Abstract
The ascorbate content of plants is usually increased in high light (HL), implying a function for ascorbate in the acclimation of plants to HL. Nevertheless, the importance of ascorbate in HL acclimation has not yet been tested directly. Here, we report on the acclimation process of an ascorbate-deficient Arabidopsis mutant to HL. The mutant vtc2 has only 10% to 30% of wild-type levels of ascorbate, and it is also slightly deficient in feedback de-excitation (qE), a photoprotective mechanism that causes the dissipation of excess light as heat. The vtc2 mutant was unable to acclimate to HL, when transferred from low light to HL. Its mature leaves bleached, and it showed an increased degree of lipid peroxidation and photoinhibition. In parallel, we tested the photosensitivity of an ascorbate-deficient xanthophyll cycle mutant, vtc2npq1, which also lacks zeaxanthin and nearly all qE. The double mutant bleached sooner and had higher degrees of lipid peroxidation and photoinhibition than the vtc2 mutant. This was in contrast to the npq1 single mutant that showed only slight deviations from the wild-type phenotype under the conditions used. These results demonstrate the antioxidant role of ascorbate in the acclimation process to HL and point to the relative importance of ascorbate in comparison with other photoprotective processes, such as specific xanthophylls or feedback de-excitation. The results also provide further support for the proposed role of zeaxanthin as an antioxidant and lipid stabilizer.
Plants have evolved several mechanisms to protect themselves from excess absorbed light energy. One important set of mechanisms against excess light are antioxidants and antioxidant enzymes, which scavenge or quench dangerous reactive oxygen species (ROS) that are produced as a result of excess light. Antioxidant enzymes include ascorbate peroxidase (APX), superoxide dismutase (SOD), and 2-Cys peroxiredoxin (2-CP). In the chloroplast, SOD and APX work together in the so-called water-water cycle to reduce superoxide, which is produced by reduction of oxygen to water by photosystem (PS) I. The water-water cycle is considered to be an important protective mechanism that also increases the electron transport rate (ETR) and dissipates excess energy (Asada, 1999). Chloroplast 2-CP belongs to a ubiquitous group of enzymes, alkyl hydroperoxide reductases, which reduce hydrogen peroxide and alkyl hydroperoxides (Baier and Dietz, 1999; Dietz et al., 2002). It is a stromal enzyme that is localized at thylakoid membranes (König et al., 2002), and it can reduce lipid hydroperoxides that are generated as a result of lipid peroxidation chain reactions (Dietz et al., 2002). Major antioxidants in the plant are the water-soluble molecules ascorbate and glutathione, which are both found in millimolar concentrations (Noctor and Foyer, 1998), and the lipid-soluble tocopherols and carotenoids.
Ascorbate is required for the production of zeaxanthin (Z) in the xanthophyll cycle as a cofactor for violaxanthin (V) de-epoxidase (VDE) and APX and plays an important role in modulating the cell cycle by stimulating cell division and by accelerating cell expansion and cell elongation (Horemans et al., 2000). It is also required for the synthesis of Hyp-rich glycoproteins such as extensins, which are components of the cell wall. Ascorbate is believed to be involved in the recycling of tocopherols (Fryer, 1992) and is itself re-reduced by dehydroascorbate reductase, which uses glutathione (Noctor and Foyer, 1998), by monodehydrosacorbate reductase, which uses NADPH, or directly by PSI via reduced ferredoxin. Thirty to 40% of the cell's ascorbate is found in the chloroplast (Foyer et al., 1983), suggesting its importance for the protection against photooxidative stress originating there. To date, no viable mutant that totally lacks ascorbate is known. A knockout of the biosynthetic enzyme gene GDP-Man pyrophosphorylase, cyt1, is an embryo-lethal mutation (Lukowitz et al., 2001). Several ascorbate-deficient mutants of Arabidopsis that have decreased levels of ascorbate (vtc1–vtc4) have been described (Conklin et al., 1996, 2000).
Another important mechanism that protects against excess light is the harmless dissipation of light energy as heat, known as feedback de-excitation or the qE component of non-photochemical quenching of chlorophyll (Chl) fluorescence. qE requires the build-up of a proton gradient across the thylakoid membrane (Müller et al., 2001), the presence of the PSII protein PsbS (Li et al., 2000), and the conversion of V to antheraxanthin (A) and Z through the xanthophyll cycle (Demmig-Adams et al., 1989). The npq1 mutant of Arabidopsis is deficient in the enzyme VDE that is responsible for the conversion of V to A and Z, and therefore it lacks Z in high light (HL) and is deficient in qE (Niyogi et al., 1998). The npq1 mutant is more sensitive to photoinhibition and lipid peroxidation than the wild type when transferred from low light (LL) to HL, but it is able to survive in HL (Havaux and Niyogi, 1999). qE has been shown to be very important for short-term photoprotection (Li et al., 2002) and plant fitness in the field (Külheim et al., 2002) by preventing over-reduction of the electron transport acceptors. Transgenic plants that have a higher qE capacity are more tolerant of short-term HL stress than the wild type (Li et al., 2002). It is still unclear if qE is also important for longer-term protection against HL and for HL acclimation.
Ascorbate has previously been found at higher levels in plants grown under full sunlight (Grace and Logan, 1996; Eskling and Åkerlund, 1998) and has therefore been implicated in HL acclimation. HL acclimation is a complex process involving many different responses, including changes in morphology, leaf anatomy, and chloroplast composition. In the present study we examined the importance of ascorbate in the acclimation process of Arabidopsis to HL during a 5-d experiment using the Arabidopsis mutant vtc2. The vtc2 mutant, which produces only 10% to 30% of wild-type ascorbate (Jander et al., 2002; Müller-Moulé et al., 2002), was originally isolated due to its sensitivity to ozone (Conklin et al., 1996). The VTC2 gene has been cloned (Jander et al., 2002), but its function is still unclear. The vtc2 mutant is also slightly qE-deficient (Noctor et al., 2000; Smirnoff, 2000), probably due to substrate limitation of V deepoxidase (Müller-Moulé et al., 2002), the enzyme required for the production of Z. We also investigated the role of ascorbate in the acclimation of npq1 to HL by testing the vtc2npq1 double mutant for acclimation to HL. In this paper, we show that ascorbate is required for HL acclimation of leaves that developed in LL, and that a lack of Z makes ascorbate-deficient plants even more susceptible to HL stress.
