Abstract
In this article, we report the isolation of plant protoporphyrinogen oxidase (PPO) genes and the isolation of herbicide-tolerant mutants. Subsequently, an Arabidopsis double mutant (Y426M + S305L) was used to develop a selectable marker system for Agrobacterium tumefaciens-mediated transformation of maize (Zea mays) and to obtain multiple events tolerant to the PPO family of herbicides. Maize transformants were produced via butafenacil selection using a flexible light regime to increase selection pressure. Butafenacil selection per se did not change transgene copy number distribution relative to other selectable marker systems, but the most tolerant events identified in the greenhouse were more likely to contain multiple copies of the introduced mutant PPO gene. To date, more than 2,500 independent transgenic maize events have been produced using butafenacil selection. The high frequency of A. tumefaciens-mediated transformation via PPO selection enabled us to obtain single-copy transgenic maize lines tolerant to field levels of butafenacil.
In the last decade, two predominant methods were developed to facilitate maize (Zea mays) transformation. The first successful method was microparticle bombardment (Klein et al., 1988a, 1988b, 1989). The bombardment method has been adopted widely by many researchers for use on different maize tissue types and on other crops (Fromm et al., 1990; Gordon-Kamm et al., 1990; Armstrong, 1999). More recently, Agrobacterium tumefaciens-mediated transformation was developed for maize (Ishida et al., 1996). This approach has been increasingly incorporated as the method of choice for reasons such as low transgene copy number, well-defined borders, and high frequency (Negrotto et al., 2000; Ingham et al., 2001; Frame et al., 2002; Miller et al., 2002). Concurrent with gene delivery methods, selectable marker development has been integral in developing efficient maize transformation. Kanamycin (Fromm et al., 1986; Rhodes et al., 1988; Lyznik et al., 1989) and hygromycin (Walters et al., 1992) were two of the earliest antibiotics used as selection agents in corn. The first herbicide used as a selection agent for maize transformation was the Glu analog phosphinothricin or, more commonly, the tripeptide bialaphos (l-phosphinothricyl-l-alanyl-l-ananine), which contains phosphinothricin as the active ingredient (Fromm et al., 1990; Gordon-Kamm et al., 1990). Maize callus selection on bialaphos, mediated by either the bar (bialaphos resistance) gene or pat (phosphinothricin acetyl transferase) gene, was found to be more efficient than selection on kanamycin (Register et al., 1994).
Although herbicide-based selectable marker systems have proven to be quite effective, a significant amount of work has also been done to develop so-called “positive” selectable marker systems. These are systems that facilitate growth of transformed tissue rather than kill non-transformed tissue (Joersbo, 2001). An example of this is the phospho-Manisomerase (PMI) system, which enables the efficient recovery of transgenic corn events on media containing Man (Joersbo et al., 1998; Negrotto et al., 2000; Wang et al., 2000; Wright et al., 2001).
Although a variety of selectable marker systems are available for maize, additional systems are desirable for several reasons. First, new selectable markers can be used for sequential corn transformations, thus facilitating the stacking of multiple traits as valuable new traits become available (Armstrong, 1999). Second, new systems that reduce selection time would be advantageous for maximizing high-throughput production of transgenic events. The process of large-scale transformation could also benefit from flexible applications of selection pressures (i.e. light or temperature) that would allow for increased selection levels without labor-intensive tissue transfer. Finally, a new herbicide tolerance gene provides a unique advantage in that non-transgenic plants can be removed from a segregating population by a simple herbicide spray in the field (Gordon-Kamm et al., 1990; Spencer et al., 1990). This is particularly important when considering the scale involved in commercial corn hybrid seed production.
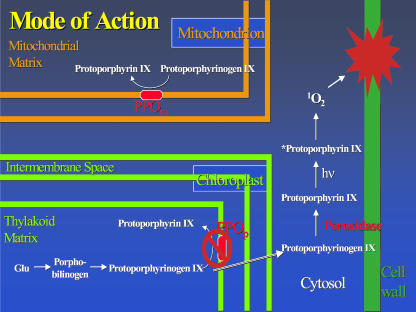
We have investigated a possible selectable marker system consisting of the herbicidal compound butafenacil (Tomlin, 2000) and its molecular target, protoporphyrinogen oxidase (PPO). PPO is a key enzyme in the chlorophyll/heme biosynthetic pathway, catalyzing the oxidation of protoporphyrinogen IX to protoporphyrin IX (Fig. 1; Smith et al., 1993). This is the last common step in the production of heme and chlorophyll. Heme is an essential cofactor in cytochromes, hemoglobin, oxygenases, peroxidases, and catalases, and, therefore, is a necessary product for all aerobic organisms. The production of chlorophyll, a light-harvesting pigment, is an essential process for all green photosynthetic organisms. This characteristic makes PPO an excellent gene target for herbicide development (Jacobs et al., 1991; Nandihalli and Duke, 1993). When PPO is inhibited by the PPO family of herbicides, protoporphyrin IX accumulates and causes light-dependent membrane damage (Fig. 1; Lee et al., 1993).
Figure 1.
Mode of action of PPO. The porphyrin pathway was proposed by Jacobs et al. (1991). When chloroplastic PPO is inhibited, protoporphyrinogen IX accumulates, leaks into the cytosol, and is oxidized to protoporphyrin IX. Protoporphyrin IX reacts with light to produce singlet oxygen, leading to lipid peroxidation, membrane disruption, and cell death.
PPO genes have been isolated from Escherichia coli (Sasarman et al., 1979, 1993), Bacillus subtilis (Dailey et al., 1994), and plants (Narita et al., 1996; Lermontova et al., 1997; Ward and Volrath, 1998; Volrath et al., 1999, 2000). Attempts have been made to improve plant tolerance to PPO inhibitory herbicides both by overexpressing native PPO genes in plants and by selecting for resistant mutants. Expression of the naturally tolerant B. subtilis PPO in transgenic tobacco (Nicotiana tabacum) and rice (Oryza sativa) showed resistance to a diphenyl ether herbicide (Lee et al., 2000). No field trials were reported in this publication. In transgenic tobacco plants overexpressing the wild-type Arabidopsis PPO-1 enzyme, the enzymatic activity increased about 5- to 7-fold, and tolerance to the diphenyl-ether herbicide acifluorfen was increased about 5-fold in a seed germination assay (Lermontova and Grimm, 2000). Watanabe et al. (1998) reported a herbicide-resistant tobacco cell line (YZI-1S) that was selected via conventional tissue culture with a PPO herbicide. The level of plastid PPO mRNA in tobacco YZI-1S cells was the same as in wild type, whereas the level of mitochondria PPO mRNA was up to 10 times higher. Other resistant cell lines of soybean (Glycine max) and tobacco have been isolated and shown to overproduce mitochondrial PPO (Warabi et al., 2001; Watanabe et al., 2002). In the herbicide-selected Chlamydomonas reinhardtii green algae mutant rs-3, resistance to the porphyric herbicide S-23142 was due to a (GTG) Val to (ATG) Met mutation at Val-389 of the C. reinhardtii plastidic PPO gene (Randolph-Anderson et al., 1998).
Our research focused on using cloned plant PPO genes to identify mutations that confer high levels of tolerance to the PPO inhibitor butafenacil (Tomlin, 2000) and on the transfer of these herbicide-tolerant genes back into plants. In this paper, we will describe the identification of PPO mutations, the development of a selectable marker system, and the production of transgenic corn events tolerant to PPO inhibitors.
RESULTS
Isolation of Plant PPO Genes
Arabidopsis PPO cDNAs were isolated by functional complementation of the E. coli PPO mutant SASX38 (Sasarman et al., 1993). This bacterial strain has a hemG (PPO) deletion and will not grow without the addition of exogenous heme in the culture medium or an alternate source of PPO activity. Sequence analysis of complementing clones revealed two classes of Arabidopsis PPO genes. The first class was designated “PPO-1” (Ward and Volrath, 1998) and corresponded to the chloroplast-targeted enzyme (Che et al., 2000). This cDNA was 1,719 bp in length and encoded a protein with a molecular mass of 57.7 kD. The N-terminal peptide sequence had features characteristic of a chloroplast transit peptide of approximately 35 to 50 amino acids. The sequence (GenBank accession no. AX084732) was described in U.S. patent 5,767,373 (Ward and Volrath, 1998) and by Narita et al. (1996). An Arabidopsis PPO-1 cDNA fragment was subsequently used as a hybridization probe to isolate full-length or partial cDNAs encoding the PPO-1 enzymes from maize, wheat (Triticum aestivum), rice, sorghum (Sorghum bicolor), soybean, cotton (Gossypium hirsutum), sugar beet (Beta vulgaris), sugarcane (Saccharum officinarum), and oilseed rape (Brassica napus; Volrath et al., 2000).
