Abstract
Large parts of agricultural soil are contaminated with lead (Pb) and cadmium (Cd). Although most environments are not heavily contaminated, the low levels observed nonetheless pose a high risk of heavy metal accumulation in the food chain. Therefore, approaches to develop plants with reduced heavy metal uptake are important. Recently, many transgenic plants with increased heavy metal resistance and uptake of heavy metals were developed for the purpose of phytoremediation. However, to reduce heavy metal in the food chain, plants that transfer less heavy metals to the shoot are required. We tested whether an Escherichia coli gene, ZntA, which encodes a Pb(II)/Cd(II)/Zn(II) pump, could be useful for developing plants with reduced heavy metal content. Yeast cells transformed with this gene had improved resistance to Pb(II) and Cd(II). In Arabidopsis plants transformed with ZntA, ZntA was localized at the plasma membrane and improved the resistance of the plants to Pb(II) and Cd(II). The shoots of the transgenic plants had decreased Pb and Cd content. Moreover, the transgenic protoplasts showed lower accumulation of Cd and faster release of preloaded Cd than wild-type protoplasts. These results show that a bacterial transporter gene, ZntA, can be functionally expressed in plant cells, and that that it may be useful for the development of crop plants that are safe from heavy metal contamination.
Large parts of agricultural soil are contaminated with heavy metals by natural and anthropogenic activities. The major anthropogenic activities that contaminate soil with heavy metals include industrial processes and agricultural practices including the use of fertilizers containing various heavy metals (Ross, 1994). Many fertilizers contain Pb and Cd, and therefore, these heavy metals are found at high concentrations in vast areas of agricultural land (Shull, 1998). Soils contaminated with heavy metals cause contamination of foodstuffs. Many fruits and grains contain Pb (Shaffer, 2001), whereas many leafy vegetables contain Cd (Mench, 1998). Even when the contamination levels are not very high, heavy metal-containing foodstuffs pose the risk of heavy metal accumulation in the food chain, thereby endangering human health. Tobacco (Nicotiana tabacum) plants, for example, naturally accumulate high quantities of Cd, and tobacco smokers are consequently exposed to a high intake of this heavy metal (Elinder CG, 1985). Fetal exposure to Cd, caused by pregnant women smoking tobacco, can result in growth retardation (Office of Environmental Health Hazard Assessment, 1996). Due to such detrimental effects of heavy metals, the decontamination of polluted sites is being actively studied worldwide. However, it is economically impossible to clean up all of the soils mildly contaminated with heavy metals, and it is technically almost impossible to decontaminate natural, rock heavy metal sources. Therefore, it is important to develop plants that have reduced uptake of heavy metals in mildly or highly contaminated soils.
Among heavy metals, Pb is the most important as an environmental pollutant (Salt et al., 1998), because it is found all over the world and damages the growth of children and the health of adults (Lanphear, 1998). Despite the enormous amount of effort that has been expended to develop heavy metal-resistant genes in plants, there has been no report of such a gene, and no plants expressing Pb(II) resistance genes from other organisms have been developed for the purpose of improving Pb(II) resistance. ZntA from Escherichia coli is a Pb(II)/Cd(II)/Zn(II)-transporting ATPase that mediates resistance to toxic concentrations of Pb(II), Cd(II), and Zn(II) by pumping these metals out of the cells (Rensing et al., 1997; Sharma et al., 2000). Therefore, the introduction of the ZntA gene into plants would be useful for development of crops resistant to Pb(II), Cd(II), or Zn(II). However, to our knowledge, no bacterial membrane transporter has yet been introduced into plants and shown to be functional. Here, we show that ZntA can be successfully expressed in the plasma membrane of Arabidopsis, where it confers resistance to Pb(II) and Cd(II) and reduces the heavy metal content of the transgenic plants.
RESULTS
ZntA Expressed in Brewer's Yeast Saccharomyces cerevisiae Enhances Resistance to Pb(II) and Cd(II)
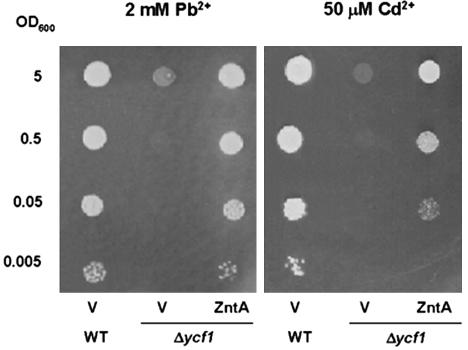
Because ZntA is a bacterial protein, we examined whether the protein could function in a eukaryotic cell using Brewer's yeast. When ZntA was overex-pressed in three lines of wild-type yeast cells, SEY6210, DTY165, and Y800, there was no clear change in Pb(II) or Cd(II) resistance (data not shown). Therefore, we decided to overexpress ZntA in DTY167, which is hypersensitive to Cd(II) due to its deficiency in the YCF1-mediated vacuolar sequestration of Cd(II)-glutathione complexes (Szczypka et al., 1994). We previously found that YCF1 contributes to Pb(II) resistance as well (Song et al., 2003). DTY167 yeast transformed with ZntA grew better on Pb(II)- or Cd(II)-containing media than control ΔYCF1 cells containing empty vector (Fig. 1). Remarkably, the growth of the ZntA transformants was comparable with the isogenic wild-type DTY165 plants when grown on media containing 2 mm Pb(II) or 50 μm Cd(II). Having shown that ZntA can enhance Pb(II) and Cd(II) resistance in a eukaryotic cell, we proceeded to developing plants with enhanced heavy metal resistance.
