Abstract
We investigated how the presence of cadmium (Cd) at the emergence of Phragmites australis Trin. (Cav.) ex Steudel plants from rhizomes interacted with leaf and chloroplast physiological and biochemical processes. About 8.5 nmol Cd mg–1 chlorophyll was found in leaves, and 0.83 nmol Cd mg–1 chlorophyll was found in chloroplasts of plants treated with 50 μm Cd. As a result, a 30% loss of chlorophyll was measured concomitantly with a comparable percentage reduction in light-saturated photosynthesis. Rubisco content and activity were lowered by 10% and 60%, respectively. Antioxidant activity was stimulated by Cd treatment and was associated with an increase in the glutathione and pyridine pools, and with a larger pool of reduced glutathione. It is suggested that the glutathione pool and its predominance in the reduced state protected the activity of many key photosynthetic enzymes against the thiophilic binding of Cd. Chloroplast ultrastructure was not significantly altered with 50 μm treatment and the efficiency of photosystem II, measured as the fluorescence ratio Fv/Fm, remained high because F0 and Fm were proportionally decreased. In plants treated with 100 μm Cd, all effects were exacerbated, but Fv/Fm remained close to that of control leaves and the glutathione and pyridine nucleotides pools were lowered. The results suggest that glutathione exerted a direct important protective role on photosynthesis in the presence of Cd.
Most plants respond to cadmium (Cd) present in the root environment: the metal ion is absorbed on cortical cell walls or it is channeled into roots, where it is then subsumed into the closest vacuoles or loaded into the xylem for transport into leaves (Sanità di Toppi and Gabbrielli, 1999). The amount of this metal retained in the roots or transported to leaves differs significantly between species. Some Brassicaceae (e.g. Thlaspi caerulescens) are known to accumulate up to 1% (w/w) Cd on a dry weight basis and tolerate Cd in the aboveground tissues (Lombi et al., 2000). Unfortunately, this tolerance capacity is limited to a restricted number of species and genera, and thus, even a few micromoles of Cd in the root environment are toxic to most plants. Cd often damages root tips (visible browning), reduces nutrient and water uptake, impairs photosynthesis, and inhibits growth (Das et al., 1998). The basis of these dys-functions include irreversible changes to protein conformation by forming metal thiolate bonds (Dafré et al., 1996) and alteration of cell wall and membrane permeability by binding to nucleophilic groups (Ramos et al., 2002). Furthermore, because Cd induces antioxidant responses in all plant organs (Iannelli et al., 2002), it is also assumed that it directly or indirectly causes the formation of active oxygen species, thus interfering with the redox status and inducing significant chlorophyll loss (i.e. visible chlorosis; Baryla et al., 2001; Schutzendubel et al., 2001). Currently, it is not known clearly how this metal, which is not capable of carrying on a Fenton-type reaction, is involved in the formation of active oxygen species and thus in the most deleterious membrane damage (Navari-Izzo and Quartacci, 2001). It could be hypothesized (Atal et al., 1991) that photochemistry is somehow altered in the presence of Cd, with the consequent formation under high light of chlorophyll triplets in the photosystem antennae and then in a cascade of singlet oxygen, superoxide ions, and other reactive oxygen species. However, this does not imply that photochemistry is the cause of the high sensitivity of photosynthesis to Cd. Results of transient fluorescence analysis are, in fact, contradictory as are the analyses of various metabolic dys-functions thought to limit photosynthetic activities (Malik et al., 1992; Mendelssohn et al., 2001).
It is well established that many plants, as soon as Cd enters roots or arrives in leaves, stimulate sulfate absorption (Nocito et al., 2002) and reduction and the synthesis of specialized peptides and proteins (reduced glutathione [GSH] and phytochelatins, and metallothionein conjugates) involved in Cd chelation and segregation into vacuoles (Clemens, 2001). However, it is still unclear as to whether these peptides, specifically phytochelatins, are involved in the metal tolerance mechanism (Clemens, 2001). Some indications seem to attribute an important role to glutathione in this mechanism. Glutathione, the base unit of phytochelatins, is very mobile and can be found at high concentrations in all cell compartments, as well as in the phloem and in roots (Foyer and Rennenberg, 2000). GSH is also an important antioxidant and forms, with oxidized glutathione (GSSG), an important redox couple (for review, see Noctor et al., 1998): it interacts with NADP/NADPH and NAD/NADH, providing the conditions to support mitochondrial oxidative phosphorylation, generation of ATP, and hence key anabolic activities (May et al., 1998; Hutchison et al., 2000). GSH also takes part in thioredoxin-related regulation of many enzymes of photosynthetic metabolism (Schürmann and Jacquot, 2000). However, its involvement with photosynthetic performance in the presence of Cd seems, so far, not adequately explored. On the other hand, glutathione synthesis and reduction are energetically very expensive for plants: eight electrons per molecule are required for the reduction of sulfate to sulfide in the chloroplasts (Leustek and Saito, 1999), and NADPH is used to maintain it in the reduced state (Noctor et al., 1998). Consequently, a large investment in resources at the expenses of other processes is necessary to make GSH adequately available in the whole plant.
We have investigated how the presence of Cd at the emergence of Phragmites australis plants from rhizomes interacted with glutathione and photosynthesis. Analysis of the redox state (including GSH and GSSG and pyridine nucleotides), chloroplast ultrastructure, antioxidant activities of chloroplasts and leaves, as well as analysis of gas exchange and fluorescence of leaves, suggest that developing leaves, and thus photosynthesis, could cope with mild Cd toxicity. Increased GSH concentration, a very mobile antioxidant and a targeted thiol, seems to be the optimal defense strategy, together with phytochelatins, in the preservation of key photosynthetic thiolic enzymes from Cd inactivation.
RESULTS
Table I shows that about 8.5 nmol Cd mg–1 total chlorophyll were translocated from roots to leaves of P. australis plants that had emerged in the presence of 50 μm Cd, and from there, 0.83 nmol Cd was passed to chloroplasts. When Cd in the root environment was 100 μm, then 21 nmol of this metal arrived in leaves and 3 nmol reached the chloroplasts (Table I). A similar distribution of Cd between leaves and chloroplasts has been found in other species (Siedleka and Krupa, 1999; Ramos et al., 2002). The leaf content of Fe, Ca, and Zn significantly increased with respect to controls in the presence of 50 μm Cd, whereas at 100 μm, Cd, Fe, and Zn did not change with respect to controls and Ca was slightly and significantly reduced (Table I).
