Abstract
In perennial plants, freeze-thaw cycles during the winter months can induce the formation of air bubbles in xylem vessels, leading to changes in their hydraulic conductivity. Refilling of embolized xylem vessels requires an osmotic force that is created by the accumulation of soluble sugars in the vessels. Low water potential leads to water movement from the parenchyma cells into the xylem vessels. The water flux gives rise to a positive pressure essential for the recovery of xylem hydraulic conductivity. We investigated the possible role of plasma membrane aquaporins in winter embolism recovery in walnut (Juglans regia). First, we established that xylem parenchyma starch is converted to sucrose in the winter months. Then, from a xylem-derived cDNA library, we isolated two PIP2 aquaporin genes (JrPIP2,1 and JrPIP2,2) that encode nearly identical proteins. The water channel activity of the JrPIP2,1 protein was demonstrated by its expression in Xenopus laevis oocytes. The expression of the two PIP2 isoforms was investigated throughout the autumn-winter period. In the winter period, high levels of PIP2 mRNA and corresponding protein occurred simultaneously with the rise in sucrose. Furthermore, immunolocalization studies in the winter period show that PIP2 aquaporins were mainly localized in vessel-associated cells, which play a major role in controlling solute flux between parenchyma cells and xylem vessels. Taken together, our data suggest that PIP2 aquaporins could play a role in water transport between xylem parenchyma cells and embolized vessels.
Winter embolism, the generation of air bubbles in xylem vessels induced by freeze-thaw cycles, often leads to a loss of hydraulic conductivity of the vessels (Cochard and Tyree, 1990; Améglio et al., 2001; Ewers et al., 2001). Vulnerability to winter embolism is related to the anatomy and vessel diameter of woody plants (Cochard and Tyree, 1990) and affects the ability of plants to survive cold climates and the geographic distribution of species (Tyree and Cochard, 1996; Pockman and Sperry, 1997; Lemoine et al., 1999).
Detailed physiological studies of the responses of temperate woody plants to winter embolism have been made. Plants minimize the impact of winter embolism by replacing embolized vessels by new functional vessels every year and/or by refilling embolized vessels by generating positive xylem pressures (Holbrook and Zwieniecki, 1999; Tyree et al., 1999; Améglio et al., 2002). Although making new vessels is common to all the plants that exhibit secondary growth, the generation of xylem pressures has only been reported in a few species such as maple (Acer pseudoplatanus; O'Malley and Milburn, 1983; Tyree, 1983; Sperry et al., 1987, 1994), grapevine (Vitis vinifera; Sperry et al., 1987), birch (Betula alleghaniensis) (Sperry et al., 1994; Zhu et al., 2000), and walnut (Juglans regia; Améglio et al., 1995, 2001; Ewers et al., 2001).
In walnut trees, depending on the temperature, two types of positive xylem pressures have been found. The first one, referred to as autumn- and spring-positive pressures in the xylem sap, is closely related to root pressure, which depends on the uptake and presence of minerals (Ewers et al., 2001). The second kind of positive xylem pressure, called winter xylem pressure, is related to low winter temperatures and originates in the stem itself (Ewers et al., 2001; Améglio et al., 2001). Evidence favoring the involvement of xylem parenchyma cells in the repair of winter embolism has been reported (Améglio and Cruiziat, 1992; Améglio et al., 2001; Ewers et al., 2001). When embolism takes place in an excised stem upon freezing, a concomitant increase in the sap volume of xylem vessels and sugar concentration is observed. The rise of sugar concentration, chiefly Suc, in the xylem vessels results from the conversion of starch-to-sugar that normally occurs in the winter in parenchyma cells (Sauter et al., 1996). Concomitant with an increase of water xylem sap, Améglio et al. (2001) found a decrease in the symplastic stem water, suggesting that water is released into the vessel lumen, at least partially, from xylem adjacent living cells. Although the exact mechanism by which winter positive pressures in the xylem sap can be generated still remains unclear, taken together, these observations consistently show that both sugar and water, two essential components of the winter positive pressure, are most likely supplied, locally, from the xylem parenchyma cells. Sperry et al. (1987, 1994) suggested that in maple trees, xylem parenchyma cells contributed to the restoration of xylem hydraulic conductivity.
Several authors (Pickard, 1989; Améglio and Cruiziat, 1992; Yang and Tyree, 1992; Améglio et al., 2001) have hypothesized that an osmotic pressure gradient between xylem sap and surrounding parenchyma cells, associated with high xylem sap osmolarity, may move the water into the vessels and give rise to a pressure that is sufficient to restore the hydraulic conductivity of the xylem. This would be possible if a low hydraulic resistance pathway between parenchyma cells and vessels existed. Aquaporins could play a role in this osmotically driven water flow between cells, although the involvement of active water transport as proposed for rapid volume changes in motor cells cannot be ruled out (Morillon et al., 2001). Since their discovery and functional characterization, water channel proteins (aquaporins) have been shown to play a central physiological role in cellular water balance (Chrispeels et al., 2001; Maurel and Chrispeels, 2001; Tyerman et al., 2002). Recent molecular and microscopic studies shown that aquaporins are highly expressed in the plasma membrane of xylem parenchyma cells (Barrieu et al., 1998; Kirch et al., 2000). Based on these observations, it has been hypothesized that aquaporins may contribute to the water release into embolized vessels and, therefore, to the restoration of xylem hydraulic conductivity (Holbrook and Zwieniecki, 1999; Tyree et al., 1999). However, no experimental evidence in woody plants supporting this hypothesis exists.
This study focuses on walnut trees, where three different mechanisms are responsible for the restoration of stem hydraulic conductivity: (a) autumn and Spring xylem pressures that seem to have a root origin; (b) winter xylem pressure, which appears to have a stem origin; and (c) formation of new functional vessels. Water flows at the cellular level have been shown to take part in the generation of winter positive pressure (Améglio et al., 2001). Here, we report that in twigs of walnut trees grown outdoors in France, sugars accumulate in the xylem sap during the winter months. We also report the identification and the functional characterization of two walnut PIP2 aquaporins that are expressed in the xylem and their possible involvement in winter embolism recovery based on the changes in the abundance of mRNA and protein during the winter months and the localization of these aquaporins in the vessel-associated cells (VACs) in winter.
RESULTS
Although it is often difficult to obtain sufficient quantities of conductive plant tissues for analysis, it is relatively easy to obtain the active xylem tissues from woody twigs. One removes the bark and then scrapes the twig with a scalpel. The cells that are collected constitute a high-quality xylem preparation containing the different cell types found in the xylem (vessels, fibers, parenchyma, and VACs).