Figure 1.
Complementation of Pb(II) or Cd(II) sensitivity of the YCF1-null mutant by ZntA. Yeast cells were grown on one-half synthetic Gal media containing 2 mm Pb(NO3)2 or 50 μm CdCl2 at 30°C for 4 d. WT, Wild type; Δycf1, YCF1-null mutant; V, vector only. Note that ZntA rescued the growth of the mutant yeast in Pb(II)- or Cd(II)-containing media almost to the level of the wild type.
ZntA Is Localized in the Plasma Membrane of Transformed Arabidopsis Cells
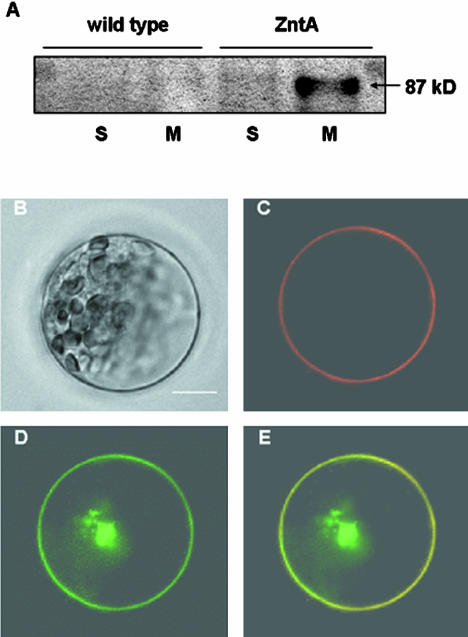
To the best of our knowledge, there has been no previous report of successful expression of a bacterial transporter in plants. Therefore, we first investigated whether ZntA can be expressed in plant cells, and if so, where in the cell the gene product would be localized. ZntA was fused with green fluorescent protein (GFP) under the control of the 35S promoter. The resulting ZntA:GFP construct was transformed into Arabidopsis protoplasts isolated from whole seedlings. We fractionated the protoplast lysates into soluble and membrane parts by ultracentrifugation, and performed western blots using a monoclonal anti-GFP antibody. ZntA:GFP was detected in the membrane fraction of ZntA transformants, whereas no band was detected in the soluble fraction of ZntA transformants or in any fractions of the wild type (Fig. 2A).
Figure 2.
Localization of the ZntA:GFP fusion protein in Arabidopsis protoplasts. A, Protein gel-blot analysis of ZntA:GFP. Soluble (S) and membrane (M) fractions prepared from the wild-type protoplasts or protoplasts transformed with ZntA:GFP were analyzed by western blot using a monoclonal anti-GFP antibody. Note that only the membrane fraction from the transformed cells contains a protein band that cross-reacts with the anti-GFP antibody. B, Bright-field images of a mesophyll cell protoplast. C and D, Fluorescence of AtAHA2:RFP and ZntA:GFP, respectively, from the same cell shown in B. E, An overlapped image of C and D. Bar = 10 μm and applies to all images.
To investigate whether ZntA is localized at the plasma membrane, Arabidopsis protoplasts transformed with ZntA:GFP were observed under a fluorescent microscope (Fig. 2B). In mesophyll (Fig. 2D) and all other cell types (data not shown), the green fluorescence of ZntA:GFP was localized at the periphery of the cells, most likely at the plasma membrane. To confirm the plasma membrane localization of ZntA:GFP, we made a DsRed-fused AtAHA2 construct encoding the plasma membrane H+-ATPase under the control of the 35S promoter. The ZntA:GFP and AtAHA2:RFP constructs were introduced into the Arabidopsis protoplasts simultaneously, and localization of the proteins were examined. The green fluorescence of ZntA:GFP was clearly colocalized with the red fluorescence of AtAHA2:RFP (Fig. 2C), confirming ZntA localization at the plasma membrane in Arabidopsis protoplasts.
