Abstract
Plant growth and development are strongly dependent on sink-source interactions. In the majority of plants, sucrose (Suc) is the dominant form in which photo-assimilate is transported from source to sinks. Although the effects of Suc on photosynthetic metabolism have been intensively studied, the effect of Suc supply on metabolism in sink organs has received relatively little attention. For this reason, we performed a detailed characterization of the metabolism of potato (Solanum tuberosum) plants in which the Suc supply to the tuber was restricted by genetic or environmental perturbation. These characterizations revealed a clear inverse relationship between the levels of Suc and free amino acids. When data obtained from this study were considered alongside our previous work, a negative correlation between tuber Suc and amino acid content became apparent. Analysis of the transcript levels of key enzymes involved in amino acid biosynthesis revealed that several of these were increased under these conditions. Taken together, these data strongly suggest that Suc regulates amino acid biosynthesis in storage tissues such as potato tubers, most probably at the level of transcription.
Suc represents the major form of translocated carbon in most plant species. Much research effort has been expended on characterizing the proteins responsible for the transfer of Suc from photosynthetically active tissues into the phloem (Giaquinta, 1983; Riesmeier et al., 1993a; Turgeon, 1996; Bush, 1999) and processes driving the phloem transport step (Canny, 1995; Ohrlich, 1998; Henton et al., 2002). Furthermore, considerable attention has been paid to defining the mechanisms underlying the unloading of Suc from the phloem in heterotrophic tissues (Patrick, 1997; Oparka and Cruz, 2000; Kühn et al., 2003).
Complementary DNAs encoding the transporter were initially isolated from spinach (Spinacia oleracea; SoSUT1; Riesmeier et al., 1992) and potato (Solanum tuberosum; StSUT1; Riesmeier et al., 1993b) and have been subsequently isolated from a wide of other plant species including Arabidopsis (AtSUC1; Sauer and Stolz, 1994), Plantago major (PmSUC2; Gahrtz et al., 1994), Ricinus communis (RcSUC1; Weig and Komor, 1996), rice (Oryza sativa; OsSUT1; Hirose et al., 1997), and tobacco (Nicotiana tabacum; NtSUT1; Burkle et al., 1998). Persuasive evidence that SUT1 class transporters function as Suc:proton symporters was provided by elegant electrophysiology experiments in which StSUT1and AtSUC1 were independently expressed in Xenopus oocytes (Boorer et al., 1996; Zhou et al., 1997).
Data from the above studies complemented compelling in vivo evidence suggesting a role for the SUT1 protein within phloem loading (Riesmeier et al., 1993b). Proof for this involvement was provided by the analysis of transgenic plants in which the expression of SUT1 in either tobacco or potato was constitutively reduced using antisense technology (Riesmeier et al., 1994; Burkle et al., 1998). Transgenics of both species were characterized by a massive accumulation of leaf carbohydrates and a curled, bleached leaf phenotype. Furthermore, the reduction of Suc export capacity resulted in a dramatic influence on the development of sink tissues, with both species exhibiting impaired root development, potato lines being characterized by a reduced tuber yield, and tobacco lines displaying a much delayed flowering. In a more recent study, a tDNA insertional mutation of SUC2, a phloem-specific Suc transporter of Arabidopsis, was observed to result in stunted growth, retarded development, and sterility (Gottwald et al., 2000).
Despite the large amount of research effort spent on the cloning, localization, and functional characterization of Suc transporters in higher plants, most reverse genetic approaches address the effects that the limitation of source export has on the leaves with relatively few studies concerned with Suc limitation of sink metabolism. Two exceptions to this are the use of transgenic plants impaired in their Suc transporter activity to address the question whether sink metabolism is source or sink limited (Sweetlove et al., 1998) and the overexpression of the potato Suc transport StSUT1 in pea (Pisum sativum) under the control of a seed-specific promoter (Rosche et al., 2002). In the same way, it was demonstrated that modulating the diurnal supply of Suc to the tuber by antisense repression of the triose phosphate transporter (Riesmeier et al., 1993) can have profound effects on the growth of sink organs (Kehr et al., 1998).
In this paper, we use metabolic profiling to perform a detailed characterization of alterations in metabolism that occur on the modification of the rate of Suc supply to a model heterotrophic organ, namely the potato tuber. To this end, we followed three independent approaches to modulate the Suc supply, exploiting previously generated transgenic lines in which the expression of the major Suc transporter (SUT1) was repressed by antisense inhibition (Riesmeier et al., 1994), growth of plants under different light qualities, and the feeding of various concentrations of Suc to the tuber via the stolon. We were able to demonstrate a clear link between the tuber Suc content and certain amino acid levels. Analysis of transcripts of key enzymes of amino acid biosynthesis strongly suggests that the increase of amino acids under conditions where Suc supply is limited is regulated at the transcriptional level. Taken together, these results strongly implicate Suc as a key regulator of amino acid biosynthesis in potato tubers.
RESULTS
Manipulation of Suc Import into the Potato Tuber. Description of the Biological Systems
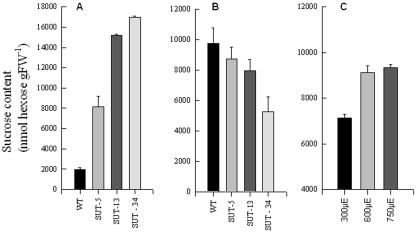
As described above, the aim of this study was to gain insight into the effects of altering the Suc supply to storage organ metabolism. We decided to concentrate this study on the potato tuber, due to the previous comprehensive experience of our group concerning this system, and to take three different approaches to modulate the Suc supply. As a first experiment, we decided to evaluate Suc content in the various experimental systems that we planned to study. For this reason, we grew transgenic potato plants, which have previously been characterized, to exhibit constitutively repressed expression of the Suc transporter SUT1 (Riesmeier et al., 1994) and harvested samples both from source leaves and from developing tubers. After having confirmed by RNA-blot analysis that the expression of SUT1 was reduced in the transformants and that the visible phenotype of these transformants was in accordance with that reported in previous studies (Riesmeier et al., 1994; Kühn et al., 1999) we determined the Suc concentration of the transgenics. As would be expected, the transformants exhibited an increased leaf Suc content (Fig. 1A) and a decreased tuber Suc content (Fig. 1B). As a second approach, we also grew wild-type potato plants in controlled environment growth cabinets in which the applied light regime was identical with the exception of the light intensity (which was set at 300, 600, and 750 μE) and harvested samples from tubers of the same developmental age. Although there was little change in the Suc content of tubers grown at 600 and 750 μE, those grown at 300 μE contained considerably less Suc than those grown at higher light intensities (Fig. 1C). Given that both manipulations we have described so far may result in pleiotropic effects in the source and sink tissues, we decided to adopt a more direct approach to elevate the supply of Suc to the tuber. To achieve this, we cut the stolon attaching the tuber to the mother plant and placed its cut end in water or 20, 200, and 500 mm Suc for a period of 24 h. Parallel experiments using radiolabeled Suc indicated that this method resulted in proportional influxes and accumulation of Suc into the potato tuber (the level of 14C recovered in equivalent sized tuber samples incubated in 20, 50, or 200 14C-Suc was 37.4, 51.6, and 92.4 KBq, respectively; in each case, only 5%–7% of the 14C was recovered in substances other than Suc). Having established that the levels of Suc were altered in all three experimental systems, we argue that they are valid for further study.