Table I.
Cd in leaves and chloroplasts on a chlorophyll basis, and Fe, Zn, and Ca on a fresh weight basis in leaves
P. australls plants were grown in the presence of 0 (control), 50, and 100 μm Cd. Different letters in the same column indicate significant differences between the treatments (P < 0.05, analysis of variance [ANOVA]; post hoc test least significant difference [lsd]. nd, Not detectable. Values are indicated ± se. n = 4.
| Cd
|
Fe
|
Zn
|
Ca
|
||
|---|---|---|---|---|---|
| Leaves | Chloroplasts | ||||
| nmol mg chlorophyll-1 | mg g-1 fresh weight | ||||
| Control | nd | nd | 0.37 ± 0.02a | 0.045 ± 0.002a | 12.07 ± 0.60a |
| Cd 50 μm | 8.5 ± 0.6a | 0.83 ± 0.06a | 0.69 ± 0.05b | 0.103 ± 0.007b | 14.79 ± 0.73b |
| Cd 100 μm | 21.0 ± 1.6b | 3.00 ± 0.21b | 0.39 ± 0.03a | 0.043 ± 0.003a | 9.48 ± 0.47c |
In Table II, we have summarized the most relevant results from photosynthesis and fluorescence measurements. Photosynthesis was measured by varying the concentration of CO2 in the leaf cuvette and maintaining the photosynthetic photon flux density (PPFD) incident on the leaf surface at 800 μmol m–2 s–1 or by varying PPFD and maintaining CO2 at 350 μbar bar–1. On a leaf area basis, Cd decreased photosynthesis at 350 μbar bar–1 CO2 and 800 μmol m–2 s–1 PPFD by around 28% of the control values (22.5 μmol m–2 s–1) in the presence of 50 μm Cd, and by about 60% in presence of 100 μm Cd. Maximum photosynthesis measured under saturating light and CO2 was reduced with respect to controls by 40% at 50 μm Cd and by 50% at 100 μm Cd. The initial slope of the photosynthesis curve at low internal CO2 (40–150 μbar bar–1) was also strongly reduced: by 60% and 83% in 50 and 100 μm Cd leaves, respectively. The slope of the photosynthetic response at low PPFD did not change between control and 50 μm Cd leaves, and was slight lower than the slope of the control in 100 μm Cd leaves. However, photosynthesis on a chlorophyll basis showed no statistically significant difference between control and 50 μm Cd, and a slight increase from control with 100 μm Cd. This indicates that photosynthesis was mostly limited by chlorophyll content and thus most likely by the photochemical capacity with 50 μm Cd. There were no changes in the CO2 compensation point or apparent photorespiration with any treatment. Dark respiration only increased strongly in leaves exposed to 100 μm Cd. Transpiration was the same in control and 50 μm Cd leaves, but decreased (but not significantly) in 100 μm Cd leaves, suggesting that stomata closed in response to high Cd concentration. The increase in the internal CO2 concentration with the increase of Cd in leaves was perhaps an indication that metabolism was limited by something other than the supply of CO2, even at the highest Cd treatment. Photochemical and nonphotochemical quenching of fluorescence decreased and increased with respect to the increase in Cd concentration, in accordance with the lower photosynthesis and higher dissipation as heat of the absorbed energy.
Table II.
Net CO2 assimilation rate (A) per leaf surface unit and per chlorophyll content, maximal net CO2 assimilation rate in saturating light and CO2 (Amax), evapotranspiration (E), CO2 compensation point (ΓCO2 int), dark respiration (Rdark), internal CO2 concentration (CO2 int), Rubisco activity (RuBPCase), initial slope of CO2 assimilation rate curve response to photosynthetic photon flux density (δA/δPPDF) and to internal CO2 concentration (δA/δCO2 int), leaf absorptance (Leafabs), fluorescence estimation of photosystem II quantum yield (ΔF/Fm), photochemical (qP) and nonphotochemical (qN and NPQ) fluorescence quenching of absorbed light energy, basal (F0), maximal (Fm), and fluorescence ratio (Fv/Fm with Fv = Fm-F0) of dark-adapted leaves
P. australis plants were grown in the presence of 0 (control), 50, or 100 μm Cd in the hydroponic nutrient solution. Different letters in the same row mean significance of difference between the treatments (P < 0.05, ANOVA; post hoc test lsd). Values are indicated ± se. n = 4.