Stable Expression of ZntA in Arabidopsis Improves the Pb(II) and Cd(II) Resistance of the Transformed Plants
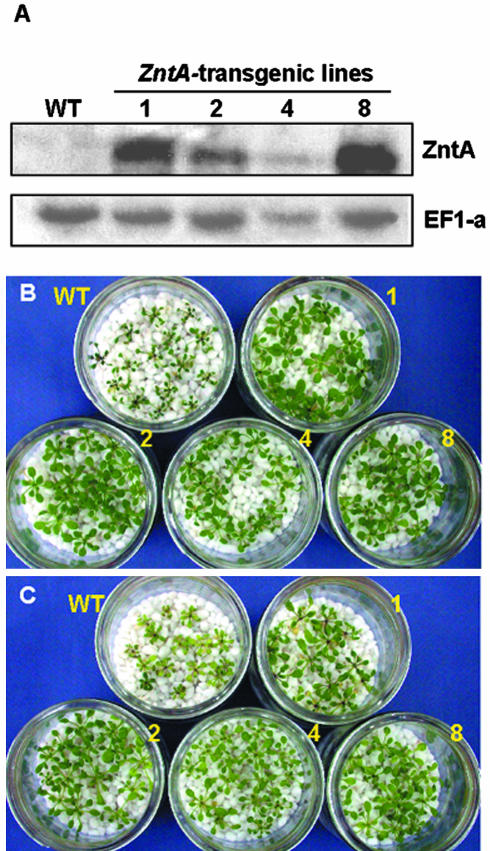
To test whether ZntA can be used to develop plants with enhanced resistance to Pb(II) and Cd(II), we introduced ZntA into Arabidopsis using a pBI121 vector containing the 35S promoter. Four independent lines of transgenic plants (1, 2, 4, and 8) were obtained from selection on kanamycin-one-half Murashige and Skoog agar plates. To detect the ZntA expression, total RNA was isolated from T3 homozygous plants and used for northern-blot analysis. All transgenic lines showed ZntA messages (Fig. 3A).
Figure 3.
Enhanced resistance of ZntA-expressing Arabidopsis plants to Pb(II) or Cd(II). A, RNA gel-blot analysis of whole seedlings of four ZntA-transgenic T3 homozygous lines. Hybridization with an EF1-α probe was used as a loading control. B and C, Photographs of wild-type and ZntA-transgenic T3 homozygous plants. Seeds were germinated and grown on one-half Murashige and Skoog-agar medium with 3% (w/v) Suc for 2 weeks and then transferred onto small gravels wetted with one-half Murashige and Skoog liquid media plus 3% (w/v) Suc containing 0.7 mm Pb-tartarate (B) or 70 μm CdCl2 (C).
To evaluate the resistance of the transformants to heavy metals, seeds of T3 homozygous ZntA-transgenic plants were germinated and grown on one-half Murashige and Skoog plus 3% (w/v) Suc agar plates for 2 weeks, after which the seedlings were transferred onto fine gravel soaked in one-half Murashige and Skoog plus 3% (w/v) Suc medium containing each metal and grown for another 2 weeks. Gravel was chosen to avoid the binding of agar to the heavy metals, which may interfere with the uptake of the metals by the plants. In the control one-half Murashige and Skoog medium without additional heavy metals, the growth of the wild-type and transgenic lines of plants were similar (data not shown). In the medium containing 0.7 mm Pb(II) or 70 μm Cd(II) (Fig. 3, B and C), the transgenic lines grew better than wild-type plants; their leaves were broader and greener, and their fresh weights were higher than those of the wild types (Fig. 4A). In Cd(II)-containing medium, wild-type plants showed chlorosis, whereas the transgenic lines did not (Fig. 3C).
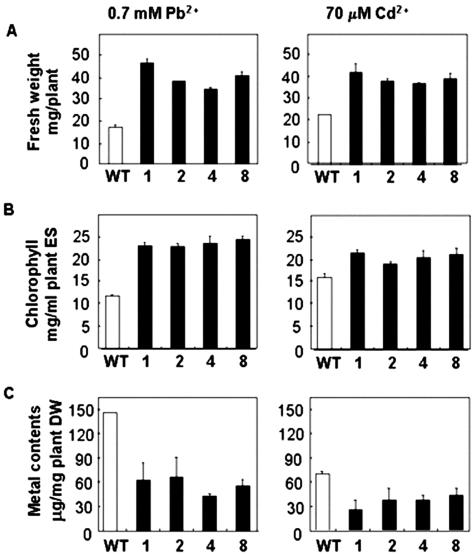
Figure 4.
Phenotypic analysis of wild-type and ZntA-transgenic plants grown in Pb-tartarate or CdCl2-containing one-half Murashige and Skoog medium for 2 weeks. A, Fresh weights. B, Chlorophyll contents. The extract solution (ES) consisted of 20 mg of leaves boiled in 1 mL of ethanol. C, Pb (left) and Cd (right) contents of shoots measured using atomic absorption spectrometer. DW, Dry weight. The average of six independent experiments is shown. Error bars represent se.
