Abstract
Presenilin 1 (PS1) plays a pivotal role in Notch signaling and the intracellular metabolism of the amyloid β-protein. To understand intracellular signaling events downstream of PS1, we investigated in this study the action of PS1 on mitogen-activated protein kinase pathways. Overexpressed PS1 suppressed the stress-induced stimulation of stress-activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK) in human embryonic kidney 293 cells. Interestingly, two functionally inactive PS1 mutants, PS1(D257A) and PS1(D385A), failed to inhibit UV-stimulated SAPK/JNK. Furthermore, H2O2- or UV-stimulated SAPK activity was higher in mouse embryonic fibroblast (MEF) cells from PS1-null mice than in MEF cells from PS+/+ mice. MEFPS1(−/−) cells were more sensitive to the H2O2-induced apoptosis than MEFPS1(+/+) cells. Ectopic expression of PS1 in MEFPS1(−/−) cells suppressed H2O2-stimulated SAPK/JNK activity and apoptotic cell death. Together, our data suggest that PS1 inhibits the stress-activated signaling by suppressing the SAPK/JNK pathway.
Keywords: apoptosis, c-Jun NH2-terminal kinase, presenilin 1, γ-secretase, stress-activated protein kinase
Introduction
Alzheimer's disease (AD) is a progressive and fatal neurodegenerative disorder characterized by the loss of neurons in brain regions involved in learning and memory. One of the early events in the development of AD is the accumulation of amyloid β-peptide (Aβ) in the cerebral cortex. Aβ1–42 is produced from amyloid β-protein precursor (APP) by both β-secretase– and γ-secrease–mediated processing (Selkoe 1998). Most cases of early-onset familial AD (FAD) are caused by mutations in the genes encoding presenilin 1 (PS1) (Sherrington et al. 1995) and presenilin 2 (PS2) (Levy-Lahad et al. 1995; Rogaev et al. 1995). Presenilins appear to be required for the processing of APP to Aβ (by γ-secretase activation) (De Strooper et al. 1998; Wolfe et al. 1999; Li et al. 2000).
PS1 is a membrane protein that contains eight putative transmembrane domains and primarily localized to intracellular membranes including the endoplasmic reticulum and Golgi apparatus (Cook et al. 1996; Doan et al. 1996; Kovacs et al. 1996; De Strooper et al. 1997). Presenilins are homologous to two Caenorhabitis elegans gene products, SEL-12 and HOP1, both of which facilitate Notch/LIN-12 signaling (Artavanis-Tsakonas et al. 1999). Indeed, PS1 plays a pivotal role in Notch signaling by regulation of the Notch processing through γ-secretase activation (Levitan and Greenwald 1995; Li and Greenwald 1997; Chan and Jan 1999; De Strooper et al. 1999; Ray et al. 1999; Li et al. 2000). Notch signaling appears to be associated with the regulatory mechanisms of a variety of cellular events including cell fate control during embryonic development, differentiation, cell growth, and apoptosis (Artavanis-Tsakonas et al. 1999).
The mitogen-activated protein kinase (MAPK) pathway is one of the major signaling pathways that transmit intracellular signals initiated by extracellular stimuli to the nucleus. The MAPK pathway regulates a variety of cellular functions, including cell proliferation, differentiation, and death (Minden and Karin 1997; Ip and Davis 1998; Schaeffer and Weber 1999). The MAPK pathway includes three distinct components: MAPKs, MAPK kinases (MAPKKs), and MAPKK kinases (MAPKKKs). MAPKKKs phosphorylate and activate MAPKKs, which in turn phosphorylate and activate MAPKs. When activated, MAPKs phosphorylate various proteins that include transcription factors, thereby regulating gene expression or other cellular functions. The family of mammalian MAPKs includes three subfamilies: extracellular signal-regulated kinase (Erk), stress-activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK) and p38 MAPK (Boulton et al. 1991; Han et al. 1994; Cobb and Goldsmith 1995; Kyriakis and Avruch 1996; Su and Karin 1996; Fanger et al. 1997). The Erk pathway consists of Erk and upstream kinases that include MAPK/Erk kinase 1 and Raf-1 (Schaeffer and Weber 1999). This pathway is often stimulated by mitogenic stimuli. The p38 MAPK pathway consists of p38 MAPK and its upstream kinases that include MAPKKs such as MKK3 or MKK6 and MAPKKK such as ASK1 or TAK1 (Schaeffer and Weber 1999). The SAPK/JNK pathway consists of SAPK/JNK and upstream kinases that include MAPKKs such as SEK1/JNKK1/MKK4 or MKK7, and MAPKKKs such as MEKK1, ASK1, or TAK1 (Minden and Karin 1997; Ip and Davis 1998). Like the p38 pathway, the SAPK/JNK pathway can be activated by a variety of cellular stresses that include genotoxic stress, free radicals, heat shock, osmotic shock, ischemia, and proinflammatory cytokines such as tumor necrosis factor-α and interleukin-1β (Minden and Karin 1997; Ip and Davis 1998). When activated, SAPK/JNK can phosphorylate and activate c-Jun or other transcription factors including ATF-2 and Elk-1 (Gupta et al. 1995; Minden et al. 1995; Yang et al. 1998). Although the physiological role of SAPK/JNK is not fully understood, SAPK/JNK has been shown to be involved in the cellular mechanism of apoptotic cell death under certain conditions (Minden and Karin 1997; Ip and Davis 1998). In particular, a series of studies using mice have demonstrated a pivotal role of SAPK/JNK in apoptotic cell death and excitotoxic neuronal death (Yang et al. 1997; Dong et al. 1998; Kuan et al. 1999; Tournier et al. 2000).