RESULTS
Ascorbate-Deficient Mutants Bleached within 2 d after Transfer to HL
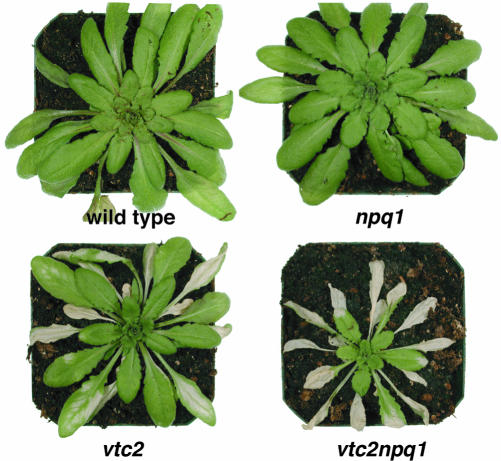
Plants were transferred to HL after 6 weeks of growth in LL. At this age, wild-type and the npq1 mutant plants were of the same size, whereas vtc2 and vtc2npq1, which had only about 20% of wild-type ascorbate levels (Müller-Moulé et al., 2002), were smaller in size and also had fewer leaves. This difference in growth has been also shown for the vtc1 mutant and is likely the result of a higher abscisic acid content (Pastori et al., 2003). Upon transfer from LL to HL, the vtc2npq1 double mutant showed obvious bleaching after only 1 d in HL (Fig. 1). After 2 d, all mature leaves of the double mutant were bleached. Bleaching occurred more slowly in the vtc2 mutant, and the npq1 mutant and the wild type did not exhibit any obvious bleaching during the entire experiment (5 d). Because vtc2 and vtc2npq1 bleached so extensively, there was insufficient mature leaf material left for sampling after the 2nd d. Therefore, all data shown are lacking d 3, 4, and 5 for the two ascorbate-deficient mutants. Interestingly, young leaves that were not yet fully developed did not bleach.
Figure 1.
Phenotype of wild-type and mutant Arabidopsis plants after 2.5 d in HL.
The Bleaching of Ascorbate-Deficient Mutants Was Paralleled by an Increase in Lipid Peroxidation
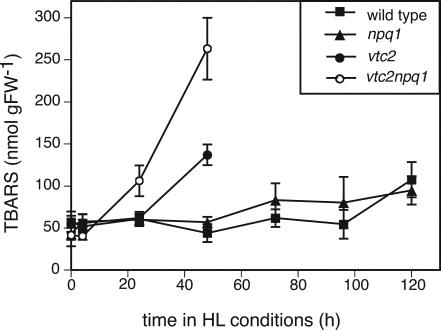
The vtc2 and vtc2npq1 mutants also showed a dramatic increase in lipid peroxidation. The vtc2npq1 mutant showed higher degrees of lipid peroxidation with the thiobarbituric acid reactive substances (TBARS) assay after only 1 d, whereas vtc2 plants had higher levels after 2 d (Fig. 2). The double mutant exhibited twice as much lipid peroxidation as vtc2. There was no clear increase in lipid peroxidation for the wild type and the npq1 mutant during the course of the experiment.
Figure 2.
Lipid peroxidation measured as TBARS. Data are the means ± se (n = 6–9). HL conditions consisted of cycles of 10 h of HL (1,800 μmol photons m–2 s–1) followed by 14 h of dark. Controls that remained in LL for 6 d showed higher values than the LL plants at t = 0, probably due to aging. The values after 6 d in LL were 63.8 ± 9.7 (wild type), 57.2 ± 10.5 (npq1), 76.1 ± 9.5 (vtc2), and 65.0 ± 8 (vtc2npq1).
Lipid peroxidation was also measured using a thermoluminescence technique. In plant leaves, lipid peroxidative damage is associated with two thermoluminescence bands peaking at 80°C to 90°C and 135°C to 140°C (Hideg and Vass, 1993; Vavilin and Ducruet, 1998), and the amplitude of the latter band was used in this study as an index of lipid peroxidation in Arabidopsis leaves. This signal is believed to originate mostly from lipid cycloperoxides that are broken during heating, resulting in the formation of triplet carbonyl species, which emit photons when they return to the ground state (Vavilin and Ducruet, 1998). Photons can also be emitted via Chl after excitation transfer from the triplet carbonyls (Hideg and Vass, 1993). Leaves taken from LL-grown plants were transferred to HL of 1,000 μmol photons m–2 s–1 at 9 ± 1°C for up to 25 h. A lower temperature was used to increase the light stress.
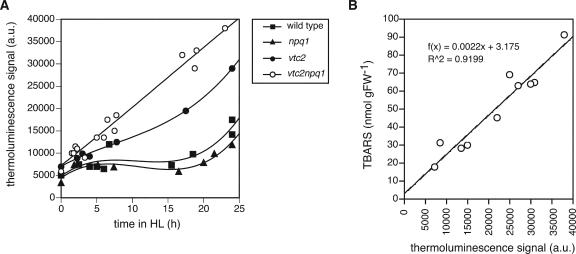
As can be seen in Figure 3A, the four genotypes showed the same differences in degree of lipid peroxidation as were observed in the previous experiment (Fig. 2). The wild type and the npq1 mutant showed a slight increase in lipid peroxidation only after 25 h, whereas both ascorbate-deficient mutants showed much stronger lipid peroxidation already at early time points. The double mutant in particular showed a doubling of lipid peroxidation after only 2.5 h. To ensure that the TBARS and thermoluminescence measurements correlated, TBARS were measured simultaneously with thermoluminescence in this experiment. Figure 3B shows that the TBARS and thermoluminescence measurements for vtc2npq1 were correlated, and that TBARS therefore can be used as an estimate of lipid peroxidation in Arabidopsis. It should be mentioned though that this strong correlation was not found for samples that were extensively bleached, possibly because the thermoluminescence measurements depend on Chl content (Vavilin et al., 1998).
Figure 3.
Lipid peroxidation in response to HL and chilling stress. Leaves were harvested from LL grown plants and exposed to 1,000 μmol photons m–2 s–1 and 9 ± 1°C. A, Thermoluminescence measurement of lipid peroxidation. Data were fitted with a smooth function to best depict differences between genotypes. B, Correlation between TBARS measurement of vtc2npq1 mutant leaves and thermoluminescence in the same experiment.
Ascorbate-Deficient Mutants Were also More Photoinhibited in HL
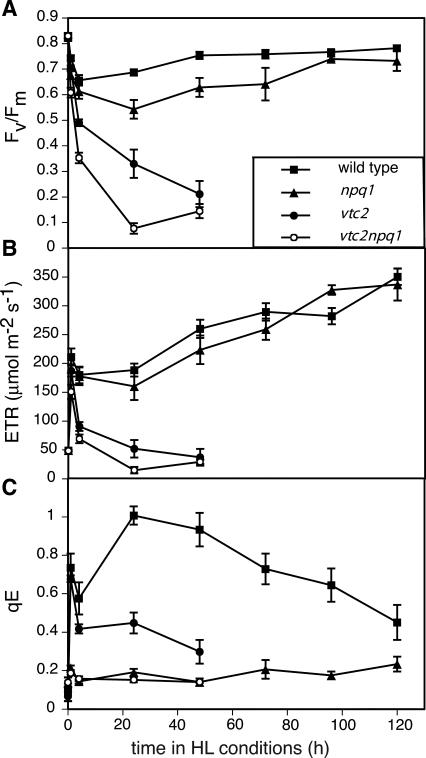
In the first 4 h, all genotypes showed a rapid decrease in Fv/Fm, a Chl fluorescence parameter that is commonly used to measure photoinhibition (Fig. 4A). Fv/Fm continued to decrease drastically in the vtc2npq1 mutant during the 1st d of HL, and also substantially in the vtc2 mutant. After 1 d of HL, the vtc2npq1 mutant had an Fv/Fm value of less than 0.1, whereas the vtc2 mutant still showed a value of 0.35. After 2 d in HL, both ascorbate-deficient mutants had the same low level of Fv/Fm. In contrast, the decline in Fv/Fm was stabilized in the npq1 mutant and the wild type, which did not show any further decrease in Fv/Fm after the 1st d of HL, but rather showed recovery during the next few days of the experiment. No increase in the Fo value were apparent in any genotypes showing that the effects on PSII activity were likely not due to heat stress.