| Control | 50 μm Cd | 100 μm Cd | |
|---|---|---|---|
| A (μmol CO2 m-2 s-1) | 22.5 ± 1.2 | 16.1 ± 1.0b | 9.2 ± 2.0c |
| A (μmol CO2 g chlorophyll-1 s-1) | 63 ± 3a | 61 ± 4a | 70 ± 8a |
| Amax (μmol CO2 m-2 s-1) | 36.0 ± 1.7a | 21.0 ± 2.1b | 17.9 ± 2.5b |
| E (mmol H2O m-2 s-1) | 5.6 ± 0.9a | 5.8 ± 0.6a | 3.6 ± 1.2a |
| ΓCO2 int (μbar bar-1) | 40 ± 1a | 40 ± 1a | 40 ± 2a |
| Rdark (μmol CO2 m-2 s-1) | -0.6 ± 0.2a | -0.5 ± 0.1* | -1.8 ± 0.3b |
| CO2 int (μbar bar-1) | 235 ± 11a | 264 ± 9ab | 285 ± 7b |
| RuBPCase (μmol CO2 m-2 s-1) | 170.2 ± 16a | 71.1 ± 8b | 51.9 ± 8b |
| RuBPCasemax/Amax | 4.7 | 3.5 | 3.5 |
| δA/δPPDF (0-150 μmol photons m-2 s-1) | 0.050 | 0.050 | 0.040 |
| δA/δCO2 int (0-200 μbar bar-1) | 0.170 | 0.068 | 0.028 |
| Leafabs (%; 400-700 nm) | 83 ± 2g | 78 ± 3a | 70 ± 2b |
| ΔF/Fm | 0.28 ± 0.02g | 0.21 ± 0.06ab | 0.13 ± 0.02b |
| A/(ΔF/Fm) × Leafabs | 66.7 | 59.8 | 49.5 |
| qP | 0.54 ± 0.11a | 0.44 ± 0.09a | 0.35 ± 0.07a |
| NPQ | 1.7 ± 0.5a | 1.95 ± 0.21a | 3.4 ± 0.20b |
| qN | 0.76 ± 0.06a | 0.80 ± 0.01a | 0.89 ± 0.09a |
| Fv/Fm | 0.78 ± 0.04a | 0.78 ± 0.03a | 0.79 ± 0.05a |
| Fo | 37 ± 6a | 27 ± 2a | 26 ± 4a |
| Fm | 171 ± 8a | 131 ± 5b | 126 ± 4b |
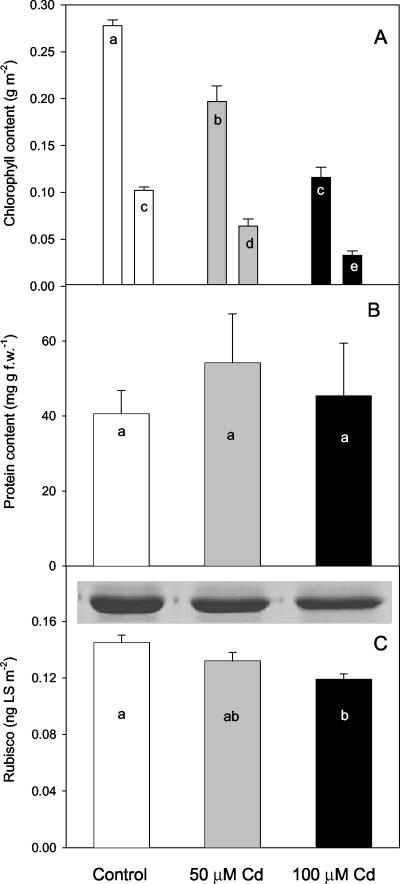
Estimation of photosystem (PS) II quantum yield and PSII efficiency by fluorescence showed that decreases in ΔF/Fm were similar to those for photosynthesis. However, Fv/Fm did not change between control and the two Cd treatments. Actually, Fm, the maximum fluorescence in the dark, and F0, the basal fluorescence in the dark, decreased similarly with respect to controls, thus maintaining this ratio constant. However, Fv/Fm measured on leaf discs from fully mature control plants exposed to 8.5 nmol Cd mg–1 chlorophyll (as found in the 50 μm Cd-treated leaves) decreased to 40% of the initial value (0.81 ± 0.04) after a few days. Leaf discs infiltrated with the same solution, but without Cd, only showed a slight statistically insignificant change in Fv/Fm (0.78 ± 0.06). The total chlorophyll content was reduced by between 30% and 60% in leaves of plants that had developed in the presence of 50 and 100 μm Cd, respectively (Fig. 1A). The reduction was less pronounced in chlorophyll a than in chlorophyll b. In fact, the ratio of chlorophyll a to chlorophyll b tended to increase with increasing Cd concentration. Leaf soluble proteins increased in both Cd treatments with respect to the control, with a peak at 50 μm Cd (Fig. 1B). However, the Rubisco large subunit content (Fig. 1C) and Rubisco activity (Table I) were reduced in both Cd treatments. In particular, Rubisco activity was reduced by 58% in 50 μm Cd leaves and by about 70% in 100 μm Cd leaves, whereas the reduction of Rubisco content was only 10% and 18%, respectively, in the two treatments. However, the ratio between the in vitro maximum Rubisco activity and maximum photosynthesis was always very high, indicating that the extractable enzyme activity could still support photosynthesis even in 100 μm Cd-treated leaves.
Figure 1.
Chlorophyll a and b (first and second column for each treatment, respectively; A), protein (B), and SDS-PAGE of the large subunit of Rubisco contents (C) measured in plants of P. australis grown in the presence of 0 μm (control, white bars), 50 μm (gray bars), and 100 μm (black bars) Cd. Bars showing the same letter within each graph are not significantly different (P = 0.05, ANOVA; post hoc test lsd). Error bars mean se. n = 4.
Table III shows a large data set, including enzymatic and nonenzymatic antioxidants, glutathione and glutathione-related enzymes, and pyridine nucleotides, all determined in chloroplasts and in leaves. Generally, Cd-treated leaves showed higher values than control leaves, with a maximum in 50 μm Cd-treated leaves, and a slight decrease in 100 μm with respect to 50 μm Cd-treated leaves. Furthermore, measurements carried out on chloroplasts showed higher values than measurements in leaves, particularly for SOD, APX, and GST activities, which were much higher than activities measured in leaves with increasing Cd concentrations. In contrast, CAT activity was not affected by the presence of Cd, GR increased only with 100 μm Cd, and GPX increased only in chloroplasts of the two Cd treatments, whereas ascorbate content was not affected by Cd. However, Cd exposure strongly increased total glutathione, particularly GSH, in chloroplasts at 50 μm Cd and to a lesser extent in leaves. Cd exposure also increased the pyridine nucleotide content, whereas total NADP (NADPH and NADP) and NAD (NADH and NAD) almost doubled in leaves and chloroplasts.
Table III.
Activity of superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR), glutathione-S-transferase (GST), GSH and GSSG, ascorbic acid (AA), NADH and NAD+, and NADPH and NADP+ contents measured on protein basis (milligrams of protein) in leaves and chloroplasts
P. australls plants were grown in the presence of 0 (control), 50, or 100 μm Cd. Different letters in the same row mean significance of difference between the treatments (P < 0.05, ANOVA; post hoc test lsd). Values are indicated ± se. n = 4. nd, Not detected.
