Abstract
Several GTPases of the Rab family, known to be regulators of membrane traffic between organelles, have been described and localized to various intracellular compartments. Rab11 has previously been reported to be associated with the pericentriolar recycling compartment, post-Golgi vesicles, and the trans-Golgi network (TGN). We compared the effect of overexpression of wild-type and mutant forms of Rab11 on the different intracellular transport steps in the endocytic/degradative and the biosynthetic/exocytic pathways in HeLa cells. We also studied transport from endosomes to the Golgi apparatus using the Shiga toxin B subunit (STxB) and TGN38 as reporter molecules. Overexpression of both Rab11 wild-type (Rab11wt) and mutants altered the localization of the transferrrin receptor (TfR), internalized Tf, the STxB, and TGN38. In cells overexpressing Rab11wt and in a GTPase-deficient Rab11 mutant (Rab11Q70L), these proteins were found in vesicles showing characteristics of sorting endosomes lacking cellubrevin (Cb). In contrast, they were redistributed into an extended tubular network, together with Cb, in cells overexpressing a dominant negative mutant of Rab11 (Rab11S25N). This tubularized compartment was not accessible to Tf internalized at temperatures <20°C, suggesting that it is of recycling endosomal origin. Overexpression of Rab11wt, Rab11Q70L, and Rab11S25N also inhibited STxB and TGN38 transport from endosomes to the TGN. These results suggest that Rab11 influences endosome to TGN trafficking primarily by regulating membrane distribution inside the early endosomal pathway.
Keywords: Rab11, intracellular trafficking, endosomes, Shiga toxin, TGN38
Introduction
The cell maintains several dynamic properties to internalize and secrete proteins in different ways. The traffic of membranes between organelles occurs through vesicular or tubular intermediates that selectively convey proteins and lipids from one compartment to another under the control of specific proteins. In this context, small GTPases of the Rab family are believed to play a role of ensuring accurate targeting or docking of transport vesicles with their acceptor membranes (Chavrier and Goud 1999). Several of these Rab proteins such as Rab4, Rab5, Rab7, Rab11, Rab17, Rab18, and Rab20 (Chavrier et al. 1990; Van der Sluijs et al. 1991, Van der Sluijs et al. 1992; Lutcke et al. 1993, Lutcke et al. 1994; Ullrich et al. 1996), have been localized to the endocytic compartments. The endosomal compartment consists of early and late endosomes, which differ from each other in their subcellular localization, morphology, and physical characteristics. Electron microscopical studies have revealed that vesicle intermediates between these two compartments exist (Gruenberg et al. 1989). An emerging concept is that early endosomes can be subdivided into sorting and recycling endosomes, the latter being presumed to function in the delivery of proteins destined for the TGN (Ghosh et al. 1998). Recently, on the basis of cellubrevin (Cb) and transferrin (Tf) intracellular distribution, it has been suggested that recycling endosomes may also be further subdivided into distinct populations (Teter et al. 1998). Recycling endosomes are more plastic than sorting or late endosomes and have been shown to possess a perinuclear tubular morphology that is maintained by microtubules (Hopkins et al. 1990, Hopkins et al. 1994; Cole and Lippincott-Schwartz 1995). The precise boundaries between the sorting and recycling endosomes are still somewhat unclear and the morphology of these compartments varies among different cell types (Sönnichsen et al. 2000).
Internalized receptors and ligands first enter the peripheral early sorting endosomes where they are separated and either designated for degradation in lysosomes, like the epidermal growth factor (EGF) and low density lipoprotein (LDL) receptors, or recycled back to the plasma membrane, as with Tf receptor (TfR). Recycling back to the plasma membrane may occur directly from sorting endosomes or via the recycling compartment (Ghosh and Maxfield 1995; Ren et al. 1998).
The biosynthetic/secretory and the endocytic/degradative pathways are interconnected. Newly synthesized soluble lysosomal enzymes are delivered from the TGN to “prelysosomes” by the mannose-6-phosphate (M6P) receptors (Kornfeld 1992). After release of their ligands, the M6P receptors are returned to the Golgi complex. The major human histocompatibility complex (MHC) class II molecules are also directly transported via the Golgi apparatus to an endosomal compartment. There, within the endosomal pathway, they encounter antigenic peptides before being expressed at the cell surface for antigen presentation (Cresswell 1994). Some other proteins, like TGN38, cycle between the plasma membrane and the TGN via an early endosomal compartment (Luzio et al. 1990; Reaves et al. 1993; Chapman and Munro 1994; Ghosh et al. 1998). Finally, some bacterial protein toxins, such as the Shiga toxin B subunit (STxB), use a retrograde transport pathway from the plasma membrane to the ER, via endosomes and the Golgi apparatus (Sandvig et al. 1991, Sandvig et al. 1992; O'Brien et al. 1992; Johannes and Goud 1998; Mallard et al. 1998).
Rab11 has been found in association with the TGN membranes, post-Golgi vesicles, and recycling endosomes (Urbe et al. 1993; Ullrich et al. 1996). In MDCK cells, Rab11 is restricted to recycling endosomal compartments, which are distinct from Rab4-positive early endosomes (Sheff et al. 1999). In parietal epithelial cells, Rab11 is enriched on tubulovesicular structures controlling the cell surface expression of H+/K+ ATPase, a function that, in these cells, is related to plasma membrane recycling (Goldenring et al. 1994). In CHO cells, Rab11 has been shown to regulate the late recycling of the TfR (Ullrich et al. 1996; Ren et al. 1998). On the basis of its localization to both constitutive and regulated secretory vesicles in PC12 cells (Urbe et al. 1993), Rab11 has also been postulated to function in exocytosis. In yeast, two Rab11 homologues, Ypt31p and Ypt32p, are thought to regulate membrane transport of proteins along the secretory pathway (Benli et al. 1996; Jedd et al. 1997). To better integrate these results, we set out to investigate the effect of Rab11 overexpression on different transport pathways in the same cell type. For this purpose we used reporter molecules, specific for particular membrane transport steps, in HeLa cells.
Overexpression of Rab11wt and Rab11Q70L caused the TfR, TGN38, and the STxB to accumulate in large structures spread throughout the cytoplasm. However, overexpression of Rab11S25N resulted in the redistribution of these markers into a distinct tubular network containing the recycling endosomal marker Cb. Cointernalization of antibodies directed against TGN38 and the CI-M6P receptor resulted in the accumulation of anti-TGN38 antibodies in tubular endosomes, whereas anti-CI-M6P receptor antibodies were transported to late endosomes, as they were in control cells. Several other events, such as EGF transport to lysosomes for degradation and MHC class II maturation in the late endocytic pathway, were not affected by overexpression of wild-type Rab11 (Rab11wt) and mutants. In contrast, overexpression of Rab11wt, a GTPase-deficient Rab11 mutant (Rab11Q70L), and a dominant negative Rab11 mutant (Rab11S25N) affected the delivery of the STxB and TGN38 to the TGN. Therefore, we hypothesize that Rab11 contributes to maintain the organization of the early endosomal pathway into distinct subcompartments, each possessing different transport functions.
Materials and Methods
Reagents and Antibodies
Rhodamine-coupled EGF was obtained from Molecular Probes. RPMI 1640, FCS, PBS, penicillin, streptomycin, and sodium pyruvate were purchased from GIBCO BRL. RPMI depleted of methionine and 35S Trans-label were from ICN Biochemicals. Protein A–sepharose was from Amersham Pharmacia Biotech. The rabbit antibody raised against full-length recombinant Rab11 expressed in Escherichia coli was affinity purified, essentially as described previously (Martinez et al. 1994). The rabbit anti-Rab6 and the anti-Cb antisera have been described previously (Galli et al. 1998; Martinez et al. 1997). The anti–human TfR antibody H68.4 was kindly provided by Dr. I. Trowbridge (The Salk Institute, San Diego, CA), rabbit antiserum against the cation-independent M6P (CI-M6P) receptor was kindly provided by Dr. B. Hoflack (Institut de Biologie de Lille, Lille, France), anti–rat TGN38 mAb by Dr. G. Banting (University of Bristol, Bristol, UK), anti-vesicular stomatis virus (VSV) G-tag mAb P5D4 by Dr. T. Kreis (University of Geneva, Geneva, Switzerland), and CTR433, an mAb recognizing a cis/medial Golgi antigen (Jasmin et al. 1989), by Dr. M. Bornens (Institut Curie, Paris, France). FITC- and Texas red–labeled donkey secondary antibodies were from Amersham Pharmacia Biotech. The mAb DA6.147 (anti–HLA-DRα chain) has been described elsewhere (Guy et al. 1982). mAbs against human placenta alkaline phosphatase were obtained from Tebu (Le Perrayen Yvelines, France). The mAb H189 against hemagglutinin (HA) was kindly provided by Dr. J. Skehel (Harvard University, Cambridge, MA). 125I-EGF was from Amersham Pharmacia Biotech. 5-([4,6-dichlorotriazin-2-yl]amino) fluorescein was obtained from Sigma-Aldrich, and coupling with the STxB was performed as described previously (Johannes et al. 1997). Iron-saturated human Tf was from Sigma-Aldrich. FITC-EGF was obtained from Molecular Probes.