Figure 4.
Chl fluorescence parameters. Data are the means ± se (n = 9). HL conditions consisted of cycles of 10 h of HL (1,800 μmol photons m–2 s–1) followed by 14 h of dark. ETR and qE were measured under the actual incident photon flux density of LL (160 μmol photons m–2 s–1 at t = 0) or HL. Controls that remained in LL for 6 d were within the se of the initial LL mean and/or differed by less than 4% from the initial LL value. A, Fv/Fm. B, ETR. C, qE.
The ETR was estimated from Chl fluorescence parameters (Genty et al., 1989). All four genotypes showed an initial increase in their ETR when plants were transferred to HL due to the higher supply of photons (Fig. 4B). The wild type and the npq1 mutant maintained a higher ETR in the HL than in LL, and it increased even more during the course of the experiment. The two ascorbate-deficient mutants, on the other hand, showed a decline in ETR as early as after 1 h in HL. After 1 d in HL, the vtc2 mutant had as low an ETR as in LL, and in the vtc2npq1 mutant, ETR had declined below that in LL.
As expected, the npq1 and the vtc2npq1 mutant exhibited only a very low level of qE (approximately 0.2) when transferred to HL (Fig. 4C). The wild type, on the other hand, showed a maximum qE of 1.0 after 1 d in HL, after which qE started to decrease again. The vtc2 mutant had less qE than the wild type, with the highest values after 1 h in HL. The vtc2 mutant did not show any further increases in qE during the next 2 d in HL.
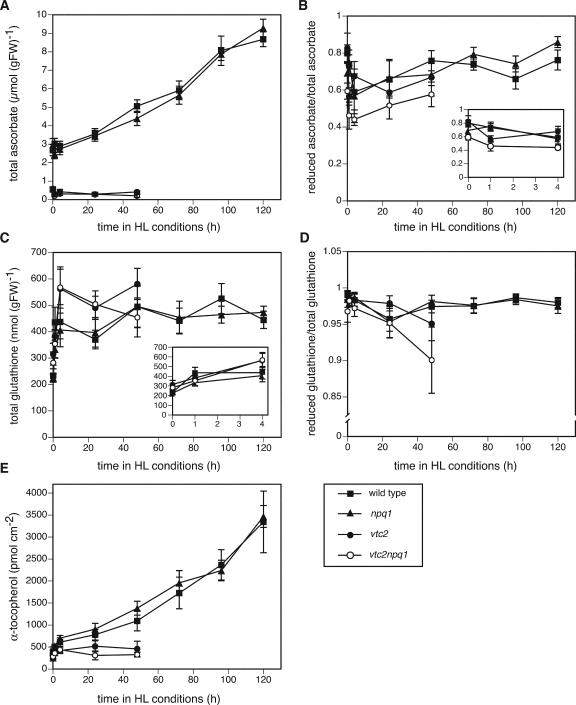
The Antioxidant Content Increased in the Wild Type and the npq1 Mutant in HL
The ascorbate content of wild type and npq1 started to increase within 1 d in HL, from 2.8 μmol g–1 fresh weight in LL to 9 μmol –1 fresh weight after 5 d in HL (Fig. 5A). In LL, the vtc2 and vtc2npq1 mutants had only about 20% of the ascorbate found in wild type and npq1, and these two mutants showed no change in ascorbate content during the first 2 d of HL. There was no major change in the reduction state of ascorbate in the wild type and the npq1 mutant, but there was a small decrease after 4 h and 1 d in HL, which showed recovery after 2 d in HL (Fig. 5B). The ascorbate pool in the vtc2 mutant was as reduced as that of the wild type in LL, but it was more oxidized after only 1 h in HL and never recovered to LL levels. In LL, the vtc2npq1 double mutant had already a more oxidized pool, which became even more oxidized after only 1 h in HL.No recovery occurred during the first 2 d of the treatment.
Figure 5.
Antioxidant levels and redox states. Data are the means ± se (A and B, n = 6–12; C, n = 6; D and E, n = 6–9). HL conditions consisted of cycles of 10 h of HL (1,800 μmol photons m–2 s–1) followed by 14 h of dark. A, Total ascorbate content. Wild-type and vtc2npq1 controls that remained in LL for 6 d showed higher values than the LL plants at t = 0. The values after 6 d in LL were 3.2 ± 0.3 (wild type) and 0.63 ± 0.1 (vtc2npq1). B, Ascorbate redox state. The values after 6 d in LL differed substantially from the LL plants at t = 0, with the ascorbate-deficient mutants showing a more oxidized pool. The values after 6 d in LL were 0.73 ± 0.04 (wild type), 0.86 ± 0.05 (npq1), 0.54 ± 0.07 (vtc2), and 0.41 ± 0.08 (vtc2npq1). C, Total glutathione. Controls that remained in LL for 6 d showed higher values than the LL plants at t = 0. The values after 6 d in LL were 257 ± 17 (wild type), 302 ± 21 (npq1), 323 ± 19 (vtc2), and 356 ± 29 (vtc2npq1). D, Glutathione redox state. All LL controls measured after the experiment differed less than 4% from the initial LL value. E, α-Tocopherol. All LL controls measured after the experiment were within the se of the initial LL mean.
Interestingly, glutathione levels in the wild type and the npq1 mutant increased only during the first 4 h in HL (Fig. 5C), whereas the redox state was not significantly affected (Fig. 5D). The ascorbate-deficient mutants also showed a significant increase in glutathione during the first 4 h of HL, after which it remained stable (vtc2) or decreased (vtc2npq1). The redox state of glutathione was similar to that of the wild type except in the vtc2npq1 mutant, which showed a decrease in reduction state after 2 d in HL.
The wild type and the npq1 mutant also exhibited an increase in α-tocopherol content, paralleling the increase in ascorbate content, after only 4 h in HL, reaching a 7-fold higher level than in LL after 5 d HL (Fig. 5E). In contrast, neither ascorbate-deficient mutant showed an increase in α-tocopherol.