To better understand the intracellular signaling downstream of PS1, we investigated whether PS1 could modulate the MAPK signaling pathways. We report in this study that PS1 inhibits the SAPK/JNK pathway and that the PS1-induced suppression of the SAPK/JNK pathway requires functionally active PS1. Our findings suggest that PS1 inhibits stress-activated signaling by suppressing the SAPK/JNK pathway.
Materials and Methods
Plasmids
cDNA clones encoding wild-type PS1 or its mutants were inserted into pcDNA3-FLAG vector as described (Kovacs et al. 1996). pcDNA3-SAPKβ-FLAG (J. Woodgett, Ontario Cancer Institute, Toronto, Canada), pcDNA3-SAPKβ-hemagglutinin (HA), pCEP4-HA-ERK2 (M.H. Cobb, University of Texas Southwestern Medical Center, Dallas, TX), pcDNA3-p38-FLAG (R.J. Ulevitch, The Scripps Research Institute, La Jolla, CA), pEBG-SEK1 (L.I. Zou, Harvard Medicaal School, Boston, MA), pcDNA3-HA-MEKK1 (G.L. Johnson, University of Colorado, Denver, CO), pcDNA3-ΔMEKK1, and pEXV-Rac1V12 (A. Hall, University College London, London, UK) were described previously (Shim et al. 1996, Shim et al. 2000).
Cell Culture and DNA Transfection
Human embryonic kidney (HEK) 293 cells, B103 rat neuroblastoma cells, and mouse embryonic fibroblasts (MEFs) from wild-type or PS1-null mice (Shen et al. 1997) were grown in DME (GIBCO BRL) supplemented with 10% fetal bovine serum. MEFPS1(+/+) and MEFPS1(−/−) cells (J. Shen, Brigham and Women's Hospital, Harvard Medical School, Boston, MA) at the passage between three and six were used, and they were in the same passage at the experiments. Cultured cells were transfected by calcium phosphate method or LipofectAMINE (GIBCO BRL). To establish cells that stably expressed ectopic PS1, B103 cells were transfected with pcDNA3 empty vector or pcDNA3-PS1. After 48 h of transfection, the cells were maintained in complete medium containing 500 μg/ml G418 to select neomycin-resistant cells. Expression of ectopic PS1 in the neomycin-resistant cells was analyzed by immunoblot using anti-PS1 antibody.
Apoptosis Assay
After the proper treatments, cultured cells were fixed with 70% ethanol on ice for 1 h and then stained with 10 μg/ml propidium iodide. The propidium iodide–stained cells were analyzed by flow cytometry (FACSCalibur®; Becton Dickinson). Alternatively, cells were transfected with indicated vector constructs plus pEGFP. After 24 h of transfection, the cells were treated with indicated apoptotic stimuli. The cells were fixed with 4% formaldehyde for 30 min and then stained with 10 μg/ml of DAPI for 10 min. The DAPI-stained nuclei were examined for apoptotic morphology with a ZEISS Axiovert fluorescence microscope. Green fluorescent protein (GFP)–expressing cells were scored for apoptotic nuclei. More than 200 cells were counted in each experiment, and data from three independent experiments were analyzed.
Immune Complex Kinase Assay
Cultured cells were lysed in a lysis buffer, and the cell lysates were subjected to immunoprecipitation using appropriate antibodies (Park et al. 2000; Shim et al. 2000). The resultant immunopellets were assayed for activities of the indicated protein kinases (Park et al. 2000). GST–c-Jun (1–79) was used as a substrate for SAPK/JNK, GST–ATF2 (1–109) for p38, myelin basic protein for Erk2, GST–SAPKβ(K55R) for SEK1, and GST–SEK1(K129R) for MEKK1. The phosphorylated proteins were resolved by SDS-PAGE on a 10% polyacrylamide gel and analyzed with a Fuji BAS 2500 PhosphorImager. The cell lysates were also subjected to immunoblot analysis using the indicated antibodies. The bands in the immunoblot were visualized using an enhanced chemiluminescence system (Amersham Pharmacia Biotech).