The second class of clone was designated “PPO-2” and corresponded to the mitochondria-targeted enzyme. The putative full-length cDNA was 1,738 bp in length, encoded a protein with a molecular mass of 55.6 kD, and possessed an amino-terminal extension that had some characteristics of a mitochondria transit peptide. The sequence of PPO-2 exhibited limited homology to PPO-1 (53% similar, 28% identical). The sequence of PPO-2 (GenBank accession no. AX084734) was also described in U.S. Patent 5,767,373 (Ward and Volrath, 1998).
Identification of Mutants Tolerant to PPO Inhibitors
Wild-type E. coli strains showed no sensitivity to butafenacil at any concentration, consistent with the reported resistance of the native bacterial enzyme to similar herbicides (Sasarman et al., 1993). In contrast, the E. coli strain SASX38 complemented by either Arabidopsis PPO-1 or PPO-2 was clearly sensitive to butafenacil, with strong growth inhibition at concentrations as low as 10 nm. This enabled us to use the bacterial system to screen large numbers of mutated plant PPO genes for mutations that could confer herbicide tolerance.
We chose to use the mutator E. coli strain XL1-Red (Greener and Callahan, 1994) for random in vivo mutagenesis of the Arabidopsis PPO-1 gene. The clone that proved most useful for these experiments was originally isolated from a plasmid library comprising Arabidopsis cDNAs cloned bidirectionally in the yeast (Saccharomyces cerevisiae) expression vector pFL61 (Minet et al., 1992). This library also expresses in E. coli. The particular truncated PPO-1 clone chosen for mutagenesis/screening was missing the first 40 amino acids of the gene, and translation apparently initiates at an ATG within the yeast PGK terminator. This PPO-1 clone was randomly mutated, transformed into E. coli strain SASX38, and screened for the ability to grow on media containing butafenacil. In a pilot round of mutagenesis/screening, we identified an amino acid change near the N terminus of this PPO-1 insert that confers herbicide “tolerance” only by increasing growth rate; this apparent increase in enzyme function appears to be an artifact of this particular truncated fusion protein. This modified clone, designated pMut-1, was subjected to a second round of random mutagenesis in XL1-Red and screened on herbicide concentrations lethal to the unmutagenized pMut-1 PPO-1 plasmid. More than 90% of the resistant colonies recovered from this second screen contained PPO-1 coding sequences with single amino acid changes that conferred significant tolerance to butafenacil. Three different mutants were identified. All amino acid numbers correspond to the full-length Arabidopsis PPO-1 cDNA. Plasmid Ala220Val had a GCT (Ala) to GTT (Val) mutation at amino acid 220. Plasmid Tyr-426-Cys had a TAC (Tyr) to TGC (Cys) mutation at amino acid 426. The third resistant clone, isolated at lower frequency, was pGly-221-Ser, with a GGT (Gly) to AGT (Ser) change at amino acid 221, adjacent to the mutation in pAla-220-Val.
Because PPO herbicides are competitive inhibitors of PPO, mutations that confer resistance to these herbicides also tend to reduce enzymatic activity. SASX38 cells relying on the original mutants Ala-220-Val and Tyr-426-Cys for PPO activity had significantly reduced growth rates (relative to pMut-1) in the absence of herbicide. In an effort to isolate “second site” changes that could mitigate or eliminate this effect, these two mutant plasmids were independently mutagenized again and screened in SASX38 on lethal herbicide concentrations. Several second site changes were identified that enhanced the growth rate of the herbicide-tolerant mutants both in the presence and absence of herbicide. These mutations failed to confer any herbicide resistance when inserted alone into wild-type PPO genes. The most interesting change arose several times in both screens and enhanced the growth rate of both mutants significantly. This mutation contained a TCA (Ser) to TTA (Leu) change at amino acid 305 and was designated Ser-305-Leu.
Amino acids Ala-220 and Tyr-426 were also subjected to site-directed mutagenesis, with every possible amino acid change assayed for both function and tolerance by growth in the presence and absence of herbicide. This led to the identification of additional herbicide tolerance mutations, some of which were more resistant than the original isolates. Ala-220 could be changed to Val, Thr, Leu, Cys, or Ile to yield a functional and herbicide-resistant PPO enzyme. Likewise, Tyr-426 could be changed to Cys, Ile, Leu, Thr, or Met to yield a functional and herbicide-resistant PPO enzyme.
Combinations of mutations identified in the site-directed studies and in the second site screens were constructed and assayed for growth ± butafenacil. This led to the identification of multiple highly tolerant, highly functional Arabidopsis PPO-1 mutant combinations, such as the Y426M + S305L mutant used for gene targeting in Arabidopsis (Hanin et al., 2001). The Tyr-426-Met resistance mutation without any additional second site mutation confers high tolerance to butafenacil as well. The transgenic constructs pWCO38 and pWCO39, derived from the double mutant Y426M + S305L, were used in all experiments reported below.
Production of Herbicide-Tolerant Plants by Expression of Mutant PPO
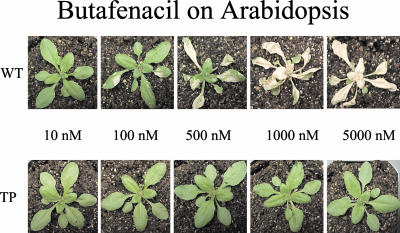
To evaluate an herbicide resistant PPO enzyme in plants, we needed an effective promoter to control expression of the transgene. For expression in Arabidopsis and potentially in other dicots, we isolated the endogenous Arabidopsis PPO-1 promoter (Johnson et al., 2000). The promoter fragment as isolated comprised 580 bp (GenBank accession no. AX084744) of Arabidopsis genomic DNA upstream from the initiating Met (ATG) of the PPO-1 coding sequence. This 580-bp fragment was fused to the double mutant PPO (Y426M + S305L) and transformed into Arabidopsis by vacuum infiltration of A. tumefaciens (Bechtold and Pelletier, 1998). Seed from the infiltrated plants was collected and plated on a range of butafenacil concentrations (10.0 nm–10.0 μm). Transgenic seedlings expressing the altered enzyme were recovered at herbicide concentrations of up to 5 μm. Wild-type Arabidopsis germination was totally inhibited by 100.0 nm butafenacil, indicating at least 50-fold higher herbicide tolerance in the transgenic seedlings. Multiple plants that germinated on butafenacil were transplanted and taken to seed. Progeny from these plants were tested in a spray assay with various concentrations of the herbicide. When compared with control plants in the spray assay, some transgenic lines were at least 500-fold more tolerant to the herbicide spray (Fig. 2). The herbicide tolerance trait appeared to be stably inherited through several generations of the plants that were tested. Seed from some of the most tolerant lines was also tested in a germination assay. In addition to butafenacil, Arabidopsis plants expressing the double mutant PPO (Y426M + S305L) were tested for cross-tolerance to a variety of other PPO inhibitors. These tests showed that the gene was capable of conferring broad-range tolerance to different classes of PPO inhibitors, although the levels varied widely among different compounds (Table I). In the germination assay, tolerance to butafenacil was at least 100-fold above wild type.
Figure 2.
Tolerance of transgenic Arabidopsis plants. Wild-type (WT, top) and transgenic plants (TP, bottom) were sprayed with 10, 100, 500, 1,000, and 5,000 nm butafenacil solutions. Photos were taken 1 week after the spray.
Table I.
