Abstract
In mitotic cells, an error in chromosome segregation occurs when a chromosome is left near the spindle equator after anaphase onset (lagging chromosome). In PtK1 cells, we found 1.16% of untreated anaphase cells exhibiting lagging chromosomes at the spindle equator, and this percentage was enhanced to 17.55% after a mitotic block with 2 μM nocodazole. A lagging chromosome seen during anaphase in control or nocodazole-treated cells was found by confocal immunofluorescence microscopy to be a single chromatid with its kinetochore attached to kinetochore microtubule bundles extending toward opposite poles. This merotelic orientation was verified by electron microscopy. The single kinetochores of lagging chromosomes in anaphase were stretched laterally (1.2–5.6-fold) in the directions of their kinetochore microtubules, indicating that they were not able to achieve anaphase poleward movement because of pulling forces toward opposite poles. They also had inactivated mitotic spindle checkpoint activities since they did not label with either Mad2 or 3F3/2 antibodies. Thus, for mammalian cultured cells, kinetochore merotelic orientation is a major mechanism of aneuploidy not detected by the mitotic spindle checkpoint. The expanded and curved crescent morphology exhibited by kinetochores during nocodazole treatment may promote the high incidence of kinetochore merotelic orientation that occurs after nocodazole washout.
Keywords: aneuploidy, kinetochores, mitosis, microtubules, mitotic spindle checkpoint
Introduction
The unequal distribution of sister chromatids during mitosis or meiosis and cell cytokinesis produces aneuploid daughter cells. The role of aneuploidy in human meiotic cells is well known for generating severe congenital syndromes (for review see Nicolaidis and Petersen 1998). In addition, recent studies have indicated that aneuploidy in somatic mitotic cells plays a significant role in tumorigenesis (for review see Sen 2000). Abnormal chromosome number is a common feature of malignant tumor cells and recent results suggest that the aneuploidy-associated chromosome instability is intimately associated with (Duesberg et al. 1998; for review see Lengauer et al. 1998) or even precedes (Li et al. 1997) cell transformation.
A potential source of aneuploidy in mitotic mammalian tissue cells are chromosomes which are left behind at the spindle equator during anaphase as the great majority of sister chromatids move to their spindle poles (Ford and Corell 1992; Cimini et al. 1999). In telophase, depending on where cytokinesis occurs, the presence of a lagging chromosome can give rise to a monosomic daughter cell and a trisomic one. Lagging chromosomes can often be seen as micronuclei separated from the main nucleus at the subsequent interphase. Normally, the percentage of anaphase cells with one or more of these lagging chromosomes ranges from <1 to 5% for several tissue cells in culture, such as human lymphocytes and Chinese hamster cells (Izzo et al. 1998; Minissi et al. 1999; Catalán et al. 2000). The occurrence of lagging chromosomes is enhanced significantly (>10%) after release of a mitotic block induced by antimitotic drugs, like nocodazole, which can reversibly disassemble mitotic spindle microtubules (Cimini et al. 1999).
These lagging chromosomes raise important questions about errors in the mechanisms of chromosome segregation and the mitotic spindle checkpoint. The first major issue is how these lagging chromosomes primarily remain near the spindle equator in anaphase if they are able by anaphase onset to congress to near the spindle equator (Cimini, D., and F. Degrassi, unpublished observations). Chromosome congression to the metaphase plate usually means that one sister kinetochore is attached to and pulled toward one pole by formation of kinetochore microtubules to that pole, whereas the other sister is attached to and pulled toward the opposite pole by formation of kinetochore microtubules to that pole (Rieder and Salmon 1998). If poleward forces toward opposite poles exist, why are these chromosomes not segregated in anaphase? A related question is how a mitotic block with nocodazole substantially enhances the occurrence of these lagging chromosomes in anaphase without disrupting their congression to the metaphase plate. A second major issue concerns limitations of the mitotic spindle checkpoint. Why does the mitotic spindle checkpoint not sense the defect which produces these lagging chromosomes in anaphase? The spindle checkpoint normally prevents errors in chromosome segregation by delaying anaphase onset when chromosomes have not achieved their full complement of kinetochore microtubules or lack the tension typical of metaphase aligned chromosomes (for review see Nicklas 1997; Rieder and Salmon 1998; Maney et al. 2000). The spindle checkpoint produces the mitotic block for cells treated with drugs like nocodazole that induce microtubule disassembly and disrupt kinetochore microtubule formation and chromosome alignment on the spindle (Canman et al. 2000).
Possible hypotheses to explain these lagging chromosomes in anaphase include sister chromatid nondisjunction, detachment of kinetochore microtubules at anaphase, inactivation of pulling forces at kinetochores in anaphase, and merotelic kinetochore orientation. The first hypothesis predicts that lagging chromosomes are paired chromatids with properly oriented sister kinetochores, with one sister kinetochore fiber to one pole and the other sister with its kinetochore fiber to the opposite pole. This has been shown for the inhibition of sister chromatid decatenation by topoisomerase II inhibitors (Gorbsky 1994; Cimini et al. 1997). The second hypothesis predicts kinetochores on lagging chromosomes in anaphase without kinetochore microtubules, and the third hypothesis predicts the loss of kinetochore tension in anaphase. Thus far there is no experimental support for either the second or third hypothesis. The last hypothesis predicts that sister chromosome disjunction at anaphase onset is normal and that a lagging chromosome is a single sister chromatid with its kinetochore attached by kinetochore microtubules to opposite poles (merotelic orientation), rather than to only one pole as normally occurs by metaphase. There is some experimental support for this hypothesis. Previous studies have shown that chromosomes with merotelically oriented kinetochores can be produced in meiosis or in mitosis by chromosome disruption (e.g., errors in replication, laser surgery, and mutant centromeres) or spindle disruption (e.g., disassembly and multipolarity). These chromosomes can congress normally to the equator of a bipolar spindle in prometaphase, not delay anaphase onset, and remain behind at the equator in anaphase with their single kinetochores connected by kinetochore microtubules to opposite poles (Heneen 1975; Ladrach and LaFountain 1986; Ouspenski and Brinkley 1993; Khodjakov et al. 1997; Sluder et al. 1997; Wise and Brinkley 1997; Yu and Dawe 2000).
We have tested the above hypotheses for the origin of lagging chromosomes during anaphase in mammalian tissue cells by analyzing PtK1 cells in culture. Our data are consistent with the merotelic orientation hypothesis and show that merotelic kinetochore orientation is a major mechanism producing chromosome loss during mitosis. A lagging chromosome was found to almost always be a single chromatid with its kinetochore attached to and stretched between bundles of microtubules oriented toward opposite poles. Apparently, antagonistic pulling forces toward opposite poles prevent normal anaphase segregation of the lagging chromosome. Merotelically oriented kinetochores are not detected by the mitotic spindle checkpoint since they exhibit no staining in anaphase for the mitotic checkpoint protein Mad2 or the 3F3/2 phosphoepitope, markers for checkpoint activation at kinetochores. The expansion of kinetochores into “crescents” around centromeres during a nocodazole mitotic block suggests that kinetochore defects in the “search and capture” mechanism of kinetochore microtubule formation contribute to merotelic kinetochore orientation.
Materials and Methods
Cell Culture and Treatment
PtK1 cells (American Type Culture Collection) were maintained in HAM's F-12 medium (Sigma-Aldrich) complemented with 10% fetal bovine serum, penicillin, streptomycin, and amphotericin B (antimycotic) and grown in a 37°C, 5% CO2 humidified incubator. For nocodazole treatment, a 10 mM stock solution in DMSO was diluted into medium to a final concentration of 2 μM nocodazole. Cells were incubated with nocodazole-containing medium and either fixed after 3 h of treatment, or washed four times with medium at 37°C and incubated in fresh medium for 1 h at 37°C to allow for spindle reassembly and entry into anaphase before fixation. Pilot experiments showed that the majority of mitotic-arrested cells accomplished anaphase ∼1 h after the release from the nocodazole block.