Results
PS1 Suppresses the SAPK/JNK Pathway
To investigate whether PS1 might modulate a function of the SAPK/JNK pathway, we transfected HEK293 cells with SAPKβ/JNK3 and PS1 constructs (Fig. 1 A). Exposure of the transfected cells to 80 J/m2 UV light resulted in stimulation of SAPK activity. Interestingly, ectopically expressed PS1 suppressed the UV-stimulated SAPK activity. Similarly, ectopic PS1 suppressed the SAPK activity stimulated by sorbitol (Fig. 1 A) or other stresses including anisomycin (data not shown). PS1 also inhibited p38 MAPK activity stimulated by UV light, whereas it did not affect the PMA-induced stimulation of Erk2 activity (Fig. 1 B). In the following experiments, we looked at the mechanism by which PS1 inhibited the SAPK/JNK pathway.
Figure 1.
PS1 suppresses the SAPK/JNK pathway. (A) Overexpressed PS1 inhibited the stress-stimulated SAPK/JNK activity in HEK293 cells. (B) Effect of PS1 on p38 or Erk2 activity. In A and B, HEK293 cells in 100-mm dishes were transiently transfected with the PS1 construct (4 μg) along with HA-SAPKβ (1 μg), HA-p38 (1 μg), or HA-Erk2 (1 μg) constructs, as indicated. After 48 h of transfection, the cells were exposed to UV light (80 J/m2), sorbitol (0.6 M), or TPA (200 nM) and further incubated for 30 min. The cell lysates were examined for the indicated protein kinase activities by immune complex kinase assays. Protein phoshorylation was quantified using a PhosphorImager. IB, immunoblot analysis of transfected cells with the indicated antibodies.
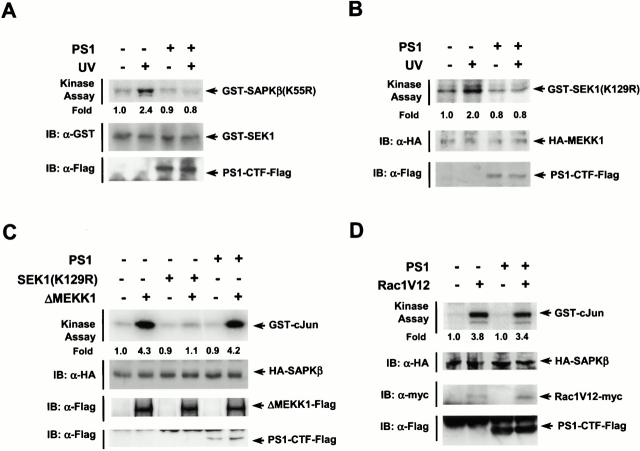
The SAPK/JNK pathway is composed of SAPK/JNK and its upstream kinases that include SEK1 and MEKK1 (Minden and Karin 1997; Ip and Davis 1998). When activated, MEKK1 can phosphorylate and activate SEK1, which then phosphorylates and activates SAPK/JNK. To better understand how PS1 suppressed the SAPK activity in cells, we examined a possible action of PS1 on SEK1 and MEKK1. Ectopic PS1 inhibited the UV-stimulated SEK1 or MEKK1 activity in transfected HEK293 cells (Fig. 2A and Fig. B). Overexpressed PS1, however, failed to inhibit SAPK activity stimulated by coexpression of a constitutively active form of MEKK1, ΔMEKK1 (Fig. 2 C). These results suggest that PS1 might suppress the SAPK/JNK pathway by acting on a site or sites upstream of MEKK1. Rac1, a member of the small GTP-binding protein Rho family, has been shown to act upstream of MEKK1 to stimulate the SAPK/JNK pathway (Coso et al. 1995; Minden et al. 1995). Overexpression of Rac1V12, a constitutively active mutant of Rac1, induced SAPK stimulation in transfected HEK293 cells (Fig. 2 D), and PS1 did not repress the Rac1V12-induced SAPK stimulation. This suggests that PS1 may inhibit the SAPK/JNK pathway by acting on a site upstream of Rac1 or by a pathway independent of the Rac1-mediated signaling.
Figure 2.
PS1 inhibits UV-stimulated activity of SEK1 or MEKK1. (A) PS1 inhibits SEK1 activity. (B) PS1 inhibits MEKK1 activity. In A and B, HEK293 cells in 100-mm dishes were transfected with pcDNA3-PS1-Flag (4 μg), pEBG-SEK1 (1 μg), and pcDNA3-HA-MEKK1 (1 μg), as indicated. After 48 h of transfection, the cells were exposed to 80 J/m2 UV light, incubated further for 30 min, and lysed. For measuring GST–SEK1 activity, GST–SEK1 was isolated from the cell lysates using glutathione–agarose beads and then assayed for phosphorylation of GST–SAPKβ(K55R). For measuring HA-MEKK1 activity, the cell lysates were subjected to immunoprecipitation using anti-HA antibody. The immunopellets were assayed for MEKK1 activity by immune complex kinase assay. (C) PS1 does not affect ΔMEKK1-stimulated SAPK activity. HEK293 cells in 100-mm dishes were cotransfected with pcDNA3-PS1-Flag (4 μg), pcDNA3-SEK1(K129R) (1 μg), and pcDNA3-ΔMEKK1-Flag (1 μg) along with pcDNA3-HA-SAPKβ (1 μg), as indicated. (D) PS1 does not change Rac1-stimulated SAPK activity. HEK293 cells in 100-mm dishes were cotransfected with pcDNA3-PS1-Flag (4 μg) and pcDNA3-Rac1V12 (1 μg) along with pcDNA3-HA-SAPKβ (1 μg). In C and D, the transfected cells were lysed after 48 h of transfection. SAPK activity in the cell lysates was measured by immune complex kinase assay using mouse anti-HA antibody. IB, immunoblot analysis of transfected cells with the indicated antibodies.