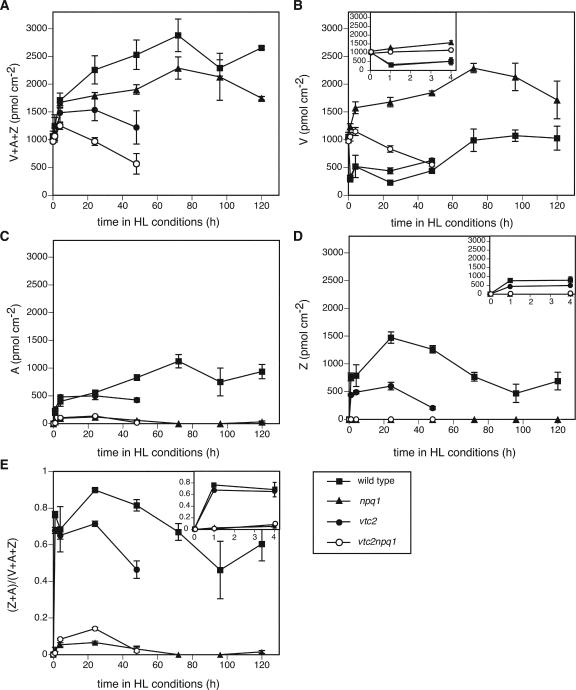
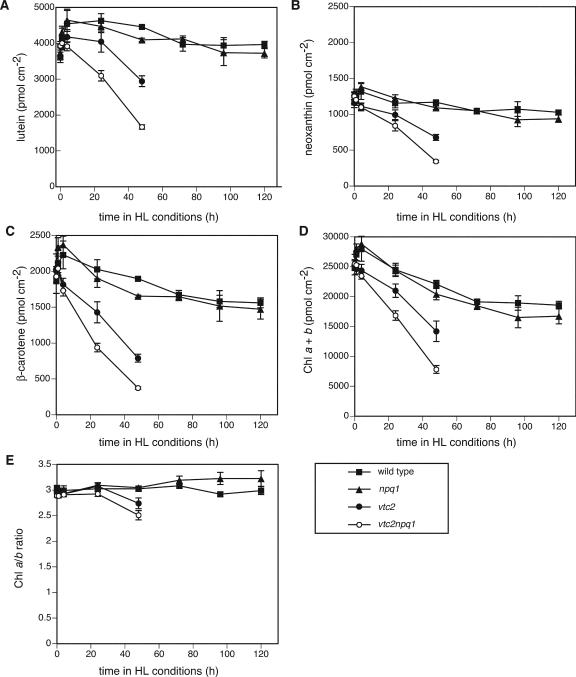
All genotypes showed the same pigment phenotype in LL (Figs. 6 and 7), with the exception of vtc2npq1, which had slightly lower Chl a/b ratios. After 1 h in HL, the wild type, which has a functional xanthophyll cycle, showed the typical decrease in V (Fig. 6B) and increase in A, Z, and the de-epoxidation state (Fig. 6, B–E). The vtc2 mutant also showed these changes, but to a lesser extent due to its lower ascorbate content (Müller-Moulé et al., 2002). For instance, the wild type had 90% of its xanthophyll cycle pool de-epoxidized after 1 d in HL, whereas the vtc2 mutant only had 71% de-epoxidized. As expected, the npq1 and the vtc2npq1 mutants did not show any changes in the xanthophyll cycle pigments due to their impaired VDE gene. During the exposure to HL, both the wild type and the npq1 mutant showed a progressive increase in their xanthophyll cycle pool size, whereas the ascorbate-deficient mutants showed a decrease in the pool due to bleaching (Fig. 6A). This decrease was more pronounced in the vtc2npq1 mutant than in the vtc2 mutant and could also be seen for each individual xanthophyll (V, A, and Z; the latter two only in the vtc2 mutant). The pigment bleaching was also apparent in the levels of lutein, neoxanthin, β-carotene, and total Chl (Fig. 7, A–D). Again, the decrease in pigments was more pronounced for the vtc2npq1 mutant than for the vtc2 mutant. The wild type and the npq1 mutant showed significant decreases in β-carotene and Chl (Fig. 7, C and D) in HL, but levels of lutein and neoxanthin remained relatively stable (Fig. 7, A and B). Interestingly, the Chl a/b ratio decreased in the two ascorbate-deficient mutants during the first 2 d of the experiment but showed a slight increase in the wild type and the npq1 mutant after 5 d in HL (Fig. 7E).
Figure 6.
Levels of xanthophyll cycle pigments. Data are the means ± se (n = 6 except for n = 4 for 4-d time point). HL conditions consisted of cycles of 10 h of HL (1,800 μmol photons m–2 s–1) followed by 14 h of dark. A, Xanthophyll cycle pool size (V+A+Z). B, V. C, A. D, Z. E, De-epoxidation state. For A and B, all LL controls measured after the experiment were within the se of the LL mean. and/or differed by 4% or less from the LL value. For C, D, and E, controls that remained in LL for 6 d were unchanged (0).
Figure 7.
Levels of other pigments. Data are the means ± se (n = 6 except for n = 4 for 4 d time point). HL conditions consisted of 10 h of HL (1,800 μmol photons m–2 s–1) followed by 14 h of dark. A, Lutein. Wild-type and npq1 controls that remained in LL for 6 d showed higher values than the LL plants at t = 0. The values after 6 d in LL were 3,938 ± 249 (wild type) and 4,236 ± 170 (npq1). B, Neoxanthin. Wild-type and npq1 controls that remained in LL for 6 d showed higher values than the LL plants at t = 0. The values after 6 d in LL were 1,289 ± 70 (wild type) and 1,425 ± 43 (npq1). C, β-Carotene. npq1 and vtc2npq1 controls that remained in LL for 6 d showed higher values than the LL plants at t = 0. The values after 6 d in LL were 2,365 ± 162 (npq1) and 2,079 ± 76 (vtc2npq1). D, Total Chl. Wild-type, npq1, and vtc2npq1 controls that remained in LL for 6 d showed higher values than the LL plants at t = 0. The values after6dinLL were 26,854 ± 1,671 (wild type), 28,465 ± 1,302 (npq1), and 25,131 ± 902 (vtc2npq1). E, Chl a/b ratio. Controls that remained in LL for 6d differed by less than 3% from the initial LL value.
