Abstract
The P2Y2 nucleotide receptor (P2Y2R) contains the integrin-binding domain arginine-glycine-aspartic acid (RGD) in its first extracellular loop, raising the possibility that this G protein–coupled receptor interacts directly with an integrin. Binding of a peptide corresponding to the first extracellular loop of the P2Y2R to K562 erythroleukemia cells was inhibited by antibodies against αVβ3/β5 integrins and the integrin-associated thrombospondin receptor, CD47. Immunofluorescence of cells transfected with epitope-tagged P2Y2Rs indicated that αV integrins colocalized 10-fold better with the wild-type P2Y2R than with a mutant P2Y2R in which the RGD sequence was replaced with RGE. Compared with the wild-type P2Y2R, the RGE mutant required 1,000-fold higher agonist concentrations to phosphorylate focal adhesion kinase, activate extracellular signal–regulated kinases, and initiate the PLC-dependent mobilization of intracellular Ca2+. Furthermore, an anti-αV integrin antibody partially inhibited these signaling events mediated by the wild-type P2Y2R. Pertussis toxin, an inhibitor of Gi/o proteins, partially inhibited Ca2+ mobilization mediated by the wild-type P2Y2R, but not by the RGE mutant, suggesting that the RGD sequence is required for P2Y2R-mediated activation of Go, but not Gq. Since CD47 has been shown to associate directly with Gi/o family proteins, these results suggest that interactions between P2Y2Rs, integrins, and CD47 may be important for coupling the P2Y2R to Go.
Keywords: purinergic receptors, cell surface receptors, integrins, GTP-binding proteins, signal transduction
Introduction
Cellular signaling by G protein–coupled receptors (GPCRs) not only involves direct coupling to heterotrimeric G proteins, but also involves the formation of larger protein complexes that assist in transmitting an extracellular signal to an intracellular response. For example, GPCRs activated by gamma-aminobutyric acid (GABA) can form a heteromeric complex with other gamma-aminobutyric acid receptors that is essential for activation of inwardly rectifying potassium channels (Jones et al. 1998). Also, several studies have proposed that GPCRs activate mitogen-activated protein (MAP) kinases, such as an extracellular regulated kinase (ERK1/2), by using β-arrestin or tyrosine kinase receptors as scaffolding for GPCR-induced Ras complex assembly (Pawson and Scott 1997; Luttrell et al. 1999). To add to the complexity, there appears to be a great deal of heterogeneity in the molecular mechanisms a particular GPCR uses to activate MAP kinases depending on the scaffolding proteins present in a particular cell type (Della Rocca et al. 1999).
In this paper, we have explored protein complex formation between integrins and the P2Y2 nucleotide receptor (P2Y2R), a Go- and Gq-coupled receptor that is stimulated by ATP or UTP and mediates activation of PLC-β and MAP kinases (Erb et al. 1994; Boarder et al. 1995). Sequence analysis and molecular modeling of mouse and human P2Y2R cDNAs predicted that the first extracellular loop of this receptor contains the integrin-binding domain RGD (Lustig et al. 1996; van Rhee et al. 1998). RGD has been shown to be the core recognition sequence for many integrins, including αVβ3, αVβ5, αVβ11, α5β1, and αIIbβ3 (Hynes 1992), and is present in a variety of integrin ligands, including collagen, fibronectin, and other extracellular matrix proteins, blood-borne adhesive proteins, viral coat proteins, bacterial membrane proteins, proteins from the IgG superfamily, snake venom proteins, and other integrins (Clark and Brugge 1995; Frenette and Wagner 1996). Also, bioactive proteins containing an RGD sequence, such as the insulin-like binding protein 1, have been shown to interact directly with integrins (Jones et al. 1993). To address the possibility that the P2Y2R interacts with a specific integrin, we used cell transfectants expressing recombinant wild-type (RGD) or RGE mutant P2Y2Rs, as well as synthetic RGD- and RGE-containing peptides modeled after the first extracellular loop of the receptor. We also examined how the RGD to RGE mutation affects P2Y2R-mediated signal transduction. The results of this study suggest that the RGD sequence of the P2Y2R is responsible for forming a complex with the integrins αVβ3 and αVβ5, and with CD47, a receptor for thrombospondin belonging to the IgG superfamily that is known to couple directly to β1, β2, and β3 integrins and to hetero-trimeric G proteins in the Gi/o family (Frazier et al. 1999).
Materials and Methods
Antibodies and Peptides
Anti–human α5 and αVβ5 mAbs, anti–human α5β1 polyclonal antibody (pAb), FITC-labeled goat anti–mouse F(ab)2 antibody, and human fibronectin were purchased from GIBCO BRL. Anti–human αV mAb was purchased from Zymed Laboratories. Anti–human leukocyte antigen class II mAb was purchased from Immunotech. The production of 7G2 (mouse anti–human β3 mAb) and B6H12 (mouse anti–human CD47 mAb) has been described previously (Gresham et al. 1989; Brown et al. 1990). Anti-hemagglutinin (HA) pAb, antiphosphotyrosine mAb, anti–human α3 mAb, and anti–mouse IgG conjugated to HRP were purchased from Santa Cruz Biotechnology, Inc. Anti–mouse IgG conjugated to Cy5 and anti–rabbit IgG conjugated to Oregon green 488 were purchased from Molecular Probes, and anti–focal adhesion kinase (FAK) mAb was purchased from Upstate Biotechnology. Human vitronectin was purchased from Calbiochem. RGDs and RGEs were purchased from Sigma-Aldrich. The peptide corresponding to amino acids 93–110 of the human P2Y2R, YARGDHWPFSTVLCKLVR (P2Y2 93–110), and the peptide YARGEHWPFSTVLCKLVR in which the aspartic acid was replaced with glutamic acid (P2Y2 93–110[E97]) were synthesized at the Eppley Core Laboratory (University of Nebraska Medical Center, Omaha, NE). P2Y2 93–110 and P2Y2 93–110(E97) were synthesized on a peptide synthesizer (431A; Applied Biosystems) using Fmoc chemistry and purified on a Waters Delta Prep 4000 system by reverse-phase HPLC with 0.1% trifluoroacetic acid in water versus 0.1% trifluoroacetic acid in acetonitrile using two Delta-Pak C18 40× 100 mm cartridges with 15 μm particle size and 300 Å pore size. Matrix-assisted laser desorption ionization (time of flight) mass spectrometry was used to verify the amino acid composition of P2Y2 93–110 and P2Y2 93–110(E97).
Cell Culture and Transfection
Human K562 erythroleukemia cells (CCL 243; American Type Culture Collection) were cultured in suspension in RPMI 1640 medium (GIBCO BRL) containing 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Human 1321N1 astrocytoma cells (Parr et al. 1994) were cultured in DME (GIBCO BRL) containing 5% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The retroviral vector, pLXSN, was used for stable expression of the various P2Y2R constructs in 1321N1 cells as described previously (Erb et al. 1995). In brief, the recombinant P2Y-pLXSN constructs were used to transfect PA317 amphotrophic packaging cells for production of the viral vectors. Then, 1321N1 cells were infected with the viral vectors and selected for neomycin resistance with 1 mg/ml G418 (GIBCO BRL).
Immunofluorescent Staining and Visualization
Fluorescence-activated Cell Sorting.
K562 cells (106 cells/ml) were centrifuged (100 g at 4°C) and the cell pellet was resuspended in 100 μl of HBSS (GIBCO BRL) with or without antibodies to α5, αVβ5, or β3 (0.2 mg/ml). After a 30-min incubation at 4°C, the cells were washed twice with 1 ml of PBS (GIBCO BRL) and incubated for 30 min at 4°C in 100 μl of HBSS containing 1:100 dilution of FITC-conjugated antimurine IgG. The cells were washed three times with 1 ml of PBS, fixed with 300 μl of 1% (wt/vol) paraformaldehyde in PBS containing 50 μM CaCl2, and stored at 4°C protected from light. The cells were then transferred to 12 × 75-mm tubes and fluorescence intensity was determined using a flow cytometer (EPICS 753; Beckman Coulter).
Dual Immunofluorescence Labeling.
1321N1 cells expressing HA-tagged P2Y2R constructs were plated on glass coverslips and grown to ∼40% confluence. The cells were incubated for 1 h at 22°C with 1:100 dilution of rabbit anti-HA pAb in DME containing 3% BSA, washed in PBS, and incubated for 1 h at 22°C with 1:100 dilution of anti–rabbit IgG conjugated to Cy5 in DME containing 3% BSA. The cells were then washed in PBS, fixed in 1% formalin in PBS for 10 min, lysed with 0.5% Triton X-100 in H2O for 1 min, and washed again in PBS. The fixed cells were incubated for 1 h at 22°C in PBS containing 3% BSA and 1:100 dilution of mouse anti-αV mAb, washed in PBS, and incubated for 1 h at 22°C in PBS containing 3% BSA and 1:100 dilution of anti–mouse IgG conjugated to Oregon green. The dual-labeled cells were washed in PBS, rinsed in H2O, and mounted on glass slides in Prolong Antifade reagent (Molecular Probes). Digital images of the dual-labeled cells were taken on a confocal microscope (MRC600; Bio-Rad Laboratories), processed with CoMOS software, and visualized with Adobe Photoshop® v5.5 in which red was assigned to Cy5 fluorescence and green was assigned to Oregon green fluorescence. Yellow pixels, representing colocalization of P2Y2Rs and αV integrins, were quantified in single cells from a Photoshop® histogram. In brief, single cell images (1.64 μm/pixel magnification) were selected from a flattened, 24-bit RGB mode document and copied to a new document in CYMK mode where curves for cyan, magenta, and black, representing image noise, were reduced to 0. Pixels within counts 1–50 of the resulting yellow histogram were recorded as the total number of yellow pixels per cell.
