Abstract
Maize (Zea mays) leaves develop basipetally (tip to base); the upper blade emerges from the shoot apical meristem (SAM) before the expansion of the lower sheath. Founder cells, leaf initials located in the periphery of the SAM, are distinguished from the SAM proper by the differential accumulation of KNOX proteins. KNOX proteins accumulate in the SAM, but are excluded from maize leaf primordia and leaf founder cells. As in Arabidopsis and tomato (Lycopersicon esculentum), maize shoots failed to initiate new leaves when cultured in the polar auxin transport inhibitor N-1-naphthylphthalamic acid (NPA). We demonstrate that NPA-induced arrest of leaf initiation in maize is correlated with the failure to down-regulate KNOX accumulation in the SAM. In addition, NPA-cultured shoots formed abnormal tubular leaf bases in which the margins failed to separate in the lower leaf zone. The tubular leaf bases always formed in the fourth leaf from the arrested meristem. Moreover, the unseparated margin domains of these tubular leaf bases accumulated ectopic KNOX protein(s). Transfer of NPA-cultured apices to NPA-free media resulted in the resumption of leaf initiation from the SAM and the restoration of normal patterns of KNOX down-regulation, accordingly. These data suggest that the lower sheath margins emerge from the leaf base late in maize leaf development and that the separation of these leaf margin domains is correlated with auxin transport and down-regulation of KNOX proteins. In addition, these results suggest that the down-regulation of KNOX accumulation in maize apices is not upstream of polar auxin transport, although a more complicated feedback network may exist. A model for L1-derived margin development in maize leaves is presented.
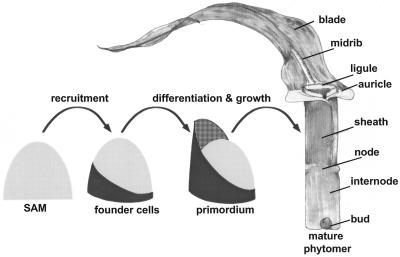
Plant shoots are comprised of repeated structural segments called phytomers (Fig. 1), each comprised of the leaf, stem, and lateral bud (Sharman, 1942; Galinat, 1959). The phytomeric structure of plants shoots is best illustrated in the morphology of grasses such as maize (Zea mays), in which reduced lateral branch length and an alternate phyllotaxy reveals the stacked arrangement of the lateral organ segments. Fate mapping analyses (Poethig, 1984) demonstrated that approximately 200 founder cells of the maize phytomer are recruited from the shoot apical meristem (SAM) as a clonally, non-distinct unit (Fig. 1). Subsequent development of the phytomer into component parts occurs basipetally, such that the apical domains of the upper leaf blade differentiate first, and the lower domains of the leaf sheath and stem differentiate last (Poethig and Szymkowiak, 1995). Thus, models of early phytomer development distinguish two stages, namely: (a) recruitment of founder cells, and (b) subsequent growth and development of distinct phytomer components (Fig. 1). Recruitment of maize leaf founder cells from the SAM is correlated with the expression of knox (knotted1-like homeobox) genes; knox genes are expressed in the SAM proper but are down-regulated in maize leaf founder cells and leaf primordia (Smith et al., 1992; Jackson et al., 1994).
Figure 1.
Maize phytomer development. Cartoon depicting the development of the maize vegetative phytomer, the repeating plant segment comprised of the leaf, internode, and lateral bud. Approximately 200 founder cells are recruited from the periphery of the SAM. Once recruited, the founder cells grow and multiply into a young primordium. Later, the primordium grows further, and differentiates the specific tissue and organ domains found in the mature phytomer (drawing by M. Mooney).
Auxin production and transport has profound effects on plant development (for review, see Berleth and Sachs, 2001). An array of plant developmental processes, including root development, lateral bud initiation, vascular patterning, and the establishment of embryonic polarity are affected by changes in auxin concentration. Although auxins are transported acropetally (toward the shoot tip) and basipetally (toward the root base) in plants, the overall directionality of auxin transport is basipetal (for review, see Estelle, 1998). This polarized movement of auxin is probably mediated by auxin efflux proteins, which localize predominantly to the basal ends of plant shoot cells (Galweiler et al., 1998). Immunohistolocalization (Avsian-Kretchmer et al., 2002) and mass spectrophotometric analyses (Ljung et al., 2001) of auxin accumulation in Arabidopsis suggest that most (if not all) of the bioactive auxin indole acetic acid (IAA) present in the smallest leaf primordia and in the SAM is produced in and transported from larger leaves. In addition, recent work has demonstrated that IAA transport is required for the initiation of lateral organs from shoot meristems in tomato (Lycopersicon esculentum) and Arabidopsis (Reinhardt et al., 2000). When grown in the presence of chemical inhibitors of auxin transport, new leaf formation is arrested. Intriguingly, direct application of IAA to the SAM of transport-inhibited tomato shoots results in the resumption of lateral organogenesis, invoking a requirement for IAA during the formation of leaf and flower primordia.
