Abstract
Skeletal muscle is one of a several adult post-mitotic tissues that retain the capacity to regenerate. This relies on a population of quiescent precursors, termed satellite cells. Here we describe two novel markers of quiescent satellite cells: CD34, an established marker of hematopoietic stem cells, and Myf5, the earliest marker of myogenic commitment. CD34+ve myoblasts can be detected in proliferating C2C12 cultures. In differentiating cultures, CD34+ve cells do not fuse into myotubes, nor express MyoD. Using isolated myofibers as a model of synchronous precursor cell activation, we show that quiescent satellite cells express CD34. An early feature of their activation is alternate splicing followed by complete transcriptional shutdown of CD34. This data implicates CD34 in the maintenance of satellite cell quiescence.
In heterozygous Myf5 nlacZ/+ mice, all CD34+ve satellite cells also express β-galactosidase, a marker of activation of Myf5, showing that quiescent satellite cells are committed to myogenesis. All such cells are positive for the accepted satellite cell marker, M-cadherin. We also show that satellite cells can be identified on isolated myofibers of the myosin light chain 3F-nlacZ-2E mouse as those that do not express the transgene. The numbers of satellite cells detected in this way are significantly greater than those identified by the other three markers.
We conclude that the expression of CD34, Myf5, and M-cadherin defines quiescent, committed precursors and speculate that the CD34−ve, Myf5−ve minority may be involved in maintaining the lineage-committed majority.
Keywords: skeletal muscle, satellite cell, Myf5, CD34, MyoD
Introduction
Tissue-specific activities are carried out by functionally competent cells that have achieved specialization through terminal differentiation, a process that involves withdrawal from the cell cycle and an appropriate configuration of gene activation and repression. With the exception of the liver, where functional cells retain a proliferative option (Michalopoulos and DeFrances 1997), terminal differentiation appears permanent so that the capacity to replace such cells during adult life depends on the persistence of a stem cell compartment. In constantly self-renewing tissues such as blood, skin, and gut, these compartments conform to a hierarchical archetype in which slowly dividing stem cells give rise to highly proliferative, lineage-restricted progenitor cells, which become committed precursors before terminal differentiation (Watt 1998; Akashi et al. 1999; Booth and Potten 2000).
Although not normally subject to rapid cell turnover, adult skeletal muscle also retains the ability to grow in response to increased work load and to repair and regenerate following damage. The mechanical functions of skeletal muscle are carried out by syncytial myofibers, each containing a highly specialized contractile apparatus maintained by large numbers of postmitotic myonuclei. The capacity to generate new myonuclei resides in a population of mononucleated precursors, termed satellite cells, which lie sequestered between the basal lamina and sarcolemma of each myofiber (reviewed in Bischoff 1994). In immature muscle, many satellite cells are cycling and differentiate after a limited number of divisions to contribute myonuclei to growing myofibers (Moss and Leblond 1971; Schultz 1996). In mature muscle, satellite cells are mitotically quiescent (Schultz et al. 1978), but can be rapidly activated to provide myonuclei for nascent or pre-existing myofibers (Grounds and McGeachie 1987; Rantanen et al. 1995). Temporal studies of satellite cell proliferation suggest that those lost to differentiation are replaced, possibly by asymmetric division, from a distinct subpopulation defined in growing muscle by an extended cycle time (Schultz 1996). The presence of populations of satellite cells with differing rates of division and proliferative capacities has been confirmed by in vitro clonal analyses (Schultz and Lipton 1982; Molnar et al. 1996). Adult skeletal muscle also appears to contain rare multipotent progenitors that can give rise to myonuclei and all hematopoietic lineages, but the identity and location of these cells have yet to be defined (Gussoni et al. 1999; Jackson et al. 1999). Although any direct lineage relationships between these putative subpopulations have yet to be established, the above observations suggest that the regenerative compartment of adult skeletal muscle may also conform to the hierarchical archetype of other self-renewing adult tissues.
Satellite cell subpopulations have thus far been defined only by behavioral criteria and there are no reports of differential expression of quiescent satellite cell protein markers such as M-cadherin (Irintchev et al. 1994), c-met (Cornelison and Wold 1997), and myocyte nuclear factor (Garry et al. 1997). Here we describe two novel markers, CD34 and Myf5, that are expressed on most but, significantly, not all quiescent satellite cells.
The first of these markers, CD34, is an accepted, clinically exploited marker of adult hematopoietic stem cells (HSCs) and early blood-cell progenitors and has become the standard criterion for the isolation of such cells from both blood and bone marrow (Krause et al. 1996). Despite a pre-eminent status in transplantation biology, CD34 remains remarkably enigmatic. Structurally, CD34 is a highly O-glycosylated, transmembrane sialomucin, expressed by HSC and progenitors (Krause et al. 1996) and by small-vessel endothelium (Baumhueter et al. 1994). Two isoforms of CD34 are translated from alternatively spliced transcripts: the full-length protein (CD34full) has an intracellular domain with three potential phosphorylation sites that are lacking in the short cytoplasmic tail of truncated CD34 (CD34trunc) (Suda et al. 1992; Nakamura et al. 1993). The significance of the two isoforms is unclear since extracellular engagement of either promotes homotypic cytoadhesion via the same tyrosine kinase–mediated pathway (Tada et al. 1999). The function of CD34 is also obscure, although its expression on HSC has been implicated in the regulation of differentiation (Fackler et al. 1995; Cheng et al. 1996) and adhesive interactions with bone marrow stroma (Healy et al. 1995). On endothelium, CD34 may be involved in L-selectin–mediated leukocyte recruitment (Puri et al. 1995). Here we show that CD34trunc is expressed on quiescent satellite cells and that activation is accompanied by a transient switch to the expression of CD34full through alternative splicing, before complete transcriptional shutdown.
The second marker, Myf5, is one of a family of muscle-specific basic helix-loop-helix transcription factors. Myf5 is the earliest marker of myogenic commitment and, together with MyoD, is integral to the determination of skeletal muscle (reviewed in Tajbakhsh and Buckingham 2000). Using the Myf5 nlacZ/+ mouse, which has a reporter gene encoding nuclear-localizing β-galactosidase (β-Gal) (nlacZ), targeted to the Myf5 locus (Tajbakhsh et al. 1996a), we show that the Myf5 locus is active in all CD34+ve quiescent satellite cells and that all Myf5+ve precursors express CD34.
We then determined the total number of satellite cells associated with individual isolated muscle fibers using the 3F-nlacZ-2E transgenic mouse carrying regulatory elements from the locus of the fast myosin light chain 1F/3F gene that drives a nlacZ reporter gene (Kelly et al. 1995). This transgene is expressed by all myonuclei in fast myofibers, but not by the associated satellite cells. We have found that the total number of satellite cells identified by a lack of 3F-nlacZ-2E transgene expression is significantly higher than the number of CD34+ve or Myf5+ve satellite cells. We therefore conclude that the satellite cell compartment consists of two populations: a majority expressing both CD34 and Myf5 and an as yet undefined minority that is negative for both markers.
The expression of CD34 on quiescent adult skeletal muscle satellite cells extends the role of CD34 in progenitor cell biology. Recently, the status of CD34 as a marker of stem cells has been called into question by the identification of CD34−/low HSCs (reviewed in Goodell 1999) and the association of CD34 expression with activation and progress towards either self-renewal or differentiation (Sato et al. 1999). Coexpression with Myf5 and M-cadherin, both of which are restricted to the myogenic lineage, suggests that in adult skeletal muscle, CD34 does not mark stem cells but is expressed by precursors that are committed to a specific fate and have become arrested and held in reserve for subsequent activation. It seems likely therefore that CD34 plays a fundamental role in the regulation of lineage-primed progenitor compartments in a range of adult tissues, including blood and skeletal muscle.
Materials and Methods
Primers and Probes
CD34 primers were designed using the sequence of Brown et al. 1991. Amplification of both transcripts was carried out using a forward primer in exon 4 (5′-CCAGGGTATCTGCCTGGAAC-3′) and a reverse primer in exon 5 (5′- GCTGGAGTTTGCTGGGAGT-3′). Primers used to distinguish transcripts for CD34full and CD34trunc were 5′-AGCACAGAACTTCCCAGCAA-3′ in exons 5/6 and 5′CCTCCACCATTCTCCG- TGTA-3′ in exon 8. For amplification of single fiber cDNA, the above exon 4 primer was used in conjunction with a second exon 8 primer (5′-tcacagttctgtgtcagccac-3′) for first round amplification, before a second round of nested PCR using the primers designed to distinguish the two splice variants. External forward and reverse primers for MyoD cDNA were 5′-CGCTCCAACTGCTCTGATGG-3′ and 5′-AAGAACCAGGGGCACCATCC-3′, respectively, and the internal primers for nested amplification were 5′-CGGCGGCAGAATGGCTACGA-3′ and 5′-GAGGGGCGGCGTCGGGAGAC-3′: the nested primers lie in different exons separated by a 327-bp intron (Zingg et al. 1991). S16 primers were as described by Foley et al. 1993.
A CD34 cDNA fragment corresponding to 442 bp of exons 4–7 was generated by PCR amplification of cDNA reverse transcribed from total RNA extracted from embryonic day (E) 17.5 mouse embryos, using 5′-GAGAATTCTGGAATCCGAGAAGTGAGGT-3′ and 5′-ACTCTA GAACCCAGCCTTTCTCCTGTAG-3′ as forward and reverse primers, respectively. An M-cadherin cDNA fragment corresponding to 502 bp of exons 2–5 (Link et al. 1998) was produced using 5′-GAGAATTCCAAACGCCTCCCCTACCC-3′ and 5′-ACTCTAGACACAGCCACCACCTCACG-3′ as forward and reverse primers. Forward and reverse primers carried a 5′ EcoR1 site and a 5′ Xba1, respectively (underlined), for cloning into pBluescript II KS(−) (Stratagene).
