Abstract
The MADS domain protein AGL15 (AGAMOUS-Like 15) has been found to preferentially accumulate in angiosperm tissues derived from double fertilization (i.e. the embryo, suspensor, and endosperm) and in apomictic, somatic, and microspore embryos. Localization to the nuclei supports a role in gene regulation during this phase of the life cycle. To test whether AGL15 is involved in the promotion and maintenance of embryo identity, the embryogenic potential of transgenic plants that constitutively express AGL15 was assessed. Expression of AGL15 was found to enhance production of secondary embryos from cultured zygotic embryos, and constitutive expression led to long-term maintenance of development in this mode. Ectopic accumulation of AGL15 also promoted somatic embryo formation after germination from the shoot apical meristem of seedlings in culture. These results indicate that AGL15 is involved in support of development in an embryonic mode.
The MADS domain protein AGL15 (AGAMOUS-Like 15) preferentially accumulates in a wide variety of tissues that are developing in an embryonic mode, suggesting that it may play an important role during this phase of the higher plant life cycle (Heck et al., 1995; Rounsley et al., 1995; Perry et al., 1996, 1999). MADS domain proteins are a family of regulatory factors that share an approximately 55- to 60-amino acid residue domain (the MADS domain) that mediates dimer formation and sequence-specific binding to DNA (for review, see Riechmann and Meyerowitz, 1997). The plant MADS box gene family is relatively large, numbering 107 in Arabidopsis (Parenicová et al., 2003). Many members of this group have been shown to play key roles in developmental decisions, as demonstrated by loss-of-function mutations resulting in homeotic transformation of organ identity (for review, see Riechmann and Meyerowitz, 1997). However, it is not unusual for members of this family to have redundant functions, making a double or even triple mutant combination necessary before a phenotype is observed (Kempin et al., 1995; Liljegren et al., 2000; Pelaz et al., 2000).
In cases where functional redundancy exists, ectopic expression studies can be particularly revealing. For example, the petunia (Petunia hybrida) MADS box gene FBP11 produces ectopic ovules on floral organ surfaces when constitutively expressed (Colombo et al., 1995), substantiating FBP11's proposed role as an ovule identity gene. MADS box genes expressed in inflorescence and floral meristems have been studied to the greatest extent, but members of the MADS box family are preferentially expressed in other tissues (Heck et al., 1995; Rounsley et al., 1995; Alvarez-Buylla et al., 2000; Burgeff et al., 2002).
AGL15 is the only MADS box gene reported to date that is preferentially expressed in developing embryos (Heck et al., 1995; Rounsley et al., 1995; Fernandez et al., 2000). Although other MADS box genes are expressed in embryos, they are also expressed at similar or higher levels at other stages of plant development. AGL15 accumulates in the nuclei of cells in the embryo beginning very early in development (by the eight-cell stage for Arabidopsis) and remains at relatively high levels throughout morphogenesis and into maturation stage (Perry et al., 1996). The level of AGL15 decreases during desiccation. AGL15 accumulates in nuclei of a wide variety of tissues developing in an embryonic mode including apomictic, somatic, and microspore embryos, embryonic tissue derived from the precocious germination system of Brassica napus, and extra-embryonic organs in mutants of Arabidopsis (Perry et al., 1999). AGL15 is expressed after germination in Arabidopsis, in the vegetative shoot apical meristem (SAM), and bases of lateral organs such as leaves, cauline leaves, and floral organs (Fernandez et al., 2000). The level of expression in these tissues is generally at least 10-fold lower than found in the embryo (Heck et al., 1995; Fernandez et al., 2000).
The presence of AGL15 in the nuclei of a wide variety of tissue types developing in an embryonic mode suggests that AGL15 may be important for this phase of the life cycle. Other transcriptional regulators that are preferentially expressed during embryogenesis have been shown to promote somatic embryo development when ectopically expressed (Lotan et al., 1998; Stone et al., 2001; Boutilier et al., 2002). In this report, evidence that AGL15 also acts to promote development in an embryonic mode is presented. Providing AGL15 via a transgene that causes constitutive expression supported maintenance of embryo development in culture for extended periods of time, over 6 years to date. The effect of AGL15 was also examined in genetic backgrounds where somatic embryos readily initiate from the SAM (Mordhorst et al., 1998). AGL15 enhanced embryo production from the meristems in these mutant backgrounds and in a wild-type background.
RESULTS
Expression of AGL15 Leads to Extension of the Period of Embryonic Development in Culture
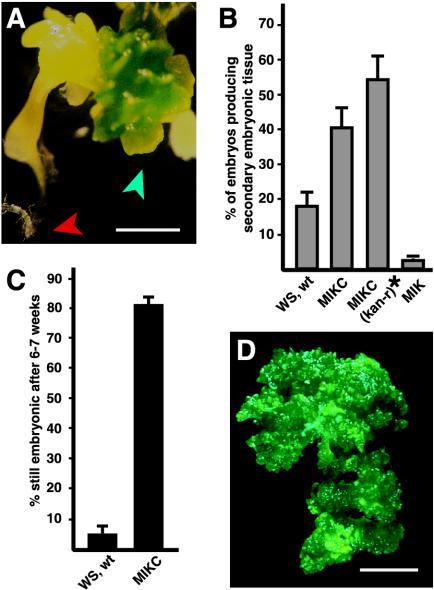
Previous results have demonstrated that in all cases tested, embryos or embryonic tissue from flowering plants accumulate AGL15 (Perry et al., 1996, 1999). The correlation is suggestive, but does AGL15 directly contribute to either the establishment or maintenance of the embryonic phase? Plants carrying transgenic constructs consisting of the cauliflower mosaic virus (CaMV) 35S promoter driving constitutive expression of full-length AGL15 (Fernandez et al., 2000) were used to address this question. The full-length transgenes, referred to in this report as MIKC constructs, encode the conserved DNA-binding MADS domain, a short linker region (called an I domain), the weakly conserved K domain, and the unique C-terminal domain. Two forms of the MIKC transgene were used to transform Arabidopsis: One contains the first three introns, whereas the other represents the cDNA (Fernandez et al., 2000). The transgene with the first three introns generally provides higher levels of expression of AGL15. Transgenic plants expressing full-length AGL15 via the 35S promoter accumulate AGL15 in many tissues, including those in inflorescence meristems, flowers, and flower buds (Perry et al., 1999; Fernandez et al., 2000), leaves (C. Zhu and S. Perry, unpublished data), and developing seedlings and siliques (S. Perry, unpublished data). When developing embryos were removed from the seed at the green bent cotyledon stage (10–11 d after flowering) and cultured on germination media (GM) without exogenous growth regulators, embryonic foci appeared on the cultured embryos within 2 to 3 weeks, as shown in Figure 1A. Often, the foci appeared at sites where the embryo had been wounded during isolation, but they also formed at the shoot apex and cotyledons and occasionally on the hypocotyl. Often, the root apical meristem activated, producing an elongated root (Fig. 1A). As shown in Figure 1B, over 40% of the embryos cultured from MIKC (with introns) plants showed developing embryonic foci within 3 weeks after the start of culture. A total of 530 cultured embryos from two independent transgenic lines expressing moderate levels of AGL15 (described by Fernandez et al., 2000) were scored for secondary embryo development. Upon subculturing, over 80% of the embryos with embryonic foci continued producing embryonic tissue for at least 6 to 7 weeks (Fig. 1C). At the time of submission of this manuscript, one set of cultured embryos has been producing additional embryonic tissue continuously for over 6 years. Appearance of the cultured tissue after approximately 1.5 years is shown in Figure 1D. Excised wild-type (Wassilewskija [Ws] ecotype) embryos also produced secondary embryos in culture, but at a lower frequency (18.2% of embryos cultured, se 3.7%, 873 embryos total scored, Fig. 1B). Over a 2-month period, these foci gradually stopped forming embryonic tissue and began forming leaves (indicated by the presence of trichomes; Fig. 1C).