Cell Culture
HeLa cells were grown in DME containing 4.5 g/liter glucose (GIBCO BRL) supplemented with 10% FCS, 4 mM glutamine, and 5 mM sodium pyruvate (GIBCO BRL) in a 6% humidified CO2 incubator. The M10 melanoma cell line was a kind gift from Dr. T. Hercend (Hospital Paul Brousse, Villejuif, France) and was cultured in RPMI medium supplemented with 2 mM glutamine, 5% sodium pyruvate, and 10% FCS. HeLa cells (SA48) stably transfected with sialyl transferase were kindly provided by Dr. T. Nilsson (EMBL, Heidelberg, Germany). HeLa cells stably transfected with rat TGN38 were a kind gift from Dr. G. Banting (University of Bristol, Bristol, UK).
Plasmids and Transfection
pGEM1-SEAP and pGEM1HA plasmids have been described elsewhere (Martinez et al. 1994). pGEM1-Rab11 was a generous gift from Dr. M. Zerial (EMBL, Heidelberg, Germany). Rab11S25N and Rab11Q70L were generated by PCR-based mutagenesis on pGEM-Rab11 plasmid. In the first amplification reaction, the mutant primers 5′-CGAGACAAGAGATTATTCTTTCC-3′ (S25N) and 5′-CGGTATCGCTCGAGCCCTGCTGTGTCC-3′ (Q70L) were used in combination with an oligonucleotide corresponding to the T7 promoter. To construct a plasmid expressing the effector loop mutant Rab11I44E, a previously described PCR-based strategy was adopted (Johannes et al. 1997). First, a pGEM-1 vector carrying the Rab11 cDNA (pGEM-Rab11) was used with PCR primers x/169-1 (5′-GAAAGTAAGAGCACCGAAGGAGTAGAGTTTGCAAC-3′) and x/169-2 (5′-GTTGCAAACTCTACTCCTTCGGTGCTCTTACTTTC-3′) and external primers x/103-1 (5′-AAGATGGGATCCGCGCAATGGGCACCCGCGACGACGA-3′) and x/103-2 (5′-GTAGGTGAACAGGGCTTACTGACGTCGAAA-3′) to produce DNA fragments that, in a second PCR with primers x/103-1 and x/103-2, yielded a fragment that was cloned into the BamHI and PstI restriction sites of pGEM-Rab11. Sequences derived by PCR were verified. HeLa cells were infected with the VT7 recombinant vaccinia virus as described (Fuerst et al. 1986). After infection, the cells were transfected by lipofection using the DOTAB reagent (Boehringer) with either an empty pGEM plasmid (control cells) or plasmids encoding for various Rab constructs (2 μg). More than 90% of the cells were usually transfected, as tested by immunofluorescence using an affinity-purified antibody against Rab11. Where indicated, HeLa cells were also cotransfected with secreted alkalin phosphatase (SEAP) or HA for overexpression of these proteins.
Internalization of Endocytic Markers
Iron-saturated human Tf (Sigma-Aldrich) was labeled with FITC (Amersham Pharmacia Biotech) or tetramethyl rhodamine isothiocyanate (TRITC; Sigma-Aldrich) as described previously (Salamero et al. 1990). For internalization of fluorophore-coupled Tf or fluorescein-EGF, HeLa cells grown on 15-mm round coverslips were transfected for 5 h before being incubated in DME containing 4.5 g/liter glucose (GIBCO BRL) supplemented with 10% FCS, 4 mM glutamine, and 5 mM sodium pyruvate (GIBCO BRL) for various periods of time. CY3-coupled STxB fragments (Johannes et al. 1997) were bound to cells at 4°C for 45 min before internalization into HeLa cells for the times indicated at 19°C or 37°C. Cells were then washed with ice cold PBS and fixed with 3% paraformaldehyde. For antibody uptake, transfected cells were incubated for 2 h at 37°C with mAbs directed against TGN38 or rabbit serum raised against the CI-M6P receptor (5 μg/ml and at a 1:1,000 dilution, respectively). Identical concentrations of irrelevant antibodies did not lead to intracellular staining.
Immunofluorescence and Confocal Microscopy
For immunofluorescence experiments, cells were grown on 12-mm round glass coverslips, transfected 5 h before fixation and quenching in 50 mM glycine. Cells were permeabilized in 0.2% saponin containing PBS and 1% BSA and were then double labeled with antibodies against various proteins. As secondary antibodies, we used donkey anti-IgG antibodies coupled to FITC or Texas red (Amersham Pharmacia Biotech). Where indicated, cells were also treated with 20 μM nocodazole or 10 μM cytochalasine D for 30 min before fixation. Coverslips were mounted in Mowiol (Hoechst AG).
Confocal laser scanning microscopy and multiple immunofluorescence analysis were performed using a TCS4D confocal microscope based on a DM microscope interfaced with a mixed gas argon/krypton laser (Leica).
Streptolysin-O Permeabilization
HeLa cells transfected with Rab11wt or Rab11 mutants were permeabilized with streptolysin-O (provided by Dr. Bhakdi, University of Mainz, Mainz, Germany; Bhakdi et al. 1993) at 4°C, essentially as described previously (Salamero et al. 1996). Pore formation was induced by incubating the cells at 37°C. After extensive washing with KOAc buffer (25 mM Hepes-KOH, pH 7.0, 115 mM potassium acetate, 25 mM MgCl2), permeabilized cells were fixed and treated for immunofluorescence with antibodies directed against Rab11 (affinity-purified) and TfR (as described above). To better preserve the tubular morphology of the early endosomal compartments in Rab11S25N, cells were incubated with streptolysin-O for 2 min at 37°C before washing and fixation.
Pulse–Chase Experiments
Intracellular transport of newly synthesized MHC class II molecules in M10 cells was monitored using metabolic labeling and immunoprecipitation protocols as described previously (Saudrais et al. 1998). Samples were boiled in Laemmli's sample buffer for electrophoresis on SDS-PAGE (PAGE) and run under reducing conditions.
The release of sulfated SEAP and the appearance of HA at the cell surface in cells overexpressing Rab11wt and mutants were monitored as described previously (Martinez et al. 1994). Bands corresponding to HA and SEAP were analyzed and quantified with a Phosphorimager (Molecular Dynamics, Amersham-Pharmacia Biotech).
Transport of the STxB
To follow sulfation of STxB in HeLa cells overexpressing Rab11wt and mutants, we used a recombinant STxB carrying a tandem of sulfation sites (Mallard et al. 1998). Cells were transfected for 3 h. Metabolic sulfate labeling was monitored as described previously (Mallard et al. 1998).
Measurement of 125I-EGF Degradation and 125I-Tf Recycling
The degradation of 125I-EGF was carried out as described previously (Mallard et al. 1998). For 125I-Tf recycling, cells were incubated with 10 nM 125I-Tf for 60 min at 37°C. Cells were then washed three times with ice cold PBS, reincubated at 37°C for the indicated times, and the recycling of iodinated Tf in the medium was estimated as described (Ullrich et al. 1996).
GTP Ligand Overlay Blotting and Immunoblotting
HeLa cells were transfected with Rab11 and mutants for 5 h before lysis in SDS-PAGE sample buffer and resolution on 10% polyacrylamide gels. Proteins were transferred overnight to nitrocellulose membranes (Immobilon; Millipore) using a Transblot cell (Bio-Rad Laboratories) at 100 mA. After transfer, the membranes were incubated in blotting buffer (50 mM Tris, pH 7.5, 5 mM MgCl2, 1 mM EGTA, 0.3% Tween 20) twice for 20 min at room temperature and were probed with 1 μCi/ml [32P]GTP in blotting buffer for 2 h. Aliquots from the same cell lysates were used for immunoblotting and probed with affinity-purified anti-Rab11 antibodies.
Results
Morphological Alterations of the TfR-containing Compartments in HeLa Cells Overexpressing Rab11wt and Mutants
In HeLa cells, endogenous Rab11 displayed a punctate vesicular pattern dispersed throughout the cytoplasm (Fig. 1 A, control, top). Only a partial colocalization was found between Rab11 staining and TfR in these cells (Fig. 1 A, control, top and bottom, respectively).
Figure 1.