Ligand-coated Bead-binding Assay
Preparation of ligand-coated beads and analysis of their binding to cells were performed as described (Lindberg et al. 1993), with modifications to mask unreacted sites on the coated beads and to reduce nonspecific bead binding. Ligand-coated beads were prepared by incubating 1.3-μm diameter aldehyde-modified fluorescent latex beads (4 × 108/ml; Molecular Probes) with 0.25 mM P2Y2 93–110, 0.25 mM P2Y2 93–110(E97), 0.04 mg/ml fibronectin, 0.02 mg/ml vitronectin, or 0.04 mg/ml human serum albumin (HSA) in 225 μl of PBS. After 1 h incubation at 37°C, the beads were sonicated for 10 s and added to 12.5 mg/ml HSA in 800 μl of PBS containing 0.1 M glycine to mask unreacted sites. After 30 min incubation at 37°C, the beads were centrifuged (2,000 g for 5 min) and resuspended in 1 ml of HBSS supplemented with 10 mM Hepes, pH 7.4, 1% HSA, 4.2 mM NaHCO3, 50 μg/ml gentamicin, and 1 mM MnCl2 (modified HBSS). The peptide-coated beads were sonicated for 5 s immediately before use with an Artek probe sonicator at the microtip limit setting. K562 cells (1.5 × 105) were preincubated in 70 μl of modified HBSS with various concentrations of indicated peptide or antibody for 15 min at 22°C, followed by the addition of 1.7 × 107 ligand-coated beads. After 1 h incubation at 37°C, the number of beads bound to cells was determined by fluorescence microscopy (Lindberg et al. 1993). Binding was calculated as the adhesion index (AI), defined as the number of beads bound per 100 cells.
Receptor Constructs
Incorporation of the HA Epitope into the P2Y2R.
Wild-type human P2Y2R cDNA was subcloned into the retrovirus expression vector pLXSN at the EcoRI/BamHI sites of the multiple cloning site. The open reading frame of the wild-type P2Y2R cDNA was modified to incorporate at the NH2 terminus of the expressed protein, the HA epitope (YPYDVPDYA) from influenza virus, by using the PCR. The forward and reverse HA primers were, respectively: 5′-GATCGTGAATTCTGATGTATCCATATGATGTTCCAGATTATGCTGCAGCAGACCTGGAACCCTGG-3′ and 5′-GATCGTGGATCCCCTGACTGAGGTGCTATAGCCG-3′. The PCR solution contained primers (0.7 μM each), 10 μl of 10× Vent polymerase buffer (New England Biolabs, Inc.), 100 ng of template DNA, 1 U Vent (exo+) polymerase, and 20 μl of dNTP mixture (0.2 mM dATP, dCTP, dTTP, and dGTP) in a final volume of 100 μl. The PCR parameters were: 96°C for 1 min, 62°C for 1 min, and 72°C for 2.5 min for 25 cycles. After verification of the PCR amplification products by agarose gel electrophoresis, the products were purified using a Promega PCR Wizard kit. The purified PCR products and pLXSN DNA were digested overnight with EcoRI and BamHI and ligated together, followed by transformation of competent Escherichia coli and identification of positive clones. All mutant DNAs were sequenced on both strands to ensure mutagenesis had occurred as predicted using an ABI Prism automated sequencer (PerkinElmer) and fluorescence dideoxy-nucleotide technology.
Site-directed Mutagenesis of P2Y Receptor cDNA.
A codon for glutamic acid (E) was substituted for D116 and D121 of the turkey P2Y1R and D97 of the human P2Y2R cDNAs by the PCR method described above, except that the forward and reverse P2Y1R-E116 primers were, respectively: 5′-GCCTCCACCAAGTGCTCCCTG-3′ and 5′-CCTCTGCAGTTTGCACATGACATCCCCGAAGATCCATTCGGTTTT-3′. The forward and reverse P2Y1E121R primers were, respectively: 5′-GCCTCCACCAAGTGCTCCCTG-3′ and 5′-CCTCTGCAGTTTGCACATGACTTCCCCGAA-3′. The 260-bp PCR products were excised from a 0.7% agarose gel, cut with the restriction enzymes PstI and DraIII, and substituted for the wild-type PstI/DraIII cDNA fragment in a P2Y1R-p bluescript construct. The P2Y1R-E116 and P2Y1R-E121 cDNAs were subcloned into the BamHI polylinker site of pLXSN for mammalian cell expression. This protocol was also used to make the F119 to R119 mutation in the turkey P2Y1R cDNA, in which the forward and reverse P2Y1R-R119 primers were, respectively: 5′-GCCTCCACCAAGTGCTCCCTG-3′ and 5′-CCTCTGCAGTTTGCACATGACATCCCCGCGGATCCAGT-3′. The forward and reverse P2Y2R-E97 primers were, respectively: 5′-CGCCCGCGGCGAACACTGGCCC-3′ and 5′-AGACACCGGTGCACG-3′. The 110-bp PCR product was excised from a 0.7% agarose gel, cut with the restriction enzymes SacII and AgeI, and substituted for the wild-type SacII/AgeI cDNA fragment in the HA-tagged P2Y2R-pLXSN construct. The mutant cDNA sequences were verified by using fluorescence dideoxy-nucleotide sequencing, as described above.
Measurement of Cell Signaling Pathways
Calcium Assay.
The intracellular free calcium concentration ([Ca2+]i) was measured by dual excitation spectrofluorometric analysis of cell suspensions loaded with fura-2 (Sigma-Aldrich) as described previously (Lustig et al. 1992) and assayed in Hepes-buffered saline (10 mM Hepes, pH 7.4, 130 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM glucose). Concentration–response data were analyzed with the Prism curve-fitting program (GraphPAD).
IP Assay.
To measure accumulation of IPs, 1321N1 cells expressing P2Y2R were plated in 12-well dishes and incubated in the presence or absence of specified antibodies for 24 h in 0.7 ml serum-free and inositol-free DME containing 2 μCi of [3H]inositol (20 Ci/mmol). The cells were washed three times and incubated for 30 min at 37°C in 1 ml of LiCl solution (15 mM glucose, 25 mM Hepes, 110 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 0.9 mM MgCl2, and 30 mM LiCl) followed by stimulation with 0.1 μM UTP for 10 min. The reactions were stopped by aspiration and cell extracts were obtained by addition of 0.3 ml of ice-cold 10% perchloric acid for 15 min. The extracts were neutralized with 0.6 ml of 2 M NaOH and 225 mM Hepes. The IPs in the neutralized extracts were separated from inositol and glycerophosphoinositol on Dowex columns as described previously (Berridge et al. 1983). Radioactivity in the IP fraction was determined by liquid scintillation spectrometry using Ecolume scintillation fluid.
FAK and ERK1/2 Phosphorylation.
1321N1 cells expressing P2Y2R constructs were grown to 80% confluence in six-well plates and incubated at 37°C in medium without serum for 24 h before the experiment. After stimulation, the cells were washed with ice-cold PBS and lysed with 250 μl of lysis buffer (25 mM Tris-HCl, pH 7.4, 25 mM NaCl, 1 mM Na3VO4, 10 mM NaF, 10 mM Na4(P2O7), 25 mM β-glycerophosphate, 25 mM p-nitrophenylphosphate, 0.5 mM EGTA, 0.5% Triton X-100, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 nM okadaic acid). The extracts were centrifuged (8,200 g for 10 min at 4°C) to remove insoluble material, solubilized in 100 μl of 2× Laemmli sample buffer (120 mM Tris-HCl, pH 6.8, 2% SDS, 10% sucrose, 1 mM EDTA, 50 mM dithiothreitol, 0.003% bromophenol blue), heated for 3 min at 96°C, subjected to 7.5% SDS-PAGE, and transferred to nitrocellulose membranes for protein immunoblotting. Immunoblotting of tyrosine phosphorylated FAK was performed by using 1:1,500 dilution of rabbit antiphospho FAK Tyr397 IgG (Upstate Biotechnology) as the primary antibody and 1:2,000 dilution of HRP-conjugated anti–rabbit IgG as the secondary antibody. Immunoblotting of phosphorylated ERK1/2 was performed by using 1:1,000 dilution of mouse antiphospho p42/p44 MAP kinase IgG (Cell Signaling) as the primary antibody and 1:2,000 dilution of HRP-conjugated anti–mouse IgG as the secondary antibody. Chemiluminescence in the blots was visualized on autoradiographic film with the LumiGlo chemiluminescence system (New England BioLabs, Inc.) and was quantitated by using a GS-525 molecular imager and MultiAnalyst software (Bio-Rad Laboratories). For normalization of the signal, the membranes were stripped of antibodies by a 30 min incubation at 60°C in stripping buffer (62.5 mM Tris-HCl, pH 6.7, 100 mM 2-mercaptoethanol, and 2% SDS), washed with TTBS, and reprobed with 1:1,000 dilution of anti-FAK or anti-p42/p44 MAP kinase as the primary antibodies, which bind to FAK or ERK1/2 independent of their phosphorylation state.
