Abstract
Background & Aims
Cancer and oncological therapy are associated with a progressive physical deterioration, malnutrition, and enhanced inflammatory burden. Our considerable data showing the strong anabolic potential of amino acids led us to test whether amino acids can acutely stimulate muscle protein synthesis in cancer patients (CA) undergoing intense chemotherapy.
Methods
Mixed muscle fractional synthesis rate (FSR), rates of phenylalanine appearance and disappearance (Ra and Rd), and net phenylalanine balance (NB) were measured during a primed constant infusion of L-[ring-2H5]phenylalanine. Blood and muscle tissue samples were collected in the basal state and following ingestion of 40 g of amino acids (AA) given in 30 mL boluses every 10 minutes for 3 hours. Serum and tissue cytokines and NF-κB expression in skeletal muscle were measured and compared to normative, healthy older controls (OC).
Results
Skeletal muscle TNF-α, IL-6, and NF-κB were elevated in CA. FSR and model-derived protein synthesis (Rd) increased significantly from basal to AA (FSR: 0.052 ± 0.009 vs. 0.120 ± 0.008 %•h-1, P<0.001; Rd: 23.1 ± 4.1 vs. 36.4 ± 5.0 nmol•min-1•100mL leg-1, P≤0.05). Model-derived protein breakdown (Ra) remained unchanged from basal to AA.. Phenylalanine NB improved from a negative basal value (-16 ± 2) to zero (0.8 ± 6 nmol•min-1•100 ml leg-1, P≤0.05) following AA.
Conclusion
Despite advanced cancer, ongoing therapy, and an enhanced inflammatory burden, amino acids were capable of acutely stimulating muscle protein synthesis in these patients.
Key terms: Oral amino acids, Muscle protein synthesis, Inflammation, Cancer
INTRODUCTION
Cachexia adds a significant burden to the health of already compromised cancer patients, decreasing quality of life and increasing mortality.1 Phenotypically, this condition is characterized by a rapid and unwanted loss in lean body mass and fat mass. In a typical individual, a loss of 30% of total body weight reflects a 75% loss of skeletal muscle protein2 and without immediate therapeutic intervention, often leads to death.3, 4 Thus, maintaining lean tissue mass and preventing loss of lean muscle tissue is a high priority in the fight against cancer.
The functional impairments characteristic of cancer-related muscle loss are similar, albeit more rapid in onset, to those consistent with age-related sarcopenia.3 Metabolic abnormalities common to both cancer cachexia and sarcopenia include hormone dysregulation,1, 5 increased insulin resistance,1, 4, 6 increased muscle proteolysis,3, 7, 8 decreased muscle protein synthesis,9 elevated synthesis of acute phase proteins,1, 4 and elevated cytokines1, 3-6, 10-15. Despite these commonalities, the mechanisms behind the rapid loss of muscle mass associated with cachexia, in contrast to the progressive declines seen with sarcopenia, remain poorly understood. Stable isotope tracer methodologies provide a unique opportunity to measure the in vivo rates of skeletal muscle protein synthesis and breakdown16 in response to physical,17, 18 nutritional,19-23 or pharmacologic interventions.24, 25 In the case of cancer cachexia, these techniques may yield critical information regarding the mechanisms underlying muscle wasting, and directly assess the efficacy of anti-cachectic interventions.
Considerable debate remains over how host- and tumor-derived factors interact with intracellular signaling mechanisms to disrupt skeletal muscle protein balance. Recently, NF-κB-dependent muscle wasting was found to be activated by late-stage cancer or by chronic inflammation.26 Further, activation of NF-κB by pathogen associated molecules such as tumor-derived factors and cytokines4, 27 is an important first step in the intracellular signaling pathway leading to activation of NF-κB mediated muscle protein breakdown.1, 4, 27 More recently, evidence suggests that expression of pro-cachectic cytokines such as TNF-α and IL-6 may also be responsible for regulating skeletal muscle protein synthesis by down regulating either IGF-I or inhibiting signaling components of anabolic pathways controlled by IGF-I, Akt and mTOR.27 However, despite this recent evidence, it is not known how cancer or the concomitant inflammatory process affects in vivo skeletal muscle metabolism. Specifically, we do not know if the skeletal muscle of cancer patients responds to acute nutritional stimuli known to be anabolic in healthy young and older individuals.