Immunostaining with CREST and Anti–α-tubulin Antibodies
Cells were rapidly rinsed in PBS, lysed for 5 min with PHEM buffer containing 0.5% Triton X-100 and then fixed in 95% methanol plus 5 mM EGTA. The cells were then rinsed in PBS and subsequently blocked in 5% boiled donkey serum in PBS for 1 h at room temperature. The coverslips were then incubated in primary antibodies (human calcinosis, Raynaud's phenomenon, esophageal dysfunction, sclerodactyly, and telangiectasia [CREST] serum and mouse anti–α-tubulin) diluted in 5% boiled donkey serum overnight at 4°C. CREST serum (a gift from Dr. B.R. Brinkley, Baylor College of Medicine, Houston, TX) was diluted 1:600. Anti–α-tubulin (DM1α; Sigma-Aldrich) was diluted 1:300. Cells were then rinsed in PBST (PBS with 0.05% Tween 20), incubated in secondary antibodies (Rhodamine red–X anti–human, diluted 1:100, and Alexa 488 anti–mouse, diluted 1:1,000) for 45 min at room temperature, rinsed again, and mounted in an antifade solution containing 90% glycerol and 0.5% N-propyl gallate. The same fixation procedure was used for experiments in which only CREST staining was performed.
Immunostaining with Anti-Mad2 and -3F3/2 Antibodies
Cells were rinsed rapidly in PHEM buffer and then lysed for 5 min in 0.5% Triton X-100 in PHEM buffer. For the 3F3/2 staining, 100 nM microcystin (Sigma-Aldrich) was included in the lysis buffer to block phosphatase activity. Cells were fixed for 20 min in PHEM plus 4% formaldehyde freshly prepared from paraformaldehyde. Cells were then rinsed in PBST and blocked in 5% boiled donkey serum in PBS for 1 h at room temperature. For Mad2 staining, a rabbit primary anti-Mad2 antibody (Waters et al. 1998) diluted 1:100 and a Rhodamine red–X anti–rabbit secondary antibody (Jackson ImmunoResearch Laboratories) diluted 1:100 were used. A Rhodamine red–X anti–mouse antibody (Jackson ImmunoResearch Laboratories) diluted 1:100 was used against the 3F3/2 antibody. The 3F3/2 antibody was provided by Dr. G.J. Gorbsky (University of Oklahoma Health Sciences Center, Oklahoma City, OK). All the antibodies were diluted in 5% boiled donkey serum. Coverslips were incubated in the primary antibody overnight at 4°C, rinsed in PBST, incubated in the secondary antibody for 45 min at room temperature, rinsed again, and mounted in an antifade solution containing 90% glycerol and 0.5% N-propyl gallate.
Fluorescence Microscopy and Image Acquisition
For analysis of CREST and α-tubulin staining, immunofluorescently stained cells were recorded with a spinning disk confocal fluorescence microscope system equipped with a 100× 1.4 NA Plan-Apochromatic phase–contrast objective lens. The microscope was an inverted microscope (TE3000; Nikon) equipped with phase–contrast transillumination or epifluorescence illumination by a Yokogawa CS10 spinning disk confocal attachment (PerkinElmer) containing filters and filter wheels for illumination at 488 or 568 nm from a 60 mW argon/krypton laser. Digital images were obtained with an Orca ER-cooled CCD camera (Hamamatsu Photonics). Image acquisition, microscope shutters, and z-axis focus were all controlled by MetaMorph (Universal Imaging Corp.) software in a PC computer. Phase–contrast microscopy was used to visualize the chromosomes. Z-series optical sections through each cell analyzed were obtained in 0.2-μm steps. To enhance resolution of merotelic kinetochore orientation in prometaphase and metaphase cells, three dimensional (3-D) deconvolution and reconstruction was performed on the z-series confocal optical sections using a Delta Vision image processing workstation (Applied Precision). For part of the analysis of Mad2 and 3F3/2 staining, immunofluorescence-stained cells were viewed by wide-field epifluorescence using a Nikon Microphot FX-A microscope equipped with a 60× 1.4 NA objective lens and a 2.0× optovar projection lens. Both differential interference contrast and fluorescence images were obtained with a cooled CCD digital camera (C4880; Hamamatsu). Z-series optical sections through each cell analyzed were obtained in 0.5-μm steps using MetaMorph image processing software and a Ludl stepping motor (Salmon et al. 1994).
Kinetochore Maximal Width
MetaMorph tracking software was calibrated with a stage micrometer and used to measure maximal width of kinetochores in normal and lagging chromosomes. In the same anaphase cell, the maximal widths of kinetochores on lagging chromosomes and on chromosomes that had moved normally to their spindle poles were measured. To facilitate accurate measurements, the images were zoomed 200–400%. 25 ana-telophase cells containing lagging chromosomes were analyzed.
Electron Microscopy
Cells released for 1 h from a mitotic block induced by 2 μM nocodazole were fixed for electron microscopy as described in Khodjakov et al. 1997. In brief, cells were fixed with 2.5% glutaraldehyde in 0.1 M Millonig's phosphate and then postfixed in 2% aqueous OsO4. The coverslips were then treated with 0.15% tannic acid, washed, and stained en bloc in 1% uranyl acetate. Subsequently, the coverslips were washed, dehydrated in graded series of ethanol, and flat-embedded in Epon. The positions of anaphase cells with lagging chromosomes were visualized on flat-embedded coverslips with a ZEISS Photomicroscope III using a 40× objective and marked by scribing a circle on the coverslip around them. Afterwards, the positions of the same cells were relocalized on the plastic surface as described in Rieder and Cassels 1999. The relocated cells were then thick-sectioned, viewed, and photographed with an intermediate voltage electron microscope (model 910; ZEISS) at 100 kV according to Khodjakov et al. 1997.
A 3-D model of a merotelically oriented kinetochore was also generated. Stereopairs for two consecutive sections containing the kinetochore were obtained at 12,500× magnification and ±16° tilt. Microtubules, kinetochore, and chromosome contours were traced in 3-D space using the Stereocon image-reconstruction system (Marko and Leith 1996).
Online Supplemental Material
A rotatable 3-D file of Fig. 4 d and the software that allowed us to rotate the image are available at http://www.jcb.org/cgi/content/full/153/3/517/DC1.
Figure 4.
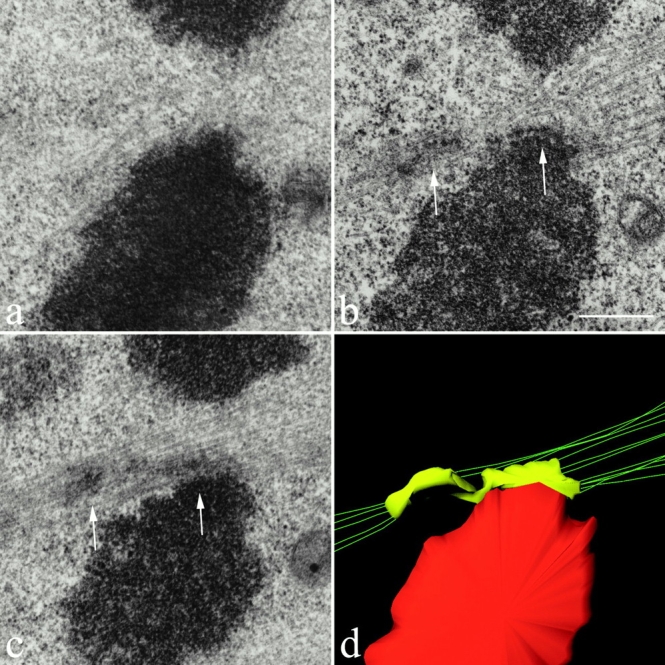
Electron micrographs showing sequential-thick sections (a–c) through the merotelically oriented kinetochore of a lagging chromosome in a cell fixed at late anaphase. In panels b and c, kinetochore microtubules can be seen extending from the left side (left arrow) of the kinetochore toward the left spindle pole and from the right side of the kinetochore (right arrow) to the right spindle pole. (d) 3-D structure of the organization of kinetochore microtubules attached to the merotelically oriented kinetochore shown in panels b and c. The reconstruction was obtained from stereopairs for the two consecutive sections containing the kinetochore. The kinetochore is color encoded in yellow, the microtubule axes are green, and the chromatin proximal to the kinetochore is red. The figure clearly shows 5 kinetochore microtubules extending toward one pole and 11 kinetochore microtubules extending toward the opposite pole. It also clearly shows that the kinetochore is stretched laterally beyond the centromere region of the chromosome. For clarity, interpolar microtubules that pass near the kinetochore are not shown. A rotatable, 3-D file of panel d is available at http://www.jcb.org/cgi/content/full/153/3/517/DC1. Bar, 0.5 μm.