Alternatively, tyrosine phosphorylation of FAK was analyzed by immunoprecipitation of FAK as described by the antibody manufacturer (Upstate Biotechnology) followed by immunoblotting of the precipitated protein with 1:1,500 dilution of mouse antiphosphotyrosine monoclonal IgG as the primary antibody and 1:1,500 dilution of HRP-conjugated anti–mouse IgG as the secondary antibody.
Results
P2Y2 93–110 Interacts Specifically with αVβ3/β5 Integrins and CD47
To investigate whether the RGD domain of the P2Y2R facilitates binding to a specific integrin, we coupled a peptide derived from the human P2Y2R, YARGDHWPFSTVLCKLVR (P2Y2 93–110) to fluorescent beads and assessed the ability of the peptide-coated beads to adhere to human K562 erythroleukemia cells. Integrin receptor expression in K562 cells has been reported to be limited to the fibronectin-binding integrin α5β1 (Hemler et al. 1987), but expression of other integrin subunits (α2, α3, αV, and β3) can be induced by treatment of the cells with the protein kinase activator phorbol dibutyrate (PDBu; Burger et al. 1992; Jarvinen et al. 1993). To examine cell surface expression of integrin receptors, K562 cells were incubated with antibodies to α5, αVβ5, or β3 and subjected to fluorescence-activated cell sorting. We found that 88, 63, and 74% of the K562 cells were positively stained by antibodies to α5, αVβ5, and β3, respectively, and that an overnight treatment of K562 cells with 100 nM PDBu did not increase the fluorescence intensity or percentage of cells stained by these antiintegrin antibodies (not shown). This indicates that the K562 cell line used in this study expresses vitronectin-binding integrins (αVβ3 and αVβ5) and that cell surface expression of these integrins does not require PDBu-mediated stimulation of protein kinase C. Results of the peptide-binding studies indicated that P2Y2 93–110-coated beads bound to K562 cells with an AI of 580 ± 71 (mean ± SEM, n = 6), whereas fibronectin- and vitronectin-coated beads exhibited AI values of 318 ± 14 (n = 3) and 300 ± 28 (n = 3), respectively (Fig. 1 A). The AI for nonspecific binding, determined with human serum albumin–coated beads, was 46 ± 10 (n = 3), and the AI for beads coated with an RGE mutant P2Y2 peptide, YARGEHWPFSTVLCKLVR (P2Y2 93–110[E97]), was 70 ± 18 (n = 3). Previous studies indicated that the RGE sequence has a low affinity for integrins (Hautanen et al. 1989). Soluble P2Y2 93–110 inhibited K562 cell binding of P2Y2 93–110- and vitronectin-coated beads (IC50 equals 30 ± 15 μM and 105 ± 35 μM, respectively), but not fibronectin-coated beads, suggesting that this peptide interacts with an integrin receptor that binds vitronectin.
Figure 1.
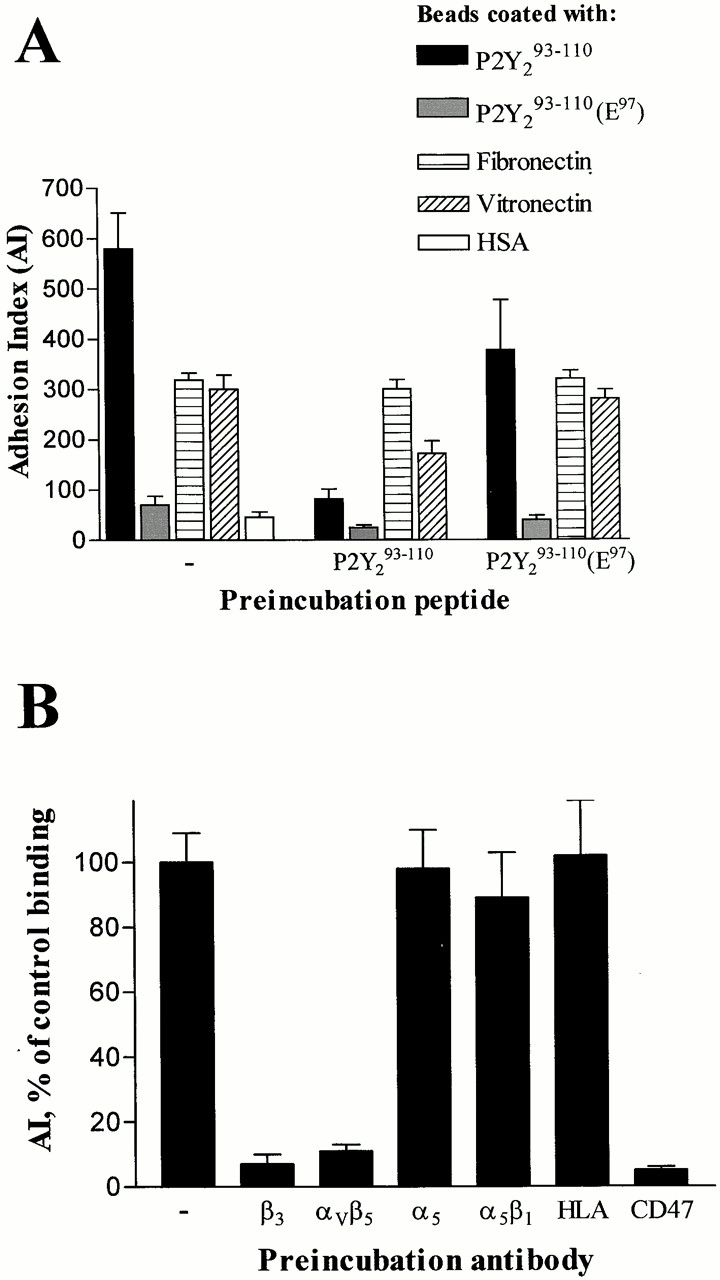
P2Y2 peptide-coated bead binding to K562 cells is inhibited by a soluble RGD peptide and antibodies to αVβ3/β5 integrins and CD47. (A) Latex beads were coated with the indicated peptide or protein and incubated with K562 cells suspended in HBSS for 1 h at 37°C in the presence or absence of preincubation peptide. The amount of bead binding to K562 cells was determined and the AI values were calculated. The data are the mean ± SEM (n = 3–6). (B) K562 cells were preincubated for 15 min in HBSS alone or in the presence of antiintegrin, control anticlass II HLA, or anti-CD47 antibodies. P2Y2 93–110 peptide-coated latex beads were added and the incubation was continued for 1 h at 37°C. The AI values were calculated and the percentage of bead binding to K562 cells compared with the control (HBSS alone) is shown. The data are the mean ± SEM (n = 3).
The specificity of binding between K562 cells and P2Y2 93–110-coated beads was determined by preincubating the cells with antibodies raised against specific integrins or integrin-associated proteins. P2Y2 93–110-coated bead binding to K562 cells was inhibited by mAbs to β3, αVβ5, or CD47, but not by antibodies to the α5β1 integrin (Fig. 1 B). These results suggest that the RGD peptide corresponding to the first extracellular loop of the P2Y2 receptor binds specifically to αVβ3/β5 integrins, but not to the α5β1 integrin expressed in K562 cells. The finding that anti-CD47 antibodies could also inhibit binding of P2Y2 93–110-coated beads to K562 cells supports the involvement of an αVβ3 integrin, since CD47 was shown previously to associate with β3 integrins and regulate vitronectin binding to the αVβ3 integrin (Lindberg et al. 1993).