Oral amino acids are acutely anabolic to skeletal muscle in healthy individuals. We recently showed that while age-related differences exist in the time course of the anabolic response, ingestion of amino acids results in muscle protein anabolism in both young and elderly.20, 21 We hypothesized that ovarian cancer patients can similarly benefit from oral amino acids administered in small boluses. In the present study we sought to determine if amino acid ingestion could acutely stimulate muscle protein synthesis in women with advanced or recurrent ovarian cancer undergoing oncological therapy. We concomitantly measured the expression of serum and skeletal muscle cytokines and NF-κB and compared our findings to healthy older controls.21
SUBJECTS AND METHODS
Subjects and Methods
Six patients with FIGO stage IIIC ovarian cancer (CA) (47±5 y, 59±6 kg, 1.60±0.03 m, 22±3 BMI) classified as cachectic based upon having lost more than 10% of their pre-morbid weight participated in this study. Informed written consent was obtained on all subjects using consent forms approved by the UTMB Institutional Review Board (IRB). Eligible patients were diagnosed with ovarian cancer, stage IIIC, either undergoing primary therapy or therapy for recurrence. All patients were at least 6-months post-surgery at the time of the study and received combination chemotherapy with Paclitaxel 175 mg/m2 and Carboplatinum, AUC= 5. All patients were on a 21-day chemotherapy cycle. Patients were admitted to the hospital on day 19, and studied on day 20. Patients received scheduled chemotherapy on day 21 and were discharged the following day (day 1 of the next cycle). To demonstrate the acute response of skeletal muscle to amino acids (AA) in CA, each patient served as their own control (i.e., basal vs. AA). For purposes of making normative comparisons about inflammatory markers, CA were compared to a group of healthy older controls (OC) previously shown to be responsive to the exact oral amino acid composition used in this experiment.21 Finally, no study related complications occurred in the performance of this clinical study protocol.
Experimental Protocol
Metabolic studies were conducted over an 8 h period following an overnight fast (Figure 1). At 0600 hours the morning of the study, polyethylene catheters were inserted into the forearm vein for infusion of labeled phenylalanine, in the wrist vein of the opposite hand for arterialized blood sampling and into the femoral artery and vein of one leg for blood sampling. Blood samples were collected for determination of background phenylalanine enrichment and concentration before starting a primed (2 μmol/kg) continuous infusion of L- [ring-2H5]phenylalanine (0.05 μmol•kg-1•min-1) from time 0 to 480 minutes (Figure 1). Muscle biopsy samples (80-100 mg) were collected from the vastus lateralis muscle using a 5 mm Bergström biopsy needle at 120, 300 and 480 min. Samples were frozen in liquid nitrogen and stored at -80°C. At 230 and 420 min a continuous infusion of indocyanine green (ICG) was started into the femoral artery and maintained for 20 min to calculate leg blood flow as previously described.28 Amino acids were ingested in small boluses (30 mL) every 10 min from 300 to 480 min (Figure 1) to facilitate gastric accommodation and to prevent bloating and nausea common in this patient population. The mixture contained 40 g of essential and nonessential amino acids dissolved in 540 mL of distilled water containing sugar-free flavor (Crystal Light, Kraft Foods, White Plains, NY) (Table 2). The proportion of amino acids in the mixture was selected because it closely resembles that of meat protein29 and because it had previously been shown to be strongly anabolic in a group of healthy older controls.21
Figure 1.
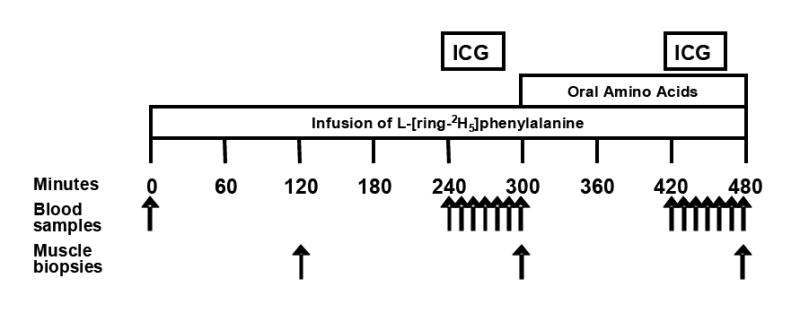
Experimental protocol. Outline of the timeline of infusion of the stable isotope L-[ring-2H5]phenylalanine, administration of the oral amino acids, collection of blood samples, and collection of muscle biopsies.
Table 2.
Amino acids content of the balanced amino acid supplement
| Alanine (g) | 2.5 ± 0.0 |
| Arginine (g) | 2.8 ± 0.0 |
| Asparagine (g) | 3.7 ± 0.0 |
| Cysteine (g) | 0.5 ± 0.0 |
| Glutamine (g) | 5.8 ± 0.0 |
| Glycine (g) | 1.9 ± 0.0 |
| Histidine (g) | 2.0 ± 0.0 |
| Isoleucine (g) | 1.9 ± 0.0 |
| Leucine (g) | 3.2 ± 0.0 |
| Lysine (g) | 3.9 ± 0.0 |
| Methionine (g) | 1.0 ± 0.0 |
| Phenylalanine (g) | 1.6 ± 0.0 |
| Proline (g) | 1.9 ± 0.0 |
| Serine (g) | 1.7 ± 0.0 |
| Threonine (g) | 2.0 ± 0.0 |
| Tryptophan (g) | 0.5 ± 0.0 |
| Tyrosine (g) | 1.5 ± 0.0 |
| Valine (g) | 2.2 ± 0.0 |
| Total amino acids (g) | 40.7 ± 0.1 |
| (kJ) | 693 ± 2 |
| (kcal) | 166 ± 0 |
Analytical Methods
Phenylalanine enrichments and concentrations in blood and muscle samples were analyzed using gas-chromatography mass-spectrometry (GCMS) (Hewlett Packard, Palo Alto, CA) as previously described.25, 30 Protein-bound phenylalanine enrichment was analyzed with GCMS after protein hydrolysis and amino acid extraction16, using the external curve approach for low enrichments.30, 31 High sensitivity C-reactive protein (hsCRP) was analyzed using an Immulite 2000 chemiluminescence immunoassay analyzer (DPC, Los Angeles, CA). Skeletal muscle cytokines were determined with commercially available multiplexed biomarker immunoassay kits (Linco Research Inc., St. Charles, MO). All other blood measures were performed by the UTMB Clinical Laboratory.