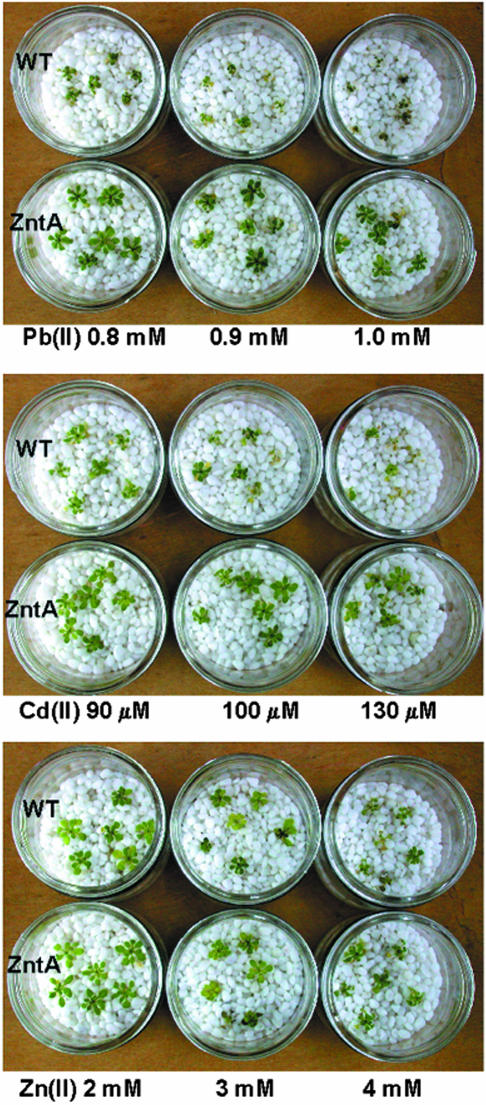
The difference in fresh weight varied among the transgenic lines and ranged from 2.0- to 2.6-fold of wild type in Pb(II)-containing medium, and 1.6- to 1.8-fold of wild type in Cd(II)-containing medium (Fig. 4A). The chlorophyll content of the transgenic lines was 1.9- to 2.0-fold of the wild type in Pb(II)-containing medium, and 1.2- to 1.4-fold of wild type in Cd(II)-containing medium (Fig. 4B). The growth of ZntA-transgenic plant line 1 was tested using various metals at different concentrations (Fig. 5, 0.8, 0.9, 1 mm Pb(II); 90, 110, 130 μm Cd(II); 2, 3, 4 mm Zn(II)). ZntA-transgenic plants showed more prominent phenotypic differences compared with wild-type plants in Pb(II)-containing medium than in Cd(II)- or Zn(II)-containing media. The difference in phenotypes was evident at all three concentrations of Pb(II) tested, but was more pronounced at higher concentrations. In Cd(II)-containing medium, ZntA-transgenic plants were also more resistant than wild type, and their phenotypic differences became more pronounced as the concentration of Cd(II) increased. In Zn(II)-containing medium, transgenic lines were only slightly more tolerant than wild-type plants. The difference in the extent of resistance to Pb(II), Cd(II), and Zn(II) of the ZntA-transgenic plants corresponds to the fact that ZntA activity is stimulated more by Pb(II) than by Cd(II) or Zn(II) (Sharma et al., 2000).
Figure 5.
Enhanced resistance of ZntA-transgenic Arabidopsis plants to various concentrations of Pb(II), Cd(II), or Zn(II). Seeds of wild-type and ZntA-transgenic T3 homozygous plants were germinated, grown on one-half Murashige and Skoog-agar medium plus 3% (w/v) Suc for 2 weeks, and then transferred onto small gravels wetted with one-half Murashige and Skoog plus 3% (w/v) Suc media containing 0.8, 0.9, or 1.0 mm Pb-tartarate (A); 90, 110, or 130 μm CdCl2 (B); or 2, 3, or 4 mm ZnCl2 (C). Photographs were taken 10 d after the onset of the heavy metal treatment.
The ZntA-Expressing Transgenic Arabidopsis Plants Have Reduced Pb and Cd Contents
In E. coli, ZntA pumps Pb(II), Cd(II), and Zn(II) from the cytoplasm into the medium. Thus, the high resistance to Pb(II) and Cd(II) observed in ZntA-expressing plants may be due to a high rate of Pb(II) and Cd(II)-pumping activity in these plants. Therefore, we measured the levels of Pb and Cd in wild-type and ZntA-transgenic plants grown in media containing heavy metals. In roots, there was no significant difference in the Pb or Cd levels of wild-type and ZntA-transgenic plants (data not shown). In shoots, the Pb content of the transgenic plants was consistently lower than that of the wild-type plants (Fig. 4C); the average Pb level in shoots of transgenic lines was about 45% of wild-type plants, and in line 4, the Pb level was only about 30% of control. The Cd contents in the shoots were also lower in transgenic lines than in the control, although the difference was not as great as for the Pb levels; ZntA-transgenic plants showed Cd levels 46% to 67% of the control level (Fig. 4C). Short-term radiotracer flux data using 109Cd were consistent with these results: The Cd content in the shoots of plants transformed with ZntA was lower than that of wild-type plants, whereas the Cd content in roots did not differ (Supplemental Fig. 1, which can be found in the online version of this article under supplemental data at http://www.plantphysiol.org.).