RGD-dependent Colocalization of the P2Y2R with αV Integrins
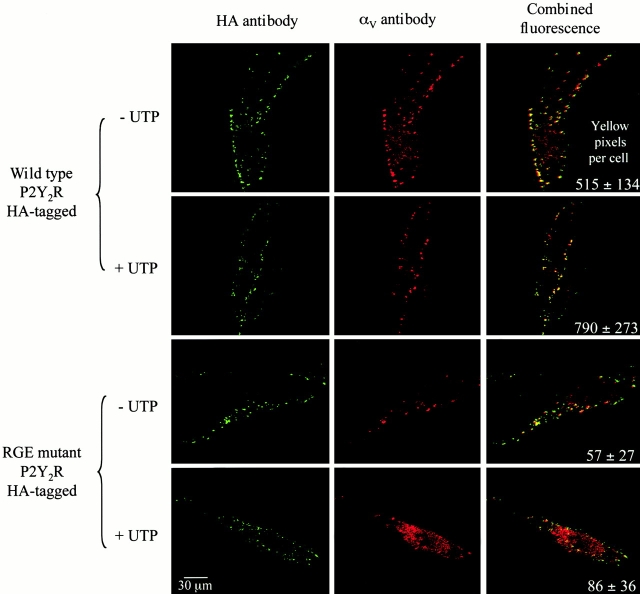
To determine whether the full-length P2Y2R interacts with αVβ3/β5 integrins, we expressed HA-tagged wild-type or RGE mutant P2Y2Rs in human 1321N1 astrocytoma cells and used immunofluorescence to analyze the distribution of these receptors relative to the αV integrin subunit. Confocal microscopy of dual-labeled 1321N1 cell transfectants showed that endogenously expressed αV exhibited ∼10-fold higher levels of colocalization with the wild-type P2Y2R than with the RGE mutant P2Y2R (Fig. 2). Furthermore, colocalization of αV and the wild-type P2Y2R occurred whether or not the cells were treated with 100 μM UTP (Fig. 2), indicating that association of these proteins is not dependent on P2Y2R activation.
Figure 2.
Colocalization of P2Y2Rs with αV integrins. Human 1321N1 cells expressing HA-tagged wild-type or RGE mutant P2Y2Rs were incubated at 37°C with or without 1 mM UTP for 5 min. The HA-tagged receptors were detected by confocal microscopy and immunofluorescence with the dye Cy5 (shown in red) and αV integrins in the same cells were detected by immunofluorescence with the dye Oregon green (shown in green). The combined fluorescence, indicative of colocalization, is shown in yellow. The data are both representative and the mean ± SEM (n = 6).
The RGD Domain of the P2Y2R Regulates Go-mediated Ca2+ Mobilization and Other Signaling Events
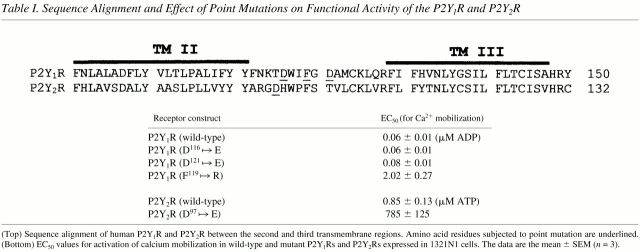
Functional studies indicated that the RGE mutant P2Y2R required ∼1,000-fold higher concentrations of the agonists ATP or UTP than the wild-type P2Y2R to stimulate PLC-dependent mobilization of intracellular Ca2+ in human 1321N1 cell transfectants (Fig. 3 A). The difference in agonist potencies between the wild-type and RGE mutant P2Y2Rs in the cell transfectants was not due to differences in the level of cell surface receptor expression, since fluorescence-activated cell sorting of intact cells labeled with anti-HA antibodies confirmed that both HA-tagged wild-type and RGE mutant P2Y2Rs had similar expression levels (not shown). It is also unlikely that the RGE mutation in the first extracellular loop of the P2Y2R affects ligand binding since receptor modeling and mutagenesis studies have indicated that the ligand-binding determinants for both the P2Y1 and P2Y2 receptor subtypes are located within the third, sixth, and seventh transmembrane domains (Erb et al. 1995; van Rhee et al. 1998). However, to address the possibility that the D to E point mutation in the P2Y2R was affecting ligand binding, we analyzed the effect of similar point mutations in the structurally related P2Y1 receptor. The ADP-selective P2Y1R does not express the RGD integrin-binding motif, but does contain an aspartic acid residue (KTD116) in almost the same position as the aspartic acid residue in the RGD97 sequence of the P2Y2R (Table ). To investigate the ability of D116 in the P2Y1R to influence agonist-induced Ca2+ mobilization, we expressed in 1321N1 cells a P2Y1R containing a KTD116 to KTE mutation. Unlike the RGE mutant P2Y2R, the KTE mutant P2Y1R exhibited the same agonist potency as the wild-type P2Y1R (Table ). Likewise, mutation of another aspartic acid residue in the first extracellular loop of the P2Y1R (FGD121 to FGE) had no effect on agonist potency (Table ). Interestingly, when we mutated FGD to RGD in the P2Y1R, it then required >30-fold higher concentrations of ADP to stimulate Ca2+ mobilization compared with the wild-type P2Y1R (Table ). Although these results do not prove whether or not ligand binding to the P2Y2R is affected by the RGD97 to RGE mutation, they do, by comparison, suggest that D to E mutations in the first extracellular loop of P2YRs are not important for receptor activity but, rather, it is expression of the RGD integrin-binding domain that influences receptor activity.
Figure 3.
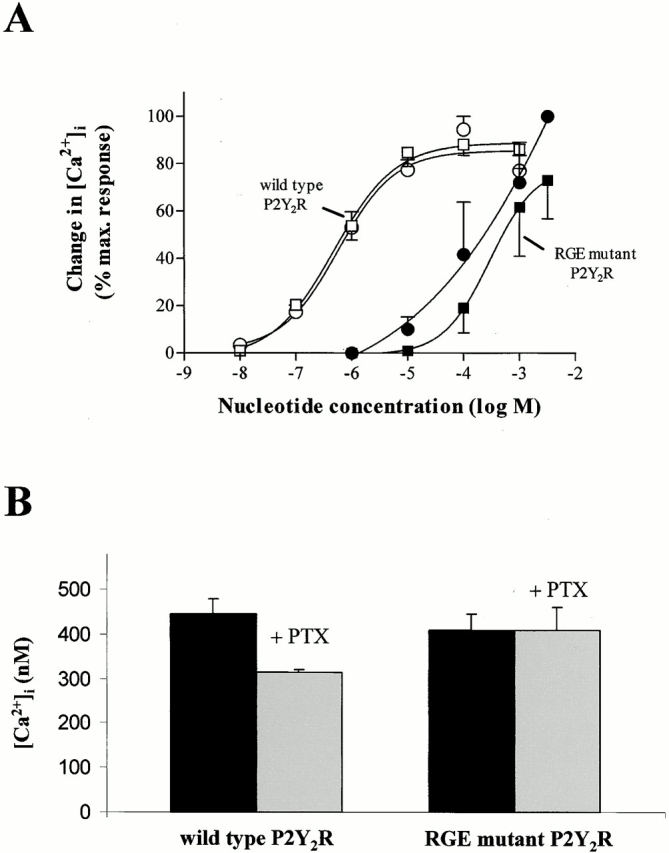
Calcium mobilization mediated by wild-type and RGE mutant P2Y2Rs. (A) Human 1321N1 cells expressing either wild-type (open symbols) or RGE mutant (filled symbols) P2Y2Rs were assayed for receptor activity by quantitating changes in the concentration of cytoplasmic free calcium ([Ca2+]i) in response to the indicated concentration of ATP (squares) or UTP (circles). Data are expressed as a percentage of the maximum increase in [Ca2+]i elicited by the most effective ligand for each receptor construct and represent the mean ± SEM (n = 3). (B) 1321N1 transfectants were treated overnight with or without 200 ng/ml Bordetella pertussis toxin (PTX). The maximum change in [Ca2+]i in response to 1 μM UTP for the wild-type P2Y2R transfectants or 100 μM UTP for the RGE mutant P2Y2R transfectants is shown as the mean ± SEM (n = 3) from one experiment and is representative of three separate experiments. The resting [Ca2+]i was ∼130 nM for each condition.
Table 1.
Sequence Alignment and Effect of Point Mutations on Functional Activity of the P2Y1R and P2Y2R
| ||
|---|---|---|
| Receptor construct | EC50 (for Ca2+ mobilization) | |
| P2Y1R (wild-type) | 0.06 ± 0.01 (μM ADP) | |
| P2Y1R (D116 ↦ E | 0.06 ± 0.01 | |
| P2Y1R (D121 ↦ E) | 0.08 ± 0.01 | |
| P2Y1R (F119 ↦ R) | 2.02 ± 0.27 | |
| P2Y2R (wild-type) | 0.85 ± 0.13 (μM ATP) | |
| P2Y2R (D97 ↦ E) | 785 ± 125 | |
(Top) Sequence alignment of human P2Y1R and P2Y2R between the second and third transmembrane regions. Amino acid residues subjected to point mutation are underlined. (Bottom) EC50 values for activation of calcium mobilization in wild-type and mutant P2Y1Rs and P2Y2Rs expressed in 1321N1 cells. The data are the mean ± SEM (n = 3).
The Gi/o inhibitor pertussis toxin caused a 30% inhibition of Ca2+ mobilization mediated by the wild-type P2Y2R expressed in 1321N1 cells, whereas Ca2+ mobilization mediated by the RGE mutant receptor was insensitive to pertussis toxin treatment (Fig. 3 B). This supports other studies indicating that the P2Y2R activates both Go- and Gq-mediated calcium signaling (Erb et al. 1994), and further indicates that substitution of the RGD integrin-binding domain with RGE preferentially affects Go-mediated calcium signaling.