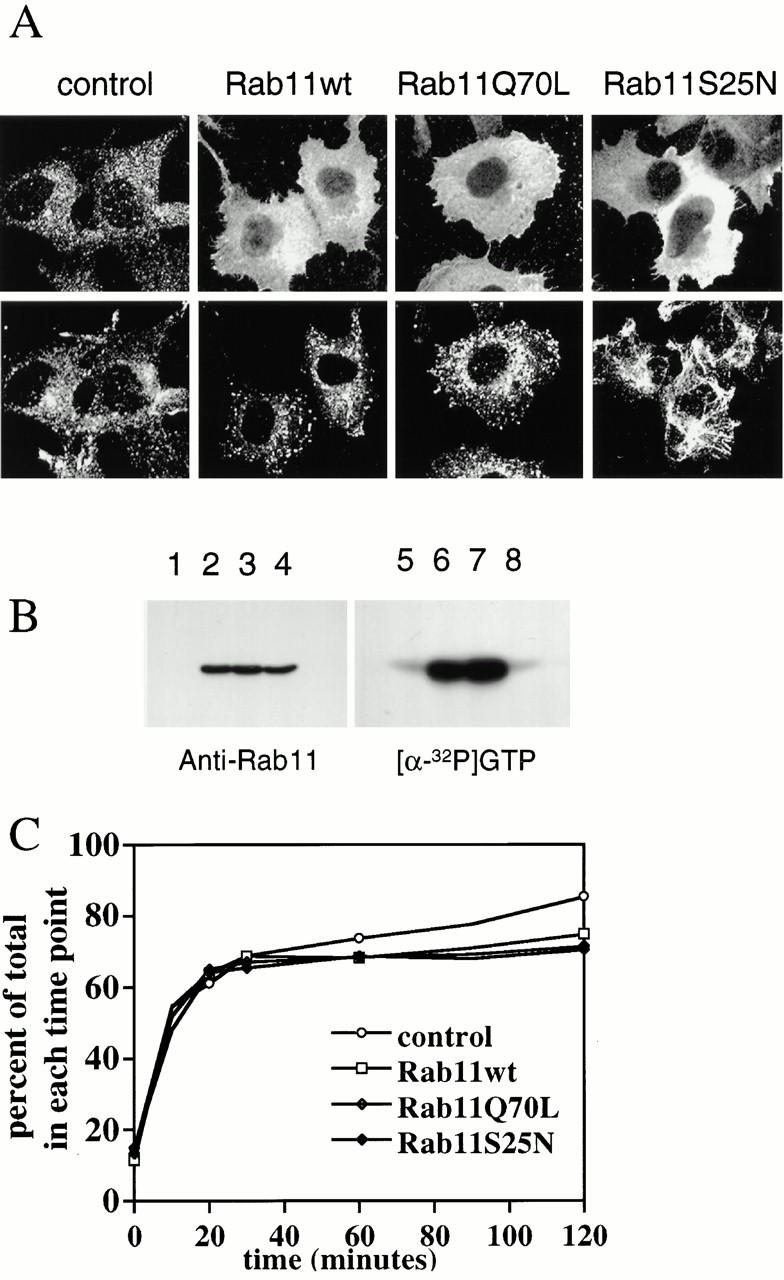
Overexpression of Rab11wt and mutant proteins alters TfR distribution in HeLa cells. (A) HeLa cells were transfected with empty pGEM plasmid (control cells), Rab11wt, Rab11Q70L, or Rab11S25N for 5 h before fixation and permeabilization. In control cells, staining for TfR was located in the perinuclear region (bottom), mostly colocalizing with the staining for Rab11 (top) and in small vesicular structures spread throughout the cells. In Rab11Q70L cells and, to a lesser extent, in Rab11wt cells, the staining pattern of the TfR was concentrated in larger vesicles. In Rab11S25N-overexpressing cells the TfR became redistributed into a distinct tubular network. Overexpressions were assessed by immunofluorescence with the anti-Rab11 antibody. (B) HeLa cells transfected with pGEM-1 (control cells), Rab11wt, Rab11Q70L, and Rab11S25N plasmids were directly lysed 5 h after transfection in 1% Triton X-100. Samples were run on SDS-PAGE gels and transferred to nitrocellulose for immunoblotting using an affinity-purified antibody to Rab11 and an anti–rabbit IgG antibody coupled to peroxidase (ECL protocol). High levels of overexpression of Rab11wt, Rab11Q70L, and Rab11S25N (lanes 2, 3, and 4, respectively) were detected compared with control cells (lane 1). GTP-binding activity was detected using the same cell lysates. The lysates were resolved in SDS-PAGE, transferred onto nitrocellulose, and probed with radiolabeled GTP ([α-32P]GTP). Rab11wt and Rab11Q70L cells (lanes 6 and 7, respectively) showed strong GTP-binding activity compared with control cells and Rab11S25N cells (lanes 5 and 8, respectively). (C) Tf internalization and recycling. Cells overexpressing empty plasmid or Rab11 mutants were allowed to internalize 125I-Tf for 60 min at 37°C. Tf was then chased for the indicated time. Nonspecific binding to the plasma membrane was determined by acidic wash and was estimated to be <5%. After the different times, cells were again placed on ice, the extracellular medium was collected, and TCA was added to 10%. Intracellular 125I-Tf was determined by measuring the cell-associated radioactivity after solubilization in 1 ml of 1% Triton and recycling was calculated as a percentage of the total 125I-Tf activity in each time point.
To study the function of Rab11 in HeLa cells, we overexpressed a dominant negative mutant of Rab11 (Rab11S25N) and a GTPase-deficient mutant of Rab11 (Rab11Q70L). In cells overexpressing Rab11wt or Rab11Q70L, the TfR was essentially found in a dispersed and punctate population of vesicles. However, in cells overexpressing Rab11S25N, the TfR was brought into an expanded tubular network (Fig. 1 A, bottom). The levels of overexpression were similar in cells transfected with Rab11wt, Rab11Q70L, or Rab11S25N, as detected by immunoblotting with affinity-purified antibodies against Rab11 (Fig. 1 B, control, lane 1; Rab11wt, lane 2; Rab11Q70L, lane 3; and Rab11S25N, lane 4). These proteins were overexpressed 30 or 40 times, depending on the experiment. In GTP overlay experiments, Rab11S25N showed low GTP-binding activity (Fig. 1 B, lane 8), as expected. In contrast, Rab11Q70L and Rab11wt showed strong GTP-binding activity (Fig. 1 B, lanes 7 and 6). In agreement with previous studies in BHK and CHO cells (Ullrich et al. 1996; Ren et al. 1998), we also detected a weak inhibition of the 125I-Tf recycling when Tf was continuously internalized for 1 h and chased for various times in cells overexpressing Rab11wt and mutants for 3 h (Fig. 1 C).
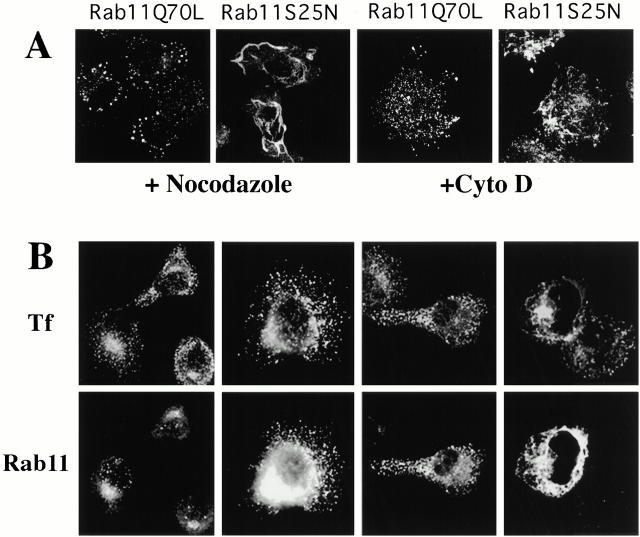
Treatment with the microtubule-depolymerizing agent nocodazole profoundly affected the TfR-positive tubular network generated by Rab11S25N overexpression, giving rise to an intriguing enlargment of this structure (Fig. 2 A, left). Careful optical sectioning by confocal microscopy indicated that this structure appeared as a unique, interconnected compartment, suggesting that the Rab11S25N-induced tubular network may correspond to an exaggerated endosomal compartment. The tubular pattern observed in untreated Rab11S25N-expressing cells (see Fig. 1 A) was no longer visible, showing that the Rab11S25N-induced tubular network is indeed organized along microtubules, similar to that reported for recycling endosomes (Yamashiro et al. 1984). In Rab11Q70L-overexpressing cells, nocodazole induced a further dispersion of the TfR staining (Fig. 2 A, left). In contrast, depolymerization of actin filaments by cytochalasine D had only a minor effect on the overall distribution of early endosomal membranes (Fig. 2 A, right).
Figure 2.
(A) Rab11Q70L- and Rab11S25N-overexpressing cells were treated with 20 μM nocodazole for 30 min before fixation, permeabilization, and immunostaining for TfR (left, + Nocodazole). The same cells were also treated with cytochalasin D (10 μM) for 30 min before fixation (right, +Cyto D). In both cases, effects on either microtubuli or actin filaments were controlled by fluorescence staining of β-tubulin and polymerized actin using a β-tubulin mAb or FITC-phalloidin, respectively (not shown). (B) Transfected Rab11 molecules associate with membranous compartments containing the TfR. HeLa cells were transfected for 5 h with empty pGEM plasmid (control cells), Rab11wt, Rab11Q70L, or Rab11S25N plasmids before permeabilization with streptolysin-O. Cells were fixed, permeabilized with saponin, and incubated with antibodies against the TfR or Rab11.
To determine the intracellular membrane localization of overexpressed Rab11, HeLa cells were transfected with Rab11wt or Rab11 mutants for 5 h and permeabilized with streptolysin-O in order to induce pore formation at the plasma membrane. After extensive washes, most of the cytosolic Rab11 molecules, which corresponded to the majority of overexpressed proteins in transfected cells, were released into the medium (not shown), allowing the detection of the Rab11 molecules that had remained associated to intracellular membranes. Double labeling with antibodies against the TfR clearly indicated a colocalization between endogenous Rab11 and the TfR only in the pericentriolar area in mock-transfected cells (Fig. 2 B, control). In cells overexpressing Rab11wt or Rab11Q70L, a strong Rab11 immunoreactivity was found in all TfR-positive compartments, including peripheral vesicles (Fig. 2 B, Rab11wt and Rab11Q70L). Membrane association of Rab11S25N molecules in streptolysin-O–permeabilized cells was more difficult to detect since only a small fraction of this mutant is associated with membranes at the steady state. This phenomenon has also been reported previously for equivalent mutants of other Rab proteins (Martinez et al. 1994). In addition, streptolysin-O treatment affected the microtubule network (not shown), and the TfR tubular network generated by Rab11S25N consistently was not fully preserved under those conditions (Fig. 2 B, Rab11S25N). However, in cells overexpressing the Rab11S25N mutant, we detected Rab11 on membranous structures that were also positive for the TfR (Fig. 2 B, Rab11S25N).
