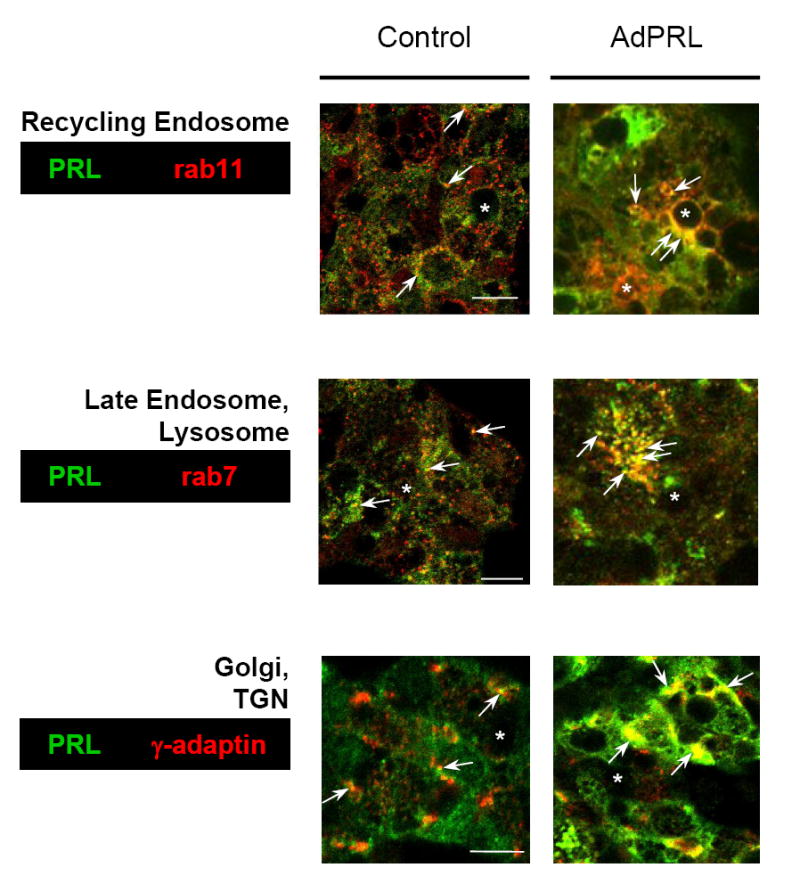
Control and AdPRL-transduced acini grown on Matrigel® coated coverslips were fixed and permeabilized with ethanol at −20°C (for rab7 and rab 11) or were fixed with 4% paraformaldehyde followed by permeabilization with 0.25% Triton X-100 (for γ-adaptin) then stained for PRL with guinea pig anti-prolactin antibody and rabbit anti-rab 7 or rab 11 or mouse anti-γ-adaptin antibody followed by FITC-conjugated donkey anti-guinea pig secondary antibody and rhodamine-conjugated goat anti-rabbit or mouse secondary antibody. In order to clearly visualize the co-localizations of PRL with rab11 and rab7 in AdPRL-transduced acini, unconjugated donkey anti-guinea pig IgG antibody was added to the FITC-conjugated donkey anti-guinea pig IgG secondary antibody at a 5: 1 ratio (*, apical/luminal regions; bar, 10 μm.)
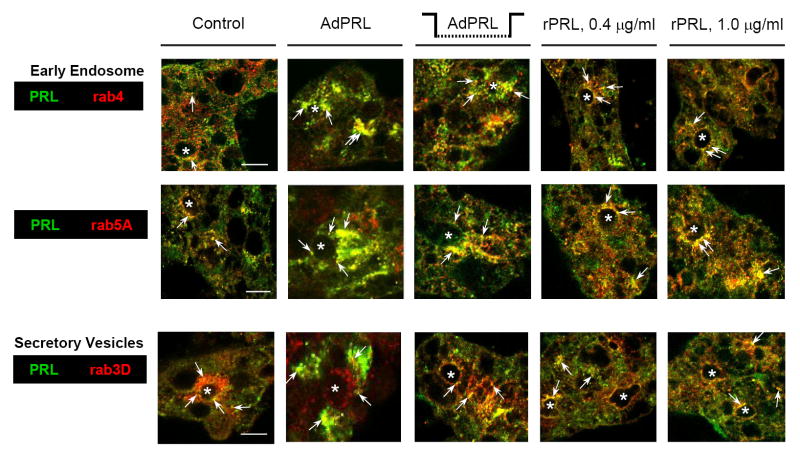
Additional studies examined the co-localizations of PRL with rab4 and rab5A, markers for the early endosome, and rab3D, a marker for mature secretory vesicles, in non-transduced cells, cells that had been transduced with AdPRL, cells that had been co-cultured with AdPRL-transduced cells, and cells that had been grown in the presence of recombinant PRL. The images in Fig. 3 reveal partial co-localization of PRL with rab3D, and they confirm the previously-reported observation that elevated levels of PRL disperse rab3D from its normal concentration in the apical cytoplasm (Wang et al., 2007). The images in Fig. 3 also demonstrate that PRL co-localized with rab4 and rab5A in control cells, AdPRL-transduced cells, and cells that had been maintained in the presence of elevated rPRL. The co-localizations of PRL with rab4 and rab5A were obviously increased in cells that had been maintained in media containing rPRL.
Figure 3. Co-localizations of PRL with rab4, rab5A, and rab3D.