The PS1-induced Suppression of the SAPK/JNK Pathway Requires Functionally Active PS1
In the following experiments, we examined the effects of several FAD-linked PS1 mutants on the SAPK pathway. The FAD-linked PS1 mutants M146V, C410Y, and L286V inhibited the UV-stimulated SAPK activity in cotransfected HEK293 cells, just as wild-type PS1 did (Fig. 3 A). In contrast, the biologically inactive transmembrane aspartate mutants of PS1 (D257A and D385A) could not inhibit the UV-induced SAPK stimulation (Fig. 3 B). The two aspartic acid residues D257 and D385 in PS1 are essential for PS1 endoproteolysis and γ-secretase activation (Wolfe et al. 1999). The UV-induced SAPK stimulation was also repressed by overexpression of PS1ΔEx9 (Fig. 3 B). PS1ΔEx9 is a functional PS1 mutant lacking exon 9 (amino acids 290–319), which contains an endoproteolytic site, M298 (Thinakaran et al. 1996; Podlisny et al. 1997). Thus, PS1ΔEx9 does not undergo endoproteolysis but is competent for γ-secretase activation (Li et al. 2000). Our data, therefore, suggest that γ-secretase activation may be important for PS1 to suppress the SAPK/JNK pathway.
Figure 3.

Effects of PS1 mutants on SAPK/JNK. (A) Effects of FAD-linked PS1 variants on UV-stimulated SAPK activity. HEK293 cells in 100-mm dishes were cotransfected with pcDNA3-HA-SAPKβ (1 μg) and pcDNA3-Flag vector (4 μg) expressing each PS1 variant (wild type, M146V, C410Y, or L286V), as indicated. After 48 h of transfection, the cells were exposed to UV light (80 J/m2) and incubated further for 30 min. The cell lysates were examined for SAPK activity by immune complex kinase assay using mouse anti-HA antibody. (B) Effects of endoproteolysis mutants of PS1 on UV-stimulated SAPK activity. HEK293 cells in 100-mm dishes were cotransfected with pcDNA3-HA-SAPKβ (1 μg) and pcDNA3-Flag vector (4 μg) expressing each PS1 variant (wild type, D257A, D385A, or ΔEx9), as indicated. The transfected cells were exposed to UV light (80 J/m2), and SAPK activity in the cell lysates was measured by immune complex kinase assay using mouse anti-HA antibody. IB, immunoblot analysis of transfected cells with anti-HA or anti-Flag antibody. CTF, the COOH-terminal fragment of PS1 after PS1 endoproteolysis.
PS1 Protects Neuroblastoma B103 Cells from Apoptosis Mediated by SAPK/JNK Activation
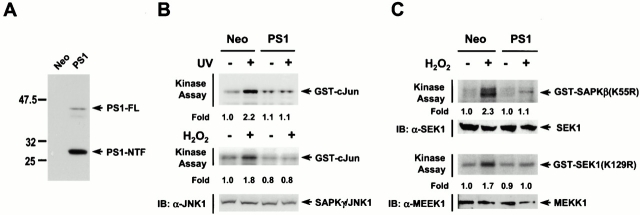
Next, we stably transfected rat neuroblastoma B103 cells with PS1 (B103-PS1 cells) or with an empty vector (B103-neo cells). We then investigated the inhibitory effect of PS1 on SAPK/JNK. Immunoblot data confirmed the expression of PS1 in B103-PS1 cells, but not in B103-neo control cells (Fig. 4 A). Exposure of the B103-neo control cells to UV light or H2O2 resulted in the stimulation of endogenous SAPK/JNK, whereas the UV- or H2O2-induced stimulation of SAPK/JNK activity was repressed in B103-PS1 cells (Fig. 4 B). Stably expressed ectopic PS1 also inhibited the H2O2-induced stimulation of endogenous SEK1 or MEKK1 in B103-PS1 cells (Fig. 4 C).
Figure 4.