Western immunoblot analysis of NF-κB
Cytosolic extracts were prepared from baseline (120 min) muscle tissue for the analysis of NF-κB. Briefly, whole cell extract was prepared from muscle biopsy samples by slicing frozen human muscle into small pieces with a clean razor blade and thawing the tissue in lysis buffer (150 mM NaCl, 10 mM Tris, 1% Triton X-100, 1% Na deoxycholate, 0.1% SDS, 5 mM EDTA) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine,10 μg/ml aprotinin, 50 μg/ml leupeptin, 1 μg/ml pepstatin A) at a concentration of 3 ml of ice cold lysis buffer per gram of tissue. Tissues were homogenized with a Dounce homogenizer (4°C) and centrifuged at 15,000 g for 20 min, and the supernatants were removed and discarded. Bradford assays were performed for determination of protein concentration of cytosolic extracts. Tissue lysates were electrophoresed on 10% SDS-PAGE gel loaded with 40 μg protein in each well. Immunoblots were incubated with NF-κB p65 (H-268) antibody (Santa Cruz Biotechnology). Chemiluminescence was recorded on Kodak film and analyzed with ImageJ software. Protein loading was evaluated using β-actin.
Calculations
Phenylalanine was selected to trace muscle protein kinetics because it is neither produced nor metabolized in skeletal muscle. Mixed muscle FSR was calculated by measuring the direct incorporation of L-[ring-2H5]phenylalanine into protein, using the precursor-product model:
where EP1 and EP2 are the enrichments of bound L-[ring-2H5]phenylalanine in consecutive muscle biopsies, t is the time interval between biopsies and EM is the mean L-[ring-2H5]phenylalanine enrichment in the muscle intracellular pool.21, 32 Data are expressed as %•h-1.
Protein synthesis (Rd) protein breakdown (Ra) and net phenylalanine balance (NB) were calculated as follows:
where Ca and Cv represent the phenylalanine concentrations in the femoral artery and vein, respectively.16 BF represents leg blood flow, as determined by the ICG dye dilution method.28 Leg volume was determined anthropometrically33. Data are expressed as nmol • min-1 • 100ml leg-1.32
Statistical Methods
Statistical analyses were performed using SPSS 13.0 (SPSS Inc.) and Microsoft Excel (Microsoft Corporation). Student t-tests were used to calculate differences between basal and amino acids (paired) and between CA and OC (unpaired). P≤0.05 was chosen to indicate statistical significance.
RESULTS
Blood Chemistry
Laboratory measures were collected to assess nutritional status and cancer inflammatory burden (Table 1). Serum albumin levels, an index of malnutrition, measured in the low normal range in CA. CA-125, a cancer tumor marker was significantly elevated above the normal reference range in CA (P=0.037). High sensitivity C-reactive protein levels measured in CA were in the high normal range, suggestive of low-grade systemic inflammation.
Table 1.
Subject characteristics.
| Normal range | CANCER | OLDER CONTROLS | |
|---|---|---|---|
| Serum Biomarkers | |||
| Albumin (g/L) | [35 – 50] | 35.8 ± 1.89 | |
| AST (U/L) | [13 – 40] | 24.2 ± 5.91 | |
| ALT (U/L) | [9 – 51] | 19.7 ± 3.37 | |
| BUN (mmol/L) | [2.5 – 8.2] | 4.03 ± 0.65 | |
| WBC (109/L) | [4.5 – 10.5] | 6.35 ± 1.26 | |
| CA-125 (U/mL) | [0 – 21] | 800 ± 762 | |
| hsCRP (mg/dL) | [0 – 0.8] | 0.77 ± 0.13† | 0.04 ± 0.007 |
| Muscle Biomarkers | |||
| TNF-α (pg/mL) | 0.26 ± 0.04† | 0.19 ± 0.01 | |
| IL-6 (pg/mL) | 5.11 ± 2.11† | 1.09 ± 0.15 |
Values are mean ± SEM.
Values are significantly greater than a representative group of healthy older controls (n=8, P<0.05).21
Leg Blood Flow
Leg blood flow in CA remained unchanged from basal to AA (3.55±0.46 vs. 3.54±0.50 mL•min-1•100mL•leg-1).
Phenylalanine Concentration and Enrichment
As expected, arterial phenylalanine enrichments fell sharply in the early portion of AA ingestion, while phenylalanine concentrations rose in the expected fashion (Figure 2).
Figure 2.