A Quicktime® movie of Fig. 5 is also available at the same location. The movie allows the cell to rotate and discriminate microtubule bundles ending on the kinetochores from bundles that are aligned with the kinetochores, but not attached to them.
Figure 5.
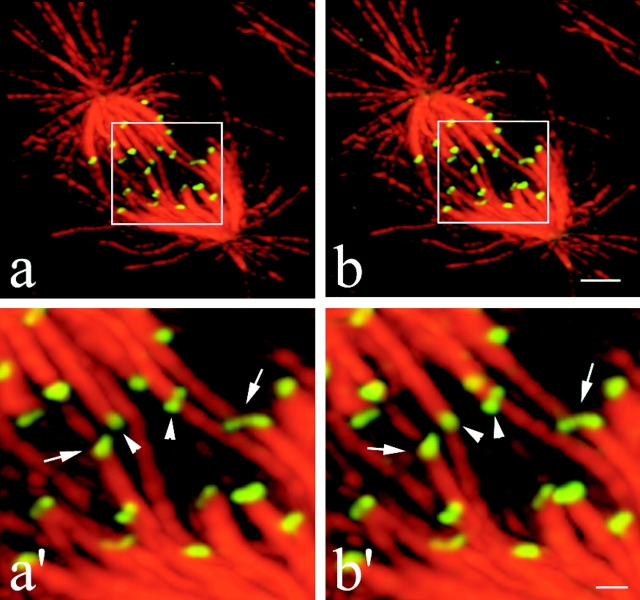
Merotelic kinetochore orientation in a late prometaphase cell observed 15 min after release from a mitotic block with 2 μM nocodazole. The figure shows that merotelic kinetochore orientation occurs by late prometaphase on chromosomes near the spindle equator. Two different angles of a projection from a deconvolved 3-D image stack are shown in panels a and b. Higher magnification views of the central region of the spindle containing the merotelically oriented kinetochores (arrows) are shown in panels a′ and b′. Spindle microtubules are red and kinetochores stained with CREST antibody are green. By rotating the 3-D reconstructed image, microtubule bundles ending at a single kinetochore (a′ and b′, arrows) could be easily discriminated from bundles of microtubules which were aligned with kinetochores, but not connected to them (a′ and b′, left arrowhead). By further rotating the 3-D image, the kinetochore indicated by the right arrowhead was seen attached only to one microtubule bundle and not to microtubules coming from both poles. Note that the kinetochore CREST staining has a bilobed kidney shape for both normal and merotelically oriented kinetochores at the higher resolution achieved by 3-D image processing and deconvolution. Also, note that the right merotelically oriented kinetochore appears stretched in comparison to kinetochores with kinetochore fibers from one pole only. A Quicktime® movie of this figure is available at http://www.jcb.org/cgi/content/full/153/3/517/DC1. Bars: (b) 2 μm; (b′) 0.5 μm.
Results
Lagging Chromosomes Are Individual Sister Chromatids Whose Frequency of Occurrence Is Highly Increased in Cells Recovering from a Nocodazole Block
Lagging chromosomes could be observed in anaphase in both control PtK1 cells (Fig. 1 a) and in PtK1 cells fixed after 1 h recovery from a nocodazole (2 μM)-induced mitotic arrest (Fig. 1 b). These chromosomes were left near the spindle equator, often with their arms nearly perpendicular to the spindle axis (Fig. 2), whereas all other chromosomes correctly moved to their spindle poles. The presence of anaphase-lagging chromosomes was analyzed in 1,631 control anaphases and 758 nocodazole-released anaphases by immunofluorescence microscopy. Kinetochores were stained with CREST antibodies, microtubules with an anti–α-tubulin antibody, and chromosomes with DAPI. We observed lagging of paired sister chromatids in only 1 of 20 control anaphase cells showing lagging chromosomes (Table ). In this case, these chromatids did not exhibit any CREST staining for kinetochores, suggesting that inactivation of the kinetochores had occurred in that chromosome or that they were acentric chromosome fragments. Lagging of paired sister chromatids was observed in only 2 out of 135 cells recovering from a nocodazole-induced mitotic block and showing lagging chromosomes (Table ). These chromatids did show CREST staining for kinetochores. In all the other cases of lagging chromosomes analyzed, the lagging chromosomes were single chromatids with only one CREST signal, indicating that lagging of paired sisters was a rare event both in untreated cells and in cells recovering from a mitotic block (Table ). The kinetochore of a lagging chromosome was often very stretched (Fig. 1 a and below), but we could always detect the weak fluorescence of the thin region between the two thicker ends. Note that CREST antibodies do not stain the centromere region between sister kinetochores (Fig. 2 a).
Figure 1.
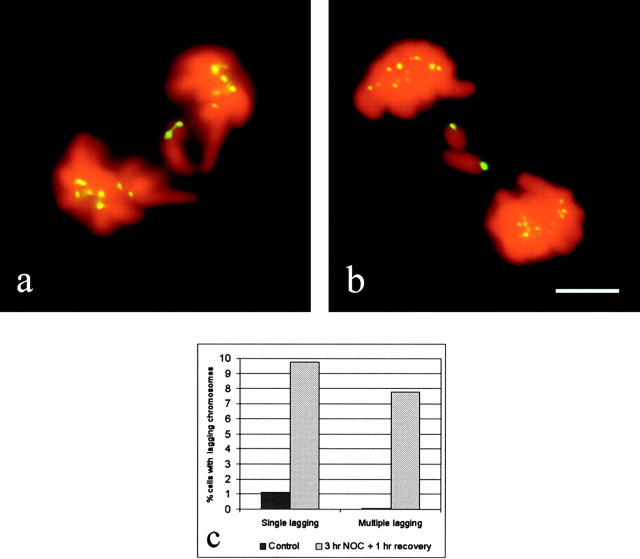
Fluorescent images of lagging chromosomes in PtK1 cells fixed at late anaphase. Kinetochores were stained with CREST antibodies (pseudocolored green), whereas chromosomal DNA was stained with DAPI (pseudocolored red). Examples of a single lagging chromosome in an untreated cell (a) and multiple lagging chromosomes in a cell recovering from a nocodazole-induced mitotic arrest (b). Lagging chromosomes are single chromatids with only one CREST signal. (c) The graph shows the frequencies of anaphase cells with lagging chromosomes in nocodazole-released and untreated cells (the cells showing lagging of two paired sister chromatids were not included in the analysis, since they represent a rarely occurring event both in untreated cells and in cells recovering from a mitotic block). The data obtained in six independent experiments, in which only CREST staining or both CREST and α-tubulin immunostaining were performed, were pooled together, since no statistically significant differences were observed (by χ2 test, P > 0.1). 1,631 control anaphases and 758 nocodazole-released anaphases were analyzed for the presence of lagging chromosomes. Bar, 5 μm.
Figure 2.