Changes in Content of Sugars during Autumn-Winter Period
The exposure of xylem parenchyma cells to low temperatures induced an increase in intracellular solute concentration, mainly in sugars (Sauter and Kloth, 1987; Sauter, 1988; Améglio and Cruiziat, 1992). This accumulation of soluble sugars is thought to play a key role in preventing damage during the chilling period (Livingston and Henson, 1998; Sauter, 1998) and has been correlated with the winter embolism recovery process in walnut xylem (Améglio et al., 2001). We studied changes of soluble sugars in the xylem tissue of walnut twigs and the expression of aquaporins to investigate the possible role of aquaporins in the water fluxes associated with the osmoticum changes occurring during the recovery of winter embolism.
The tissues collected when the temperatures were lower, i.e. from December to February (Fig. 1B), exhibited higher content of soluble sugars (Fig. 1) than those harvested at early autumn or spring, when the minimum temperatures rose to 10°C and above.
Figure 1.
A, Dynamics of starch, Suc, and soluble sugar (Suc + Fru + Glc) content in xylem parenchyma cells. B, Minimum daily air temperatures in the field. The experiments were performed from October 2000 to March 2001.
From October to November, the total soluble sugars (Suc, Glc, and Fru) content of xylem cells rose from about 28 to 34 mg g–1 dry weight to 66 mg g–1 dry weight in the early winter (December and January; Fig. 1A). During the same period, a decrease in starch content was observed (105–30 mg g–1 dry weight). Later, from January to March, a concomitant decrease of soluble sugars with an increase of starch was observed again. Suc accounted for most of the soluble sugars at all times examined. These observations are in agreement with a possible conversion between starch and soluble sugars.
Isolation and Structure of Two Aquaporin cDNAs of Walnut Xylem
Degenerated oligonucleotide primers from conserved regions of plant aquaporins (WCA/WCB) were used to clone a PCR-amplified fragment in walnut (about 450 pb). The screening of a xylem cDNA library with the isolated fragment allowed us to clone two full-length cDNA of 1,278 bp.
The complete nucleotide sequences of the clone JrPIP2,1 (EMBL accession no. AY189973) and clone JrPIP2,2 (EMBL accession no. AY189974) contained an open reading frame of 864 nucleotides that encoded a 287-amino acid polypeptide. Based on their sequence homologies, both cDNAs belong to the membrane intrinsic proteins (MIPs) superfamily (Weig et al., 1997), and the highest similarity was found with the members of the PIP2 aquaporin subgroup (Fig. 2; Johanson et al., 2001).
Figure 2.
Comparison of the deduced amino acid sequences of JrPIP2,1 and JrPIP2,2 with that of AtPIP2,3.
At the nucleotide sequence level, JrPIP2,1 and JrPIP2,2 have 96% of similarity within the coding region but only 54% in the 3′-untranslated region (UTR). At the protein level, the deduced amino acid sequences of JrPIP2,1 and JrPIP2,2 differ only by three amino acid residues. The identity between JrPIP2(1 and 2) and the other members of the PIP2 family ranges from 70% to 81%, and the greatest identity was found with the Samanea saman isoform SsAQP2 (Moshelion et al., 2002).
Like other MIP family proteins, hydrophobicity profiles of the predicted JrPIP2,1 and JrPIP2,2 polypeptides are consistent with the existence of six transmembrane domains and five connecting loops. These two polypeptides also contain the conserved NPA motifs known to be involved in the selectivity filter of the water channel.
Jr-PIP2,1 Is a Water Channel
Because of the very high level of amino acid sequence identity between JrPIP2,1 and JrPIP2,2, JrPIP2,1 was the only clone used for oocyte swelling assays. Functional analysis of the JrPIP2,1-encoded protein was carried out by injection of the cRNA into Xenopus laevis oocytes. The increase in Pos (osmotic water permeability) of oocytes injected with cRNAs of JrPIP2,1 or AtPIP2;2 (Arabidopsis aquaporin) were, respectively, 10- and 12-fold higher than the water-injected control, demonstrating the aquaporin activity of JrPIP2,1 (Fig. 3).
Figure 3.
Osmotic water permeability (Pos) of oocytes injected with water (Pos = 9 ± 3 μm s–1), JrPIP2,1 (Pos = 125 ± 17 μm s–1), and AtPIP2,2 (Pos = 170 ± 23 μm s–1) cRNAs. The results are expressed as the mean of at least three independent experiments ± sd.
Expression Analysis of JrPIP2,1 and JrPIP2,2 in Different Organs
Two specific primer couples (WC11/WC12 and WC21/WC22) were designed based on JrPIP2,1 and JrPIP2,2 3′-UTR, respectively. Because 3′-UTR sequences are usually the most divergent region within genes (Duval et al., 2002; Marin-Olivier et al., 2002), both of these primer couples could allow us to isolate gene-specific probes by PCR. Figure 4A shows that each primer pair amplified only one product, even when both aquaporin genes were present in the mixture. Using WC21/WC22 primers, a 160-pb fragment was amplified from JrPIP2,2 full-length cDNA (Fig. 4A, lane 2) or from JrPIP2,2 together with JrPIP2,1 (Fig. 4A, lane 3), but nothing was amplified from the JrPIP2,1 cDNA clone alone (Fig. 4A, lane 4). Conversely, WC11/WC12 primers PCR-amplified a 240-bp fragment from JrPIP2,1 (Fig. 4A, lane 6) or from JrPIP2,2 together with JrPIP2,1 (Fig. 4A, lane 7) but not from JrPIP2,2 alone (Fig. 4A, lane 8). Furthermore, the presence of both JrPIP2,1 and JrPIP2,2 and both couples of primers mixed in the PCR reaction did not affect the intensity of the amplified fragments (Fig. 4A, lane 9).
Figure 4.