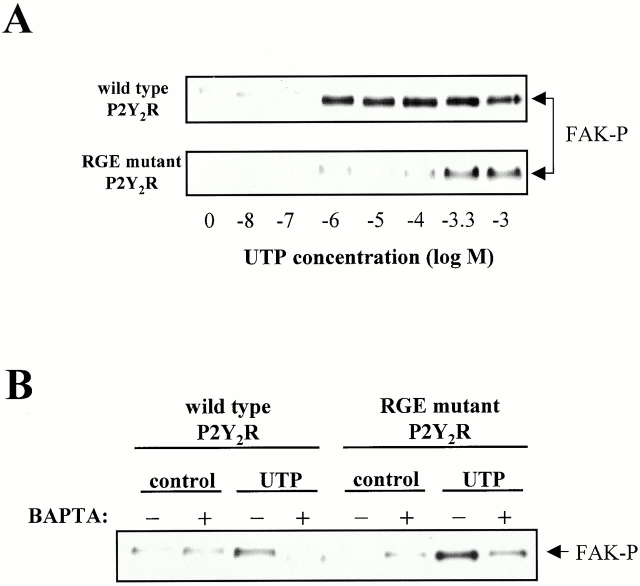
Previous studies indicated that the P2Y2R mediates tyrosine phosphorylation of FAK (Soltoff et al. 1998a), a protein known to be associated with integrins, focal adhesions, and cytoskeletal proteins (Clark and Brugge 1995). Fig. 4 A shows that FAK phosphorylation by the RGE mutant P2Y2R requires ∼1,000-fold higher concentrations of the agonist UTP, as compared with the wild-type P2Y2R. FAK phosphorylation mediated by the wild-type and RGE mutant P2Y2Rs was dependent on intracellular Ca2+ mobilization, since preincubation with the calcium chelating agent 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) inhibited UTP-induced FAK phosphorylation in 1321N1 cell transfectants (Fig. 4 B). These results are consistent with the conclusion that the RGD sequence of the P2Y2R promotes efficient activation of the calcium-dependent signaling pathway.
Figure 4.
FAK phosphorylation mediated by the wild-type and RGE mutant P2Y2Rs. Human 1321N1 cells expressing either wild-type or RGE mutant P2Y2Rs were stimulated with (A) the indicated concentration of UTP for 5 min at 37°C or with (B) 1 mM UTP after a 30 min preincubation with buffer or 10 μM BAPTA-AM. The cells were lysed in RIPA buffer and FAK was immunoprecipitated from the lysate. Phosphorylation of FAK was detected by Western blot analysis with an antiphosphotyrosine antibody. The data are representative of results from three to six experiments.
P2Y2R activation in 1321N1 cells also leads to phosphorylation of the MAP kinases, ERK1/2. Similar to results from experiments on calcium mobilization and calcium-dependent FAK phosphorylation (Fig. 3 and Fig. 4), ERK1/2 phosphorylation by the RGE mutant P2Y2R requires ∼1,000-fold higher concentrations of UTP, as compared with the wild-type P2Y2R (Fig. 5 A). Depending on the cell type, ERK1/2 phosphorylation by the P2Y2R has been found to occur by the calcium-independent activation of protein kinase Cδ and phospholipase D (Neary et al. 1999) and/or by the calcium-dependent transactivation of the EGF receptor (Soltoff 1998b). In 1321N1 cell transfectants, we found that ERK1/2 phosphorylation by the wild-type P2Y2R was partially inhibited by the calcium chelator BAPTA, whereas ERK1/2 phosphorylation by the RGE mutant was not affected by BAPTA treatment (Fig. 5 B). Thus, it appears that activation of ERK1/2 by the P2Y2R in 1321N1 cells involves both calcium-dependent and -independent events and that the RGD sequence of the P2Y2R primarily assists in activating the calcium-dependent ERK1/2 signaling pathway.
Figure 5.
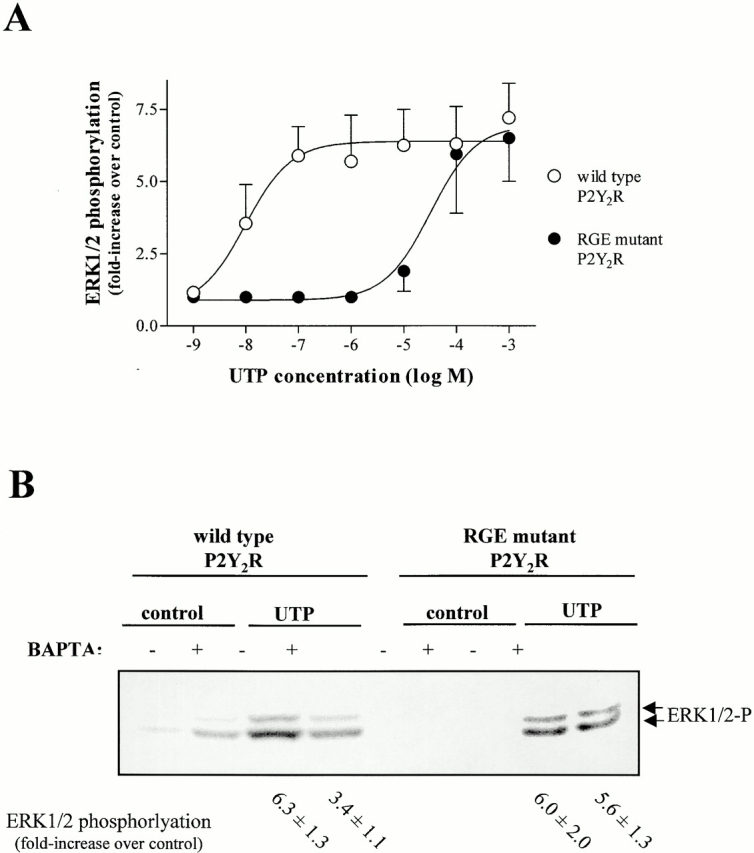
MAP kinase activation mediated by the wild-type and RGE mutant P2Y2Rs. Human 1321N1 cells expressing either wild-type or RGE mutant P2Y2Rs were stimulated with (A) the indicated concentration of UTP for 5 min at 37°C or with (B) 100 μM UTP after a 30 min preincubation with buffer or 10 μM BAPTA-AM. MAP kinase activation (ERK1 and ERK2 phosphorylation) was detected by Western blot analysis with an antiphospho ERK1/2 antibody as described in Materials and Methods. The data are representative of results from four to six experiments.
P2Y2R-mediated Signaling Is Inhibited by Anti-αV Antibodies and by RGDS
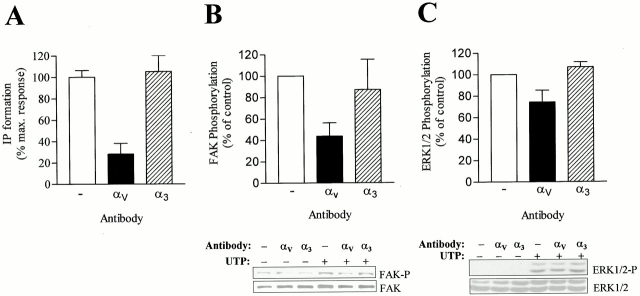
We investigated whether an antibody raised against the αV integrin subunit could interfere with UTP-stimulated P2Y2R signal transduction. To analyze the affect of this antibody on the P2Y2R calcium signaling pathway, we measured IP formation since inositol 1,4,5-trisphosphate (IP3) is the second messenger for intracellular calcium mobilization (Weisman et al. 1998). The results indicated that overnight treatment with anti-αV antibody partially inhibited IP formation induced by UTP (Fig. 6 A), consistent with the decreased calcium response seen for RGE mutant receptors as compared with wild-type P2Y2R (Fig. 3). Similarly, phosphorylation of FAK, a calcium-dependent response (Fig. 4 B), was partially inhibited by anti-αV antibody (Fig. 6 B). ERK1/2 phosphorylation was inhibited by anti-αV antibody to a lesser extent than FAK phosphorylation or IP formation (Fig. 6 C), consistent with the presence of both calcium-dependent and calcium-independent pathways for ERK1/2 phosphorylation (Fig. 5 B). Anti-α3 integrin antibody had no effect on the ability of the P2Y2R to stimulate any of these signaling events (Fig. 6, A–C). These findings suggest that interaction of αV integrin with the P2Y2R is important for optimum P2Y2R activation.
Figure 6.