Cross-tolerance of transgenic Arabidopsis to PPO herbicides
Background tolerance of wild-type Arabidopsis to butafenacil was <100 nm. The degree of transgenic tolerance was expressed as folds of background tolerance. A value of >100× indicated good germination on 10 μm butafenacil. Aclonifen as negative control.
| Name | Brand Name | Tolerance Level |
|---|---|---|
| Butafenacil (CGA 276′854) | — | ≥100× |
| Flupropazil (276 analog) | — | ≥100× |
| Pentoxazone | — | ≥100× |
| Carfentrazone | — | ≥100× |
| Fluazolate | — | ≥100× |
| Pyraflufen | — | ≥10× |
| Aclonifen | — | — |
| Azafenidin | Milestone | ≥100× |
| BAY 11340 | — | ≥10× |
| Fluthiacet-methyl (CGA 248′757) | Action | ≥100× |
| Flumioxazin | Sumisoya | ≥1,000× |
| Flumiclorac | Resource | ≥10× |
| Sulfentrazone | Authority | ≥10× |
| Fluoroglycofen | Compete | ≤10× |
| Fomesafen | Reflex | ≤10× |
| Lactofen | Cobra | ≤10× |
| Acifluorfen | Blazer | ≥10× |
| Oxyfluorfen | Goal | ≥10× |
| Bifenox | Modown | ≥10× |
Having validated the transgene in Arabidopsis, a vector was constructed to test whether the double mutated PPO enzyme (Y426M + S305L) could confer similar herbicide tolerance in maize plants. For maize expression, the gene was fused to the maize Ubiquitin promoter (Christensen and Quail, 1996) and cloned into the co-integrating transformation vector pSB12 (Komari et al., 1996) to create pWCO38. For initial experiments, the cassette was also cloned into a pSB12 vector already containing a maize ubiquitin/PMI cassette (Negrotto et al., 2000) to create the vector pWCO39. This vector allowed cotransformation of the validated PMI selectable marker and the PPO expression cassette on a single T-DNA.
The selection initially used for maize transformation was the PMI system (Negrotto et al., 2000). Two dozen pWCO39 maize events were generated by A. tumefaciens-mediated transformation and Man selection, with approximately 90% containing the double mutated gene. Plants from primary events were evaluated for tolerance to butafenacil by spray application. Twelve of the events were tolerant to butafenacil at a concentration of 1.0 to 2.5 μm, whereas the others were tolerant to concentrations of 5 to 25 μm. Untransformed control plants exhibited injury at 500 nm butafenacil. The herbicide damage was seen initially as localized areas of chlorosis and culminated in severe necrosis and collapse of the leaves (Fig. 3).
Figure 3.
Tolerance of transgenic maize plants. Wild-type maize plants (left) and transgenic T0 plants (right) were sprayed with a 5 μm solution of butafenacil. The photo was taken 10 d after spraying.
Development of the Mutated PPO as a Selection Marker in Maize
It was clear that the double mutant was able to confer tolerance to an entire maize plant. However, the question remained whether the gene/herbicide combination could be used as a selectable marker system. It was particularly unclear how effective the herbicide would be on maize callus tissue grown in the dark because PPO herbicides require light for full activity (Sherman et al., 1991). The first step was to determine the effect of the compound on maize callus tissue. Kill curves of wild-type maize callus showed that increasing butafenacil concentrations reduced the amount of callus produced, even in the absence of light. The average of three replicates gave 6.3, 6.2, 5.3, 3.6, 2.1, and 2.0 g of wild-type callus, respectively, at 0, 5, 50, 250, 500, and 750 nm butafenacil, after 1 month of growth on selection medium. Based on the toxicity of butafenacil on untransformed callus, selection Scheme 1 was designed as follows: 250, 500, and 750 nm for the first, second, and third rounds of selection, respectively, after initially culturing the callus on 5 nm for 2 weeks. After 2 weeks on the 5 nm medium, callus was white to yellowish and appeared healthy and almost identical to callus lines without 5 nm butafenacil, except with slight browning at the surface contacting medium. Although we did not refer to this step as the first selection step in the process, we believe it did impose selective pressure on the tissue and that it particularly inhibited callus proliferation from the embryo axis side, thus increasing the callus proportion from the scutellum. Our observation, consistent with the report from Frame et al. (2002), indicated that the scutellum-derived callus was most likely the producer of transgenic events.
All tissue (embryo and emerging callus) was transferred to the first round of selection. Each round of selection spanned 2 weeks with all callus being transferred, without any subjective dissection, until events emerged. During the initial stage of selection, the callus turned brown during selection, although there was some growth at the lower levels. Typically, after about 45 d on selection, transformed sectors emerged with a distinguished phenotype: whitish to yellowish color on a brown background (see the emerging callus at the far right of middle row of Fig. 4). The transformed sectors were isolated and grown separately on 750 nm without further browning. Those transformants appeared as small (1–2 mm) blond masses with no browning tissue attached. The callus was predominately type I callus if A188, A188xHiII, or HiIIxA188 was used. However, if pure HiII was used for transformation, the predominate callus was type II. In all genotypes above, the browning occurred in all callus lines during the first round of selection. In contrast, butafenacil only killed the contacting portion of highly compact Type I callus derived from other genotypes we tested. In the later example, a more labor-intensive dissection of the surviving tissue was required. This indicates that the efficacy of butafenacil selection is callus type independent, although highly compact Type I callus required longer selections (one to two rounds more) at higher concentrations (up to 1,500 nm). The color of the PPO-transformed tissue allowed for accurate identification of events at an early stage. This callus was allowed to proliferate for an additional 2 to 4 weeks before being transferred to regeneration media, at which point no further herbicide selection was necessary. On the regeneration media, the callus differentiated into small plantlets, which were transferred to soil after reaching a height of 3 to 4 inches. The plants were sprayed with butafenacil 1 week after transplantation to soil.
Figure 4.
Transgenic callus emerging from the selection medium. The callus photo was taken 45 d after A. tumefaciens inoculation for experiment AP89. A transgenic event in the far right side of the middle row is emerging from the untransformed brown callus lines. The diameter of the Petri dish was 9 cm. IE, Immature embryos.
Additional work was done to optimize butafenacil concentrations used for selection. Three selection schemes were compared in their ability to produce transformants. One-third of the embryos from each transformation plate were transferred to one of three media containing different amounts of butafenacil. The selection Scheme 1 was as described above. Selection Scheme 2 was designed as 500, 750, and 750 nm for the three rounds of selection. Selection Scheme 3 was at 750 nm for all three rounds. Because light enhances PPO inhibitor activity, all experiments were kept in the dark during selection. Four replications were done for each scheme with a total of 950 embryos. The average transformation frequency (TF) was 14.9%, 16.5%, and 19.2% for selection Schemes 1, 2, and 3, respectively. Although transformation variability was high among experiments, the trend in all four was that the TF tended to be higher with increased butafenacil levels (Table II). Maize ear quality, including factors such as uniformity of the size of immature embryos, probably contributed to the large differences in TF among the four experiments, which were initiated during the transition from fall to winter. In addition to somewhat higher TF, selection Scheme 3 showed a significant reduction in the total amount of callus material transferred in each round. This reduction was a result of early death of untransformed tissue.
Table II.
The effect of selection schemes on TF
TF is defined as the percentage of embryos producing events. One-third of cocultivated embryos from each experiment were transferred to one of three selection media containing different concentrations of butafenacil (nanomolar). All selection steps were kept in the dark. The decrease in TF from Experiments 1 to 4 may be due to inferior embryo quality at the beginning of the winter season. Significant differences are detected between selection schemes 1 and 2, 2 and 3, and 3 and 1 based on paired Student's t test at P = 0.05.
| Selections | Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | Average |
|---|---|---|---|---|---|
| 250-500-750 | 33/153(21.6%) | 11/45(24.4%) | 1/51(2.0%) | 2/67(3.0%) | 47/316(14.9%) |
| 500-750-750 | 35/153(22.9%) | 12/45(26.7%) | 2/51(3.9%) | 3/67(4.4%) | 52/316(16.5%) |
| 750-750-750 | 38/155(24.5%) | 15/45(33.3%) | 4/51(7.8%) | 4/67(5.9%) | 61/318(19.2%) |
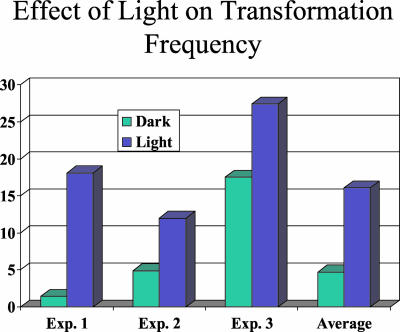
Our normal practice minimized the tissue exposure to light by maintaining all cultures in a darkroom except during physical transfer to fresh media. Even during this subculture process, the plates were kept under cover. The only time the plates were exposed to light was during transfer in the laminar flow hood. Because PPO inhibitors are more active in the presence of light (Wright et al., 1995), experiments were performed to determine the effect of light treatments on selection. The light treatment was added to the optimal selection scheme (Scheme 3, described above), by exposing cultures to a regular cool-white fluorescent light at 75 μmol m–2 s–1 for 8 h, 1 d after the initial transfer. During the initial 750 nm butafenacil selection, exposure of callus material to light reduced the amount of tissue by 70% and led to increased TF (Fig. 5). At such light-enhanced stringency, only transformed calli continued to proliferate. Therefore, it was not necessary to provide an additional light treatment for the later transfers. Further, the time necessary to detect transformation events decreased from 6 to 8 weeks to about 4 weeks. Increasing the concentration of butafenacil to 1,500 nm also resulted in a comparable TF but did not decrease the amount of time needed to detect transgenic events.