ZntA-Expressing Arabidopsis Cells Accumulate Less Cd and Release Cd Faster Than Wild-Type Cells
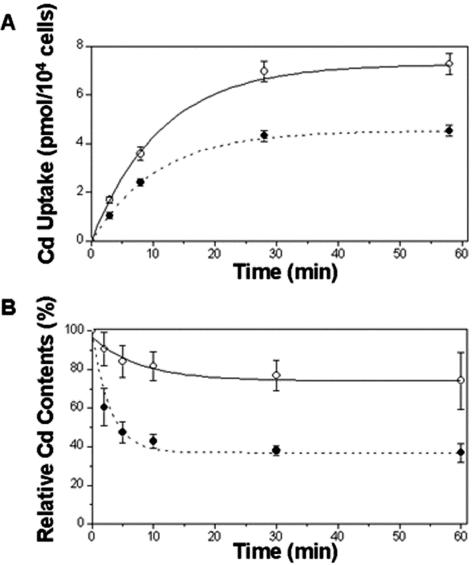
To obtain direct evidence for the mechanism leading to heavy metal resistance in the ZntA-transgenic plants, we performed flux studies using radioactive Cd(II) and protoplasts isolated from the ZntA-transgenic and wild-type plants. Cd accumulation in wild-type cells was higher than in ZntA transformants throughout the 60 min of incubation in 109Cd(II)-containing medium (Fig. 6A). Both types of cells seemed to approach saturation in 1 h, when the Cd content of control cells was 1.6-fold of that of ZntA transformants. The transformed protoplasts may export Cd actively via ZntA, and therefore cannot accumulate as much Cd within the cells as wild-type protoplasts do. Cd efflux was faster in ZntA-transgenic protoplasts than in wild-type protoplasts during the initial phase until 10 min (Fig. 6B). The curves shown in Figure 6B represent first order exponential functions obtained by fitting the data with a software (Origin 7.0, OriginLab, Northampton, MA). The slopes of the tangent lines in the Cd content graphs were –2.74 (T = 2 min) and –1.56 (T = 5 min) for the wild type and –11.0 (T = 2 min) and –2.60 (T = 5 min) for the ZntA transformant. After 10 min, the Cd content did not decrease much further in either the transformant or the wild-type protoplasts (Fig. 6B). The portion of Cd remaining in the protoplasts after 60 min of washing was 74% of the initial amount for the wild type and 37% of the initial amount for the ZntA-transgenic protoplasts (Fig. 6B). These results show that ZntA-transgenic cells release Cd faster and store less Cd than wild-type cells.
Figure 6.
Uptake and release of Cd by ZntA-transgenic and wild-type protoplasts. A, Time-dependent increase of Cd content in protoplasts of wild-type and ZntA-transgenic plants incubated in 10 μm CdCl2. B, Time-dependent decrease of Cd content in protoplasts of wild-type and ZntA-transgenic plants preloaded with 10 μm CdCl2, and then washed with Cd-free bathing solution. Cd content was normalized as the percentage of the Cd content at T = 0 min for each cell type. •, ZntA-expressing cells; ○, wild-type protoplasts. Assays were performed in triplicate and repeated three times. Error bars represent se.
DISCUSSION
In this study, we showed that a bacterial heavy metal pump, ZntA, can be expressed in Arabidopsis, resulting in increased resistance to Pb(II) and Cd(II) and reduced heavy metal contents in the shoots of the transgenic plants (Figs. 3 and 5). The enhanced resistance is probably due to the exclusion of heavy metals, as suggested by the decreased metal contents (Fig. 4C). ZntA-transgenic plants most likely exclude heavy metals at the cellular level by pumping the heavy metals from the plasma membrane to the extracellular space, because the transgenic protoplasts accumulated less Cd (Fig. 6A) and released Cd faster than wild-type protoplasts (Fig. 6B). This explanation is further supported by the fact that ZntA is localized at the plasma membrane in transgenic Arabidopsis cells (Fig. 2), and ZntA exports Cd(II)-thiolate complexes from the plasma membrane of E. coli (Sharma et al., 2000). Only a small portion of the total Cd was released from the protoplasts during our efflux study, which may have occurred because Cd interacts very strongly with -SH moieties such as those found in phytochelatins, proteins, and other groups of macromolecules. Therefore, only a minor fraction of the total Cd may have been available in the transportable, low Mr Cd-thiolate form. However, the initial efflux was faster in the transgenic lines than that in wild type. These results suggest that ZntA can function as a heavy metal export pump when expressed in plant cells, as it does in E. coli. Thus, ZntA can be used to develop plants with enhanced resistance to and reduced uptake of heavy metals.