Figure 1.
Production and maintenance of embryonic culture tissue (ECT). A, Cultured zygotic embryo carrying the MIKC transgene. Developing embryonic foci are indicated with a blue arrowhead. The developing root of the cultured zygotic embryo is indicated with a red arrowhead. Bar = 1 mm. B, Percentage of cultured zygotic embryos that produced embryonic foci. Results shown are means and se of the mean derived from six to eight experiments. Ws, wt, Wild-type zygotic embryos cultured on GM. MIKC, Zygotic embryos from a hemizygous transgenic plant carrying one copy of the 35S: AGL15 transgene cultured on GM plus kanamycin. MIKC (kan-r)*, Recalculated percentage of the MIKC embryos that produced secondary embryos estimating that only approximately 75% of cultured MIKC zygotic embryos carried the transgene. MIK, Zygotic embryos from a transgenic plant with a transgene consisting of the 35S promoter driving expression of a truncated form of AGL15 lacking the C-terminal domain. C, Percentage of the embryonic foci that continued to develop in embryonic mode after 6 to 7 weeks in culture. Results shown are means and ses from at least four experiments. D, Appearance of the ECT from an MIKC embryo after approximately 1.5 years in culture. Bar = 2.5 mm.
The MIKC (with introns) transgenic plants used in these experiments were hemizygous for the transgene because the homozygous individuals in these lines are sterile. Therefore, the percentage of MIKC embryos that produce secondary embryos is likely to be an underestimate because 25% of the cultured embryos arrest for another reason, i.e. they are kanamycin sensitive. If the efficiency of production of secondary embryos is recalculated based on the total kanamycin-resistant embryos cultured (estimated as 75% of the total), then over one-half (54.8%, se 7.5%) of the cultured embryos showed secondary embryonic tissue development (Fig. 1B, MIKC [kan-r, kanamycin resistant]). Transgenic plants that expressed a truncated form of AGL15 lacking the C-terminal domain were also generated. These plants expressed a form of AGL15 consisting of only the DNA-binding MADS domain, the I-linker domain, and the K domain. Similar constructs with AGAMOUS and Serum Response Factor sequences produce dominant negative effects (Gauthierrouviere et al., 1993; Mizukami et al., 1996; Belaguli et al., 1999). In general, plants carrying the transgene encoding truncated AGL15, referred to as the MIK transgene, showed phenotypic changes that were opposite of those in plants carrying the full-length form of AGL15. For example, the MIKC transgenic plants flower later than wild type (Fernandez et al., 2000), whereas the MIK transgenic plants flower earlier than wild type (K.W. Nichols and D.E. Fernandez, unpublished data). However, MIKC and MIK zygotic embryos progressed through morphogenesis at the same rate as wild-type embryos (Fernandez et al., 2000; data not shown). Cultured embryos carrying the MIK transgene produced embryonic foci at a very low frequency (Fig. 1B, 2.4%, se 0.7%, 714 embryos total scored from three independent MIK transgenic lines). No instances of continued embryonic tissue production were observed after 2 months.
What is the evidence that the MIKC culture tissue was developing in an embryonic mode? The organs produced more closely resembled cotyledons than leaves. The margins of the organs were smooth, and no trichomes were present (Fig. 1, A and D). The organs also had a simple vasculature, much like that found in cotyledons from seedlings (compare Fig. 2A, cotyledon from a seedling, with Fig. 2C, organ from the ECT), rather than the complex network of vasculature found in leaves from seedlings (Fig. 2B). During the first few months in culture, leaves with serrated margins, trichomes, and a more complex vasculature were also found at low frequency (Fig. 2D). Besides cotyledon-like organs, what appeared to be fused embryonic axes and root meristems, which occasionally produced an elongating root, were often present (Fig. 2, E, F, and I). In addition, whole embryos were occasionally observed on the surface of the cotyledon-like organs (Fig. 2F). New embryonic structures originated from a variety of regions, including axils of older organs (Fig. 2G) and lamina of cotyledon-like organs (Fig. 2H).
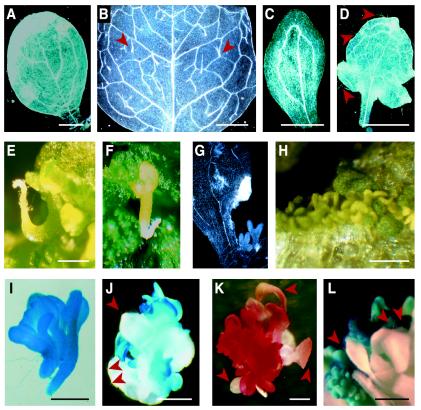
Figure 2.
Characterization of the ECT. A to D, Cleared organs observed using dark-field optics to examine vasculature. Cotyledon (A) and leaf (B) from a germinated seedling with the MIKC transgene. C, Cotyledon-like organ from the embryonic cultures. D, Leaf-like organ from the embryonic cultures. E and F, Examples of activated root apical meristems (E) and intact embryos (F) occasionally produced from embryonic cultures. G and H, Embryonic-like structures produced in the axils (G) or on the surface (H) of lateral organs. I and J, β-Glucuronidase (GUS) activity in the ECT carrying a soybean (Glycine max) β-conglycinin promoter:GUS construct. K, Sudan red 7B staining of ECT indicating accumulation of triacylglycerols. L, GUS activity in the ECT carrying an AGL15 promoter:GUS construct. Arrowheads in B, D, J, K, and L indicate trichomes. Bars in A to D and J to L = 1 mm; in E, H, and I, bars = 0.5 mm.
To test whether the tissues with embryonic features expressed seed-specific programs, a transgene consisting of the soybean β-conglycinin promoter driving expression of the reporter gene GUS (Hirai et al., 1994) was introduced into the MIKC transgenic plants. Developing embryos containing both transgenes were removed from the seeds at the green bent cotyledon stage and placed into culture. Embryonic foci appeared within 3 weeks and were stained for GUS activity. As shown in Figure 2I, GUS activity was associated with all parts of fused embryonic axes and cotyledons. In cases where leaves were also produced (scored by the presence of trichomes), GUS staining was apparent only in the cotyledon-like organs that did not have trichomes and not in organs resembling leaves (Fig. 2J). Developing foci also stained intensely with the dye Sudan Red 7B (fat red, Fig. 2K), which specifically stains neutral lipids (Brundrett et al., 1991), such as the triacylglycerols that accumulate during Arabidopsis embryogenesis (Ogas et al., 1997). Little staining was observed in leaf-like organs produced in culture (Fig. 2K).