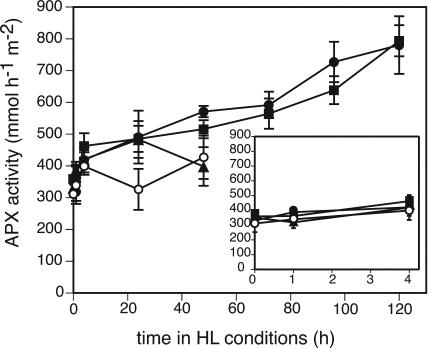
Antioxidant Enzymes Showed an Increase in Protein Levels or Activity in HL
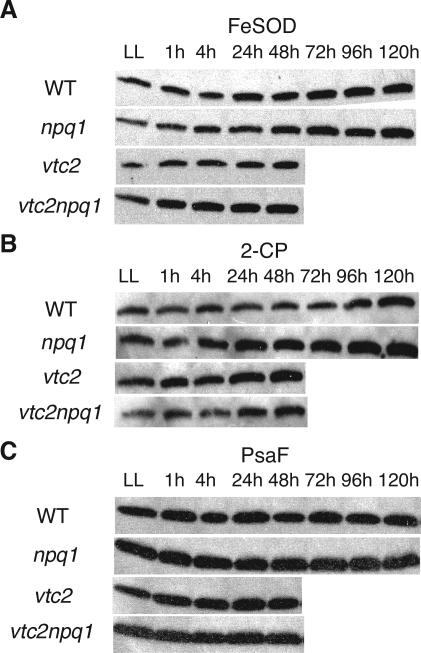
APX activity increased for all genotypes after transfer to HL (Fig. 8). There was no difference between the genotypes with regard to APX activity in LL or during the first 4 h of the treatment. After 1 d in HL, APX activity of vtc2npq1 was significantly lower than the activities of the wild type, vtc2, and npq1. After 2 d in HL, the APX activity of vtc2 was as low as that of vtc2npq1. Total SOD activity did not change during the course of the experiment, although the data were somewhat variable (data not shown). Plants possess several isozymes of SOD. Arabidopsis has iron (Fe) SOD isozymes and one copper/zinc (CuZn) SOD isozyme in the chloroplast, another CuZnSOD in the cytosol, and a manganese (Mn) SOD in the mitochondria (Kliebenstein et al., 1998). Because the protein level of chloroplastic Cu/ZnSOD has been shown to be unaffected by HL treatment (Kliebenstein et al., 1998), only the protein levels of FeSOD were tested. FeSOD levels increased slightly in the wild type after 3 d in HL, and even more in the npq1 mutant, which already showed an increase after 2 d in HL (Fig. 9A). The ascorbate-deficient mutants did not show a significant increase in FeSOD levels within 2 d, although a very small increase was apparent after 1 h. All genotypes showed also an increase in protein levels of 2-CP, with all genotypes but the wild type showing an increase after only 1 d in HL. The wild type showed an increase in 2-CP levels after 4 d in HL (Fig. 9B). The protein levels of PsaF, a PSI protein, which was expected to be stable in the wild type, were also determined (Fig. 9C). PsaF levels stayed constant during the whole experiment, and no differences were observed among the four genotypes (Fig. 9C).
Figure 8.
Total APX activity. Data are the means ± sd (n = 3). HL conditions consisted of cycles of 10 h of HL (1,800 μmol photons m–2 s–1) followed by 14 h of dark. Symbols are the same as in Figures 2, 3, 4, 5, 6, 7. Controls that remained in LL for 6 d were within the sd of the initial LL mean.
Figure 9.
Immunoblot analysis of protein levels. HL conditions consisted of cycles of 10 h of HL (1,800 μmol photons m–2 s–1) followed by 14 h of dark. A, FeSOD. B, 2-CP. C, PsaF. Equal amounts of protein were loaded in each lane. A different membrane was used for each genotype. Representative blots from one of two experiments are shown.
DISCUSSION
Wild-Type Mutant Plants Acclimated Well to HL
Wild-type Arabidopsis plants grown in LL were able to acclimate to HL conditions. They showed a transient decrease in Fv/Fm (Fig. 4A) and a small increase in lipid peroxidation after 5 d (Fig. 2), but otherwise no visible damage (Fig. 1). Plants also acclimated by decreasing their antenna (total Chl decreased, Chl a/b ratio increased; Fig. 7, D and E) and increasing their ETR (Fig. 4B), ascorbate (Fig. 5A), glutathione (Fig. 5C), α-tocopherol content (Fig. 5E), and xanthophyll cycle pool size (Fig. 6A). Because the fresh weight per area of the leaves increased by approximately 50% during the course of the experiment, the changes in ascorbate and glutathione, which are expressed on a fresh weight basis (Fig. 5, A and C), would be even more pronounced if expressed on a per area basis. All of these changes have previously been reported during HL acclimation in other species (Grace and Logan, 1996; Leipner et al., 1997; Munné-Bosch and Alegre, 2000). In contrast to findings with other plant species (Grace and Logan, 1996), the amount of glutathione did not increase as much in response to HL in Arabidopsis (Fig. 5C). Two of the three species measured in the abovementioned study increased their glutathione content two to three times, whereas one of the species examined showed an increase more comparable with that reported here, from 450 to 600 nmol g–1 fresh weight (Grace and Logan, 1996). Other species, such as beech (Fagus spp.), do not show a difference in glutathione content between sun and shade leaves but showed a 2-fold higher content of ascorbate in sun leaves (Polle, 1997). Interestingly, no significant changes in oxidation states of the ascorbate and the glutathione pools were found (Fig. 5, B and D), although both the ascorbate and the glutathione redox state have been implicated in signaling light stress (Noctor and Foyer, 1998).
Plants increased their antioxidant capacity also by increasing their antioxidant enzyme content. Total APX activity levels increased already after 1 h and by more than 2-fold after 5 d in HL (Fig. 8), and a small increase in FeSOD protein levels was also seen (Fig. 9A). An increase in FeSOD protein level in response to HL has been reported previously (Kliebenstein et al., 1998). Higher expression of mRNAs for stromal APX, cytosolic APX1, peroxisomal APX3, and chloroplastic FeSOD3 after 4 h in HL was also apparent in a DNA microarray experiment using Arabidopsis ecotype Col and various npq mutants (T. Golan and K.K. Niyogi. unpublished data). In spinach (Spinacia oleracea), cytosolic APX was the only isozyme to show increased mRNA expression in response to HL (Yoshimura et al., 2000). 2-CP levels also increased in Arabidopsis in response to HL (Fig. 9B), consistent with a previous report (Horling et al., 2003). All of the enzymes found to be increased after transfer to HL (APX, FeSOD, and 2-CP) are important for the scavenging of ROS produced in the chloroplast. In addition, APX and 2-CP are both involved in dissipating excess light energy through two alternative water-water cycles (Asada, 1999; Dietz et al., 2002).
The npq1 Mutant Was Slightly More Sensitive to HL Than the Wild Type
The npq1 mutant behaved similarly to the wild type, but was somewhat more photoinhibited and showed lipid peroxidation slightly earlier than the wild type. No differences were found in regard to ETR, antioxidant, or pigment levels (Figs. 4,5,6,7), except for the lack of V de-epoxidation (Fig. 6). Compared with the wild type, the npq1 mutant showed a faster increase in 2-CP levels (Fig. 9B). This increase in 2-CP might be necessary to compensate for the lack of Z, which functions as an antioxidant (Havaux, 1998; Havaux and Niyogi, 1999; Baroli et al., 2003). A decrease in Z might cause a higher rate of lipid peroxidation that is counteracted by a higher rate of lipid peroxide reduction through increased levels of 2-CP.
The small difference in photosensitivity between the wild type and the npq1 mutant is somewhat different from a previous result, where the npq1 mutant showed more pronounced lipid peroxidation than the wild type when transferred to HL (Havaux and Niyogi, 1999). In contrast to the previous experiment, the photoperiod in this experiment was not changed after transfer to HL, and plants were treated with a 10-h light period instead of a 15-h light period. The smaller total daily photon exposure (micromoles of photons per square meter per second times the duration of light) is a likely reason why the npq1 mutant was better able to acclimate to HL in this experiment. The increased sensitivity of the npq1 mutant to HL in the previous experiment was found to be due mainly to the lack of Z rather than to the lack of qE (Havaux and Niyogi, 1999). Both experiments therefore indicate that the role of qE in long-term photoprotection in Arabidopsis is relatively minor, because qE-deficient plants could acclimate to HL. qE appears to be most important during short-term changes in light intensity (Külheim et al., 2002; Li et al., 2002).