Figure 1.
Effect of genetic and environmental perturbations on Suc content. Transformant and wild-type potato plants were grown in the greenhouse in 3.5-L pots. The Suc content was determined in either leaf tissue harvested after 6 weeks of growth from fully expanded source leaves (A) or tuber tissue from developing tubers after 10 weeks of growth (B). In a separate experiment, wild-type plants were grown in controlled environment growth chambers under different light intensities, tuber tissue from developing tubers after 10 weeks of growth, and the Suc content was determined (C). Data represent the mean ± se of determinations on six individual plants per line/condition.
Tubers of Transgenic Plants Displaying a Reduced Suc Transporter Expression Display Significant Increases in Amino Acids
The levels of amino acids, Krebs cycle intermediates, sugars, sugar alcohols, and several representative compounds of secondary metabolism were analyzed using a gas chromatography (GC)-mass spectrometry (MS) protocol, in the same samples that were used for the Suc determinations presented in Figure 1. The data are presented at http://www.mpimp-golm.mpg.de. In total, the relative levels of more than 60 molecules of known chemical structure were determined. A substantial number of these metabolites were altered in the transgenic lines the most notable being a general increase in amino acids including Ala, Arg, Asn, Gln, Gly, Ser, Thr, and Val and in the Krebs cycle intermediates citrate and isocitrate. Minor changes were also observed in Asp, β-Ala, and Pro and in the secondary metabolites shikimate and a reduction in sugars such as Gal, Man, and raffinose. The increase in the level of certain amino acid pools in parallel to the decrease in Suc is also reflected on the total amino acid content (42.8 ± 3.2, 46.2 ± 5.1, 47.4 ± 2.1, and 53.4 ± 4.2 μmol g fresh weight–1 for wild type, SUT-34, SUT-13, and SUT-5, respectively) coincident to the decrease in Suc levels. Although the data seem to be in agreement with out previous suggestions that the amount of Suc in tubers regulates their amino acid contents (Roessner et al., 2001a, 2001b), it remains possible that the changes in amino acid levels may merely reflect changes in the levels of these metabolites in the leaves. To distinguish between these possibilities, we profiled the metabolite levels in the leaves of the wild-type and transgenic lines (again presented at http://www.mpimp-golm.mpg.de). Although many changes were observed in the levels of the organic and amino acid pools, the pattern of change in the leaf was not the same as that observed in tubers, indicating that the changes in tuber metabolism were unlikely to be overly influenced by changes in leaf metabolism.
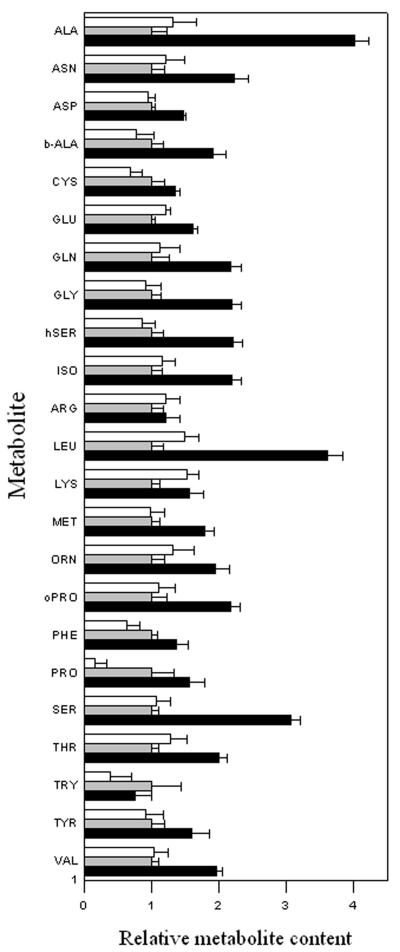
Metabolite Contents of Tubers Harvested from Plants Grown under Different Light Intensities
The data obtained from the transgenic plants impaired in the expression of the Suc transporter strongly suggest that reduction in tuber Suc content lead to an increase in the levels of certain amino acids. However, these changes may be due to indirect mm effects resulting from the long-term adaptation of the transgenics to what constitutes a dramatic morphological change. For this reason, we decided to test this hypothesis further by evaluating the effect on the tuber metabolism of modifying growth conditions of wild-type plants. As mentioned earlier, we observed a clear trend of increasing tuber Suc content in plants grown continuously under conditions of increasing light intensity. We studied the levels of other metabolites in these samples to determine if metabolite pool sizes differed in instances where the level of tuber Suc was only slightly reduced. We paid particular attention to amino acid biosynthesis (Fig. 2); however, the full data set obtained from the metabolic profiling of these samples can be accessed at http://www.mpimp-golm.mpg.de. Intriguingly, under conditions in which the tuber Suc content is significantly lower, there is an increase in the level of most amino acids. In particular, there are between 2- and 4-fold increases in the levels of Ala, Asn, β-Ala, Gln, Gly, homo-Ser, 5-oxo-Pro, Orn, Ser, Thr, and Val (metabolites that were found to increase to a similar magnitude in the antisense lines). In addition, there were relatively large changes in the contents of Ile, Leu, and Met, which were relatively unchanged in the transformants. That said, the general pattern of change is remarkable conserved between the two approaches. In effect, we can essentially mirror the results obtained from the Suc transporter antisense tubers by elevating the Suc supply environmentally. We also have recently demonstrated that potato plants heterologously expressing a Suc transporter from spinach (SoSUT1; Riesmeier et al., 1993b) and, consequently, exhibiting elevated Suc content, were characterized by a reduced amino acid content (Leggewie et al., 2003).