| Control
|
50 μm Cd
|
100 μm Cd
|
||||
|---|---|---|---|---|---|---|
| leaves | chloroplasts | leaves | chloroplasts | leaves | chloroplasts | |
| SOD (U mg-1 protein) | 12.5 ± 1.8a | 21.7 ± 1.2bc | 19.3 ± 0.4b | 43.6 ± 1.06d | 23.5 ± 1.0c | 51.6 ± 1.55c |
| APX (μmol AA mg-1 protein min-1) | 0.10 ± 0.001a | 5.99 ± 0.07b | 0.32 ± 0.033c | 13.6 ± 0.19d | 0.57 ± 0.03c | 12.3 ± 1.15d |
| CAT (μmol H2O2 mg-1 protein min-1) | 0.47 ± 0.015a | nd | 0.50 ± 0.02a | nd | 0.52 ± 0.02a | nd |
| GPX (μmol H2O2 mg-1 protein min-1) | 21.4 ± 1.6a | 12.5 ± 0.3bc | 11.5 ± 2.4bc | 24.5 ± 0.12a | 10.6 ± 1.5b | 14.3 ± 0.1c |
| GR (U mg-1 protein) | 0.092 ± 0.02ab | 0.07 ± 0.008a | 0.10 ± 0.019ab | 0.05 ± 0.007a | 0.16 ± 0.03b | 0.04 ± 0.01a |
| GST (μmol conjugate mg-1 protein min-1) | 0.17 ± 0.02a | 0.13 ± 0.01a | 0.31 ± 0.07b | 0.30 ± 0.05h | 0.22 ± 0.06ab | 0.16 ± 0.01a |
| GSH (nmol GSH mg-1 protein) | 0.74 ± 0.01a | 12.2 ± 1.2b | 27.87 ± 3.5c | 75.0 ± 4.5d | 23.8 ± 4.2c | 13.5 ± 2.1b |
| GSSG (nmol GSH mg-1 protein) | 0.09 ± 0.02a | 1.4 ± 0.60b | 7.5 ± 1.2c | 19.8 ± 0.8d | 9.6 ± 1.2c | 9.9 ± 1.4c |
| AA (mg AA mg-1 protein) | 0.29 ± 0.05a | nd | 0.24 ± 0.03a | nd | 0.14 ± 0.1b | nd |
| NADH (nmol mg-1 protein) | 0.43 ± 0.01a | 1.55 ± 0.31b | 0.68 ± 0.3a | 1.94 ± 0.20b | 0.35 ± 0.10a | 2.3 ± 0.5a |
| NAD+ (nmol mg-1 protein) | 1.00 ± 0.04a | 1.71 ± 0.02b | 1.51 ± 0.4h | 2.40 ± 0.07c | 1.8 ± 0.5bc | 2.9 ± 0.9c |
| NADPH (nmol mg-1 protein) | 0.81 ± 0.06a | 3.75 ± 0.23b | 1.95 ± 0.5c | 5.69 ± 0.15d | 1.2 ± 0.3ac | 6.1 ± 1.1d |
| NADP+ (nmol mg-1 protein) | 2.50 ± 0.09a | 1.75 ± 0.16b | 4.68 ± 1.6c | 2.58 ± 0.02a | 11.9 ± 1.5d | 3.7 ± 0.9ac |
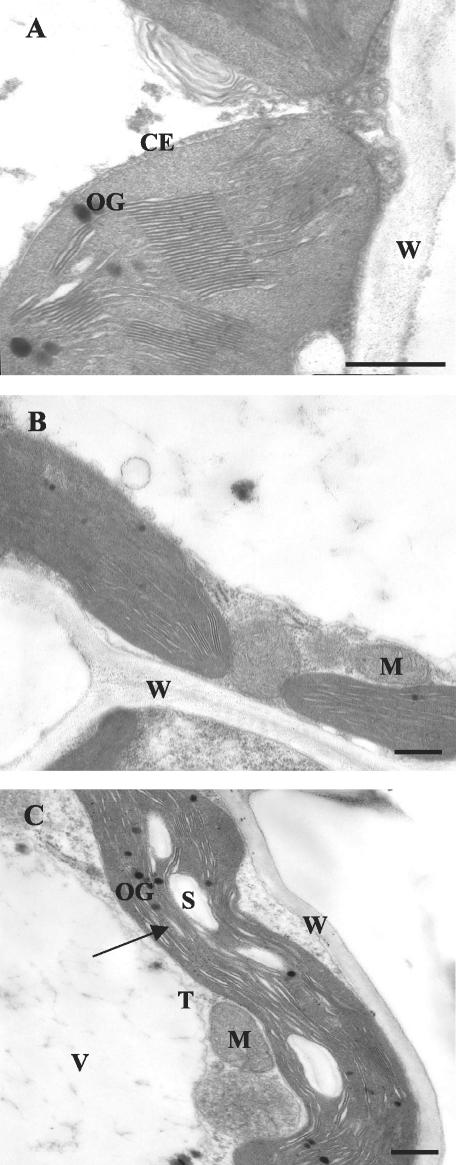
Figure 2 shows transmission electron microscopy micrographs of developing leaves of control and Cd-treated plants. The chloroplasts from Cd-untreated plants were typical mesophyll chloroplasts (Fig. 2A) with a well-organized internal membrane structure and normally developed grana and stroma thylakoids. Little or no starch was present. In 50 μm Cd-treated plants (Fig. 2B), most of the chloroplasts were undamaged or only slightly damaged. A small proportion of chloroplasts were lenticular in shape. In 100 μm Cd-treated plants, the chloroplast thylakoid system was affected (Fig. 2C), with an apparent swelling of the thylakoid membrane, fewer grana, and abundant starch grains.
Figure 2.
Electron micrographs of transverse ultrathin sections from 0 μm (control, A), 50 μm (B), and 100 μm Cd-treated leaves (C) of P. australis plants. All bars = 1 μm. CE, Chloroplast envelope; M, mitochondria; OG, osmiophilic globules; S, starch; T, tonoplast; V, vacuole; W, cell wall. Arrow in C indicates swelling of thylakoid membranes.