Interestingly, the decrease in the heavy metal content of the ZntA plants was evident in the shoots but not in the roots (Fig. 4C and Supplemental Fig. 1). Because ZntA is under the control of the 35S promoter for expression in plants in this study, the gene is most likely expressed in the roots as well as in the shoots. It is possible that the heavy metal contents were not different in the transgenic roots because they are directly exposed to high concentrations of the heavy metals in the medium, and therefore are likely to have high levels of Cd(II) and Pb(II) in sequestered and precipitated forms and in the apoplast. The decreased heavy metal content in the shoots of ZntA-transgenic lines show that less of the heavy metals is translocated to the shoots from the roots of ZntA plants. Therefore, we can conclude that the outer root cells of the ZntA-transgenic plants efficiently exported heavy metals from symplasm before the metals reach the cytoplasm of endodermis. The Casparian strip in endodermis ensures that only heavy metals that enter the cytoplasm of the endodermal cells can be loaded into the xylem for long-distance transport to the shoot. Therefore shoots usually contain only a minor portion of the total heavy metals, and in rice grown in high Pb(II), less than 5% of the total Pb was found in the shoots (Kim et al., 2002). In the shoot, heavy metals can be excluded from each cell by ZntA and can remain in the apoplast, thus decreasing their toxicity. This results in less deposition of the metals in cellular compartments such as vacuoles and a consequent reduction of the shoot sink of heavy metals, which may in turn have led to a decrease in metal translocation and accumulation.
There have been reports of transgenic plants with improved resistance to mercury (Rugh et al., 1998; Bizily et al., 1999), Cd (Cobbett, 2000), or Al (Ma et al., 1997). Our ZntA-transgenic plants conferred resistance not only to Cd(II) but also to Pb(II), an important advance in the field of biotechnology of heavy metal resistance. The improved growth of the ZntA-transgenic plants compared with the wild-type plant was more pronounced in Pb(II)-containing medium than in Cd(II)- or Zn(II)-containing media (Fig. 5). This differential response of ZntA to different heavy metals is reminiscent of an observation made in E. coli where ZntA activity was found to be stimulated more by Pb(II) than by Cd(II) or Zn(II) (Sharma et al., 2000).
Transgenic ZntA Arabidopsis plants show that it is feasible to develop plants with enhanced resistance and reduced uptake of heavy metals. If ZntA can be introduced into crop plants, the resulting plants would be safer because they would contain less heavy metal in their shoots. Moreover, the productivity of the plant would remain high even in heavy metal-contaminated soils. The enhanced heavy metal resistance of the ZntA-transgenic plants offers another exciting possibility. If ZntA can be introduced into perennial plants of high biomass, like poplar (Populus spp.) for example, they could serve for phytostabilization. The transgenic plants would survive in soils contaminated by Pb(II) and/or Cd(II), thereby efficiently holding the contaminated soil, reducing erosion, and preventing the migration of the contaminated soil into the water table. Therefore, ZntA-transgenic plants have the potential both as crops with reduced levels of toxic metals and as stabilizing elements in heavy metal-contaminated environments.
Currently, the normal range of heavy metal concentration of soils is considered to be between 2 and 200 mg kg–1 for Pb and between 0.01 and 7 mg kg–1 for Cd (Ross, 1994), although the amounts available for the roots may differ depending on the soil composition. ZntA-transgenic plants showed enhanced resistance to solutions containing up to 207 mg kg–1 Pb(II) and 14.6 mg kg–1 Cd(II), which are slightly above the normal range. Therefore, ZntA-transgenic plants may give both a higher yield and reduced heavy metal content in soils considered slightly above normal with respect to heavy metal content. This would make them relevant for the agriculture of crops that are safe from heavy metals.
In summary, we present for the first time a plant with enhanced resistance to Pb(II). Furthermore, to our knowledge this is the first transgenic plant that functionally expresses a bacterial transporter. Therefore, our study opens the possibility of using bacterial transporters to develop plants with improved resistance to and reduced uptake of heavy metals. To test this possibility, we are currently developing ZntA-transgenic tobacco and poplar plants for use in the field.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis wild-type seeds (Arabidopsis ecotype Columbia 0) and transgenic 35S-ZntA seeds were surface sterilized, placed in the dark at 4°C for 2 d, and then sown on plates of one-half Murashige and Skoog with 3% (w/v) Suc (Murashige and Skoog, 1962) or in soil.
Cloning of ZntA and Plasmid Constructions
DNA manipulations were performed according to standard methods (Sambrook and Russell, 2001). ZntA (GenBank accession no. NP_417926) was isolated by PCR using the genomic DNA of the Escherichia coli K-12 strain as a template and two primers (5′-GGATCCAAAATAAAGAAGAACAATGTCGACTCCTGACAAT-3′ and 5′-GGATCCCTCTCCTGCGCAACAATCT-3′). The fidelity of all constructs was confirmed by sequencing.
Expression of ZntA in Brewer's Yeast (Saccharomyces cerevisiae)
ZntA was inserted into the BamHI site of pESC-URA (Stratagene, La Jolla, CA), yielding pESC-ZntA. pESC-ZntA and pESC were transformed into the Brewer's yeast ycf1Δ strain DTY167 (MATura3-52 leu2-3,-112 his-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 ycf1::hisG; Szczypka et al., 1994) and its isogenic wild type, DTY165, by the lithium acetate method (Ito et al., 1983). For heavy metal-resistance tests, the yeast strains were grown on one-half synthetic Gal media without (control) or with 2 mm Pb(NO3)2 or 50 μm CdCl2 at 30°C for 4 d.