Non-transduced acini grown on Matrigel® coated coverslips were cultured in the absence of added PRL; in the presence of either 0.4 μg or 1.0 μg recombinant rabbit prolactin; or in the presence of microporous culture inserts containing AdPRL-transduced acini. Additional acini were on Matrigel® coated coverslips were transduced with AdPRL. After rinsing, acini were fixed and permeabilized with ethanol at −20°C, then labeled with guinea pig anti-prolactin antibody and with rabbit anti-rab 4, anti-rab 5A, or anti-rab3D antibody. Secondary antibodies were FITC-conjugated donkey anti-guinea pig IgG and rhodamine-conjugated goat anti-rabbit IgG. For dual staining of AdPRL-transduced acini, unconjugated plain donkey anti-guinea pig antibody was added to the FITC-conjugated donkey anti-guinea pig IgG antibody at a 5: 1 ratio. (*, apical/luminal regions; bar, 10 μm.)
When lacrimal acinar cells take up [125I]-BSA, a marker for fluid phase endocytosis, [125I] enters via the transcytotic - paracrine apparatus and is preferentially directed to the autolysosome; most of the cells’ steady-state content of [125I] is recovered in partial proteolytic products [Rose et al. 2005]. The presence of significant amounts of PRL immunoreactivity evident in Fig. 3 suggested that acinar cells that have internalized PRL from their ambient medium might retain relatively large steady-state contents of intact PRL. Experiments were designed to test this hypothesis, as well as the hypothesis that cells release internalized, stored PRL in response to stimulation with CCh. These experiments used the same co-culture system as in Fig. 3 to expose non-transduced acinar cells to elevated levels of rPRL secreted by AdPRL-transduced cells. The cells were then placed in PRL-free media, either without CCh, or with 100 μM CCh, and incubated for up to 5 hr. Supernatant media were collected after various intervals and analyzed by Western blot. The data presented in Fig. 4 indicate that the cells released intact PRL, as well as apparent conjugate forms of 53 kDa and 80 kDa, to their ambient medium, and that 100 μM CCh accelerated the initial phase of PRL release.
Figure 4. Secretion of internalized PRL by non-transduced cells.
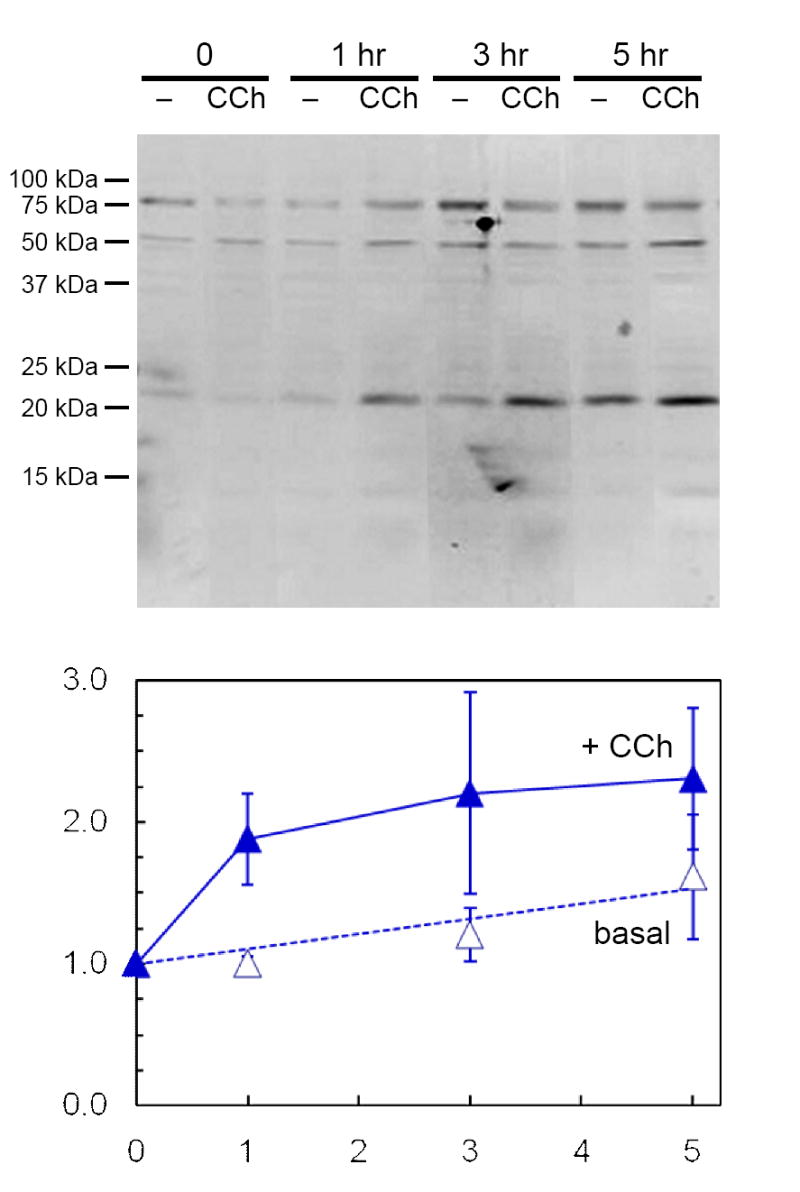
Non-transduced acini on Matrigel® coated 12-well plates (2×106 cells/well) were incubated for 18 hr in the presence of microporous culture well inserts containing AdPRL-transduced acini. Following removal of the inserts and washing with PBS, fresh culture media with or without 100 μM CCh were added. After additional incubations for 1, 3 or 5 hr, the media were collected, centrifuged to remove debris, and concentrated 10-fold for analysis by Western blotting. Blots were probed with anti-prolactin antibody and IRDye 800-conjugated anti-guinea pig IgG antibody, then scanned and quantified as described in Methods. Amounts of 23 kDa PRL released are expressed relative to the 0 time value in the absence of CCh and represent the means ± sem for three separate preparations.