Tubular TfR-positive Compartments in Rab11S25N-overexpressing HeLa Cells Are Different from Sorting Endosomes and Contain Cb
Temperatures <20°C (16°C) have been shown to prevent the progression of endocytosed Tf from sorting endosomes to the recycling compartment in CHO cells (Ren et al. 1998). The TfR–Tf complexes leave early sorting endosomes for the recycling endosomes within a half-time of 2–3 min (Mayor et al. 1993; Ghosh et al. 1994). In an attempt to investigate the transport of Tf to TfR-containing compartments in HeLa cells overexpressing Rab11 mutants, TRITC-coupled Tf (red) was internalized for 45 min at 19°C before fixation and labeling with antibodies against the TfR (green) (Fig. 3 A). Similarly, cells were washed and chased for 5 min in 37°C before fixation (Fig. 3 B). At 19°C, TRITC-Tf was mainly colocalized with TfR in peripheral structures in mock-transfected cells and not in the pericentriolar area. In Rab11Q70L-overexpressing cells, Tf colocalized with TfR in large peripheral structures at both temperatures. In the Rab11S25N-overexpressing cells, TRITC-Tf appeared largely excluded from the TfR-positive tubular network at 19°C (Fig. 3 A). However, after 5 min of chase at 37°C, this tubular compartment became labeled with TRITC-Tf (Fig. 3 B), highlighting its recycling endosome-like characteristics.
Figure 3.
Accessibility of internalized Tf to the TfR-positive structures generated by overexpression of Rab11 mutants at different temperatures. (A) HeLa cells were transfected for 5 h with empty pGEM plasmid (control cells), Rab11Q70L, or Rab11S25N plasmids. TRITC-Tf (red fluorescence) was then internalized for 45 min at 19°C before fixation. (B) TRITC-Tf internalized at 19°C was subsequently chased for 5 min at 37°C. In both cases, cells were stained with an antibody directed against the TfR (green fluorescence). At 19°C, the cells showed a punctate staining for the TfR in control and Rab11Q70L-expressing cells and a distinct tubular network in the Rab11S25N cells (green fluorescence), whereas the internalized TRITC-Tf stained the same structures but only to a very low extent (red fluorescence). However, in cells warmed up to 37°C for 5 min (B), the TfR and the internalized Tf were almost completely colocalized.
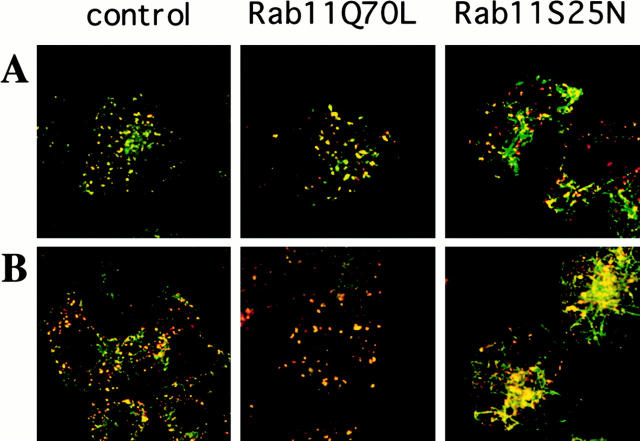
We further characterized the endosomal structures generated on overexpression of Rab11 mutants. Cb is a v-SNARE protein associated with recycling endosomes involved in TfR recycling. However, Cb has also been reported to be associated with vesicles that do not contain TfR (McMahon et al. 1993; Galli et al. 1994; Teter et al. 1998). In mock-transfected cells loaded with TRITC-Tf at 37°C for 1 h and chased for 10 min, Cb was colocalized with internal Tf in the pericentriolar area (Fig. 4, control). In Rab11S25N-overexpressing cells, both markers became largely colocalized in tubular structures (Fig. 4, Rab11S25N), whereas in Rab11Q70L-overexpressing cells, Cb became segregated from TRITC-Tf (Fig. 4, Rab11Q70L). Also, the steady state distribution of Cb was altered in these latter cells compared with mock-transfected cells.
Figure 4.
Intracellular localization of cellubrevin and Tf. HeLa overexpressing Rab11wt and mutant cells were allowed to internalize Tf for 1 h at 37°C followed by washes and further chase for another 10 min at 37°C before being fixed and stained for Cb (CbV). In control cells, Cb colocalized with Tf in the pericentriolar area, but was also in vesicles that were negative for internalized Tf. In cells overexpressing Rab11Q70L, Cb mainly was segregated from Tf. The steady state distribution of Cb was also altered in these cells.
To investigate the putative role of Rab11 at the exit of sorting endosomes, we followed the internalization of EGF, a protein that is engaged in the late endocytic pathway. When TRITC-Tf (red) and fluorescein-EGF (green) were cointernalized at 19°C, these two proteins localized in close apposition in early endosomal structures in both control cells and Rab11 mutant–overexpressing cells (Fig. 5 A). When chased at 37°C, both markers became separated (Fig. 5 B). In control cells and in Rab11Q70L-overexpressing cells, TRITC-Tf labeled punctate vesicular structures devoid of fluorescein-EGF. In the Rab11S25N-overexpressing cells, the TRITC-Tf was, however, transferred to the tubular network and the fluorescein-EGF–labeled vesicular structures were also clearly separated from this network. Some of the fluorescein-EGF–positive compartments were identified as late endosomal and/or lysosomal structures by double staining with the lysosomal marker LAMP-2 (data not shown). We also monitored the effect of Rab11 mutants on the 125I-EGF transport to lysosomal and/or degradative compartments by following its intracellular degradation. In control cells, up to 80% of the 125I-EGF specifically bound to the plasma membrane was degraded after 2 h, and similar results were obtained in cells overexpressing Rab11wt or mutants, suggesting that Rab11 does not control access to the late endocytic pathway (Fig. 5 C).
Figure 5.
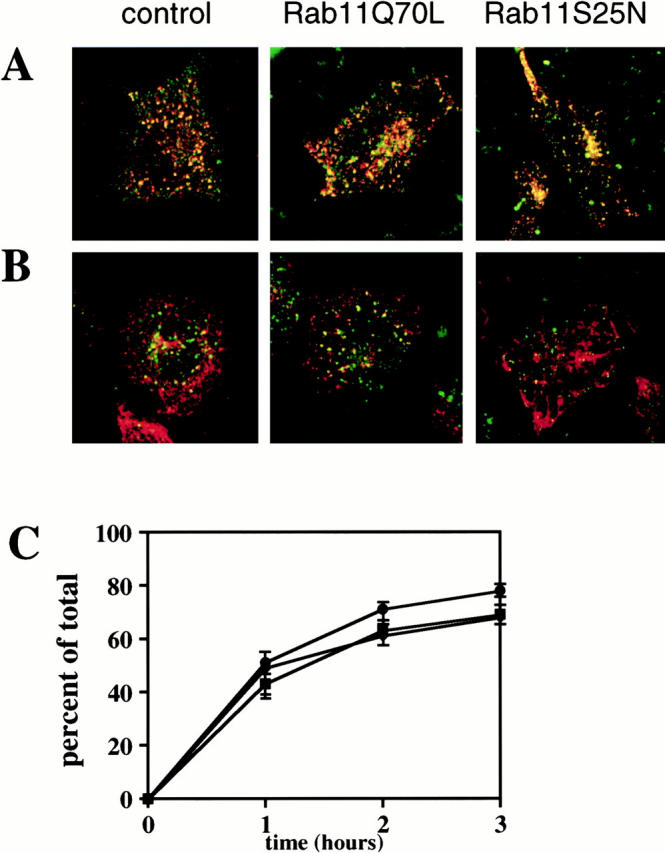
Overexpression of Rab11 mutant proteins does not affect transport to late endosomal compartments. HeLa cells were transfected for 5 h with empty pGEM plasmid (control cells), Rab11Q70L, or Rab11S25N plasmids. (A) EGF (green fluorescence) and Tf (red fluorescence) were continuously internalized at 19°C for 45 min. In control cells and cells transfected with Rab11 mutants, EGF and Tf mostly colocalized in vesicular structures. (B) The cells were further chased at 37°C. After 5 min at 37°C, the staining for EGF (green fluorescence) appeared in vesicles separate from the vesicles containing Tf (red fluorescence) in control cells and Rab11Q70L cells. In Rab11S25N cells, internalized Tf reached the tubular network, as already seen in Fig. 3. Internalized EGF is located in vesicles separate from this network. (C) Measurement of 125I-EGF degradation. After 5 h of transfection with empty pGEM plasmid (control cells, •), Rab11wt (♦), Rab11Q72L (▪), and Rab11S25N (□) plasmids, HeLa cells were incubated with 125I-EGF on ice for 1 h. The cells were then washed in ice cold serum-free medium and incubated at 37°C for various times. Cell lysates and extracellular medium were collected and TCA precipitated. Extracellular TCA-soluble 125I-EGF was calculated as a percentage of the total. No differences were observed in cells overexpressing Rab11wt and mutants compared with control cells.
Golgi Complex Morphology, Secretion, and Cell Surface Transport Are Unaffected in HeLa Cells Overexpressing Rab11wt and Mutants
Earlier studies show that Rab11 is also associated with Golgi membranes and post-Golgi vesicles (Urbe et al. 1993). In HeLa cells, Rab11 did not seem to be associated with the cis and/or medial Golgi complexes (Fig. 6 A). Overexpression of mutants of other Rab proteins associated with Golgi membranes (Goud et al. 1990; Antony et al. 1992) has been shown to alter the Golgi morphology (Martinez et al. 1994, Martinez et al. 1997). However, the Golgi complex remained unaffected in HeLa cells overexpressing Rab11wt and mutants (Fig. 6 A).