Figure 2.
Relative tuber amino acid levels of potato plants grown under different light conditions. Metabolites were determined as described in “Materials and Methods” from plants grown under low (300 μE, white bar), medium (600 μE, gray bar), or high (750 μE, black bar) light intensities. Data are normalized to the mean response calculated for the 600-μE light intensity samples (to allow statistical assessment, individual samples from this set of plants were normalized in the same way). Values presented are mean ± se of determinations on six individual plants.
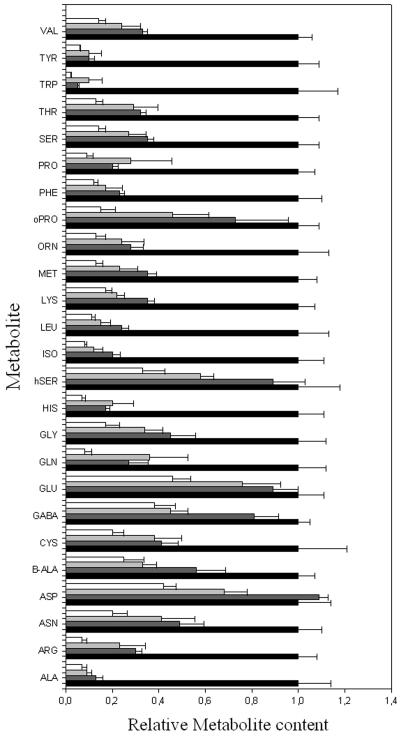
Direct Manipulation of Suc Supply to the Stolon
Although we managed minor manipulations in tuber sugar content, we did not want to overly stress the plants by varying the light intensities over two broad a range because it is clear that very high light intensities would lead to photoinhibition (Long et al., 1994), whereas very low light intensities would preclude tuber initiation (Burton 1989; Fernie and Willmitzer, 2001). Furthermore, despite performing a detailed analysis of leaf metabolism, we cannot rule out that some of the effects seen in the transgenic tubers are somehow pleiotropic to effects on leaf metabolism. For these reasons, we decided to study the effect of perturbing the Suc supply in isolated tubers. To achieve this, we cut the stolon attaching the tuber to the mother plant and placed the cut end of the stolon in water or 20, 200, and 500 mm Suc. After 24 h of incubation, we rapidly harvested tissue from defined areas along the stolon apex axis of the potato tuber and determined the levels of metabolites by GC-MS (Fig. 3; http://www.mpimp-golm.mpg.de). Even the supply of relatively low concentrations of Suc caused a large decrease in the amino acid content; however, the restriction of amino acid biosynthesis was progressively heightened under higher concentrations. Notably, the metabolites Glu, Ser, and Thr decreased markedly (these metabolites had increased sharply in the transgenic lines exhibiting reduced Suc import), and the amino acids β-Ala, Cys, Orn, Pro, and Tyr (these metabolites had also increased in lines in which the Suc supply was altered by changing the light conditions). Most interestingly, Arg, Tyr, and Val were dramatically altered under every condition in which the Suc content was altered. It is important to note that although these metabolites showed a negative correlation to Suc, they have shown no clear trend with respect to the levels of Glc, Fru, or any other sugar.
Figure 3.
Relative tuber amino acid levels of potato plants fed via the stolon with various concentrations of Suc. Metabolites were determined as described in “Materials and Methods” from tubers fed with 0 (black bar), 20 (dark-gray bar), 200 (light gray bar), or 500 (white bar) mm Suc. Data are normalized to the mean response calculated for the control (to allow statistical assessment, individual samples from this set of plants were normalized in the same way). Values presented are mean ± se of determinations on six individual tubers.
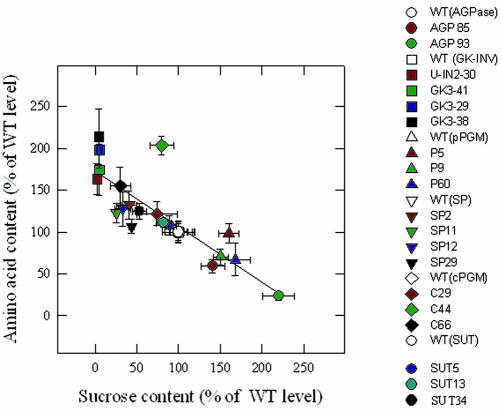
The data obtained from three different systems displaying an altered Suc level in tubers strongly suggest a negative correlation between Suc concentration and amino acid content. To test the generality of this conclusion, we took advantage of the large data set we have accumulated during research on carbon metabolism in the potato tuber (Fig. 4). There is a strong negative correlation between the Suc and amino acid content (r2 = 0.70) in the various transgenic lines, suggesting a strong linkage between Suc content and amino acid content. This relationship is conserved in instances in which the Suc level is reduced with respect to wild type (Trethewey et al., 1998, 1999; Fernie et al., 2002b) and when it is increased with respect to wild type (Trethewey et al., 1999; Fernie et al., 2001a, 2002a). Furthermore, it is maintained in lines that display a modified level of Suc but unaltered levels of other sugars (Fernie et al., 2002b).
Figure 4.
Relation between Suc and total amino acid content in transgenic tubers. Total amino acid content for the SUT1 tubers was determined as described in “Materials and Methods” in the same extracts as used for determining the Suc content presented in Figure 1. Data sets for the other transgenics are taken from the following publications: AGPase and cytosolic invertase lines (Trethewey et al., 1999), GK3 lines (Trethewey et al., 1998), cytosolic PGM lines (Fernie et al., 2002a), plastidial PGM lines (Fernie et al., 2001b), and Suc phosphorylase lines (Fernie et al., 2002b). To account for different growth conditions between these experiments, data are normalized with respect to the wild-type control plants that were grown alongside.