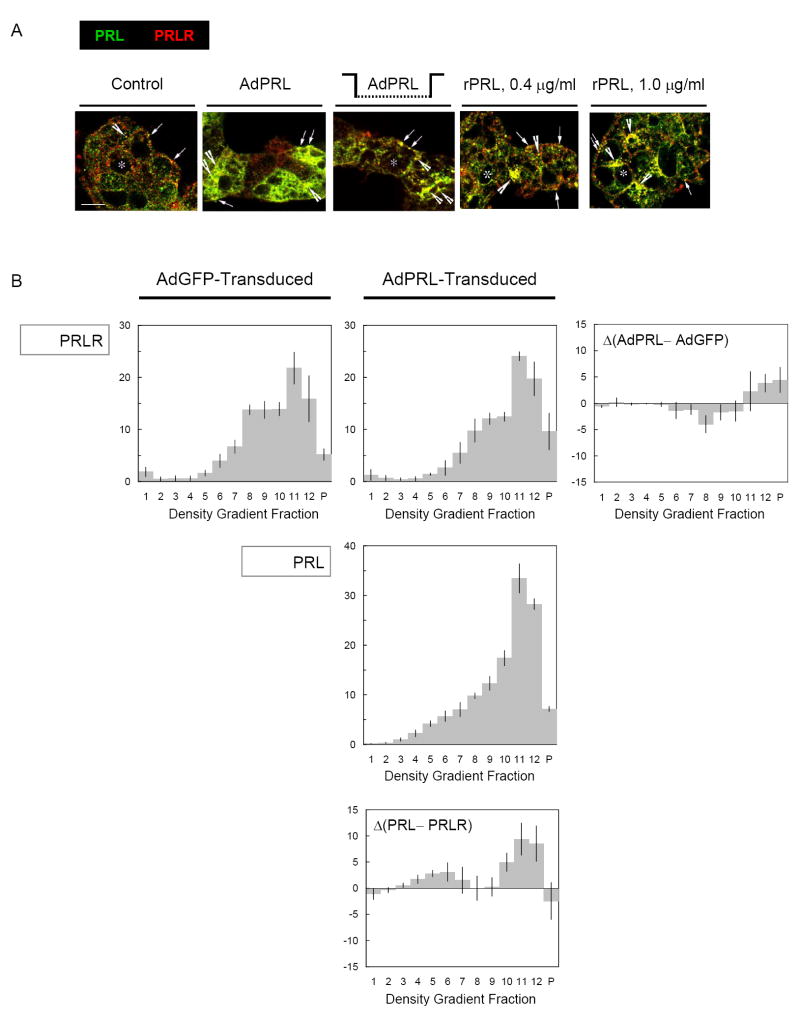
Lacrimal acinar cells express receptors for EGF, and EGF stimulation activates a biphasic membrane traffic program in which EGF receptors and internalized [125I]-EGF are initially accumulated in the apical recycling endosome, then subsequently re-directed to the lysosome (Xie et al., 2004). The observations that PRL influences acinar cell physiology imply that the cells must contain functional PRL receptors (PRLR), and Wood et al. (1999) have confirmed the presence of PRLR with immunocytochemical methods. The observations that acinar cells maintain pools of intact, endocytosed PRL and release it in response to CCh stimulation (Fig. 4) suggested that PRLR divert a significant component of the internalized PRL away from the bulk fluid-phase traffic to the autolysosome. The co-localization of PRL with PRLR was examined by confocal immunofluorescence microscopy. As illustrated in Fig. 5A, PRL co-localized extensively with PRLR in control cells, AdPRL-transduced cells, and cells that had been cultured in media containing rPRL. Analytical subcellular fractionation methods also were used to survey the localizations of PRL and PRLR in AdPRL-transduced cells and Ad-LacZ-transduced cells. As illustrated in Fig. 5B, transduction with AdPRL did not significantly alter the abundance of PRLR, but it shifted PRLR away from fraction 8, which contains the Golgi complex and early endosome, and toward fractions 11 and 12, which contain glycerophospholipid-rich elements of the secretory vesicle membrane, the pre-lysosome, and the lysosome. The compartmental distribution of PRL in AdPRL-transduced cells generally resembled the distribution of PRLR. However, there were relative excesses of PRL over PRLR in fractions 4-6, which contain the recycling endosome [Xie et al. 2004], fraction 10, which contains elements of the late endosome, and fractions 11-12 [Rose et al., 2005].
Figure 5. Co-localization of PRL and PRLR.
A. Confocal immunofluorescence microscopy. Acini grown on Matrigel® coated coverslips were incubated in various configurations as in Figure 3, then washed, fixed with 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, then stained guinea pig anti-prolactin antibody and mouse anti-prolactin receptor antibody followed by a FITC-conjugated donkey anti-guinea pig IgG secondary antibody and rhodamine-conjugated goat anti-mouse IgG secondary antibody. (*, apical/luminal regions; bar, 10 μm.) B. Analytical Subcellular Fractionation. Subcellular fractionation analyses were performed by isopycnic centrifugation on sorbitol density gradients. Membranes sedimented from the 13 fractions were analyzed by SDS-PAGE, and parallel Western blots were probed with rabbit antibodies to PRL and PRLR followed by appropriate secondary antibody conjugated with IRDye™ 800. The immunoreactive signals were quantified with an Odyssey Scanning Infrared Fluorescence Imaging System.