Here, we demonstrate that, as in tomato and Arabidopsis, maize leaf initiation is blocked by N-1-naphthylphthalamic acid (NPA), a chemical inhibitor of polar auxin transport (PAT). In addition, we show that NPA treatment causes a failure to separate the margins of the lowermost region of the leaf, resulting in the formation of a tubular lower leaf zone. Intriguingly, the arrest of leaf initiation is correlated with the failure to down-regulate KNOX accumulation in the peripheral zone of the SAM, whereas ectopic KNOX accumulation occurs in the vicinity of the fused leaf margins. Movement of treated shoots to NPA-free media results in the resumption of leaf initiation and normal patterns of KNOX down-regulation. Likewise, exogenous application of auxin to NPA-treated meristems is correlated with the initiation of a new leaf buttress. These data provide evidence against a simple model in which knox gene regulation occurs upstream of auxin transport (Tsiantis et al., 1999) and suggest that margin separation requires KNOX down-regulation and auxin transport. A model for maize leaf margin development is presented.
RESULTS
PAT Inhibition Prevents New Leaf Initiation and Affects Growth of Existing Leaves
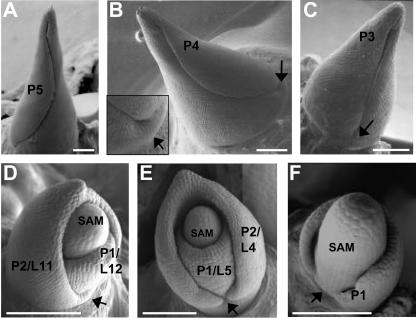
Maize seedlings were harvested 14 d after germination, and the shoots were dissected to remove all but the last five leaf primordia (developing leaves are designated by a plastochron number and/or a leaf no., as described by Sylvester et al., 1990, and in “Materials and Methods”). Subsequently, the P5 apices were placed in maize tissue culture media (MCM; Irish and Nelson, 1988) with and without the inhibitor of PAT, NPA. In addition to its inhibitory effects on PAT, NPA (and all other known inhibitors of PAT) disrupts membrane trafficking in general (Geldner et al., 2001). Development of maize leaf hairs is stage specific (Sharman, 1942; Sylvester et al., 1990); P5 leaves are devoid of large hairs, whereas P6 leaves contain hairs near the leaf tip. Thus, the presence of hairs, in addition to the size of the primordium, are used as a marker of leaf developmental stage. At the P5 stage of development (Fig. 2A), maize leaves are approximately 6 mm high, and the margins surround and obscure the disc of insertion (Sharman, 1942), where the leaf emerges from the stem. This disc of insertion can be observed in the next smallest leaf (P4) and forms a collar at the base of the leaf primordium (Fig. 2B). Likewise, the collar is evident at the bases of early P3 and P2 leaves (Fig. 2, C and D), well before the edges of the developing leaf primordium grow to surround the apex.
Figure 2.
Initiation of maize leaf primordia is arrested by NPA. Scanning electron micrographs of successive stages in development of normal maize leaf primordia (A–D). Arrows, Disc of insertion, a collar-like structure at the leaf base. Maize shoots containing five leaf primordia were cultured for 20 d without NPA (D); 12 total leaves were formed. In contrast, equivalent shoots cultured in NPA (E and F) failed to generate new leaf primordia and were developmentally arrested at five total leaves. Note that the NPA-treated P2 primordium in D lacks a prominent collar in the space between the leaf margins (arrow), whereas the P1 primordium in E shows incomplete lateral development. SAM, Vegetative SAM; L, leaf number; P, plastochron stage of development, wherein a P1/L12 leaf designates the 12th total leaf made by the SAM (L12) at the developmental stage when it is the closest primordium to the SAM (P1), as explained in “Materials and Methods.” All bars = 200 μm.
After 20 d in culture, P5-dissected maize shoots grown in 30 μm NPA failed to initiate any new leaf primordia (Figs. 2, E and F, and 3, B and D), whereas equivalent siblings grown in MCM alone produced five to seven additional leaves (Figs. 2D and 3, A and C) and averaged 10.19 total leaves (Table I). Frequently, the NPA-cultured shoot meristems continued to grow and formed an enlarged SAM, whereas new leaf initiation was arrested (Figs. 2, 3, 4, and 5; Table I). Careful analyses of the leaf primordia contained in the NPA-treated apices revealed unequal growth and development among the five extant leaves (Table II; Fig. 3). In particular, the older leaf primordia (P5–P4) achieved much greater growth in the presence of the PAT inhibitor than did the smaller primordia (P3–P1), whereas formation of new leaf primordia (P0) was abrogated (Tables I and II; Figs. 2 and 3). Growth of the oldest three NPA-treated leaf primordia is not significantly different from that observed in untreated controls (Table II). In contrast, the fourth and fifth leaf primordia of NPA cultured shoots exhibited far less growth than those of control shoots (Fig. 3). Therefore, the extent of leaf development during PAT inhibition is correlated with the size of the leaf primordium. Moreover, SEM analyses of PAT-arrested P1 and P2 leaves reveal the absence of a prominent collar at the primordial leaf bases, in addition to a reduction in lateral growth of the lower domain of the youngest leaf primordia (Fig. 2, E and F).
Figure 3.