Overexpressed PS1 inhibits the stress-induced stimulation of the SAPK/JNK pathway in B103 rat neuroblastoma cells. (A) Establishment of B103 cells stably expressing PS1. B103 cells were transfected with pcDNA3-PS1 or pcDNA3 empty vector, and expression of PS1 in isolated clones was examined by immunoblot analysis with mouse anti-PS1 monoclonal antibody. FL, full-length; NTF, the NH2-terminal fragment of PS1 after PS1 endoproteolysis. (B) Overexpressed PS1 inhibits the UV- or H2O2-stimulated activity of endogenous SAPK/JNK in B103 cells. Either B103-neo or B103-PS1 cells were exposed to UV light (80 J/m2) or H2O2 (200 μM for 2 hr). The cell lysates were subjected to immunoprecipitation using mouse anti–SAPKγ/JNK1 antibody. The resultant immunopellets were examined for SAPKγ/JNK1 activity by immune complex kinase assay. (C) Overexpressed PS1 inhibits the H2O2-induced stimulation of endogenous SEK1 or MEKK1. B103-neo or B103-PS1 cells were exposed to 200 μM H2O2 for 2 h. Enzymatic activity of SEK1 or MEKK1 in B103-neo or B103-PS1 cells were examined by immune complex kinase assay using anti-SEK1 or anti-MEKK1 antibody.
SAPK/JNK has been shown to mediate stress-induced apoptotic cell death under certain conditions (Xia et al. 1995; Verheij et al. 1996; Minden and Karin 1997; Ip and Davis 1998). We examined whether PS1 could modulate SAPK-involved apoptotic cell death in B103 cells (Fig. 5). Exposure of B103 cells to H2O2 resulted in an increase in apoptosis (Fig. 5 A). The H2O2-induced apoptotic cell death was markedly reduced in cells transfected with SEK1(K129R), a dominant negative form of SEK1. Apoptosis induced by ΔMEKK1 overexpression was also repressed in B103 cells transfected with SEK1(K129R) (Fig. 5 B). These results suggest that the SAPK/JNK signaling cascade is involved in the mechanism of the H2O2- or ΔMEKK1-induced apoptosis in B103 cells. Overexpressed PS1 prevented apoptotic cell death induced by H2O2 (Fig. 5 A), but not by ΔMEKK1 (Fig. 5 B). These data suggest that PS1 might prevent the H2O2-induced apoptotic cell death through inhibiting the SAPK/JNK pathway and that the inhibition occurs a site upstream of MEKK1 in the SAPK/JNK pathway. Our data also show that PS1ΔEx9, which retains an ability of γ-secretase activation (Li et al. 2000) and suppressed the SAPK/JNK pathway (Fig. 3), also inhibited H2O2-induced apoptotic cell death (Fig. 5 A). In contrast, PS1(D257A), which lacks an ability of γ-secretase activation (Wolfe et al. 1999) and could not suppress the SAPK pathway (Fig. 3), failed to block the H2O2-induced apoptotic cell death.
Figure 5.

PS1 suppresses H2O2-induced apoptotic cell death in B103 cells. B103 cells in 35-mm dishes were transfected with pEGFP (0.5 μg) along with pcDNA3-Flag expressing each PS1 variant (wild type, D257A, or ΔEx9) (1 μg), pcDNA3-HA-SEK1(K129A) (0.5 μg), or pCMV5-ΔMEKK1 (0.5 μg) (B), as indicated. Where indicated, after 48 h of transfection, the cells were exposed to H2O2 (0.5 mM) for 6 h (A). The cells were fixed with 4% formaldehyde and stained with DAPI (10 μg/ml) for 30 min. GFP-expressing cells were counted for apoptotic nuclei. More than 200 cells were counted in each experiment. The data represent results from three independent experiments.
Elevated SAPK/JNK Activity in Cells from PS1-null Mice
Next, we examined the H2O2-induced JNK1/SAPKγ stimulation in MEF cells from PS1+/+ and PS1−/− mice (Shen et al. 1997). Interestingly, the endogenous JNK1/SAPKγ activity both at the basal state and in the H2O2-stimulated state was higher in MEFPS1(−/−) cells than in MEFPS1(+/+) cells (Fig. 6 A). Ectopic expression of PS1 in the MEFPS1(−/−) cells resulted in a decrease in the H2O2-stimulated SAPK/JNK activity (Fig. 6 B). We also examined H2O2-induced apoptotic cell death in MEFPS1(+/+) and MEFPS1(−/−) cells. MEFPS1(−/−) cells were more sensitive to H2O2-induced apoptosis than MEFPS1(+/+) cells (Fig. 6 C). Furthermore, overexpressed PS1 converted MEFPS1(−/−) cells to be more resistant to H2O2-induced apoptosis (Fig. 6 D). On the other hand, biologically inactive PS1(D257A), which failed to suppress the SAPK pathway (Fig. 3), did not lower the H2O2-induced apoptotic cell death in the MEFPS1(−/−) cells. Together, these data strongly suggest that PS1 functions as a negative regulator of the SAPK/JNK pathway and that PS1 suppresses H2O2-induced apoptosis by inhibiting the SAPK/JNK pathway.
Figure 6.