Figure 6.
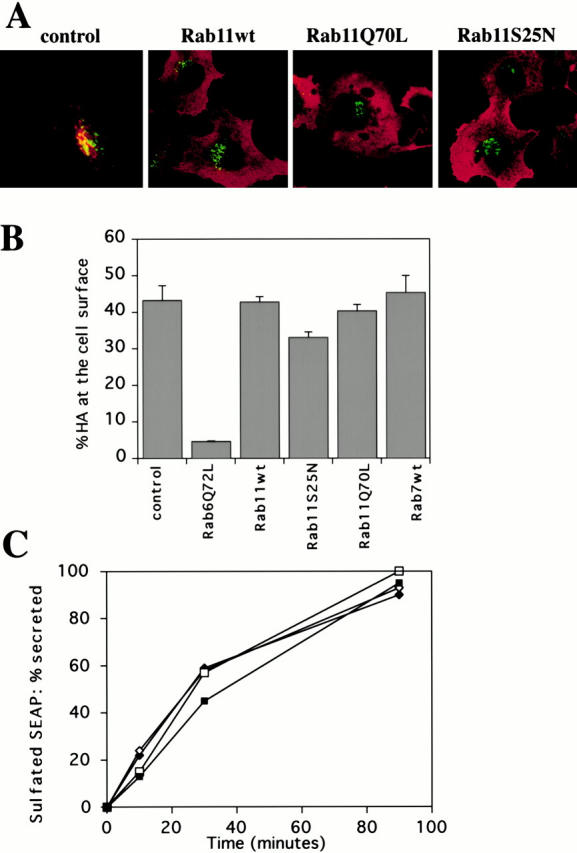
Overexpression of Rab11wt and mutant proteins does not alter Golgi morphology and the constitutive exocytotic pathway. (A) Control (empty pGEM plasmid), Rab11wt, Rab11Q70L, or Rab11S25N transfected cells (from left to right) were labeled for Rab11 (red fluorescence) and a cis/medial Golgi marker, CTR433 (green fluorescence). None of the overexpressing cells showed an affected morphology of the Golgi compartment. (B) To test the effects of the overexpression of Rab11wt and mutants on the appearance of HA at the plasma membrane, HeLa cells were transfected for 5 h before metabolic labeling for 10 min, chase for 75 min in serum-free medium, and trypsin treatment, as described in Materials and Methods. Cell-associated HA was then immunoprecipitated and quantified. Two trypsin cleavage products were detected (HA1 and HA2). The amount of HA molecules present at the cell surface was calculated by the ratio (HA1 + HA2)/(HA0 + HA1 + HA2), HA0 representing uncleaved intracellular HA. We express the results as a percentage of the value found in control cells. Data represents the mean of three separate experiments ±SD. (C) Overexpression of Rab11wt and mutants does not alter the secretion of sulfated SEAP into the extracellular medium. HeLa cells cotransfected with SEAP plasmid and with empty pGEM plasmid (control cells, ♦), Rab11wt (⋄), Rab11Q70L (□), and Rab11S25N (▪) constructs were pulsed for 5 min with [35S]sulfate and chased for various periods of time, as described in Materials and Methods. At the indicated times, both SEAP present in the extracellular medium and in cells was immunoprecipitated and quantified by scanning the specific bands using a Phosphorimager. Sulfated SEAP present in the extracellular medium at each time point is expressed as a percentage of total labeled SEAP. Neither the kinetics of secretion, nor the level of sulfation was affected by overexpression of Rab11 or mutants. The graph illustrates a calculation of secreted SEAP compared with total and is a representative of three different experiments.
To investigate the possible role of Rab11 at the interface between the Golgi and the plasma membrane, we followed the cell surface appearance of an exocytic membrane marker, hemaglutinin from the influenza virus (HA). We compared Rab11wt and mutant-overexpressing cells with cells overexpressing Rab6Q72L, as this Rab protein has been shown previously to modify the rate of HA transport to the cell surface (Martinez et al. 1994). As expected, Rab6Q72L caused a strong inhibition of the appearance of the HA at the cell surface (Fig. 6 B), whereas Rab11Q70L and Rab11wt did not. A small but reproducible effect was observed with Rab11S25N. This could be explained if a small fraction of the newly synthesized HA molecules engaged an alternative pathway for routing to the plasma membrane, e.g., a pathway via the early/recycling endosomes, where Rab11S25N may exert an inhibitory effect. In fact, the exocytic pathway of membrane proteins in nonpolarized cells has recently been shown to be far more complex than described before (Yoshimori et al. 1996). It cannot be formally excluded that this effect of Rab11S25N might correspond to a direct and weak effect of the mutant on the exit of membrane proteins at the Golgi level. We thus tested another marker of the secretory pathway, the soluble SEAP. SEAP is transported to the TGN where the protein is sulfated by TGN sulfotransferase. Sulfated SEAP is then transferred to the plasma membrane and secreted into the external medium. As shown in Fig. 6 C, overexpression of Rab11wt and mutants did not affect the extent of secretion of sulfated SEAP into the external medium. We also verified that the sulfation efficiency on SEAP was not affected by overexpression of Rab11 mutants, indicating that sulfotransferase activity was not impaired, and that SEAP was efficiently transported to the TGN under all conditions (not shown).
Transport from Golgi to Endosomal Compartments of MHC Class II Molecules Is Unaffected by Overexpression of Rab11wt and Mutants
We concluded from the above data that Rab11 does not function as a regulator of the constitutive exocytic pathway. However, there was still the possibility that Rab11 acts on a transport step from the Golgi to endosomal compartments. Therefore, we investigated the effect of overexpressing Rab11wt and mutants on the transport of MHC class II molecules from the TGN to the endosomes and/or lysosomes in a human melanoma cell line (M10) constitutively expressing these molecules. In fact, MHC class II molecules consist of α and β chains that associate with the invariant chain (Ii) upon entry into the ER (Cresswell 1994). The formation of this complex is a prerequisite for transport to the Golgi complex. After exiting the TGN, the αβIi complexes deviate from the constitutive secretory pathway and target directly to the endosomal pathway. Once there, the Ii is degraded (Riberdy et al. 1994).
Compared with HeLa cells, mock-transfected M10 cells displayed a more pronounced pericentriolar staining for the TfR (Fig. 7 A). However, overexpression of Rab11wt and mutants induced the same morphological changes in the TfR compartments as those observed in HeLa cells (Fig. 7 A; see also Fig. 1 A). In pulse–chase experiments, we tested the kinetics of Ii degradation in the endocytic pathway, providing a direct measure of αβIi transfer from the TGN to the endosomes and/or lysosomes. No difference could be detected in the kinetics of degradation of the Ii (p33) chain associated with αβ dimers in M10 cells overexpressing Rab11wt and mutants compared with control cells (Fig. 7B and Fig. C), suggesting that Golgi to endosome and/or lysosome transport of these molecules is not regulated by Rab11.
Figure 7.
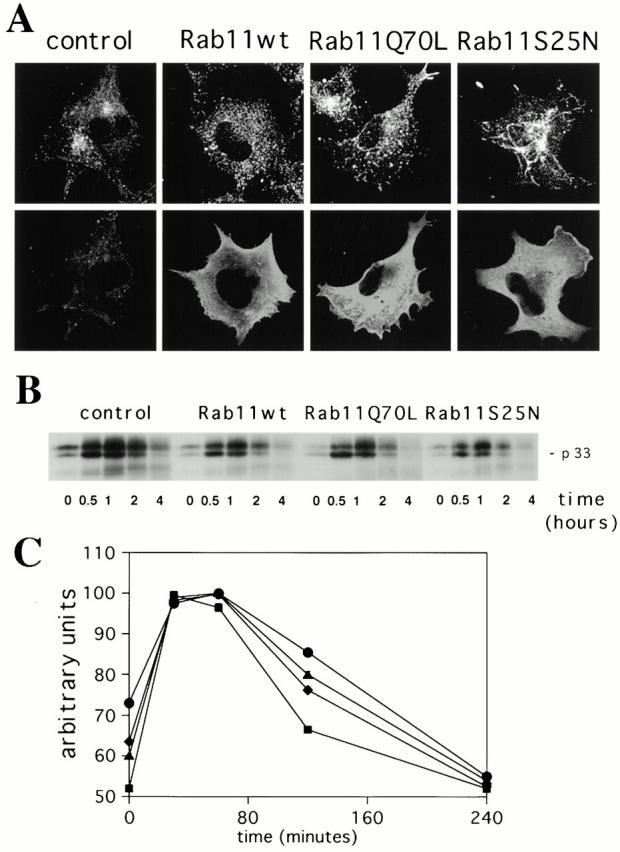
Overexpression of Rab11wt and mutant proteins does not alter the transport of the αβIi precursor forms of MHC class II molecules from the TGN to degradative compartments in the M10 melanoma cell line. (A) M10 cells were transfected for 5 h with empty pGEM plasmid (control cells), Rab11wt, Rab11Q70L, or Rab11S25N plasmids. Cells were double labeled for TfR (top) and Rab11 (bottom) as described in the legend to Fig. 1. (B) Cells were pulse labeled for 10 min. After the indicated times of chase, the L243 mAb was used for the first immunoprecipitation in order to remove newly generated αβ dimers. Proteins in the supernatants of the first immunoprecipitation were then immunoprecipitated with the DA6.147 mAb. DA6.147 immunoprecipitates, which contain αβ dimers associated with Ii, were treated for SDS-PAGE. Coprecipitated Ii (p33) is indicated. No differences were detected among the cell types tested. The figure is representative of three different experiments. Note that oligomerization of the αβIi complexes was complete only 30 min after the pulse and that degradation of p33-associated Ii did not vary significantly within the different overexpressing cells. (C) The absence of an effect from Rab11wt and mutants overexpression in M10 cells on the degradation of Ii was confirmed by the quantification of the signal detected for the p33 Ii in each lane (control, •; Rab11Q70L, ♦; Rab11wt, ▴; Rab11S25N, ▪). Results are representative of three distinct experiments. Data are expressed as a percentage of the maximum Ii associated with αβ dimers found at the 30-min time point.