Reverse Transcription-PCR and Northern Blotting
Total RNA was prepared according to the method of Chomczynski and Sacchi 1981. For reverse transcription (RT)-PCR analysis of cell lines, 2 μg of RNA were reverse transcribed in a 20-μl reaction, using Superscript RNase H− reverse transcriptase (Life Technologies) primed with oligo-dT12-18. 2-μl aliquots of cDNA were used as template for 24 cycles of PCR using Red Hot DNA polymerase (Advanced Biotechnologies) in the presence of 1 μCi of [α-32P]dCTP (3,000 Ci/mmol; Amersham Pharmacia Biotech). PCR products were separated on polyacrylamide gels and analyzed using a PhosphorImager 445 SI equipped with ImageQuant software (Molecular Dynamics). For RT-PCR analysis of isolated fibers, individual muscle fibers were lysed in 10 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 1 mM MgCl2, 0.5% NP-40, and the entire lysate was reverse transcribed as above. 2-μl aliquots of cDNA were used as template for 35 cycles of PCR amplification, and 2 μl of product were then amplified through a further 35 cycles, using fully nested primers. For Northern blotting, total RNA was denatured at 50°C in 6.5% glyoxal, fractionated on a 1.5% agarose gel in 10 mM sodium phosphate, pH 7.0, and then transferred onto Hybond-N+ membrane (Amersham Pharmacia Biotech) and hybridized according to manufacturer's instructions.
Myf5nlacZ/+ Mice
The Myf5 nlacZ/+ mouse has nlacZ-SV40poly(A) RNApolII/Neo targeted to the first exon of the Myf5 gene such that β-Gal is produced as a fusion protein with the first 13 amino acids of Myf5. The Myf5 gene is also disrupted and a small deletion is introduced. Homozygous Myf5 nlacZ/+ animals die shortly after birth due to respiratory problems caused by abnormal rib development, whereas the heterozygous Myf5 nlacZ/+ mice used in the studies described here are viable (Tajbakhsh et al. 1996a).
3F-nlacZ-2E-transgenic Mice
The 3F-nlacZ-2E transgenic mouse contains seven copies of a construct consisting of 2 kb upstream of the myosin light chain (MLC)–3F transcriptional start site, nlacZ-SV40 poly(A) in frame in the second MLC3F-specific exon, 1 kb of MLC3F sequence 3′ of nlacZ, and a 260-bp 3′ MLC1F/3F enhancer (Kelly et al. 1995).
In Situ Hybridization
Hybridizations were carried out using (CBA × C57Bl/10) F1 embryos. Noon of the day of the vaginal plug was designated E 0.5. Digoxygenin-UTP–labeled riboprobes were generated and hybridized to headless, eviscerated embryos as described (Zammit et al. 2000). The CD34 riboprobe was derived from the 442-bp cDNA fragment of exons 4–7; the M-cadherin riboprobe was synthesized from the 502-bp cDNA fragment of exons 2–5.
Whole Muscle Preparation
Mice were killed by cervical dislocation, muscles were removed complete with tendons, rinsed in PBS, and then fixed for 5 min in freshly prepared 4% paraformaldehyde in PBS. For cryostat sectioning, unfixed muscles were mounted in OCT (Raymond Lamb) compound and frozen in liquid nitrogen. Muscles were fixed or frozen within 10 min of the animal being killed.
Cell Culture and Single Fiber Preparation
Primary muscle cells were obtained by enzymatic disaggregation of leg muscle from 1-d-old C57Bl/10 mice and cultured as described previously (Beauchamp et al. 1999). The ICR/IAn myogenic line was cloned from a primary culture prepared by enzymatic disaggregation of a crush-injured tibialis anterior (TA) muscle of a 6-wk old, ICR/IAn phosphorylase kinase-deficient mouse and was grown as a primary culture. Derivation and maintenance of I28, C2C12, and H-2Kb-tsa58 cell lines have been described previously (Blau et al. 1983; Morgan et al. 1994; Irintchev et al. 1997). To induce myogenic differentiation, cultures were allowed to reach 70% confluence before transfer into differentiation medium consisting of DME, 4 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, supplemented with 10% (primary, ICR/IAn cells and H-2Kb–tsa58 clones) or 2% (C2C12 and I28 cells) horse serum. All cultures were maintained on plastic pre-coated with 0.01% gelatin. sEND.1 endothelial cells, a polyoma virus-transformed line derived from a subcutaneous hemangioma induced in a 3-wk-old ICR mouse (Williams et al. 1988), were cultured in DME containing 4 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin, supplemented with 10% fetal calf serum.
Single muscle fibers were isolated from collagenase-digested extensor digitorum longus (EDL) muscles of ∼6-wk-old mice, as described by Rosenblatt et al. 1995, except that plastic and glassware were coated with 5% BSA in PBS rather than horse serum to minimize exposure to mitogens that could potentially activate satellite cells. Fibers were put into culture, fixed, or lysed within 2 h of the mouse being killed.
Histochemical Detection of β-Galactosidase Activity
nlacZ reporter gene-derived β-Gal activity was detected using the chromogenic substrate 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-Gal). X-Gal was used at a final concentration of 400 μg/ml in PBS containing 4 mM potassium ferrocyanide, 4 mM potassium ferricyanide, 2 mM MgCl2, and 0.02% NP-40. Whole muscles or fibers isolated from 3F-nlacZ-2E and Myf5/nlacZ mice were incubated in X-Gal solution for 2 h and overnight, respectively, at 37°C, and then rinsed in PBS. Isolated fibers were mounted in Dako Faramount aqueous mounting medium containing the fluorescent nuclear counterstain 4′,6-diamidino-2-phenylindole (DAPI) at 100 ng/ml.
Immunofluorescent Staining
Cultures were incubated for 1 h at 37°C with biotinylated, rat anti–mouse CD34 monoclonal antibody (clone RAM34; PharMingen) before fixation in 4% paraformaldehyde in PBS for 5 min at 37°C. Cells were permeabilized with 0.1% Triton X-100 in PBS at room temperature, and then incubated in 20% normal goat serum for at least 30 min to block nonspecific antibody binding. Mouse anti–human MyoD1 (clone 5.8a; Dako) monoclonal antibody and rabbit anti–mouse Myf5 polyclonal antibody (Yablonka-Reuveni et al. 1999) were applied for 90 min at room temperature in 5% horse serum in PBS. Anti–CD34 antibody was detected using biotin-conjugated goat anti–rat IgG polyclonal antibody (Sigma-Aldrich) followed by streptavidin conjugated to Alexa Fluor 594. Mouse monoclonal and rabbit polyclonal primary antibodies were detected using Alexa Fluor 488–conjugated goat anti–mouse or rabbit IgG polyclonal antibodies (fluorescent-labeled antibodies were from Molecular Probes).
Isolated muscle fibers were stained with the anti–CD34 and –MyoD1 antibodies, rabbit anti–mouse M-cadherin polyclonal antibody (Irintchev et al. 1994), and mouse anti–Escherichia coli β-Gal monoclonal antibody (clone GAL-13; Sigma-Aldrich). Fibers were fixed and treated as above except that permeabilization was carried out using 0.5% Triton X-100 and all primary antibodies were applied in 0.35% type IV lambda carrageenan (Sigma-Aldrich) in PBS for 16–40 h at 4°C. Primary antibodies were detected with appropriate species-specific Alexa Fluor–conjugated secondary antibodies, diluted in 0.35% carrageenan and applied for 2 h at room temperature. The same biotin-conjugated intermediate was used in conjunction with the anti–CD34 antibody.
8-μm-thick cryosections were fixed as described for cultured cells and incubated in PBS containing 20% normal goat serum and 0.5% blocking reagent (Roche Diagnostics Ltd.). Primary antibodies were applied for 16 h at 4°C, secondary antibodies for 2 h at room temperature. All antibodies were diluted in 6% horse serum in PBS.
All preparations were mounted in Dako Faramount aqueous mounting medium containing 100 ng/ml DAPI and examined using an Axiophot microscope (Carl Zeiss, Inc.).
Results
Transcripts for both CD34full and CD34trunc Are Expressed by Skeletal Muscle Precursor Cells In Vitro
To investigate the expression of CD34 in skeletal muscle precursor cells, total RNA from primary myogenic cultures and established myogenic cell lines was screened by RT-PCR. CD34 mRNA was present in all of the myogenic cultures examined, both in undifferentiated myoblasts and after differentiation into myotubes, although levels of expression varied considerably (Fig. 1 A). The highest levels were found in primary myogenic cultures prepared by enzymatic disaggregation of limb muscles dissected from newborn mice. Although primary muscle precursor cells are closest to their in vivo counterparts, there is an undefined contribution from contaminating fibroblasts and endothelial cells, both of which express CD34 (Brown et al. 1991; Baumhueter et al. 1994). However, CD34 transcript was also present in I28 cells (an expanded primary culture of myogenic cells purified by a selective replating; Irintchev et al. 1997), a spontaneous, cloned myogenic cell line from the phosphorylase kinase–deficient mouse ICR/IAn, and in the immortal myogenic line C2C12 (Yaffe and Saxel 1977; Blau et al. 1983), all derived from adult skeletal muscle satellite cells. The levels of transcript in these lines were 10- to 20-fold less than in the endothelial cell line, sEND.1 (Williams et al. 1988) (data not shown) and were slightly, but reproducibly, lower in differentiated cultures compared with proliferating myoblasts. The lowest levels of CD34 mRNA (∼100-fold less than in sEND.1 cells) were present in two conditionally immortal cell lines and the relative expression was unchanged after differentiation. These lines were derived from an adult H-2Kb-tsa58 heterozygote mouse, and are maintained under permissive conditions as proliferating cells by the activity of the thermolabile tsA58 mutant SV40 large T antigen, rather than by endogenous mechanisms that normally operate to maintain the undifferentiated state (Morgan et al. 1994). Northern analysis of C2C12 cultures confirmed the presence of CD34 transcript (Fig. 1 B). Using a CD34 cDNA probe extending from exon 4 into exon 7, a single band of ∼2.7 kb was obtained using target RNA from myoblasts, fusing cultures, and differentiated myotubes. The band was the same size as that obtained with total RNA from endothelial cells, brain, and spleen, but was present at considerably lower levels compared with these control samples.
Figure 1.