DISCUSSION
In this experiment, we grew P. australis plants from rhizomes in the presence of 50 μm Cd and, after 4 weeks, we found 8.5 nmol Cd mg–1 chlorophyll in leaves and 0.83 nmol Cd mg–1 chlorophyll in chloroplasts. In general, a typical sign of Cd toxicity is visible loss of chlorophylls, as also observed in our Cd-treated leaves. This has been interpreted as the effect of strong oxidation on the photochemical apparatus (Somashekaraiah et al., 1992), the effect of interference of Cd with Fe root uptake (Siedleka and Krupa, 1999), or reduction of chloroplast density and size (Baryla et al., 2001). Our measurements, reported in Table I, excluded the possibility of loss of chlorophylls being caused by low levels of Fe in leaves, but did not exclude that Cd could have replaced Mg in chlorophylls (Küpper et al., 1998). The reduced amount of active chlorophylls in our Cd-treated leaves with respect to control leaves resulted in a decrease in F0 values. This parameter is used to obtain the Fv/Fm ratio, an estimation of PSII efficiency in dark-adapted leaves (Fracheboud et al., 1999). Surprisingly, this ratio, in our experiment, remained high, even after exposure of chloroplasts to high Cd concentrations. In fact, Fm decreased proportional to F0 (Table II), thus explaining the maintenance of a high ratio and possibly indicating balanced damage to, or close regulation of, energy harvesting and energy conversion capacities. However, in a short-term Cd treatment, we took small leaf discs from fully mature control plants and exposed them to 8.5 nmol Cd mg–1 chlorophyll, the amount found in the 50 μm Cd-treated leaves. After a few days, Fv/Fm decreased to 40% of the initial value and of the control value. Thus, in contrast to the previous result, Cd was very damaging for PSII, and chlorosis occurred on leaf surfaces in association with the decrease in Fv/Fm. It must be stressed that the two experiments were performed with newly emerged leaves in contact with Cd from their emergence, or with fully mature leaves. This confirmed that P. australis had an intrinsic capacity to defend chloroplasts against toxicity caused by nanomolar amounts of Cd (Fediuc and Erdei, 2002), but also indicated that this capacity was higher in developing than in mature leaves. A comparable growth-stage response of toxicity to Cd has been found in different sections of a single leaf of rye (Secale cereale; Krupa and Moniak, 1998).
Although Cd did not cause a reduction in Fv/Fm, it did induce a reduction in photosynthetic electron transport. Differences in the fluorescence parameters, specifically of ΔF/Fm and qP, but also indirectly qN and NPQ, are indications of reduced electron transport between Cd-treated and control leaves (Table II). Varying light and CO2 levels around leaves was used to investigate in detail the consequences of this electron transport reduction on photosynthesis. The response of photosynthesis at high light was limited in Cd-treated leaves with respect to controls. However, photosynthesis differences disappeared when rates were expressed on a chlorophyll basis, indicating that electron transport is closely coupled to photosynthesis. An interesting interpretation of the above results is suggested by the work of Küpper et al. (2002) on the formation of copper- and zinc-substituted chlorophylls in the PSII antenna and reaction centers. In our experiment, it is possible that Cd-substituted chlorophylls were formed in the PSII antenna and reaction centers of P. australis. This could have seriously damaged entire photochemical units, leaving only a few still active. In a previous paper, Küpper et al. (1996) also found that chlorophylls substituted with Cd are unstable and completely degraded. This would explain the apparent constancy of Fv/Fm and of photosynthesis calculated on a chlorophyll basis and the increase of SOD and APX activities associated to chloroplasts. In fact, the damaged units could have been oxidatively degraded inducing this antioxidant response in the chloroplasts. In contrast, the extrachloroplastic CAT was not significantly affected by Cd.
On the other hand, analysis of parameters elaborated from gas exchange and biochemical measurements, reported in Table II, indicated that the major interference of Cd with photosynthesis was a reduction in maximal extractable Rubisco activity (60% less than controls), which could only slightly be attributed to a reduction in Rubisco content (10% less than controls). Because Cd is typically associated with widespread oxidative activity, as indirectly confirmed in this experiment by antioxidant stimulation (Table III), the effects on Rubisco content could be considered a result of oxidative activity (Romero-Puertas et al., 2002) or of proteases induced by reduced oxygen species (Prasad, 1996). Alternatively, it is not excluded that the Cd-binding mechanism limiting chlorophyll synthesis could also be responsible for the reduction in Rubisco activity. It is known that Rubisco activase has Cys thiol residues in the carboxy terminus of the larger isoform that takes part in Rubisco down-regulation at low light levels (Portis, 2003), and it is not excluded that these thiols could have bound Cd, causing inactivation of the enzyme. Because maximum extractable Rubisco activity was largely in excess of maximum measured photosynthesis (Table II), it is unlikely that Rubisco limited photosynthetic performance under the growth conditions. In contrast, at low CO2 concentrations, photosynthetic responses could be Rubisco limited (Stitt and Schulze, 1994) and, in fact, the initial slopes of the photosynthetic curve was very different between Cd-treated and control leaves.
Another major effect observed in this investigation was that nanomoles of Cd in leaves and in chloroplasts increased the GSH level by about 37 and six times, respectively, above levels in the controls. This was a predictable result because it is known that Cd acts as a strong sink for thiols, which increases the demand for sulfate absorption (Nocito et al., 2002). What is unexpected is that Cd specifically stimulated the predominance of the reduced form over GSSG. This confirms the suggestion that thiols can trap Cd only when they are in the reduced state (Ow, 1996). It also explains why, in Cd-treated leaves, the pool of pyridine nucleotides increased: They are quantitatively needed to maintain as much as possible of the glutathione and phytochelatins in reduced forms. The high cost of this activity could be mitigated by using glutathione in antioxidant activity against the effects of Cd. Glutathione can diffuse to many leaf compartments and this characteristic is very useful for an antioxidant. Furthermore, GSH efficiently lowers the probability that Cd will bind to thiols in the active sites of many photosynthetic enzymes, which would alter their functionality and inhibit photosynthesis. Despite the ability of the plant to reduce Cd toxicity, this metal caused visible swelling of thylakoids on ultrastructural analysis.
Doubling (100 μm Cd) the concentration of Cd in the growth medium almost doubled the concentration of Cd in leaves, and in chloroplasts, Cd concentrations were as high as 3 nmol mg–1 chlorophyll. This high metal concentration reduced photosynthesis and chlorophylls (50%–60%), as well as Rubisco content (35%) and activity (70%). Notably, the decrease in photosynthesis seems to follow (as with 50 μm Cd) that of chlorophyll and of maximum extractable Rubisco activity. Despite this, the efficiency of PSII was still close to control values. In contrast, chloroplasts had visible alterations in thylakoids and a large starch accumulation. It is likely, as previously argued, that Cd bound to the cytosolic thiol-dependent enzyme F-1,6-P2ase and reduced sucrose synthesis, thus limiting phosphate recycling between cytosol and chloroplasts (Sharkey, 1990). A consequence of this lower phosphate exchange could be starch accumulation in chloroplasts, as shown in similar situations when triggered by high CO2 and low temperatures in C3 plants. Most of the studies on the inhibitory interaction of Cd on photosynthesis could be interpreted as Cd binding to thiols in key enzymes. At 100 μm Cd, however, Cd-induced oxidative activity is more widespread, as suggested by the increase in activity and concentration of antioxidant molecules and, as a consequence, senescence processes are initiated and become dominant.