In addition to triggering exocytotic fusion of secretory vesicles with the apical plasma membrane, stimulation with CCh accelerates traffic through the transcytotic - paracrine apparatus (Gierow et al., 1995, Lambert et al., 1993). The observations that PRL is present in the apical recycling endosome, and that it is present in this compartment in relative excess over PRLR suggest that the transcytotic - paracrine apparatus might mediate the constitutive secretion of PRL, and that this apparatus also might mediate some component of the CCh-stimulated secretion. Since elevated levels of PRL disperse the secretory vesicle marker, rab3D, away from its normal concentration in the apical cytoplasm, it seemed possible that PRL might have entered the population of large, paracrine secretory vesicles which it induced. To test this hypothesis, EM-gold immunocytochemical methods were used to survey the localization of PRL at a level of resolution permitting visualization of the secretory vesicles. The images in Fig. 6 confirm that PRL immunopositivity is present in smaller vesicles consistent with endosomes or secondary lysosomes, and transport vesicles of the transcytotic - paracrine apparatus. They also demonstrate that PRL immunopositivity is present within large secretory vesicles at the basal cell surface. Notably, the electron densities of the secretory granule interiors often are frequently heterogeneous, and the immunogold labeling of PRL typically is associated with the more electron dense areas.
Figure 6. EM-gold microscopy.
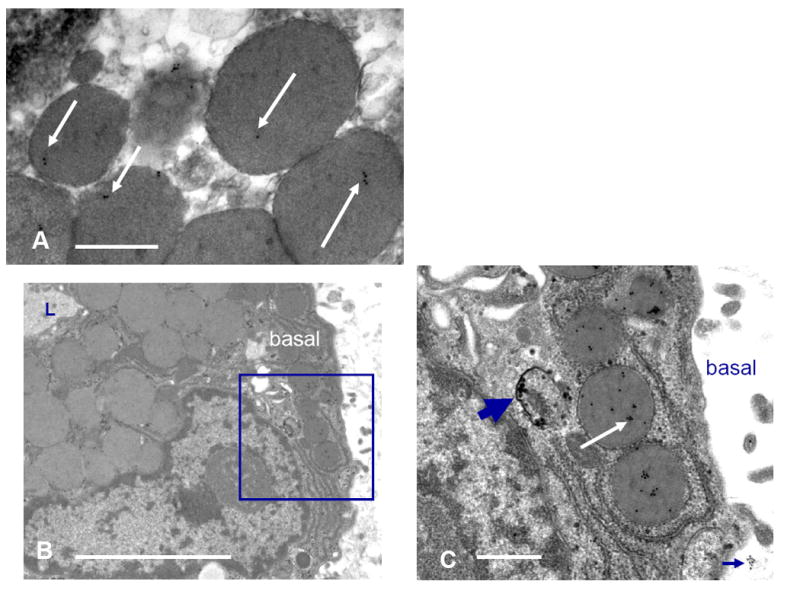
A. Control raft. Gold particles overlie secretory vesicles near the apical surface of acinar cells. B. and C. AdPRL-transduced raft. Micrograph B shows an acinar cell raft at low magnification with a portion of lumen (L) evident at upper left. Gold labeling is essentially absent from the apical secretory vesicles. The enclosed area is the basal cytoplasm of the acinar cell that is seen at higher magnification in micrograph C. The thick arrow indicates immunogold particles localizing PRL within a small vesicle, and the white arrow shows PRL localized within secretory vesicles. Occasionally small clusters of gold were evident just beyond the acinar cell basal surface (cluster at lower right), suggesting that PRL released at the basal surface associates with the subjacent extracellular matrix. (B Bar = 10 μ. C Bar =2 μ).
DISCUSSION
The results we obtained in this study demonstrate that when lacrimal epithelial cells either synthesize PRL or take it from their ambient medium, they traffic it into both the constitutive transcytotic - paracrine apparatus and a regulated secretory apparatus. Moreover, the level of PRL determines which of two alternative regulated secretory apparatus the cells express. Under normal conditions, they employ the classic, regulated exocrine secretory apparatus to release products into the nascent fluid secretion. When PRL is elevated, they employ a novel, regulated paracrine apparatus to release products into the underlying tissue space.
Our current cellular model for the lacrimal epithelial membrane traffic apparatus is illustrated in Fig. 7. It is based on findings from subcellular analyses of the compartmental organizations of intrinsic biochemical markers and of endocytosed markers, horseradish peroxidase (Gierow et al, 1996), [125I]-EGF (Xie et al, 2004), and [125I]-BSA (Rose et al., 2005), as well as on findings from light- and electron microscope imaging analyses. Most recently, a live cell imaging study employed the chimeric secretory protein construct syncollin-GFP, which is targeted to regulated secretory vesicles in both endocrine and exocrine cells (Hodel and Edwardson, 2000) to demonstrate that increased levels of PRL induced acinar cells to express the regulated paracrine apparatus, in which vesicles accumulated in the basal cytoplasm and fused with the basal-lateral membrane in response to acute stimulation with CCh (Wang et al., 2007).
CCh stimulation accelerates secretion of PRL from cells that over-express it (Wang et al., 2007), as well as from cells that have internalized it (Fig. 4), and in both cases the rate of secretion subsides to basal values after an initial burst. The lacrimal gland fluid elicited from normal, non-pregnant female rabbits by stimulation with pilocarpine contains PRL. In principle, such secretion might be mediated by either the regulated exocrine apparatus or the transcytotic - paracrine apparatus, which operates constitutively but is accelerated by reflex (Fullard and Tucker, 1991) and pharmacologic stimulation. EM studies have demonstrated that PRL is present in secretory vesicles of intact lacrimal gland (Wood et al., 1999). The EM localization studies of the ex vivo acinar cell model illustrated in Fig. 6 demonstrate that PRL reaches large vesicles with morphology and subcellular localizations consistent with secretory vesicles and that it also reaches small endosomal or secondary lysosomal vesicles. In the presence of elevated PRL, both types of structure are preferentially positioned in the basal cytoplasm
The TGN is the central sorting nexus for products newly emerging from the biosynthetic apparatus (Farquhar, 1981). Our working assumption, based on analogy to the regulated exocrine apparatus of pancreatic- and salivary epithelial cells, is that transport vesicles emerge from a specialized domain of the TGN and undergo homotypic fusion to form an immature secretory vesicle. Transport vesicles return to the TGN from the immature secretory vesicle to remove membrane in excess over the surface area required for a spherical volume, and the immature vesicle matures to the static form that resides in the apical cytoplasm until stimulated to undergo exocytotic fusion with the apical plasma membrane. Immature secretory vesicles have not been imaged in lacrimal epithelial cells as morphologically distinct structures, such as exist in pancreas and salivary glands. However, the observations that the substance within the secretory vesicles is heterogeneous and that PRL is consistently associated with the more electron dense regions (Fig. 6) suggest that some condensation occurs during their formation. It also is notable that the concentration of PRL in more electron dense areas of the secretory vesicle lumen contrasts with the uniformly electron dense content of PRL secretory vesicles in the pituitary gland. This marked difference likely reflects the fact that lacrimal epithelial cells secrete a complex mixture of products, while pituitary lactotrophs’ secretory product is highly enriched in PRL.