Effect of antiintegrin antibodies on IP formation and FAK and ERK1/2 phosphorylation by the P2Y2R. Human 1321N1 cells expressing wild-type P2Y2Rs were incubated for 24 h with or without 100 μg/ml of the indicated antibody. The cells were then stimulated with 1 μM UTP for 10 min (A), 1 μM UTP for 5 min (B), or 10 nM UTP for 5 min (C). For A, generation of IP is expressed as the percentage of maximum stimulation with UTP after background subtraction (backgrounds for control, α3-treated, and αV-treated samples were 18,462 ± 1,090, 15,670 ± 1,000, and 30,450 ± 3,650 dpm, respectively). For B, FAK phosphorylation was assayed by Western blot analysis with antiphospho FAK antibody as described in Materials and Methods. For C, ERK1/2 phosphorylation was assayed as described in the legend to Fig. 5. The data for A are the mean ± SEM (n = 3). The data for B and C are both representative (bottom) and the mean ± SEM (n = 3–5) (top).
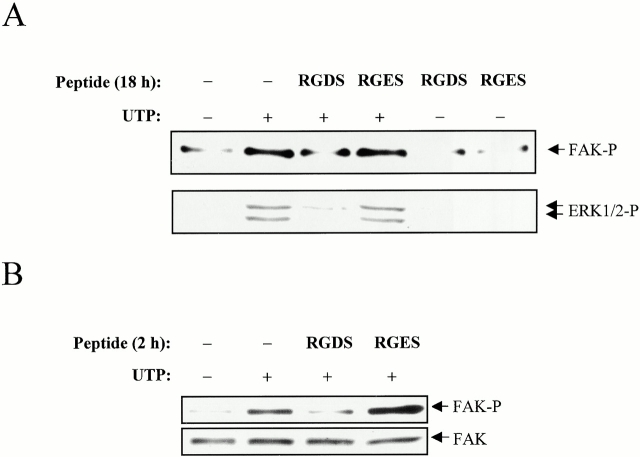
Previous studies have indicated that overnight treatment with the tetrapeptide RGDS, which inhibits integrin dimerization and disrupts the formation of focal adhesions, can also inhibit GPCR-mediated ERK1/2 activation and FAK phosphorylation in certain cell types (Della Rocca et al. 1999). We tested whether RGDS could similarly influence FAK and ERK1/2 phosphorylation mediated by the P2Y2R in 1321N1 cell transfectants. The results indicated that overnight treatment with RGDS inhibited FAK and ERK1/2 phosphorylation, whereas the control peptide, RGES, had no effect on the ability of the P2Y2R to induce phosphorylation of these kinases (Fig. 7 A). P2Y2R-mediated Ca2+ mobilization and IP formation were not inhibited by overnight treatment with RGDS, and shorter incubation periods with RGDS did not cause inhibition of FAK or ERK1/2 phosphorylation (not shown). However, a 2-h incubation with RGDS could inhibit P2Y2R-mediated FAK phosphorylation (Fig. 7 B), but only if the P2Y2Rs were subjected to agonist-induced sequestration (Garrad et al. 1998), in which the peptide was added during the time period when the internalized receptors were being recycled to the plasma membrane. Thus, the RGDS peptide is less effective as a modulator of P2Y2R activity than anti-αV antibody.
Figure 7.
Effect of RGDS and RGES peptides on FAK and ERK1/2 phosphorylation by the P2Y2R. Human 1321N1 cells expressing wild-type P2Y2Rs were (A) incubated for 18 h with 100 μM of the indicated peptide or (B) treated with 100 μM UTP for 10 min to induce internalization of the P2Y2Rs, rinsed, and incubated for 2 h with 100 μM of the indicated peptide during the time period when internalized P2Y2Rs are being recycled to the plasma membrane (Garrad et al. 1998). The cells were then stimulated with 1 μM UTP (for FAK experiments) or 10 nM UTP (for ERK1/2 experiments) for 5 min at 37°C. Phosphorylation of FAK and ERK1/2 were assayed as described in the legends to Fig. 4 and Fig. 5, respectively. The data are representative of results from three to five experiments.
Discussion
The results of this study suggest that an integrin-binding domain (RGD) in the P2Y2R allows this receptor to associate with αVβ3/β5 integrins and the integrin-associated thrombospondin receptor, CD47. Furthermore, mutation of the RGD sequence to RGE in the P2Y2R or addition of an anti-αV integrin antibody was found to inhibit P2Y2R signal transduction (i.e., IP formation and phosphorylation of FAK and ERK1/2). This suggests that interaction between P2Y2Rs and integrins either shifts the P2Y2R into a high affinity state and/or allows efficient coupling of this receptor to its intracellular signaling proteins. Currently, there is no reliable assay for measuring ligand binding to the P2Y2R, making it difficult to distinguish between these two possibilities. However, there is evidence that the latter is affected. We found that the Gi/o inhibitor, pertussis toxin, partially inhibited Ca2+ signaling by the wild-type P2Y2R, but not by the RGE mutant receptor. Since CD47 has been found to form a complex with integrins and proteins in the Gi/o family (Frazier et al. 1999), these data suggest that the P2Y2R requires integrin–CD47 interactions to access Go.
Activation of CD47 has been shown to shift several integrins into a high affinity state. For example, the CD47 agonist 4N1K stimulates platelet spreading on a fibrinogen-coated surface and causes platelet aggregation by activating the integrin αIIbβ3 (Chung et al. 1997). In melanoma cells, activation of CD47 stimulates αVβ3 integrin-dependent cell spreading on a vitronectin-coated surface (Gao et al. 1996). And in vascular smooth muscle cells, activation of CD47 inhibits adenylate cyclase and ERK1/2 activity which stimulates α2β1 integrin-dependent chemotactic migration toward soluble collagen (Wang et al. 1999). In turn, there is evidence that integrin activation can transactivate CD47. In melanoma cells, activation of the αVβ3 integrin with peptides derived from type IV collagen causes Ca2+ mobilization and chemotaxis, both of which require CD47 (Shahan et al. 2000). Whether the P2Y2R can also influence integrin or CD47 activity remains to be seen. Nonetheless, the results presented here suggest that signaling by the P2Y2R may depend on interaction with αVβ3/β5 integrins and CD47 and support the idea that functional diversity among members of the GPCR superfamily is provided by differential linkage to scaffolding proteins involved in the formation of a protein-signaling complex (Montell 1998).
The presence of an RGD sequence in an extracellular domain of a GPCR is rare, occurring only in the first extracellular loop of several species homologues of the P2Y2Rs and P2Y6Rs and the third extracellular loop of the H2 histamine receptor (GPCR database: http://www.gpcr.org). In contrast to the human and murine P2Y2Rs, the rat homologue contains a QGD instead of an RGD sequence (Rice et al. 1995). However, this change in the arginine position of the RGD integrin-binding domain is considered to be a conservative substitution that maintains integrin binding (Gresham et al. 1992).
Although the potential for a GPCR to physically interact with an integrin has not been explored previously, there is precedence for interactions between integrins and other types of receptors containing an RGD sequence. For example, the urokinase-type plasminogen-activated receptor has been found to coimmunoprecipitate with the αMβ2 integrin (Wei et al. 1996). Also, activated PDGFb receptors and insulin receptors have been found to coimmunoprecipitate with αVβ3 integrins (Schneller and Ruoslahti 1997), and the synergistic actions of αVβ3 and the PDGF receptor increase endothelial cell migration (Woodard et al. 1998). Attempts to coimmunoprecipitate the HA-tagged P2Y2R with the αV integrin thus far have been unsuccessful. Studies that have successfully coimmunoprecipitated GPCRs and other proteins have found that (a) covalent crosslinking of the proteins is necessary because of the harsh conditions required to solubilize GPCRs and (b) overexpression of both proteins is necessary for Western blot detection (Luttrell et al. 1999). Therefore, we are currently developing these techniques for coimmunoprecipition of proteins with P2Y2Rs. In this study, we have shown by immunofluorescence microscopy that the HA-tagged P2Y2R and the αV integrin colocalize in the plasma membrane of 1321N1 astrocytoma cells and that the extent of colocalization between these proteins is ∼10-fold greater than the level of colocalization between αV and the RGE mutant receptor (Fig. 2). Although these data are suggestive of a direct interaction between an integrin and the RGD sequence of the P2Y2R, it is possible that the interaction observed by microscopy reflects a transient, low-affinity interaction or an interaction with the detergent insoluble actin cytoskeleton. If this is the case, it will require more sophisticated techniques to demonstrate this interaction by coimmunoprecipitation. Whatever the nature of this RGD-dependent interaction, our data strongly suggest that it is directly relevant to the regulation of P2Y2R-mediated signal transduction.