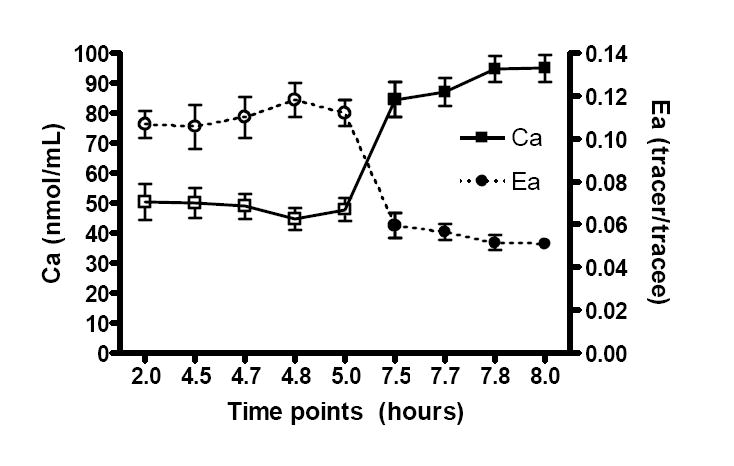
Arterial phenylalanine concentrations (Ca) and enrichment (Ea) in the basal state (□Ca, ○Ea) and in response to amino acids (■ Ca, ●Ea).
Mixed Muscle Fractional Synthesis Rate (FSR)
Mixed muscle FSR increased significantly from a basal value of 0.052 ± 0.009 to 0.120 ± 0.008 %•h-1 over the 3 h AA period (P=0.001) (Figure 3).
Figure 3.
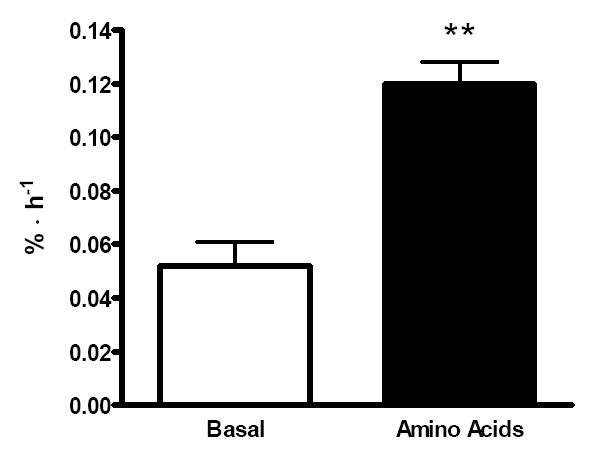
Mixed muscle fractional synthetic rate (FSR) in the basal state (white bars) and in response to amino acids (black bars). **Significantly different than basal, P≤0.001.
Protein synthesis (Rd)
Rd, a model-derived index of muscle protein synthesis increased from basal to AA in CA (23.1 ± 4.1 vs. 36.4 ± 5.0 nmol•min-1•100 mL leg-1, P≤0.05) (Figure 4).
Figure 4.
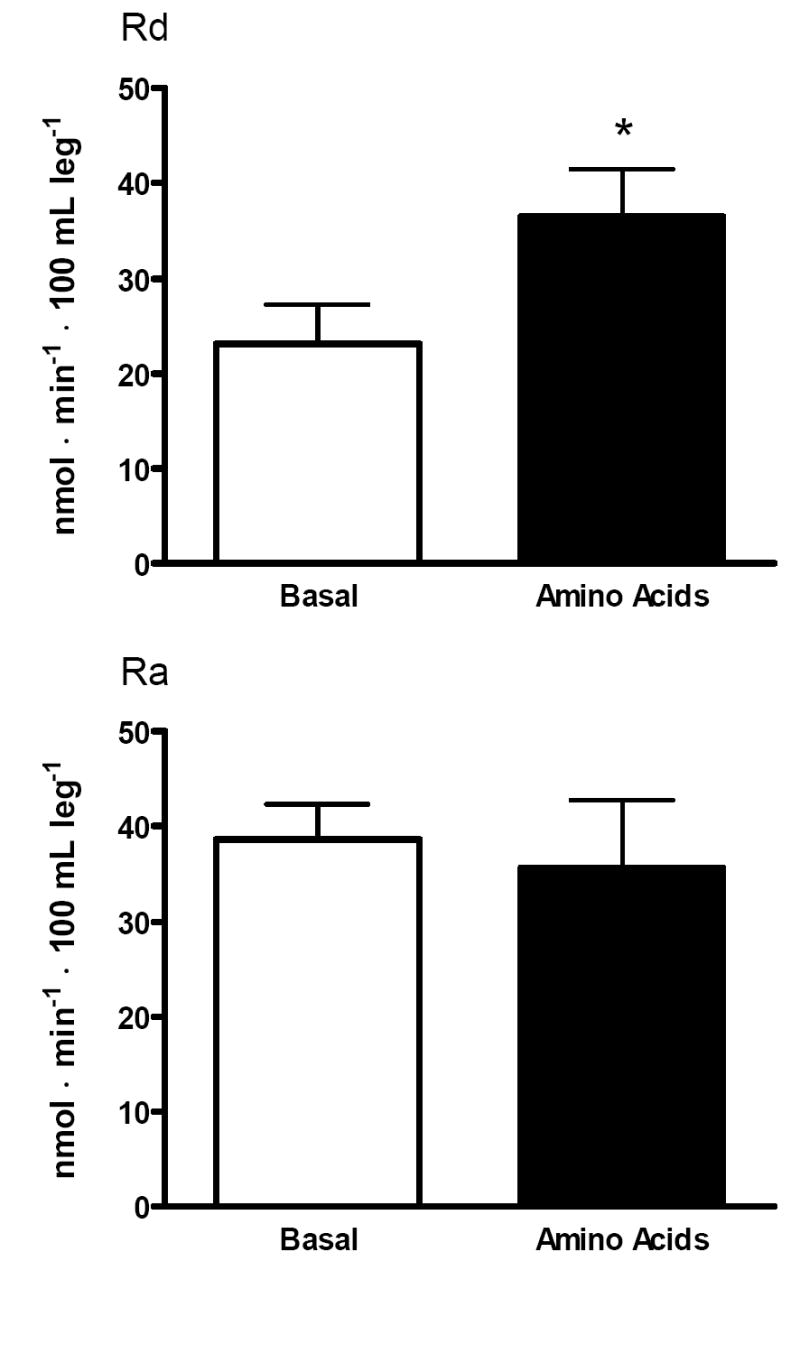
Muscle protein synthesis (Rd) and degradation (Ra) in the basal state (white bars) and in response to amino acids (black bars). *Significantly different than basal, P≤0.05.