The secretory vesicles of the PRL-induced regulated paracrine apparatus may form in the same manner. They appear to contain a relatively smaller amount of SC; evidence suggests that this is because elevated PRL levels cause increased amounts of pIgR to be directed into the specialized TGN domain associated with the constitutive, transcytotic - paracrine pathway (Mircheff et al., 2006). On the other hand, the observation that PRL is associated with the more electron dense regions within the regulated paracrine secretory vesicles suggests that they also are generated from immature vesicles which recycle excess membrane to the TGN. An alternative hypothesis, not depicted in Fig. 7, but not yet excluded, is that the regulated paracrine vesicles emerge from the same TGN domain as exocrine vesicles but subsequently are targeted differently. A third hypothesis, proposed as the pathway for transcytotic secretion of PRL in mammary alveolar epithelial cells, is that PRL reaches secretory vesicles after trafficking to the late endosome and autophagosome or pre-lysosome (Seddiki et al., 2002). This hypothesis also is not excluded, but it seems unlikely for lacrimal acinar cells because exposure to elevated PRL decreases their content of rab7, which mediates traffic in the autophagic - lysosomal apparatus (Wang et al., 2007).
The observations that both PRL emerging from the biosynthetic apparatus and internalized recombinant PRL co-localize extensively with rab4 and rab5A (Fig. 3), rab11 (Fig. 2), and rab7, indicate that PRL traffics to the early endosome, principally marked by rab4 and rab5A; the recycling endosome, principally marked by rab11; and the autophagic - lysosomal apparatus, principally marked by rab7. Since the early endosome and recycling endosome communicate with the TGN (Gierow et al., 1996, Xie et al, 2004, Rose et al, 2005), the TGN is likely to be the sorting nexus through which newly-synthesized PRL reaches the endosomes comprising the constitutive transcytotic apparatus. The TGN also is likely to be the sorting nexus and through which endocytosed PRL reaches the regulated secretory apparatus. The observation that PRL co-localizes with PRLR is consistent with the hypothesis that the receptor plays an important role in mediating PRL’s traffic to the recycling endosome and the regulated secretory vesicles, then releases it into the lumenal fluid phases of these compartments.
In comparison with β-hexosaminidase, a protein that they secrete via the regulated exocrine apparatus (Anderson et al., 2006), acinar cells secrete PRL at a relatively high basal rate (Wang et al., 2007). This observation indicates that PRL is secreted by way of the constitutive transcytotic - paracrine apparatus in addition to the regulated apparatus. However, we are not able to discern whether constitutive PRL secretion is directed symmetrically, or preferentially directed to one plasma membrane domain or the other.
The regulated exocrine secretory apparatus is the characteristic functional specialization of exocrine epithelial cells. In contrast, like the biosynthetic apparatus and the autophagic - lysosomal apparatus, the constitutive transcytotic - paracrine apparatus is assembled from generic components, i.e., the apical recycling endosome and the early endosome. It is possible that the regulated paracrine apparatus induced by PRL results from a reorganization of the exocrine secretory apparatus. This interpretation might account for the continued partial co-localization of PRL with rab3D despite the dispersion of rab3D away from the apical cytoplasm. However, that PRL would induce such an apparatus appears a rather unique phenomenon. While PRL is well known to induce expression of differentiated functions in a range of tissues (Bole-Feysot et al., 1998), including mammary gland (Akers, 1985, Houdebine et al., 1985, Forsyth, 1986, Clevenger and Plank, 1998), uterine glands (Gray, et al., 2001), prostate (Reiter et al., 1999), intestine (Mainoya , 1975, Pahuja and DeLuca, 1981), hematopoietic cells (Hooghe et al., 1993), thymus (De Mello-Coelho et al., 1998), B cells (Grimaldi et al., 2005), and dendritic cells (Matera et al., 2001), it has not to our knowledge, however, previously been reported to induce expression of a regulated paracrine apparatus. This apparatus seems to us reminiscent of the regulated secretory apparatus characteristically expressed by endocrine cells. At this point, the closest analogy that we see is the epithelial cells of the thyroid gland, which secrete thyroglobulin and iodine into the follicular lumen under resting conditions, then transcytose thyroglobulin to the interstitial space in response to stimulation with thyrotropin (Herzog, 1983).
Lacrimal epithelial cells’ mechanism for recycling ambient PRL as a paracrine mediator contrasts strikingly with the lactating mammary epithelium, which transcytoses PRL as an exocrine secretory product (Seddiki et al., 2002). However, it is strongly reminiscent of pituitary lactotrophs, which internalize PRL from their ambient medium and traffic it into their regulated endocrine secretory apparatus (Giss and Walker, 1985). This may give them the capacity to buffer the level of PRL, depending on the circumstances, in the ocular surface fluid or in the underlying tissue space despite fluctuations in the serum PRL level caused by the episodic and pulsatile nature of PRL release from the pituitary gland. Moreover, PRL’s ability to induce expression of a unique, regulated paracrine apparatus, which secretes PRL to the underlying tissue space, may be central to the immunophysiological transformation the lacrimal gland undergoes during pregnancy. That is, PRL may exert influences on ductal epithelial cells, which appear to be the major sources of TGF-β and EGF in addition to PRL, that parallel the influences documented in the ex vivo acinar cell model.