Expression of ZntA:GFP in Arabidopsis Protoplasts
For transient expression of ZntA in Arabidopsis protoplasts, ZntA was fused with GFP and inserted into an mGFP vector, and AtAHA2:RFP was used to identify the plasma membrane (Kim et al., 2001). Arabidopsis protoplasts were prepared as described (Abel and Theologis, 1998) and were transformed with each plasmid by the PEG method (Jin et al., 2001). Expression of protein was monitored after 2 to 3 d, and images were captured with a cooled CCD camera using an Axioplan fluorescence microscope (Zeiss, Oberkochen, Germany). The filter sets used were Filter set 44 (excitation; BP 455–495, beamsplitter; FT500, emission; BP 505–555) for GFP and Filter set 20 (excitation; BP 540–552, beamsplitter; FT560, emission; BP 575–640) for red fluorescent protein (Zeiss).
To confirm the expression of ZntA-GFP in the Arabidopsis protoplasts, membrane and soluble fractions were obtained from crude homogenates of the protoplasts by ultracentrifugation at 100,000g for 1 h, and western blots were performed as described (Sambrook and Russell, 2001) using a monoclonal anti-GFP antibody (BD Biosciences Clontech, Palo Alto, CA).
Stable Transformation of Arabidopsis with ZntA
For stable transformation, ZntA was inserted in the BamHI site of pBI121 (Jefferson et al., 1987), which contains a 35S promoter, and the LBA4404 strain of Agrobacterium tumefaciens was used to transform Arabidopsis by the floral dipping method (Clough and Bent, 1988). Transformed seeds were tested for kanamycin resistance on solid Murashige and Skoog medium containing 50 μg μL–1 kanamycin. T3-generation seeds were used for tests.
To detect the expression level of ZntA in transgenic plants, total RNA was extracted from Arabidopsis plants grown on one-half Murashige and Skoog-agar medium supplemented with 3% (w/v) Suc for 3 weeks. Subsequent RNA preparation and northern hybridization were carried out as described (Sambrook and Russell, 2001).
Measurements of Fresh Weight, Chlorophyll Content, and Heavy Metal Contents in Arabidopsis
Arabidopsis plants were grown on one-half Murashige and Skoog-agar medium supplemented with 3% (w/v) Suc for 2 weeks and then transferred to one-half Murashige and Skoog liquid medium supported by small gravel with or without CdCl2 or Pb(NO3)2. After growing for an additional 2 weeks, the plants were harvested, rinsed in ice-cold 1 mm tartarate solution, and blot-dried. Thirteen plants of each T3 homozygous line were collected, weighed, and digested with 11N HNO3 at 200°C overnight. Digested samples were diluted with 0.5 n HNO3 and analyzed using an atomic absorption spectrometer (SpectrAA-800, Varian, Palo Alto, CA).
For determination of chlorophyll content, the leaves were harvested, and chlorophyll was extracted with 95% (v/v) ethanol for 20 min at 80°C. A664 and A648 were measured, and the chlorophyll A and B contents were calculated as described (Oh et al., 1997).
Cd(II) Flux Assay in Arabidopsis Protoplasts
Wild-type and transgenic protoplasts were isolated, purified, and concentrated using a percoll gradient (Cline et al., 1985). Uptake assays followed the protocol described previously (Martinoia et al., 1993) with slight modifications. Approximately 5 × 105 protoplasts were incubated in 500 μL of a bathing solution (0.5 m Gly betaine, 10 μm CaCl2, 10 mm MES-KOH [pH 5.6], and 10 μm CdCl2) containing 18.5 kBq of 3H2O and 4.6 kBq of 109Cd for 0, 2, 10, 30, or 60 min, and then healthy protoplasts were collected by centrifugation at 10,000g for 20 s using percoll and silicon oil gradients. Only the healthy protoplasts thus obtained were counted for their 109Cd and 3H2O contents using a γ-ray counter (1470 Wizard, Wallac, Boston) and a liquid scintillation counter (Tri-CARB2100TR, Packard BioScience, Meriden, CT), respectively. To obtain only the time-dependent uptake into the cells, the radioactivity at T = 0 min was subtracted from all data values.
For efflux assays, purified Arabidopsis protoplasts were first loaded with109Cd and 3H2O by incubation in a bathing solution (0.5 m Gly betaine, 100 μm CaCl2, 10 mm MES-KOH [pH 5.6], and 10 μm CdCl2) containing 18.5 kBq of 3H2O and 4.6 kBq of 109Cd for 60 min and then centrifuged for 5 min at 46g. The bathing solution was discarded, and the cells were resuspended and incubated in a Cd-free bathing solution consisting of 0.5 m Gly betaine, 100 μm CaCl2, and 10 mm MES-KOH (pH 5.6) for 0, 2, 5, 10, 30, or 60 min. After each incubation period, healthy protoplasts were collected, and their 109Cd and 3H2O contents were measured as described above. The number of protoplasts collected was quantified from the radioactivity of 3H2O as described previously (Martinoia et al., 1993), and the protoplast number was used to normalize the 109Cd contents.