If the culture tissues have embryo identity, relatively high levels of AGL15 promoter activity might be expected. Immature zygotic embryos that contain a transgene consisting of 2.5-kb 5′- and 2.5-kb 3′-regulatory regions of AGL15 driving expression of the reporter gene GUS (Fernandez et al., 2000) were excised and cultured. These embryos, like wild-type embryos, will transiently produce new embryonic tissues in culture. As shown in Figure 2L, GUS activity was detected in these tissues, but not in leaf-like organs produced in culture, indicating that AGL15 promoter activity was associated with development in an embryonic mode.
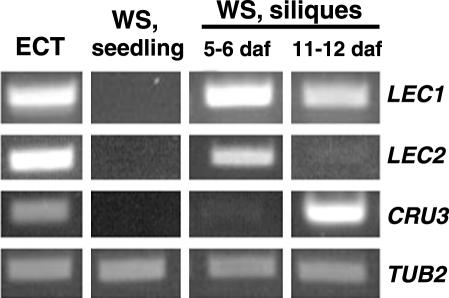
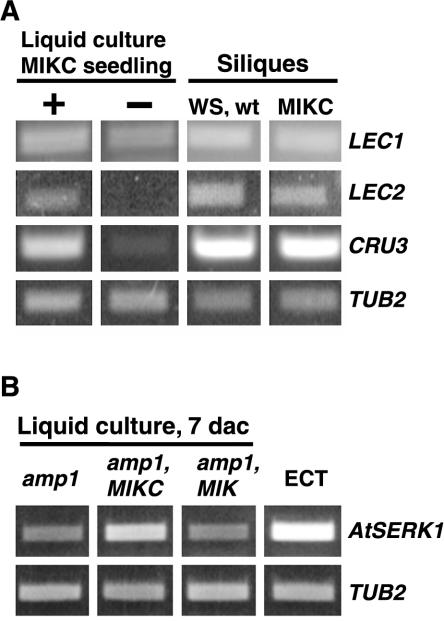
The mode of development of the culture tissue was further investigated at a molecular level. Cultures that had been maintained for extended periods (over 5 years) were used for these experiments. Reverse transcription (RT)-PCR and gene-specific oligonucleotide primers were used to compare expression of several embryo markers in the culture tissue with expression in seedlings and siliques. LEC1 (LEAFY COTYLEDON1) and LEC2 (LEAFY COTYLEDON2) both encode transcriptional regulators that are expressed primarily during embryonic development (Lotan et al., 1998; Stone et al., 2001). As shown in Figure 3, both LEC1 and LEC2 were expressed in the ECT. As expected, expression was also observed in silique tissue, particularly at earlier stages of development, but not in wild-type seedlings (Fig. 3) or in MIKC seedlings (data not shown). In addition, one of the Arabidopsis 12S cruciferin seed storage protein genes (AtCRU3) was expressed in the culture tissue and in older stage siliques but not in seedlings (Fig. 3). The control, β-2-tubulin (TUB2; Snustad et al., 1992), was expressed in all tissues.
Figure 3.
Expression of embryo-specific genes in the ECT. Gene-specific primers and RT-PCR were used to examine expression of LEAFY COTYLEDON1, LEAFY COTYLEDON2, and a 12S cruciferin gene in the ECT and in Ws seedlings and staged silique tissue. Products were analyzed on an agarose gel. Tubulin (TUB2) served as a control.
Expression of AGL15 Stimulates Production of Embryos from SAMs
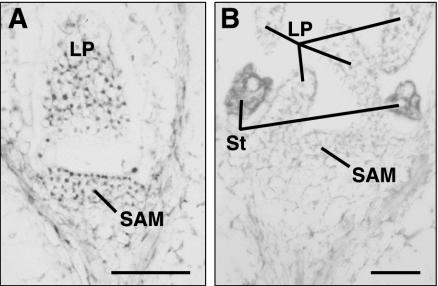
A group of Arabidopsis mutants with enlarged SAMs showed enhanced production of embryos from the SAM when seedlings were grown in liquid culture media containing 2,4-dichlorophenoxyacetic acid (2,4-D; Mordhorst et al., 1998; von Recklinghausen et al., 2000). This group of mutants includes amp1 (altered meristem program; allelic to pt1, cop2, and hpt), clavata1, and clavata3. Increased size of the SAM is correlated with increased frequency of somatic embryo development in this system (Mordhorst et al., 1998). In the amp1 background, where the SAM is approximately twice the size of wild type, 30% of the seedlings produce secondary embryos (Mordhorst et al., 1998). This finding was intriguing because the very young SAM is one of two places where AGL15 can be detected immunologically after germination in Arabidopsis (Fig. 4; Fernandez et al., 2000). Figure 4A shows immunohistochemical localization of AGL15 in the SAM of a 4-d-old seedling. AGL15 was detected in the nuclei of the cells in the SAM and in the young leaf. However, by 6 d after germination, nuclear localized AGL15 could no longer be detected in the SAM and leaves (Fig. 4B).
Figure 4.
Accumulation of AGL15 in the shoot apex of seedlings. A, Four-day-old Ws wild-type seedling. B, Six-day-old Ws wild-type seedling. LP, Leaf primordia; St, stipule primordia. Bars = 50 μm.
To test whether altering AGL15 levels affects production of somatic embryos in the amp1 background, the MIKC and MIK transgenes were introduced into the amp1 mutant background via genetic crosses. Homozygous amp1 progeny were identified in the F2 generation by screening for seedlings with three cotyledons, and the transgene was selected by kanamycin resistance. Seed was germinated in liquid culture with 2,4-D as described by Mordhorst et al. (1998), with kanamycin present to eliminate any seedlings lacking the MIKC or MIK transgenes. amp1 homozygous mutants were germinated in media without kanamycin.
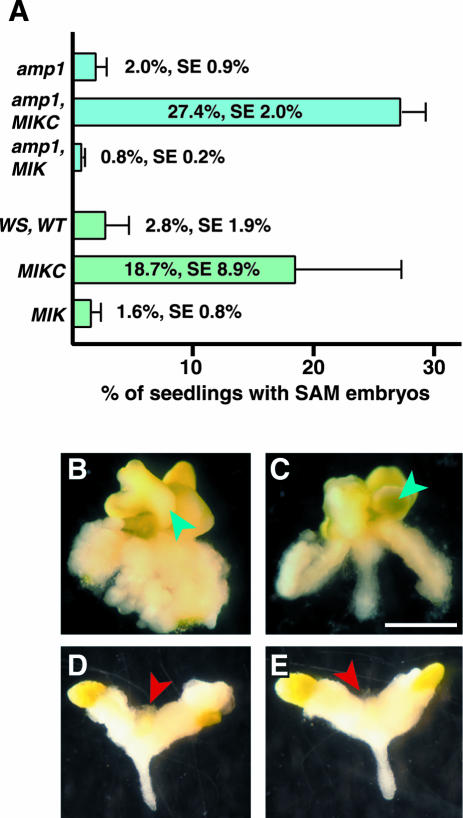
As shown in Figure 5A, under our culture conditions, only 2% (se 0.9%, n = 5 experiments, 891 total seedlings scored) of the amp1 homozygous seedlings produced new embryos at the SAM. However, when the MIKC transgene was present, 27.4% of the amp1 seedlings produced new structures at the SAM as shown in Figure 5, B and C (se 2.0%, n = 5 experiments, 1,265 total kanamycin-resistant seedlings scored). The new growth lacked trichomes and appeared to be similar to the shoot meristem embryos reported previously (Mordhorst et al., 1998). Approximately another one-third had green smooth meristem development (data not shown) that might correspond to the embryogenic callus reported by von Recklinghausen et al. (2000). amp1 homozygous seedlings carrying the MIK transgene did not produce embryo-like structures in the culture system (Fig. 5A, 0.8%, se 0.2%, n = 5 experiments, 1,857 total kanamycin-resistant seedlings scored). Typically, the meristem did not develop in these seedlings (Fig. 5, D and E). The seedlings callused and looked much like the amp1 seedlings, except that the tips of the cotyledons often remained green.