Acknowledgments
Support: EY 013720 (akm), EY 010550 (jes), EY 005801 (akm), EY 011386 (sfh-a), EY 012689 and Core Grant (mdt), and DK 048522 (sfh-a and akm)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akers RM. Lactogenic hormones: binding sites, mammary growth, secretory cell differentiation, and milk biosynthesis in ruminants. J Dairy Sci. 1985;68:501–519. doi: 10.3168/jds.s0022-0302(85)80849-3. [DOI] [PubMed] [Google Scholar]
- Allansmith MR, Kajiyama G, Abelson MB, Simon MA. Plasma cell content of main and accessory lacrimal glands and conjunctiva. Am J Ophthalmol. 1976;82:819–826. doi: 10.1016/0002-9394(76)90056-8. [DOI] [PubMed] [Google Scholar]
- Ariga H, Edwards J, Sullivan DA. Androgen control of autoimmune expression in lacrimal glands of MRL/Mp-lpr/lpr mice. Clinical Immunol Immunopath. 1989;53:499–508. doi: 10.1016/0090-1229(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Andersson SV, Edman MC, Bekmezian A, Holmberg J, Mircheff AK, Gierow JP. Characterization of β-hexosaminidase secretion in rabbit lacrimal gland. Exp Eye Res. 2006;83:1081–1088. doi: 10.1016/j.exer.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Azzarolo A-M, Bjerrum K, Maves CA, Becker L, Wood RL, Mircheff AK, Warren DW. Hypophysectomy-induced regression of female rat lacrimal glands: Partial restoration and maintenance by dihydrotestosterone and prolactin. Invest Ophthalmol Vis Sci. 1995;36:216–226. [PubMed] [Google Scholar]
- Azzarolo A-M, Mazaheri AH, Mircheff AK, Warren DW. Sex-dependent parameters related to electrolyte, water, and glycoprotein secretion in rabbit lacrimal glands. Curr Eye Res. 1993;12:795–802. doi: 10.3109/02713689309020384. [DOI] [PubMed] [Google Scholar]
- Azzarolo AM, Mircheff AK, Kaswan RL, Stanczyk FZ, Gentschein E, Becker L, Nassir B, Warren DW. Androgen support of lacrimal gland function. Endocrine. 1997;6:39–45. doi: 10.1007/BF02738800. [DOI] [PubMed] [Google Scholar]
- Beagley KW, Eldridge JH, Lee F, Kiyono H, Everson MP, Koopman WJ, Hirano T, Kishimoto T, McGhee JR. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169:2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endoc Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Plank TL. Prolactin as an autocrine/paracrine factor in breast tissue. J Mammary Gland Biol Neoplas. 1997;2:59–68. doi: 10.1023/a:1026325630359. [DOI] [PubMed] [Google Scholar]
- De Mello-Coelho V, Savino W, Postel-Vinay W, Dardenne M. Role of prolactin and growth hormone on thymus physiology. Dev Immunol. 1998;6:317–323. doi: 10.1155/1998/89782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Chang N, Fong YC, Wang Y, Trousdale MD, Mircheff AK, Schechter JE. Interacting influences of pregnancy and corneal injury on rabbit lacrimal gland immunoarchitecture and function. Invest Ophthalmol Vis Sci. 2006;47:1368–1375. doi: 10.1167/iovs.05-1034. [DOI] [PubMed] [Google Scholar]
- Farquhar MG. Membrane recycling in secretory cells: implications for traffic of products and specialized membranes within the Golgi complex. Methods Cell Biol. 1981;23:399–427. doi: 10.1016/s0091-679x(08)61511-3. [DOI] [PubMed] [Google Scholar]
- Forsyth IA. Variation among species in the endocrine control of mammary epithelial growth and function: the roles of prolactin, growth hormone, and placental lactogen. J Dairy Sci. 1986;69:886–893. doi: 10.3168/jds.S0022-0302(86)80479-9. [DOI] [PubMed] [Google Scholar]
- Franklin RM, Kenyon KR, Tomasi TB., Jr Immunohistologic studies of human lacrimal gland: localization of immunoglobulins, secretory component, and lactoferrin. J Immunol. 1973;110:984–992. [Google Scholar]
- Franklin RM, Prendergast RA, Silverstein AM. Secretory immune system of rabbit ocular adnexa. Invest Ophthalmol Vis Sci. 1979;18:1093–1096. [PubMed] [Google Scholar]
- Fullard RJ, Tucker DL. Invest Ophthalmol Vis Sci. 1991;32:2290–2301. [PubMed] [Google Scholar]
- Gao J, Lambert RW, Wickham LA, Banting G, Sullivan DA. Androgen control of secretory component mRNA levels in the rat lacrimal gland. J Steroid Biochem Mol Biol. 1995;52:239–249. doi: 10.1016/0960-0760(94)00172-i. [DOI] [PubMed] [Google Scholar]
- Gierow JP, Lambert RW, Mircheff AK. Fluid phase endocytosis by isolated rabbit lacrimal acinar cells. Exp Eye Res. 1995;60:511–525. doi: 10.1016/s0014-4835(05)80066-1. [DOI] [PubMed] [Google Scholar]
- Gierow JP, Yang T, Bekmezian A, Liu N, Norian JM, Kim SA, Rafisolyman S, Zeng H, Okamoto CT, Wood RL, Mircheff AK. Na,K-ATPase in lacrimal gland acinar cells endosomal system. Correcting a case of mistaken identity. Am J Physiol. 1996;271:C1685–C1698. doi: 10.1152/ajpcell.1996.271.5.C1685. [DOI] [PubMed] [Google Scholar]
- Giss BJ, Walker AM. Mammotroph autoregulation: intracellular fate of internalized prolactin. Molec Cell Endocrinol. 1985;42:259–267. doi: 10.1016/0303-7207(85)90057-7. [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW. Developmental biology of uterine glands. Biol Reprod. 2001;65:1311–1323. doi: 10.1095/biolreprod65.5.1311. [DOI] [PubMed] [Google Scholar]