The αVβ3 integrin is expressed in vascular endothelial cells and has been shown to play a vital role in angiogenesis, the de novo growth of blood vessels, a process essential to the growth of solid tumors that has become an important target in cancer therapy (Brooks et al. 1994a,Brooks et al. 1994b). The P2Y2R is also expressed in vascular endothelial cells, where it has been recognized for its antithrombotic and vasodilatory properties (Pearson et al. 1983; Boarder et al. 1995). Activation of the P2Y2R has been shown to increase prostacyclin and nitric oxide release from endothelial cells (Lustig et al. 1992; Weisman et al. 1998), surfactant phospholipid secretion from alveolar type II cells (Rice et al. 1995), and inducible nitric oxide synthase expression in macrophages (Denlinger et al. 1996). In addition to these cells, the P2Y2R is expressed in kidney, liver, spleen, heart, brain, and placental tissue (Lustig et al. 1993; Parr et al. 1994). Recent studies have indicated that the P2Y2R is up-regulated in activated thymocytes as an immediate early gene response (Koshiba et al. 1997) and in epithelial cells in response to ligation of the salivary gland duct (Turner et al. 1998), whereas it is down-regulated during differentiation of human myeloid leukocytes (Martin et al. 1997). Also, activation of the P2Y2R in human umbilical vein endothelial cells has been found to induce a transient, 10-fold increase in αv integrin expression that is inhibited by the protein kinase C inhibitor GF109238 (Erb, L., C. Clamp, and G.A. Weisman. 1998. International Society for Heart Research–XVI World Congress. J. Mol. Cell. Cardiol. 30:499). In endothelial cell cultures, P2Y2R expression is readily apparent (Pearson et al. 1983; Lustig et al. 1992), although it is more difficult to detect in mature blood vessels (B. Krugh, unpublished observation). One hypothesis is that the level of P2Y2R expression increases in response to tissue damage or to cell perturbations associated with culture procedures. If this hypothesis holds with endothelial cell damage in vivo, the up-regulation of P2Y2Rs, like αVβ3, may occur under conditions that promote angiogenesis or atherosclerosis. Considering the wide-spread distribution of P2Y2Rs, it seems likely that interactions between P2Y2Rs and integrins, such as αVβ3, could have a range of physiological consequences yet to be delineated.
Here we have presented data suggesting that P2Y2Rs, αVβ3/β5 integrins, and CD47 interact within the same cell. Is it also possible for these receptors to interact intercellularly and affect cell–cell adhesion? Several studies have indicated that extracellular ATP and UTP can stimulate cell–cell adhesion, although the molecular/biochemical basis for this interaction has not been identified. For example, ATP and UTP have been shown to stimulate cell adhesion in a monocyte/macrophage lineage (Ventura and Thomopoulos 1995) and enhance neutrophil adherence to endothelial cell monolayers (Parker et al. 1996). Antibodies to P-selectin, ICAM-1, and the β2 integrin subunit did not prevent ATP-induced intercellular adherence between neutrophils and endothelial cells (Parker et al. 1996), although the contribution of αVβ3/β5 integrins was not explored. The αVβ3 integrin on monocytes mediates their adherence to endothelial and epithelial cells (Beekhuizen and Van Furth 1993; Murphy et al. 1994), an early event in the acute inflammatory response. Also, αVβ3 and CD47 play a role in transendothelial monocyte and neutrophil migration (Lindberg et al. 1996; Weerasinghe et al. 1998), although a role for the P2Y2R as a counter-receptor for αVβ3 or CD47 has not been assessed. Studies by our group have found that activated polymorphonuclear cells bind more readily to K562 cells transfected with human P2Y2R cDNA than to untransfected or vector-transfected K562 cells (H. Gresham, unpublished observation). Therefore, cell–cell interactions between P2Y2Rs, αVβ3/β5 integrins, and CD47 are likely to have physiological consequences and should be further investigated. Since peptides modeled after the first extracellular loop of the P2Y2R bind selectively to αVβ3/β5 integrins and CD47 (Fig. 1 B), the potential for P2Y2R-derived peptides to inhibit intercellular interactions between these receptors also should be investigated. Such inhibitors of αVβ3 integrin, CD47, and P2Y2R interactions could prove useful as pharmacotherapeutic agents in the treatment of atherosclerosis, diabetes, cancer and inflammation.
Acknowledgments
We thank Simon Robson (Harvard University, Cambridge, MA) for helpful discussions and advice.
This work was supported by grants from the American Diabetes Association, American Heart Association, Cancer Research Center of Missouri, Monsanto, National Institutes of Health, North American Cystic Fibrosis Foundation, and the University of Missouri-Columbia F21C Program.
Footnotes
Abbreviations used in this paper: AI, adhesion index; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; ERK1/2, extracellular regulated kinase; FAK, focal adhesion kinase; GPCR, G protein–coupled receptor; HSA, human serum albumin; IP, inositol phosphate; HA, hemagglutinin; MAP, mitogen-activated protein; pAb, polyclonal Ab; P2YR, P2Y nucleotide receptor; RGD, arginine-glycine-aspartic acid.
References
- Beekhuizen H., Van Furth R. Monocyte adherence to human vascular endothelium. J. Leukoc. Biol. 1993;54:363–378. [PubMed] [Google Scholar]
- Berridge M.J., Dawson R.M.C., Downes C.P., Heslop J.P., Irvine R.F. Changes in the level of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem. J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarder M.R., Weisman G.A., Turner J.T., Wilkinson G.F. G protein-coupled P2 purinoceptorsfrom molecular biology to functional responses. Trends Pharmacol. Sci. 1995;16:133–139. doi: 10.1016/s0165-6147(00)89001-x. [DOI] [PubMed] [Google Scholar]
- Brooks P.C., Clark R.A., Cheresh D.A. Integrin αVβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels Cell. 79 1994. 1157 1165a [DOI] [PubMed] [Google Scholar]
- Brooks P.C., Clark R.A., Cheresh D.A. Requirement of vascular integrin αVβ3 for angiogenesis Science. 264 1994. 569 571b [DOI] [PubMed] [Google Scholar]
- Brown E., Hooper L., Ho T., Gresham H. Integrin-associated proteina 50-kD plasma membrane antigen physically and functionally associated with integrins. J. Cell Biol. 1990;111:2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger S.R., Zutter M.M., Sturgill-Koszycki S., Santoro S.A. Induced cell surface expression of functional α2β1 integrin during megakaryocytic differentiation of K562 leukemic cells. Exp. Cell Res. 1992;202:28–35. doi: 10.1016/0014-4827(92)90400-3. [DOI] [PubMed] [Google Scholar]
- Chung J., Gao A.G., Frazier W.A. Thrombspondin acts via integrin-associated protein to activate the platelet integrin alphaIIbbeta3. J. Biol. Chem. 1997;272:14740–14746. doi: 10.1074/jbc.272.23.14740. [DOI] [PubMed] [Google Scholar]
- Clark E.A., Brugge J.S. Integrins and signal transduction pathwaysthe road taken. Science. 1995;268:233–238. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Della Rocca G.J., Maudsley S., Daaka Y., Lefkowitz R.J., Luttrell L.M. Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. J. Biol. Chem. 1999;274:13978–13984. doi: 10.1074/jbc.274.20.13978. [DOI] [PubMed] [Google Scholar]
- Denlinger L.C., Fisette P.L., Garis K.A., Kwon G., Vazquez-Torres A., Simon A.D., Nguyen B., Proctor R.A., Bertics P.J., Corbett J.A. Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. J. Biol. Chem. 1996;271:337–342. doi: 10.1074/jbc.271.1.337. [DOI] [PubMed] [Google Scholar]
- Erb L., Lustig K.D., Sullivan D.M., Turner J.T., Weisman G.A. Functional expression and photoaffinity labeling of a cloned P2U purinergic receptor. Proc. Natl. Acad. Sci. USA. 1994;90:10449–10453. doi: 10.1073/pnas.90.22.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb L., Garrad R.A., Wang Y., Quinn T., Turner J.T., Weisman G.A. Site-directed mutagenesis of P2U purinoceptorspositively charged amino acids in transmembrane helices 6 and 7 affect agonist potency and specificity. J. Biol. Chem. 1995;270:4185–4188. doi: 10.1074/jbc.270.9.4185. [DOI] [PubMed] [Google Scholar]
- Frazier W.A., Gao A., Dimitry J., Chung J., Brown E.J., Lindberg F.P., Linder M.E. The thrombospondin receptor integrin-associated protein (CD47) functionally couples to heterotrimeric Gi . J. Biol. Chem. 1999;274:8554–8560. doi: 10.1074/jbc.274.13.8554. [DOI] [PubMed] [Google Scholar]
- Frenette P.S., Wagner D.D. Adhesion molecules. N. Engl. J. Med. 1996;334:1526–1529. doi: 10.1056/NEJM199606063342308. [DOI] [PubMed] [Google Scholar]