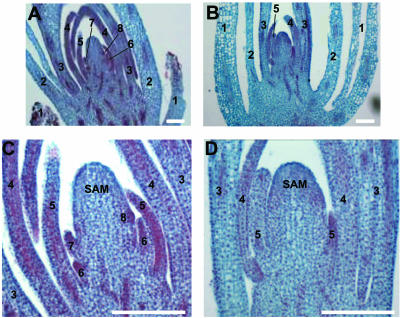
Effects of NPA on growth of young leaf primordia. A and C, Light micrographs of shoots containing five leaf primordia cultured for 14 d without NPA. Note that three new leaf primordia (6–8, a small protrusion on the right flank of the SAM) have developed in culture, and the first leaf primordium (1) was dissected to aid in tissue fixation. B and D, Equivalent shoots cultured in 30 μm NPA. Note that five total leaf primordia are present, and no new leaves are initiated. In addition, the NPA-treated fourth and fifth leaves (4 and 5 in B and D) are significantly smaller than corresponding leaves of shoots cultured without NPA (4 and 5 in A and B). Thus, NPA treatment induces the arrest of new leaf initiation and retards growth of existing young leaf primordia. Numbers refer to the leaf number (as opposed to the plastochron number) as described in Figure 2 and in “Materials and Methods.” Bars in C and D = 100 μm and A and B = 250 μm.
Table I.
Effects of NPA on leaf initiation in cultured P5 maize shoot apices
Data represent total leaf counts of maize shoots dissected to contain five leaf primordia and grown in culture.
| Time in Culture | MCM | NPA |
|---|---|---|
| 12 d | ||
| No. of samples | 7 | 9 |
| No. of leaves | 7.29, sd = 0.49 | 5.11, sd = 0.33 |
| 14 d | ||
| No. of samples | 15 | 20 |
| No. of leaves | 7.80, sd = 0.56 | 5.15, sd = 0.37 |
| 20 d | ||
| No. of samples | 16 | 19 |
| No. of leaves | 10.19, sd = 0.91 | 5.32, sd = 0.58 |
Figure 4.
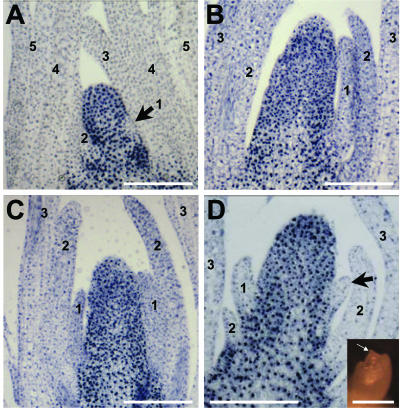
NPA induces tubular leaf bases in P4 leaves. A through C, Light micrographs of representative serial, transverse sections through maize shoot apices cultured for 14 d in NPA-free media. A, Above the SAM; B, at the level of the first leaf primordium; C, below the first leaf primordium. Note that leaf primordia have separated, thin, tapered margins. Arrows show P4 (plastochron stage 4, such that a P4 leaf is the fourth leaf removed from the SAM as described in “Materials and Methods”) margins in A through C and in G. Equivalent shoots cultured in NPA show tubular leaf bases in P4 leaves (arrows in D through F and H) that result from the fusion of margin domains. I, Samples cultured for 14 d in NPA and switched to NPA-free media for 20 d have initiated three new leaf primordia (labeled 6–8 according to total leaf number as described in Fig. 2 and “Materials and Methods”) yet retain the fused, lower leaf margins of leaf 2, which was at the P4 stage of development when placed in NPA culture. The outer leaf (leaf 1) was removed to aid in fixation. Bars in A through F and in I = 100 μm. Bars in G and H= 250 μm.
Figure 5.
NPA disrupts KNOX accumulation in the maize SAM. KNOX immunolocalization analyses of longitudinal sections of maize shoots. A, In shoots cultured for 20 d without NPA, KNOX proteins accumulate in the SAM and developing stems but are excluded from leaves and leaf founder cells located in the periphery of the SAM (arrow in A). B and C, In shoots cultured 20 d in NPA, leaf initiation is arrested, whereas SAMs often become enlarged. Note that the size of the smallest leaf primordia (leaf labeled 1 in B and C) is much larger in the arrested shoots than in the unarrested shoot (leaf labeled 2 in A). Correlated with the lack of leaf initiation is the failure to down-regulate KNOX accumulation in the SAM (B and C). Application of 10 mm IAA dissolved in lanolin paste to NPA-cultured shoots (arrow in inset in D) resulted in the formation of a new lateral organ buttress (arrow in D) after 3 additional d in NPA. Leaf labels (1–5) in this figure denote the developmental stage as Plastochron number (as opposed to total leaf no.) as described previously.
Table II.