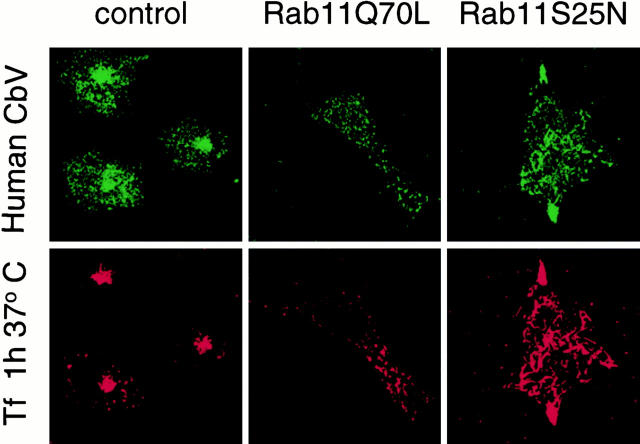
Overexpression of Rab11 Mutants Affects TGN38 Distribution and Recycling to the TGN Without Changing the Distribution of Sialyltransferase and the Accessibility of the M6P Receptor to Late Endosomal Compartments
The TGN marker protein TGN38 is known to cycle between the plasma membrane, endosomes, and the TGN (Reaves et al. 1993; Chapman and Munro 1994). More precisely, TGN38 was recently shown to be delivered to the TGN after trafficking through the recycling compartment without entering late endosomes (Ghosh et al. 1998). HeLa cells stably transfected with rat TGN38 were allowed to internalize TRITC-Tf (red) for 45 min at 37°C before fixation and labeling with an mAb against TGN38 (green) (Fig. 8 A). Strikingly, in cells overexpressing Rab11Q70L, TGN38 became partially accumulated, together with the internalized TRITC-Tf, in the large vesicular structures described above (Fig. 8 A). Even more evident was the colocalization of TGN38 with the tubular structures labeled with internalized TRITC-Tf in Rab11S25N-overexpressing cells. We also noticed that these effects were observed only after >5 h of overexpression, reflecting a relatively slow rate of TGN38 exit from the TGN (Reaves et al. 1993; Ghosh et al. 1998), whereas tubularized endosomes labeled with internalized Tf were already visible within 3–4 h. These changes in the distribution of TGN38 upon Rab11wt and mutants overexpression suggest that this Rab protein may act on the homeostasis of the TGN membranes. In contrast to TGN38, the localization of another protein of the trans-Golgi/TGN, α-2,6-sialyltransferase (ST) (Roth et al. 1985; Rabouille et al. 1995), was largely unaffected in cells overexpressing Rab11Q70L and Rab11S25N (Fig. 8 B). This is consistent with the fact that the cellular location of ST (Colley et al. 1992; Dahdal and Colley 1993) and TGN38 (Bos et al. 1993; Humphrey et al. 1993; Wong and Hong 1993) depends on different mechanisms.
Figure 8.
Distribution and recycling to the TGN of TGN38 in cells overexpressing Rab11 mutants. (A) HeLa cells expressing rat TGN38 were transfected for 5 h with empty pGEM plasmid (control cells), Rab11Q70L, or Rab11S25N plasmids. TRITC-Tf (red fluorescence) was internalized for 45 min at 37°C before fixation and labeling with an mAb directed against TGN38 (green fluorescence). (B) SA48 HeLa cells, a stable transfectant for sialyltransferase with an epitope tag from the VSV G protein, were transfected for 5 h with empty pGEM plasmid (control cells), Rab11wt, Rab11Q70L, or Rab11S25N plasmids before fixation and labeling with P5D4 mAbs directed against the VSV G epitope (green fluorescence). Overexpression was assessed by double staining for Rab11 (red fluorescence). The staining pattern of sialyltransferase did not change in Rab11 mutant–overexpressing cells compared with control cells. (C) These antibody uptake assays were performed using monoclonal and polyclonal antibodies directed against the luminal domains of rat TGN38 and the CI-M6P receptor, respectively. M6P receptor–specific antibodies (green fluorescence) and anti-TGN38 mAb (red fluorescence) were allowed to continuously internalize in cells for 2 h at 37°C. In control cells, this allowed decoration of structures indistinguishable from those stained by the same antibodies in fixed cells (not shown for CI-M6P receptor and in A for TGN38). In the Rab11Q70L cells, the staining pattern of the internalized TGN38 became redistributed in punctate vesicles, whereas in Rab11S25N cells the staining of the TGN38 was redistributed in a tubular network. Although they appear more dispersed in Rab11Q70L cells than in control cells, M6P receptor antibodies do not stain these structures.
Also, M6P receptors cycle between the TGN and endosomes (Kornfeld 1992). The overall distribution of this molecule did not seem to change after overexpression with Rab11wt or mutants, and no localization to the TfR-positive structures generated was found (not shown). Therefore, we compared the intracellular trafficking in living cells of antibodies directed against the luminal domains of TGN38 and CI-M6P receptor (Fig. 8 C). In mock-transfected cells, continuous internalization of both antibodies for 2 h at 37°C was sufficient to label structures that were otherwise indistinguishable when labeled by the same antibodies in fixed cells (not shown). Internalized TGN38 antibodies labeled cisternal perinuclear structures, whereas internalized M6P receptors appeared vesicular and distinct from the TGN. This is consistent with the fact that in HeLa cells the CI-M6P receptor is essentially concentrated in late endosomes. In cells overexpressing Rab11Q70L or Rab11S25N, anti-TGN38 and anti–CI-M6P receptor largely remained segregated into different compartments, the anti–CI-M6P receptor still labeling vesicular structures, as in mock-transfected cells (Fig. 8 C). In contrast, the anti-TGN38 antibodies failed to concentrate in the TGN and decorated structures identical to those observed for the steady state distribution of this molecule in cells overexpressing the corresponding mutants (Fig. 8 A). Interestingly, although the steady state distribution of TGN38 was markedly affected by overexpression of Rab11 mutants, this molecule can still access the plasma membrane, showing that overexpression of Rab11 mutants does not preclude the transport of TGN38 from the TGN to the plasma membrane or its efficient recycling from early endosomal compartments to the cell surface (Ghosh et al. 1998).
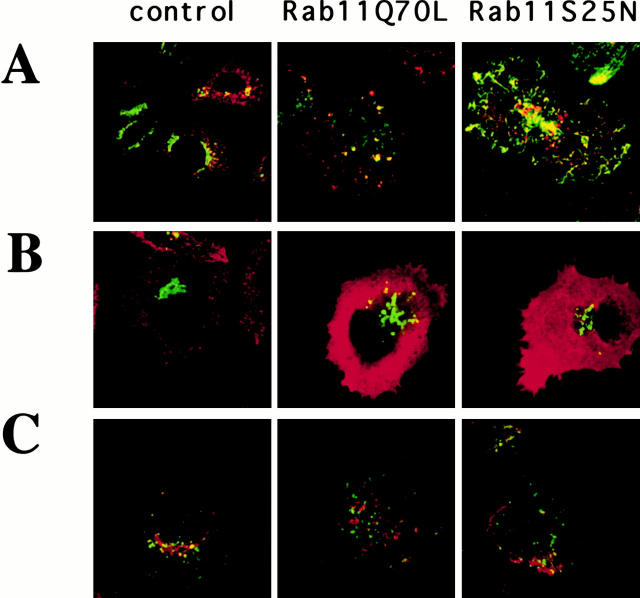
Overexpression of Rab11wt, Rab11S25N, and Rab11 Q70L Inhibits the Delivery of the STxB from Early Endosomes to the TGN
Although the above data suggest that Rab11 is involved in the regulation of transport between early endosomes and the TGN, the low amount of TGN38 at the plasma membrane and in endosomes did not allow us to assess precisely at which stage of the endocytic pathway this Rab protein acts. Therefore, we took advantage of the property of certain bacterial protein toxins, such as STxB, to be delivered from the plasma membrane to the biosynthetic pathway (O'Brien et al. 1992; Sandvig and van Deurs 1996; Johannes and Goud 1998). We recently found that STxB is transported directly from early endosomes/recycling endosomes to the TGN, bypassing late endosomes (Mallard et al. 1998). To determine whether transport from the endosomes to the Golgi complex was affected by overexpression of Rab11wt and mutants, we investigated the distribution of internalized STxB in HeLa cells transfected with various Rab11 constructs. As shown above in Fig. 3, low temperatures did not allow internalized Tf to reach the TfR-containing extended tubular structures generated by overexpression of Rab11S25N. However, even at low temperatures, Tf and TfR were mainly found colocalized in the same structures in Rab11Q70L-overexpressing cells. Similarly, internalized STxB at low temperature was found in the same structures with the TfR in Rab11Q70L-overexpressing cells, whereas the tubular, TfR-positive network in Rab11S25N-overexpressing cells was largely inaccessible to STxB (Fig. 9 A). In another set of experiments, fluorescent STxB was bound on ice to HeLa cells that overexpressed Rab11wt and mutants. The cells were then shifted to 37°C, fixed, and stained for TfR and Rab6 (Fig. 9 B). In control cells (Fig. 9 B, CTL), STxB accumulated in the Golgi area as described previously (Mallard et al. 1998). In Rab11wt- (not shown) and mutant-overexpressing cells, STxB remained largely confined to TfR-containing structures, even though some STxB was also transferred to the Golgi as assessed by a partial colocalization with Rab6.