Figure 5.
TF under different light treatments. □, TF for three independent experiments was compared between dark (green) and light (blue) treatments. Cultures were maintained in the growth chamber in the dark at all times except transfers in the hood. For the light treatment, the callus was exposed to regular fluorescent light at 75 μmol m–2 s–1 for 8 h 1 d after the initial transfer to the fresh medium for Scheme 3. The effect of dark and light treatment on TF is not significant based on paired Student's t test (t = 3.941, degrees of freedom =2, P value = 0.0588), maybe due to limited experiments.
The Effect of PPO Selection on Transgene Copy Number
Selection based on herbicide tolerance could potentially lead to an increase in gene copy number as a result of strong selection for high gene expression (Shyr et al., 1992). The stability of transgenes has been shown to be related to the copy number of insertions (Sidorenko and Peterson, 2001). Simple insertions also facilitate the regulatory approval process for transgenic products. Thus, it was important to determine if the PPO selectable marker system resulted in plants with an increased transgene copy number. Analysis of PPO events found the majority (71%) to be single-copy events (Table III), similar to the results seen with PMI selection (70%) when using the same transformation method (Ingham et al., 2001).
Table III.
Transgene copy no. distribution (percentage of total)a
| Category | PMIb | PPOc | HT-PPOd |
|---|---|---|---|
| Total events | 287 | 45 | 46 |
| One copy | 201 (70%) | 32 (71%) | 26 (57%) |
| Two copies | 62 (21%) | 7.0 (16%) | 11 (24%) |
| >Two copies | 24 (8.0%) | 6.0 (13%) | 9.0 (20%) |
a No. of samples determined to have the indicated copy no. among total events as determined by TaqMan assay. bPMI data cited from Ingham et al. (2001). cEvents containing the pWCO38 construct and tolerant to 1 μm butafenacil or above. dHighly tolerant (>25 μm) pWCO38 events identified from approximately 2,500 events by greenhouse spray. Oligos used for PPO copy no. assay for all pWCO38 events were AtPPO63F, R, and P, respectively, as forward primer (GGACAGAATTCCGGTGTTTGTAG), reverse primer (GCACCGCCCGGAAGA), and probe (CCCGCCAA TCATGTTCAACAGCAAA).
Further analysis was done for a subset of 46 highly tolerant pWCO38 events (Table III) identified from about 2,500 transformants by a greenhouse spray assay. About 57% of our highly tolerant events had a single copy of the PPO gene, indicating that multiple copies were not absolutely required for high tolerance. Forty-four percent of these highly tolerant events were found to have multiple copies, in contrast to 29% of a random pool of transgenic events selected by the primary assay at 1 μm butafenacil. This indicates that multiple copies do tend to render transgenic plants more tolerant to the herbicide.
Development of Herbicide-Tolerant Transgenic Maize Plants
In addition to developing PPO/butafenacil as a selectable marker system, we also wanted to produce maize plants tolerant to field rates of butafenacil. Due to the variable tolerance of transgenic events, a two-step screen was used to identify the most highly tolerant events. Approximately 2,500 transgenic T0 events were produced, and eight to 10 plants were regenerated from each event. This primary screen identified approximately 100 events that were tolerant to greater than 50 μm butafenacil. The secondary screen, performed on T1 progeny from these events, consisted of a greenhouse screen using field rates of butafenacil. This resulted in the identification of 12 events with field-effective levels of tolerance. Those events passing the second screen were promoted for field trials. The results of field trials were correlated with the greenhouse results, and several events were identified that exhibited acceptable levels of tolerance (Fig. 6). The combination of the tolerant PPO genes and the process of using these genes to produce herbicide-tolerant plants was branded as Acuron Technology (Holmberg, 2000).
Figure 6.
Corn tolerance under field application conditions. Corn plants at the three-leaves stage were sprayed with butafenacil at 400 g of active ingredient (AI) per hectare (3× effective field rate). Photos were taken 8 d after treatment (8DAT). Wild-type (left) versus transgenic (right) plants.
DISCUSSION
Isolation of Herbicide-Tolerant PPO Genes
Complementation of E. coli hemG mutants has proved to be a routinely successful method for the isolation of eukaryotic PPO genes (Dailey et al., 1995; Nishimura et al., 1995; Narita et al., 1996; Lermontova et al., 1997) despite the fact that there is no sequence similarity between the hemG protein (Sasarman et al., 1993) and either plant or mammalian PPO enzymes. The ability to complement bacteria with plant enzymes also provides a method of isolating herbicide-tolerant variants of these enzymes. The PPO inhibitor butafenacil, in contrast to some other PPO herbicides, appears to have no uptake problems in E. coli. The XL1-Red mutagenesis strain enabled us to create huge pools of independent mutants with minimal effort, and the advertised 1/2,000-bp mutation rate was ideal for the purpose of mutagenizing a 1.7-kb PPO cDNA. Although transitions were strongly favored in our mutant populations, the system generated all possible base changes. Subsequent mutagenesis using other methodology (data not shown) has failed to identify any additional single base changes in the Arabidopsis PPO-1 gene that confer significant herbicide tolerance. With this conservative method of mutagenesis, the probability of picking up amino acid changes requiring multiple base changes is very low. We have addressed this by saturation site-directed mutagenesis of identified resistance sites and by using other plant PPO cDNAs for screening. The plant enzymes are quite conserved at the amino acid level, but different codon usage creates enough sequence diversity (Table IV) to greatly increase the opportunities for identifying resistance sites from single base mutations.
Table IV.
PPO gene homology
| Maize | Sugarcane | Sorghum | Rice | Wheat | Cotton | Soybean | Sugar beet | Arabidopsis | |
|---|---|---|---|---|---|---|---|---|---|
| Maize | 100 | - | - | - | - | - | - | - | - |
| Sugarcane | 97 | 100 | - | - | - | - | - | - | - |
| Sorghum | 96 | 97 | 100 | - | - | - | - | - | - |
| Rice | 87 | 89 | 88 | 100 | - | - | - | - | - |
| Wheat | 86 | 82 | 86 | 86 | 100 | - | - | - | - |
| Cotton | 73 | 69 | 74 | 72 | 72 | 100 | - | - | - |
| Soybean | 72 | 67 | 72 | 71 | 72 | 79 | 100 | - | - |
| Sugar beet | 69 | 64 | 70 | 68 | 70 | 72 | 73 | 100 | - |
| Arabidopsis | 70 | 66 | 70 | 69 | 70 | 76 | 75 | 72 | 100 |
The cDNAs (not including the putative chloroplast transit peptide sequence) were aligned with the maize PPO-1 cDNA using the GAP program described in Devereux et al., 1984. Sequences are described in US patent #5,939,602 (Volrath et al., 1999). Sugarcane, sorghum and rice sequences were only partial cDNAs.
There were two primary sources of background in the mutant screening process. Many plasmid vectors can mutate easily to give tolerance via higher expression of the PPO gene product. Our choice of the pMut-1 plasmid as a screening vector greatly reduced this problem. Despite the fact that SASX38 is a hemG deletion strain, the second problem was mutation to resistance by the E. coli strain. We believe that SASX38 may adapt to the herbicide by overexpressing the coproporphyrinogen oxidase (hemF) enzyme, which is involved in the step of the porphyrin pathway immediately before PPO. The ability of this enzyme to oxidize protoporphyrinogen IX was published by Narita et al. (1999), and the enzyme would be unaffected by PPO inhibitors. This adaptation of the cells to the herbicide poses a particular problem when attempting to characterize herbicide-tolerant mutants by traditional growth curves in liquid culture. This is the primary reason for performing screens and assays of mutants on solid plates, where the appearance of an occasional large colony can be ignored. A hemF knockout mutant of the SASX38 could be constructed and might serve to eliminate this problem.
PPO as a Selectable Marker
Identification of the PPO mutants has enabled the development of a new and effective selectable marker system. Transgenic maize events were easily detected using the PPO selection system for A. tumefaciens transformation, with the TF being comparable with that reported for both the phosphinothricin Acetyl transferase (PAT) and PMI systems. For our initial experiments, the average TF was 10.4%, 12.2%, and 13.6% for PAT, PMI, and PPO, respectively. The TF via PMI selection was similar to the earlier phase of developing A. tumefaciens-mediated transformation by our colleagues (Negrotto et al., 2000).