A, Primers couples (WC11/WC12) and (WC21/WC22) are specific for JrPIP2,1 and JrPIP2,2, respectively. Regarding JrPIP2,2-specific primers (WC21/WC22), PCR reactions were performed either with JrPIP2,2 alone (row 2), together with JrPIP2,1 (row 3), or with JrPIP2,1 alone (row 4). Similarly, for the JrPIP2,1-specific primer (WC11/WC12), PCR reactions were performed either with JrPIP2,1 alone (row 6), together with JrPIP2,2 (row 7), or with JrPIP2,2 alone (row 8). JrPIP2,1 and JrPIP2,2 were co-amplified in the presence of both these primer couples (row 9). PCR reactions were also carried out without JrPIP2,2 (row 1) and JrPIP2,1 (row 5). B, Expression pattern of JrPIP2,1 and JrPIP2,2 in various plants organs. Semiquantitative reverse transcriptase (RT)-PCR was used to investigate the expression of these walnut aquaporins relative to the constitutively expressed Actin (JrAct1). JrPIP2,1 and JrPIP2,2 were amplified individually or co-amplified in the same tube. At least three individual sets of RT-PCR reactions were performed in each case.
Transcripts level analysis was carried out by this semiquantitative RT-PCR approach, using RNAs from different tissues and organs: xylem, bark, buds (dormant and nondormant), leaves, roots, and flowers (male and female). JrPIP2,1 transcripts were found in all organs (Fig. 4B) but to the lowest extent in roots and dormant buds. JrPIP2,2 transcripts were strongly detected in roots and leaves, slightly less in female flowers and bark, and at a very much lower level in xylem and nondormant buds. No JrPIP2,2 transcript was detectable in male flowers and dormant buds. In addition, when both JrPIP2,1 and JrPIP2,2 were co-amplified together, the same expression patterns were obtained than for each respective individual gene.
Accumulation Pattern of JrPIP2,1, JrPIP2,2, and JrPIP2(1 and 2) Transcripts in the Xylem Tissue during the Autumn-Winter Period
Using the specific primers for JrPIP2,1 (WC11/WC12) and JrPIP2,2 (WC21/WC22), we investigated the expression pattern for each isoform throughout the autumn-winter period on samples collected from October to March. As shown in Figure 5A, JrPIP2,1 and JrPIP2,2 were differentially expressed, especially from October to January. JrPIP2,1 transcript accumulation increased significantly throughout the winter, reaching a maximum in February, and then dropped to a very low level in March. JrPIP2,2 transcripts were substantial in October and decreased significantly until January. Like JrPIP2,1, JrPIP2,2 had a maximum accumulation in February, followed by a dramatic decreasing in March (Fig. 5A).
Figure 5.
A, Expression pattern of JrPIP2,1 and JrPIP2,2 in xylem parenchyma cells throughout autumn-winter period. Semiquantitative RT-PCR was performed with either JrPIP2,1-specific primers (WC11/WC12), JrPIP2,2-specific primers (WC21/WC22), or with both couples of primers. In the last PCR condition, JrPIP2,1 and JrPIP2,2 were co-amplified in the same tube. B, Expression of JrPIP2(1 and 2) genes in xylem parenchyma cells throughout autumn-winter period. Semiquantitative RT-PCR was performed with specific primers (WC1/WC2) common to JrPIP2,1 and JrPIP2,2 in the presence of actin-specific primers (A1/A2). JrPIP2 and JrAct1 were co-amplified in the same tube.
Using a primer couple (WC1 and WC2) designed from conserved coding sequences to JrPIP2,1 and JrPIP2,2, we then investigated the simultaneous change in their transcripts. Therefore, the amplified band (450 pb) is referred to as JrPIP2(1 and 2) (Fig. 5B). The expression pattern obtained in Figure 5B is strongly similar to that of JrPIP2,1. The level of JrPIP2(1 and 2) transcripts began to increase in October, reached a maximum in January and February, and decreased significantly in March.
Quantitation of the JrPIP2(1 and 2) Protein Levels in the Xylem Tissue during the Autumn-Winter Period
Because the MIP mRNA expression pattern might not always reflect protein accumulation (Suga et al., 2001), we monitored the PIP2 protein expression profile. We used an antiserum raised against the carboxy-terminal region, the AtPIP2,3 aquaporin (Arabidopsis) that has sequence identity with both walnut aquaporin isoforms (Fig. 6A). The serum detected a single band of 30 kD in an extract of E. coli cells that express a fusion protein from a pET-His-Tag-JrPIP2,1 plasmid (Fig. 6B). Likewise, AtPIP2 serum also cross-reacted with His-Tag-JrPIP2,2 (data not shown). We initially made the His-tag fusion construct because we were unsure if the heterologous serum would recognize the walnut protein. However, as shown in Figure 6A, the walnut and Arabidopsis aquaporins have shared C-terminal epitopes.
Figure 6.
A, Alignment of the conserved carboxy-terminal region of JrPIP2,1, JrPIP2,2, and AtPIP2,3. B, Specificity of the AtPIP2 polyclonal antibodies against JrPIP2,1. A JrPIP2,1-His-tag fusion protein was constructed and expressed in Escherichia coli JM109 using the pET-15b vector, relative to control (E. coli JM109 transformed with pET-15b vector). Fifty micrograms of protein-soluble fraction was subjected to SDS. C, Immunoblot of microsomal fraction proteins of xylem tissue harvested from November 2000 to March 2001 incubated with the polyclonal antibodies raised against either AtPIP2 [JrPIP2(1 and 2)] or plasma membrane H+-ATPase (H+-ATPase).
Protein extracts of walnut xylem tissue collected from November to March were then analyzed by immunoblotting. The AtPIP2 antiserum cross-reacted with a 28-kD protein, presumably the gene product of JrPIP2,1 and JrPIP2,2. Therefore, the 28-kD protein is referred to as JrPIP2(1 and 2). The JrPIP2(1 and 2) amount rose significantly from a very low level in November to reach a maximum in January and February before dropping in March (Fig. 6C). During the winter period, we did not observe any change in the level of the plasma membrane H+-ATPase.