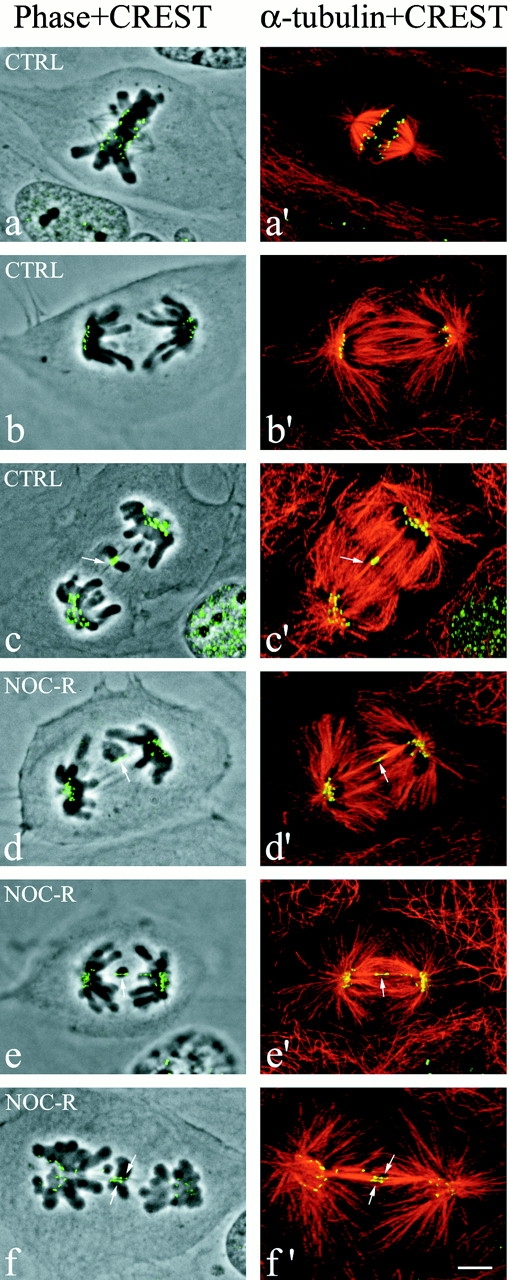
Fluorescent and phase–contrast images of PtK1 cells fixed at anaphase and immunostained for kinetochores with CREST antibody and for microtubules with anti–α-tubulin antibody. The overlay of phase–contrast and CREST images (left column) and α-tubulin and CREST images (right column) is shown. The CREST and microtubule fluorescent images were obtained by projecting into a single image the maximal brightness at each pixel location through a stack of optical sections acquired at 0.2-μm intervals through the immunostained cells by confocal fluorescence microscopy. (a and a′) Normal metaphase. Sister kinetochores exhibit punctuate CREST fluorescence and they appear completely separated from each other because of the stretching induced on centromeric chromatin. (b and b′) Normal anaphase. (c and c′) Example of a lagging chromosome in an untreated anaphase cell (arrows point at the kinetochore of the lagging chromosome). (d–f′) Examples of single or multiple lagging chromosomes in anaphase cells during recovery from a nocodazole-induced mitotic block (arrows point at the kinetochores of lagging chromosomes). The CREST- and α-tubulin–merged images show clearly that the kinetochores of lagging chromosomes in anaphase are connected to microtubules coming from both poles (merotelic attachment) and that the CREST-stained region is stretched compared with the kinetochores correctly localized to the spindle poles. Bar, 5 μm.
Table 1.
Occurrence of Single Lagging, Multiple Lagging, or Loss of Paired Sister Chromatids in Ana-telophase Cells, as Shown by CREST Staining
| Cells analyzed | Single chromatids | ≥2 chromatids | Paired sister chromatids | Total | Percentage | |
|---|---|---|---|---|---|---|
| Control cells | 1631 | 18 | 1 | 1 | 20 | 1.16 |
| 1 h recovery | 758 | 74 | 59 | 2 | 135 | 17.55 |
Counts of CREST-stained kinetochores also verified that lagging chromosomes were single chromatids. Kinetochore count was performed on only 31 anaphase cells, because in these cells the kinetochores near the poles were sufficiently separated from each other to allow accurate counts. The chromosome number in our PtK1 cells in culture is slightly variable above 12 sister pairs (13.5 ± 1.5). In each anaphase cell analyzed with one or more lagging chromosomes (31 total), the counts always resulted in an even number of kinetochores. For all 11 anaphases analyzed with a single lagging chromosome (with 1 CREST-stained kinetochore), the sum of kinetochores at both poles was always an odd number. A similar result was found for three anaphases with three lagging chromosomes. For the 17 anaphases that had 2 lagging chromosomes, the sum of all CREST-stained kinetochores at the poles was an even number, as predicted by each lagging chromosome having only one kinetochore. Although lagging chromosomes were the major source of aneuploidy in the cells analyzed, there was also evidence that sister pairs might missegregate. In 6 of the 31 anaphases with lagging chromosomes, the number of kinetochores segregated to one pole was greater than the sum of the number at the opposite pole plus the number of lagging kinetochores by two and, in one case, by six. This inequality indicates that occasionally both sisters of one or more sister pairs can migrate toward the same pole, as reported for other mammalian cells (Cimini et al. 1999). Taken together, these results confirm that the lagging of paired sisters is a rare event (Table ).
The data summarized in Fig. 1 c and Table show that the mean frequency of anaphases with one or more lagging chromosomes (each showing only one CREST signal; i.e., a single chromatid) was 1.16% in untreated cells and 17.55% in cells released from the nocodazole block. The frequencies of lagging chromosomes in six independent experiments, in which only CREST or both CREST and α-tubulin immunostaining were performed, did not show statistically significant differences. The frequency of anaphase cells containing a single lagging chromosome was about ninefold higher in cells released from the mitotic arrest than in untreated cells, whereas the frequency of anaphase cells showing multiple (two or more) lagging chromosomes was increased >100 times, since this event was rarely observed in control cells (Fig. 1 c and Table ). A χ2 test confirmed that in cells recovering from the nocodazole block, the frequencies of both single and multiple lagging chromosomes were highly increased compared with control cells (P < 0.001 in both cases). For this reason we used the nocodazole treatment as a tool to increase the number of cells containing lagging chromosomes for analysis.
A Lagging Chromosome Is Connected at Its Kinetochore to Bundles of Microtubules from Opposite Spindle Poles (Merotelic Orientation)
To analyze the connections between mitotic spindle microtubules and the kinetochore of lagging chromosomes during mitosis, we performed immunostaining for centromeric proteins (CREST staining) and microtubules (α-tubulin immunostaining) and obtained high resolution optical sections through anaphase cells with lagging chromosomes by fluorescence confocal microscopy. Fig. 2, a and b show normal metaphase and anaphase, respectively. The overlay of the phase–contrast image with the CREST staining (Fig. 2, left column) confirmed that lagging chromosomes in cells recovering from a mitotic block were single chromatids with one CREST-staining kinetochore (stretched, see below) (Fig. 2, c–e), whereas the merged images of CREST and tubulin staining (Fig. 2, right column) revealed that a single kinetochore was connected to bundles of microtubules coming from opposite spindle poles (merotelic attachment) (Fig. 2c′–e′). In late anaphase and telophase the connection of microtubules to the kinetochore of a lagging chromosome was not detectable because of the presence of a large number of microtubules in the spindle midzone. However, in 26 cells recovering from a mitotic block in which the connection of microtubules to the kinetochore of a lagging chromosome was clearly detectable, microtubule bundles were seen extending toward both poles (i.e., merotelic attachment).
To test if merotelic attachment is a general cellular mechanism responsible for chromosome loss and not only an abnormal behavior induced by nocodazole treatment, we performed the same immunostaining on cells not treated with nocodazole. We could clearly see for all the 11 lagging chromosomes analyzed in untreated cells that the attachment of kinetochore microtubule bundles to kinetochores was merotelic (Fig. 2c and Fig. c′), except in the case of the two paired sisters with no CREST signals that did not show any microtubule binding. Thus, merotelic attachment appeared to be responsible for lagging chromosomes during mitosis in untreated cells as well as cells recovering from the nocodazole block.