Supplementary Material
Acknowledgments
We thank Drs. Ildoo Hwang and Nava Moran for critical reading of the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.021972.
This work was supported by Pohang Iron and Steel Company, by the National Research Laboratory program and the Israel-Korea collaborative research program of the Ministry of Science and Technology of Korea (to Y.L.), by the Körber Stiftung (to E.M.), and by the Creative Research Initiative Program of the Ministry of Science and Technology of Korea (grant no. M10116000005–02F0000–00310 to I.H.).
The online version of this article contains Web-only data.
References
- Abel S, Theologis A (1998) Transient gene expression in protoplasts of Arabidopsis thaliana. Methods Mol Biol 82: 209–217 [DOI] [PubMed] [Google Scholar]
- Bizily SP, Rugh CL, Summers AO, Meagher RB (1999) Phytoremediation of methylmercury pollution: merB expression in Arabidopsis thaliana confers resistance to organomercurials. Proc Natl Acad Sci USA 96: 6808–6813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Lubben TH, Keegstra K (1985) Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem 260: 3691–3696 [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123: 825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder CG (1985) Cadmium: Uses, Occurrence, and Intake. Cadmium and Health. CRC Press, Boca Raton, FL
- Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153: 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong G-W, Hwang I (2001) A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13: 1511–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Eu YJ, Yoo CM, Kim YW, Pih KT, Jin JB, Kim SJ, Stenmark H, Hwang I (2001) Trafficking of phosphatidylinositol 3-phosphate from the trans-golgi network to the lumen of the central vacuole in plant cells. Plant Cell 13: 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YY, Yang YY, Lee Y (2002) Pb and Cd uptake in rice root. Physiol Plant 116: 368–372 [Google Scholar]
- Lanphear BP (1998) The paradox of lead poisoning prevention. Science 281: 1617–1618 [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H, Hiradate S (1997) Detoxifying aluminium with buckwheat. Nature 390: 569–5709403684 [Google Scholar]
- Martinoia E, Grill E, Tommasini R, Kreuz K, Amrhein N (1993) ATP-dependent glutathione S-conjugate `export' pump in the vacuolar membrane of plants. Nature 364: 247–249 [Google Scholar]
- Mench MJ (1998) Cadmium availability to plants in relation to major long-term changes in agronomy systems. Agric Ecosyst Environ 67: 175–187 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Office of Environmental Health Hazard Assessment (1996) Reproductive and Cancer Hazard Assessment, Evidence of Developmental and Reproductive Toxicity of Cadmium. California Environmental Protection Agency, Sacramento, CA
- Oh SA, Park JH, Lee GI, Paek KH, Park SK, Nam HG (1997) Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J 12: 527–535 [DOI] [PubMed] [Google Scholar]
- Rensing C, Mitra B, Rosen BP (1997) The ZntA gene of Escherichia coli encodes a Zn(II)-translocating P-type ATPase. Proc Natl Acad Sci USA 94: 14326–14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SM (1994) Sources and forms of potentially toxic metal in soil-plant system. In SM Ross, eds, Toxic Metals in Soil-Plant Systems. John Wiley & Sons, New York, pp 3–25
- Rugh CL, Senecoff JF, Meagher RB, Mercle SA (1998) Development of transgenic yellow poplar for mercury phytoremediation. Nat Biotechnol 16: 925–928 [DOI] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I (1998) Phytoremediation. Annu Rev Plant Physiol Plant Mol Biol 49: 643–668 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual, Ed 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Shaffer M (2001) Waste lands: the threat of toxic fertilizer. California's Advocate for the Public Interest, Los Angeles, CA
- Sharma R, Rensing C, Rosen BP, Mitra B (2000) The ATP hydrolytic activity of purified ZntA, a Pb(II)/Cd(II)/Zn(II)-translocating ATPase from Escherichia coli. J Biol Chem 275: 3873–3878 [DOI] [PubMed] [Google Scholar]
- Shull LR (1998) Non-Technical Summary: Development of Risk-Based Concentration for Arsenic, Cadmium, and Lead in Inorganic Commercial Fertilizers. Report Prepared for the California Department of Food and Agriculture and the Heavy Metal Task Force. Newfields Inc., West Sacramento, CA
- Song W-Y, Sohn EJ, Martinoia E, Lee YJ, Yang Y-Y, Jasinski M, Forestier C, Hwang I, Lee Y (2003) Engineering tolerance and accumulation of lead and cadmium in transgenic plants. Nat Biotechnol (in press) [DOI] [PubMed]
- Szczypka MS, Wemmie JA, Moye-Rowley WS, Thiele DJ (1994) A yeast metal resistance protein similar to human cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance-associated protein J Biol Chem 269: 22853–22857 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.