- Gao A.G., Lindberg F.P., Dimitry J.M., Brown E.J., Frazier W.A. Thrombospondin modulates αVβ3 function through integrin-associated protein. J. Cell Biol. 1996;135:533–544. doi: 10.1083/jcb.135.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrad R.C., Otero M.A., Erb L., Theiss P.M., Clarke L.L., González F.A., Turner J.T., Weisman G.A. Structural basis of agonist-induced desensitization and sequestration of the P2Y2 nucleotide receptor. J. Biol. Chem. 1998;273:29437–29444. doi: 10.1074/jbc.273.45.29437. [DOI] [PubMed] [Google Scholar]
- Gresham H., Goodwin J.L., Allen P.M., Anderson D.C., Brown E. A novel member of the integrin receptor family mediates Arg-Gly-Asp-stimulated neutrophil phagocytosis. J. Cell Biol. 1989;108:1935–1943. doi: 10.1083/jcb.108.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham H., Adams S.P., Brown E.J. Ligand binding specificity of the leukocyte response integrin expressed by human neutrophils. J. Biol. Chem. 1992;267:13895–13902. [PubMed] [Google Scholar]
- Hautanen A., Gailit J., Mann D.M., Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J. Biol. Chem. 1989;264:1437–1442. [PubMed] [Google Scholar]
- Hemler M.E., Huang C., Schwarz L. The VLA protein family. J. Biol. Chem. 1987;262:3300–3309. [PubMed] [Google Scholar]
- Hynes R.O. Integrinsversatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jarvinen M., Ylanne J., Virtanen I. The effect of differentiation inducers on the integrin expression of K562 erythroleukemia cells. Cell Biol. Int. 1993;17:399–407. doi: 10.1006/cbir.1993.1078. [DOI] [PubMed] [Google Scholar]
- Jones J.I., Gockerman A., Busby W.H., Wright G., Clemmons D.R. Insulin-like growth factor binding protein 1 stimulates cell migration and binds to the α5β1 integrin by means of its Arg-Gly-Asp sequence. Proc. Natl. Acad. Sci. USA. 1993;90:10553–10557. doi: 10.1073/pnas.90.22.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.A., Borowsky B., Tamm J.A., Craig D.A., Durkin M.M., Dai M., Yao W.J., Johnson M., Gunwaldsen C., Huang L.Y. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Koshiba M., Apasov S., Sverdlov P., Chen P., Erb L., Turner J.T., Weisman G.A., Sitkovsky M.V. Transient up-regulation of P2Y2 nucleotide receptor mRNA expression is an immediate early gene response in activated thymocytes. Proc. Natl. Acad. Sci. USA. 1997;94:831–836. doi: 10.1073/pnas.94.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F.P., Gresham H.D., Schwarz E., Brown E.J. J. Cell Biol. 1993;123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F.P., Gresham H.D., Reinhold M.I., Brown E.J. Integrin-associated protein immunoglobulin domain is necessary for efficient vitronectin bead binding. J. Cell Biol. 1996;134:1313–1322. doi: 10.1083/jcb.134.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig K.D., Landis D.M., Hicks-Taylor C.S., Zhang X., Erb L., Sportiello M., Weisman G.A. Mechanisms by which extracellular ATP and UTP stimulate the release of prostacyclin from bovine pulmonary artery endothelial cells. Biochim. Biophys. Acta. 1992;1134:61–72. doi: 10.1016/0167-4889(92)90028-a. [DOI] [PubMed] [Google Scholar]
- Lustig K.D., Shiau A.K., Brake A.J., Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl. Acad. Sci. USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig, K.D., G.A. Weisman, J.T. Turner, R. Garrad, A.K. Shiau, and L. Erb. 1996. P2U purinoceptors: cDNA cloning, signal transduction mechanisms and structure-function analysis. D.J. Chadwick and J.A. Goode, editors. John Wiley & Sons, New York, NY. 193–204. [DOI] [PubMed]
- Luttrell L.M., Ferguson S.S.G., Daaka Y., Miller W.E., Maudsley S., Della Rocca G.J., Lin F.T., Kawakatsu H., Owada K., Luttrell D.K., Caron M.G., Leflowitz R.J. β-arrestin-dependent formation of β2 adrenergic receptor-src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- Martin K.A., Kertesy S.B., Dubyak G.R. Down-regulation of P2U-purinergic nucleotide receptor messenger RNA expression during in vitro differentiation of human myeloid leukocytes by phorbol esters or inflammatory activators. Mol. Pharmacol. 1997;51:97–108. doi: 10.1124/mol.51.1.97. [DOI] [PubMed] [Google Scholar]
- Montell C. TRP trapped in fly signaling web. Curr. Opin. Neurobiol. 1998;8:389–397. doi: 10.1016/s0959-4388(98)80066-4. [DOI] [PubMed] [Google Scholar]
- Murphy J.F., Bordet J.C., Wyler B., Rissoan M.C., Chomarat P., Defrance T., Miossec P., McGregor J.L. The vitronectin receptor (αVβ3) is implicated, in cooperation with P-selectin and platelet-activating factor, in the adhesion of monocytes to activated endothelial cells. Biochem. J. 1994;304:537–542. doi: 10.1042/bj3040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary J.T., Kang Y., Bu Y., Yu E., Akong K., Peters C.M. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytesinvolvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J. Neurosci. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A.L., Likar L.L., Dawicki D.D., Rounds S. Mechanism of ATP-induced leukocyte adherence to cultured pulmonary artery endothelial cells. Am. J. Phys. 1996;270:695–703. doi: 10.1152/ajplung.1996.270.5.L695. [DOI] [PubMed] [Google Scholar]
- Parr C.E., Sullivan D.M., Paradiso A.M., Lazarowski E.R., Burch L.H., Olsen J.C., Erb L., Weisman G.A., Boucher R.C., Turner J.T. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Natl. Acad. Sci. USA. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Scott J.D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Pearson J.D., Slakey L.L., Gordon J.L. Stimulation of prostaglandin production through purinoceptors on cultured procine endothelial cells. Biochem. J. 1983;214:273–276. doi: 10.1042/bj2140273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R., Burton F.M., Fiedeldey D.T. Cloning and expression of the alveolar type II cell P2u purinergic receptor. Am. J. Respir. Cell Mol. Biol. 1995;12:27–32. doi: 10.1165/ajrcmb.12.1.7811468. [DOI] [PubMed] [Google Scholar]
- Schneller M., Ruoslahti E. αVβ3 integrin associates with activated insulin and PDGFb receptors and potentiates the biological activity of PDGF. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahan T.A., Fawzi A., Bellon G., Monboisse J., Kefalides N.A. Regulation of tumor cell chemotaxis by type IV collagen is mediated by a Ca2+-dependent mechanism requiring CD47 and the integrin αVβ3 . J. Biol. Chem. 2000;275:4796–4802. doi: 10.1074/jbc.275.7.4796. [DOI] [PubMed] [Google Scholar]
- Soltoff S.P., Avraham H., Avraham S., Cantley L.C. Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C J. Biol. Chem. 273 1998. 2653 2660a [DOI] [PubMed] [Google Scholar]
- Soltoff S.P. Related adhesion focal tyrosine kinase and the epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G protein-coupled P2Y2 receptor J. Biol. Chem. 273 1998. 23110 23117b [DOI] [PubMed] [Google Scholar]
- Turner J.T., Park M., Camden J.M., Weisman G.A. Salivary gland nucleotide receptorschanges in expression and activity related to development and tissue damage. Ann. NY Acad. Sci. 1998;842:70–75. doi: 10.1111/j.1749-6632.1998.tb09633.x. [DOI] [PubMed] [Google Scholar]
- van Rhee A.M., Jacobson K.A., Garrad R., Weisman G.A., Erb L. P2 receptor modeling and identification of ligand binding sites. In: Turner J.T., Weisman G.A., Fedan J.S., editors. P2 Nucleotide Receptors. Humana Press; Totowa, NJ: 1998. pp. 135–166. [Google Scholar]
- Ventura M.A., Thomopoulos P. ADP and ATP activate distinct signaling pathways in human promonocytic U-937 cells differentiated with 1,25-dihydroxy-vitamin D3 . Mol. Pharmacol. 1995;47:104–114. [PubMed] [Google Scholar]
- Wang X.Q., Lindberg F.P., Frazier W.A. Integrin-associated protein stimulates alpha2beta1-dependent chemotaxis via Gi-mediated inhibition of adenylate cyclase and extracellular-regulated kinases. J. Cell Biol. 1999;147:389–399. doi: 10.1083/jcb.147.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasinghe D., McHugh K.P., Ross F.P., Brown E.J., Gisler R.H., Imhof B.A. A role for the αVβ3 integrin in the transmigration of monocytes. J. Cell Biol. 1998;142:595–607. doi: 10.1083/jcb.142.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Lukashev M., Simon D.I., Bodary S.C., Rosenberg S., Doyle M.V., Chapman H.A. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- Weisman G.A., Gonzalez F.A., Erb L., Garrad R.C., Turner J.T. The cloning and expression of G protein-coupled P2Y nucleotide receptors. In: Turner J.T., Weisman G.A., Fedan J.S., editors. P2 Nucleotide Receptors. Humana Press; Totowa, NJ: 1998. pp. 63–79. [Google Scholar]
- Woodard A.S., Garcia-Cardena G., Leong M., Madri J.A., Sessa W.C., Languino L.R. The synergistic activity of αVβ3 integrin and PDGF receptor increases cell migration. J. Cell Sci. 1998;111:469–478. doi: 10.1242/jcs.111.4.469. [DOI] [PubMed] [Google Scholar]