SAPK/JNK activity and H2O2-induced apoptotic cell death was higher in MEFPS1(−/−) cells than in MEFPS1(+/+) cells. (A) SAPK/JNK activity in MEF cells from PS1+/+ and PS1−/− mice. MEFPS1(+/+) and MEFPS1(−/−) cells were exposed to 200 μM H2O2 for 2 h. The cell lysates were subjected to immunoprecipitation using anti–SAPKγ/JNK1 antibody. The resultant immunopellets were examined for SAPKγ/JNK1 activity by immune complex kinase assay. (B) Overexpression of PS1 results in a decrease in the H2O2-stimulated SAPK/JNK activity in MEFPS1(−/−) cells. MEF cells from PS1−/− mice were transiently transfected with pcDNA3-HA-SAPKβ (1 μg) and pcDNA3-PS1-Flag (4 μg), as indicated. After 48 h of transfection, the cells were exposed to 200 μM H2O2 for 2 h and then examined for SAPK/JNK activity by immune complex kinase assay using anti-HA antibody. (C) H2O2-induced apoptotic cell death in MEFPS1(+/+) and MEFPS1(−/−) cells. MEFPS1(+/+) or MEFPS1(−/−) cells were exposed to 500 μM H2O2 for 12 h, fixed with 70% ethanol, and then stained with 10 μg/ml propidium iodide. The percentage of apoptotic cells with sub-G1 DNA content was determined by measuring the fluorescence of the propidium iodide-stained cells using FACScan®. (D) Overexpressed PS1 suppresses H2O2-induced apoptosis in MEFPS1(−/−) cells. MEFPS1(−/−) cells were transfected with plasmids expressing the indicated proteins along with pEGFP. After 40 h of transfection, the cells were exposed to 500 μM H2O2 for 12 h and then stained with DAPI. GFP-positive cells were scored for DAPI-stained apoptotic nuclei with a ZEISS Axiovert fluorescence microscope.
Discussion
In this study, we demonstrate that PS1 inhibits the SAPK/JNK pathway. Ectopically expressed PS1 blocked the stress-induced stimulation of SAPK/JNK and its upstream kinases including SEK1 and MEKK1. FAD-linked PS1 mutants, M146V, C410Y, and L286V, were also able to inhibit the SAPK stimulation. Interestingly, biologically inactive PS1 mutants D257A and D385A, both of which have been shown to lack γ-secretase activation and PS1 endoproteolysis (Wolfe et al. 1999), failed to inhibit SAPK stimulation. Furthermore, PS1ΔEx9, which lacks the endoproteolysis site but is competent for activation of γ-secretase activity (Li et al. 2000), retained the inhibitory effect on the SAPK/JNK pathway. These data suggest that the γ-secretase activation, rather than the PS1 endoproteolysis, is required for the PS1-induced inhibition of the SAPK/JNK pathway. γ-Secretase has two major substrates, APP and Notch (De Strooper et al. 1998, De Strooper et al. 1999). The cleavage of APP or Notch by γ-secretase produces Aβ or the intracellular domain of Notch, Notch-IC, respectively. Aβ1–42 did not inhibit the SAPK/JNK activity (data not shown). In comparison, our preliminary data showed that overexpression of the Notch intracellular domain, which is the active form of intracellular Notch, resulted in suppression of SAPK/JNK activation (data not shown). These findings imply that PS1-mediated cleavage of Notch might be involved in the mechanism of PS1-induced suppression of the SAPK pathway. In this regard, Notch has been previously proposed to play a role in the regulation of the SAPK/JNK pathway (Ordentlich et al. 1998; Zecchini et al. 1999).
Several lines of evidence suggest that presenilins are involved in apoptosis (Kim and Tanzi 1997; Kim et al. 1997). Overexpression of PS2 has been shown to potentiate apoptosis of PC12 cells induced by NGF withdrawal or neurotoxic Aβ1–42 (Wolozin et al. 1998). Another study showed that ALG3, a truncated form of murine PS2, reduced T cell receptor– or Fas-induced apoptosis in a mouse T cell hybridoma (Vito et al. 1996). Studies using PS1-null mice have demonstrated that PS1 is involved in neuronal survival (Shen et al. 1997). In this study, we show that ectopic PS1 suppressed the H2O2-induced apoptosis in B103 neuroblastoma cells. Moreover, deficiency of PS1 caused an elevation in the H2O2-induced apoptosis in MEF cells from PS1-null mice, as compared with MEF cells from PS1+/+ wild-type mice. The H2O2-induced apoptosis was blocked by overexpression of SEK1(K129R), suggesting that the SAPK/JNK pathway is involved in the mechanism of the H2O2-induced apoptosis. Thus, our findings suggest that PS1, by inhibiting the SAPK pathway, can protect cells from stress-induced apoptotic cell death. The precise mechanism by which PS1 inhibits the SAPK/JNK pathway, however, needs to be further studied.
Acknowledgments
We thank Drs. R.J. Davis (University of Massachusetts, Worcester, MA), J. Woodgett, G.L. Johnson, L.I. Zon, M.H. Cobb, R.J. Ulevitch, and A. Hall for providing JNK1, SAPKβ, MEKK1, SEK1, ERK2, p38, and Rac1V12 cDNA clones, respectively; Dr. J. Shen for MEFPS1(+/+) and MEFPS1(−/−) cells; and Dr. G. Hoschek for critical reading of the manuscript.