Immunolocalization of JrPIP2(1 and 2) in the Xylem Tissue in February (Winter Period)
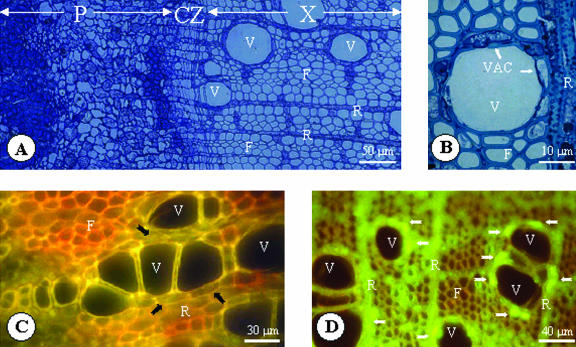
To determine in which cells of the xylem JrPIP2(1 and 2) is localized, we carried out an immunolocalization study on sections of xylem collected in February [i.e. when a high level of both JrPIP2(1 and 2) transcripts and corresponding proteins were found; Figs. 5B and 6C]. As shown in Figure 7A, walnut xylem contains different cell types (parenchyma cells including VACs), which are living cells, and vessels and fibers, which are dead cells. Xylem vessels are in direct contact with many VACs (Fig. 7B), indicating that these cells could be directly involved in the regulation of water flux for winter embolism recovery. After incubation of sections with AtPIP2 saturated by the purified JrPIP2,1 protein (control), the only fluorescence seen is associated with the cell walls (autofluorescence). Fluorescence is strongest in the lignified walls of the fibers and the vessels and in the cell wall corners between some vessels and parenchyma cells (Fig. 7C). Incubation of the sections with an AtPIP2 serum (Fig. 7D) shows immunolabeling of living cells: The strongest immunolabeling was observed in VACs and very low immunolabeling was detected in ray parenchyma cells (Fig. 7D). These results demonstrate that during the winter months, the JrPIP2(1 and 2) protein is located in VACs, and we suggest their possible involvement in the regulation of the water flux from parenchyma cells to xylem vessels in winter.
Figure 7.
Transversal section showing the general organization of the stem in walnut tree. A, Cambium zone appears at the phloem-xylem interface. Xylem contains lignified xylem vessels and fibers (died cells) associated with parenchyma cells (living cells). The support tissue is constituted of fibers emptied of their content. B, Cell types in walnut xylem. VACs (white arrows) are specialized cells that surround the xylem vessels. From their localization in the tissue, VACs have the ability to control nutrient exchanges between the parenchyma cells and the xylem vessels. Localization of aquaporins in xylem tissue of walnut tree sampled in February (winter period). C, No recognition in control (AtPIP2 antiserum saturated by the purified JrPIP2,1 protein). Black arrows indicated the localization of VACs. D, Section showing extensive green immunofluorescence (white arrows) in VACs. X, Xylem; P, phloem; CZ, cambial zone; V, xylem vessel; F, fiber; R, ray cell.
DISCUSSION
The mechanism of refilling of winter embolized xylem vessels in trees has been a matter of debate. To date, all proposed mechanisms can be categorized into two models, either “physical models” or “vitalistic models.” According to the “physical models,” winter-positive stem pressures would be strictly due to freeze-thaw events (Tyree, 1983; Milburn and O'Mally, 1984). In contrast, according to the “vitalistic models,” activities of xylem living cells and the presence of Suc in xylem sap are required for pressure buildup (Johnson et al., 1987; Yang and Tyree, 1992). In walnut tree, experimental evidences supporting the vitalistic models were recently obtained by Améglio et al., 2001. These authors showed that the winter-positive stem pressures could be induced by low nonfreezing temperatures and correlated with increased Suc concentration in xylem sap. These data indicate the importance of living cells in the mechanism of winter embolism recovery in walnut tree. In this context, we investigated here the possible role of aquaporins of xylem parenchyma in this process.
To restore hydraulic conductivity after winter embolism, walnut xylem generates local positive pressures that are tightly correlated with high Suc concentrations in xylem vessels (Améglio et al., 2001, 2002). To date, it is proposed that the Suc released from parenchyma cells into xylem vessels results from winter starch mobilization in xylem parenchyma cells. In walnut, we show that the starch breakdown and increase of Suc content in xylem parenchyma cells occurred at the same time (Fig. 1A) and when the daily minimum temperatures are lowest (Fig. 1B). Moreover, parallel to the increase in Suc concentration in parenchyma cells, the Suc released into xylem sap is considerably enhanced (data not shown). For instance, Suc concentration in the harvested xylem sap in February was found to be at least 10 times higher than that harvested in October. Consistent with previous data (Améglio and Cruiziat, 1992; Améglio et al., 2001), these findings mean that the starch that is stored in parenchyma cells is hydrolyzed to Suc, which is then released into xylem sap. During the winter months, there is an increase in xylem volume sap concomitant with a decrease in the parenchyma water content, suggesting that water that enters the embolized vessels comes, at least partially, from the xylem parenchyma cells (Améglio et al., 2001). Thus, we examined the expression of aquaporin genes, the abundance of the respective proteins, and, finally, their immunolocalization in the xylem.
To identify the aquaporins putatively expressed in the xylem tissue, we screened a xylem-derived cDNA library and isolated two different PIP-like genes that cluster only with the plant PIP2 aquaporin subfamily. The PIP2 aquaporin clones differ by only three amino acids. However, at the nucleotide sequence level, these two isoforms differ in their 3′-UTR. In Brassica oleracea, it has been shown that two aquaporin isoforms (BoPIP1b1 and BoPIP1b2) largely differ in their 3′-UTR, whereas their protein sequences differ only by one amino acid (Marin-Olivier et al., 2002). JrPIP2,1 and JrPIP2,2 have a very strong sequence identity with AtPIP2,2 and AtPIP2,3, two homologous genes in Arabidopsis that encode functional water channels (Daniels et al., 1994). Our functional assays with X. laevis oocytes demonstrate that JrPIP2,1 and JrPIP2,2 are two new members belonging to the PIP2 subfamily (Fig. 3). The absence of PIP1 aquaporins suggests that this subfamily may be poorly expressed in xylem tissue. In addition, the fact that all living cells of the xylem tissue are parenchyma cell (VACs and axial and ray parenchyma cells) mRNA; therefore, their respective proteins extracted from the xylem tissue come mainly from xylem parenchyma cells.