- Grimaldi CM, Hill L, Xu X, Peeva E, Diamond B. Hormonal modulation of B cell development and repertoire selection. Mol Immunol. 2005;42:811–820. doi: 10.1016/j.molimm.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Hamm-Alvarez SF, da Costa S, Yang T, Wei X-H, Gierow P, Mircheff AK. Cholinergic stimulation of lacrimal acinar cells promotes redistribution of membrane associated kinesin and the secretory protein, β-hexosaminidase, and increases kinesin motor activity. Exp Eye Res. 1997;64:141–156. doi: 10.1006/exer.1996.0198. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog V. Transcytosis in thyroid follicle cells. J Cell Biol. 1983;97:607–617. doi: 10.1083/jcb.97.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel A, Edwardson JM. Targeting of the zymogen-granule protein syncollin in AR42J and AtT-20 cells. Biochem J. 2000;350:637–643. [PMC free article] [PubMed] [Google Scholar]
- Hooghe R, Delhase M, Vergani P, Malur A, Hooghe-Peters EL. Growth hormone and prolactin are paracrine growth and differentiation factors in the haemopoietic system. Immunol Today. 1993;14:212–214. doi: 10.1016/0167-5699(93)90165-h. [DOI] [PubMed] [Google Scholar]
- Houdebine LM, Dijane J, Dusanter-Fourt I, Martel P, Kelly PA, Devinoy E, Servely JL. Hormonal action controlling mammary activity. J Dairy Sci. 1985;68:489–500. doi: 10.3168/jds.S0022-0302(85)80848-1. [DOI] [PubMed] [Google Scholar]
- Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci USA. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. Laboratory reference values. N Engl J Med. 2004;351:1548–1563. doi: 10.1056/NEJMcpc049016. [DOI] [PubMed] [Google Scholar]
- Kuhn LC, Kraehenbuhl JP. Role of secretory component, a secreted glycoprotein, in the specific uptake of IgA dimer by epithelial cells. J Biol Chem. 1979;254:11072–11081. [PubMed] [Google Scholar]
- Lambert RW, Maves CA, Gierow JP, Wood RL, Mircheff AK. Plasma membrane internalization and recycling in rabbit lacrimal acinar cells. Invest Ophthalmol Vis Sci. 1993;34:305–316. [PubMed] [Google Scholar]
- Mainoya JR. Effects of bovine growth hormone, human placental lactogen and ovine prolactin on intestinal fluid and ion transport in the rat. Endocrinology. 1975;96:1165–1170. doi: 10.1210/endo-96-5-1165. [DOI] [PubMed] [Google Scholar]
- Matera L, Mori M, Galetto A. Effect of prolactin on antigen function of monocyte-derived dendritic cells. Lupus. 2001;20:728–734. doi: 10.1191/096120301717164967. [DOI] [PubMed] [Google Scholar]
- McGee DW, Beagley KW, Aicher WK, McGhee JR. Transforming growth factor-beta enhances interleukin-6 secretion by intestinal epithelial cells. Immunology. 1992;77:7–12. [PMC free article] [PubMed] [Google Scholar]
- Mircheff AK, Hamm-Alvarez SF, Kaslow HR, Schechter JE, Trousdale MD. Epithelial cells in lymphocyte regulation in the lacrimal gland. In: Zierhut M, Stern ME, Sullivan DA, editors. Immunology of the Lacrimal Gland, Tear Film and Ocular Surface. Taylor and Francis; London: 2005. pp. 43–70. [Google Scholar]
- Mircheff AK, Wang Y, de Saint Jean M, Ding C, Schechter JE. Lacrimal epithelium mediates hormonal influences on APC and lymphocyte cycles in the ocular surface system. In: Zierhut M, Rammensee HG, Streilein JW, editors. Antigen-Presenting Cells and the Eye. Taylor and Francis; London: 2007. in press. [Google Scholar]
- Mircheff AK, Wang Y, de Saint Jean M, Ding C, Trousdale MD, Hamm-Alvarez SF, Schechter JE. Mucosal immunity and self-tolerance in the ocular surface system. The Ocular Surface. 2005;4:182–193. doi: 10.1016/s1542-0124(12)70204-5. [DOI] [PubMed] [Google Scholar]
- Mircheff AK, Wang Y, Nakamura T, Ding C, Schechter JE. Enhanced lacrimal gland mucosal immune function associated with pregnancy. Invest Ophthalmol Vis Sci. 2006;47 doi: 10.1167/iovs.05-1034. ARVO E-Abstract 1944. [DOI] [PubMed] [Google Scholar]
- Mircheff AK, Warren DW, Wood RL. Hormonal support of lacrimal function, primary lacrimal deficiency, autoimmunity, and peripheral tolerance in the lacrimal gland. Ocular Immunol Inflamm. 1996;4:145–172. doi: 10.3109/09273949609079648. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986;46:613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: Profile of chemokine receptor expression on human plasma cells ac-counts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–1140. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- Pahuja DN, DeLuca HF. Stimulation of intestinal calcium transport and bone calcium mobilization by prolactin in Vitamin D-deficient rats. Science. 1981;214:1038–1039. doi: 10.1126/science.7302575. [DOI] [PubMed] [Google Scholar]