Ascorbate-Deficient Mutants Experienced Significant Photodamage in HL
In contrast to the npq1 mutant, the ascorbate-deficient mutants were much more sensitive to the HL. The vtc2 and vtc2npq1 mutants showed extensive bleaching (Figs. 1, 6, and 7), and higher degrees of lipid peroxidation (Figs. 2 and 3A) and photoinhibition (Fig. 4A) than the wild type or the npq1 mutant. All (vtc2npq1) or most (vtc2) mature leaves were fully bleached and dried up by the 3rd d of HL, making it impossible to collect tissue at later time points. The vtc2 and vtc2npq1 mutants also had lowered ETR in HL instead of the increase seen in the other two genotypes (Fig. 4B). This could be due to a low operation of the Calvin-Benson cycle due to inactivation of several H2O2-sensitive enzymes, such as Rubisco, glyceraldehyde-3-dehydrogenase, and Fru bisphosphatase (Asada, 1994). Interestingly, the levels of the PSI protein PsaF (on a protein basis) were not affected (Fig. 9C), even though the leaves experienced drastic bleaching. This result is difficult to explain, but it is possible that the decrease in PsaF was paralleled by a decrease in the total protein content. Rubisco, which accounts for a large amount of the total protein, declines preferentially during senescence (Matile, 2001).
Which of the many functions of ascorbate might explain the HL sensitivity of the ascorbate-deficient mutants? It most likely was not the lowered amount of Z (Fig. 6D), due to substrate limitation of VDE (Müller-Moulé et al., 2002), that caused the HL sensitivity, because the npq1 mutant did not show any bleaching in this experiment, whereas vtc2 did. It also seems unlikely that the cell cycle plays a direct role in acclimation, at least in the fully developed mature leaves of Arabidopsis that were measured. Ascorbate can function as an electron acceptor for PSI in the water-water cycle, thereby increasing ETRs. The role of ascorbate in the water-water-cycle is closely connected with its role as an antioxidant, and therefore it seems most likely that the HL sensitivity of the ascorbate mutants is due to the roles of ascorbate as an antioxidant, and possibly also due to its role as electron transport acceptor in the water-water cycle. The ascorbate-deficient mutants might not have had the necessary protective antioxidant content required to counteract the increased generation of ROS after transfer to the HL. The ROS therefore would cause more damage to PSII and destroy more pigments and lipids than in the wild type. This damage was evident as decreases in Fv/Fm (Fig. 4A) and ETR (Fig. 4B) and increases in lipid peroxidation (Fig. 2). An increase in oxidative stress due to the lack of ascorbate was also apparent in a different study looking at water-stressed Arabidopsis plants (Munné-Bosch and Alegre, 2002), strongly supporting the hypothesis that it is the role of ascorbate as an antioxidant that made the plants more sensitive to stresses.
Plants generally respond to an increase in light intensity and the subsequent rise in ROS by increasing the ascorbate and the xanthophyll cycle pool sizes (Grace and Logan, 1996; Logan et al., 1998) and in some species, the α-tocopherol content (Leipner et al., 1997; Munné-Bosch and Alegre, 2000), as was the case in this experiment for the wild type and the npq1 mutant (Fig. 5E). For the ascorbate-deficient mutants, however, no increase in ascorbate, α-tocopherol (Fig. 5), or the xanthophyll cycle pool (Fig. 6A) occurred within the first 2 d of HL. In water-stressed vtc1 plants, a decrease in α-tocopherol content was seen (Munné-Bosch and Alegre, 2002). The lack of increase in α-tocopherol could be either due to the photooxidation of tocopherols or to the inability of the ascorbate-deficient mutants to efficiently regenerate oxidized α-tocopherol. An inability to regenerate α-tocopherol would render the plants even more susceptible to HL or other stresses that produce ROS in the thylakoid membrane.
A mutant totally devoid of all tocopherols, vte1, on the other hand shows no apparent growth defect under optimal conditions (Porfirova et al., 2002). Even under HL conditions (850 μmol photons m–2 s–1), this mutant only showed small reductions in Chl content and photosynthetic quantum yield. This study, however, used HL of only one-half the intensity as was used in our experiments, and levels of other antioxidants were not determined. It is therefore possible that other antioxidants or photoprotective mechanisms are up-regulated in this mutant. In a very recent study, chlP transgenic tobacco (Nicotiana tabacum) plants, which only have half of the tocopherol levels of wild type, were more sensitive to a treatment with cold and HL (Havaux et al., 2003). Both of these experiments underscore a role for tocopherol in maintaining photosynthesis under HL stress.
The ascorbate-deficient mutants showed a rapid oxidation of the ascorbate pool within 1 h of HL (Fig. 5B) but showed a difference in regard to the redox state of the glutathione pool only after 2 d in HL (Fig. 5D). It is conceivable that the ascorbate pool was oxidized more rapidly than in the wild type because of the smaller ascorbate pool size. Interestingly, an oxidation of the pool was also observed during aging (see legend to Fig. 5). APX levels increased in the two ascorbate-deficient mutants, but this increase was smaller than in the wild type or the npq1 mutant after 1 d (vtc2npq1) or 2 d (vtc2) in HL. This is probably due to the fact that some regions of the leaf were already bleached, whereas other regions still remained functional and were able to increase APX activity. The ascorbate-deficient mutants also showed an increase in 2-CP levels after 1 d in HL, similar to the npq1 mutant (Fig. 9).
Interestingly, young, developing leaves of both ascorbate-deficient mutants did not show any signs of bleaching (Fig. 1), suggesting that young leaves have a higher capacity for photoprotection. Because leaf temperatures of young and mature leaves of the vtc2npq1 mutant were similar (data not shown), this phenomenon cannot be explained by differences in evaporative cooling between young and mature leaves. However, one likely cause for the increased protection is an increased antioxidant content. For example, young leaves of the npq1 mutant have been shown to be more resistant to HL or oxidative stress, and these leaves had higher levels of α-tocopherol than older, mature leaves (Havaux et al., 2000). Second, the ascorbate content also is generally higher in younger tissues, and it accumulates in actively growing tissues such as meristems (Horemans et al., 2000).
The Combined Lack of Ascorbate and Z Increases the Photosensitivity of Arabidopsis
The vtc2npq1 mutant showed more bleaching (Figs. 1, 6, and 7), earlier and higher degrees of lipid peroxidation (Figs. 2 and 3A), lower Fv/Fm values (Fig. 4A), lower ETRs (Fig. 4B), and a more oxidized ascorbate pool in LL than the vtc2 mutant (Fig. 5B). The lack of Z in the vtc2npq1 mutant could conceivably affect a Z-dependent sustained form of thermal energy dissipation (Demmig et al., 1987), thereby increasing the light sensitivity of vtc2. The increased damage in the vtc2npq1 mutant is also consistent with an antioxidant function of Z. Z has been implicated in previous studies to function as an antioxidant or in membrane stabilization (Havaux, 1998; Havaux and Niyogi, 1999; Baroli et al., 2003). In one of these studies, the HL sensitivity of npq1 was compared with that of a qE-deficient mutant with a normal xanthophyll cycle (npq4). The npq1 mutant showed higher degrees of lipid peroxidation, showing that it is Z itself rather than the role it has in qE that rendered the plants HL sensitive. Together, these studies emphasize the function of Z as an antioxidant during HL stress.