TF typically has been highly variable for maize transformation. During their early stages of development, A. tumefaciens TF with PMI was reported to range from 0.7% to 32% (Negrotto et al., 2000), whereas the frequency with PAT varied from 0% to 30.6% (Ishida et al., 1996). This variation has been attributed to a number of factors that include overall health and vigor of donor plants, the quality and size of immature embryos, maize genotype, the timely conduction of experiments, and the consistency of prepared media. This type of variation can make comparison of treatments during optimization of transformation very difficult. To minimize the impact of this variation on the comparisons of the PPO, PAT, and PMI selection systems, our experiments were conducted with embryos taken from the same ear and were replicated over time. The results showed that although the TF varied over time, the general trend within a side-by-side experiment was the same for the three selection systems.
In addition to acceptable TF, the PPO selection system offered other benefits. First, transformants showed a unique phenotype that facilitated the identification and subculture of transformed callus tissue. Second, the time for whole callus selection was significantly reduced. Finally, and most importantly, selection pressure could be easily increased during the selection process by exposing the callus to light. An increase of selection stringency could be achieved by increasing light intensity, lengthening light exposure, or both. The light treatment resulted in a significant reduction in the amount of callus tissue produced during selection and in increased TF. This increase may be due to more rapid death of untransformed tissues caused by the formation of singlet oxygen in the presence of the PPO inhibitor and light (Sherman et al., 1991). The combination of light with the highly active PPO inhibitor chemistry significantly reduces the labor-intensive practice of tissue transfer and the amount of untransformed tissue surviving selection. In fact, this PPO selection system could potentially provide a system where only a single plate is required to carry out selection before regeneration. Thus, the properties of PPO selection come close to fulfilling the requirements of an optimal maize selection system: one characterized by a short selection period and minimal proliferation of viable callus. Additional flexibility exists because the cross-tolerance of PPO mutants to other compounds provides a large palette of potential selection agents to choose from. Extension to other crops should also be possible, although the concentration spectrum for dicot transformation might be more stringent as a result of increased plant sensitivity.
PPO as a Mechanism for Crop Tolerance
Herbicides targeted to PPO characteristically have a very rapid contact action causing leaf burning, desiccation, and growth inhibition. Inhibition of the normal enzymatic reaction leads to the accumulation of protoporphyrinogen IX in the chloroplast, which then leaks out to the cytoplasm and is oxidized by peroxidases. Exposure to light causes formation of singlet oxygen and other oxidative species resulting in membrane disruption and subsequent cell death (Smith et al., 1993). Natural crop tolerance to compounds from this class of herbicide is often limited compared with other types of herbicides (Gressel, 2000; Owen, 2000). Although it is known that the PPO herbicides are more active on broadleaved species (Witkowski and Halling, 1989), the enzyme target appears to be equally sensitive to the herbicides. To date, natural occurrence of weed tolerance to PPO inhibitors has not been reported, although this type of herbicide was developed 40 years ago. Our observation that resistance mutations tend to reduce enzymatic function may help to explain the lack of naturally occurring resistant enzymes.
Multiple chemical families have been classified as PPO inhibitors. These herbicidal compounds include diphenylethers, oxidiazoles, cyclic imides, phenyl pyrazoles, pyridine derivatives, and phenopylates. All of these compounds are thought to act as substrate analogs; therefore, cross-resistance of mutations selected using a given inhibitor is expected. Our cross-tolerance assays using the SASX38/plant PPO system have shown that all of the mutations tested can confer tolerance to a variety of PPO-inhibiting compounds, both commercial and experimental (Ward and Volrath, 1998). An Arabidopsis germination assay also showed the cross-tolerance of mutant PPO transgenic plants to various commercial PPO inhibitors such as acifluorfen, fomesafen, fluoroglycofen, bifenox, oxyfluorfen, lactofen, fluthiacetmethyl, sulfentrazone, and flupropazil (Table I). Similar tests were conducted for transgenic corn, and wide cross-tolerance was also observed (data not shown). Multiple mutations, individually or in combination, can confer tolerance to a wide range of inhibitors. Therefore, Acuron technology is not dependent on any single herbicide or any single mutant PPO gene. In our work, we have concentrated on butafenacil as a selection agent with mutant PPO genes. We expect that other compounds could work either as selective agents or as PPO-inhibitory herbicides for the field application to transgenic corn lines, although further optimization may be required for any particular compound (Theodoridis et al., 2000).
Acuron technology could be useful in the development of a variety of PPO herbicide-tolerant crops. We have isolated complete PPO-1 cDNA sequences from maize, wheat, sugar beet, cotton, and soybean. Table IV shows the significant homology of the maize PPO cDNA to other species. Corresponding herbicidetolerant mutants for many of these cDNAs have been described (Volrath et al., 1999; de Marco et al., 2000). We have tested many mutations isolated in one gene at the homologous position in other genes. Although most mutations occur at sites where gene homology is very high, a given mutation frequently does not behave identically when inserted into a different plant enzyme. The strong correlation of resistant mutations with compromised enzyme activity tends to make many mutation/gene combinations poor candidates for engineering crop tolerance. Fortunately, the availability of multiple resistance mutations and multiple plant PPO genes should serve to easily overcome this potential limitation on the use of this technology. Although PPO is an efficient selectable marker, the frequency of transgenic plants with field level tolerance was low. This issue could be addressed by further optimization of transgene expression, such as by alteration of the Arabidopsis double mutant gene to maize codon usage.
MATERIALS AND METHODS
Bacterial Complementation and Selection
An Arabidopsis (Landsberg) cDNA library in the plasmid vector pFL61 (Minet et al., 1992) was kindly supplied by Dr. Michele Minet (Centre de Genetique Moleculaire, Gif sur Yvette, France) and amplified as colonies on solid media to maximize clone representation. A second Arabidopsis (Columbia) cDNA library in the Lambda UniZap vector was purchased from Stratagene (La Jolla, CA) and amplified as pBluescript plasmids by mass in vivo excision of the phage library according to the manual. The Escherichia coli hemG mutant SASX38 (Sasarman et al., 1979) was kindly provided by Dr. Alex Sasarman (Department of Microbiology and Immunology, Universite de Montreal, Quebec, Canada) and maintained on Luria-Bertani media containing 20 μg mL–1 hematin (United States Biochemicals, USB Corporation, Cleveland OH). Plasmid libraries were transformed into SASX38 by electroporation. Electrocompetent cells were prepared according to the Gene Pulser (Bio-Rad Laboratories, Hercules, CA) manual with the addition of 20 μg mL–1 hematin to all solutions, including storage buffer. Transformed cells were plated on L agar containing 100 μg mL–1 ampicillin at a density of approximately 500,000 transformants per 10-cm plate. The cells were incubated at 37°C for 40 h in low light and selected for the ability to grow without the addition of exogenous heme. Plasmid DNA was isolated from heme prototrophs and transformed back into SASX38 to verify complementation before sequence analysis.
The Arabidopsis PPO-1 clone initially chosen for mutagenesis was designated SLV17. This truncated PPO gene was inserted in reverse orientation in the pFL61 vector (relative to the yeast [Saccharomyces cerevisiae] PGK promoter). Translation of PPO-1 apparently initiates at an ATG within the yeast PGK terminator to create a fusion protein. The coding sequence after the first round of mutagenesis/screening contained two changes, a silent AGT (Ser) to AGC (Ser) change at amino acid 343 and an ACG (Thr) to AAG (Lys) change at amino acid 56, which leads to higher enzyme activity and/or expression. An example of the pMut-1 construct, containing the Tyr-426-Cys resistance mutation, was deposited with the Agricultural Research Center, Patent Culture Collection, Northern Regional Research Center (NRRL; Peoria, IL) on November 14, 1994 as pWDC-7 with the deposit designation NRRL 21339N.