The analysis of α-tubulin immunostaining showed a major decrease in microtubule fluorescence at the kinetochore of the lagging chromosome (Fig. 3, middle column). This indicates that the majority of microtubules from opposite poles do not run past the kinetochore, but rather terminate on it. However, there was a low level of microtubule fluorescence at the kinetochore region. This fluorescence could be produced by microtubules attached to different positions along the extended kinetochore or by interpolar microtubules clustered in the vicinity of kinetochore microtubule bundles as already shown by electron microscopy in PtK1 cells (Mastronarde et al. 1993).
Figure 3.
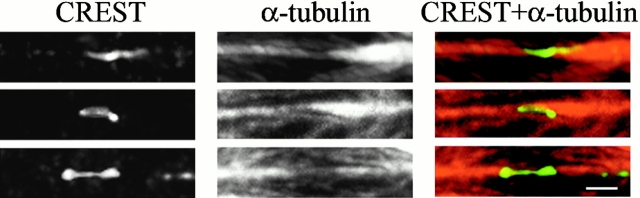
CREST (left) and α-tubulin (middle) immunostaining of the kinetochore region of three lagging chromosomes observed in anaphase cells. α-Tubulin immunostaining (middle) clearly shows a lack of fluorescence in the region where the kinetochore is localized, indicating that most of the microtubules coming from the opposite spindle poles end on the kinetochore. A few microtubules run through the kinetochore region, indicating that interpolar microtubules are clustered near kinetochore microtubule bundles. The overlay of CREST and α-tubulin immunostaining is shown in the right column. Bar, 1 μm.
We were also able to show by electron microscopy that the single kinetochore of an anaphase-lagging chromosome has kinetochore microtubules extending towards opposite poles. Nocodazole-treated cells were released from a mitotic block for 1 h and then fixed for electron microscopy. Samples were plastic-embedded and anaphases with lagging chromosomes were localized by phase–contrast microscopy. Selected cells were relocalized on the plastic after the coverslip was removed and the samples were sectioned. For three lagging chromosomes in two cells, the kinetochore on the lagging chromosome had microtubules extending towards opposite spindle poles as shown in Fig. 4 (i.e., merotelic attachment). The figure shows three serial-thick sections through a lagging chromosome in an anaphase cell with its kinetochore connected by kinetochore microtubule bundles to both spindle poles. In the two sections shown in Fig. 4b and Fig. c, the kinetochore can be seen stretched laterally between microtubule bundles extending toward opposite poles (arrows point at opposite sides of the kinetochore). In these thick sections, the sites of microtubule attachment to the kinetochore are difficult to see because of the section thickness, the attachment of plus ends at various positions along the stretched kinetochore, and the presence of interpolar microtubules which extend pass the kinetochore without attachment. To clearly see the distribution of kinetochore microtubules and the attachment position of their plus ends, a 3-D Stereocon reconstruction (Fig. 4 d) was generated by stacking the pertinent information contained in the two sections shown in Fig. 4b and Fig. c. The resulting image (Fig. 4 d) clearly shows that 5 kinetochore microtubules extend toward one pole and 11 extend toward the opposite pole. There were 10–12 microtubules in the vicinity of the kinetochore that did not terminate in the kinetochore and these microtubules were not included in the reconstruction so that the kinetochore microtubules are clearly visible.
Since lagging chromosomes remain behind at the spindle equator as chromosomes move to the spindle poles during anaphase, we expected to find chromosomes in late prometaphase or metaphase cells with one of the sister kinetochores merotelically oriented. To test this possibility, we fixed cells ∼15 min after release from a nocodazole-induced mitotic block at a time when most of the cells had already reestablished bipolar spindle assembly and their chromosomes were partially aligned on a metaphase plate. We needed higher resolution procedures to help clearly see the orientation of microtubule attachment to kinetochores near the metaphase plate, where chromosomes are very crowded, as compared with the resolution we needed for the analysis of one or a few lagging chromosomes at mid- to late anaphase. This was achieved by acquiring z-series stacks of confocal images at 0.2-μm intervals through each cell analyzed and processing the image stacks to obtain 3-D projections after digital image deconvolution (Agard et al. 1989). Fig. 5 shows two angles of a 3-D projection through a late prometaphase cell with two kinetochores with merotelic orientation (arrows). By rotating reconstructed 3-D images, we could easily discriminate merotelic orientation of microtubule bundles ending at a single kinetochore (Fig. 5, arrows) from bundles of microtubules which were aligned with kinetochores, but not connected to them (Fig. 5, arrowheads). From this analysis, we concluded that merotelic kinetochore orientation occurs during the period when kinetochores normally form their kinetochore microtubules in prometaphase and early metaphase (McEwen et al. 1997).
Analysis of the Kinetochore Stretching in Anaphase Induced by Merotelic Attachment
During mitosis, kinetochore microtubules exert a net pulling force on their chromatids (Khodjakov and Rieder 1996; Waters et al. 1996, Waters et al. 1998). These forces stretch the centromere-proximal chromatin so that at metaphase the distance between two sister kinetochores is often more than twice the separation of sister kinetochores on unattached, relaxed centromeres. Although these metaphase kinetochores are under tension, the kinetochores seen by CREST immunofluorescence staining do not appear stretched, since their size is not different between attached metaphase kinetochores and unattached prometaphase kinetochores (Waters et al. 1998). By wide-field epifluorescence microscopy, sister kinetochores of metaphase chromosomes appear as two intensely stained and unstretched spots completely separated from each other; their widths were only slightly larger than their thicknesses (Fig. 2, a and a′, and 6 a, left). In contrast, CREST staining of anaphase cells indicates that merotelic attachment induces a stretching of the single kinetochore of lagging chromosomes during anaphase. These kinetochores are much wider than normal kinetochores (Fig. 1 a; 2, c–f′; 3; and 4) and sometimes they appear as two intensely stained spots connected by a less stained region (Fig. 1 a; 2, e–f′; 3; and 6 a, right). This pattern of kinetochore stretching is consistent with the structure of the stretched kinetochore seen in the electron microscope reconstruction in Fig. 4 d. The kinetochore is extended laterally into two domains by clusters of kinetochore microtubules extending toward opposite poles. The left domain is pulled away from the centromere region, but remains connected to the right domain by a thin kinetochore extension.
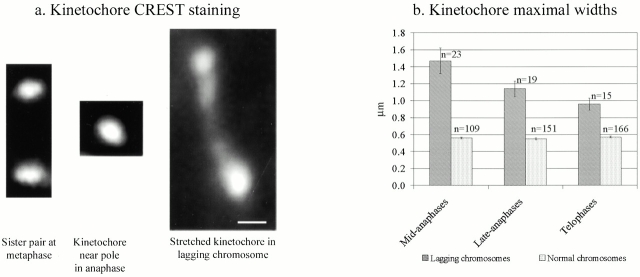
We evaluated the difference in the size of kinetochores between normal and lagging chromosomes by measuring the maximal width of the CREST signal. In the same anaphase cell, the maximal width of the kinetochores on lagging chromosomes and on chromosomes that had moved normally to the spindle poles was measured. On average, the maximal width of kinetochores of lagging chromosomes was >2-fold greater than normal kinetochores, but could be stretched laterally up to 5.6-fold (an example of a stretched kinetochore, >5-fold wider than the average, is shown in Fig. 6 a, right; see also Fig. 1 a and 2, c–f′). The stretching observed ranged between 1.2- and 5.6-fold, but we also observed a correlation between the mitotic phase and the degree of stretching by comparing the data for different stages of ana-telophase. In mid-anaphase, the kinetochores of lagging chromosomes were on average 2.6-fold wider (1.47 vs. 0.56 μm) than normal kinetochores. In late anaphase the ratio between lagging and normal kinetochores was 2.1 (1.14 vs. 0.55 μm) and it was reduced to 1.7 in telophase (0.96 vs. 0.57 μm; Fig. 6 b). This comparison suggests that during anaphase a merotelically oriented kinetochore is stretched laterally by pulling forces generated by kinetochore microtubules extending to opposite spindle poles. During the later stages of mitosis stretching is reduced, indicating that the pulling forces are decreased. Statistical analysis of the data showed that the differences in kinetochore width between normal and lagging chromosomes were statistically significant for all three mitotic phases analyzed (P < 0.001, Student's t test).