One major problem linked to xylem is the low transcript level, compared with other plant organs. Therefore, to overcome this obstacle, the expression patterns of JrPIP2,1 and JrPIP2,2 were monitored by semiquantitative RT-PCR strategy, which is widely used in many recent studies (Taybi and Cushman, 1999; Duval et al., 2002; Miesak and Coruzzi, 2002; Orsel et al., 2002). JrPIP2,1 and JrPIP2,2 were expressed in vegetative and reproductive organs, indicating that they are not xylem-specific aquaporins (Fig. 4B). Other aquaporins also have been found to be expressed in multiple tissues or cell types. For instance, expression of BoPIP1b1 and BoPIP1b2, which were respectively isolated from the stigma and the anther-derived cDNA library, were expressed in reproductive organs and in vegetative tissues. In addition, evidence that high levels of PIP aquaporin mRNA can be associated with high rates of water flow through cells has been reported previously in the literature. In S. saman, Moshelion et al. (2002) suggested that there is a tight correlation between the change of SsAQP2 (PIP2 aquaporin) transcripts abundance and the motor cell volume changes in leaf-moving organs. Based on the data presented in Figures 4B and 5A, we may expect, therefore, that JrPIP2,1 and JrPIP2,2 could be involved in various physiological processes, and at least one of both could play an important role in refilling of embolized xylem vessels. Judging by their transcript levels throughout the autumn-winter period, it is more likely that JrPIP2,1, rather than JrPIP2,2, may be involved in the hydraulic mechanism of this process (Fig. 5A). The level of JrPIP2,1 transcripts was higher during the winter (from December–February) than either during the autumn (October and November) or at the beginning of spring (March), whereas the level of JrPIP2,2 transcripts decreased during the autumn-winter period, except in February. The reason for this sudden accumulation of JrPIP2,2 transcripts in February is unknown. It might be due, at least partially, to the lowest freezing temperatures measured shortly before xylem tissues were collected (down to –10°C; Fig. 1B). In herbaceous plants, stress conditions such as chilling and salt have been shown to affect PIP mRNAs expression (Yamaguchi-Shinozaki et al., 1992; Ruiter et al., 1997; Mariaux et al., 1998) or PIP protein abundance (Kirch et al., 2000).
One feature of PIPs in higher plants is the expression of multiple isoforms within single species (Johanson et al., 2001), and we cannot rule out the possibility that the walnut xylem may have other PIP2 isoforms as well and that these were not picked up in our cDNA library screen or detected by the antibodies. For the rest of the discussion, we will refer to JrPIP2 and to JrPIP2 to indicate WC1/WC2 primers PCR amplified (450 pb, Fig. 5B) and AtPIP2 cross-reacted protein (28 kD, Fig. 6C), respectively. We propose that JrPIP2 aquaporins (as defined above) play an essential role in winter embolism recovery. When positive pressure in the xylem sap was related to root pressure, i.e. before the beginning of the winter (October and November) and the spring (March), the amount of JrPIP2 transcripts and proteins was low (Figs. 5A and 6C) and coincided with reduced Suc content in parenchyma cells (Fig. 1A). This situation is completely different from that in winter (from December–February). During this period, xylem tissues have a high level of JrPIP2 transcripts (Fig. 5A), proteins (Fig. 6C), and Suc content (Fig. 1A). In fact, the relative abundance of JrPIP2 transcripts and proteins when stem pressure has a local origin, compared with their low abundance when stem pressure has a root origin, suggest that PIP2 aquaporins may be essential for water transport from parenchyma cells to embolized vessels in winter. On the other hand, it is worth noting that, in contrast to PIP2 aquaporins, there was no increase in H+-ATPase level during the winter in the same xylem tissues, further supporting that the transient accumulation of JrPIP2 transcripts and proteins cannot result simply from a general stimulation of all plasma membrane proteins but is rather specific to PIP2 aquaporins (Fig. 6C). Taken together, the winterinduced up-regulation of JrPIP2 aquaporin in walnut xylem may be required for the regulation and the facilitation of water release into embolized vessels.
In herbaceous plants, previous studies indicate that aquaporin immunolocalization can provide further clues about their role in various physiological processes (Fleurat-Lessard et al., 1997; Barrieu et al., 1998; Kirch et al., 2000; Otto and Kaldenhoff, 2000). In tree xylem, the few localization studies that have been carried out have emphasized the control of the nutrient flux between parenchyma cells and xylem vessels by VACs, which are not connected to vessels by plasmodesmata (Fromard et al., 1995; Lachaud and Maurousset, 1996; Alves et al., 2001). Therefore, water or solute moving from VACs to xylem vessels or in the opposite direction must cross the plasmalemma of the VACs. In Robinia pseudoacacia, xylem, Fromard et al. (1995) showed that the plasma membrane H+-ATPase, which is involved in solute transport through the plasma membrane, is mainly localized in VACs. In walnut tree, when stem pressure has a local origin (winter period), an increase in xylem volume sap correlates with a decrease in parenchyma cells water content (Améglio et al., 2001). We also show here an accumulation of JrPIP2 transcripts (Fig. 5B) and corresponding proteins (Fig. 6C) in xylem. We assumed that a higher plasma membrane water permeability of the cells close to the vessels would facilitate the refilling of embolized vessels in winter. The data of the Figure 7D support this assumption. When the transcripts and protein amount of aquaporin are higher (i.e. in February), the immunostaining is much stronger in the cells close to vessels (VACs) than in other living cell types (ray parenchyma elements). These findings suggest that the water movement from parenchyma cells to vessels, which is required for the winter embolism repair, is probably closely related to the presence of the PIP2 aquaporins in VACs. In the context of “vitalistic models,” our data sustain the importance of xylem living cells in winter embolism recovery in walnut tree.
In conclusion, our work provides an insight into the role of aquaporins in perennial plant species. We describe the first example, to our knowledge, of possible involvement of PIP2 aquaporins in refilling of embolized vessels. To elucidate how the PIP2 aquaporins (JrPIP2,1 and JrPIP2,2) are differentially regulated during the winter period, it will be interesting to isolate their respective promoters and determine whether they contain response elements that interact with specific transcription factors that are either cold induced or osmoticum induced.
MATERIALS AND METHODS
Plant Material
One-year-old twigs were cut from 8-year-old walnut (Juglans regia L. cv Franquette) trees (grown outdoors near Clermont-Ferrand, France). The xylem tissue that contains living cells (parenchyma cells including VACs) and dead cells (fibers and xylem vessels; Fig. 7A) was harvested at different times (the end of each month from October 2000–March 2001) and frozen immediately in liquid nitrogen. Xylem tissue was collected after peeling the bark and scraping the outside xylem with a scalpel. Therefore, the xylem contamination with other tissues (i.e. cambium) was avoided. The means of the minimum temperatures during this period are given in Figure 1B.
Determination of Sugars and Starch Content
Xylem tissues (10-cm-long segments) collected from 1-year-old twigs over this period (from October 2000–March 2001) were frozen in liquid nitrogen. Soluble sugars (Suc, Glc, and Fru) and starch content in xylem parenchyma cells (living cells) were determined using the hexokinase method as described according to the manufacturer's instructions (Boehringer Mannheim GmbH, Mannheim, Germany) and Kunst et al. (1984).