Figure 5.
Embryonic development from the shoot apex of seedlings in culture. A, Percentage of seedlings that showed embryonic development at the SAM when germinated in liquid media containing 2,4-D. Results shown are means and ses of four to six independent experiments. amp1, amp1 mutant without any transgenes. amp1, MIKC, amp1 mutant containing a transgene consisting of the 35S promoter driving expression of full-length AGL15. amp1, MIK, amp1 mutant containing a transgene consisting of the 35S promoter driving expression of a truncated form of AGL15 lacking the C-terminal domain. WS, wt, Wild-type Arabidopsis seedlings. MIKC and MIK, Transgenic Ws seedlings expressing the different forms of AGL15. B to E, SAM development in seedlings germinated in liquid culture containing 2,4-D. B and C, amp1 mutant carrying a transgene consisting of the 35S promoter driving expression of full-length AGL15. D and E, amp1 mutant carrying a transgene consisting of the 35S promoter driving expression of a truncated form of AGL15 lacking the C-terminal domain. Blue arrowheads indicate development of embryo-like tissue at the shoot apex; red arrowheads indicate lack of development at the shoot apex. Bar = 1 mm.
The MIKC transgene was also able to enhance production of embryos from the SAM in the absence of the amp1 mutation. Although the frequency of production was somewhat variable, the MIKC seedlings produced meristem-derived embryos at a higher frequency on average than Ws wild-type or MIK seedlings. For MIKC, 18.7% (se 8.9%) of seedlings produced embryo structures, compared with 2.8% (se 1.9%) for Ws wild-type seedlings and 1.6% (se 0.8%) for MIK seedlings (Fig. 5A). All numbers are means from at least four independent experiments scoring a total of 810 to 1,114 seedlings.
The seedlings bearing embryonic structures were assessed further to confirm that development was occurring in an embryonic mode. MIKC seedlings that produced new structures at the SAM were separated from MIKC seedlings that did not, and RNA was extracted from both sets of seedlings for RT-PCR. As shown in Figure 6A, liquid culture grown seedlings with SAM development showed increased accumulation of LEC1, LEC2, and AtCRU3 mRNA relative to seedlings lacking SAM development. Wild-type and MIKC seedlings grown on GM plates did not produce new embryos at the SAM, and none of the embryo markers accumulated in these tissues (Fig. 3; data not shown).
Figure 6.
Expression of embryo-specific genes in seedlings germinated in liquid culture with 2,4-D. A, MIKC seedlings with SAM development (+) were separated from MIKC seedlings lacking SAM development (–) after 21 d in culture and assessed for expression of embryo-specific genes. Gene-specific primers and RT-PCR were used to assess expression of LEAFY COTYLEDON1, LEAFY COTYLEDON2, and a 12S cruciferin gene in the liquid culture seedlings and in staged 11- to 12-d-old silique tissue. B, amp1 liquid culture seedlings with the MIKC or MIK transgene or lacking any transgene were assessed for expression of AtSERK1 7 d after culture. ECT was also assessed for expression of AtSERK1. Tubulin (TUB2) served as a control. RT-PCR products were analyzed on an agarose gel.
To examine the effect of AGL15 accumulation on early stages of somatic embryo development, expression of AtSERK1 was assessed shortly after culture initiation (7 d). AtSERK1 is expressed in tissues with increased embryogenic competence, and ectopic expression of this gene enhances efficiency of somatic embryo initiation in culture (Hecht et al., 2001). As shown in Figure 6B, amp1 seedlings containing the MIKC transgene showed increased accumulation of AtSERK1 mRNA compared with amp1 seedlings with or without the MIK transgene. AtSERK1 was also expressed in the ECT derived from cultured MIKC zygotic embryos (Fig. 6B).
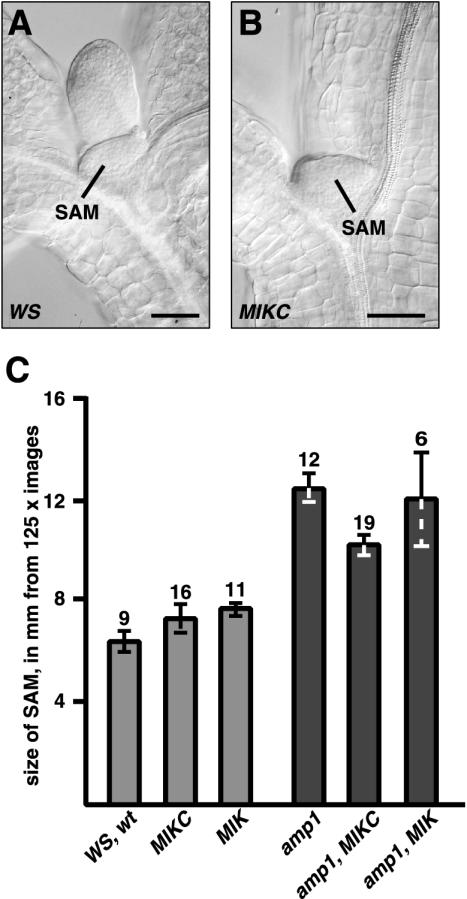
Because Mordhorst et al. (1998) found that there is a correlation between SAM size and ability to produce SAM somatic embryos in culture, the meristems of MIKC and MIK in wild-type and amp1 mutant backgrounds and Ws and amp1 were examined. Seedlings grown on GM plates and mature embryos were cleared in Hoyer's solution and examined using differential interference contrast (DIC) optics. As shown in Figure 7, A and B, the meristem of an MIKC seedling was similar in size to that of a wild type (Ws seedling). As shown in Figure 7C, when images of meristems were measured, no significant differences were observed between Ws, MIKC and MIK seedlings. amp1, as expected, had a larger meristem, but the addition of the AGL15 transgenes did not increase meristem size over that found for amp1 alone. Likewise, comparative meristem sizes in mature embryos were similar (data not shown).
Figure 7.
Relative size of the SAM of seedlings with and without MIKC and MIK transgenes in wild-type and amp1 mutant backgrounds. A and B, Wild-type and MIKC seedlings were cleared and examined under DIC optics. A, Ws wild-type seedling; B, seedling containing a transgene consisting of the 35S promoter driving expression of full-length AGL15. C, Average size (diameter) of the SAM measured in millimeters from pictures of seedlings that were at a final magnification of 125×. Bar = mean size with se; the number above the bar indicates the number of seedlings photographed and measured. Bar in A and B = 50 μm.