In conclusion, this experiment showed that leaves of P. australis can avoid Cd-induced irreversible damage to photochemistry when they emerge in the presence of 50 μm of the metal and can develop defense mechanisms to cope with the affinity of Cd for thiols. The high concentration of GSH, a very mobile molecule, seems to be an optimal response defense strategy: it provides widespread antioxidant protection to leaves and throughout the plant and, most importantly, it increases the thiol concentration in the cytoplasm and chloroplasts. Enhancing the number of targetable thiols probably helps to preserve the activity of key photosynthetic enzymes and to sustain photosynthesis until a higher Cd concentration (100 μm) overloads this defense capacity.
MATERIALS AND METHODS
Plant Material
Phragmites australis (Cav.) Trin. ex Steud rhizomes were collected from a clean site in the Trasimeno Lake (Perugia, Italy), potted in three groups of four with expanded argyll, and watered with 2 liters of full-strength Hoagland nutrient solution, pH 6.0.
Growth in the Presence of Cd
The Cd was added as CdSO4 to the nutrient solution to give concentrations of 0, 50, or 100 μm Cd. Air was continuously and gently bubbled through all pots, and solutions were replaced twice a week. Pots were located inside a growth chamber with controlled temperature (27°C/22°C day/night), humidity (95%/85% day/night), PPFD (800 μmol m–2 s–1 at 40 cm from white lamps), and a 16-h photoperiod.
Four large leaf samples from plants that had emerged in pots containing 0, 50, and 100 μm Cd were taken after 21 d, weighed, immediately frozen in liquid nitrogen, and stored at –80°C.
Cd Treatment of Mature Control Leaves
Leaf discs (1 cm2) were taken from mature leaves of plants grown under the conditions of the previous experiment (growth in presence of Cd). Chlorophylls were measured on three replicate discs as described below. Six leaf discs were floated on small plates in a 50 mm phosphate buffer containing 8.5 nmol Cd mg–1 leaf disc total chlorophyll, and six replicates were placed in the same buffer solution without Cd as controls. The amount of Cd used corresponds to the amount measured in the leaves of plants exposed to 50 μm Cd (see Table I). After 3 d, the ratio of variable (Fv = Fm – F0) to maximum fluorescence (Fm), Fv/Fm, was measured on dark-adapted discs (30 min in darkness) from all treatments using the fluorometer (PAM 2000; Walz, Effeltrich, Germany).
Chloroplast Isolation
Fresh leaf samples were ground in an ice-cold isolation medium containing 0.33 m sorbitol, 50 mm HEPES-KOH, 2 mm EDTA, 2 mm EGTA, 1 mm MgCl2, 1 mm MnCl2, and 0.2% (w/v) bovine serum albumin at pH 7.3. The homogenate was centrifuged at 2,000g for 10 min and the pellet was suspended in a second aliquot of the isolation medium and centrifuged at 6,500g for 20 min in a Percoll discontinuous gradient (40%–80%, v/v). The lower dark green band containing the intact chloroplasts was collected, after first carefully removing the upper part, and then by diluting the dark green band with an ice-cold import buffer containing 0.33 m sorbitol and 50 mm HEPES-KOH, pH 7.3, to obtain the purified fraction containing intact chloroplasts (Cline et al., 1985).
Metal Content
Determination of Cd was carried out on leaves and chloroplasts of P. australis plants. Ca, Zn, and Fe contents were also measured in P. australis leaves. After 21 d of Cd treatment, leaf samples and chloroplasts pellets were dried at 80°C for 48 h and the dry weight was measured. Determinations of metals were made by atomic absorption spectrophotometry (Analyst 300; Perkin Elmer, Germany) on nitric-perchloric acid (3:1, v/v) digests of four replicate samples from plant tissue for Cd, Ca, Zn, and Fe and from pelleted chloroplasts for Cd.
Soluble Proteins
Between 0.2 and 0.5 g of fresh leaf was ground to a fine powder with a mortar and pestle under liquid nitrogen. Proteins were then extracted at 4°C by grinding with 50 mm of ice-cold phosphate buffer, pH 7.0, containing 0.1% (w/v) AA, 0.1% (v/v) Triton X-100, and 1% (w/v) polyvinyl pyrrolidine. The homogenate was centrifuged at 4°C for 20 min at 12,000g. The clear supernatant fraction was used for the enzyme assays. Protein concentration was quantified as described by Bradford (1976), using bovine serum albumin as a standard.
Rubisco Content and Activity
Rubisco was extracted from frozen samples, separated, and identified by SDS-PAGE using Rubisco from spinach (Spinacia oleracea; Sigma, St. Louis) as standard, as described by Laemmli (1970). Rubisco (EC 4.1.1.39) activity was measured on leaf extracts according to Sharkey et al. (1991).
Chlorophylls
Chloroplasts and frozen leaf tissue were ground with a mortar and pestle under liquid N2. Eighty percent (v/v) acetone was added to extract pigments and, after centrifugation of the supernatant for 10 min at 10,000g, O.D. was measured at 470, 646.8, and 663.2 nm using a spectrophotometer (Perkin Elmer). The extinction coefficients and the equations reported by Lichtenthaler (1987) were used to calculate the amounts of chlorophyll a and b.
AA
The AA content was determined by HPLC according to Olmos and Hellin (1996) at 254 nm. Leaf tissues were homogenized in liquid nitrogen and were extracted in 5% (w/v) ice-cold metaphosphoric acid. The extract was centrifuged for 10 min at 6,000g. The column used was the Alltima C18 column (4.6 × 250 mm, 5 μm; Alltech, Italia). The mobile phase consists of a solvent of 0.05 m sodium acetate and acetonitrile (95:5, v/v). The temperature of the column was adjusted to 26°C and the flow rate was 1 mL min–1. Calibration was achieved using purified AA as standard.
GSH and GSSG
The concentrations of GSH and GSSG were spectrophotometrically determined with an enzyme-recycling assay at 412 nm (Griffith, 1980). The assay was based on sequential oxidation of glutathione by 5,5′-dithiobis-(2-nitrobenzoic acid) and reduction by NADPH in the presence of known amounts of GR. To quantify GSSG content, 2-vinylpyridine was added to the extract. Standard curves were generated with reduced and oxidized glutathione.