Figure 6.
Analysis of kinetochore stretching. (a) Kinetochores seen by CREST staining. (Left) Example of sister kinetochores on a bioriented chromosome aligned at the equator in a metaphase PtK1 cell. The two sister kinetochores are completely separated from each other because of the stretching induced on centromeric chromatin, and the interkinetochore distance is ∼2 μm. (Middle) Kinetochore correctly transported to the pole in an anaphase cell recovering from a nocodazole-induced mitotic block. The maximal width of this kinetochore is typical of kinetochores near the pole at anaphase, ∼0.6 μm. (Right) Stretched kinetochore on a lagging chromosome left behind after anaphase onset in a cell recovering from a nocodazole-induced mitotic block. The length of this kinetochore is 3.14 μm. These images are all oriented with the spindle axis approximately vertical. (b) Measurements of maximal kinetochore width for normal anaphase chromosomes (correctly moved to the spindle poles) or lagging chromosomes in ana-telophase PtK1 cells after 1 h release from a nocodazole-induced mitotic arrest. The histograms show the means ± SE of the maximal kinetochore width. n, number of measured kinetochores. Bar, 0.5 μm.
Mad2 and the 3F3/2 Phosphoepitope Are Not Present on the Kinetochore of a Lagging Chromosome at Anaphase
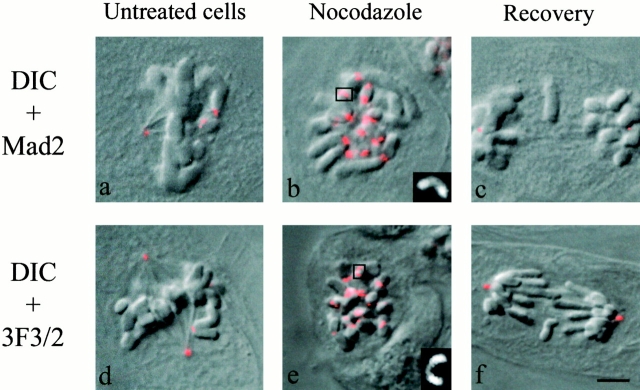
Untreated cells, nocodazole-treated cells, and cells fixed after 1 h recovery from the nocodazole-induced mitotic arrest were examined to investigate the state of the mitotic spindle checkpoint proteins on lagging chromosomes. We immunostained cells with anti-Mad2 or 3F3/2 antibodies. As shown previously (for review see Rieder and Salmon 1998; Maney et al. 2000), both the mitotic checkpoint protein Mad2 and the 3F3/2 phosphoepitope are highly accumulated on unattached kinetochores lacking kinetochore microtubules at prophase and prometaphase (Fig. 7, a and d). Mad2 and 3F3/2 staining of kinetochores disappear as they attach kinetochore microtubules, develop tension, and become correctly aligned on the metaphase plate before anaphase onset (Gorbsky and Ricketts 1993; Waters et al. 1998). Kinetochores in cells depleted of microtubules by nocodazole treatment expand into crescent shapes as seen by both Mad2 and 3F3/2 staining (Fig. 7b and Fig. e; see higher magnification images in the insets). In our study, by 1 h after washing out nocodazole, spindles reassembled, chromosomes aligned at the spindle equator, and cells entered anaphase, including those that exhibited lagging chromosomes. The entry into anaphase of cells with lagging chromosomes predicted that the kinetochore of a lagging chromosome should be depleted of Mad2 and 3F3/2. We found that kinetochores on lagging chromosomes did not stain for either Mad2 (44 lagging chromosomes analyzed) or 3F3/2 (25 lagging chromosomes analyzed) (Fig. 7c and Fig. f, respectively), which is typical of late metaphase and anaphase kinetochores (Gorbsky and Ricketts 1993; Waters et al. 1998). These results indicate that merotelic microtubule attachment at the kinetochore of each lagging chromosome depletes Mad2 and dephosphorylates the epitope recognized by the 3F3/2 antibody as occurs for properly attached chromosomes.
Figure 7.
Mad2 (a–c) and 3F3/2 (d–f) immunostaining in untreated, nocodazole-treated cells and cells fixed after 1 h recovery from a nocodazole-induced mitotic arrest. Overlays of differential interference contrast (DIC) and fluorescence images are shown. Mad2 and 3F3/2 staining are present on the kinetochores of unattached and unaligned chromosomes in prometaphase (a and d), are enhanced on the kinetochores of nocodazole-arrested cells (b and e), but are not present on the kinetochores of lagging chromosomes (or chromosomes normally moved to the spindle poles) in anaphase cells (c and f). The insets in the bottom right corners of panels b and e show higher magnifications of crescent-shaped kinetochores highlighted by a square in the pictures. Bar, 5 μm.
Discussion
A Major Mechanism of Aneuploidy Is the Formation of Lagging Chromosomes with Merotelically Oriented Kinetochores
The 1% occurrence of lagging chromosomes at the spindle equator of untreated anaphase PtK1 cells and the order of magnitude higher frequency after mitotic arrest with nocodazole treatment are values similar to those reported previously by in situ hybridization analysis for other types of mammalian tissue cells in culture (Cimini et al. 1999). For PtK1 cells, we have shown that lagging chromosomes in anaphase are single chromatids with their kinetochores stretched between kinetochore microtubule bundles oriented towards opposite poles as diagrammed in Fig. 8 c. In addition, we found that this kinetochore merotelic orientation is exhibited before anaphase when chromosomes have become aligned near the spindle equator in late prometaphase and metaphase (Fig. 8 b). These data clearly indicate that merotelic orientation of individual kinetochores is the major mechanism responsible for lagging chromosomes at anaphase. Merotelic kinetochore orientation does not block chromosome congression to the spindle equator (Khodjakov et al. 1997). In anaphase, a merotelically oriented kinetochore remains under tension and becomes stretched laterally by poleward forces directed along its kinetochore microtubules to opposite poles.
Figure 8.
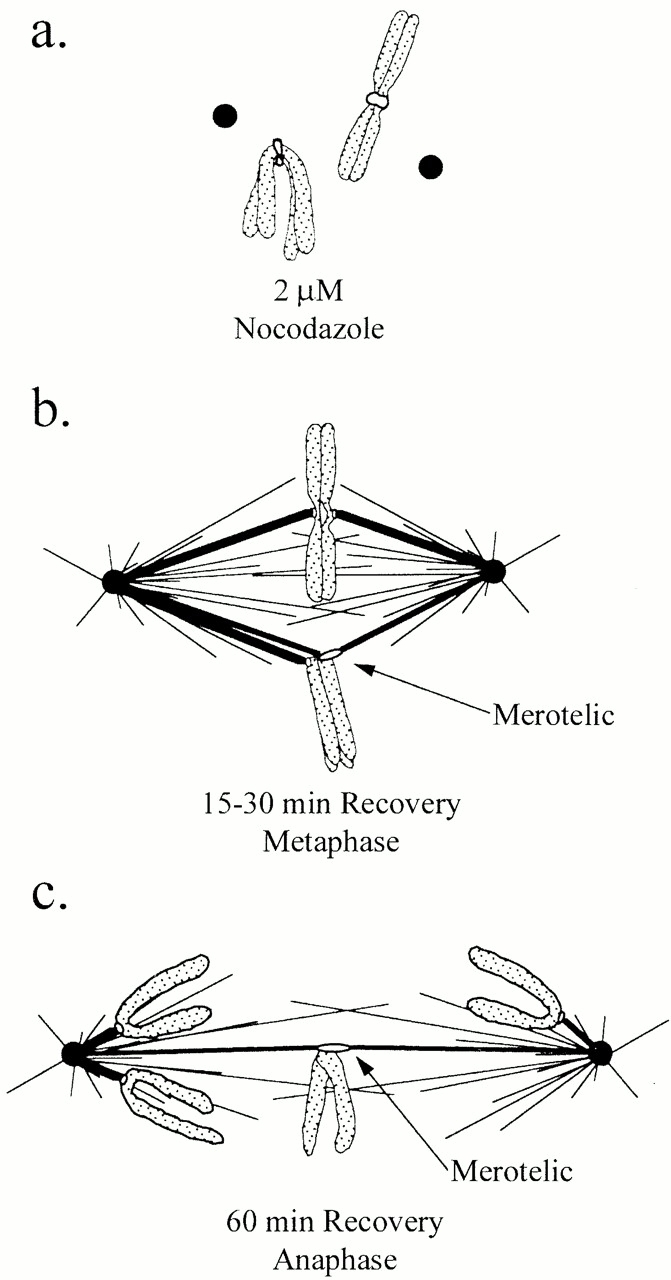
Proposed events that drive the production of lagging chromosomes in anaphase through merotelic kinetochore orientation. (a) Spindle disassembly induced by the nocodazole mitotic block promotes the expansion and curvature of the outer domain of kinetochores around their centromeric DNA. (b) A slow compaction of the kinetochore outer domain when the spindle reassembles after nocodazole removal may promote the unusually high frequency of merotelic kinetochore orientation that occurs by the time chromosomes have moved to near the spindle equator in prometaphase. (c) A merotelically oriented kinetochore does not move poleward like normal kinetochores at anaphase because it does not split apart like sister kinetochores at anaphase onset, but instead remains under tension and becomes further stretched by poleward forces directed along its kinetochore microtubules to opposite poles.