Figure 9.
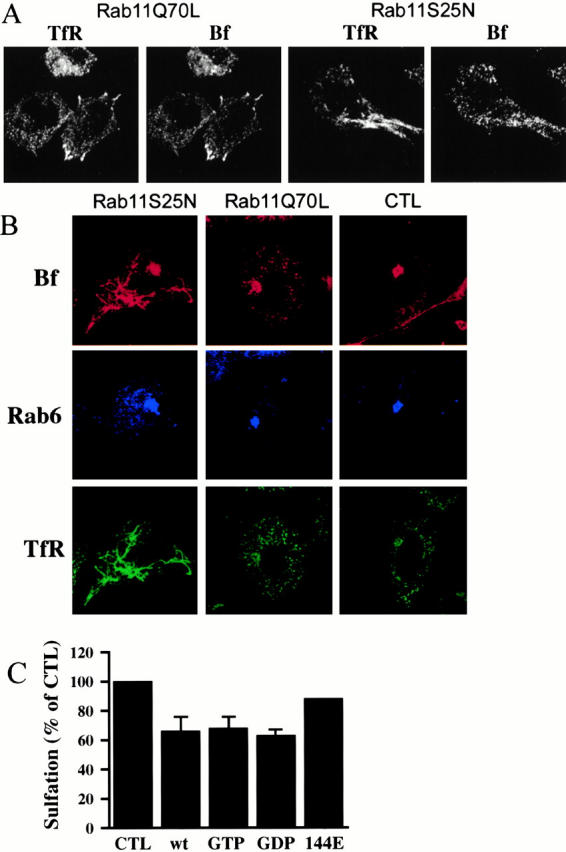
Overexpression of Rab11wt and mutants alters the distribution and the transport of internalized STxB. (A) HeLa cells were transfected with Rab11Q70L and Rab11S25N for 5 h before incubation with CY3-conjugated STxB (Bf) on ice for 30 min. After washes, cells were warmed up to 19°C for 40 min, fixed, permeabilized, and stained for TfR. (B) HeLa cells were transfected with Rab11 mutants for 3 h before incubation with CY3-STxB on ice for 45 min. After 45 min of internalization at 37°C of prebound STxB, cells were fixed and labeled with antibodies against the TfR and Rab6. In control cells (right), STxB displayed a typical Golgi staining, whereas in Rab11Q70L-expressing cells (middle), it also accumulated in distinct punctuate vesicles which were colabeled with the TfR. In the Rab11S25N cells (left), STxB was also found in the tubular network labeled for TfR. Note that a Golgi staining was often seen with internalized STxB in Rab11Q70L and Rab11S25N cells. (C) Recombinant STxB-Sulf2 was bound to sulfate-starved HeLa cells after 3 h of transfection with Rab11wt and mutants. After 30 min at 37°C, [35S]sulfate was added and the cells were then incubated for another 15 min before lysis. STxB was then immunoprecipitated before gel electrophoresis. Quantification was performed with a Phosphorimager. Particular attention was paid to the relative levels of Rab expression and to the rates of cell transfection, which were calculated in parallel. Data were normalized to the rate of protein and proteoglycan sulfation in the different cells. The results represent the mean ± SD of two (for Rab11I44E) or five independent experiments (all the other transfectants). We express the results as a percentage of the value found in control cells.
To further examine the effect of Rab11wt and mutant overexpression on the transport from endosomes to the TGN, we followed the sulfation of internalized STxB in these transfected cells. As already mentioned, protein sulfation is a TGN-specific protein modification (Niehrs and Huttner 1990), and we have recently shown that STxB with sulfation sites (STxB-sulf) can be sulfated after internalization in HeLa cells (Johannes et al. 1997; Mallard et al. 1998), thus allowing us to measure the transfer of the protein from endosomes to the TGN. After 3 h of transfection, HeLa cells were incubated on ice with STxB-sulf for 45 min, and the cells were then shifted to 37°C for 45 min in the presence of [35S]sulfate. After lysis, STxB-sulf was immunoprecipitated and analyzed by SDS-PAGE before quantification by comparing the level of STxB sulfation to that of the sulfated cellular proteins (Fig. 9 C). Compared with mock-transfected cells or with cells transfected with an effector mutant of Rab11 (Rab11I44L), sulfation of STxB-Sulf was reduced by 35% in Rab11wt-, Rab11Q70L-, and Rab11S25N-overexpressing cells (Fig. 9 C).
Discussion
Our results indicate that Rab11 is likely to be associated with recycling endosomes in HeLa cells as found previously in other cell types (Ullrich et al. 1996; Sönnichsen et al. 2000). In addition to recycling endosomes, Rab11 has also been localized to the TGN and post-Golgi vesicles in some cell types (Urbe et al. 1993). In HeLa cells, we were unable to detect colocalization between endogenous Rab11 and Golgi or TGN markers by immunofluorescence. In addition, biochemical and morphological data obtained in this study argue against a role for Rab11 in the biosynthetic/secretory pathway. Based on our results, a role for Rab11 in transport from early endosomes to late endosomes and from the TGN to late endosomes can also be excluded.
Modifications of TfR-containing Compartments in Cells Overexpressing Rab11wt and Mutants
Internalized Tf, EGF, or STxB feebly accessed the tubular endosome generated by Rab11S25N at low temperature, indicating that this tubular endosome is different from the sorting endosome. In addition, further chase at 37°C allowed both Tf and STxB (but not EGF) to rapidly label these tubular endosomes. Since Rab11S25N did not block the access to the tubular endosomes, Rab11 is probably not directly involved in transport from sorting to recycling endosomes. The exaggeration of this recycling endosome suggests instead that Rab11 plays a role in the exit of membranes from this compartment. Depolymerization of microtubules, rather than fragmenting the tubular endosome induced by Rab11S25N overexpression, leads to swelling and the loss of the pericentriolar organization of this structure. This suggests that the extension of the tubular endosome upon Rab11S25N overexpression results from the inhibition of the formation of transport intermediates from the recycling endosome. Although the generally accepted model describes a role for Rab proteins in vesicle docking and fusion (Novick and Zerial 1997), there is also evidence that certain Rabs, including the yeast Rab11 homologues, may function in promoting vesicle budding (Riederer et al. 1994; Nuoffer et al. 1994; Tisdale and Balch 1996; Jedd et al. 1997).
Upon overexpression of Rab11wt and Rab11Q70L, both types of Rab11 molecules substantially colocalized with the TfR compared with untransfected cells. Surprisingly, these enlarged peripheral structures contained most of the intracellular TfR and seemed to correspond to sorting endosomes. However, the EGF rapidly segregated from other internalized markers, implying that Rab11 does not influence the exit of this molecule from sorting endosomes. One possible explanation would be that strong overexpression of Rab11wt or Rab11Q70L leads to a partial fusion between recycling and sorting endosomes. In support of this hypothesis, it has been suggested that Rab5, a marker of sorting endosomes, and Rab11 partially colocalize in cells overexpressing both molecules (Ullrich et al. 1996). It was also recently postulated that the active form of Rab11 is required for the formation of Tf-containing vesicles, which emerge from sorting endosomes en route to either the plasma membrane or pericentriolar recycling endosomes (Ren et al. 1998). However, direct evidence of distinct roles for early and/or sorting endosomes and recycling endosomes have now been provided in MDCK cells, showing that Rab11-positive compartments do not seem to be essential for recycling from early and/or sorting endosomes to the basolateral plasma membrane, although they may be involved in specific sorting of apical to basolateral proteins (Sheff et al. 1999). Our results do not favor a direct role of Rab11 at the exit of the sorting endosome, although the overexpression of the mutants may have modified the dynamic homeostasis of both the recycling and sorting endosomes.
Modifications of Cb-positive Recycling Endosomes in Cells Overexpressing Rab11 Mutants
Several membrane proteins that cycle through the endosomal membranes have overlapping but distinct distributions, suggesting that they may not always follow the same path (Wei et al. 1998). The v-SNARE protein Cb, which is a constituent of recycling endosomes, has been suggested in previous studies to codistribute only partially with Tf (Galli et al. 1994). More precisely, this heterogeneity in Tf and Cb distribution reveals that the recycling endosomal network is constituted in different subcompartments (Teter et al. 1998).
The strong increase in the colocalization of Tf and Cb in cells overexpressing the dominant negative mutant of Rab11 implies a reduced segregation of Cb from the Tf-positive recycling compartment and is consistent with the hypothesis that Rab11 plays a role in sorting events that take place at this site. Moreover, overexpression of Rab11Q70L induced further segregation between Tf and Cb, and these distinct redistributions may reflect the disappearance of the tubular recycling endosomes themselves. This also reinforces the assumption that Rab11 acts as a sorting modulator at the exit of the recycling compartment.