Pyridine Nucleotides
Frozen leaf samples were ground with a mortar and pestle under liquid nitrogen and were extracted with ethanol-water (1:1, v/v) containing 0.1 m NaOH or 0.1 m HCl (Carrier and Neve, 1979). The oxidized (NADP+ and NAD+) and reduced (NADPH and NADH) forms of the nucleotides were determined using the enzymatic cycling assay according to Matsumura and Miyachi (1980).
Enzyme Activities
Total APX (EC 1.11.1.11) activity was determined by measuring the oxidation rate of ascorbate at 290 nm according to Asada (1992). APX activity was expressed as micromoles ascorbate oxidized per milligram of protein per minute. Total SOD (EC 1.15.1.1) activity was assayed according to its ability to inhibit ferric cytochrome c reduction using a constant flux of O–2 generated by the xanthine-xanthine oxidase system (McCord and Fridovich, 1969). The reaction mixture contained 10 μm KCN to inhibit cytochrome c oxidase. One unit of SOD was defined as the quantity of enzyme required to inhibit the reduction of cytochrome c by 50% in a 1-mL reaction volume. CAT (EC 1.11.1.6) activity was assayed spectrophotometrically at 240 nm after the decomposition of hydrogen peroxide (ε = 39.4 mm–1 cm–1) and was expressed as micromoles H2O2 per milligram of protein per minute according to Chance and Maehly (1955).
GST (EC 2.5.1.18) was measured spectrophotometrically at 340 nm by monitoring the increase in absorbance due to the formation of the conjugate, S-2,4-dinitro (phenylglutathione; ε = 10 mm–1 cm–1), between GSH and 1-chloro-2,4-dinitrobenzene. The GST-specific activity was expressed as micromoles conjugate per milligram of protein per minute, as reported by Mannervik and Guthenberg (1981).
GR (EC 1.6.4.2) activity was assayed spectrophotometrically after the formation of thiobenzoic acid at 412 nm, according to Smith et al. (1988). GR activity was expressed as units per milligram of protein, referring to a calibration curve of known amounts of yeast GR (Sigma) as standard.
GPX (EC 1.11.1.9) activity was measured spectrophotometrically after the decrease in A340 of NADPH (ε = 6.2 mm–1 cm–1). GPX activity was expressed as micromoles NADPH per milligram of protein per minute according to Klapheck et al. (1990).
Photosynthesis and Fluorescence Measurements
The central part of a P. australis leaf was enclosed in the cuvette of a gas exchange system (HCM 1000; Walz), configured for simultaneous measurement of chlorophyll fluorescence (PAM 101 modulated fluorometer; Walz). The relative humidity of air entering the cuvette was set at 50% and air and cuvette temperatures were 25°C or 7°C. CO2 partial pressure was varied between 0 and 700 μbar bar–1 at 50 μbar bar–1. A white light source (KL 1500; Schott, Mainz, Germany) was used to vary the incident PPFD on the leaf surface between 0 and 2,000 μmol m–2 s–1 PPFD. The leaf absorbance (Aleaf) was measured with a spectroradiometer (Li-1800; Li-Cor, Lincoln, NE). Photosynthesis and stomatal conductance were calculated according to von Caemmerer and Farquhar (1981). The fluorescence quantum yield of electron transport through PSII (ΔF/Fm) was estimated by dividing the difference between the maximum fluorescence (Fm) and the steady-state fluorescence (Fs) in the illuminated leaf (ΔF = Fm – Fs) by Fm, as reported in Genty et al. (1989). The efficiency of PSII (Fv/Fm) was estimated from the ratio of variable (Fv = Fm – F0) to maximum fluorescence (Fm) measured on 30 min dark-adapted leaves.
Ultrastructural Analysis
Fresh tissues (1–2 mm2) were taken from last fully expanded leaves and were immediately treated for microscopy observations (TEM 400 T; Philips, Eindhoven, The Netherlands), as reported in Loreto et al. (2001).
Statistical Analysis
Data reported in the tables and figures were all analyzed using the statistical program SPSS 6.0 (SPSS, Chicago). Significance of difference was tested at P = 0.05 using ANOVA and post hoc lsd. The data are means ± se from four determinations.
Acknowledgments
The authors wish to thank Dr. Lucia Fiore and Angelo De Martino for helping with the biochemical analyses.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026518.
This work was supported by the Consiglio Nazionale delle Ricerche of Italy.