For these merotelically oriented single kinetochores in anaphase, we measured a 1.2–5.6-fold lateral stretching. This lateral direction of stretching is not typically experienced by kinetochores attached only to microtubules from one pole. Normally oriented kinetochores are pulled by kinetochore microtubule-associated forces along the kinetochore-centromere axis (Waters et al. 1996, Waters et al. 1998), without noticeable kinetochore stretching (Fig. 6 a). For these normally oriented kinetochores, there is no obvious stretching of the kinetochore along the microtubule–centromere axis seen in CREST stained cells (Fig. 6 a). The lateral stretching of a merotelically oriented kinetochore in anaphase was first reported by Khodjakov et al. 1997 in a study where chromosome fragments possessing only one kinetochore were obtained by cutting prometaphase PtK1 chromosomes using laser microsurgery. A similar result has also been reported recently for chromosomes with single kinetochores in meiosis II maize cells (Yu and Dawe 2000). Our results and these previous findings indicate that kinetochores are much more compliant laterally than along the normal kinetochore microtubule–centromere axis. Kinetochores can also be stretched along the chromosome longitudinal axis by chemical and mechanical treatments on isolated chromosomes (Zinkowski et al. 1991). However, this has not been observed in intact cells, indicating that kinetochores are not normally pulled along the longitudinal axis.
Abnormal Kinetochore Expansion and Curvature May Promote Merotelic Kinetochore Orientation
Kinetochore microtubule formation is thought to occur by a “search and capture mechanism,” where kinetochores recruit kinetochore microtubules by capturing the plus growing ends of polar spindle microtubules into binding sites in the kinetochore outer plate (for review see Rieder and Salmon 1998). In normal prometaphase spindles, unattached kinetochores can be larger in width by about a factor of two and appear more curved by conventional electron microscopy than kinetochores on chromosomes at the spindle equator, with a full complement of kinetochore microtubules extending to only one pole (Rieder 1982; Salmon 1989). The kinetochore outer domain, including the outer plate and the fibrous corona, contains microtubule motor proteins CENP-E and cytoplasmic dynein, which help recruit kinetochore microtubules as well as mitotic spindle checkpoint proteins (for review see Rieder and Salmon 1998; Maney et al. 2000).
What produces the substantial increase in merotelic kinetochore orientation after release of the mitotic block? One possibility is that the nocodazole-induced mitotic block may somehow inhibit, in cells recovering from the mitotic block, the normal mechanisms that correct merotelic orientation when it naturally occurs during prometaphase. On the other hand, the substantial increase in merotelic orientation is correlated with the previous expansion of the kinetochore outer domain into expanded crescents around the centromere during nocodazole treatment (Fig. 7b and Fig. e, and Fig. 8 a). Kinetochore crescent formation is typical of the response of kinetochores in mammalian tissue cells to depletion of spindle microtubules (Echeverri et al. 1996; Thrower et al. 1996; Martinez-Exposito et al. 1999). The expanded crescent morphology of the kinetochore outer domain disappears upon spindle reassembly and kinetochores regain a more punctuate appearance by immunofluorescence labeling (Thrower et al. 1996). Nevertheless, persistence of some crescent morphology during spindle reassembly would increase the solid angle faced by the kinetochore, increasing the probability that it could acquire kinetochore microtubules by capturing the plus ends of polar microtubules from opposite poles (diagrammed in Fig. 8 b), instead of from only one pole as typically occurs in prometaphase (for review see Rieder and Salmon 1998).
Merotelically Oriented Kinetochores Are Not Detected by the Mitotic Spindle Checkpoint
Mitotic tissue cells containing chromosomes with merotelically oriented kinetochores, induced either by multiple spindle poles (Sluder et al. 1997), laser microsurgery of chromosomes (Khodjakov et al. 1997), or release from a nocodazole block (our results), will enter anaphase normally, with no indications that the mitotic spindle checkpoint is activated. Khodjakov et al. 1997 in particular found similar results to ours when prometaphase chromosomes in PtK1 tissue cells were cut by laser microbeam irradiation to produce chromosome fragments possessing only one sister kinetochore. This single kinetochore acquired kinetochore microtubules from opposite poles, congressed to near the spindle equator, did not block anaphase onset, and remained stretched laterally near the spindle equator during anaphase segregation.
Our results also extend these studies by providing biochemical evidence that merotelically oriented kinetochores have inactivated checkpoint activity in cells that enter anaphase. Much as occurs for kinetochores of properly aligned metaphase chromosomes (Gorbsky and Ricketts 1993; Nicklas 1997; Waters et al. 1998), we found no Mad2 or 3F3/2 staining on the merotelically oriented kinetochore of lagging chromosomes in anaphase (Fig. 7). Thus, the microtubule attachment and tension generated at merotelically oriented kinetochores are able to inactivate mitotic checkpoint activity measured by depletion of Mad2 and 3F3/2 staining of kinetochores. The absence of 3F3/2 staining on merotelically oriented kinetochores suggests that the tension which laterally stretches the kinetochore must have a component in a direction that is able to inactivate the phosphorylation detected by the 3F3/2 phosphoepitope antibody. Like the kinetochores on lagging chromosomes in our PtK1 studies, a very recent report from Yu and Dawe 2000 has shown that, during maize meiosis II, chromosomes with a single kinetochore originated by premature sister separation during meiosis I exhibit merotelic orientation, kinetochore stretching, and depletion of Mad2 and 3F3/2 in anaphase. Thus for both mitosis and meiosis in animals and higher plants, kinetochore merotelic orientation is a major mechanism of aneuploidy not detected by the mitotic spindle checkpoint.
Supplemental Material
Acknowledgments
Thanks to G. Cassels for her expert assistance with electron microscopy.
Supported by Nationsl Institutes of Health grants GM24364 to E.D. Salmon and GM59363 to A. Khodjakov. D. Cimini is a Ph.D. fellow of the University of Rome “La Sapienza.” Stereocon is a part of National Resource supported by National Center for Research Resources grant RR012019.
Footnotes
The online version of this article contains supplemental material.
Abbreviation used in this paper: 3-D, three dimensional.