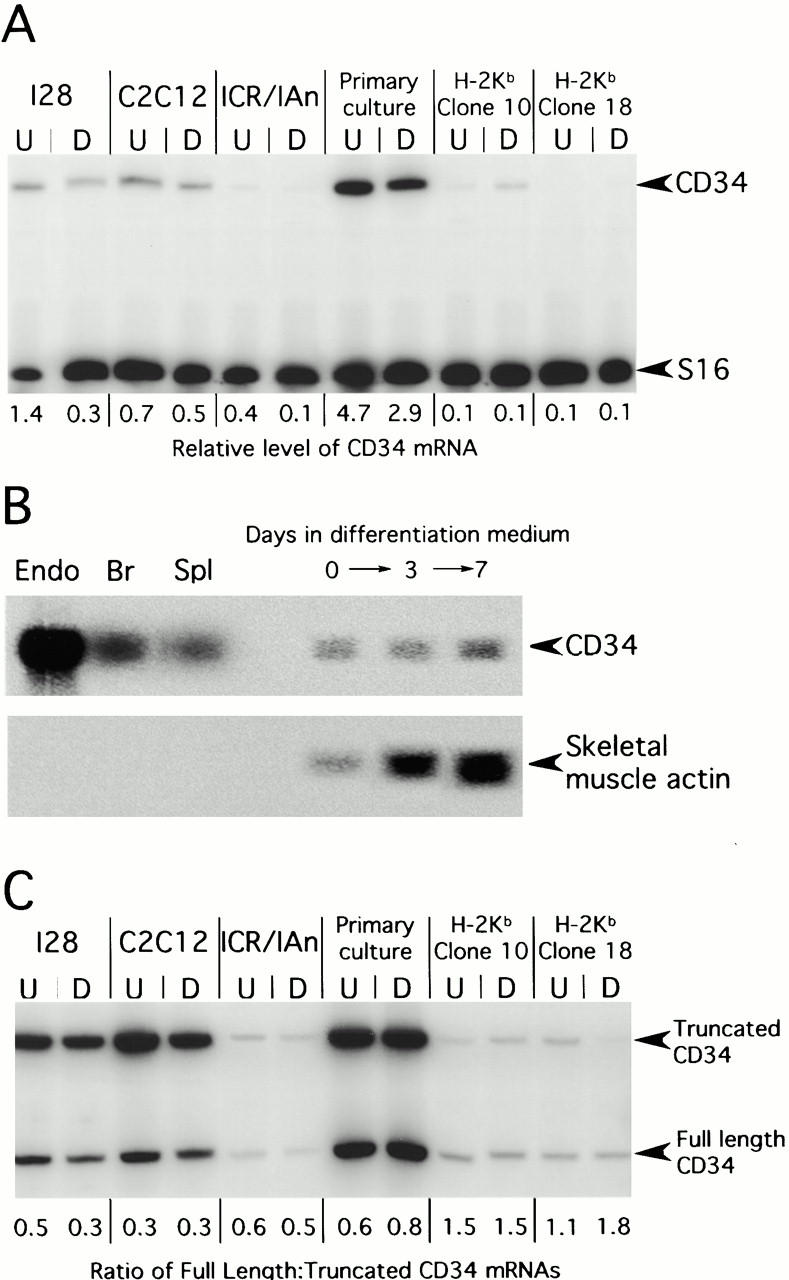
CD34 expression in primary muscle cultures and clonal myogenic cell lines. (A) RT-PCR analysis of CD34 expression in skeletal muscle cultures. Levels of CD34 mRNA in undifferentiated (U) and differentiated (D) cultures were calculated relative to transcript for S16 ribosomal protein. (B) Northern blot analysis of CD34 mRNA in differentiating C2C12 cultures. 30 μg of total RNA isolated from myoblasts (day 0) and from cultures induced to differentiate for 3 or 7 d were analyzed. Progressive differentiation was confirmed by reprobing for skeletal-muscle actin expression. Only 5 μg of total RNA from sEND.1 cells (Endo), brain (Br) and spleen (Spl) were run. (C) RT-PCR analysis of the relative levels of expression of the two CD34 isoform transcripts in undifferentiated (U) and differentiated (D) skeletal muscle cultures.
RT-PCR was also used to determine whether both isoforms of CD34 are expressed in skeletal muscle cultures. CD34trunc is translated from a 2.7-kb mRNA consisting of nine exons, whereas CD34full is derived from a shorter transcript from which a single exon (exon X situated between exons 7 and 8) has been spliced out. The paradoxical relationship between RNA and protein size is due to the presence of a stop codon in exon X, which terminates translation of CD34trunc (Suda et al. 1992; Nakamura et al. 1993). Forward and reverse primers in exon 5/6 and exon 8, respectively, were used to generate splice variant-specific PCR products of 416 bp for CD34trunc (including exon X) and 250 bp for CD34full (lacking exon X). Transcripts for both isoforms were present in all of the myogenic cultures examined (Fig. 1 C). In primary cultures and the I28, C2C12, and ICR/IAn cell lines, CD34trunc mRNA was two- to threefold more abundant than that for CD34full, whereas the transcript for CD34full was the more abundant in both lines derived from the H-2Kb-tsa58 mouse. No significant changes in relative expression were observed after differentiation (Fig. 1 C).
Expression of CD34 Is Associated with Skeletal Muscle Precursors that Do Not Differentiate In Vitro
Expression of CD34 protein in skeletal muscle cultures was investigated by immunofluorescent staining using an antibody raised against an extracellular epitope that recognizes both the full-length and truncated isoforms of CD34. In sEND.1 cultures, virtually all cells showed intense cell-surface expression (Fig. 2 A). In contrast, C2C12 myoblasts maintained in proliferation medium showed highly variable levels of expression, with most cells either negative or very faintly positive and only a small percentage (estimated as <5%) of strongly CD34+ve cells (Fig. 2 B). Heterogeneous expression of the myogenic regulatory factors Myf5 (Fig. 2 C) and MyoD (D) was also observed in proliferating C2C12 cultures, as reported previously in asynchronous cultures (Tapscott et al. 1988; Lindon et al. 1998). Although there was no apparent correlation between expression of CD34 and Myf5 (i.e., some CD34+ve myoblasts expressed Myf5, whereas others were Myf5−ve; Fig. 2 C), strongly CD34+ve myoblasts were invariably MyoD−ve (D).
Figure 2.
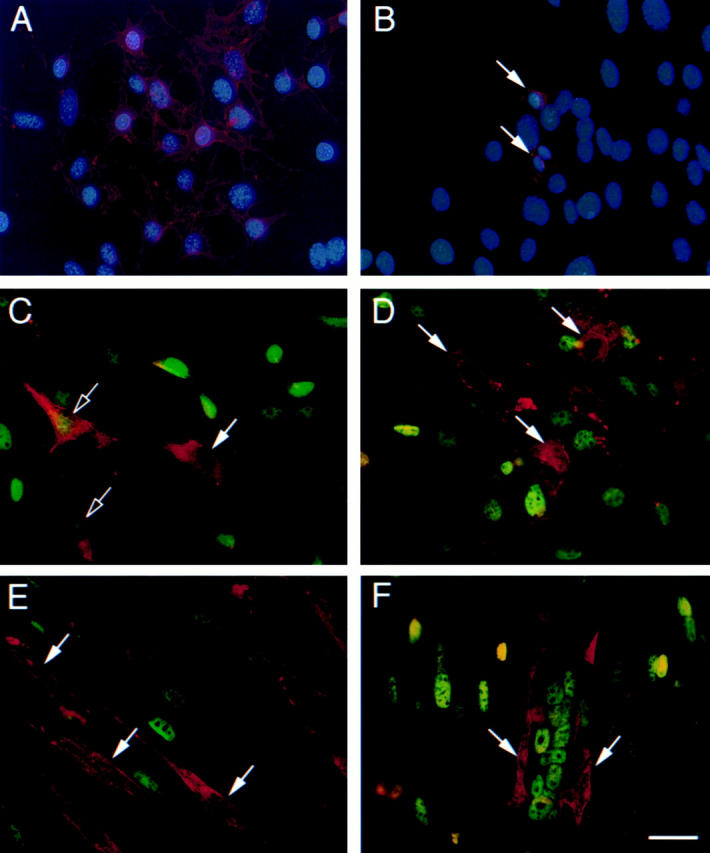
CD34 expression on a subset of muscle cells that are phenotypically segregated after induced differentiation. (A and B) Cultures of sEND.1 endothelial cells (A) and proliferating C2C12 myoblasts (B) immunostained for expression of CD34 (red) and counterstained with DAPI (blue). (Arrows) CD34+ve myoblasts. (C and D) Double immunostaining of proliferating C2C12 myoblasts for expression of CD34 (red) and either Myf5 (C, green) or MyoD (D, green). Strongly CD34-expressing cells were either negative (arrow) or faintly positive (open arrow) for Myf5 (C), but negative for MyoD (D, arrow). (E and F) Differentiated C2C12 cultures double immunostained for CD34 (red) and either Myf5 (E, green) or MyoD (F, green). CD34+ve cells (arrow) remained morphologically undifferentiated and did not express Myf5 (E) or MyoD (F). Bar, 30 μm.
In C2C12 cultures that had been allowed to differentiate for 5 d, strong expression of CD34 was maintained on a small number of mononucleated cells, usually closely associated with multinucleated myotubes (Fig. 2E and Fig. F). In contrast to the variable levels of Myf5 expression observed among the CD34+ve population in proliferating cultures, those that persisted after 5 d in differentiation medium were consistently Myf5−ve (Fig. 2 E). Furthermore, the CD34+ve cells did not express MyoD (Fig. 2 F), even though the majority of the culture had withdrawn from the cell cycle and entered terminal differentiation as defined by expression of p21 and myogenin (data not shown). Expression of CD34 therefore defines a subset of precursors that do not differentiate in culture, in contrast to the CD34−ve majority.
CD34 mRNA Is Absent during Primary Myogenesis, Expressed during Secondary Myogenesis, and Maintained after Birth
In the mouse embryo, myogenesis occurs in distinct waves: an initial phase of primary muscle fiber formation is followed ∼2 d later by the generation of secondary muscle fibers (Ontell and Kozeka 1984). Distinct populations of muscle precursor cells are involved in each event: embryonic myoblasts fuse during primary myogenesis, whereas fetal or secondary myoblasts differentiate during secondary myogenesis. A further population of precursors, the satellite cells, persists in the postnatal animal and is involved in growth and regeneration (reviewed in Miller et al. 1999). Whole mount in situ hybridization of mouse embryos and dissected 3-d postnatal tissue was used to determine when CD34 transcript is expressed in skeletal muscle with respect to primary and secondary myogenesis. At E 11.5, staining for CD34 transcript was observed in the outflow tract of the heart and as a diffuse network throughout the embryo presumably reflecting expression in the developing circulatory system (Young et al. 1995) (Fig. 3 A). Although intersomitic blood vessels were clearly positive, no CD34 transcript was detected in the somites or in the limb buds. In marked contrast, transcript for M-cadherin, which marks all myogenic cells at this stage (Rose et al. 1994), was restricted to the myotome of the somites and the proximal region of the limb buds (Fig. 3 B) at E 11.5. The mutually exclusive patterns of expression at E 11.5 shows that CD34 is not present in skeletal muscle during primary myogenesis. However, at E 16.5, hybridization with either CD34 (Fig. 3 C) or M-cadherin (D) riboprobes produced strikingly similar lines of punctate staining, apparently in register with the underlying fibers. Previous developmental studies have shown that M-cadherin protein is uniformly expressed in the myotome at E 11.5, but becomes clustered at points of contact between primary and secondary fibers during secondary myogenesis between E 16 and 18 (Rose et al. 1994). In postnatal muscle, M-cadherin protein is restricted to satellite cells and the adjacent region of the underlying fiber (Irintchev et al. 1994). Since both transcriptional activation and downregulation of M-cadherin occur before changes in protein (Moore and Walsh 1993; Rose et al. 1994), it is likely that the discrete hybridization pattern obtained for M-cadherin (and by inference, that obtained for CD34) at E 16.5 reflects the distribution of satellite cells that continue to express M-cadherin protein after birth. Identical results were obtained with the CD34 probe on muscles from 3-d postnatal mice as on E 16.5 embryonic tissues (Fig. 3 E) and, when gently teased apart, at least some of the staining was clearly localized to putative satellite cells, closely applied to individual muscle fibers (F).