Assessment of the Transcriptional Regulation of Amino Acid Biosynthesis within the Transgenic Lines
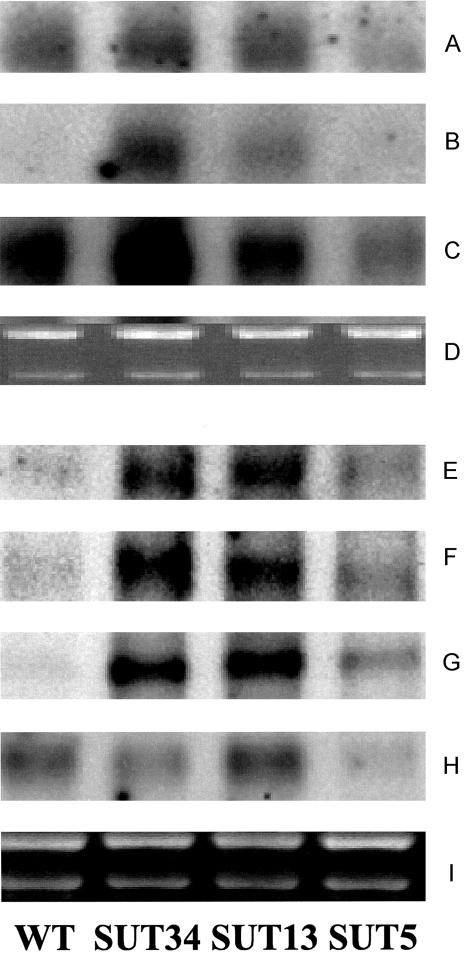
To see whether or not the increase in certain amino acids is at least partly reflected on the expression level of the genes of key enzymes of their biosynthetic pathways, we performed a series of RNA-blot experiments. The level of SUT1 transcript was reduced in the tuber (Fig. 5A); however, not to as great an extent as has been observed previously in the leaf (Riesmeier et al., 1994). It is also important to note that the SUT transcript level in the tuber does not absolutely correlate with the tuber Suc level (see Fig. 1). The transcription of Gln synthetase, which has previously been shown to be induced in Arabidopsis seedlings grown in the presence of abundant Suc (Oliveira and Coruzzi, 1999), was markedly enhanced in the transgenic lines (Fig. 5E) in which Suc content is decreased. Interestingly, the transcription of NADH Glu synthase, the isoform of this enzyme that is most abundant in sink tissues (Lancien et al., 2000), was also enhanced in these lines (Fig. 5F). There have been no reports of Suc repression of NADH Glu synthase expression to date; however, the ferredoxin-dependent isoform of Arabidopsis leaf is conversely Suc inducible (Coschigano et al., 1998). Furthermore, the transcript levels of one isoform of Asp aminotransferase, the enzyme that converts asparagines to Asp, was dramatically induced in the transformants (Fig. 5G). For practical reasons, we did not investigate further isoforms of the enzyme; however, because the five functionally identified isoforms of Arabidopsis have been demonstrated to exhibit a high level of functional redundancy (Schultz et al., 1998), we believe that the result presented here is most probably representative of other possible potato Asp amino transferases. The transcript levels of Asp kinase, a key enzyme in the synthesis of Leu, Ile, Thr, and Met (Azevedo, 2002), also increased markedly in the transgenic lines, which is consistent with the changes in the metabolite levels of this pathway (Fig. 5C). Several attempts to measure the level of dihydrodipicolinate synthase, the key enzyme of Lys biosynthesis (Galili, 2002), revealed no major changes in the transcript level of this enzyme (data not shown); however, it should be noted that the transcript is only present at the level of detection. However, the transcription of Trp synthase was markedly enhanced in line SUT-13 (Fig. 5B). Conversely, the transcription of Pro-5-carboxylate reductase, the final enzyme in the Pro biosynthetic pathway, is repressed in the transgenic lines (Fig. 5I), correlating with the reduced level of Pro in tubers from lines SUT-34 and SUT-13 but strangely not with the level of Pro in line SUT-5.
Figure 5.
A to I, Northern-blot analysis in growing tubers of wild-type and transgenic potato plants. RNA extracts were prepared from the same samples as the metabolite measurements presented in Figure 1 and Supplementary Data Table I. Transcript level for SUT1 (A), Trp synthase (B), Asp kinase (C), Gln syntethase (E), NADH-dependent Glu synthase (F), Asp aminotransferase (G), and pyrroline-5-carboxylate reductase (H) are presented above their respective RNA loading controls (D and I).
DISCUSSION
Genetic and Environmental Manipulation of Levels of Suc within the Potato Tuber Are Accompanied by Opposing Changes in the Levels of Amino Acids
The main objective of this study was to analyze the influence of changes in the level of Suc on storage sink metabolism using the potato tuber as a model system. To approach this problem, we applied three different biological manipulations targeted at modifying the content of the disaccharide—transgenic lines exhibiting a constitutive repression of the major Suc transporter, wild-type plants grown under different light qualities, and detached wild-type tubers that were fed with varying amounts of Suc through the stolon. All three manipulations produced the desired changes in Suc content, confirming their suitability for use in this study.
The most important observation made, irrespective of the experimental system, was the increase of specific amino acids under conditions of decreased supply of Suc to the tuber (and the increase of the same amino acids under conditions of increased Suc supply). Although in principle the increase in amino acids could be mediated by increased uptake from the leaf tissues mediated via one of the many amino acid transporters identified in plants (for example, see Ortiz-Lopez et al., 2000; Fischer et al., 2002), this seems unlikely for a number of reasons. The pattern of change in the amino acid levels does not correspond with that observed in the leaves. For example Ser, Val, and Ala all significantly increase in the tuber but are unaltered in the leaf. Furthermore, the levels of organic acid intermediates in the pathway between Suc and amino acids are also elevated in the tubers. Moreover, the total level of amino acids is enhanced in many transgenic lines in which the Suc level is depressed and reduced in many transgenic lines in which the Suc level is increased (see Fig. 4). Following a similar line of thought, the metabolic profile of the SUT tubers is broadly similar to those of a range of transgenics expressing a more efficient pathway of Suc catabolism in a tuber-specific manner (Roessner et al., 2001a, 2001b). However, perhaps the most important evidence that the changes are the result of perturbation of tuber metabolism per se is provided by the fact that environmental manipulation of Suc content in the tuber had qualitatively similar effects on amino acid biosynthesis. Varying the light intensity and the experiments in which Suc was fed through the stolon both resulted in altered Suc content and the corresponding change in amino acid contents: low light enhancing amino acid synthesis in the tuber, whereas high Suc supply strongly inhibited these pathways. When taken together, we believe that these observations allow us to exclude the possibility that these changes are merely reflections of changes that occur in the leaves and subsequently transported to the tuber.