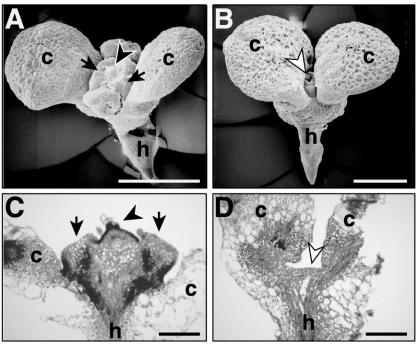
Differences at the meristem became apparent shortly after initiation of culture in media containing 2,4-D. By 5 d in culture, the SAM of amp1 with MIKC seedlings was enlarged compared with amp1 alone (Fig. 8, A and B, respectively). A “collar” of tissue, as described by von Recklinghausen et al. (2000), perhaps derived from the outer SAM, was clearly apparent in seedlings carrying the MIKC transgene, as was development at the central region of the SAM. Cell proliferation at the SAM continued, and, by 10 d in culture, the SAM of amp1 with MIKC was smooth, enlarged, and green in color (data not shown). By 7 d after culture, the SAM of amp1 seedlings carrying the MIKC transgene was enlarged and showed cytoplasmically dense protrusions in the central part of the meristem, as revealed by staining of tissue sections (arrowhead, Fig. 8C). In amp1, only callus development was obvious at the meristem (data not shown). The meristem region of amp1 seedlings with the MIK transgene had a relatively flat meristem (Fig. 8D) or a dome near the apex that did not have any cytoplasmically dense protrusions (data not shown).
Figure 8.
Appearance of the SAM of seedlings in liquid culture containing 2,4-D. A and B, Appearance of the apical region of amp1 seedlings with the MIKC transgene (A) or amp1 seedlings lacking a transgene (B) after 5 d in culture. C and D, Tissue sections (7 μm) stained with toluidine blue O of amp1 seedlings containing either the MIKC (C) or the MIK (D) transgene after 7 d in culture. Arrows in A and C, Ring of tissue proliferation; black arrowheads, proliferation at the central zone of the SAM. White arrowheads in B and D, Little to no proliferation at the meristem. Bar in A and B = 500 μm; bar in C and D = 200 μm.
DISCUSSION
In this report, results are presented demonstrating that continuous expression of AGL15 driven by the CaMV 35S promoter (MIKC construct) enhances first the production of secondary embryonic tissue from cultured zygotic embryos and then supports the long-term maintenance of the embryonic phase. There are several published reports of induction of Arabidopsis somatic embryo cultures from immature zygotic embryos (Sangwan et al., 1992; Wu et al., 1992; Pillon et al., 1996; Luo and Koop, 1997; Ikeda-Iwai et al., 2002) or from leaf protoplasts (O'Neill and Mathias, 1993; Luo and Koop, 1997). These cultures require the addition of exogenous auxins and, in some cases, cytokinins. Auxin is thought to induce embryogenic competence, but how this occurs is not yet understood (Harada et al., 1998). The synthetic auxin 2,4-D has been found to promote recurrent embryony on the surface of cultured zygotic embryos, perhaps by generating cells that recapitulate the current developmental program. However, 2,4-D leads to dedifferentiation and formation of embryogenic callus in the case of the somatic embryos derived from the zygotic embryos (Pillon et al., 1996). In the absence of a sufficiently high level of 2,4-D, non-embryogenic callus results (Pillon et al., 1996). Notably, development of secondary embryos in our culture system occurred in the absence of exogenous growth regulators, and continued embryonic development with MIKC tissues was sustained for extensive periods of time.
Whereas both wild-type and MIKC transgenic developing embryos gave rise to secondary embryos, MIKC transgenic embryos produced embryonic structures at a significantly higher frequency. Over one-half of cultured MIKC embryos produced secondary embryos; however, only 18% of cultured wild-type embryos showed this development. Transgenic embryos expressing a truncated form of AGL15 lacking the C-terminal domain (MIK construct) were unable to efficiently promote secondary embryo development. This type of truncated construct has been found to have dominant negative effects for other MADS domain proteins (Gauthierrouviere et al., 1993; Mizukami et al., 1996; Belaguli et al., 1999), possibly by blocking endogenous protein activity either by formation of nonfunctional heterodimers or occupation of DNA-binding sites.
Although the majority of MIKC embryos that began embryonic development continued producing embryonic tissue (cotyledons) over extended periods of time, wild-type cultures shortly switched to generating vegetative tissues (leaves). The wild-type embryos contain endogenous AGL15 at the time of excision (Perry et al., 1996), which may contribute to competency to promote development of secondary embryos. However, the endogenous promoter may be unable to maintain expression at the levels needed to support development in an embryonic mode over extended periods of time. On the other hand, when AGL15 is constitutively expressed at high levels using the 35S promoter, AGL15 protein is maintained at levels comparable with that found in B. napus and Arabidopsis zygotic embryos (Wang et al., 2002). In these cultures, development continues as embryonic for extended time periods (over 6 years to date). The culture tissue was examined at a morphological and molecular level and found to express embryonic features and gene expression programs for all tests performed. Embryonic development proceeded in the absence of exogenous growth regulators and without any obvious callus phase. The tissue was very prolific, and gram amounts could be easily collected (E.W. Harding and S.E. Perry, unpublished data).
Ectopic expression of several other transcriptional regulators (LEC1, LEC2, BABY BOOM, and WUSCHEL) have been shown to promote embryonic programs (Lotan et al., 1998; Stone et al., 2001; Boutilier et al., 2002; Zuo et al., 2002). In the case of LEC1 and LEC2, expression via the CaMV 35S promoter results in germinating seedlings with embryo-like features (Lotan et al., 1998; Stone et al., 2001). Ectopic expression of LEC1 in vegetative tissues through a 17-β-estradiol-inducible XVE promoter is not sufficient to drive somatic embryo formation (Zuo et al., 2002). However, ectopic expression of WUS through this promoter is sufficient to induce somatic embryo development, with embryos forming most frequently on the roots (Zuo et al., 2002). Development of somatic embryos correlates with expression of LEC1; however, this expression is observed only after WUS mRNA levels decrease. When WUS is expressed via the 35S promoter, a somewhat different phenotype is obtained with some shoot-like and some embryo-like features but not the more extensive embryonic calli and somatic embryos observed with the inducible promoter. Somatic embryo production by BABY BOOM also relies on constitutive expression, with embryonic tissue formation occurring on cotyledon, leaf petioles, and margins and from the SAM (Boutilier et al., 2002). It remains to be determined whether inducible expression is sufficient to cause switches in developmental programs.
The effect of 35S:AGL15 can be distinguished from the effect of these other factors. Neither constitutive ectopic (Fernandez et al., 2000; S. Perry, unpublished data) nor inducible (Fang and Fernandez, 2002) expression of AGL15 were sufficient to drive embryonic developmental programs in tissues after germination under normal conditions. For the MIKC constructs, 37 independent transgenic lines carrying the construct that included the first three introns, and 48 independent transgenic lines with the cDNA version were examined. In addition, 73 independent transgenic lines carrying the MIK construct were generated. None of these transgenic lines produced embryo-like structures on organs after germination under normal conditions (D.E. Fernandez, unpublished data). Therefore, other factors or conditions found in the contexts of embryos and SAMs in culture conditions are also involved, and AGL15 appears to act primarily on maintenance or support of development in embryonic mode, rather than a change in identity.