Figure 3.
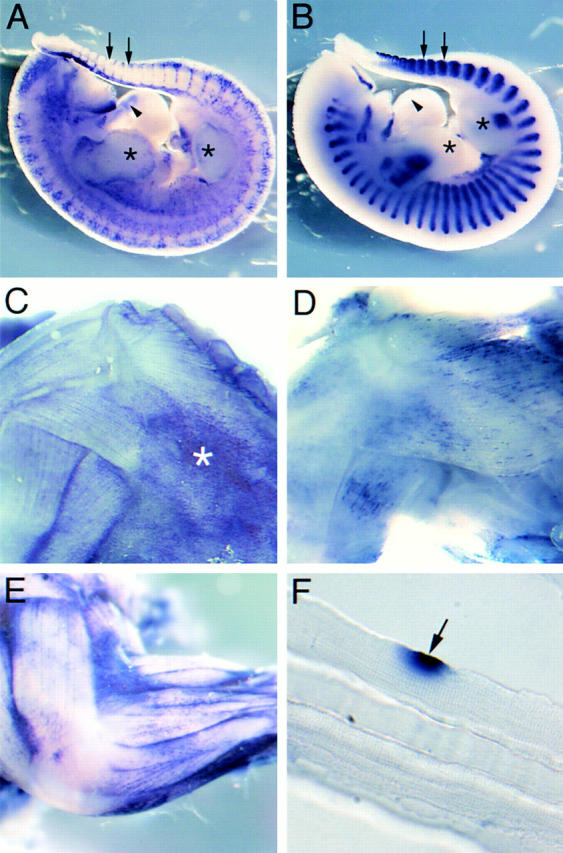
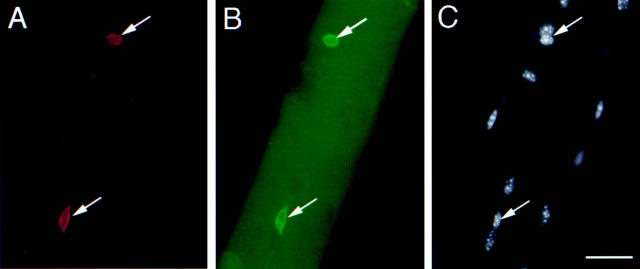
CD34 expression during skeletal muscle development. (A and B) Whole mount in situ hybridization of E 11.5 embryos for CD34 (A) and M-cadherin (B) transcripts. At E 11.5, M-cadherin was expressed at the major sites of primary myogenesis; i.e., the myotome of the somites (two representative somites are arrowed) and the limb buds (*). In contrast, CD34 transcript was present as a diffuse network throughout the embryo and in the outflow tract of the heart (arrowhead), but not in the myotome or limb buds. (C and D) Whole-mount in situ hybridization of the musculature of the shoulder region of E 16.5 embryos for CD34 (C) and M-cadherin (D). Both showed similar patterns of expression, although overlying fascia also expressed CD34 transcript (*). (E) Whole-mount in situ hybridization of the upper limb of a day 3 postnatal mouse showing CD34 expression in muscle, apparently in register with the underlying fibers. (F) A single fiber teased from day 3 postnatal muscle after hybridization with the CD34 riboprobe showing expression in a presumptive satellite cell (arrow).
CD34 Is Expressed on Adult Skeletal Muscle Satellite Cells Associated with Isolated Single Fibers
The expression of CD34 protein on adult skeletal muscle satellite cells was confirmed using isolated single muscle fibers prepared by collagenase digestion and mechanical disruption of EDL muscles of 5–8-wk-old mice (Rosenblatt et al. 1995). This procedure produces viable single fibers together with their associated satellite cells, surrounded by an intact basal lamina but free of blood vessels, nerves, and connective tissue. Immunostaining of freshly isolated fibers that had been fixed within 2 h of the death of the animal revealed the presence of CD34+ve mononucleated cells attached to the sarcolemma of the muscle fibers. The same cells also expressed M-cadherin, a marker of quiescent myogenic precursors in adult skeletal muscle (Irintchev et al. 1994), thereby confirming their identity as satellite cells (Fig. 4, A–C).
Figure 4.
Expression of CD34 and M-cadherin on satellite cells associated with isolated single muscle fibers. (A–C) A single EDL fiber stained for M-cadherin (A, red), CD34 (B, green), and counterstained with DAPI (C). (Arrows) Two satellite cells expressing both M-cadherin and CD34. The myonuclei, visualized by counterstaining with DAPI, did not express M-cadherin or CD34. Bar, 30 μm.
Some of the CD34+ve cells associated with freshly isolated fibers were also found to express low levels of MyoD (Fig. 5, A–C). In preparations that had been maintained for 48 h in conditions that promote satellite cell activation and proliferation, all satellite cells showed strong coexpression of CD34 and MyoD, although the CD34 staining appeared more punctate compared with the uniform surface staining observed on freshly isolated cells (Fig. 5, D–F). CD34 was not detected in preparations maintained for longer than 48 h, when many satellite cells had migrated from the parent fiber and begun to proliferate on the surrounding dish (data not shown).
Figure 5.

Expression of CD34 and MyoD on satellite cells associated with isolated single muscle fibers. (A–C) A freshly isolated EDL single fiber immunostained for CD34 (A, red), MyoD (B, green), and counterstained with DAPI (C). (Arrow) A single CD34+ve, MyoD+ve satellite cell closely associated with an underlying myonucleus. (D–F) A single EDL fiber immunostained for CD34 (D, red), MyoD (E, green), and counterstained with DAPI (F) after 48 h in culture. Two CD34+ve, MyoD+ve satellite cells (arrows) are shown migrating off the parent fiber. The CD34 staining was punctate, in contrast to the continuous surface staining observed on quiescent cells. Bar, 30 μm. (G) RT-nested PCR analysis of CD34 isoform expression in isolated single fibers during activation in vitro. Each pair of gels shows the PCR products obtained from 12 individual fibers using primers for CD34 (left) and MyoD (right). Groups of fibers were taken immediately after isolation (0 h) and after 3 or 6 h of culture in the presence of horse serum. PCR products derived from the CD34trunc and CD34full alternately spliced transcripts are arrowed T (416 bp) and F (250 bp), respectively.
The expression of CD34 transcripts in adult satellite cells was investigated by fully nested PCR of cDNA prepared from individual muscle fibers. At the earliest time point after isolation, transcript for CD34trunc, but not CD34full, was detected in all preparations (Fig. 5 G). 3-h later, both isoforms were present, with different preparations containing transcript for either CD34f ull or CD34trunc, or both. After a further 3 h, all preparations contained transcript for CD34full, although a proportion continued to express low levels of transcript for the CD34trunc and by 24 h, no CD34 mRNA was detected in the isolated fiber preparations (data not shown). The same cDNA samples were also screened for the presence of MyoD transcript. Although the protein was clearly present in a small percentage of satellite cells associated with freshly isolated fibers albeit at low levels (Fig. 5 B), few samples were positive for MyoD mRNA either at the earliest time point or after 3 h in culture (Fig. 5 G). This suggests that either MyoD protein is highly stable in quiescent satellite cells or that the amount of transcript was below the level of detection. However, after 6 h, MyoD transcript was detected in almost all of the samples analyzed, attributable to satellite cell activation resulting in either the initiation or upregulation of MyoD transcription. Although the precise timings of the switch in CD34 transcripts and their subsequent disappearance varied between experiments, the transition from CD34trunc to CD34full always accompanied the initiation of satellite cell activation before transcriptional upregulation of MyoD.
Myf5 Is Expressed on Adult Skeletal Muscle Satellite Cells Associated with Isolated Single Fibers
Myf5 nlacZ/+ heterozygous mice were used to determine whether the CD34+ve satellite cells associated with freshly isolated fibers also express Myf5. The Myf5 nlacZ/+ mouse has nlacZ targeted to the Myf5 locus such that activation of Myf5, and therefore expression of endogenous Myf5 from the untargeted allele, is reported by β-Gal activity (Tajbakhsh et al. 1996a). When freshly isolated fibers from Myf5 nlacZ/+ mice were incubated in X-Gal solution, rare β-Gal+ve nuclei were observed, sometimes closely apposed to β-Gal−ve nuclei (Fig. 6A and Fig. B). Since β-Gal can translocate from nuclei carrying nlacZ to other nontransgenic nuclei within a muscle fiber (Yang et al. 1997), the presence of adjacent β-Gal−ve nuclei suggests that each β-Gal+ve nucleus was contained within its own cytoplasm, isolated from the underlying syncytial fiber. This was confirmed by immunostaining of fibers isolated from heterozygous Myf5 nlacZ/+ mice, which demonstrated that the β-Gal+ve nuclei were in satellite cells, as defined by surface expression of M-cadherin (Fig. 6C and Fig. D) and CD34 (E and F). Furthermore, analysis of fibers prepared from mice of more than 6 mo of age, demonstrated that β-Gal is produced by satellite cells in mature muscle and is not a relic from earlier developmental stages (data not shown). Previous attempts to detect Myf5 protein in isolated fiber preparations were unsuccessful due to the high levels of nonspecific binding encountered with the available antibodies (Yablonka-Reuveni et al. 1999). However, fully nested RT-PCR analysis revealed the presence of transcript for Myf5 in all single-fiber preparations from C57Bl/10 mice analyzed immediately after isolation, or after 24 h in culture (data not shown), confirming the fidelity of the Myf5 nlacZ/+ as a reporter of endogenous Myf5 transcription. These observations show that Myf5 is expressed by quiescent satellite cells associated with fibers obtained from normal adult skeletal muscle.