References
- Agard D.A., Hiraoka Y., Shaw P., Sedat J.W. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. doi: 10.1016/s0091-679x(08)60986-3. [DOI] [PubMed] [Google Scholar]
- Canman J.C., Hoffman D., Salmon E.D. The role of pre- and post-anaphase microtubules in the cytokinetic phase, or C-phase, of the cell cycle. Curr. Biol. 2000;10:611–614. doi: 10.1016/s0960-9822(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Catalán J., Falck G.C.-M., Norppa H. The X chromosome frequently lags behind in female lymphocyte anaphase. Am. J. Hum. Genet. 2000;66:687–691. doi: 10.1086/302769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D., Antoccia A., Tanzarella C., Degrassi F. Topoisomerase II inhibition in mitosis produces numerical and structural chromosomal aberrations in human fibroblasts. Cytogenet. Cell Genet. 1997;76:61–67. doi: 10.1159/000134517. [DOI] [PubMed] [Google Scholar]
- Cimini D., Tanzarella C., Degrassi F. Differences in malsegregation rates obtained by scoring ana-telophase or binucleate cells. Mutagenesis. 1999;14:563–568. doi: 10.1093/mutage/14.6.563. [DOI] [PubMed] [Google Scholar]
- Duesberg P., Rausch C., Rasnik D., Helman R. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proc. Natl. Acad. Sci. USA. 1998;95:13692–13697. doi: 10.1073/pnas.95.23.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri C.J., Paschal B.M., Vaughan K.T., Vallee R.B. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J.H., Corell A.T. Chromosome errors at mitotic anaphase. Genome. 1992;35:702–705. doi: 10.1139/g92-107. [DOI] [PubMed] [Google Scholar]
- Gorbsky G.J. Cell cycle progression and chromosome segregation in mammalian cells cultured in the presence of the topoisomerase II inhibitors ICRF-187 [(+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane; ADR-529] and ICRF-159 (Razoxane) Cancer Res. 1994;54:1042–1048. [PubMed] [Google Scholar]
- Gorbsky G.J., Ricketts W.A. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J. Cell Biol. 1993;122:1311–1321. doi: 10.1083/jcb.122.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneen W.K. Kinetohcores and microtubules in multipolar mitosis and chromosome orientation. Exp. Cell Res. 1975;91:57–62. doi: 10.1016/0014-4827(75)90140-8. [DOI] [PubMed] [Google Scholar]
- Izzo M., Antoccia A., Degrassi F., Tanzarella C. Immunofluorescence analysis of diazepam-induced mitotic apparatus anomalies and chromosome loss in Chinese hamster cells. Mutagenesis. 1998;13:445–451. doi: 10.1093/mutage/13.5.445. [DOI] [PubMed] [Google Scholar]
- Khodjakov A., Rieder C.L. Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J. Cell Biol. 1996;135:315–327. doi: 10.1083/jcb.135.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A., Cole R.W., McEwen B.F., Buttle K.F., Rieder C.L. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J. Cell Biol. 1997;136:229–240. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladrach K.S., LaFountain J.R. Malorientation and abnormal segregation of chromosomes during recovery from colcemid and nocodazole. Cell Motil. Cytoskeleton. 1986;6:419–427. doi: 10.1002/cm.970060407. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Kinzler K.W., Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- Li R., Yerganian G., Duesberg P., Kraemer A., Willer A., Rausch C., Hehlmann R. Aneuploidy correlated 100% with chemical transformation of Chinese hamster cells. Proc. Natl. Acad. Sci. USA. 1997;94:14506–14511. doi: 10.1073/pnas.94.26.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney T., Ginkel L.M., Hunter A.W., Wordeman L. The kinetochore of higher eucaryotesa molecular view. Int. Rev. Cytol. 2000;194:67–131. doi: 10.1016/s0074-7696(08)62395-5. [DOI] [PubMed] [Google Scholar]
- Marko M., Leith A. Stereocon-three-dimensional reconstructions from stereoscopic contouring. J. Struct. Biol. 1996;116:93–98. doi: 10.1006/jsbi.1996.0016. [DOI] [PubMed] [Google Scholar]
- Martinez-Exposito M.J., Kaplan B.K., Copeland J., Sorger P. Retention of the Bub3 checkpoint protein on lagging chromosomes. Proc. Natl. Acad. Sci. USA. 1999;96:8493–8498. doi: 10.1073/pnas.96.15.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde D.N., McDonald K.L., Ding R., McIntosh J.R. Interpolar spindle microtubules in PtK cells. J. Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.F., Heagle A.B., Cassels G.O., Buttle K.F., Rieder C.L. Kinetochore fiber maturation in PtK1 cells and its implications for the mechanism of chromosome congression and anaphase onset. J. Cell Biol. 1997;137:1567–1580. doi: 10.1083/jcb.137.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minissi S., Gustavino B., Degrassi F., Tanzarella C., Rizzoni M. Effect of cytochalasin B on the induction of chromosome missegregation by colchicine at low concentrations in human lymphocytes. Mutagenesis. 1999;14:43–49. doi: 10.1093/mutage/14.1.43. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Nicolaidis P., Petersen M.B. Origin and mechanisms of non-disjunction in human autosomal trisomies. Hum. Reprod. 1998;13:313–319. doi: 10.1093/humrep/13.2.313. [DOI] [PubMed] [Google Scholar]
- Ouspenski I.I., Brinkley B.R. Centromeric DNA cloned from functional kinetochore fragments in mitotic cells with unreplicated genomes. J. Cell Sci. 1993;105:359–367. doi: 10.1242/jcs.105.2.359. [DOI] [PubMed] [Google Scholar]
- Rieder C.L. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int. Rev. Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Cassels G. Correlative light and electron microscopy of mitotic cells in monolayer cultures. Methods Cell Biol. 1999;61:297–315. doi: 10.1016/s0091-679x(08)61987-1. [DOI] [PubMed] [Google Scholar]
- Rieder C.L., Salmon E.D. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E.D. Metaphase chromosome congression and anaphase poleward movement. In: Warner F.D., McIntosh J.R., editors. Cell Movement. Vol. 2. Kinesin, Dynein, and Microtubule Dynamics. Alan R. Liss; NY: 1989. pp. 431–440. [Google Scholar]
- Salmon E.D., Inoue T., Desai A., Murray A.W. High resolution multimode digital imaging system for mitosis studies in vivo and in vitro. Biol. Bull. 1994;187:231–232. doi: 10.1086/BBLv187n2p231. [DOI] [PubMed] [Google Scholar]
- Sen S. Aneuploidy and cancer. Curr. Opin. Oncol. 2000;12:82–88. doi: 10.1097/00001622-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sluder G., Thompson E.A., Miller F.J., Hayes J., Rieder C.L. The checkpoint control for anaphase onset does not monitor excess numbers of spindle poles or bipolar spindle symmetry. J. Cell Sci. 1997;110:421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- Thrower D.A., Jordan M.A., Wilson L. Modulation of CENP-E organization at kinetochores by spindle microtubule attachment. Cell Motil. Cytoskeleton. 1996;35:121–133. doi: 10.1002/(SICI)1097-0169(1996)35:2<121::AID-CM5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Waters J.C., Chen R.-H., Murray A.W., Salmon E.D. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 1998;141:1181–1191. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J.C., Skibbens R.V., Salmon E.D. Oscillating mitotic newt lung cell kinetochores are, on average, under tension and rarely push. J. Cell Sci. 1996;109:2823–2831. doi: 10.1242/jcs.109.12.2823. [DOI] [PubMed] [Google Scholar]
- Wise D.A., Brinkley B.R. Mitosis in cells with unreplicated genomes (MUGs)spindle assembly and behavior of centromere fragments. Cell Motil. Cytoskeleton. 1997;36:291–302. doi: 10.1002/(SICI)1097-0169(1997)36:3<291::AID-CM9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Yu H.-G., Dawe R.K. Functional redundancy in the maize meiotic kinetochore. J. Cell Biol. 2000;151:131–141. doi: 10.1083/jcb.151.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkowski R.P., Meyne J., Brinkley B.R. The centromere–kinetochore complexa repeat subunit model. J. Cell Biol. 1991;113:1091–1110. doi: 10.1083/jcb.113.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.