PPO plasmids were transformed into the E. coli strain XL1-Red (Stratagene) for random in vivo mutagenesis during growth. Plasmid DNA was extracted from XL1-Red colonies that had been incubated on plates at high density (100,000 colony forming units [cfu] per 15-cm plate) for approximately 24 h. The mutated DNA was electroporated into SASX38. The transformations were grown out in Luria-Bertani broth (no hematin) for 1 h and then plated onto L media containing sufficient PPO-inhibiting herbicide to completely kill cells containing the wild-type pMut-1 clone. Plates were incubated at 37°C for up to 48 h in low light. Plasmid DNA was isolated from colonies that grew on herbicide, transformed back into SASX38, and screened to verify that the resistance was plasmid borne before sequence analysis. Subsequent rounds of selection on existing mutants were performed identically, using herbicide concentrations sufficient to completely inhibit the original mutant. In vitro mutagenesis to create site-specific changes was carried out using the Quik-Change kit (Stratagene). All mutants and mutant combinations were characterized by screening on solid media. Isolated clones were transformed into SASX38 and plated at medium/high density (several thousand cfu per 10-cm plate) in the presence and absence of herbicide. Plates were scored visually for the appearance of colonies/lawns over a period of 6 to 48 h post-plating.
Arabidopsis Transformation and Characterization
The protocols for transformation and plant manipulation for Arabidopsis are described by Molina et al. (1999) and Hanin et al. (2001). To isolate the native PPO promoter, the Arabidopsis PPO-1 cDNA was used to probe an Arabidopsis (Columbia, whole plant) Lambda Zap II genomic library purchased from Stratagene. Details are described by Johnson et al. (2000). One clone, ArabPT1Pro, was determined to contain 580 bp of Arabidopsis sequence upstream from the initiating Met (ATG) of the PPO-1 coding sequence in addition to coding sequence (plus introns) extending to bp 1,241 of the cDNA. This pBluescript clone was excised from the lambda vector and was deposited with the NRRL (see above) as pWDC-11, NRRL B-21515.
A full length cDNA of the wild-type Arabidopsis PPO-1 gene was isolated by complementation from the UniZap lambda library described above. This pBluescript clone was subjected to site-directed mutagenesis using the Quik-Change kit (Stratagene) to create mutant PPO genes. An EcoRI-XhoI partial digest fragment was excised from this construct and ligated into the plant expression vector pCGN1761ENX (see Example 9 of International Application No. PCT/IB95/00452 filed June 8, 1995 and published Dec. 21, 1995 as WO 95/34659). This plasmid was digested with NcoI and BamHI to produce a fragment comprised of the complete PPO-1 cDNA plus a transcription terminator from the 3′-untranslated sequence of the tml gene of Agrobacterium tumefaciens. The AraPT1Pro plasmid was digested with NcoI and BamHI to produce a fragment comprised of pBluescript and the 580-bp putative Arabidopsis PPO-1 promoter. Ligation of these two fragments produced a fusion of a full-length altered PPO cDNA to the native promoter. The expression cassette containing the PPO-1 promoter/mutant PPO/tml terminator fusion was excised by digestion with KpnI and cloned into a binary T-DNA vector that also contained a nos/kanamycin plant selectable marker. The binary plasmid was transformed by electroporation into A. tumefaciens and then into Arabidopsis using vacuum infiltration as in Bechtold and Pelletier, 1998. Transformants expressing altered PPO genes could be selected either on kanamycin or on various concentrations of PPO-inhibiting herbicide or on both. For butafenacil, background tolerance of wild-type Arabidopsis was <100 nm. The degree of transgenic tolerance was expressed as folds of background tolerance. A value of ≥100× indicated good germination on 10 μm butafenacil.
Maize (Zea mays) Transformation and Selection
A. tumefaciens strain LBA4404 (pAL4404, pSBI) was used for maize transformation and selection development. Detailed information about the disarmed helper plasmid and the virulence region is described by Ishida et al. (1996) and Negrotto et al. (2000). The cointegrate vector pSB12 is described by Komari et al. (1996). Plasmid constructs pWCO38 and pWCO39 were used in experiments reported in this paper. Plasmid WCO38 consisted of a cassette containing the Arabidopsis mutant PPO gene, Y426M + S305L, flanked by the maize ubiquitin promoter (Christensen et al., 1996) and the 35S terminator, and cloned into the HindIII site of vector pSB12. Plasmid pWCO39 has the same PPO cassette adjacent to a maize ubiquitin/PMI/nos cassette (Wright et al., 2001) between the same T-DNA borders in vector pSB12.
A. tumefaciens strains were plated from glycerol stocks onto YPC/Spec100/Tet10 plates (5.0 g L–1 yeast extract, 10.0 g L–1 peptone, 5.0 g L–1 NaCl, 1.0 g L–1 CaCl2, 15.0 g L–1 bactoagar, 100 mg L–1 spectinomycin, and 10 mg L–1 tetracycline [pH 6.8]) 1–2 d before the experiment. Plates were incubated at 28°C. A. tumefaciens suspensions were made by adding 1 loop of bacteria mL–1 LSinf+As (see below) liquid. The suspension was diluted to an optical density of 0.5 to 1.0 × 109 cfu mL–1.
For maize transformation, genotypes A188, Hi-II and the crosses (A188 × Hi-II and Hi-II × A188) were used as starting material. Dr. Ronald Phillips and the National Institute of Agribiological Resource of Japan (Tsukuba 305–8602) kindly provided the A188 material. Hi-II and its parental lines (Hi-II Parent A and B, Armstrong et al., 1991) were obtained from the Maize Genetics Cooperation Stock Center (Urbana, IL). Plants were grown in a greenhouse, and ears were pollinated several days after silks had emerged. Eight to 10 d after pollination, the ears were picked, dehusked, placed in a 20% (v/v) commercial bleach, and shaken at 150 rpm for 15 min on a VWR orbital shaker. Ears were then rinsed in 6 volumes of sterile water. Immature embryos (0.5–1.5 mm) were aseptically isolated from the ears.
Immature embryos were placed in LSinf+As (Linsmaier and Skoog, 1965; Linsmaier and Skoog [LS] major salts, LS minor salts, 0.5 mg L–1 nicotinic acid, 0.5 mg L–1 pyridoxine HCl, 1.0 mg L–1 thiamine HCl, 100 mg L–1 myo-inositol, 1.0 g L–1 casamino acids [Difco Bacto 0230-01, DIFCO Laboratories, Detroit], 1.5 mg L–1 2,4-dichlorophenoxyacetic acid, 68.5 g L–1 Suc, 36.0 g L–1 Glc [pH 5.2], and 100 μm acetosyringone) liquid in microfuge tubes (see Ishida et al., 1996; Negrotto et al., 2000). Embryos were vortexed for 10 s, followed by removal of the LSinf+As liquid with a sterile thin-tip pipette. The A. tumefaciens suspension was added. Embryos were vortexed for 30 s and allowed to stand for 5 min. Microfuge tubes were shaken to suspend the embryos, and the contents were decanted onto LSAs plates (LS major salts, LS minor salts, 0.5 mg L–1 nicotinic acid, 0.5 mg L–1 pyridoxine HCl, 1.0 mg L–1 thiamine HCl, 100 mg L–1 myo-inositol, 700 mg L–1 l-Pro, 1.5 mg L–1 2,4-dichlorophenoxyacetic acid, 20.0 g L–1 Suc, 10.0 g L–1 Glc, 500 mg L–1 MES, 100 μm acetosyringone [pH 5.8], and 8 g L–1 purified agar [Sigma, St. Louis]). Excess liquid was removed with a sterile pipette. Plates were allowed to dry in a laminar flow hood for 15 to 20 min. All embryos were oriented so that the embryo axis contacted the media. Immature embryos were cultured in the dark for 2 to 3 d at room temperature (22°C–23°C).
Regeneration and Analysis of Maize Transformants
After cocultivation, embryos were transferred to LS5Dc media containing 5 nm butafenacil and incubated for 2 weeks. LS5Dc contains LS major salts, LS minor salts, 700 mg L–1 Pro, 20 g L–1 Suc, 500 mg L–1 MES, 5 mg L–1 dicamba, 0.5 mg L–1 nicotinic acid, 0.5 mg L–1 pyridoxine HCl, 1.0 mg L–1 thiamine HCl, 100 mg L–1 myo-inositol, 100 mg L–1 carbenicillin, 1 mg L–1 AgNO3, and 8 g L–1 purified agar (pH 5.8; Ishida et al., 1996; see Negrotto et al., 2000).
For this herbicide selection, butafenacil was always added to medium post-autoclave. Three schemes were designed for butafenacil selection. Each scheme consisted of three rounds of selections. Each round spanned 2 weeks. These schemes were 250-500-750 nm for Scheme 1, 500-500-750 nm for Scheme 2, and 750-750-750 nm for Scheme 3. After 6 weeks on selection, subcultures onto 750 nm were done every 2 weeks until events were large enough to be transferred onto regeneration media. Most of the events were identified between 6 and 10 weeks. Optionally, a light treatment at an intensity of 75 μmol m–2 s–1 for 8 h was applied 1 d after the initial transfer to the fresh medium for Scheme 3. The selection time was reduced to 4 weeks because a light treatment allowed quicker identification of transformants and less growth of untransformed tissues due to increased potency of PPO herbicides.