Figure 6.
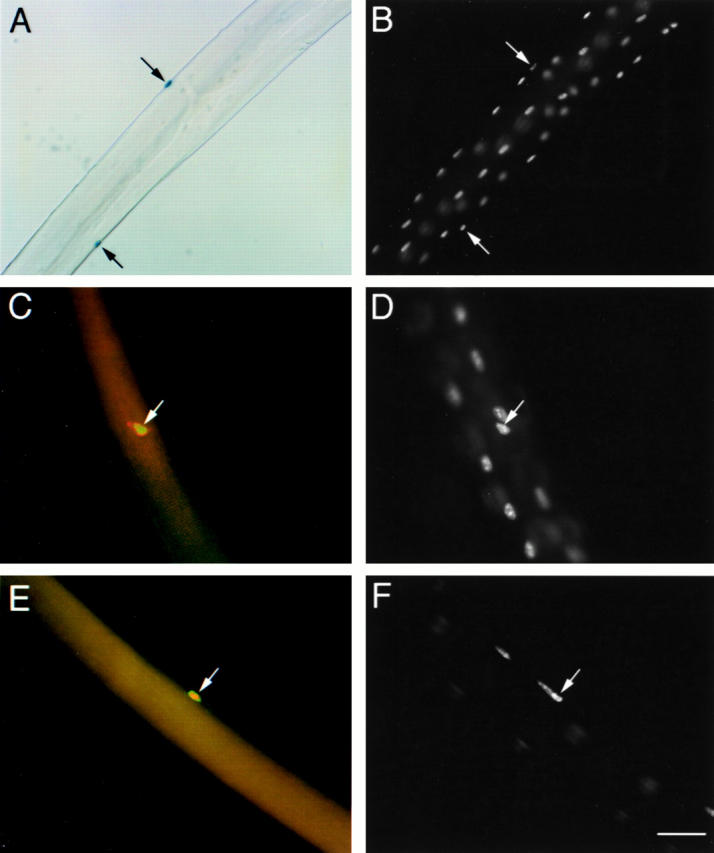
Expression of Myf5 by CD34+ve, M-cadherin+ve satellite cells associated with isolated single fibers. (A and B) A freshly isolated single fiber from a Myf5 nlacZ/+ mouse incubated in X-Gal solution (A) and counterstained with DAPI (B). (C and D) A Myf5 nlacZ/+ mouse single fiber stained for M-cadherin (C, red) and β-Gal (C, green), counterstained with DAPI (D). (E and F) A Myf5 nlacZ/+ mouse single fiber stained for CD34 (E, green) and β-Gal (E, red), counterstained with DAPI (F). The associated satellite cells (arrows) contained β-Gal activity, in contrast to the β-Gal−ve myofiber nuclei, and expressed M-cadherin and CD34. All fibers were isolated from the EDL muscle of a 6-wk-old animal. Bar: 60 μm (A and B) and 30 μm (C–F).
Quiescent Satellite Cells Express both CD34 and Myf5 In Vivo
Whole muscle preparations and cryosections were analyzed to confirm that CD34 and Myf5 are present on quiescent satellite cells in vivo and that expression on satellite cells associated with single muscle fibers was not due to activation during isolation. When intact muscles from 6-wk-old Myf5 nlacZ/+ mice were incubated in X-Gal solution immediately after dissection, either with or without prior fixation, all showed a punctate pattern of β-Gal reaction product associated with myofibers (Fig. 7 A). That this pattern reflecting the distribution of satellite cells was confirmed when cryosections of TA muscle were immunostained or incubated in X-Gal (data not shown), both of which showed that the β-Gal activity was restricted to a few nuclei associated with the edges of muscle fibers (Fig. 7 B). Furthermore, when the same sections were immunostained with anti–CD34 antibody, the β-Gal+ve satellite cells were also clearly CD34+ve (Fig. 7, C–G).
Figure 7.
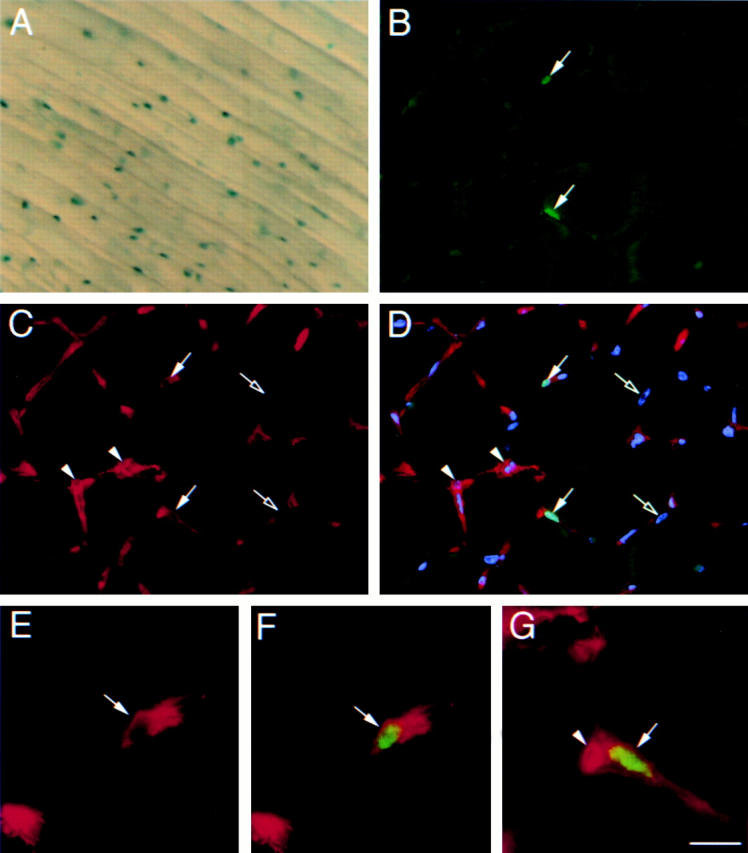
Expression of Myf5 and CD34 in satellite cells in vivo. (A) An intact EDL muscle from a 6-wk-old Myf5 nlacZ/+ mouse after incubation in X-Gal solution, showing a punctate pattern of β-Gal activity. (B–D) A transverse cryosection of the TA muscle of a 6-wk-old Myf5 nlacZ/+ mouse immunostained for β-Gal (B, D, F, and G, green) and CD34 (C–G, red) and counterstained with DAPI (D, blue). β-Gal+ve satellite cells (arrows) also expressed CD34, whereas the myonuclei (D, open arrows) were β-Gal−ve and CD34−ve. Extensive interstitial CD34 expression was observed, particularly on capillaries (C, D, and G, arrowheads). Bar: 30 μm (A–D) and 9.5 μm (E–G).
CD34 and Myf5 Are Expressed by Most, but Not All, Quiescent Satellite Cells Associated with Isolated Single Fibers
To investigate whether all satellite cells express CD34 and Myf5, the numbers of cells defined by these markers were compared with the total number of satellite cells associated with isolated fibers. Total satellite cell numbers were determined using fast fibers isolated from the EDL muscles of 3F-nlacZ-2E transgenic mouse, the myonuclei of which express nlacZ (Kelly et al. 1995). After incubation in X-Gal solution, the myonuclei were identified by the accumulation of β-Gal reaction product (Fig. 8 A). Any myonuclei that may have lost or inactivated the transgene would still be expected to contain β-Gal activity due to translocation of transcript or protein produced from nlacZ-expressing nuclei (Yang et al. 1997). Therefore, only satellite cell nuclei remained unstained as they do not express the transgene (Kelly et al. 1995) and are physically segregated from the nlacZ-expressing myonuclei. Satellite cells could therefore be identified after mounting in DAPI since the presence of β-Gal reaction precipitate quenched fluorescence from the myonuclei (Fig. 8A and Fig. B). The identity of the β-Gal−ve cells was confirmed in independent experiments in which fibers were incubated with anti–β-Gal antibody to identify the nlacZ-expressing myonuclei, together with anti–M-cadherin or anti–CD34 antibodies. As expected, cells that were M-cadherin+ve (Fig. 8C and Fig. D) or CD34+ve (E and F) did not express β-Gal, confirming that the nuclei identified as DAPI fluorescent after incubation in X-Gal included those of the M-cadherin+ve, CD34+ve satellite-cell population.
Figure 8.
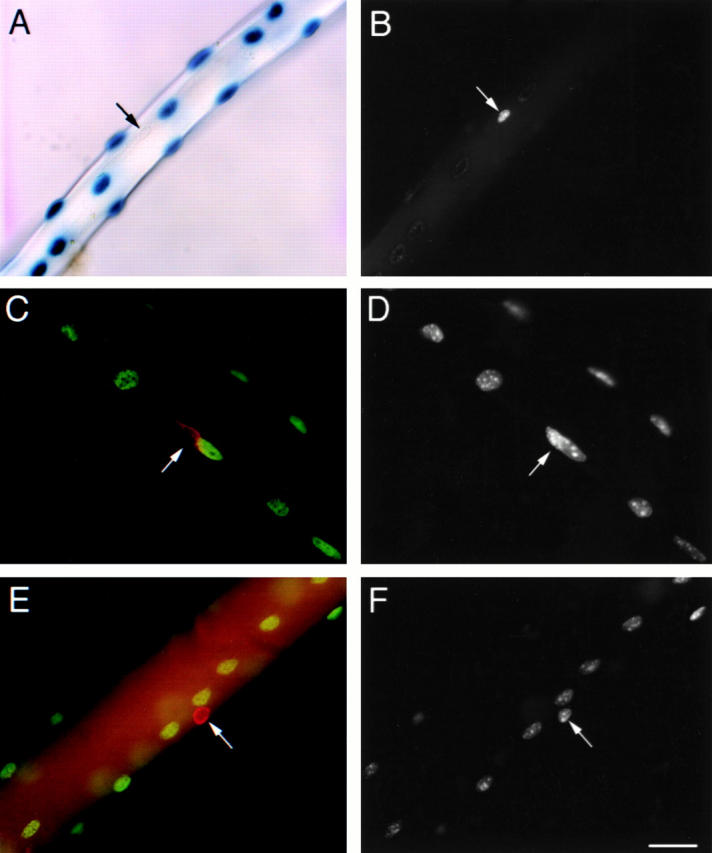
Expression of the 3F-nlacZ-2E transgene in myonuclei, but not associated satellite cells of isolated EDL single fibers. (A and B) A freshly isolated single fiber from a 3F-nlacZ-2E mouse after incubation in X-Gal (A), counterstained with DAPI (B). The 3F-nlacZ-2E transgene is expressed in all myonuclei. DAPI fluorescence is masked in β-Gal+ve nuclei, leaving non–transgene-expressing nuclei readily detectable (A and B, arrow). (C and D) A single 3F-nlacZ-2E muscle fiber stained for M-cadherin (C, red) and β-Gal (C, green), counterstained with DAPI (D). (E and F) A single 3F-nlacZ-2E muscle fiber stained for CD34 (E, red) and β-Gal (E, green), counterstained with DAPI (F). CD34+ve and M-cadherin+ve satellite cells (arrows) did not express the transgene. Bar: 30 μm (A, B, E, and F) and 19 μm (C and D).