That such a large number of metabolites change in both leaves and tubers of the transgenics was somewhat surprising with glycolysis seemingly elevated in both tissues. Although it is easy to rationalize the benefits of enhancing glycolysis and amino acid biosynthesis in carbohydrate-replete conditions, and the results of many studies of photosynthetic metabolism have revealed tight positive correlations between high carbohydrate contents, high glycolytic rates, and high amino acid levels (for example, see Krapp and Stitt, 1994, 1995; Matt et al., 1998; Morcuende et al., 1998), this is considerably more difficult in conditions where the availability of carbohydrate is limited. One possibility that has been postulated to explain the dramatic increase of amino acids in tubers cytosolically expressing invertase or Suc phosphorylase is that the synthesis of amino acids is a mechanism to compensate for the low Suc and meet the requirement for cellular osmoticum (Hare et al., 1998; Trethewey et al., 1999; Fernie et al., 2002b). In keeping with this hypothesis, it is interesting to note that there is also a significant increase in sugar alcohol content in the SUT tubers, similar to that also observed in tubers cytosolically expressing invertase or Suc phosphorylase (Roessner et al., 2001a). Moreover, both the absolute cellular levels and the subcellular concentrations of these metabolites are consistent with Suc and amino acids being the principal osmolytes of tuber storage parenchyma (Farré et al., 2001). Interestingly, these authors showed that despite the fact that the majority of the Suc is compartmented in the vacuole, its estimated subcellular concentration is remarkably similar between the cytosol and vacuole.
If the increase in amino acids is to maintain cellular osmoticum, the question remains as to whether the amino acids are synthesized de novo (which would cost carbon) or are merely generated by the degradation of proteins (which would not). Intriguingly, both transgenic (Trethewey et al., 1999; Fernie et al., 2002b) and diurnal (Geigenberger and Stitt, 2000) variation of tuber Suc levels has revealed previously that low Suc correlates with a reduced rate of starch synthesis but a largely unaltered rate of protein synthesis. In keeping with these findings, the results of a previous study demonstrated that the increase in every amino acid in tubers of transformants in which transgene expression is driven by a tissue-specific promoter (and in which no changes were observed in the amino acid in the leaves) demonstrated that the tuber contained all the biosynthetic machinery required for de novo biosynthesis. Further evidence that this is the case was provided by the demonstration that short-term incubations of isolated tuber discs in various carbon sources resulted in elevated amino acids (Roessner et al., 2001b) and that nitrate treatment of potato tubers resulted in a large increase in tuber amino acids (Mack and Schjoerring, 2002). Although we cannot directly exclude the possibility that protein degradation meets some of the demand for amino acids, it is clearly not a large provider of these metabolites. First, the level of protein is unaltered in the transgenics—a fact that was somewhat surprising given the fact that Suc stimulates expression of patatin. However, such a lack of change was previously observed in both the invertase (Trethewey et al., 1999) and Suc phosphorylase plants (Fernie et al., 2002b). The rate of protein synthesis is also unaltered in the lines studied here (T. Czechowski and A.R. Fernie, unpublished data), indicating that it is unlikely that the rate of protein turnover is altered. Because tuber protein levels are similar in magnitude to the total amino acid pool size (corresponding to approximately 1.8 mg g fresh weight–1 and 1 mg g fresh weight–1, respectively), it is unlikely that changes in protein turnover that are below the level of detection in this experiment could account for the total changes in amino acid content observed in the transgenic lines. Second, as described in detail below, the transcript level of a number of key enzymes of amino acid biosynthesis is elevated in tubers from the transformants. Taking both factors into account, it seems reasonable to postulate that Suc regulates the rate of amino acid biosynthesis per se. Although the availability of stored nitrate in the tuber vacuole most probably affords the necessary nitrate to fuel this biosynthesis, the source of the carbon in conditions in which the supply of photosynthate is limiting is likely come from mobilization of starch.
Given that the results of a recent study suggest that certain amino acid pool sizes are significantly elevated after antisense inhibition of the plastidial isoform of adenylate kinase (Regierer et al., 2002), most probably as a result of an increase in the level of cellular ATP, we also investigated the adenylate status of tuber material from the transgenic lines. However, we found no differences in the levels of any of the nucleotides or nucleotide sugars (T. Czechowski and A.R. Fernie, unpublished data), leading us to believe that the reasons for the increase in amino acid content are quite different in the current study.
Modifying Suc Import Has Dramatic Effects on Transcription of Enzymes of Amino Acid Biosynthesis
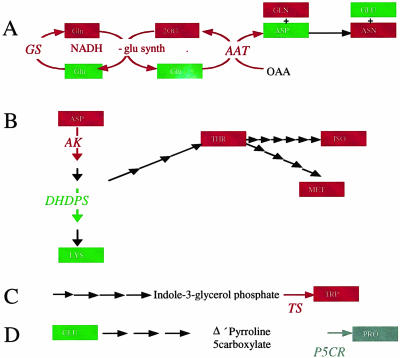
A schematic representation of the metabolic and transcriptional situation observed in the amino acid biosynthetic pathways is presented in Figure 6. Intriguingly, the pattern of change in metabolite levels is generally, yet not absolutely, mirrored in the level of transcription of key enzymes of amino acid biosynthesis. Taking these pathway by pathway, the GS/GOGAT pathway (Fig. 6A) shows some correlation, with the increased level of Gln corresponding to an elevated transcription of both Gln synthase and Asp aminotransferase. In keeping with the elevated expression of Asp aminotransferase, the level of Asn increased in the antisense lines (however, that of Asp was unchanged, similar despite an increased in NADH-dependent Glu synthetase expression the levels of Glu were unaltered). In the Asp pathway (Fig. 6B), the transcript level of Asp kinase was elevated in the transgenics, whereas that of dihydropicolinate synthase was unchanged, consistent with these expression patterns the levels of Thr, Ile, and Met were elevated in these lines, and the level of Lys was stable. Other examples of these correlations are provided by analyses of the final steps of the Trp and Pro biosynthetic pathways (Fig. 6, C and D)—with the levels of both Trp synthase mRNA and Trp increasing in the transgenics and levels of Pro 5-carboxylate reductase and Pro decreasing.