Leaf growth in B73 maize shoot apices cultured for 12 d
Data represent mean of total leaf length (in centimeters) of 10 leaves from shoots cultured for 12 d.
| 12 d after Pollination | MCM | NPA |
|---|---|---|
| L1 | 1.89, sd = 0.24 | 2.38, sd = 0.47 |
| L2 | 0.46, sd = 0.16 | 1.52, sd = 0.60 |
| L3 | 0.13, sd = 0.03 | 0.15, sd = 0.07 |
Tubular, Marginless Leaf Bases Form in PAT-Arrested Maize Shoots
Serial sections of NPA-treated maize shoots reveal the formation of marginless leaf bases not observed in apices cultured without NPA (Fig. 4). Invariably, the tubular leaf bases developed in the proximal sheath region of leaves that were at the P4 stage of development when transferred to NPA culture (Fig. 4, D–F and H). No fused margins are observed in older (P5) or younger (P3–P1) leaf primordia after 10 to 14 d in NPA culture (Fig. 4, D–F). In contrast to the proximal leaf domains, separated margins are observed in the distal leaf domains of NPA-cultured P4 leaves. Serial sectioning reveals that just above the zone of margin fusion, the free margins of the P4 leaf primordia are abnormally thick (Fig. 6, E and F). This type of altered margin morphology also is observed in leaves of the marginless mutant narrow sheath (Scanlon et al., 1996; Scanlon, 2000). In contrast, the edges of P4 leaf primordia cultured in the absence of NPA are thin and tapered (Fig. 4, A–C and G).
Figure 6.
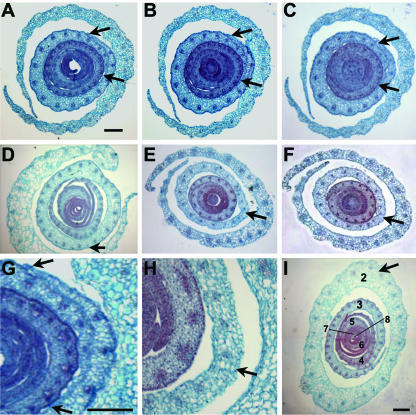
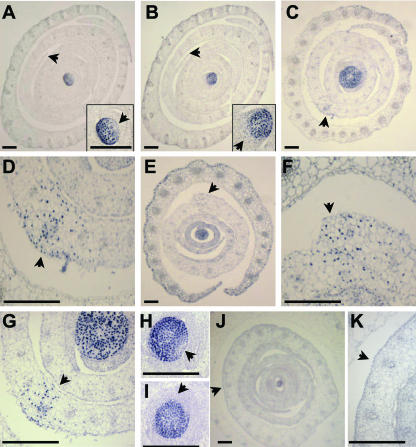
NPA-induced fused margins accumulate ectopic KNOX protein. KNOX immunolocalization of transverse sections of maize shoots cultured without NPA (A–C) and with NPA (D--G). KNOX proteins accumulate in the SAM and are down-regulated in the leaf founder cells of the P0 (arrow in inset A); a larger area of down-regulation is seen in the developing P1 leaf (arrow in inset B). Ectopic KNOX accumulation is seen in the fused margin domains of the P4 leaves in NPA-treated shoots (arrows in C–G). C and close-up in D, From a sample sectioned at the level of the second leaf. Closer to the tip of the SAM (E and close-up in F), the fused margins of an NPA-treated sample begin to separate, although the margins are thicker than in untreated samples (A and B), and KNOX accumulation is retained (F). G is another sample taken at the P2 level. When NPA-treated samples are switched to NPA-free culture for 20 d, normal down-regulation of KNOX protein at the P0 (H) and P1 (I) domains is correlated with resumption of leaf initiation. Fusion of the margins in the fourth leaf is retained after samples are switched to NPA-free culture (J), although ectopic KNOX accumulation does not persist (close-up in I). P, Plastochron number. Bars in A to C, E, and J = 100 μm. Bars in D, F, G, H, I, K, and insets in A and B = 250 μm.
Shoots cultured for 14 d in NPA reinitiate leaf production when transferred to NPA-free culture media. As shown in Figure 4I, 16 d after the switch away from NPA media, the cultured shoots had formed three additional leaf primordia. At 20 d after the transfer of NPA-cultured shoots to NPA-free media, shoots produced as few as two and as many as eight new leaf primordia. However, lateral roots of NPA cultured shoots were severely underdeveloped, even after the switch back to NPA-free media (data not shown). Therefore, none of the NPA-treated shoots analyzed in this study were grown to adulthood, and the mature plant phenotype of the marginless, tube-formed leaves could not be analyzed.
Inhibition of Auxin Transport Results in Altered Accumulation of KNOX Proteins
Immunohistolocalization analyses of PAT-inhibited shoots reveals altered accumulation patterns of maize KNOX (knotted-like homeobox) proteins. Apices cultured in the absence of NPA down-regulate KNOX proteins in leaf primordia and in founder cells of the incipient leaf, located within the peripheral zone of the SAM (Fig. 5A; Smith et al., 1992; Jackson et al., 1994). In contrast, the enlarged meristems of PAT-inhibited shoots fail to down-regulate KNOX protein accumulation (Fig. 5, B and C); this altered KNOX regulation correlates with the failure to initiate new leaf primordia from the SAM (Figs. 2F, 3, and 5, B and C).