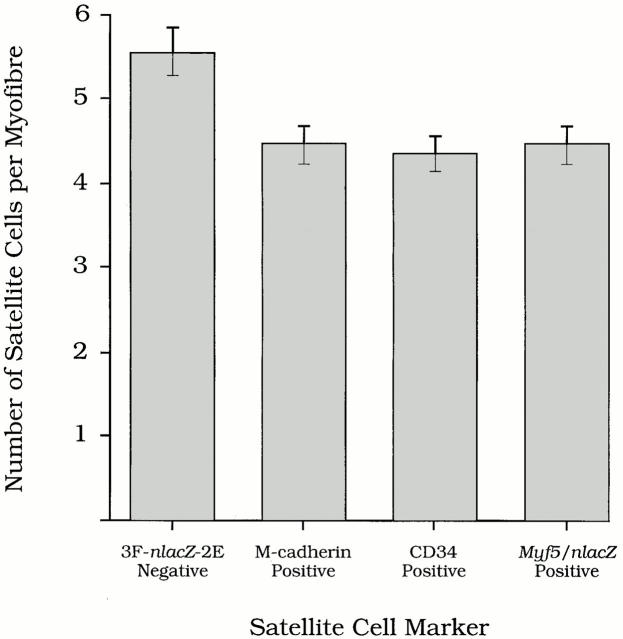
Using the above approach, EDL fibers from 5- to 8-wk-old 3F-nlacZ-2E mice were found to contain an average of 5.6 ± 0.3 satellite cells (±SEM, n = 125 fibers from six mice) in which DAPI fluorescence remained unquenched. However, using fibers isolated from the same six muscles, significantly fewer satellite cells were identified by expression of CD34 [4.5 ± 0.2 (±SEM, n = 114 fibers)] or M-cadherin [4.4 ± 0.2 (±SEM, n = 124 fibers)] (Fig. 9). A value of 4.4 ± 0.3 (±SEM, n = 122 fibers from six animals) was also obtained by counting the number of β-Gal+ve satellite cells associated with EDL fibers prepared from age-matched Myf5 nlacZ/+ heterozygous mice. Therefore, there were no significant differences between satellite cell numbers obtained by counting CD34+ve, M-cadherin+ve, or Myf5+ve cells, but all were significantly lower than the total number of satellite cells associated with the fibers. Furthermore, when fibers were immunostained for any two of the three markers (such as in Fig. 4, A–C, and 6, C–F), all satellite cells were found to express both markers. Together, this strongly suggests that the satellite cell population comprises a majority that is CD34+ve, Myf5+ve, and M-cadherin+ve and an as yet undefined minority that is negative for all three markers.
Figure 9.
Comparison of satellite cell numbers associated with isolated EDL muscle fibers determined by counting non–3F-nlacZ-2E transgene-expressing nuclei, and M-cadherin+ve, CD34+ve, and Myf5+ve cells. Although the mean numbers of M-cadherin (4.5 ± 0.2, n = 114), CD34 (4.4 ± 0.2, n = 124), and Myf5-expressing cells (4.4 ± 0.3, n = 122) per EDL myofiber were not significantly different from each other (P < 0.05), the mean number of non–3F-nlacZ-2E transgene-expressing nuclei (5.56 ± 0.28) was significantly higher (P < 0.05). Values are expressed as population means ± SEM of the pooled data from six age-matched 3F-nlacZ-2E transgenic mice, and from six age-matched Myf5 nlacZ/+mice.
Discussion
The renewal of several terminally differentiated adult tissues is sustained by populations of stem cells that both self-renew and generate a hierarchy of progressively lineage-restricted progenitors culminating in lineage-committed precursors fated to undergo terminal differentiation (Watt and Hogan 2000; Weissman 2000). In tissues with a high rate of turnover, such as blood, skin, and gut, the demands of replacement require a constant supply of precursors for terminal differentiation, such that progression from progenitor to functional, post-mitotic cell appears continuous. In adult skeletal muscle, however, new myonuclei are only required for growth and repair. Accordingly, the satellite cell compartment is normally quiescent and activated only in response to signals elicited by increased work load or damage (Seale and Rudnicki 2000). Here we report two novel markers of the majority of quiescent satellite cells: CD34, a marker of stem cells and early progenitors in the hematopoietic system (Krause et al. 1996), and Myf5, the earliest marker of myogenic commitment (Tajbakhsh and Buckingham 2000). This combination of markers suggests that most satellite cells become quiescent after committing to the skeletal muscle lineage and raises the possibility that CD34 may play a fundamental role in regulating progenitor cell differentiation.
Expression of CD34 transcript in several nonhematopoietic adult tissues has been attributed to the presence of small-vessel endothelium (Krause et al. 1996). Indeed, although CD34 transcript is present in extracts of whole skeletal muscle (Nakamura et al. 1993), CD34 protein has only been reported on capillaries, muscle spindle capsule, and axons (Baumhueter et al. 1994). Our results show that CD34 is also expressed by satellite cells in normal adult skeletal muscle, the identity of which was suggested by their morphology and distribution and confirmed by the coexpression of M-cadherin (Irintchev et al. 1994). Furthermore, using heterozygous Myf5 nlacZ/+ mice, we were also able to show that CD34+ve satellite cells are clearly committed to the myogenic lineage by the criterion of transcriptional activation of Myf5. Developmental studies of Myf5 nlacZ/+ heterozygous mice have confirmed that the distribution of β-Gal faithfully reports endogenous expression of Myf5 transcript from the wild-type allele (Tajbakhsh et al. 1996a). Although Myf5 is active in nuclei within muscle fibers at birth (Tajbakhsh et al. 1996a), our results show that the gene is switched off during early postnatal development such that Myf5 is not expressed by myonuclei at 6 wk of age, but remains active in satellite cells. This is in contrast to a previous study that concluded that Myf5 is not expressed by quiescent satellite cells in sections of undamaged muscle (Cooper et al. 1999). However, we observed clear expression of Myf5 by satellite cells both in whole muscles and in sections of normal muscle that had been fixed or frozen only minutes after dissection. This discrepancy was probably due to differences in the sensitivity of the experimental procedures for β-Gal detection used in our studies compared with those of Cooper et al. 1999. We were also able to detect CD34 protein on satellite cells in sections of normal muscle and on freshly prepared single fibers, even when isolated in the presence of 100 μM cycloheximide (data not shown). These observations confirm that both CD34 and Myf5 are expressed by quiescent satellite cells and that their presence is not the result of de novo synthesis in response to experimentally induced activation.
A small percentage of CD34+ve, Myf5 +ve satellite cells associated with freshly isolated fibers were also found to express MyoD protein, although apparently at low levels. In contrast, after 48 h of culture in the presence of serum, the vast majority of CD34+ve, Myf5 +ve satellite cells were strongly MyoD+ve. This suggests that most adult skeletal muscle satellite cells are quiescent and express both CD34 and Myf5, but little or no MyoD, and become CD34+ve, Myf5+ve, MyoD+ve on activation. Although normal adult mouse muscle contains very few, if any, dividing satellite cells (Schultz et al. 1978; Irintchev et al. 1994), sporadic expression of MyoD has been reported and attributed to satellite cell activation, presumably in response to local stimuli (Grounds et al. 1992; Creuzet et al. 1998). It is therefore likely that expression of MyoD by a proportion of satellite cells associated with freshly isolated fibers defines those that had been activated in vivo before isolation.
Our method of obtaining quiescent satellite cells associated with isolated single muscle fibers affords a unique in vitro system to study synchronous activation. Using fully nested RT-PCR, we detected a rapid increase in MyoD transcription that preceded the increase in MyoD protein expression observed by immunostaining, as described previously during satellite cell activation both in vivo (Cooper et al. 1999) and in vitro (Kitzmann et al. 1998). Although most quiescent satellite cells contained undetectable amounts of MyoD mRNA, they did contain transcripts for Myf5 (data not shown) and CD34. Quiescent satellite cells expressed transcript for the truncated, but not the full-length isoform of, CD34. Intriguingly, within hours of activation, the alternatively spliced transcript for CD34full had become the predominant isoform. After 24 h in culture, neither CD34 transcript was present, although the protein could still be detected up to 48 h, presumably due to its long half life (Krause et al. 1996).
CD34 is also expressed by skeletal muscle precursors in culture. Using the C2C12 cell line, we found that at any given time <5% of proliferating myoblasts expressed CD34 protein and, although the CD34+ve cells showed variable levels of expression of Myf5, they were consistently MyoD−ve. The variable expression of Myf5 and MyoD observed within our proliferating cultures is probably the result of asynchronicity, as both are cell-cycle regulated (Kitzmann et al. 1998). Using synchronous cultures, Kitzmann et al. 1998 showed that cells arrested in G0 are MyoD−ve, Myf5+ve, and that release into G1 is accompanied by a loss of Myf5 expression before upregulation of MyoD; it is therefore possible that expression of CD34 is associated with cells at the G0/G1 boundary. We also observed that, when induced to differentiate, the CD34+ve cells remained morphologically undifferentiated, became uniformly Myf5−ve, and, in contrast to the CD34−ve majority, showed no induction of MyoD or myogenin. These cells probably correspond to the slowly dividing, MyoD−ve, Myf5−ve ‘reserve’ cells described by Yoshida et al. 1998 that on return to appropriate conditions are able to resume proliferation and give rise to both MyoD+ve differentiation-competent cells and more reserve cells. Behaviorally similar cells have been identified in primary cultures of human satellite cells (Baroffio et al. 1996). These ‘reserve’ or stem-like cells were described in cultures polarized by differentiation and so it is unclear whether they are a permanent subpopulation or a transitory phenotype in proliferating myogenic cultures. However, their presence in clonally derived cultures suggests plasticity of myoblast behavior, and that some of those that are CD34+ve, MyoD−ve (and probably at the G0/G1 boundary at the time of induction) become quiescent in response to differentiation cues.