RT-PCR-Mediated Cloning of Aquaporin cDNAs from Xylem Tissue
Degenerate primers were deduced from conserved regions of plant aquaporin cDNAs (EMBL data library). The following primers were used: primer WCA, 5′-TAC(AG)(AT)AGA(AT)CC(AT)CCAGC-3′; and primer WCB, 5′-GT(CT)AC(AT)GCTGG(AG)TT(AGT)ATGTGAC-3′. One microgram of total RNAs was extracted from xylem tissue in February (winter period) as previously described by Gévaudant et al. (1999), reverse transcribed (“Ready-To-Go T-Primed First-Strand Kit,” Amersham, Buckinghamshire, UK), and amplified by PCR using the degenerative primers WCA and WCB. The amplified fragment is a 450-bp sequence, including only the coding region of an aquaporin-like gene. This cDNA fragment (450 pb) was then used as PIP homologous probe to screen under low-stringency conditions a cDNA library constructed only from mRNA xylem tissue, harvested over the autumn-winter period. The xylem cDNA library was constructed using the ZAP Express cDNA Synthesis Kit (Stratagene, La Jolla, CA). After screening, only two different cDNA clones (JrPIP2,1 and JrPIP2,2) were found and isolated. Their respective sequences have the following EMBL/GenBank/DDBJ accession numbers: AY189973 and AY189974.
Quantitation of Aquaporin mRNAs by Semiquantitative RT-PCR
A semiquantitative RT-PCR strategy was used to overcome the low transcript level of the xylem tissue. Four microliters of the synthesized first strand cDNA was used as target template for PCR as described above. PCR reactions were carried out as follows: 5 min at 94°C for denaturation, 30 s at 94°C, 30 s at 60°C, and 30 s at 72°C for only 18 or 23 cycles, and 10 min at 72°C for final extension. The optimal number of PCR cycles was established to generate unsaturated (linear phase) but detectable signals for every sequence. For the amplification of JrPIP2(1 and 2), each PCR tube contained common primers to JrPIP2,1 and JrPIP2,2: WC1, 5′ primer, 5′-GGCATTTCAGGAGGACACAT-3′; and WC2, 3′ primer 5′-CCTCAAAATCCATTGGTCAT-3′. For the amplification of JrPIP21, WC11 5′ primer (5′ GCACTTTGATGTGGTTTGGG-3′)andWC123′primer(5′-CATGCACGAATGGACTGAGG-3′) were used. Finally, for the amplification of JrPIP2,2, WC21 5′ primer (5′-CCCCTCTGCTCACCGATTTA-3′) and WC22 3′ primer (5′-TAGGCAAATGGACATCCTCG-3′) were chosen. Specific primers of actin (A1) 5′ primer (5′-ATGAAGCCCAATCAAAGAGGGGT3′) and A2 3′ primer (5′-TGTCCATCACCAGAATCCAGCAC3′) were used to monitor the expression of this constitutive gene. PCRs were performed in parallel on the same cDNA and under the same conditions.
Expression of JrPIP2,2 in Xenopus laevis Oocytes and Osmotic Water Permeability Assay
The JrPIP2,2 open reading frame was amplified by PCR using the following primers: 1, 5′-ACGCGTCGACATGGCCAAGGACATTGAAGCTGCCG-3′; and 2, 5′ ATAAGAATGCGGCCGCTTATATGGTGGAAGAGCTT-3′. The DNA was cut by SalI and NotI and subcloned in a pZL1 vector as in Ciavatta et al. (2001). The resulting plasmid DNA was sequenced to verify fidelity and linearized with NotI. Capped RNA was made with the T3 or T7 RNA polymerase from the Message Machine kit according to the manufacturer's instructions (Ambion, Austin, TX).
Oocyte injection with in vitro-synthesized transcripts JrPIP2,2, AtPIP2;3, or water were performed, and osmotic water permeability measurements were performed according to Ciavatta et al. (2001). Injections and subsequent swelling assays were conducted on three to four separate replicates of oocyte preparations, and five to seven oocytes were measured in each replicate.
Preparation of JrPIP2,1-His-Tag Fusion Protein
To check whether the AtPIP2 antiserum (Daniels et al., 1994) raised against the 12-amino acid residues of the Arabidopsis PIP2 aquaporin C terminus end recognizes the walnut xylem JrPIP2,1 protein, a JrPIP2,1-His-tag fusion protein was made and expressed in Escherichia coli JM109 using the pET-15b vector. The cDNA fragment encoding JrPIP2,1 was amplified using the following primers: 5′-AGCCATATGGCCAAGGACATT-3′ and 5′-GATCCTCGAGTATGGTGGAAGAGCT-3′. For directional cloning into the pET-15b vector, the primers contained an NdeI and XhoI restriction site, respectively. The PCR-derived cDNA fragment was digested and cloned into the NdeI-XhoI site of the vector. E. coli JM109 strain was transformed with trpET-15b vector (control) or pET-15b-JrPIP2,1 vector. Gene expression was monitored as by Herbette et al. (2002). The JrPIP2,1-His-tagged protein was isolated according to the manufacturer's instructions (Qiagen, Courtaboeuf, France) and used to check cross-reactivity with the Arabidopsis PIP2 antiserum.
Isolation of Microsomal Fractions, Electrophoresis, and Immunoblotting
Isolation of microsomal fractions from xylem tissue was performed according to Alves et al. (2001). Electrophoresis under denaturing conditions was performed as described by Laemmli (1970) using a 15% (w/v) SDS-polyacrylamide gel. Immunodetection was achieved with the AtP-PIP2 antiserum diluted 1/200 as the primary antibody and anti-rabbit IgG (H+L) (P.A.R.I.S., 1/10,000 dilution) as the secondary antibody. The protein-antibody complex was detected with a chemiluminescence protein gel blotting detection system (ECL, Amersham-Pharmacia Biotech, Uppsala). To check whether the accumulation of JrPIP2 protein amount may result from a general change in all the membrane proteins, a western blot was made on the same microsomal fractions using purified polyclonal antibodies raised against a walnut plasma membrane H+-ATPase peptide (CDPKEARAGIREVHF).