Protein breakdown (Ra)
Ra, a model-derived index of muscle protein breakdown remained unchanged in CA following AA (38.6 ± 3.7 vs. 35.6 ± 7.2 nmol•min-1•100 mL leg-1) (Figure 4).
Net Phenylalanine Balance (NB)
Net phenylalanine balance shifted from net negative to zero (-16±2 vs. 0.8±6 nmol•min-1•100 mL•leg-1, P≤0.05) over the last hour of AA ingestion (Figure 5).
Figure 5.
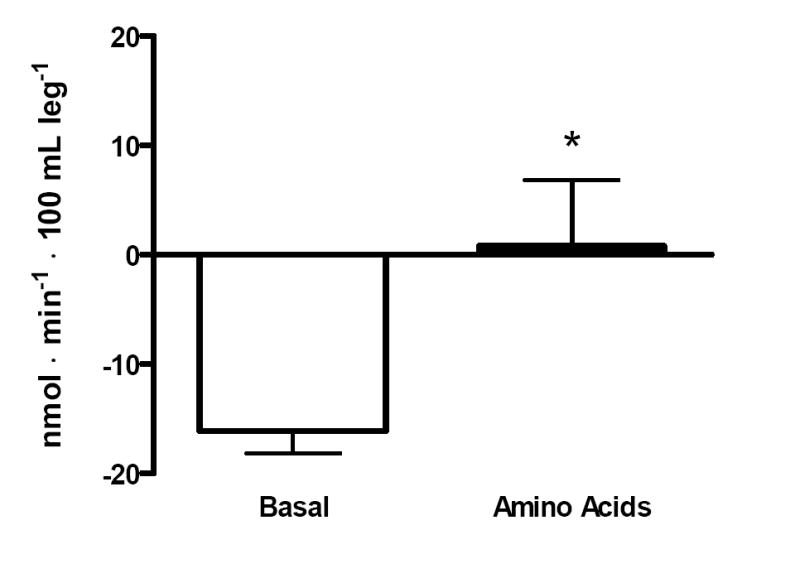
Phenylalanine net balance across the leg. Net balance in the basal state (white bars) and in response to amino acids (black bars). *Significantly different than basal, P≤0.05.
Western blot of NF-κB p65
NF-κB p65 expression was significantly greater in CA as compared to OC (P=0.0000001) (Figure 6).
Figure 6.
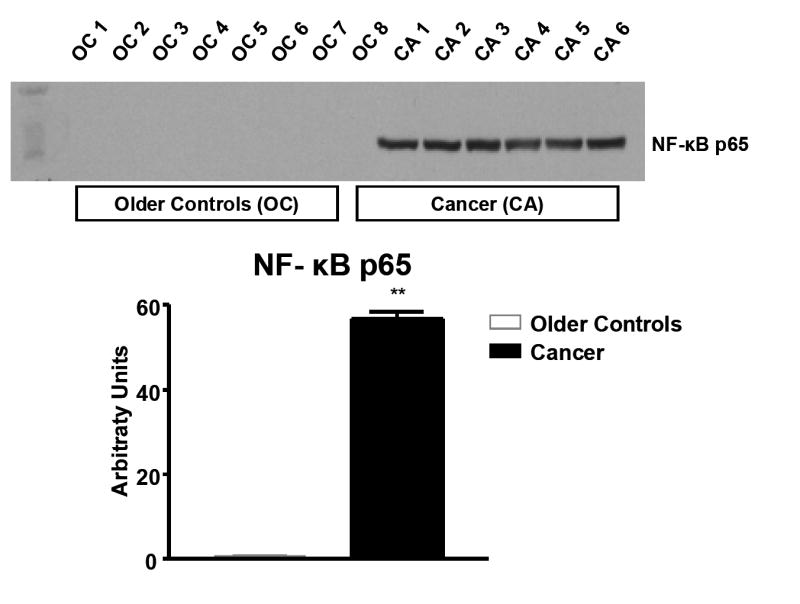
Comparison of NF-κB p65 protein expression in skeletal muscle from six stage IIIB ovarian cancer patients and eight healthy controls. *Significantly different from healthy older controls (Volpi et al.21), P<0.0001.