This work was supported by the Creative Research Initiatives Program of the Korean Ministry of Science and Technology (to E.-J. Choi).
Footnotes
J.W. Kim and T.-S. Chang contributed equally to this work and should be considered co-first authors.
T.-W. Kim's present address is Taub Institute for Research on Alzheimer's Disease and the Aging Brain, Department of Pathology, College of Physicians and Surgeons, Columbia University, New York, NY 10032.
Abbreviations used in this paper: Aβ, amyloid β-peptide; AD, Alzheimer's disease; APP, amyloid β-protein precursor; Erk, extracellular signal-regulated kinase; FAD, familial AD; HA, hemagglutinin; HEK, human embryonic kidney; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MAPKK, MAPK kinase; MAPKKK, MAPKK kinase; MEF, mouse embryonic fibroblast; PS1 and PS2, presenilins 1 and 2; SAPK, stress-activated protein kinase.
References
- Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signalingcell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Boulton T.G., Nye S.H., Robbins D.J., Ip N.Y., Radziejewska E., Morgenbesser S.D., DePinho R.A., Panayotatos N., Cobb M.H., Yancopoulos G.D. ERKsa family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Chan Y.M., Jan Y.N. Presenilins, processing of β-amyloid precursor protein, and notch signaling. Neuron. 1999;23:201–204. doi: 10.1016/s0896-6273(00)80771-0. [DOI] [PubMed] [Google Scholar]
- Cobb M.H., Goldsmith E.J. How MAP kinases are regulated. J. Biol. Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Cook D.G., Sung J.C., Golde T.E., Felsenstein K.M., Wojczyk B.S., Tanzi R.E., Trojanowski J.Q., Lee V.M., Doms R.W. Expression and analysis of presenilin 1 in a human neuronal systemlocalization in cell bodies and dendrites. Proc. Natl. Acad. Sci. USA. 1996;93:9223–9228. doi: 10.1073/pnas.93.17.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coso O.A., Chiariello M., Yu J.C., Teramoto H., Crespo P., Xu N., Miki T., Gutkind J.S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Beullens M., Contreras B., Levesque L., Craessaerts K., Cordell B., Moechars D., Bollen M., Fraser P., George-Hyslop P.S., Van Leuven F. Phosphorylation, subcellular localization, and membrane orientation of the Alzheimer's disease-associated presenilins. J. Biol. Chem. 1997;272:3590–3598. doi: 10.1074/jbc.272.6.3590. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Saftig P., Craessaerts K., Vanderstichele H., Guhde G., Annaert W., Von Figura K., Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J.S., Schroeter E.H., Schrijvers V., Wolfe M.S., Ray W.J. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- Doan A., Thinakaran G., Borchelt D.R., Slunt H.H., Ratovitsky T., Podlisny M., Selkoe D.J., Seeger M., Gandy S.E., Price D.L., Sisodia S.S. Protein topology of presenilin 1. Neuron. 1996;17:1023–1030. doi: 10.1016/s0896-6273(00)80232-9. [DOI] [PubMed] [Google Scholar]
- Dong C., Yang D.D., Wysk M., Whitmarsh A.J., Davis R.J., Flavell R.A. Defective T cell differentiation in the absence of Jnk1 . Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- Fanger G.R., Gerwins P., Widmann C., Jarpe M.B., Johnson G.L. MEKKs, GCKs, MLKs, PAKs, TAKs, and tplsupstream regulators of the c-Jun amino-terminal kinases? Curr. Opin. Genet. Dev. 1997;7:67–74. doi: 10.1016/s0959-437x(97)80111-6. [DOI] [PubMed] [Google Scholar]
- Gupta S., Campbell D., Derijard B., Davis R.J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Han J., Lee J.D., Bibbs L., Ulevitch R.J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Ip Y.T., Davis R.J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr. Opin. Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Tanzi R.E. Presenilins and Alzheimer's disease. Curr. Opin. Neurobiol. 1997;7:683–688. doi: 10.1016/s0959-4388(97)80089-x. [DOI] [PubMed] [Google Scholar]
- Kim T.W., Pettingell W.H., Jung Y.K., Kovacs D.M., Tanzi R.E. Alternative cleavage of Alzheimer-associated presenilins during apoptosis by a caspase-3 family protease. Science. 1997;277:373–376. doi: 10.1126/science.277.5324.373. [DOI] [PubMed] [Google Scholar]