Exogenous application of IAA to NPA-treated tomato shoots can result in the formation of lateral organs from PAT-arrested meristems (Reinhardt et al., 2000). Similarly, when IAA (10 mm in lanolin paste) was applied to the apical regions of 10 NPA-arrested maize shoots (Fig. 5D, inset), four shoots generated lateral outgrowths or primordia (Fig. 5D), three showed no difference from controls (application of lanolin only), and three shoots became necrotic and died. Notably, no small, buttress-sized leaf primordia (as seen after IAA treatment, Fig. 5D) were observed in any of the 48 shoots cultured for over 12 d in NPA alone (Figs. 3, B and D, and 5, B and C).
Moreover, analyses of PAT-inhibited tubular leaf bases revealed ectopic accumulation of KNOX proteins in the domain of margin fusion of P4 primordia (Fig. 6, C–G). Serial transverse sections of NPA-cultured shoots demonstrate that ectopic KNOX accumulation also occurs in the overtly thickened yet separated margins of P4 leaves distal to the region of margin fusion (Fig. 6, E and F). Except for the fused P4 margins, no other leaf domains of PAT-arrested primordia showed ectopic KNOX expression. Intriguingly, although margin fusion persists in affected leaf primordia even after shoots are switched to NPA-free media, ectopic KNOX accumulation is no longer evident in the fused leaf base after the media switch (Fig. 6, J and K). Thus, after 20 d in NPA followed by 20 additional d of culture in NPA-free media, no KNOX accumulation is seen in the fused margins of leaf primordia. Notably, after 32 d in NPA culture, the fused margins of P4 primordia retained ectopic KNOX expression (data not shown). In addition, KNOX down-regulation in the peripheral zone is restored after NPA-treated shoots are switched to NPA-free media (Fig. 6, H and I); this restoration of normal KNOX accumulation pattern is correlated with the resumption of new leaf initiation from the SAM.
DISCUSSION
Auxin Transport Is Required for Initiation of Leaves and for Normal Development of Small Leaf Primordia
As previously reported in the dicots Arabidopsis and tomato (Reinhardt et al., 2000), inhibition of PAT prevents lateral organ initiation from the maize shoot meristem (Table I; Figs. 2F and 3). In addition, overall growth and the development of medio-lateral domains of maize leaf primordia is adversely affected by auxin transport inhibitors (Figs. 2 and 3). Our results reveal that the adverse growth effects of PAT inhibition are dependent upon the age of the leaf primordium; growth of older leaf primordia is affected far less by PAT inhibition than the growth of younger leaf primordia (Fig. 3; Table II). Therefore, the ultimate effect is seen in the P0 domain of the SAM, such that PAT inhibition completely abolishes recruitment of founder cells to become a new leaf. These data complement results reported by Avsian-Kretchmer et al., 2002, who showed that inhibition of PAT caused a great reduction in free IAA accumulation in the SAM and young leaf primordia, whereas older leaf primordia still exhibited auxin accumulation during auxin transport inhibition. The accumulated data suggest that the SAM and young primordia do not synthesize abundant auxin. Instead, auxins are predominantly synthesized in older leaf primordia and are transported to younger primordia and the SAM. Extending this model, our data corroborate previous studies that implicate a requirement for auxin and auxin transport during leaf initiation and development of young leaf primordia (Reinhardt et al., 2000; for review, see Berleth and Sachs, 2001). In particular, PAT-inhibited P1 and P2 maize leaf primordia failed to complete recruitment and/or development of lateral leaf domains (Fig. 2, E and F). However, NPA does not specifically disrupt PAT but is known to be a general inhibitor of membrane trafficking (Geldner et al., 2001). Therefore, it is possible that the described effects of NPA treatment on leaf development and KNOX regulation (discussed below) are not solely a result of PAT inhibition. However, exogenous application of auxin to NPA-arrested maize apices can result in the elaboration of a new leaf primordium (Fig. 5D). These data suggest that the observed effect of NPA on leaf initiation is a result of auxin transport inhibition, rather than a generalized disruption of vesicle trafficking.
Inhibition of Auxin Transport Correlates with Altered Accumulation of KNOX Protein(s)
Previous studies of maize leaf mutations demonstrated a link between the regulation of knox gene expression and PAT (Tsiantis et al., 1999; Scanlon et al., 2002). In general, ectopic KNOX accumulation is correlated with defective PAT. In this study, we demonstrate that treatment of maize shoots with the PAT inhibitor NPA results in the loss of KNOX down-regulation in the peripheral zone of the SAM (Fig. 5, B and C) and ectopic KNOX accumulation in fused margins of P4 leaf primordia (Fig. 6, C–G). These results represent the first demonstration, to our knowledge, of altered KNOX protein accumulation in plant shoots treated with an inhibitor of auxin transport. Although a mechanistic link between knox regulation and PAT is still unexplained, these data do not support a simple relationship in which knox gene regulation occurs upstream of PAT. Instead, these results are not inconsistent with models in which PAT mechanisms function upstream of knox gene regulation, although a more complicated feedback network may exist. Moreover, our data reaffirm that down-regulation of KNOX proteins is intimately correlated with leaf initiation in maize; arrested meristems, which make no new leaves, also fail to down-regulate KNOXs in the SAM.