For shoot regeneration, Type I and/or Type II callus tissue was transferred to LS3S.AK+50 nm butafenacil (LS major salts, LS minor salts, 30 g L–1 Suc, 0.5 mg L–1 Ancimidol [Se-Pro Corporation, Carmel, IN], 1 mg L–1 Kinetin, 50 nm butafenacil, and 2.4 g L–1 Gelrite [pH 5.8]) and placed in the dark for 2 weeks. Callus was then transferred to LS3S.AK without butafenacil and placed in the light for about 2 weeks. Plantlets were transferred to LS3S (LS major salts, LS minor salts, 30 g L–1 Suc, and 6.0 g L–1 phytagar [pH 5.8]) to allow for better root formation and plant growth (Wright et al., 2001). After 1 to 2 weeks, plants were transferred to soil and placed in the greenhouse for the butafenacil spray assay and seed production. TF was defined as the percentage of immature embryos producing transgenic events (see Table II and Fig. 5). Usually, only one event was picked from one embryo-derived callus line even if there appeared to be multiple events. For transgene copy number determination, the Taqman assay was as described by our colleagues Ingham et al. (2001).
Greenhouse Spray Assays and Tolerant Plant Production
For greenhouse testing, butafenacil was diluted into sterile water, and the surfactant Silwet was added to 0.01% (v/v). Greenhouse sprays were done using Preval Sprayers (Precision Valve Corporation, Yonkers, NY). The more stringent spray was done at 50 or 100 μm. For tolerant event production, only events that were completely undamaged at 50 μm or higher were saved and transferred to the greenhouse to produce seeds. In many cases, the plants could be successfully self-fertilized; events were also recovered by outcross or backcross.
Acknowledgments
We thank many Syngenta colleagues for assistance during the course of these experiments. Particularly, we thank David Negrotto, Erik Dunder, John Dawson, Janet Suttie, and Allan Wenck for sharing their expertise in maize transformation; Chong Vang, Jacqueline Holmann, Olguitza Guzman, Cathy Tomanny, and Bernadette Cooney for tissue culture work; the plant analysis group, the media lab, and the greenhouse staff for their services; and the Crop Protection Sector and Seeds Sector (Moez Meghji and Rakesh Jain) for chemical sprays and field trials.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026245.
References
- Armstrong CL, Green CE, Phillips RL (1991) Development and availability of germplasm with high Type II culture formation response. Maize Genet Coop Newslett 65: 92–93 [Google Scholar]
- Armstrong CL (1999) The first decade of maize transformation: a review and future perspective. Maydica 44: 101–109 [Google Scholar]
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82: 259–266 [DOI] [PubMed] [Google Scholar]
- Che F, Watanabe N, Iwano M, Inokuchi H, Takayama S, Yoshida S, Isogai AK (2000) Molecular characterization and subcellular localization of protoporphyrinogen oxidase in spinach chloroplasts. Plant Physiol 124: 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5: 213–218 [DOI] [PubMed] [Google Scholar]
- Dailey TA, Dailey HA, Meissner P, Prasad ARK (1995) Cloning, sequence, and expression of mouse protoporphyrinogen oxidase. Arch Biochem Biophys 324: 379–384 [DOI] [PubMed] [Google Scholar]
- Dailey TA, Meissner P, Dailey HA (1994) Expression of a cloned protoporphyrinogen oxidase. J Biol Chem 269: 813–815 [PubMed] [Google Scholar]
- de Marco A, Volrath S, Bruyere T, Law M, Fonne-Pfister (2000) Recombinant maize protoporphyrinogen IX oxidase expressed in Escherichia coli forms complexes with GroEL and DnaK chaperones. Protein Expr Purif 20: 81–86 [DOI] [PubMed] [Google Scholar]
- Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame BR, Shou H, Chikwamba RK, Zhang Z, Xiang C, Fonger TM, Pegg SE, Li B, Nettleton DS, Pei D et al. (2002) Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol 129: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm ME, Morrish F, Armstrong C, Williams R, Thomas J, Klein TM (1990) Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Biotechnology 8: 833–839 [DOI] [PubMed] [Google Scholar]
- Fromm ME, Taylor LP, Walbot V (1986) Stable transformation of maize after gene transfer by electroporation. Nature 319: 791–793 [DOI] [PubMed] [Google Scholar]
- Gordon-Kamm WJ, Spencer TM, Mangano ML, Adams TR, Daines RJ, Start WG, O'Brien JV, Chambers SA, Adams Jr WR, Willetts NG et al. (1990) Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 2: 603–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greener A, Callahan M (1994) XL1-Red: a highly efficient random mutagenesis strain. Strategies 7: 32–34 [Google Scholar]
- Gressel J (2000) Molecular biology of weed control. Transgenic Res 9: 355–382 [DOI] [PubMed] [Google Scholar]
- Hanin M, Volrath S, Bogucki A, Briker M, Ward E, Paszkowski J (2001) Gene targeting in Arabidopsis. Plant J 28: 671–677 [DOI] [PubMed] [Google Scholar]
- Holmberg M (2000) A fine line. Successful Farming 3: 25–26 [Google Scholar]
- Ingham DJ, Beer S, Money S, Hansen G (2001) Quantitative real-time PCR assay for determining transgene copy number in transformed plants. Biotechniques 31: 132–140 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T (1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol 14: 745–750 [DOI] [PubMed] [Google Scholar]
- Jacobs JM, Jacobs NJ, Sherman TD, Duke SO (1991) Effect of diphenyl ether herbicide on oxidation of protoporphyrinogen to protoporphyrin in organellar and plasma membrane enriched fractions of barley. Plant Physiol 97: 197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joersbo M, Donaldson I, Kreiberg J, Peterson S, Brunstedt J, Okkels F (1998) Analysis of mannose selection used for transformation of sugar beet. Mol Breed 4: 111–117 [Google Scholar]
- Joersbo M (2001) Advances in the selection of transgenic plants using non-antibiotic marker genes. Physiol Plant 111: 269–272 [DOI] [PubMed] [Google Scholar]
- Johnson MA, Volrath SL, Ward ER, inventors. January 25, 2000. Promoters from plant protoporphyrinogen oxidase genes. U.S. Patent Application No. 6,018,105
- Klein TM, Fromm M, Weissinger A, Tomes D, Schaaf S, Sletten M, Sanford JC (1988a) Transfer of foreign genes into intact maize cells with high-velocity microprojectiles. Proc Natl Acad Sci USA 85: 4305–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TM, Gradziel T, Fromm ME, Sanford JC (1988b) Factors influencing gene delivery into Zea mays cells by high-velocity microprojectiles. Bio/Technology 6: 559–563 [Google Scholar]
- Klein TM, Kornstein L, Sanford JC, Fromm ME (1989) Genetic transformation of maize cells by particle bombardment. Plant Physiol 91: 440–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T (1996) Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J 10: 165–74 [DOI] [PubMed] [Google Scholar]
- Lee HJ, Duke MV, Duke SO (1993) Cellular localization of protoporphyrinogen-oxidizing activities of etiolated barley (Hordeum vulgare L.) leaves (Relationship to mechanism of action of protoporphyrinogen oxidase-inhibiting herbicides). Plant Physiol 102: 881–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Lee SB, Chung JS, Han SU, Han O, Guh JO, Jeon JS, An G, Back K (2000) Transgenic rice plants expressing a Bacillus subtilis protoporphyrinogen oxidase gene are resistant to diphenyl ether herbicide oxyfluorfen. Plant Cell Physiol 41: 743–749 [DOI] [PubMed] [Google Scholar]