- Kovacs D.M., Fausett H.J., Page K.J., Kim T.W., Moir R.D., Merriam D.E., Hollister R.D., Hallmark O.G., Mancini R., Felsenstein K.M. Alzheimer-associated presenilins 1 and 2neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat. Med. 1996;2:224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- Kuan C.Y., Yang D.D., Samanta Roy D.R., Davis R.J., Rakic P., Flavell R.A. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Kyriakis J.M., Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- Levitan D., Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995;377:351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wasco W., Poorkaj P., Romano D.M., Oshima J., Pettingell W.H., Yu C.E., Jondro P.D., Schmidt S.D., Wang K. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Li X., Greenwald I. HOP-1, a Caenorhabditis elegans presenilin, appears to be functionally redundant with SEL-12 presenilin and to facilitate LIN-12 and GLP-1 signaling. Proc. Natl. Acad. Sci. USA. 1997;94:12204–12209. doi: 10.1073/pnas.94.22.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-M., Xu M., Lai M.-T., Huang Q., Casto J.L., DiMuzio-Mower J., Harrison T., Lellis C., Nadin A., Neduvelil J.G. Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- Minden A., Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim. Biophys. Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- Minden A., Lin A., Claret F.X., Abo A., Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Ordentlich P., Lin A., Shen C.P., Blaumueller C., Matsuno K., Artavanis-Tsakonas S., Kadesch T. Notch inhibition of E47 supports the existence of a novel signaling pathway. Mol. Cell. Biol. 1998;18:2230–2239. doi: 10.1128/mcb.18.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.S., Park E., Kim M.S., Ahn K., Kim I.Y., Choi E.J. Selenite inhibits the c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) through a thiol redox mechanism. J. Biol. Chem. 2000;275:2527–2531. doi: 10.1074/jbc.275.4.2527. [DOI] [PubMed] [Google Scholar]
- Podlisny M.B., Citron M., Amarante P., Sherrington R., Xia W., Zhang J., Diehl T., Levesque G., Fraser P., Haass C. Presenilin proteins undergo heterogeneous endoproteolysis between Thr291 and Ala299 and occur as stable N- and C-terminal fragments in normal and Alzheimer brain tissue. Neurobiol. Dis. 1997;3:325–337. doi: 10.1006/nbdi.1997.0129. [DOI] [PubMed] [Google Scholar]
- Ray W.J., Yao M., Nowotny P., Mumm J., Zhang W., Wu J.Y., Kopan R., Goate A.M. Evidence for a physical interaction between presenilin and Notch. Proc. Natl. Acad. Sci. USA. 1999;96:3263–3268. doi: 10.1073/pnas.96.6.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaev E.I., Sherrington R., Rogaeva E.A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer H.J., Weber M.J. Mitogen-activated protein kinasesspecific messages from ubiquitous messengers. Mol. Cell. Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe E.J. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- Shen J., Bronson R.T., Chen D.F., Xia W., Selkoe D.J., Tonegawa S. Skeletal and CNS defects in presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Shim J., Lee H., Park J., Kim H., Choi E.J. A non-enzymatic p21 protein inhibitor of stress-activated protein kinases. Nature. 1996;381:804–806. doi: 10.1038/381804a0. [DOI] [PubMed] [Google Scholar]
- Shim J., Park H.S., Kim M.J., Park J., Park E., Cho S.G., Eom S.J., Lee H.W., Joe C.O., Choi E.J. Rb protein down-regulates the stress-activated signals through inhibiting c-Jun N-terminal kinase/stress-activated protein kinase. J. Biol. Chem. 2000;275:14107–14111. doi: 10.1074/jbc.275.19.14107. [DOI] [PubMed] [Google Scholar]
- Su B., Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- Thinakaran G., Borchelt D.R., Lee M.K., Slunt H.H., Spitzer L., Kim G., Ratovitsky T., Davenport F., Nordstedt C., Seeger M. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Tournier C., Hess P., Yang D.D., Xu J., Turner T.K., Nimnual A., Bar-Sagi D., Jones S.N., Flavell R.A., Davis R.J. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Verheij M., Bose R., Lin X.H., Yao B., Jarvis W.D., Grant S., Birrer M.J., Szabo E., Zon L.I., Kyriakis J.M. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Vito P., Lacana E., D'Adamio L. Interfering with apoptosisCa2+-binding protein ALG-2 and Alzheimer's disease gene ALG-3. Science. 1996;271:521–525. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- Wolfe M.S., Xia W., Ostaszewski B.L., Diehl T.S., Kimberly W.T., Selkoe D.J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Wolozin B., Alexander P., Palacino J. Regulation of apoptosis by presenilin 1. Neurobiol. Aging. 1998;19:S23–S27. doi: 10.1016/s0197-4580(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Xia Z., Dickens M., Raingeaud J., Davis R.J., Greenberg M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yang D.D., Kuan C.-Y., Whitmarsh A.J., Rincon M., Zheng T.S., Davis R.J., Rakic P., Flavell R.A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Yang S.H., Whitmarsh A.J., Davis R.J., Sharrocks A.D. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1740–1749. doi: 10.1093/emboj/17.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecchini V., Brennan K., Martinez-Arias A. An activity of Notch regulates JNK signaling and affects dorsal closure in Drosophila . Curr. Biol. 1999;9:460–469. doi: 10.1016/s0960-9822(99)80211-5. [DOI] [PubMed] [Google Scholar]