In addition to genes where ectopic expression maintains or induces embryo development, loss-of-function mutants that promote embryo identity have also been isolated. The loss-of-function mutant pickle causes somatic embryo production from roots (Ogas et al., 1997). LEC1 is ectopically expressed in the pkl roots, suggesting that PKL, a member of a protein family implicated in repression of transcription, may normally contribute to repression of LEC1 (Ogas et al., 1999). The pkl phenotype is enhanced by inhibition of GA biosynthesis and rescued by application of GA, although LEC1 expression is not increased in the presence of the GA biosynthesis inhibitor uniconazole-P (Ogas et al., 1999). Intriguingly, anti-AGL15-specific antiserum detects nuclear localized immunoreactive protein in pkl roots (S. Perry, unpublished data), and one direct downstream target that is expressed in response to AGL15 is a protein with similarity to GA-2-oxidases (Wang et al., 2002), a group of enzymes that convert bioactive GA to an inactive form.
Other loss-of-function mutants that result in somatic embryo production constitute a group that have enlarged SAMs (amp1, clv1, and clv3). In these cases, somatic embryo development is induced by culture of either immature embryos or germination of seeds in liquid media containing 2,4-D (Mordhorst et al., 1998, 2002; von Recklinghausen et al., 2000). Somatic embryos originate from the SAM and cotyledon axils of the germinating seedlings. This observation was particularly interesting because the very young SAM accumulates relatively high levels of AGL15, comparable with levels observed in embryos (Fig. 4A; Fernandez et al., 2000). The MIKC and MIK transgenes were introduced into the amp1 background, and efficiency of SAM embryo production was compared with amp1. Under our culture conditions, we were unable to obtain efficient SAM embryo production with amp1 alone. A recent report indicates that this is a somewhat variable phenotype depending on seed batch (Hecht et al., 2001). However, the MIKC transgene in the amp1 background reproducibly, and among many seed batches tested, produced SAM embryos. AMP1 has been cloned recently and found to encode a putative Glu carboxypeptidase that may have a role modulating levels of a signaling molecule involved in meristem size (Helliwell et al., 2001).
Is the amp1 mutant background necessary for the SAM embryo production by the MIKC transgene? Constitutive expression of AGL15 in a nonmutant background was able to produce somatic embryos at higher frequency than wild-type or MIK transgenic seeds but not to the level observed for MIKC amp1 seedlings, and production of SAM embryos by MIKC was somewhat more variable in the wild-type background. amp1 has been reported as having elevated cytokinin levels (Chaudhury et al., 1993; Nogué et al., 2000); however, we observed no enhancement of SAM embryo production of MIKC seedlings with the addition of exogenous kinetin to the liquid culture (data not shown). As found for AGL15, AtSERK1 (SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE 1) promotes somatic embryo production from the SAM in liquid culture when expressed via the 35S promoter. After 3 weeks in culture, 16% of 35S: SERK1 seedlings were scored as producing SAM embryos (Hecht et al., 2001). Interestingly, cultures of amp1 carrying the MIKC transgene had elevated expression of AtSERK1 (Fig. 6B).
Mordhorst et al. (1998) proposed that the increased size of the SAM in the amp1, clv1, and clv3 mutants may provide a larger pool of undifferentiated or uncommitted cells to follow a “default” pathway of embryogenesis when exposed to 2,4-D. The MIKC transgenes increase the frequency of SAM embryos in the amp1 background but do not increase the SAM size either in the developing embryo (data not shown) or in the normally grown seedling (Fig. 7, A–C). In addition, MIKC transgenic seedlings produced embryos at higher frequency than amp1 seedlings, even though the SAM size was smaller and similar to Ws and MIK. Therefore, increased production of somatic embryos in MIKC seedlings cannot be explained simply by an initially larger meristem, suggesting that a different pathway is involved in the MIKC effect. MIKC seedlings do produce SAM embryos at higher frequency on average and with less variability in the amp1 background (Fig. 5A), suggesting that there is some interaction between meristem size and presence of AGL15 in promotion of SAM embryo development. At early stages of development in liquid culture in 2,4-D, amp1 seedlings carrying the MIKC transgene show increased proliferation at the SAM (Fig. 8, A and C) beyond that observed in amp1 seedlings without any transgene (Fig. 8B) or carrying the MIK transgene (Fig. 8D).
In summary, results presented in this report support a role for AGL15, a MADS domain factor that accumulates during embryogenesis, in long-term maintenance of this phase of plant development. Furthermore, production of SAM embryos from mature seed germinated in liquid culture indicates that constitutive expression of AGL15 can support embryo development in the context of an SAM. Our challenge for the future is to understand the mechanism by which AGL15 achieves this effect.
MATERIALS AND METHODS
Plant Material
Arabidopsis ecotype Ws wild-type and transgenic plants were sown on GM (Murashige and Skoog, 1962, supplemented with 10 g L–1 Suc, 0.5 g L–1 MES, and 7 g L–1 agar (pH 5.6–5.7), with 50 μg mL–1 kanamycin for transgenic seed, chilled for 2 d at 4°C, and transferred to a growth room with a 23°C to 24°C, 23-h-light/1-h-dark regime. The MIKC transgenes were as described by Fernandez et al. (2000). The MIK transgene consisted of 18 bp of the 5′-untranslated region, and the coding sequence for the first 162-amino acid residues of AGL15 fused to the promoter for the 35S gene of CaMV in the pBI121 vector. No introns were included in the MIK construct. After 7 to 10 d, seedlings were transplanted to potting mix (ProMix BX, Premier Brands, Inc., Rivière-du-Loup, Quebec, Canada). Plants were grown at 20°C/18°C under a 16-h-light/8-h-dark regime in a growth chamber. To stage siliques, flowers were tagged on the day that they opened, and siliques were collected for tissue or for dissections at the desired time afterward.
Embryo Culture System
Developing zygotic embryos were removed at 10 to 11 d after flowering (green bent cotyledon stage) and placed into culture on GM. For transgenic embryos, the media was supplemented with 50 μg mL–1 kanamycin. After approximately 3 weeks, cultures were scored for formation of secondary embryonic tissue by examining the cultured embryos for new green tissue that lacked trichomes. This secondary tissue was continuously subcultured on GM or GM plus kanamycin at approximately 2.5- to 3-week intervals. The tissue was maintained at 23°C to 24°C, with a 23-h-light/1-h-dark regime. Culture development was documented using a Zeiss Stemi 2000-C stereomicroscope equipped with a 35-mm camera (Carl Zeiss, Inc., Thornwood, NY). Slides were scanned using a Nikon LS-2000 scanner (Nikon Corp., Tokyo) and assembled using Photoshop 5.0 and Illustrator 7.0 (Adobe Systems, Mountain View, CA).
Clearing of Organs and Meristem Measurement
Organs, seedlings, and mature embryos were cleared to examine vasculature and meristem size using Hoyer's solution (Schwartz et al., 1994). Mature seeds were soaked overnight in 15% (v/v) ethanol, and the embryos were dissected the following day. The isolated embryos were dehydrated through an ethanol series, followed by a Hemo-De (Fisher, Pittsburgh) series (25%, 50%, 75%, and 100% [v/v] Hemo-De in ethanol), and then placed into Hoyer's solution. All other tissues were placed directly in Hoyer's solution. Incubation in Hoyer's solutions was typically overnight, followed by examination on a Zeiss Axioplan2 (Carl Zeiss, Inc.) using dark-field or DIC optics. Documentation was as described above. Meristems were measured in millimeters directly on the 35-mm slides to compare sizes between transgenic, mutant, and wild-type plants. The final magnification on the slide was 125×.