The temporal appearance of CD34 during muscle development also suggests a potential role in establishing and/or maintaining the satellite cell compartment. We found that CD34 is not expressed in the somites during primary myogenesis, demonstrating that the earliest precursors committed to myogenesis are CD34− ve. However, transcript was detected at E 16.5, coincident with the appearance of satellite cells (Cossu et al. 1983). Although satellite cells are generally assumed to be of somitic origin, recent evidence suggests that some may ultimately be derived from primordial endothelial cells that enter skeletal muscle either via the circulation or as perithelial cells associated with developing vessels, before becoming committed to the myogenic lineage through environmental influences (Bianco and Cossu 1999; De Angelis et al. 1999). Intriguingly, this implies a common ancestor with hematopoietic and endothelial cells, lineages with which CD34 is primarily associated. Whether satellite cells are a population sequestered within the somites or derived from elsewhere, their appearance coincides with the onset of CD34 expression in skeletal muscle.
Recent data suggest that in the adult hematopoietic system, expression of CD34 marks activated stem cells either about to self-renew and return to a state of CD34− ve quiescence, or to initiate commitment to differentiation, in which case CD34 expression is maintained (Sato et al. 1999). In skeletal muscle, we have also found that CD34 does not define lineage-negative stem cells; instead, the truncated isoform of CD34 is expressed by quiescent precursors that are committed to myogenesis by the criteria of Myf5 and M-cadherin expression. During development, Myf5 is unable to initiate muscle differentiation in the absence of MyoD, myogenin, and MRF4 (Renee Valdez et al. 2000) such that the expression of Myf5 should not instigate precocious myogenic differentiation. However, expression of Myf5 is required to prevent muscle cells from adopting alternative fates (Tajbakhsh et al. 1996b) and may act to restrict quiescent satellite cells to myogenesis. When satellite cells emerge from quiescence, alternate splicing and subsequent downregulation of CD34 means that MyoD is transiently coexpressed with CD34full, whereas neither CD34 isoform is expressed once the cell has withdrawn from the cell cycle and entered terminal differentiation. The contrast between the quiescent CD34+ve satellite cell and the proliferating CD34+ve hematopoietic progenitor probably reflects the sporadic requirement for muscle repair and growth compared with the constant renewal of blood cells. However, in both cases, CD34 expression may play a role in preventing temporally or spatially premature differentiation.
Although our results show that the majority of quiescent satellite cells are CD34+ve, Myf5+ve, M-cadherin+ve, and MyoD−ve, a proportion do not appear to conform to this phenotype. When CD34, Myf5 and M-cadherin were used independently to count the numbers of satellite cells associated with single fibers isolated from the EDL muscles of adult mice, essentially the same values were obtained. This is consistent with the fact that in double-labeling experiments, all satellite cells expressed both markers. However, the CD34+ve, Myf5+ve, and M-cadherin+ve satellite cells account for only ∼80% of the total number of satellite cells as determined using the 3F-nlacZ-2E transgenic mouse, strongly suggesting that the satellite cell compartment consists of at least two phenotypically distinct populations. In studies of muscle growth (Schultz 1996) and regeneration (Rantanen et al. 1995; Heslop et al. 2000), the ability of precursors to repopulate host muscle after myoblast transplantation (Qu et al. 1998; Beauchamp et al. 1999) and in vitro clonal analyses (Schultz and Lipton 1982; Baroffio et al. 1996; Molnar et al. 1996) all suggest that satellite cells are not a homogenous population. We have shown that CD34 and Myf5 define the majority of satellite cells that are primed for activation and rapid differentiation. The CD34− ve, Myf5−ve, and M-cadherin− ve minority could represent a more stem-cell–like population responsible for replenishing the primed population, although markers for the as yet undefined minority will be required to determine whether or not they correspond to the stem-cell–like precursors previously defined by behavioral criteria.
Although CD34 is primarily associated with HSCs and early progenitors, previous studies have shown that CD34 is also expressed in the dermis, notably by perifollicular spindle cells (Nickoloff 1990) and on oval cells, bile ductular epithelium, and early hepatocytes in the liver (Omori et al. 1997), all of which have been implicated in the regeneration of their respective tissues. Our finding that CD34 is also present on the quiescent precursors of adult skeletal muscle suggests that CD34 is likely to play a fundamental role in adult tissue regeneration. Furthermore, we have been able to exploit skeletal muscle as a model of synchronous precursor activation to show that quiescent satellite cells express CD34trunc and that a switch to the expression of CD34full accompanies the onset of activation. This regulated expression of CD34 through alternate splicing suggests that the two isoforms could have distinct roles in the maintenance and activation of quiescent, lineage-primed progenitors during adult tissue renewal and regeneration.
Acknowledgments
I28 cells and the anti–M-cadherin antibody were generous gifts from Prof. A. Wernig (University of Bonn, Bonn, Germany). The myogenic line ICR/IAn was derived in our laboratory by Dr. J.E. Morgan, and the sEND.1 endothelial cell line was provided by Dr. A. Ager (National Institute for Medical Research, Mill Hill, London, UK).
This work was supported by European Community (EC) Biotechnology grant BIO4 CT 95-0228 and The British Council/EGIDE Alliance 2000 grant PN 00.172. The Muscle Cell Biology Group was supported by The Medical Research Council, EC Biotechnology grants BIO4 CT95-0284 and BMH4 CT97-2767. P.S. Zammit, L. Heslop, and D.S.W. Yu were supported by The Leopold Muller Foundation. Margaret Buckingham's laboratory was supported by grants from the Pasteur Institute, Centre National de la Recherche Scientifique, and Association Française contre les Myopathies.
Footnotes
Abbreviations used in this paper: β-Gal, β-galactosidase; DAPI, 4′,6-diamidino-2-phenylindole; E, embryonic day; EDL, extensor digitorum longus; HSC, hematopoietic stem cell; RT, reverse transcription; TA, tibialis anterior; X-Gal, 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside.
References
- Akashi K., Kondo M., Cheshier S., Shizuru J., Gandy K., Domen J., Medius R., Traver D., Weissman I.L. Lymphoid development from stem cells and the common lymphoid progenitors. Cold Spring Harbor Symp. Quant. Biol. 1999;64:1–12. doi: 10.1101/sqb.1999.64.1. [DOI] [PubMed] [Google Scholar]
- Baroffio A., Hamann M., Bernheim L., Bochaton-Piallat M.-L., Gabbiani G., Bader C.R. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60:47–57. doi: 10.1046/j.1432-0436.1996.6010047.x. [DOI] [PubMed] [Google Scholar]
- Baumhueter S., Dybdal N., Kyle C., Lasky L.A. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for L-selectin. Blood. 1994;84:2554–2565. [PubMed] [Google Scholar]
- Beauchamp J.R., Morgan J.E., Pagel C.N., Partridge T.A. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell–like properties as the myogenic source. J. Cell Biol. 1999;144:113–1121. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P., Cossu G. Uno, nessuno e centomilasearching for the identity of mesodermal progenitors. Exp. Cell Res. 1999;251:257–263. doi: 10.1006/excr.1999.4592. [DOI] [PubMed] [Google Scholar]
- Bischoff R. The satellite cell and muscle regeneration. In: Engel A.G., Franzini-Armstrong C., editors. Myology. Vol. 1. McGraw-Hill, Inc.; New York, NY: 1994. pp. 97–118. [Google Scholar]
- Blau H.M., Chiu C.-P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Booth C., Potten C.S. Gut instinctsthoughts on intestinal stem cells. J. Clin. Invest. 2000;105:1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Greaves M.F., Molgaard H.V. The gene encoding the stem cell antigen, CD34, is conserved in mouse and expressed in haemopoietic progenitor cell lines, brain, and embryonic fibroblasts. Int. Immunol. 1991;3:175–184. doi: 10.1093/intimm/3.2.175. [DOI] [PubMed] [Google Scholar]
- Cheng J., Baumhueter S., Cacalano G., Carver-Moore K., Thibodeaux H., Thomas R., Broxmeyer H.E., Cooper S., Hague N., Moore M., Lasky L.A. Hematopoietic defects in mice lacking the sialomucin CD34. Blood. 1996;87:479–490. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1981;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooper R.N., Tajbakhsh S., Mouly V., Cossu G., Buckingham M., Butler-Browne G.S. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J. Cell Sci. 1999;112:2895–2901. doi: 10.1242/jcs.112.17.2895. [DOI] [PubMed] [Google Scholar]
- Cornelison D.D.W., Wold B.J. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle cells. Dev. Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Cossu G., Molinaro M., Pacifici M. Differential response of satellite cells and embryonic myoblasts to a tumor promoter. Dev. Biol. 1983;98:520–524. doi: 10.1016/0012-1606(83)90382-2. [DOI] [PubMed] [Google Scholar]
- Creuzet S., Lescaudron L., Li Z., Fontaine-Pérus J. MyoD, myogenin, and desmin-nls-lacZ transgene emphasize the distinct patterns of satellite cell activation in growth and regeneration. Exp. Cell Res. 1998;243:241–253. doi: 10.1006/excr.1998.4100. [DOI] [PubMed] [Google Scholar]
- De Angelis L., Berghella L., Coletta M., Lattanzi L., Zanchi M., Cusella de Angelis M.G., Ponzetto C., Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta co-express endothelial and myogenic markers and contribute to post-natal muscle growth and regeneration. J. Cell Biol. 1999;147:869–877. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler M.J., Krause D.S., Smith O.M., Civin C.I., May W.S. Full-length but not truncated CD34 inhibits hematopoietic cell differentiation of M1 cells. Blood. 1995;85:3040–3047. [PubMed] [Google Scholar]
- Foley K.P., Leonard M.W., Engel J.D. Quantitation of RNA using the polymerase chain reaction. Trends Genet. 1993;9:380–385. doi: 10.1016/0168-9525(93)90137-7. [DOI] [PubMed] [Google Scholar]
- Garry D.J., Yang Q., Bassel-Duby R., Williams R.S. Persistent expression of MNF identifies myogenic stem cells in postnatal muscles. Dev. Biol. 1997;188:280–294. doi: 10.1006/dbio.1997.8657. [DOI] [PubMed] [Google Scholar]