- Lermontova I, Grimm B (2000) Overexpression of plastidic protoporphyrinogen IX oxidase leads to resistance to the diphenyl-ether herbicide acifluorfen. Plant Physiol 122: 75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lermontova I, Kruse E, Mock HP, Grimm B (1997) Cloning and characterization of a plastidal and a mitochondrial isoform of tobacco protoporphyrinogen IX oxidase. Proc Natl Acad Sci USA 94: 8895–8900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18: 100–126 [Google Scholar]
- Lyznik LA, Ryan RD, Ritchie SW, Hodges TK (1989) Stable cotransformation of maize protoplasts with gusA and neo genes. Plant Mol Biol 13: 151–161 [DOI] [PubMed] [Google Scholar]
- Miller M, Tagliani L, Wang N, Berka B, Bidney D, Zhao ZY (2002) High efficiency transgene segregation in co-transformed maize plants using an Agrobacterium tumefaciens 2 T-DNA binary system. Transgenic Res 11: 381–396 [DOI] [PubMed] [Google Scholar]
- Minet M, Dufour ME, Lacroute F (1992) Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J 2: 417–422 [DOI] [PubMed] [Google Scholar]
- Molina A, Volrath S, Guyer D, Maleck K, Ryals J, Ward E (1999) Inhibition of protoporphyrinogen oxidase expression in Arabidopsis causes a lesionmimic phenotype that induces systemic acquired resistance. Plant J 17: 667–678 [DOI] [PubMed] [Google Scholar]
- Nandihalli UB, Duke SO (1993) The porphyrin pathway as a herbicide target site. In SO Duke, JJ Menn, JR Plimmer, eds, Pest Control with Enhanced Environmental Safety: American Chemical Society Symposium Series 524. American Chemical Society, Washington DC, pp 62–78
- Narita S, Taketani S, Inokuchi H (1999) Oxidation of protoporphyrinogen IX in Escherichia coli is mediated by the aerobic coproporphyrinogen oxidase. Mol Gen Genet 261: 1012–1020 [DOI] [PubMed] [Google Scholar]
- Narita S, Tanaka R, Ito T, Okada K, Taketani S, Inokuchi H (1996) Molecular cloning and characterization of a cDNA that encodes protoporphyrinogen oxidase of Arabidopsis thaliana. Gene, 182: 169–175 [DOI] [PubMed] [Google Scholar]
- Negrotto D, Jolley M, Beer S, Wenck AR, Hansen G (2000) The use of phosphomannose-isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via Agrobacterium transformation. Plant Cell Rep 19: 798–803 [DOI] [PubMed] [Google Scholar]
- Nishimura K, Taketani S, Inokuchi H (1995) Cloning of a human cDNA for protoporphyrinogen oxidase by complementation in vivo of a hemG mutant of Escherichia coli. J Biol Chem 270: 8076–8080 [DOI] [PubMed] [Google Scholar]
- Owen MK (2000) Current use of transgenic herbicide-resistant soybean and corn in the USA. Crop Protect 19: 765–771 [Google Scholar]
- Randolph-Anderson BL, Sato R, Johnson AM, Harris EH, Hauser CR, Oeda K, Ishige F, Nishio S, Gillham NW, Boynton E (1998) Isolation and characterization of a mutant protoporphyrinogen oxidase gene from Chlamydomonas reinhardtii conferring resistance to porphyric herbicides. Plant Mol Biol 38: 839–859 [DOI] [PubMed] [Google Scholar]
- Register JC, Peterson DJ, Bell PJ, Bullock WP, Evans IJ, Frame B, Greenland AJ, Higgs NS, Jepson I, Jiao S (1994) Structure and function of selectable and non-selectable transgenes in maize after introduction by particle bombardment. Plant Mol Biol 25: 951–961 [DOI] [PubMed] [Google Scholar]
- Rhodes CA, Pierce DA, Mettler IJ, Mascarenhas D, Detmer JJ (1988) Genetically transformed maize plants from protoplasts. Science 240: 204–207 [DOI] [PubMed] [Google Scholar]
- Sasarman A, Chartrand P, Lavoie M, Tardif D, Proschek R, Lapointe C (1979) Mapping of a new heme gene in Escherichia coli K12. J Gen Microbiol 113: 297–303 [DOI] [PubMed] [Google Scholar]
- Sasarman A, Letowski J, Czaika G, Ramirez V, Nead MA, Jacobs JM, Morais R (1993) Nucleotide sequence of the hemG gene involved in the protoporphyrinogen oxidase activity of Escherichia coli K12 Can J Microbiol 39: 1155–1161 [DOI] [PubMed] [Google Scholar]
- Sidorenko LV, Peterson T (2001) Transgene-induced silencing identifies sequences involved in the establishment of paramutation of the maize P1 gene. Plant Cell 13: 319–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman TD, Becerril JM, Matsmoto H, Duke MV, Jacobs JM, Jacobs NJ, Duke SO (1991) Physiological basis for differential sensitivities of plant species to protoporphyrinogen oxidase inhibitory herbicides. Plant Physiol 97: 280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyr YYJ, Hepburn AG, Windholm JM (1992) Glyphosate selected amplification of the 5-enolpyruvylshikimate-3-phosphate synthase gene in cultured carrot cells. Mol Gen Genet 232: 377–382 [DOI] [PubMed] [Google Scholar]
- Smith AG, Marsh O, Elder GH (1993) Investigation of the subcellular location of the tetrapyrrole-biosynthesis enzyme coproporphyrinogen oxidase in higher plants. Biochem J 292: 503–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TM, Gordon-Kamm WJ, Daines RJ, Start WG, Lemaux PG (1990) Bialaphos selection of stable transformants from maize cell culture. Theor Appl Genet 79: 625–631 [DOI] [PubMed] [Google Scholar]
- Theodoridis G, Bahr JT, Hotzman FW, Sehgel S, Suarez DP (2000) New generation of protox-inhibiting herbicides. Crop Protect 19: 533–535 [Google Scholar]
- Tomlin C (2000) The Pesticide Manual, Ed 12. The British Crop Protection Council, Alton, Hampshire, GU34, 2QD, UK
- Volrath SL, Johnson MA, Potter SL, Ward ER, Heifetz PB, inventors. February 8, 2000. DNA molecules encoding plant protoporphyrinogen oxidase. U.S. Patent Application No. 6,023,012
- Volrath SL, Johnson MA, Ward ER, Heifetz PB, inventors. August 17, 1999. DNA molecules encoding plant protoporphyrinogen oxidase and inhibitor-resistant mutants thereof. U.S. Patent Application No. 5,939,602
- Walters DA, Vetsch CS, Potts DE, Lundquist RC (1992) Transformation and inheritance of a hygromycin phosphotransferase gene in maize plants. Plant Mol Biol 18: 189–200 [DOI] [PubMed] [Google Scholar]
- Wang AS, Evans RA, Altendorf PR, Hanten JA, Doyle MC, Rosichan JL (2000) A mannose selection system for production of fertile transgenic maize plants from protoplasts. Plant Cell Rep 19: 654–660 [DOI] [PubMed] [Google Scholar]
- Warabi E, Usui K, Tanaka Y, Matsumoto H (2001) Resistance of a soybean cell line to oxyfluorfen by overproduction of mitochondrial protoporphyrinogen oxidase. Pest Manag Sci 57: 743–748 [DOI] [PubMed] [Google Scholar]
- Ward ER, Volrath SL, inventors. June 16, 1998. Manipulation of protoporphyrinogen oxidase enzyme activity in eukaryotic organisms. U.S. Patent Application No. 5,767,373
- Watanabe N, Che F, Iwano M, Takayama S, Nakano T, Yoshida S, Isogai A (1998) Molecular characterization of photomixotrophic tobacco cells resistant to protoporphyrinogen oxidase-inhibiting herbicides. Plant Physiol 118: 751–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Takayama S, Yoshida S, Isogai A, Che FS (2002) Resistance to protoporphyrinogen oxidase-inhibiting compound S23142 from overproduction of mitochondrial protoporphyrinogen oxidase by gene amplification in photomixotrophic tobacco cells. Biosci Biotechnol Biochem 66: 1799–1805 [DOI] [PubMed] [Google Scholar]
- Witkowski DA, Halling BP (1989) Inhibition of plant protoporphyrinogen oxidase by the herbicide acifluorfen-methyl. Plant Physiol 90: 1239–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M, Dawson J, Dunder E, Suttie J, Reed J, Kramer C, Chang Y, Novitzky R, Wang H, Artim-Moore L (2001) Efficient biolistic transformation of maize (Zea mays L.) and wheat (Triticum aestivum L.) using the phosphomannose isomerase gene, pmi, as the selectable marker. Plant Cell Rep 20: 429–436 [DOI] [PubMed] [Google Scholar]
- Wright TR, Fuerst EP, Ogg AG, Handihall UB, Lee HJ (1995) Herbicide activity of UCC-C4243 and acifluorfen is due to inhibition of protoporphyrinogen oxidase. Weed Sci 43: 47–54 [Google Scholar]