Paraffin (Paraplast Plus, Fisher)-embedded sections (7 μm) of seedlings from liquid culture were dewaxed, rehydrated, and stained with 0.1% (w/v) toluidine blue O (Sigma, St. Louis) for 10 min at room temperature, washed briefly in water, rapidly dehydrated, and permanently mounted as described previously (Perry et al., 1996).
For scanning electron microscopy, seedlings from liquid culture were fixed in 4% (w/v) glutaraldehyde in 50 mm potassium phosphate buffer (pH 7.2) overnight at 4°C. The tissue was washed with buffer, dehydrated through an ethanol series, critically point dried, coated with platinum, and examined in a scanning electron microscope.
Reporter Constructs and Staining for GUS Activity
The GUS reporter constructs were as in Hirai et al. (1994) for the fusion of the β-conglycinin promoter to the reporter gene (β-conglycinin:GUS construct) and as in Fernandez et al. (2000) for the fusion of the AGL15 promoter to the reporter gene (AGL15:GUS). The β-conglycinin:GUS construct was crossed into the MIKC transgenic background, and embryonic cultures were initiated as described above. For AGL15:GUS, cultures were initiated directly from excised transgenic embryos. GUS staining was performed as described by Fernandez et al. (2000).
Antibody Preparation and Immunohistochemistry
Production and characterization of anti-AGL15 specific antibodies has been described previously (Heck et al., 1995; Perry et al., 1996, 1999; Fernandez et al., 2000). AGL15 was immunohistochemically localized on 7-μm tissue sections of material embedded in paraffin (Paraplast Plus) as described previously (Perry et al., 1996). Images were assembled as described above.
Sudan Red 7B (Fat Red 7B) Staining
Sudan red staining was as described by Brundrett et al. (1991). In brief, a 0.1% (w/v) solution of Sudan red 7B (Sigma) was prepared by dissolving the dye in polyethylene glycol (average molecular mass = 400 D, Sigma) with heating for 1 h at 90°C. An equal volume of 90% (v/v) glycerol was added. Chlorophyll was removed from culture and leaf tissue before fat red staining by incubating in 70% (v/v) ethanol overnight. Tissue was stained for 1 h at room temperature, rinsed several times in water, mounted in glycerol, and examined using a stereomicroscope.
RT-PCR
Total RNA was extracted from culture tissue, and seedlings germinated on GM and in liquid culture using TRIzol Reagent (Life Technologies, Rockville, MD). The hot-borate method was used to extract total RNA from staged siliques (Wilkins and Smart, 1996). cDNA was generated by RT using oligo(dT)15 primer (Promega, Madison, WI), M-MLV Reverse Transcriptase (Promega), and 2.5 μg of total RNA following the manufacturer's instructions. Semiquantitative PCR was performed after an initial denaturation at 94°C for 5 min, with the following program: 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s to 1 min, for 38 cycles for LEC1 and LEC2, 32 cycles for AtSERK1, 28 cycles for AtCRU3, and 26 cycles for TUB2. A final extension for 7 min at 72°C followed. The primers were as follows: for LEC1, 5′ and 3′ primers, GACGACGCCAAAGAAACGAT and CGACCACCTCCCATAACCAT; for LEC2, GTTCTTCAATTCCTTCTCACACAA and GCACTTCACAACAGTCCCTACTTA; for AtCRU3, TATACCTTGCCCATCTTGGAGTA and TGAACTTGATCTTCCTAGCTTCCT; for TUB2, CTCAAGAGGTTCTCAGCAGTA and TCACCTTCTTCATCCGCAGTT; and for AtSERK1, primers were as described by Hecht et al. (2001). The PCR products were visualized on a 1% (w/v) agarose gel, and the image was captured using a Chemilmager (Alpha Innotech Corporation, San Leandro, CA).
Seedling Liquid Culture System
The liquid culture system was essentially as described by Mordhorst et al. (1998). In brief, mature seeds were imbibed for 30 min in water plus 0.1% (v/v) Tween 20, surface sterilized by incubating for 5 min in 95% (v/v) ethanol followed by 5 min in 10% (v/v) commercial bleach (final 0.6% [w/v] sodium hypochlorite), and then rinsed five times with sterile distilled water. Seeds were chilled for 2 d at 4°C and introduced into liquid culture media as described previously (Mordhorst et al., 1998). Cultures were incubated on a rotary shaker at 23°C to 24°C with a 23-h-light/1-h-dark regime. Cultures were scored at 20 to 21 d.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Sonja Moe and Drs. Randy Dinkins and Huai Wang for critical reading of the manuscript and helpful comments on the results. We gratefully acknowledge Henry H. Southgate for his kind help and technical expertise in acquiring the scanning electron microscopy images. We also thank Dr. R. Scott Poethig for the amp1 seed and Dr. Joe Ogas for pkl seed and members of the University of Kentucky Seed Biology Group and the Perry lab for sharing materials and expertise.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.023499.
This work was supported by the National Science Foundation (grant no. IBN–9984274 to S.E.P.), by the U.S. Department of Agriculture (grant no. 96–35304–3699 to D.E.F.), by the University of Wisconsin Graduate School, and by the University of Kentucky. This paper (no. 03–06–036) is published with the approval of the Director of the Kentucky Agricultural Experiment Station.