A Model for the Development of Maize Leaf Margins
Models describing the development of plant lateral organs commonly invoke an early initiation step that involves founder cell recruitment of organ initials, followed by an elaboration step during which organ morphology becomes distinguishable (modeled in Fig. 1). The down-regulation of KNOX accumulation in the founder cell domain occurs early in lateral organ development, before morphological development of the leaf buttress (Smith et al., 1992; Long et al., 1996; Fig. 5A). Current models suggest that KNOX down-regulation in the meristematic peripheral zone of simple-leaved plants is correlated with recruitment of leaf founder cells (for review, see Tsiantis and Langdale, 1998). In maize leaves, down-regulation of KNOX transcription factors begins in the midrib region of the P0 and extends laterally toward the margin domain of the incipient leaf, located at the opposite flank of the SAM (Jackson et al., 1994; Scanlon et al., 1996). Furthermore, founder cell recruitment along the lateral axis is a progressive process, whereby different lateral leaf domains are recruited at different developmental times. In fact, by the time KNOX down-regulation is completed in the margin domain, the midrib region develops a welldefined leaf buttress (Jackson et al., 1994; Scanlon et al., 1996). Thus, these data suggest that recruitment of all maize phytomer founder cell domains does not occur simultaneously; initialization (as indicated by KNOX down-regulation) of the margin domain may occur a full plastochron later than initialization of the midrib region of the same phytomer. Moreover, our data further suggest that the morphological elaboration of the lower leaf margins (that are recruited at P1) may occur much later in leaf development, during P4.
Newly formed maize phytomers contain a tubular base; this disc of insertion (Sharman, 1942) is readily apparent in the margin domains of P1 to P4 leaf primordia (Fig. 2, B–D). NPA-induced inhibition of PAT causes a failure to separate the margins of the lower leaf, and results in the formation of tubular leaf sheaths in P4-staged primordia (Figs. 4 and 6). These data reveal that at the P4 stage of leaf development, the disc of insertion includes the as-yet unemerged and unseparated margins of the lower leaf sheath. Therefore, the margins of the lower leaf sheath do not emerge from the leaf base until after the P4 stage of development, and the complete separation of right and left leaf margins requires auxin transport. A role for PAT in organ separation is well documented in many dicot species. Inhibition of auxin transport is correlated with the formation of fused cotyledons (Hadfi et al., 1998) and collar-like leaf primordia (Lyndon, 1994). Likewise, mutations in PIN-FORMED1, which encodes the putative auxin efflux transporter of Arabidopsis (Galweiler et al., 1998), cause fusion of lateral organ primordia and altered expression of the organ boundary marker CUPSHAPED COTYLEDON2 (Vernoux et al., 2000). Intriguingly, a reduced ratio of auxin to cytokinin is shown to promote cell adhesion in plant tissue culture (Gamborg et al., 1968; Schiavone and Cooke, 1987), whereas auxin is implicated in providing positional cues during leaf abscission (McManus et al., 1998). Similarly, our data suggest that transport of auxin to lateral domains of the developing leaf primordium may be required for the separation of leaf margins in maize. Furthermore, failure to separate the lower sheath margins is accompanied by ectopic KNOX accumulation in the presumptive margin domain of P4-staged leaves (Fig. 6, C–G).
When examined in the context of previous analyses of maize leaf development, our data suggest a model for leaf margin development. In particular, these results illustrate a mechanism for the development of multilayered right and left margins from a single cell-layer of the maize meristem. Fate mapping studies reveal that the edges of maize leaves are derived from the L1 (i.e. protodermal) layer of the SAM (Poethig and Szymkowiak, 1995). The L1 origin of leaf margin founder cells is also supported by down-regulation patterns of KNOX proteins, which indicate that the sheath margins of maize leaves are initialized from the outer cell layer of the apex (Smith et al., 1992; Jackson et al., 1994; Scanlon et al., 1996). Moreover, clonal sectors induced on the margin flank of maize founder cells mark both margins of the mature leaf sheath, whereas only one side of the leaf blade is sectored (Scanlon and Freeling, 1997; Scanlon, 2000). These data illustrate that, at the time of their recruitment, the right and left margins of the founder cell domains that will eventually give rise to the leaf sheath are not yet separated. Thus, the left and right margins of the sheath are derived from overlapping domains in the maize apex (Scanlon and Freeling, 1997; Scanlon, 2000). In contrast, leaf blade founder cells do not overlap. Moreover, Poethig and colleagues (Poethig, 1984; Poethig and Szymkowiak, 1995) showed convincingly that the very edges of the maize margins are completely L1 derived. Taken together, these results imply that a single meristematic tissue layer (the L1) gives rise to both edges of the maize leaf sheath.