DISCUSSION
Cancer demands aggressive chemotherapy often resulting in a progressive deterioration in nutritional status, severe protein loss, muscle wasting, and generalized weight loss. Preventing or reversing muscle loss and the cachexia syndrome is a critical part of the intervention process and requires ongoing vigilance throughout the phases of cancer treatment. In the past few years, we have demonstrated a strong theoretical rationale for the use of amino acids in clinical populations suffering from muscle wasting.19-21 Our data in both healthy young and old show amino acids capable of acutely stimulating muscle protein synthesis.19-21 More importantly, we have shown that these acute anabolic benefits translate into the maintenance of lean muscle mass during an otherwise catabolic period of prolonged inactivity.34 Nevertheless, we did not know if a simple amino acid supplement is capable of acutely stimulating muscle protein synthesis in women undergoing aggressive oncological therapy for advanced and recurrent ovarian cancer.
Several similarities were observed between these cancer patients and healthy older subjects that followed the identical study protocol. 21. Leg blood flow and FSR were similar between OC and CA in both the basal state and after AA ingestion. While arterial phenylalanine enrichments were similar, we observed lower arterial concentrations in CA when compared to the OC in both the basal (48.3 ± 3.8 vs. 59.1 ± 2.1 nmol/mL, CA vs. OC) and AA periods (92.8 ± 3.3 vs. 120 ± 8.3 nmol/mL, CA vs. OC) (P≤0.05). Additionally, the increase in muscle protein synthesis (Rd) from basal to AA was smaller in CA (23.1 ± 4.1 vs. 36.4 ± 5.0 nmol•min-1•100 mL leg-1, P≤0.05), than seen in OC (32.76 ± 7.93 vs. 57.15 ± 8.79 nmol•min-1•100 mL leg-1, P≤0.01). Muscle protein breakdown (Ra) however was unchanged in response to AA and similar to that previously noted in OC (48.61 ± 9.58 vs. 41.11 ± 8.81 nmol•min-1•100 mL leg-1. Together, these findings resulted in a slightly less positive shift in the NB of CA following AA (-16±2 vs. 0.8±6 nmol•min-1•100 mL•leg-1) compared to OC (-16 ± 5 vs. 16 ± 4 nmol•min-1•100 mL•leg-1, P≤0.05).
Our findings suggest that amino acids are acutely anabolic to the skeletal muscle of these metabolically dynamic cancer patients, albeit to a smaller extent than seen in healthy older subjects. Additionally, we showed that this amino acid-induced anabolism in cancer patients could be attributed to a stimulation of muscle protein synthesis, because muscle protein breakdown remained unchanged. These data are essential in understanding the mechanisms that regulate the loss of muscle mass with cancer and its associated therapies.
In broadest terms, changes in lean muscle mass are a function of the relationship between muscle protein synthesis and breakdown. With cancer, disruption of this kinetic balance is affected not only by the inflammatory burden that accompanies cancer, but also by the various therapeutic interventions. Indeed, some have suggested that the primary factor driving cancer cachexia is enhanced protein degradation occurring through the ubiquitin-proteasome pathway.4, 35-37 This pathway is stimulated by inflammatory biomarkers such as TNF-α and NF-κB, both found to be highly elevated and expressed in our cancer group in comparison to healthy older controls. Our data show that the anabolic stimulus afforded by amino acid supplementation occurred despite the catabolic potential of inflammatory biomarkers such as NF-κB p65, TNF-α and IL-6. TNF-α, IL-6 and NF-κB are each known to negatively affect pathways involved in the amino acid mediated induction of protein synthesis such as the PI3K/Akt/mTOR pathway and activity of initiation factors eIF2 and eIF2B.38, 39 NF-κB has also been shown to inhibit mTOR activation3, 27, which is the pathway by which amino acids, particularly leucine, stimulate initiation of protein translation.40, 41 With this evidence, it would seem unlikely that amino acids could acutely stimulate muscle protein synthesis in cancer patients with an enhanced inflammatory burden. While we did not look at the mTOR signaling pathway directly as it was outside the scope of this study, the fact that FSR and Rd in these cancer patients was significantly elevated despite the demonstrated hyperactivation of NF-κB p65 and elevated TNF-α and IL-6 suggests that some portion of the PI3K/Akt/mTOR pathway is intact.