- Goodell M. CD34+ or CD34−does it really matter? Blood. 1999;94:2545–2547. [PubMed] [Google Scholar]
- Grounds M.D., Garrett K.L., Lai M.C., Wright W.E., Beilharz M.W. Identification of muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tiss. Res. 1992;267:99–104. doi: 10.1007/BF00318695. [DOI] [PubMed] [Google Scholar]
- Grounds M.D., McGeachie J.K. A model of myogenesis in vivo, derived from detailed autoradiographic studies of regenerating skeletal muscle, challenges the concept of quantal mitosis. Cell Tiss. Res. 1987;250:563–569. doi: 10.1007/BF00218947. [DOI] [PubMed] [Google Scholar]
- Gussoni E., Soneoka Y., Strickland C.D., Buzney E.A., Khan M.K., Flint A.F., Kunkel L.M., Mulligan R.C. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;410:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Healy L., May G., Gale K., Grosveld F., Greaves M., Enver T. The stem cell antigen CD34 functions as a regulator of hematopoietic cell adhesion. Proc. Natl. Acad. Sci. USA. 1995;92:12240–12244. doi: 10.1073/pnas.92.26.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop L., Morgan J.E., Partridge T.A. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J. Cell Sci. 2000;113:2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Zeschnigk M., Starzinski-Powitz A., Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dynam. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Langer M., Zweyer M., Theisen R., Wernig A. Functional improvement of damaged adult mouse muscle by implantation of primary myoblasts. J. Physiol. 1997;500:775–785. doi: 10.1113/jphysiol.1997.sp022057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson K.A., Mi T., Goodell M.A. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc. Natl. Acad. Sci. USA. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Alonso S., Tajbakhsh S., Cossu G., Buckingham M. Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice. J. Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmann M., Carnac G., Vandromme M., Primig M., Lamb N.J.C., Fernandez A. The muscle regulatory factors MyoD and Myf-5 undergo distinct cell cycle–specific expression in muscle cells. J. Cell Biol. 1998;142:1447–1459. doi: 10.1083/jcb.142.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D.S., Fackler M.J., Civin C.I., May W.S. CD34structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- Lindon C., Montarras D., Pinset C. Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J. Cell Biol. 1998;140:111–118. doi: 10.1083/jcb.140.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link D., Winnekendonk D., Kaufmann U., Palinkas J., Heuser C., Kammann M., Starzinski-Powitz A. Intercellular adhesion in developing and adult skeletal muscleanalysis of M-cadherin. Basic Appl. Myol. 1998;8:315–323. [Google Scholar]
- Michalopoulos G.K., DeFrances M.C. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Miller J.B., Schaefer L., Dominov J.A. Seeking muscle stem cells. Curr. Top. Dev. Biol. 1999;43:191–219. doi: 10.1016/s0070-2153(08)60382-8. [DOI] [PubMed] [Google Scholar]
- Molnar G., Ho M.L., Schroedl N.A. Evidence for multiple satellite cell populations and a non-myogenic cell type that is regulated differently in regenerating and growing skeletal muscle. Tissue Cell. 1996;28:547–556. doi: 10.1016/s0040-8166(96)80057-7. [DOI] [PubMed] [Google Scholar]
- Moore R., Walsh F.S. The cell adhesion molecule M-cadherin is specifically expressed in developing and regenerating, but not denervated skeletal muscle. Development (Camb.) 1993;117:1409–1420. doi: 10.1242/dev.117.4.1409. [DOI] [PubMed] [Google Scholar]
- Morgan J.E., Beauchamp J.R., Pagel C.N., Peckham M., Ataliotis P., Jat P.S., Noble M.D., Farmer K., Partridge T.A. Myogenic cell lines derived from transgenic mice carrying a thermolabile T antigena model system for the derivation of tissue-specific and mutation-specific cell lines. Dev. Biol. 1994;162:486–498. doi: 10.1006/dbio.1994.1103. [DOI] [PubMed] [Google Scholar]
- Moss F.P., Leblond C.P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Komano H., Nakauchi H. Two alternative forms of cDNA encoding CD34. Exp. Hematol. 1993;21:236–242. [PubMed] [Google Scholar]
- Nickoloff B.J. The human progenitor cell antigen (CD34) is localized on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in Karposi's sarcoma. Adv. Dermatol. 1990;127:523–529. [PubMed] [Google Scholar]
- Omori N., Omori M., Evarts R.P., Teramoto T., Miller M.J., Hoang T.N., Thorgeirsson S.S. Partial cloning of rat CD34 cDNA and expression during stem cell-dependent liver regeneration in the adult rat. Hepatology. 1997;26:720–727. doi: 10.1002/hep.510260325. [DOI] [PubMed] [Google Scholar]
- Ontell M., Kozeka K. The organogenesis of murine striated musclea cytoarchitectural study. Am. J. Anat. 1984;171:133–148. doi: 10.1002/aja.1001710202. [DOI] [PubMed] [Google Scholar]
- Puri K.D., Finger E.B., Gaudernack G., Springer T.A. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J. Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z., Balir L., van Deutekom J.C.T., Robbins P.D., Pruchnic R., Huard J. Development of approaches to improve cell survival in myoblast transplantation therapy. J. Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen J., Hurme T., Lukka R., Heino J., Kalimo H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscleevidence for two different populations of satellite cells. Lab. Invest. 1995;72:341–347. [PubMed] [Google Scholar]
- Renee Valdez M., Richardson J.A., Klein W.H., Olsen E.N. Failure of Myf5 to support myogenic differentiation without myogenin, MyoD and MRF4. Dev. Biol. 2000;219:287–298. doi: 10.1006/dbio.2000.9621. [DOI] [PubMed] [Google Scholar]
- Rose O., Rohwedel J., Reinhardt S., Bachmann M., Cramer M., Rotter M., Wobus A., Starzinski-Powitz A. Expression of M-cadherin protein in myogenic cells during prenatal mouse development and differentiation of embryonic stem cells in culture. Dev. Dynam. 1994;201:245–259. doi: 10.1002/aja.1002010308. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J.D., Lunt A.I., Parry D.J., Partridge T.A. Culturing satellite cells from single muscle fiber explants. In Vitro Cell. Dev. Biol. 1995;31:773–779. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
- Sato T., Laver J.H., Ogawa M. Reversible expression of CD34 by murine hematopoietic stem cells. Blood. 1999;94:2548–2554. [PubMed] [Google Scholar]
- Schultz E. Satellite cell proliferative compartments in growing skeletal muscles. Dev. Biol. 1996;175:84–94. doi: 10.1006/dbio.1996.0097. [DOI] [PubMed] [Google Scholar]
- Schultz E., Gibson M.E., Champion T. Satellite cells are mitotically quiescent in mature mouse musclean EM and radioautographic study. J. Exp. Zool. 1978;206:451–456. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- Schultz E., Lipton B.H. Skeletal muscle satellite cellschanges in proliferation potential as a function of age. Mech. Ageing Dev. 1982;20:377–383. doi: 10.1016/0047-6374(82)90105-1. [DOI] [PubMed] [Google Scholar]
- Seale P., Rudnicki M.A. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev. Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- Suda J., Sudo T., Ito M., Ohno N., Yamaguchi Y., Suda T. Two types of murine CD34 mRNA generated by alternative splicing. Blood. 1992;79:2288–2295. [PubMed] [Google Scholar]
- Tada J.-I., Omine M., Suda T., Yamaguchi N. A common signaling pathway via Syk and Lyn tyrosine kinases generated from capping of the sialomucins CD34 and CD43 in immature hematopoietic cells. Blood. 1999;93:3723–3735. [PubMed] [Google Scholar]
- Tajbakhsh S., Buckingham M. The birth of muscle progenitor cells in the mousespatiotemporal considerations. Curr. Top. Dev. Biol. 2000;48:225–268. doi: 10.1016/s0070-2153(08)60758-9. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Bober E., Babinet C., Pournin S., Arnold H., Buckingham M. Gene targeting the myf-5 locus with nlacZ reveals expression of this myogenic factor in mature skeletal muscle fibres as well as early embryonic muscle Dev. Dynam. 206 1996. 291 300a [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S., Rocancourt D., Buckingham M. Muscle progenitor cells failing to respond to positional cues adopt non-myogenic fates in myf-5 null mice Nature 384 1996. 266 270b [DOI] [PubMed] [Google Scholar]
- Tapscott S.J., Davis R.L., Thayer M.J., Cheng P.-F., Weintraub H., Lassar A.B. MyoD1a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Watt F.M. Epidermal stem cellsmarkers, patterning and the control of stem cell fate. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1998;353:831–837. doi: 10.1098/rstb.1998.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F.M., Hogan B.L.M. Out of edenstem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Weissman I.L. Stem cellsunits of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Williams R.L., Courtneidge S.A., Wagner E.F. Embryonic lethalities and endothelial tumors in chimeric mice expressing polyoma virus middle T oncogene. Cell. 1988;52:121–131. doi: 10.1016/0092-8674(88)90536-3. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z., Rudnicki M.A., Rivera A.J., Primig M., Anderson J.E., Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev. Biol. 1999;210:440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yang J., Ontell M.P., Kelly R., Watkins S.C., Ontell M. Limitations of nlsβ-galactosidase as a marker for studying myogenic lineage or the efficacy of myoblast transfer. Anat. Rec. 1997;248:40–50. doi: 10.1002/(SICI)1097-0185(199705)248:1<40::AID-AR5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Yoshida S., Koishi K., Masuda K., Nabeshima Y.-I. Cell heterogeneity upon myogenic differentiationdown-regulation of MyoD and Myf-5 generates ‘reserve cells.’. J. Cell Sci. 1998;111:769–779. doi: 10.1242/jcs.111.6.769. [DOI] [PubMed] [Google Scholar]
- Young P.E., Baumhueter S., Lasky L.A. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood. 1995;85:96–105. [PubMed] [Google Scholar]
- Zammit P.S., Kelly R.G., Franco D., Brown N., Moorman A.F.M., Buckingham M.E. Suppression of atrial myosin gene expression occurs independently in the left and right ventricles of the developing mouse heart. Dev. Dynam. 2000;217:75–85. doi: 10.1002/(SICI)1097-0177(200001)217:1<75::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Zingg J.-M., Alva G.P., Jost J.-P. Characterisation of a genomic clone covering the structural mouse MyoD1 gene and its promoter region. Nucleic Acids Res. 1991;19:6433–6439. doi: 10.1093/nar/19.23.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]