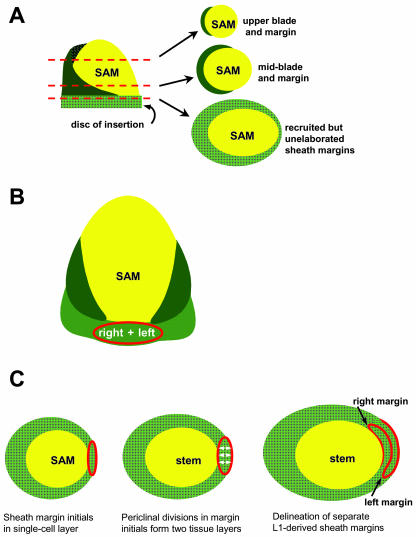
We suggest that both the right and left margins of the maize sheath are derived from a single cell layer on the flank of the maize meristem (Fig. 7, B and C), a process that requires down-regulation of KNOX protein accumulation. Once recruited, the L1 layer divides periclinaly (new cell walls parallel to the existing cell layer), resulting in the formation of two distinct cell layers. This periclinal division ultimately results in the separation of the left and right sheath margins, although each margin is clonally related and L1 derived. These margin precursor cells occupy the disc of insertion and are elaborated in a basipetal (distal to proximal) fashion during maize leaf development. Thus, the margins of the blade component of the maize leaf are elaborated early, whereas the lowermost margins of the leaf sheath are elaborated and separated very late in the development of maize leaf primordia. Moreover, elaboration and separation of the lower sheath margins correlates with PAT and KNOX down-regulation. Mutational analyses of maize margin development promise to elucidate further the specific roles of hormone transport and KNOX regulation in the formation of lateral domains of plant phytomers.
Figure 7.
Model for development of maize sheath margins. A, Cartoon of a maize shoot apex, showing the SAM (yellow) and blade (dark green) and sheath (light green) domains of a young primordium. The disc of insertion contains, in addition to the unelaborated node and stem, the recruited but as yet unexpanded domains of the lower leaf sheath. B, Fate mapping and sector analyses of maize leaf development (Scanlon and Freeling, 1997) reveal that the founder cells that give rise to the margins of the maize leaf blade (dark green) do not overlap the meristem flank; however, the founder cells that give rise to both the right and left margins of the leaf sheath (light green) are derived from a common domain that overlaps the SAM (yellow). Clonal analyses also indicate that the outermost margins of the maize leaf are derived from the L1 (protodermal) cell layer of the SAM (Poethig and Szymkowiak, 1995). C, Model for the development of left and right sheath margins from a single cell layer of the SAM. Sheath margins are initialized as a single cell layer; the right and left domains are not yet resolved (left). Periclinal divisions generate multiple cell layers from the L1 (middle). Later, the right and left margin domains are specified and emerge as separate edges of the leaf during the P4 stage of development (right).
MATERIALS AND METHODS
Nomenclature of Leaf Primordia
Leaves are designated by a plastochron number (P) and/or a leaf number (L), as described by Sylvester et al. (1990). In this scheme, leaf number refers to the sequential elaboration of leaves from the SAM, wherein the first leaf to emerge is L1 and the second is L2. Plastocron number refers to the developmental stage of the leaf, such that the youngest leaf, closest to the meristem, is named P1 and the next oldest leaf is designated P2. The leaf number for any leaf remains constant, whereas the plastochron number increases by 1 after successive new leaf initiations. Therefore, the most descriptive designation of a developing leaf primordium utilizes both the leaf number and the plastochron number. Thus, after six leaves have developed, the oldest leaf (L1) is a P6 leaf, whereas the youngest leaf (L6) is a P1 leaf. After initiation of a new leaf (L7/P1) from the SAM, the L6 progresses to the P2 developmental stage, and so on.
Maize (Zea mays) Tissue Culture
For use in tissue culture, maize B73 seedlings were harvested 14 d after germination and dissected to remove all but five to six remaining leaves. Dissected apices were placed in culture dishes containing MCM (Irish and Nelson, 1988) containing 30 μmol NPA dissolved in DMSO or in MCM containing equal amounts of DMSO but no NPA. Defects in leaf initiation were observed in shoots cultured at 10 μm NPA, 20 μm NPA, 30 μm NPA, and 100 μm NPA; most consistent results were obtained using 30 μm NPA. No effects on leaf initiation were observed after B73 maize seed were germinated and grown for over 20 d in tissue culture media containing 10 μm NPA, 30 μm NPA, or 100 μm NPA.
Microscopy
Cultured shoots of the maize inbred B73 (described above) were fixed in paraformaldehyde, paraffin embedded, and sectioned at 12 μm as described (Sylvester and Ruzin, 1994, and refs. therein). After deparaffinization, slides were stained in Johansson's safranin/fast green schedule as described (Sylvester and Ruzin, 1994). Images were obtained with an Axioplan II (Zeiss, Jena, Germany) equipped with a MicroPublisher 3.3 mega-pixel CCD camera (Southern Micro Instruments, Pompano Beach, FL).
Scanning Electron Microscopy
Cryo-scanning electron microscopy was performed with technical assistance from Dr. John Shields (Center for Ultrastructural Research, University of Georgia, Athens). Dissected maize apices were flash frozen in liquid nitrogen slush, sublimated, and sputter coated with 25 nm of palladium in the cryoprep chamber of a LEO 982 Field emission scanning electron microscope (Thornwood, NY). Images of frozen, treated specimens were taken at an accelerating voltage of 15 kV.
Immunohistolocalization of KNOX Proteins
Immunohistochemistry was performed as described by Scanlon et al. (1996); immunopositive nuclei were visualized using colorimetric methods as described therein. Images were obtained with a Zeiss Axioplan II equipped with a MicroPublisher 3.3 mega-pixel CCD camera (Southern Micro Instruments).