Tissue Preparation and Immunofluorescence Microscopy
The highly lignified walls of xylem prevented the use of the immunocytolocalization technique widely and easily used in herbaceous plants. Therefore, we used the immunohistolocalization of Fromard et al. (1995). Xylem segments (0.5 cm long) of 1-year-old twigs of walnut tree were cut and fixed for 45 min at 4°C with 1.5% (w/v) formaldehyde and 0.5% (v/v) glutaraldehyde in 0.1 m phosphate-buffered saline (PBS; pH 7.4) containing 0.5% (v/v) dimethylsulfoxide. Several washings in 0.1 m phosphate buffer (pH 7.4) were followed by abundant washing in 8 mm NaH2PO4, 1.5 mm KH2PO4, 140 mm NaCl, and 2.7 mm KCl (pH 7.2; PBS). Sections of 5- to 20-μm thickness were cut with a cryomicrotome. Sections were rinsed in PBS (pH 7.2) and were laid for 15 min on PBS, 0.1% (v/v) Triton X-100, and 0.2% (v/v) Gly (pH 7.2). After washing in a 0.1% (w/v) PBS-Triton medium, nonspecific sites were saturated for 45 min by normal goat serum in PBS, 0.2% (v/v) Triton X-100, 0.2% (v/v) Tween, and 0.1% (w/v) bovine serum albumin (BSA) and incubated overnight, with 1/40 diluted either AtPIP2 antiserum or AtPIP2 antiserum saturated by the purified JrPIP2,1 protein (control), as previously used in other studies (Bouché-Pillon et al., 1994; Fleurat-lessard et al., 1997). JrPIP2,1 was purified as described above. After washing in PBS, 1% (w/v) BSA, and the secondary mouse anti-rabbit IgG-antibody + fluorescein isothiocyanate conjugate (Sigma, St. Louis), diluted 1/200 in PBS, 1% (w/v) BSA was applied for 3 h in darkness. Specimens were observed using a Zeiss Axiovert 405 microscope (Zeiss, Jena, Germany).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027797.
This work was supported in part by the Ministère de la Recherche et de l'Education Nationale and Institut National de la Recherche Agronomique (to S.S., M.D., A.G., and J.-L.J.) and by the U.S. Department of Agriculture, National Research Initiative Competitive Grants Program (to R.M. and M.J.C.).
References
- Alves G, Sauter JJ, Julien JL, Fleurat-Lessard, Ameglio T, Guilliot A, Pétel G, Lacointe A (2001) Plasma membrane H+-ATPase, succinate and isocitrate dehydrogenases activities of vessel-associated cells in walnut trees. J Plant Physiol 158: 1263–1271 [Google Scholar]
- Améglio T, Bodet C, Lacointe A, Cochard H (2002) Winter embolism, mechanism of xylem hydraulic conductivity recovery and springtime growth patterns in walnut an peach trees. Tree Physiol 22: 1211–1220 [DOI] [PubMed] [Google Scholar]
- Améglio T, Cruiziat P (1992) Alternance tension/pression de la sève dans le xylème chez le noyer pendant l'hiver: rôle des températures. C R Acad Sci Paris Ser III 315: 429–435 [Google Scholar]
- Améglio T, Cruiziat P, Béraud S (1995) Alternance tension/pressure de la sève dans le xylème chez le noyer pendant l'hiver: conséquences sur la conductance hydraulique des rameaux. C R Acad Sci Paris Ser III 318: 351–357 [Google Scholar]
- Améglio T, Ewers FW, Cochard H, Martignac M, Vandame M, Bodet C, Cruiziat P (2001) Winter stem pressures in walnut trees: effects of carbohydrates, cooling and freezing. Tree Physiol 21: 384–394 [DOI] [PubMed] [Google Scholar]
- Barrieu F, Chaumont F, Chrispeels MJ (1998) High expression of the tonoplast aquaporin ZmTIP1 in epidermal and conducting tissues of maize. Plant Physiol 117: 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché-Pillon S, Fleurat-Lessard P, Serrano R, Bonnemain JL (1994) Asymmetric distribution of the plasma-membrane H+-ATPase in embryos of Vicia faba L. with special reference to transfer cells. Planta 19: 392–397 [Google Scholar]
- Chrispeels MJ, Morillon R, Maurel C, Gerbeau P, Kjelbom P, Johanson I (2001) Aquaporins in plants: structure, function, regulation and roles in plant water relations. Curr Top Membr 51: 277–334 [Google Scholar]
- Ciavatta VT, Morillon R, Pullman GS, Chrispeels MJ, Cairney J (2001) An aquaglyceroporin is abundantly expressed early in the development of the suspensor and the embryo proper of loblolly pine. Plant Physiol 127: 1556–1567 [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Tyree MT (1990) Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation and seasonal changes in embolism. Tree Physiol 6: 393–407 [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Mirkov TE, Chrispeels MJ (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is homolog of the tonoplast water channel protein TIP. Plant Physiol 106: 1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval FD, Renard M, Jaquinod M, Biuo V, Montrichard F, Macherel D (2002) Differential expression and functional analysis of three calmodulin isoforms in germinating pea (Pisum sativum L.) seeds. Plant J 32: 481–493 [DOI] [PubMed] [Google Scholar]
- Ewers FW, Améglio T, Cochard H, Martignac M, Vandame M, Bodet C, Cruiziat P (2001) Seasonal variation of xylem pressure in walnut trees: root and stem pressure. Tree Physiol 21: 1123–1132 [DOI] [PubMed] [Google Scholar]
- Fleurat-Lessard P, Frangne N, Maeshima M, Ratajczak R, Bonnemain J, Martinoia E (1997) Increased expression of vacuolar aquaporin and H+-ATPase related to motor cell function in Mimosa pudica L. Plant Physiol 144: 827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromard L, Babin V, Fleurat-Lessard P, Fromont JC, Serrano R, Bonnemain JL (1995) Control of vascular sap pH by the vessel-associated cells in woody species: physiological and immunological studies. Plant Physiol 108: 913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gévaudant F, Samson I, Guilliot A, Pétel G (1999) An improved method for isolating polyphenol-free RNA from woody plant tissues. J Trace Microprobe 17: 445–450 [Google Scholar]
- Herbette S, Lenne C, Leblanc N, Julien JL, Drevet JR, Roeckel-Drevet P (2002) Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur J Biochem 269: 1–7 [DOI] [PubMed] [Google Scholar]
- Holbrook NM, Zwieniecki MA (1999) Embolism repair and xylem tension: do we need a miracle? Plant Physiol 120: 7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126: 1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RW, Tyree MT, Dixon MA (1987) A requirement for sucrose in xylem sap flow from dormant maple trees. Plant Physiol 84: 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch HH, Vera-Estrella R, Golldack D, Quigley F, Michalowski CB, Barkla BJ, Bohnert HJ (2000) Expression of water channel proteins in Mesembryanthemum crystallinum. Plant Physiol 123: 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst A, Draeger B, Ziegenhorn J (1984). Carbohydrates, U.V. methods with hexokinase and glucose-6-phosphate dehydrogenase. In HU Bergmeyer, ed, Methods in Enzymatic Analysis: Metabolites, Ed 3, Vol VI. Verlag Chemie, Basel, pp 162–172 [Google Scholar]