Only a small negative impact on performance was noticed in the npq1 mutant relative to the wild type. On the other hand, the lack of Z clearly had a negative effect in the vtc2 mutant background, where the antioxidant content was already reduced compared with the wild type. This clearly points to a synergism between two different photoprotective mechanisms. It also underscores the complexity and multitude of photoprotective mechanisms that have evolved in plants to keep them alive. In conclusion, we have demonstrated the importance of ascorbate for the acclimation to HL in Arabidopsis, and an additional antioxidant role of Z that was especially evident in an ascorbate-deficient background.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All genotypes used (wild type, npq1, vtc2, and vtc2npq1) were of the Arabidopsis ecotype Col-0. The double mutant (vtc2npq1) was constructed by crossing vtc2-1 with npq1-2. The plants were grown in 10 h of light, 22°C/14 h of dark, 18°C cycle with a photon flux density of 160 μmol photons m–2 s–1 (LL) for 5 weeks and then transferred to a high-intensity discharge lighting chamber (E15, Conviron, Winnipeg, Canada) with a photon flux density of 1,800 μmol photons m–2 s–1 and a day temperature of 20°C. Samples were taken after 1 h in the LL before transfer, and at 1, 4, 24, 48, 72, 96, and 120 h after transfer to the HL. In addition, control plants were measured that had remained in the LL for 144 h. Any significant differences between the control plants and the LL-grown plants before the experiments are noted in the figure legends. Only fully expanded leaves were sampled. Severely bleached leaves were taken only if they were not dried out yet. The experiment was repeated two to four times with independently grown sets of plants. As a precaution, leaf temperatures of young and mature leaves of the wild type and the vtc2npq1 mutant were measured. Leaf temperatures increased by approximately 5°C after transfer to HL and did not differ between young and mature leaves or between genotypes, either in LL or after transfer to HL (data not shown).
Light stress was also imposed on detached leaves (Fig. 3). The leaves placed on wet filter paper were exposed to white light (1,000 μmol photon m–2 s–1) produced by 150-W metal halide lamps equipped with two infrared suppressor filters. Leaf temperature was maintained constant at 9°C ± 1°C.
Fluorescence Measurements
A total of nine samples (three sets with three samples each) were measured for each time point. Standard modulated Chl fluorescence parameters were measured with an FMS2 instrument (Hansatech, King's Lynn, UK). qE was calculated as (Fm – Fm′)/Fm′ and ΦPSII as (Fm′ – Fs)/Fm′, with Fm′ being measured in the light and Fm being defined as the value measured after 15 min dark-adaptation. ETR was calculated as photosynthetically active radiation * 0.5 * 0.84 * ΦPSII.
Pigment and Tocopherol Analysis
A total of six samples (three sets with two samples each) were measured for each time point following the protocol described by Li et al. (2002). The pigments were expressed on a per leaf area basis rather than per Chl because of Chl bleaching in the ascorbate-deficient mutants. For consistency and to facilitate comparison with previously published work, the lipid-soluble α-tocopherol was also expressed on a per leaf area basis.
Ascorbate Measurements
Total and reduced ascorbate were determined by a spectrophotometric method using the UV absorption of reduced ascorbate at 265 nm (Conklin et al., 1996). Six to 12 samples from different sets were measured for each time point.
Glutathione Measurements
Total and reduced glutathione were determined by HPLC followed by electrochemical detection (CoulArray, ESA, Chelmsford, MA). A total of six to nine leaf-disc samples (three sets with two–three samples each) were taken for each time point and immediately frozen in liquid nitrogen. The frozen discs were ground to a fine powder and extracted with 300 μL of 2% (w/v) metaphosphoric acid/2 mm EDTA, followed by 30 s of vortexing. The extract was centrifuged at 15,300g for 3 min, and 20 μL of the filtered supernatant was subjected to HPLC and separated on a TSK-GEL ODS-80Tm column at 30°C with a flow rate of 1 mL min–1 for 20 min. The mobile phase consisted of 0.2 m potassium phosphate (pH 3), 100 mg L–1 1-pentanesulfonic acid, and 1% (v/v) acetonitrile. The eight channels of the electrochemical detector were set at –200, –100, 0, +450, +650, +750, +880, and +900 mV. GSH was detected at +650 and +750 mV with +650 mV being the major channel. Oxidized glutathione was detected at +650, +750, and +880 mV with +750 mV being the major channel.
Lipid Peroxidation Measurements
Lipid peroxidation was determined as the level of TBARS (Hodges et al., 1999) or as the amplitude of the thermoluminescence signal at 135°C (Vavilin and Ducruet, 1998; Havaux and Niyogi, 1999). The TBARS assay measures a secondary end-product of lipid peroxidation, malondialdehyde (Hodges et al., 1999), whereas the high-temperature thermoluminescence signal reflects singlet oxygen and triplet carbonyls generated during lipid peroxidation (Hideg and Vass, 1993; Vavilin and Ducruet, 1998). For TBARS, data were expressed on a fresh weight basis. A total of six to nine samples from three sets were measured for each time point. Thermoluminescence was measured on leaf discs with a custom-built apparatus, as described previously (Havaux et al., 2000).
Immunoblot Analysis
Leaves were harvested and immediately frozen in liquid nitrogen. For total protein extraction, leaves were ground in extraction buffer (4% [w/v] SDS, 25 mm Tris-HCl [pH 8.8], and 2.5% [v/v] glycerol). Samples were then boiled in solubilization buffer (2× buffer: 16% [w/v] SDS, 100 mm Tris-HCl, pH 8.8, and 10% [v/v] glycerol) followed by a 30-min incubation after the addition of 10% [v/v] β-mercaptoethanol. Samples were then centrifuged for 5 min at 15,300g, and the supernatant was kept for analysis. A sample containing 1 to 2 μg of protein was loaded in each lane of a precast polyacrylamide gel (10%–20% gradient) with Tris-Gly buffer (NOVEX, San Diego), and SDS-PAGE was performed under denaturing conditions. Proteins were blotted to a polyvinylidene difluoride membrane and detected using an enhanced chemiluminescence western blotting kit (Amersham Biosciences, Piscataway, NJ). A different membrane was used for each genotype. Antibodies against the following proteins were used: FeSOD (kindly provided by Prof. Patricia Conklin), 2-CP (kindly provided by Dr. Margarete Baier and Prof. Karl-Josef Dietz), and PsaF (kindly provided by Prof. Anna Haldrup).