Increased muscle protein breakdown is a major concern in cancer and aging. In cancer cachexia, visceral protein is relatively preserved in comparison to skeletal muscle. This phenomenon is largely due to metabolic recycling of amino acids liberated from skeletal muscle for splanchnic utilization. While administration of oral amino acids to cancer patients in the present study resulted in an overall increase in the FSR of skeletal muscle over the 3 h absorptive period, the net phenylalanine balance measured in the last hour of amino acid ingestion failed to achieve a strong positive net balance as seen in previous studies of healthy individuals.20, 21 This suggests that some portion of these exogenous amino acids were subjected to an alternative metabolic fate, perhaps toward enhanced acute phase protein synthesis or tumor cell growth. In support of this notion, we showed 20-fold higher concentrations of C-reactive protein among the cancer patients when compared to the healthy older controls. While hsCRP remained within normal limits in the cancer patients (0.77 ± 0.13 mg/dL), these levels were significantly higher than those measured in the older controls (0.04 ± 0.007 mg/dL). Similar to that observed in the older controls, there was no response in protein breakdown (Ra) in the cancer patients following amino acid ingestion. Taken together, these data indicate that while the cancer patients were able to mount an acute anabolic response to the amino acids during the absorptive period, anabolism may be attenuated over time as amino acids are shunted to sustain a low-grade inflammatory response and/or tumor growth. Further outcome-based research is needed to determine if targeted amino acids such as leucine can provide a more robust stimulation of muscle protein synthesis. Leucine, and to a lesser extend the two other branched chain amino acids valine and isoleucine, is known to stimulate protein synthesis through increased phosphorylation of mTOR, S6K1, and 4E-BP1. While our balanced amino acid supplement contained 3.2 ± 0.0 g leucine, higher concentrations may be required to produce a sufficiently strong anabolic signal in cancer patients. Enhanced tumor growth is a potential risk involved with anabolic interventions. Future outcome based studies are needed to establish whether the use of amino acid supplements as an anticachectic intervention increases tumor growth. While the results of this study are encouraging with the finding that amino acids are acutely anabolic to cancer patients, these patients demonstrated a mild inflammatory burden. Conversely, in patients with more pronounced inflammatory states, such as progressive cachexia and unstable disease, a less anabolic response may be expected.
In conclusion, we have demonstrated that amino acid ingestion acutely stimulates muscle protein synthesis in ovarian cancer patients with advanced disease and inflammatory syndrome undergoing oncological therapy. The efficacy and potential clinical benefit of amino acid supplementation in cancer patients was highlighted by the similar anabolic response observed in a cohort of healthy individuals who received the same amino acid supplement.21 These data represent a positive first step toward preservation of nutritional status and prevention of cancer cachexia. Additional studies are needed to determine if long-term administration of targeted nutritional therapies such as with leucine are capable of preventing the skeletal ravages of cancer and its associated therapies.
Acknowledgments
Our sincere thanks are extended to all the cancer patients who unselfishly participated in this clinical research project. Additionally, we thank Joseph T. Santoso, M.D. for his assistance with patient recruitment, the UTMB GCRC nursing and dietary staff for assisting with the conduct of these studies and Dr. Judah Rosenblatt for his statistical assistance. This research was funded by NIH/NIA Claude D. Pepper Older Americans Independence Center Grant # P60 AG17231 (to J.S. Goodwin and R.R. Wolfe) and R01 AG21539 (M. Sheffield-Moore). Studies were conducted on the GCRC at UTMB and funded by grant M01 RR 00073 from the National Center for Research Resources, NIH, USPHS. M.S.-M. was the principal investigator. M.S.-M., E.V., R.R.W. and R.J.U. were responsible for study design and implementation. E.L.D., D.P.-J., and M.S.-M. performed data analysis and interpretation. S.L.C. and S.S. conducted sample processing and analyses. C.D.A. was the physician for the gynecological cancer patient volunteers. A.P.S. and E.V. performed the surgical procedures involved in the experiment.