Figure 6.
A to D, Scheme of the regulation of tuber amino acid biosynthesis. Superimposition of the changes observed at the transcription and metabolite level in the transformants of several key pathways of amino acid biosynthesis: the GS/GOGAT pathway (A), Asp family pathway (B), Trp pathway (C), and Pro pathway (D). Red boxes, Metabolites that increase in the transgenics; green boxes, metabolites that do not change; gray boxes, metabolites that decrease. The same color scheme is applied to the arrows to denote (lack of) change in transcripts.
In summary, we have used multiparallel metabolite analysis in conjuncture with transcript analyses and experimental manipulation of Suc levels to investigate the method of regulation of amino acid biosynthesis within the potato. The results of this study illustrate that the regulatory role that Suc plays in amino acid biosynthesis in sink tissues differs appreciably from that described previously in source tissues. We conclude that Suc coordinately inhibits amino acid biosynthesis within the tuber, most probably at the level of transcription of the key enzymes of the pathways concerned.
MATERIALS AND METHODS
Plant Material
Potato (Solanum tuberosum L. cv Desiree) was supplied by Saatzucht Lange AG (Bad Schwartau, Germany). The generation of transgenic lines exhibiting constitutive repression of the Suc transporter SUT1 has been described previously (Riesmeier et al., 1994). Plants were maintained in tissue culture with a 16-h-light, 8-h-dark regime on Murashige and Skoog medium (Murashige and Skoog, 1962), which contained 2% (w/v) Suc. Unless otherwise stated, plants were grown in the greenhouse under the same light regime with a minimum of 250 μmol photons m–2 s–1 at 22°C. Developing tubers (over 10 g fresh weight) were harvested, 6 h into the light period, from healthy 10-week old plants, whereas leaf samples were taken 6 h into the light period from mature fully developed leaves of 6-week-old plants.
Chemicals
The starch determination kit and biochemical enzymes were purchased from Boehringer Mannheim (Mannheim, Germany). All other chemicals were purchased from Sigma (St. Louis) or Merck (Damstadt, Germany).
Biochemical Analysis
Tuber slices were rapidly frozen in liquid N2 and were extracted in trichloroacetic acid or ethanol extracts as described previously (Fernie et al., 2001b). Because water and protein content of the tubers were not significantly altered in the transgenic lines, all data are expressed on a gram per fresh weight basis. Starch, sugars, and glycolytic metabolites were determined spectrophotometrically as described by Fernie et al. (2001b). Recoveries of metabolites in the trichloroacetic acid extracts throughout the extraction, storage, and assay procedure have been documented previously (Sweetlove et al., 1996; Fernie et al., 2001b).
Feeding of Suc via the Stolon Wild-Type Potato Tubers
Equivalent tubers of rapidly growing plants that had just started to flower were cut from the plant by severing the stolon with a sharp razor (Geiger et al., 1998). The cut end was then submerged in water and cut again 2 to 3 mm closer to the tuber to circumvent the occurrence of embolism. The cut end was then placed in a solution containing 20, 50, or 200 mm Suc for 24 h. To assess the rate of uptake of Suc replicate, incubations were carried out in [U-14C]Suc (specific activity 7 MBq μmol–1). All incubations took place at the same time in the same room and at the same temperature and humidity. After the incubation, a concentric core (10 mm) was taken surrounding the stolon-apex axis was taken. Single discs (2 mm thick) were then cut from this core at both stolon and apex ends of the tuber and from the middle of the tuber. The metabolites in the non-labeled experiments were then analyzed as described by Roessner et al. (2001a).
GC-MS
GC-MS was carried out on tuber and leaf tissue exactly as described by Roessner et al. (2000) and Lytovchenko et al. (2002), respectively. Data are normalized to the mean response calculated for the wild type of each measure batch as described by Roessner et al. (2001a).
HPLC
HPLC was carried out on trichloroacetic acid extracts of potato tuber to determine the adenylate and uridinlylate contents exactly as described by Regierer et al. (2002).
Extraction of RNA and Northern-Blot Analysis
Total RNA was isolated from 2g fresh weight of tuber tissue as described by Logemann et al. (1987). Standard conditions were used for the transfer of RNA to membranes. Hybridization to the Suc transporter SoSUT1 (Riesmeier et al., 1994) was preformed with standard conditions (Sambrook et al., 1989). For checking transcript level of selected enzymes, the membranes were hybridized with tomato expressed sequence tag (EST) from the Clemson State University collection. For the probes, we used the following ESTs: Asp kinase (accession no. BE353821), Trp synthase (accession no. AW625162), Asp aminotransferase (accession no. AI484604), dihydropicolinate synthase (accession no. AW623776), Gln synthetase (accession no. BG129590), NADH-dependent Glu synthase (accession no. BE449812), and pyrroline-5-carboxylate reductase (accession no. AI485470). Because the source of the EST probes was a heterologous system, the temperature of hybridization was reduced to 60°C.
Statistical Analysis of Data
The Student's t tests were performed using the algorithm incorporated into Microsoft Excel (Microsoft, Redmond, WA). The word significant is used in the text solely when the change in question has been confirmed to be statistically significant (P < 0.05) with the Student's t test.
Supplementary Material
Acknowledgments
We thank Prof. Wolf B. Frommer for supplying the SUT1 potato plants. Discussions with Drs Charles Baxter and Rainer Höfgen were of great use in the design of the northern-blot analysis experiments described here. Equally deep discussion of various aspects of Suc metabolism with Dr. Peter Geigenberger is gratefully acknowledged.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.024802.