References
- Alvarez-Buylla ER, Liljegren SJ, Pelaz S, Gold SE, Burgeff C, Ditta GS, Vergara-Silva F, Yanofsky MF (2000) MADS-box gene evolution beyond flowers: expression in pollen, endosperm, guard cells, roots and trichomes. Plant J 24: 457–466 [DOI] [PubMed] [Google Scholar]
- Belaguli NS, Zhou W, Trinh THT, Majesky MW, Schwartz RJ (1999) Dominant negative murine serum response factor: alternative splicing within the activation domain inhibits transactivation of serum response factor binding targets. Mol Cell Biol 19: 4582–4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu C-M, van Lammeren AAM, Miki BLA et al. (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14: 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA (1991) Efficient lipid staining in plant material with Sudan Red 7B or Floral Yellow 088 in polyethylene glycol-glycerol. In Biotechnic and Histochemistry: Official Publication of the Biological Stain Commission. 66: 111–116 [DOI] [PubMed] [Google Scholar]
- Burgeff C, Liljegren SJ, Tapia-Lopez R, Yanofsky MF, Alvarez-Buylla ER (2002) MADS-box gene expression in lateral primordia, meristems and differentiated tissues of Arabidopsis thaliana roots. Planta 214: 365–372 [DOI] [PubMed] [Google Scholar]
- Chaudhury AM, Letham S, Craig S, Dennis ES (1993) amp1: A mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J 4: 907–916 [Google Scholar]
- Colombo L, Franken J, Koetje E, van Went J, Dons HJM, Angenent GC, van Tunen AJ (1995) The petunia MADS box gene FBP11 determines ovule identity. Plant Cell 7: 1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S-C, Fernandez DE (2002) Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation. Plant Physiol 130: 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DE, Heck GR, Perry SE, Patterson SE, Bleecker AB, Fang S-C (2000) The embryo MADS domain factor AGL15 acts postembryonically: inhibition of perianth senescence and abscission via constitutive expression. Plant Cell 12: 183–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthierrouviere C, Cai QQ, Lautredou N, Fernandez A, Blanchard JM, Lamb NJC (1993) Expression and purification of the DNA-binding domain of SRF - SRF-Db, a part of a DNA-binding protein which can act as a dominant-negative mutant in-vivo. Exp Cell Res 209: 208–215 [DOI] [PubMed] [Google Scholar]
- Harada JJ, Lotan T, Fischer RL, Goldberg RB (1998). Response: embryos without sex. Trends Plant Sci 3: 452–453 [Google Scholar]
- Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt ED, Boutilier K, Grossniklaus U, de Vries SC (2001) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127: 803–816 [PMC free article] [PubMed] [Google Scholar]
- Heck GR, Perry SE, Nichols KW, Fernandez DE (1995) AGL15, a MADS domain protein expressed in developing embryos. Plant Cell 7: 1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Chin-Atkins AN, Wilson IW, Chapple R, Dennis ES, Chaudhury A (2001) The Arabidopsis AMP1 gene encodes a putative glutamate carboxypeptidase. Plant Cell 13: 2115–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai MY, Fujiwara T, Goto K, Komeda Y, Chino M, Naito S (1994) Differential regulation of soybean seed storage protein gene promoter-GUS fusions by exogenously applied methionine in transgenic Arabidopsis thaliana. Plant Cell Physiol 35: 927–934 [Google Scholar]
- Ikeda-Iwai M, Satoh S, Kamada H (2002) Establishment of a reproducible tissue culture system for the induction of Arabidopsis somatic embryos. J Exp Bot 53: 1575–1580 [DOI] [PubMed] [Google Scholar]
- Kempin SA, Savidge B, Yanofsky MF (1995) Molecular basis of the cauliflower phenotype in Arabidopsis. Science 267: 522–525 [DOI] [PubMed] [Google Scholar]
- Liljegren SJ, Ditta GS, Eshed HY, Savidge B, Bowman JL, Yanofsky MF (2000) SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404: 766–770 [DOI] [PubMed] [Google Scholar]
- Lotan T, Ohto M-a, Yee KM, West MAL, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93: 1195–1205 [DOI] [PubMed] [Google Scholar]
- Luo Y, Koop H-U (1997) Somatic embryogenesis in cultured immature zygotic embryos and leaf protoplasts of Arabidopsis thaliana ecotypes. Planta 202: 387–396 [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Huang H, Tudor M, Hu Y, Ma H (1996) Functional domains of the floral regulator AGAMOUS: Characterization of the DNA binding domain and analysis of dominant negative mutations. Plant Cell 8: 831–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordhorst AP, Hartog MV, Tamer MKE, Laux T, de Vries SC (2002) Somatic embryogenesis from Arabidopsis shoot apical meristem mutants. Planta 214: 829–836 [DOI] [PubMed] [Google Scholar]
- Mordhorst AP, Voerman KJ, Hartog MV, Meijer EA, van Went J, Koornneef M, de Vries SC (1998) Somatic embryogenesis in Arabidopsis thaliana is facilitated by mutations in genes repressing meristematic cell divisions. Genetics 149: 549–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nogué F, Hocart C, Letham DS, Dennis ES, Chaudhury AM (2000) Cytokinin biosynthesis is higher in the Arabidopsis amp1 mutant. Plant Growth Regul 32: 267–273 [Google Scholar]
- Ogas J, Cheng J-C, Sung ZR, Somerville C (1997) Cellular differentiation regulated by gibberellin in the Arabidopsis thaliana pickle mutant. Science 277: 91–94 [DOI] [PubMed] [Google Scholar]
- Ogas J, Kaufmann S, Henderson J, Somerville C (1999) PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc Natl Acad Sci USA 96: 13839–13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill CM, Mathias RJ (1993) Regeneration of plants from protoplasts of Arabidopsis thaliana L. cv. Columbia (C24), via direct embryogenesis. J Exp Bot 44: 1579–1585 [Google Scholar]
- Parenicová L, de Folter S, Kieffer M, Horner DS, Favalli C, Busscher J, Cook HE, Ingram RM, Kater MM, Davies B et al. (2003) Molecular and phylogenetic analysis of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell 15: 1538–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405: 200–203 [DOI] [PubMed] [Google Scholar]
- Perry SE, Lehti MD, Fernandez DE (1999) The MADS-domain protein AGAMOUS-like 15 accumulates in embryonic tissues with diverse origins. Plant Physiol 120: 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SE, Nichols KW, Fernandez DE (1996) The MADS domain protein AGL15 localizes to the nucleus during early stages of seed development. Plant Cell 8: 1977–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon E, Terzi M, Baldan B, Mariani P, Schiavo FL (1996) A protocol for obtaining embryonic cell lines from Arabidopsis. Plant J 9: 573–577 [DOI] [PubMed] [Google Scholar]
- Riechmann JL, Meyerowitz EM (1997) MADS domain proteins in plant development. Biol Chem 378: 1079–1101 [PubMed] [Google Scholar]
- Rounsley SD, Ditta GS, Yanofsky MF (1995) Diverse roles for MADS box genes in Arabidopsis development. Plant Cell 7: 1259–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan RS, Bourgeois Y, Dubois F, Sangwannorreel BS (1992) In vitro regeneration of Arabidopsis thaliana from cultured zygotic embryos and analysis of regenerants. J Plant Physiol 140: 588–595 [Google Scholar]
- Schwartz BW, Yeung EC, Meinke DW (1994) Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development 120: 3235–3245 [DOI] [PubMed] [Google Scholar]
- Snustad DP, Haas NA, Kopczak SD, Silflow CD (1992) The small genome of Arabidopsis contains at least nine expressed beta-tubulin genes. Plant Cell 4: 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 98: 11806–11811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Recklinghausen IR, Iwanowska A, Kieft H, Mordhorst AP, Schel JHN, van Lammeren AAM (2000) Structure and development of somatic embryos formed in Arabidopsis thaliana pt mutant callus cultures derived from seedlings. Protoplasma 211: 217–224 [Google Scholar]
- Wang H, Tang W, Zhu C, Perry SE (2002) A chromatin immunoprecipitation (ChIP) approach to isolate genes regulated by AGL15, a MADS-domain protein that preferentially accumulates in embryos. Plant J 32: 831–843 [DOI] [PubMed] [Google Scholar]
- Wilkins TA, Smart LB (1996) Isolation of RNA from plant tissue. In PA Krieg, ed, A Laboratory Guide to RNA: Isolation, Analysis, and Synthesis. Wiley-Liss, Inc., New York, pp 21–41
- Wu Y, Haberland G, Zhou C, Koop H-U (1992) Somatic embryogenesis, formation of morphogenetic callus and normal development in zygotic embryos of Arabidopsis thaliana in vitro. Protoplasma 169: 89–96 [Google Scholar]
- Zuo JR, Niu QW, Frugis G, Chua N-H (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30: 349–359 [DOI] [PubMed] [Google Scholar]