E.L.D., E.V., R.R.W., S.S., A.P.S., C.D.A., R.J.U., S.L.C., D.P.-J., M.S.-M. have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon JN, Green SR, Goggin PM. Cancer cachexia. Qjm. 2005;98:779–88. doi: 10.1093/qjmed/hci127. [DOI] [PubMed] [Google Scholar]
- 2.Fearon KC. The Sir David Cuthbertson Medal Lecture 1991. The mechanisms and treatment of weight loss in cancer. Proc Nutr Soc. 1992;51:251–65. doi: 10.1079/pns19920036. [DOI] [PubMed] [Google Scholar]
- 3.Argiles JM, Busquets S, Garcia-Martinez C, Lopez-Soriano FJ. Mediators involved in the cancer anorexia-cachexia syndrome: past, present, and future. Nutrition. 2005;21:977–85. doi: 10.1016/j.nut.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Molecular pathways leading to cancer cachexia. Physiology (Bethesda) 2005;20:340–8. doi: 10.1152/physiol.00019.2005. [DOI] [PubMed] [Google Scholar]
- 5.Argiles JM, Busquets S, Lopez-Soriano FJ. The pivotal role of cytokines in muscle wasting during cancer. Int J Biochem Cell Biol. 2005;37:2036–46. doi: 10.1016/j.biocel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Tisdale MJ. Wasting in cancer. J Nutr. 1999;129:243S–246S. doi: 10.1093/jn/129.1.243S. [DOI] [PubMed] [Google Scholar]
- 7.Lundholm K, Bylund AC, Holm J, Schersten T. Skeletal muscle metabolism in patients with malignant tumor. Eur J Cancer. 1976;12:465–73. doi: 10.1016/0014-2964(76)90036-0. [DOI] [PubMed] [Google Scholar]
- 8.McMillan DC, Scott HR, Watson WS, Preston T, Milroy R, McArdle CS. Longitudinal study of body cell mass depletion and the inflammatory response in cancer patients. Nutr Cancer. 1998;31:101–5. doi: 10.1080/01635589809514687. [DOI] [PubMed] [Google Scholar]
- 9.Emery PW, Edwards RH, Rennie MJ, Souhami RL, Halliday D. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J (Clin Res Ed) 1984;289:584–6. doi: 10.1136/bmj.289.6445.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argiles JM. Cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S39–50. doi: 10.1016/j.ejon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Argiles JM, Busquets S, Felipe A, Lopez-Soriano FJ. Molecular mechanisms involved in muscle wasting in cancer and ageing: cachexia versus sarcopenia. Int J Biochem Cell Biol. 2005;37:1084–104. doi: 10.1016/j.biocel.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain JS. Cachexia in cancer--zeroing in on myosin. N Engl J Med. 2004;351:2124–5. doi: 10.1056/NEJMcibr042889. [DOI] [PubMed] [Google Scholar]
- 13.Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8:265–9. doi: 10.1097/01.mco.0000165004.93707.88. [DOI] [PubMed] [Google Scholar]
- 14.DeJong CH, Busquets S, Moses AG, et al. Systemic inflammation correlates with increased expression of skeletal muscle ubiquitin but not uncoupling proteins in cancer cachexia. Oncol Rep. 2005;14:257–63. [PubMed] [Google Scholar]
- 15.Martignoni ME, Kunze P, Hildebrandt W, et al. Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin Cancer Res. 2005;11:5802–8. doi: 10.1158/1078-0432.CCR-05-0185. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research : principles and practice of kinetic analysis. 2. Hoboken, N.J.: Wiley-Liss; 2005. [Google Scholar]
- 17.Sheffield-Moore M, Yeckel CW, Volpi E, et al. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287:E513–22. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- 18.Sheffield-Moore M, Paddon-Jones D, Sanford AP, et al. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab. 2005;288:E922–9. doi: 10.1152/ajpendo.00358.2004. [DOI] [PubMed] [Google Scholar]
- 19.Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA. Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. Am J Physiol Endocrinol Metab. 2005;288:E761–7. doi: 10.1152/ajpendo.00291.2004. [DOI] [PubMed] [Google Scholar]
- 20.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–8. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 21.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. Jama. 2001;286:1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–7. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 25.Sheffield-Moore M, Urban RJ, Wolf SE, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999;84:2705–11. doi: 10.1210/jcem.84.8.5923. [DOI] [PubMed] [Google Scholar]
- 26.McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004;119:907–10. doi: 10.1016/j.cell.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin Clin Nutr Metab Care. 2005;8:255–63. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- 28.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–73. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 29.Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994;124:906–10. doi: 10.1093/jn/124.6.906. [DOI] [PubMed] [Google Scholar]
- 30.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002-0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom. 1992;6:421–4. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 31.Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism. 1997;46:943–8. doi: 10.1016/s0026-0495(97)90084-6. [DOI] [PubMed] [Google Scholar]
- 32.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol. 1995;268:E75–84. doi: 10.1152/ajpendo.1995.268.1.E75. [DOI] [PubMed] [Google Scholar]
- 33.Katch V, Weltman A. Predictability of body segment volumes in living subjects. Hum Biol. 1975;47:203–18. [PubMed] [Google Scholar]
- 34.Paddon-Jones D, Sheffield-Moore M, Urban RJ, et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89:4351–8. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- 35.Bossola M, Muscaritoli M, Costelli P, et al. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann Surg. 2003;237:384–9. doi: 10.1097/01.SLA.0000055225.96357.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norton JA, Stein TP, Brennan MF. Whole body protein synthesis and turnover in normal man and malnourished patients with and without known cancer. Ann Surg. 1981;194:123–8. doi: 10.1097/00000658-198108000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rennie MJ, Edwards RH, Emery PW, Halliday D, Lundholm K, Millward DJ. Depressed protein synthesis is the dominant characteristic of muscle wasting and cachexia. Clin Physiol. 1983;3:387–98. doi: 10.1111/j.1475-097x.1983.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 38.Vary TC, Deiter G, Kimball SR. Phosphorylation of eukaryotic initiation factor eIF2Bepsilon in skeletal muscle during sepsis. Am J Physiol Endocrinol Metab. 2002;283:E1032–9. doi: 10.1152/ajpendo.00171.2002. [DOI] [PubMed] [Google Scholar]
- 39.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–7. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 40.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–45. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 41.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–31S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]