The online version of this article contains Web-only data.
References
- Azevedo RA (2002) Analysis of aspartic acid metabolic pathway using mutant genes. Amino Acids 22: 217–230 [DOI] [PubMed] [Google Scholar]
- Boorer KJ, Loo DDF, Frommer WB, Wright EM (1996) Transport mechanism of the cloned potato H+/sucrose cotransporter StSUT1. J Biol Chem 271: 25139–25144 [DOI] [PubMed] [Google Scholar]
- Burkle L, Hibberd JM, Quick WP, Kuhn C, Hirner B, Frommer WB (1998) The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol 118: 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton WG (1989) The Potato. Longmann, New York
- Bush DR (1999) Sugar transporters in plant biology. Curr Opin Plant Sci 2: 187–191 [DOI] [PubMed] [Google Scholar]
- Canny MJ (1995) Apoplastic water and solute movement: new rules for an old space. Annu Rev Plant Physiol Plant Mol Biol 46: 215–236 [Google Scholar]
- Coschigano KT, Melo-Oliveira R, Lim J, Coruzzi GM (1998) Arabidopsis gls mutants and distinct Fd-GOGAT genes: implications for photorespiration and primary nitrogen assimilation. Plant Cell 10: 741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L (2001) Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids and sugar alcohols in potato tubers using a non-aqueous fractionation method. Plant Physiol 127: 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Roessner U, Trethewey RN, Willmitzer L (2001a) The contribution of plastidial phosphoglucomutase to the control of starch synthesis within the potato tuber. Planta 213: 418–426 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Roscher A, Ratcliffe RG, Kruger NJ (2001b) Activation of pyrophosphate: fructose-6-phosphate 1-phosphotransferase by fructose 2, 6-bisphosphate stimulates conversion of hexose phosphates to triose phosphates but does not influence accumulation of carbohydrates in phosphate-deficient tobacco cells. Planta 212: 250–263 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Tauberger E, Lytovchenko A, Roessner U, Willmitzer L, Trethewey RN (2002a) Antisense repression of cytosolic phosphoglucomutase in potato (Solanum tuberosum) results in severe growth retardation, reduction in tuber number and altered carbon metabolism. Planta 214: 510–520 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Tiessen A, Stitt M, Willmitzer L, Geigenberger P (2002b) Altered metabolic fluxes result from shifts in metabolite levels in sucrose phosphorylase expressing potato tubers. Plant Cell Environ 25: 1219–1232 [Google Scholar]
- Fernie AR, Willmitzer L (2001) Molecular and biochemical triggers of potato tuber development. Plant Physiol 127: 1459–1465 [PMC free article] [PubMed] [Google Scholar]
- Fischer WD, Loo DDF, Koch W, Ludewig U, Boorer KJ, Tegeder M, Rentsch D, Wright EM, Frommer WB (2002) Low and high affinity amino acid H+-cotransporters for cellular import of neutral and charged amino acids. Plant J 29: 717–731 [DOI] [PubMed] [Google Scholar]
- Gahrtz M, Stolz J, Sauer N (1994) A phloem-specific sucrose-H+ symporter from Plantago major L. supports the model of apoplastic phloem loading. Plant J 6: 697–706 [DOI] [PubMed] [Google Scholar]
- Galili G (2002) New insights into the regulation and functional significance of lysine metabolism in plants. Annu Rev Plant Biol 53: 27–43 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M (2000) Diurnal changes in sucrose, nucleotides, starch synthesis and AGPS transcript in growing potato tubers that are suppressed by decreased expression of sucrose phosphate synthase. Plant J 23: 795–806 [DOI] [PubMed] [Google Scholar]
- Geiger M, Stitt M, Geigenberger P (1998) Metabolism in potato tuber slices responds differently after addition of sucrose and glucose. Planta 206: 245–252 [Google Scholar]
- Giaquinta RT (1983) Phloem loading of sucrose. Annu Rev Plant Physiol 34: 347–387 [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97: 13979–13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare PD, Cress WA, van Staden J (1998) Dissecting the role of osmolyte accumulation during stress. Plant Cell Environ 21: 535–553 [Google Scholar]
- Henton SM, Greaves AJ, Piller GJ, Minchin PEH (2002) Revisiting the Munch pressure-flow hypothesis for long-distance transport of carbohydrates: modelling the dynamics of solute transport inside a semipermeable tube. J Exp Bot 53: 1411–1419 [PubMed] [Google Scholar]
- Hirose T, Imaizumi N, Scofield GN, Furbank RT, Ohsugi R (1997) cDNA cloning and tissue specific expression of a gene for sucrose transporter from rice (Oryza sativa L.). Plant Cell Environ 38: 1389–1396 [DOI] [PubMed] [Google Scholar]
- Kehr J, Hustiak F, Walz C, Willmitzer L, Fisahn J (1998) Transgenic plants changed in carbon allocation pattern display a shift in diurnal growth pattern. Plant J 16: 497–503 [DOI] [PubMed] [Google Scholar]
- Krapp A, Stitt M (1994) Influence of high-carbohydrate content on the activity of plastidic and cytosolic isoenzyme pairs in photosynthetic tissues. Plant Cell Environ 17: 861–866 [Google Scholar]
- Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanism for the sink-regulation of photosynthesis in spinach-changes in gasexchange, carbohydrates, metabolites, enzyme-activities and steady-state transcript levels after cold girdling source leaves. Planta 195: 313–323 [Google Scholar]
- Kühn C, Barker L, Burkle L, Frommer WB (1999) Update on sucrose transport in higher plants. J Exp Bot 50: 935–953 [Google Scholar]
- Kühn C, Hajirezaei MR, Fernie AR, Roessner-Tunali U, Czechowski T, Hirner B, Frommer WB (2003) The sucrose transporter StSUT1 localizes to sieve elements in potato tuber phloem and influences tuber physiology and development. Plant Physiol 131: 102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancien M, Gadal P, Hodges M (2000) Enzyme redundancy and the importance of 2-oxoglutarate in higher plant ammonium assimilation. Plant Physiol 123: 817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggewie G, Kolbe A, Lemoine R, Roessner U, Lytovchenko A, Frommer WB, Riesmeier JW, Willmitzer L, Fernie AR (2003) Overexpression of the sucrose transporter SoSUT1 in potato results in alterations in leaf carbon partitioning but has little impact on tuber morphology. Planta 217: 158–167 [DOI] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L (1987) Improved methods for the isolation of RNA from plant tissues. Ann Biochem 163: 16–20 [DOI] [PubMed] [Google Scholar]