References
- Asada K (1992) Ascorbate peroxidase-hydrogen peroxide scavenging enzyme in plants. Physiol Plant 85: 235–241 [Google Scholar]
- Atal N, Saradhi PP, Mohanty P (1991) Inhibition of chloroplast photochemical reactions by treatments of wheat seedlings with low concentration of cadmium: analysis of electron transport activities and changes in fluorescence yield. Plant Cell Physiol 32: 943–951 [Google Scholar]
- Baryla A, Carrier P, Franck F, Coulomb C, Sahut C, Havaux M (2001) Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: causes and consequences for photosynthesis and growth. Planta 212: 696–709 [DOI] [PubMed] [Google Scholar]
- Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Carrier JM, Neve N (1979) Oxidation-reduction states of pyridine nucleotides measured by an adapted enzymatic cycling method in maize leaves submitted to anoxia. Aust J Plant Physiol 23: 331–340 [Google Scholar]
- Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2: 764–817 [DOI] [PubMed] [Google Scholar]
- Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212: 475–486 [DOI] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Lubben TH, Keegstra K (1985) Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into the chloroplasts. J Biol Chem 260: 3691–3696 [PubMed] [Google Scholar]
- Dafré AL, Sies H, Akerboom T (1996) Protein S-thiolation and regulation of microsomal glutathione transferase activity by the glutathione redox couple. Arch Biochem Biophys 332: 288–294 [DOI] [PubMed] [Google Scholar]
- Das P, Samantaray S, Rout GR (1998) Studies on cadmium toxicity in plants: a review. Environ Poll 96: 29–36 [DOI] [PubMed] [Google Scholar]
- Fediuc E, Erdei L (2002) Physiological and biochemical aspects of cadmium toxicity and protective mechanisms induced in Phragmites australis and Typha latifolia. J Plant Physiol 159: 265–271 [Google Scholar]
- Foyer CH, Rennenberg H (2000) Regulation of glutathione synthesis and its role in abiotic and biotic stress. In H Rennenberg, C Brunold, LJ De Kok, I Stulen, eds, Sulfur Nutrition and Sulfur Assimilation in Higher Plants. SPB Academic Publishing, The Hague, The Netherlands, pp 127–153
- Fracheboud Y, Haldimann P, Leipner J, Stamp P (1999) Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). J Exp Bot 50: 1533–1540 [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) Relationship between the quantum yield of photosynthetic electron transport and the quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Griffith OW (1980) Determination of glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106: 207–212 [DOI] [PubMed] [Google Scholar]
- Hutchison RS, Groom Q, Ort DR (2000) Differential effects of chilling-induced photooxidation on the redox regulation of photosynthetic enzymes. Biochemistry 39: 6679–6688 [DOI] [PubMed] [Google Scholar]
- Iannelli MA, Pietrini F, Fiore L, Petrilli L, Massacci A (2002) Antioxidant response to cadmium in Phragmites australis plants. Plant Physiol Biochem 40: 977–982 [Google Scholar]
- Klapheck S, Zimmer I, Cosse H (1990) Scavenging of hydrogen peroxide in the endosperm of Ricinus communis by ascorbate peroxidase. Plant Cell Physiol 31: 1005–1013 [Google Scholar]
- Krupa Z, Moniak M (1998) The stage of leaf maturity implicates the response of the photosynthetic apparatus to cadmium toxicity. Plant Sci 138: 149–156 [Google Scholar]
- Küpper H, Küpper F, Spiller M (1996) Environmental relevance of heavy metal substituted chlorophylls using the example of water plants. J Exp Bot 47: 259–266 [Google Scholar]
- Küpper H, Küpper F, Spiller M (1998) In situ detection of heavy metal substituted chlorophylls in water plants. Photosynth Res 58: 123–133 [Google Scholar]
- Küpper H, Šetlík I, Küpper F, Spiller M, Prášil O (2002) Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. J Phycol 38: 429–441 [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Leustek K, Saito K (1999) Sulfate transport and assimilation in plants. Plant Physiol 120: 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In M Baltscheffsky, ed, Current Research in Photosynthesis. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 471–474
- Lombi E, Zhao FJ, Dunham SJ, McGrath SP (2000) Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol 145: 11–20 [Google Scholar]
- Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126: 993–1000, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik D, Sheoran IS, Singh R (1992) Carbon metabolism in leaves of cadmium-treated wheat seedlings. Plant Physiol Biochem 30: 223–229 [Google Scholar]
- Mannervik B, Guthenberg C (1981) Glutathione transferase (human placenta). Methods Enzymol 77: 231–235 [DOI] [PubMed] [Google Scholar]
- Matsumura H, Miyachi S (1980) Cycling assay for nicotinamide adenine dinucleotides. Methods Enzymol 69: 465–470 [Google Scholar]
- May MJ, Vernoux T, Leaver C, van Montagu M, Inzé D (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49: 649–667 [Google Scholar]
- McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein. J Biol Chem 224: 6049–6055 [PubMed] [Google Scholar]
- Mendelssohn IA, McKee KL, Kong T (2001) A comparison of physiological indicators of sublethal cadmium stress in wetland plants. Environ Exp Bot 46: 263–275 [Google Scholar]
- Navari-Izzo F, Quartacci MF (2001) Phytoremediation of metals. Tolerance mechanisms against oxidative stress. Minerva Biotechnol 13: 73–85 [Google Scholar]
- Nocito FF, Pirovano L, Cocucci M, Sacchi GA (2002) Cadmium-induced sulfate uptake in maize roots. Plant Physiol 129: 1872–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Arisi A, Jouanin L, Kunert K, Rennenberg H, Foyer C (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49: 623–647 [Google Scholar]
- Olmos E, Hellin E (1996) Mechanisms of salt tolerance in a cell line of Pisum sativum: biochemical and physiological aspects. Plant Sci 120: 37–45 [Google Scholar]
- Ow DW (1996) Heavy metal tolerance genes: prospective tools for phytoremediation. Res Conserv Recycl 18: 135–149 [Google Scholar]
- Portis AR (2003) Rubisco activase: Rubisco's catalytic chaperone. Photosynth Res 75: 11–27 [DOI] [PubMed] [Google Scholar]
- Prasad TK (1996) Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: changes in antioxidant system, oxidation of proteins and lipids, and proteases activities. Plant J 10: 1017–1026 [Google Scholar]
- Ramos I, Esteban E, Lucena JJ, Garate A (2002) Cadmium uptake and subcellular distribution in plants of Lactica sp. Cd-Mn interaction. Plant Sci 162: 761–767 [Google Scholar]
- Romero-Puertas MC, Palma JM, Gomez M, Del Rio LA, Sandalio LM (2002) Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ 25: 677–686 [Google Scholar]
- Sanità di Toppi L, Gabbrielli R (1999) Responses to cadmium in higher plants. Environ Exp Bot 41: 105–130 [Google Scholar]
- Schürmann P, Jacquot J-P (2000) Plant thioredoxin systems revisited. Annu Rev Plant Biol 51: 371–400 [DOI] [PubMed] [Google Scholar]
- Schutzendubel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127: 887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD (1990) Feedback limitation of photosynthesis and the physiological role of ribulose bisphosphate carboxylase carbamylation. Bot Magazine Tokyo 2: 87–105 [Google Scholar]
- Sharkey TD, Savitch LV, Butz ND (1991) Photometric method for routine determination of Kcat and carbamylation of Rubisco. Photosynth Res 28: 41–48 [DOI] [PubMed] [Google Scholar]
- Siedleka A, Krupa Z (1999) Cd/Fe interaction in higher plants: its consequences for the photosynthetic apparatus. Photosynthetica 36: 321–331 [Google Scholar]
- Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis(2-nitrobenzoic acid). Anal Biochem 175: 408–413 [DOI] [PubMed] [Google Scholar]
- Somashekaraiah BV, Padmaja K, Prasad ARK (1992) Phytoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorophyll degradation. Physiol Plant 85: 85–89 [Google Scholar]
- Stitt M, Schulze D (1994) Does Rubisco control the rate of photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ 17: 465–487 [Google Scholar]
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]