- Palkowetz KH, Royer CL, Garofalo R, Rudloff HE, Schmalstieg FC, Jr, Goldman AS. Production of interleukin-6 and interleukin-8 by human mammary gland epithelial cells. J Reprod Immunol. 1994;26:57–64. doi: 10.1016/0165-0378(93)00867-s. [DOI] [PubMed] [Google Scholar]
- Reiter E, Hennuy B, Bruyninx M, Cornet A, Klug M, McNamara M, Closset J, Hennen G. Effects of pituitary hormones on the prostate. Prostate. 1999;38:159–165. doi: 10.1002/(sici)1097-0045(19990201)38:2<159::aid-pros10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rigg LA, Lein A, Yen SS. Pattern of increase in circulating prolactin levels during human gestation. Am J of Obstet Gynecol. 1977;129:454–456. doi: 10.1016/0002-9378(77)90594-4. [DOI] [PubMed] [Google Scholar]
- Rincheval-Arnold A, Belair L, Djiane J. Developmental expression of pIgR gene in sheep mammary gland and hormonal regulation. J Dairy Res. 2002;69:13–26. [PubMed] [Google Scholar]
- Rosato R, Jammes H, Belair L, Puissant C, Kraehenbuhl JP, Djiane J. Polymeric-Ig receptor gene expression in rabbit mammary gland during pregnancy and lactation: evolution and hormonal regulation. Mol Cell Endocrinol. 1995;110:81–87. doi: 10.1016/0303-7207(95)03519-d. [DOI] [PubMed] [Google Scholar]
- Rose CM, Qian L, Hakim L, Wang Y, Jerdeva GY, Marchelletta R, Nakamura T, Hamm-Alvarez SF, Mircheff AK. Accumulation of catalytically active proteases in lacrimal gland acinar cell endosomes during chronic ex vivo muscarinic receptor stimulation. Scand J Immunol. 2005;61:36–50. doi: 10.1111/j.0300-9475.2005.01527.x. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286:2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- Schechter J, Carey J, Wallace M, Wood R. Distribution of growth factors and immune cells are altered in the lacrimal gland during pregnancy and lactation. Exp Eye Res. 2000;71:129–142. doi: 10.1006/exer.2000.0859. [DOI] [PubMed] [Google Scholar]
- Schönthal AH, Warren DW, Stevenson D, Schechter JE, Azzarolo AM, Mircheff AK, Trousdale MD. Proliferation of lacrimal gland acinar cells in primary culture. Stimulation by extracellular matrix, EGF, and DHT. Exp Eye Res. 2000;70:639–649. doi: 10.1006/exer.2000.0824. [DOI] [PubMed] [Google Scholar]
- Seddiki T, Delpal S, Auborg A, Durand G, Ollivier-Bousquet M. Endocytic prolactin routes to the secretory pathway in mammary epithelial cells. Biol Cell. 2002;24:173–185. doi: 10.1016/s0248-4900(02)01188-7. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Hann LE. Hormonal influence on the secretory immune system of the eye: endocrine impact on the lacrimal gland accumulation and secretion of IgG and IgA. J Steroid Biochem. 1989;34:253–262. doi: 10.1016/0022-4731(89)90089-7. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Sato EH. Potential therapeutic approach for the hormonal treatment of lacrimal gland dysfunction in Sjögren’s syndrome. Clin Immunol Immunopath. 1992;64:9–16. doi: 10.1016/0090-1229(92)90052-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Watari E, Mabuchi A, Yokomuro K. Production of B cell differentiation factors by mouse parenchymal liver cells. Immunol Cell Biol. 1997;75:575–579. doi: 10.1038/icb.1997.89. [DOI] [PubMed] [Google Scholar]
- Tanneau GM, Hibrand-Saint-Oyant L, Chevaleyre CC, Salmon HP. Differential recruitment of T and IgA B-lymphocytes in the developing mammary gland in relation to homing receptors and vascular addressins. J Histochem Cytochem. 1999;47:1581–1592. doi: 10.1177/002215549904701210. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chiu CT, Nakamura T, Walker AM, Petridou B, Trousdale MD, Hamm-Alvarez SF, Schechter JE, Mircheff AK. Elevated prolactin redirects secretory vesicle traffic in rabbit lacrimal acinar cells. Am J Physiol Endocrinol Metab. 2007;292:E1122–E1134. doi: 10.1152/ajpendo.00381.2006. [DOI] [PubMed] [Google Scholar]
- Warren DW. Hormonal influences on the lacrimal gland. Int Ophthalmol Clin. 1994;34:19–25. doi: 10.1097/00004397-199403410-00004. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P, Roux ME, McWilliams M, Phillips-Quagliata JM, Lamm ME. Hormonal induction of the secretory immune system in the mammary gland. Proc Natl Acad Sci USA. 1978;75:2928–2932. doi: 10.1073/pnas.75.6.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RL, Zhang J, Gierow JP, Mircheff AK, Warren DW. Prolactin and prolactin receptors in the lacrimal gland. Exp Eye Res. 1999;69:213–226. doi: 10.1006/exer.1999.0690. [DOI] [PubMed] [Google Scholar]
- Xie J, Qian L, Wang Y, Rose CM, Yang T, Nakamura T, Hamm-Alvarez SF, Mircheff AK. Novel biphasic traffic of endocytosed EGF to recycling and degradative compartments in lacrimal gland acinar cells. J Cell Physiol. 2004;199:108–125. doi: 10.1002/jcp.10458. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xie J, Qian L, Schechter JE, Mircheff AK. IL-2 immunoreactive proteins in lacrimal acinar cells. Adv Exp Med Biol. 2002;506:795–799. doi: 10.1007/978-1-4615-0717-8_112. [DOI] [PubMed] [Google Scholar]