- Lachaud S, Maurousset L (1996) Occurrence of plasmodesmata between differentiating vessels and other xylem cells in Sorbus torminalis L. Crantz and their fate during xylem maturation. Protoplasma 191: 220–226 [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lemoine D, Granier A, Cochard H (1999) Mechanism of freeze-induced embolism in Fagus sylvatica L. Trees 13: 206–210 [Google Scholar]
- Livingston DPIII, Henson CA (1998) Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol 116: 403–408 [Google Scholar]
- Mariaux JB, Bockel C, Salamoni F, Bartels D (1998) Dessication and abscisic acid-responsive genes encoding major intrinsic proteins (MIPs) from the resurrection plant Craterostigma plantagineum. Plant Mol Biol 38: 1089–1099 [DOI] [PubMed] [Google Scholar]
- Marin-Olivier, Chevalier T, Fobis-Loisy I, Dumas C, Gaude T (2002) Aquaporin PIP genes are not expressed in the stigma papillae in Brassica oleracea. Plant J 24: 231–240 [DOI] [PubMed] [Google Scholar]
- Maurel C, Chrispeels MJ (2001) Aquaporins: a molecular entry into plant water relations. Plant Physiol 125: 135–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesak BH, Coruzzi GM (2002) Molecular and physiological analysis of Arabidopsis mutants defective in cytosolic or chloroplastic aspartate aminotransferase. Plant Physiol 129: 650–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn JA, O'Mally PER (1984) Freeze-induced sap absorption in Acer pseudoplantanus: a possible explanation. Can J Bot 62: 2101–2106 [Google Scholar]
- Morillon R, Liénard D, Chrispeels MJ, Lasalles JP (2001) Rapid movements of plants organs require solute-water co-transporters or contractile proteins. Plant Physiol 127: 720–723 [PMC free article] [PubMed] [Google Scholar]
- Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, Levi H, Moran N, Kaldenhoff R (2002) Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. Plant Cell 14: 727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley PER, Milburn JA (1983) Freeze-induced fluctuation in xylem sap pressure in Acer pseudoplatanus. Can J Bot 61: 3100–3106 [Google Scholar]
- Orsel M, Krapp A, Daniel-Vedele F (2002) Analysis of the NRT2 nitrate transporter family in Arabidopsis structure and gene expression. Plant Physiol 129: 886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto B, Kaldenhoff (2000) Cell-specific expression on the mercury-insensitive plasma-membrane aquaporin NtAQP1 from Nicotiana tabacum. Planta 211: 167–172 [DOI] [PubMed] [Google Scholar]
- Pickard WF (1989) How might a tracheary element which is embolized by day be healed by night. J Theor Biol 141: 259–279 [Google Scholar]
- Pockman WT, Sperry JS (1997) freezing-induced xylem cavitation and the northern limit of Larrea tridentata. Oecologia 109: 19–27 [DOI] [PubMed] [Google Scholar]
- Ruiter RK, Van Eldik GJ, Van Herpen MMA, Schrauwen JAM, Wullems GJ (1997) Expression in anthers of two genes encoding Brassica oleracea transmembrane channel proteins. Plant Mol Biol 34: 163–168 [DOI] [PubMed] [Google Scholar]
- Sauter JJ, Kloth S (1987) Changes in carbohydrates and ultrastructure in xylem ray cells of Populus in response to chilling. Protoplasma 137: 45–55 [Google Scholar]
- Sauter JJ (1988) Temperature-induced changes in starch and sugars in the stem of Populus × canadensis “robusta.” J Plant Physiol 132: 608–612 [Google Scholar]
- Sauter JJ, Wisneiwski M, Witt W (1996) Interrelationships between ultrastructure, sugar levels, and frost hardiness of ray parenchyma cells during frost acclimation and deacclimation in poplar (Populus × canadensis Moench [robusta]) wood. J Plant Physiol 149: 451–461 [Google Scholar]
- Sperry JS, Holbrook M, Zimmermann MH, Tyree MT (1987) Spring filling of xylem vessels in wild grapevine. Plant Physiol 83: 414–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Nichols KL, Sullivan JEM, Eastlack SE (1994) Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 75: 1736–1752 [Google Scholar]
- Suga S, Imagawa S, Maeshima M (2001) Specificity of the accumulation of mRNAs and proteins of the plasma membrane and tonoplast aquaporins in radish organs. Planta 212: 294–304 [DOI] [PubMed] [Google Scholar]
- Taybi T, Cushman JC (1999) Signaling events leading to crassulacean acid metabolism induction in the common ice plant. Plant Physiol 121: 545–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman SD, Niemietz CM, Bramley H (2002) Plants aquaporins: multi-functional water and solute channels with expanding roles. Plant Cell Environ 25: 173–194 [DOI] [PubMed] [Google Scholar]
- Tyree MT (1983) Maple sap uptake, exudation and pressure changes correlated with freezing exotherms and thawing endotherms. Plant Physiol 73: 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Cochard H (1996) Summer and winter embolism in oak: impact on water relations. Ann Sci For 53: 173–180 [Google Scholar]
- Tyree MT, Salleo S, Nardini A, Lo Gullo MA, Mosca R (1999) Refilling of embolized vessels in young stems of laurel: do we need a new paradigm? Plant Physiol 120: 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weig A, Deswarte C, Chrispeels MJ (1997) The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol 114: 1347–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Koizumi M, Urao S, Shinozaki K (1992) Molecular cloning and characterization of 9 cDNAs for genes that are responsive to desiccation in Arabidopsis thaliana: sequence analysis of one cDNA that encodes a putative transmembrane channel protein. Plant Cell Physiol 33: 217–224 [Google Scholar]
- Yang SC, Tyree MT (1992) A theoretical model of hydraulic conductivity recovery from embolism with comparison to experimental data on Acer saccharum. Plant Cell Environ 15: 633–643 [Google Scholar]
- Zhu XB, Cox RM, Arp PA (2000) Effects of xylem cavitation and freezing injury on dieback of yellow birch (Betula alleghaniensis) in relation to a simulated winter thaw. Tree Physiol 20: 541–547 [DOI] [PubMed] [Google Scholar]