- Long SP, Humpries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45: 633–662 [Google Scholar]
- Lytovchenko A, Bieberich K, Willmitzer L, Fernie AR (2002) Carbon assimilation and metabolism in potato leaves deficient in plastidial phosphoglucomutase. Planta 215: 802–811 [DOI] [PubMed] [Google Scholar]
- Mack G, Schjoerring JK (2002) Effect of NO3-supply on N metabolism of potato plants (Solanum tuberosum L.) with special focus on the tubers. Plant Cell Environ 25: 999–1009 [Google Scholar]
- Matt P, Schurr U, Klein D, Krapp A, Stitt M (1998) Growth of tobacco in short-day conditions leads to high starch, low sugars, altered diurnal changes in the Nia transcript and low nitrate reductase activity, and inhibition of amino acid synthesis. Planta 207: 27–41 [DOI] [PubMed] [Google Scholar]
- Morcuende R, Krapp A, Hurry V, Stitt M (1998) Sucrose-feeding leads to increased rates of nitrate assimilation, increased rates of α-oxogluterate synthesis, and increased synthesis of a wide spectrum of amino acids in tobacco leaves. Planta 206: 394–409 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Ohrlich G (1998) Analysis of the driving forces of phloem transport in Ricinus seedlings: sucrose export and volume flow are determined by the source. Planta 206: 266–271 [Google Scholar]
- Oliveira IC, Coruzzi GM (1999) Carbon and amino acids reciprocally modulate the expression of glutamine synthetase in Arabidopsis. Plant Physiol 121: 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka KJ, Cruz SS (2000) The great escape: phloem transport and unloading of macromolecules. Annu Rev Plant Physiol Plant Mol Biol 51: 323–347 [DOI] [PubMed] [Google Scholar]
- Ortiz-Lopez A, Chang H, Bush DR (2000) Amino acids transporters in plants. Biochim Biophys Acta 1465: 275–280 [DOI] [PubMed] [Google Scholar]
- Patrick JW (1997) Phloem unloading: sieve element unloading and postsieve element transport. Annu Rev Plant Phys 48: 191–222 [DOI] [PubMed] [Google Scholar]
- Regierer B, Fernie AR, Springer F, Perez-Melis A, Leisse A, Koehl K, Willmitzer L, Geigenberger P, Kossmann J (2002) Starch content and yield increase as a result of altering adenylate pools in transgenic plants. Nat Biotechnol 20: 1256–1260 [DOI] [PubMed] [Google Scholar]
- Riesmeier JW, Fluegge UI, Schulz B, Heinke D, Heldt HW, Willmitzer L, Frommer WB (1993a) Antisense repression of the chloroplastic triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proc Natl Acad Sci USA 90: 160–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Hirner B, Frommer WB (1993b) Potato sucrose transporter expression in minor veins indicates a role in phloem loading. Plant Cell 5: 1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterisation of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11: 4705–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1994) Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J 13: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR (2001a) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13: 11–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner U, Wagner C, Kopka J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J 23: 131–142 [DOI] [PubMed] [Google Scholar]
- Roessner U, Willmitzer L, Fernie AR (2001b) High-resolution metabolic phenotyping of genetically and environmentally diverse potato tuber systems: identification of phenocopies. Plant Physiol 127: 749–764 [PMC free article] [PubMed] [Google Scholar]
- Rosche E, Blackmore D, Tegeder M, Richardson T, Schroeder H, Higgins TJV, Frommer WB, Offler CE, Patrick JW (2002) Seed-specific overexpression of a potato sucrose transporter increases sucrose uptake and growth rates of developing pea cotyledons. Plant J 30: 165–175 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sauer N, Stolz J (1994) Suc1 and suc2–2 sucrose transporters from Arabidopsis thaliana: Expression and characterisation in bakers yeast and identification of the histidine-tagged protein. Plant J 6: 67–77 [DOI] [PubMed] [Google Scholar]
- Schultz CJ, Hsu M, Miesak B, Coruzzi GM (1998) Arabidopsis mutants define an in vivo role for isoenzymes of aspartate aminotransferase in plant nitrogen assimilation. Genetics 149: 491–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Kossmann J, Riesmeier JW, Trethewey RN, Hill SA (1998) The control of source to sink carbon flux during tuber development in potato. Plant J 15: 697–706 [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Burrell MM, apRees T (1996) Starch metabolism in tubers of transgenic potato (Solanum tuberosum) with increased ADPglucose pyrophosphorylase. Biochem J 320: 493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trethewey RN, Geigenberger P, Henning A, Müller-Röber B, Fleischer-Notter H, Willmitzer L (1999) Induction of the activity of glycolytic enzymes correlates with enhanced hydrolysis of sucrose in the cytosol of transgenic potato tubers. Plant Cell Environ 22: 71–79 [Google Scholar]
- Trethewey RN, Geigenberger P, Riedel K, Hajirezaei MR, Sonnewald U, Stitt M, Riemeier JW, Willmitzer L (1998) Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J 15: 109–118 [DOI] [PubMed] [Google Scholar]
- Turgeon R (1996) Phloem loading and plasmodesmata. Trends Plant Sci 1: 418–423 [Google Scholar]
- Weig A, Komor E (1996) An active sucrose carrier (Scr1) that is predominantly expressed in the seedling of Ricinus communis L. J Plant Physiol 147: 685–690 [Google Scholar]
- Zhou JJ, Theoduolou F, Sauer N, Sanders D, Miller AJ (1997) A kinetic model with ordered cytoplasmic dissociation for SUC1, an Arabidopsis H+/sucrose cotransporter expressed in Xenopus oocytes. J Membr Biol 159: 113.125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.