Acknowledgments
We thank Dr. John Shields (University of Georgia Center for Ultrastructural Analysis, Athens) for expert assistance in cryo-scanning electron microscopy. Richard Schneeberger (Ceres, Malibu, CA) provided the KNOX antibody. We thank Jonathan Willis (Athens, GA) for assistance in dissection and culturing of maize shoots. David Henderson (Athens, GA) provided assistance in cryo-scanning electron microscopy. David Henderson and Suneng Fu (Athens, GA) provided stimulating discussions of leaf margin development.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026880.
References
- Avisian-Kretchmer O, Cheng JC, Chen L, Moctezuma E, Sung ZR (2002) Indole acetic acid distribution pattern coincides with vascular differentiation pattern during Arabidopsis leaf ontogeny. Plant Physiol 130: 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berleth T, Sachs T (2001) Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol 4: 57–62 [DOI] [PubMed] [Google Scholar]
- Estelle M (1998) Polar auxin transport: new support for an old model. Plant Cell 10: 1775–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galinat WC (1959) The phytomer in relation to the floral homologies in the American Maydea. Bot Mus Leafl Harv Univ 19: 1–32 [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palmer K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vasculature tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K (2001) Auxin inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Hadfi K, Speth V, Neuhas G (1998) Auxin-induced developmental patterns in Brassica juncea embryos. Development 125: 879–887 [DOI] [PubMed] [Google Scholar]
- Irish E, Nelson TM (1988)Development of maize plants from cultured shoot apices Planta 175: 9–12 [DOI] [PubMed] [Google Scholar]
- Jackson D, Veit B, Hake S (1994) Expression of the maize KNOTTED-1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120: 405–413 [Google Scholar]
- Ljung K, Bhalerao R, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 84: 465–474 [DOI] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton KA (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379: 66–69 [DOI] [PubMed] [Google Scholar]
- Lyndon RF (1994) Tansley review no. 74: control of organogenesis at the shoot apex. New Phytol 128: 1–18 [DOI] [PubMed] [Google Scholar]
- McManus MT, Thompson DS, Merriman, Cledwyn LL, Osbourne DJ (1998) Transdifferentiation of mature cortical cells to functional abscission cells in bean. Plant Physiol 116: 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig RS (1984) Cellular parameters of leaf morphogenesis in maize and tobacco. In RA White, WC Dickinson, eds, Contemporary Problems of Plant Anatomy. Academic Press, New York, pp 235–238
- Poethig RS, Szymkowiak EJ (1995) Clonal analysis of leaf development in maize. Maydica 40: 67–76 [Google Scholar]
- Reinhardt D, Mandele T, Kuhlenmeier C (2000) Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12: 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon MJ (2000) NARROW SHEATH1 functions from two meristematic foci during founder-cell recruitment in maize leaf development. Development 127: 4573–4585 [DOI] [PubMed] [Google Scholar]
- Scanlon MJ, Freeling M (1997) Clonal sectors reveal that a specific meristematic domain is not utilized in the maize mutant narrow sheath. Dev Biol 182: 52–66 [DOI] [PubMed] [Google Scholar]
- Scanlon MJ, Henderson D, Bernstein B (2002) SEMAPHORE1 functions during the regulation of ancestrally-duplicated knox genes and polar auxin transport in maize. Development 129: 2663–2673 [DOI] [PubMed] [Google Scholar]
- Scanlon MJ, Schneeberger RG, Freeling M (1996) The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development 122: 11683–11691 [DOI] [PubMed] [Google Scholar]
- Schiavone FM, Cooke TJ (1987) Unusual patterns of somatic embryogenesis in the domesticated carrot: developmental effects of exogenous auxins and auxin transport inhibitors. Cell Diff 2: 53–62 [DOI] [PubMed] [Google Scholar]
- Sharman BC (1942) Developmental anatomy of the shoot of Zea mays L. Ann Bot 6: 245–284 [Google Scholar]
- Smith L, Greene B, Veit B, Hake S (1992) A dominant mutation in the maize homeobox gene Knotted1 causes its ectopic expression in leaf cells with altered fates. Development 116: 21–30 [DOI] [PubMed] [Google Scholar]
- Sylvester AC, Ruzin SE (1994) Light microscopy: I. Dissection and microtechnique. In M Freeling, V Walbot, The Maize Handbook. Springer-Verlag New York, pp 83–95
- Sylvester AW, Cande WZ, Freeling M (1990) Division and differentiation during normal and liguless-1 maize leaf development. Development 110: 985–1000 [DOI] [PubMed] [Google Scholar]
- Tsiantis M, Brown MIN, Skibinski G, Langdale JA (1999) Disruptions of auxin transport is associated with aberrant leaf development in maize. Plant Physiol 121: 1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantis M, Langdale JA (1998) The formation of leaves. Curr Opin Plant Biol 1: 43–48 [DOI] [PubMed] [Google Scholar]
- Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J (2000) PIN-FORMED1 regulates cell fate at the periphery of the shoot apical meristem. Development 127: 5157–5165 [DOI] [PubMed] [Google Scholar]