APX and SOD Activity Measurements
Two leaf discs were taken and immediately frozen in liquid nitrogen. The leaf discs were ground to a fine powder in 1.5 mL of extraction buffer using mortar and pestle. A previously described, APX assay was used (Grace and Logan, 1996), but 1.3 mm ascorbate was added to the extraction buffer, and the assay as done as a plate assay using a SPECTRAmax PLUS microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). Eight samples were measured simultaneously with nonspecific rates measured in neighboring wells. A total of three samples were measured for each time point.
For SOD, the reduction of cytochrome c by superoxide was determined after treatment of the extracts with ascorbate oxidase. The same extraction buffer as for APX was used. Superoxide was generated by a phenazine methosulfate/NADH system using 100 μm NADH, 50 nm phenazine methosulfate, and 10 μm cytochrome c. SOD activity was also measured as a plate assay. One control rate and seven samples were measured simultaneously with nonspecific rates measured in neighboring wells. One unit of SOD activity causes a half-maximal inhibition of cytochrome c reduction at a rate of 0.025 A550 units min–1 in a volume of 300 μL.
Acknowledgments
We thank Margarete Baier, Patricia Conklin, Karl-Josef Dietz, and Anna Haldrup for providing us with antibodies. We also thank Heidi Ledford for critical reading of the manuscript and Barry Logan for assistance with the APX activity measurements and critical reading of the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026252.
This work was supported by the U.S. Department of Agriculture National Research Initiative (grant no. 98–35306–6600), by the Director, Office of Science, Office of Basic Energy Sciences, Chemical Science Division, U.S. Department of Energy (contract no. DE–AC03–765F00098), by the Searle Scholars Program/The Chicago Community Trust, and by the France-Berkeley Fund.
References
- Asada K (1994) Production and action of active oxygen species in photosynthetic tissue. In CH Foyer, PM Mullineaux, eds, Causes of Photooxidative Stress and Amelioration of Defense System in Plants. CRC Press, Boca Raton, FL, pp 74–104
- Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Baier M, Dietz KJ (1999) Alkyl hydroperoxide reductases: the way out of the oxidative breakdown of lipids in chloroplasts. Trends Plant Sci 4: 166–168 [DOI] [PubMed] [Google Scholar]
- Baroli I, Do AD, Yamane T, Niyogi KK (2003) Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell 15: 992–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL (2000) Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154: 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL (1996) Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc Natl Acad Sci USA 93: 9970–9974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B, Winter K, Krueger A, Czygan F-C (1987) Photoinhibition and zeaxanthin formation in intact leaves. Plant Physiol 84: 218–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Winter K, Krüger A, Czygan F-C (1989) Light response of carbon dioxide assimilation dissipation of excess excitation energy and zeaxanthin content of sun and shade leaves. Plant Physiol 90: 881–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz KJ, Horling F, König J, Baier M (2002) The function of the chloroplast 2-cysteine peroxiredoxin in peroxide detoxification and its regulation. J Exp Bot 53: 1321–1329 [PubMed] [Google Scholar]
- Eskling M, Åkerlund H-E (1998) Changes in the quantities of violaxanthin de-epoxidase, xanthophylls and ascorbate in spinach upon shift from low to high light. Photosynth Res 57: 41–50 [Google Scholar]
- Foyer C, Rowell J, Walker D (1983) Measurement of ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta 157: 239–244 [DOI] [PubMed] [Google Scholar]
- Fryer MJ (1992) The antioxidant effects of thylakoid vitamin-E (alphatocopherol). Plant Cell Environ 15: 381–392 [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Grace SC, Logan BA (1996) Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol 112: 1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M (1998) Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 3: 147–151 [Google Scholar]
- Havaux M, Bonfils J-P, Lütz C, Niyogi KK (2000) Photodamage of the photosynthetic apparatus and its dependence on the leaf developmental stage in the npq1 Arabidopsis mutant deficient in the xanthophyll cycle enzyme violaxanthin de-epoxidase. Plant Physiol 124: 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Lütz C, Grimm B (2003) Chloroplast membrane stability in chlP transgenic tobacco plants deficient in tocopherols. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96: 8762–8767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg E, Vass I (1993) The 75°C thermoluminescence band of green tissues: chemiluminescence from membrane-chlorophyll interaction. Photochem Photobiol 58: 280–283 [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611 [DOI] [PubMed] [Google Scholar]
- Horemans N, Foyer CH, Potters G, Asard H (2000) Ascorbate function and associated transport systems in plants. Plant Physiol Biochem 38: 531–540 [Google Scholar]
- Horling F, Lamkemeyer P, König J, Finkemeier I, Kandlbinder A, Baier M, Dietz KJ (2003) Divergent light-, ascorbate-, and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiol 131: 317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL (2002) Arabidopsis map-based cloning in the post-genome era. Plant Physiol 129: 440–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Monde R-A, Last RL (1998) Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant Physiol 118: 637–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J, Baier M, Horling F, Kahmann U, Harris G, Schurmann P, Dietz KJ (2002) The plant-specific function of 2-Cys peroxiredoxin-mediated detoxification of peroxides in the redox-hierarchy of photosynthetic electron flux. Proc Natl Acad Sci USA 99: 5738–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Külheim C, Agren J, Jansson S (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297: 91–93 [DOI] [PubMed] [Google Scholar]
- Leipner J, Fracheboud Y, Stamp P (1997) Acclimation by suboptimal growth temperature diminishes photooxidative damage in maize leaves. Plant Cell Environ 20: 366–372 [Google Scholar]
- Li X-P, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- Li X-P, Müller-Moulé P, Gilmore AM, Niyogi KK (2002) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99: 15222–15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BA, Demmig-Adams B, Adams WW III (1998) Antioxidants and xanthophyll cycle-dependent energy dissipation in Cucurbita pepo L. and Vinca major L. upon a sudden increase in growth PPFD in the field. J Exp Bot 49: 1881–1888 [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR (2001) Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci USA 98: 2262–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P (2001) Senescence and cell death in plant development: chloroplast senescence and its regulation. In E-M Aro, B Andersson, eds, Regulation of Photosynthesis, Vol 11. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 277–296 [Google Scholar]
- Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Moulé P, Conklin PL, Niyogi KK (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128: 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L (2000) Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 210: 925–931 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L (2002) Interplay between ascorbic acid and lipophilic antioxidant defences in chloroplasts of water-stressed Arabidopsis plants. FEBS Lett 524: 145–148 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279 [DOI] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic S, Foyer CH (2000) Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Philos Trans R Soc Lond B Biol Sci 355: 1465–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljvic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH (2003) Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 15: 939–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polle A (1997) Defense against photooxidative damage in plants. In JG Scandalios, ed, Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 623–666
- Porfirova S, Bergmüller E, Tropf S, Lemke R, Dormann P (2002) Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA 99: 12495–12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci 355: 1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavilin DV, Ducruet J-M (1998) The origin of the 115–130°C thermoluminescence bands in chlorophyll containing material. Photochem Photobiol 68: 191–198 [Google Scholar]
- Yoshimura K, Yabuta Y, Ishikawa T, Shigeoka S (2000) Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol 123: 223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]