Rab11 Is Also Involved in the Sorting of Proteins from Endosomes towards the Biosynthetic Pathway
We show that Rab11 may function in the exit of proteins out of the recycling endosomes. These proteins include both a TGN resident, TGN38, which cycles between plasma, the membrane and the TGN (Ladinsky and Howell 1992; Reaves et al. 1993), and STxB. Previous reports on TGN38 transport have suggested that acidic endosomes may participate in the delivery of TGN38 to the TGN (Chapman and Munro 1994). However, the precise characterization of this endosomal/acidic compartment, and whether this effect was direct or indirect, is difficult to evaluate. More recently, it was clearly demonstrated that most of endocytosed TGN38 chimeric protein is first delivered to the endocytic recycling compartment, before being sorted to the TGN (Ghosh et al. 1998). The redistribution of endogenous TGN38 into TfR-positive structures in cells overexpressing Rab11S25N thus suggests that TGN38 transport back to the TGN is dependent on a transport step regulated by Rab11. Evidence also exists that a small proportion of internalized Tf can reach the TGN and that cell surface TfR can be modified by TGN-specific enzymes during recycling (Snider and Rogers 1985; Fishman and Fine 1987), showing that early/recycling endosomes and the TGN may be closely related. The relative distribution and uptake of specific antibodies in the different transfected cells strongly suggest that the CI-M6P receptor and TGN38 are cycling to the TGN from different endosomal compartments.
Recent studies from our laboratory showed that TGN38 and STxB colocalized during their transport from the early endosomes to the TGN (Mallard et al. 1998). Our biochemical data indicate that sulfation of internalized STxB is indeed inhibited in cells overexpressing Rab11wt, Rab11S25N, and Rab11Q70L, but not in cells overexpressing the effector loop mutant Rab11I44E, whereas in the same cells the sulfation of a secreted protein was unaltered. The fact that not only Rab11S25N overexpression but also the overexpression of Rab11wt and Rab11Q70L led to a partial inhibition of sulfation remains a striking observation. Whether this reflects the depletion of the recycling compartment itself or an alteration in the membrane dynamic between sorting and recycling endosomes leading to the fusion of these two compartments remains to be evaluated. However, these observations suggest that for efficient transport to the TGN, at least a portion of STxB needs to pass through a recycling endosome on which Rab11 exerts its function. As a whole, these findings indicate that, in HeLa cells, Rab11 is involved in the sorting of proteins from recycling endosomes to the TGN.
In conclusion, our study emphasizes the importance of recycling endosomes in the delivery of proteins to different parts of the cell. In this context, Rab11 is likely to act as a modulator of sorting functions in recycling endosomes.
Acknowledgments
We are grateful to Dr. Ray McDermott and Mrs. Sarah Hunter for critically reading the manuscript. We thank Dr. Olivier Martinez for helpful suggestions. We thank Dominique Morineau and Daniel Meur for photographic works.
This work was supported by grants from the European Union (ERB FMRX CT 96 0020) and Human Frontier Science Program to B. Goud and M. Wilcke. M. Wilcke was supported by a postdoctoral grant from the Swedish Foundation for International Cooperation and Higher Education.
Footnotes
T. Galli's present address is UMR CNSR 144, Membrane Traffic and Neuronal Plasticity, Institut Curie, F-75248 Paris Cedex 05, France.
Abbreviations used in this paper: CI-M6P, cation-independent M6P; Cb, human cellubrevin; HA, hemagglutinin; Ii, MHC class II–associated invariant chain; M6P, mannose-6-phosphate; MHC, major histocompatibility complex; SEAP, secreted alkalin phosphatase; STxB, Shiga toxin B subunit; STxB-sulf, STxB with sulfation sites; Tf, transferrin; TfR, Tf receptor; TRITC, tetramethyl rhodamine isothiocyanate; VSV, vesicular stomatis virus.
References
- Antony C., Cibert C., Geraud G., Santa Maria A., Maro B., Mayau V., Goud B. The small GTP-binding protein rab6p is distributed from medial Golgi to the trans-Golgi network as determined by a confocal microscopic approach. J. Cell Sci. 1992;103:785–796. doi: 10.1242/jcs.103.3.785. [DOI] [PubMed] [Google Scholar]
- Benli M., Doring F., Robinson D.G., Yang X., Gallwitz D. Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Weller U., Walev I., Martin E., Jonas D., Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med. Microbiol. Immunol. 1993;182:167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- Bos K., Wraight C., Stanley K.K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R.E., Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Goud B. The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 1999;11:466–475. doi: 10.1016/S0955-0674(99)80067-2. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R.G., Hauri H.P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Cole N.B., Lippincott-Schwartz J. Organization of organelles and membrane traffic by microtubules. Curr. Opin. Cell Biol. 1995;7:55–64. doi: 10.1016/0955-0674(95)80045-x. [DOI] [PubMed] [Google Scholar]
- Colley K.J., Lee E.U., Paulson J.C. The signal anchor and stem regions of the beta-galactoside alpha 2,6-sialyltransferase may each act to localize the enzyme to the Golgi apparatus. J. Biol. Chem. 1992;267:7784–7793. [PubMed] [Google Scholar]
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu. Rev. Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- Dahdal R.Y., Colley K.J. Specific sequences in the signal anchor of the beta-galactoside alpha-2,6-sialyltransferase are not essential for Golgi localization. Membrane flanking sequences may specify Golgi retention. J. Biol. Chem. 1993;268:26310–26319. [PubMed] [Google Scholar]
- Fishman J.B., Fine R.E. A trans Golgi-derived exocytic coated vesicle can contain both newly synthesized cholinesterase and internalized transferrin. Cell. 1987;48:157–164. doi: 10.1016/0092-8674(87)90366-7. [DOI] [PubMed] [Google Scholar]
- Fuerst T., Niles E., Studier F., Moss B. Eukaryotic transcient-expression system based on recombinant vaccinia virus that synthetizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli T., Chilcote T., Mundigl O., Binz T., Niemann H., De Camilli P. Tetanus toxin–mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor–containing vesicles in CHO cells. J. Cell Biol. 1994;125:1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli T., Zahraoui A., Vaidyanathan V.V., Raposo G., Tian J.M., Karin M., Neimann H., Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol. Biol. Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R.N., Maxfield F.R. Evidence for nonvectorial retrograde transferrin trafficking in the early endosomes of HEp2 cells. J. Cell Biol. 1995;128:549–561. doi: 10.1083/jcb.128.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R.N., Gelman D.L., Maxfield F.R. Quantification of low density lipoprootein and transferrin endocytic sorting in HEp2 cells using confocal microscopy. J. Cell Sci. 1994;107:2177–2189. doi: 10.1242/jcs.107.8.2177. [DOI] [PubMed] [Google Scholar]
- Ghosh R.N., Mallet W.G, Soe T.T., McGraw T.E., Maxfield F.R. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J. Cell Biol. 1998;142:923–936. doi: 10.1083/jcb.142.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J.R., Soroka C.J., Shen K.R., Tang L.H., Rodriguez W., Vaughan H.D., Stoch S.A., Modlin I.M. Enrichment of rab11, a small GTP-binding protein, in gastric parietal cells. Am. J. Physiol. 1994;267:G187–G194. doi: 10.1152/ajpgi.1994.267.2.G187. [DOI] [PubMed] [Google Scholar]
- Goud B., Zahraoui A., Tavitian A., Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990;345:553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Griffiths G., Howell K.E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo with an assay of vesicle fusion in vitro. J. Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy K., Van Heyningen V., Cohen B.B., Deane D.L., Steel C.M. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur. J. Immunol. 1982;12:942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- Hopkins C.R., Gibson A., Shipman M., Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990;339:335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Hopkins C.R., Gibson A., Shipman M., Strickland D.K., Trowbridge I.S. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J. Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J.S., Peters P.J., Yuan L.C., Bonifacino J.S. Localization of TGN38 to the trans-Golgi networkinvolvement of a cytoplasmic tyrosine–containing sequence. J. Cell Biol. 1993;120:1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin B.J., Cartaud J., Bornens M., Changeux J.P. Golgi apparatus in chick skeletal musclechanges in its distribution during end plate development and after denervation. Proc. Natl. Acad. Sci. USA. 1989;86:7218–7222. doi: 10.1073/pnas.86.18.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G., Mulholland J., Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. J. Cell Biol. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L., Goud B. Surfing on a retrograde wavehow does Shiga toxin reach the endoplasmic reticulum? Trends Cell Biol. 1998;8:158–162. doi: 10.1016/s0962-8924(97)01209-9. [DOI] [PubMed] [Google Scholar]
- Johannes L., Tenza D., Antony C., Goud B. Retrograde transport of KDEL-bearing B-subunit of Shiga toxin. J. Biol. Chem. 1997;272:19554–19561. doi: 10.1074/jbc.272.31.19554. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose-6-phosphate/insulin-like growth factor receptors. Annu. Rev. Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Ladinsky M., Howell K. The trans-Golgi network can be dissected structurally and functionally from the cisternae of the Golgi complex by brefeldin A. Eur. J. Cell Biol. 1992;59:92–105. [PubMed] [Google Scholar]
- Luzio J.P., Brake B., Banting G., Howell K.E., Braghetta P., Stanley K.K. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38) Biochem. J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutcke A., Jansson S., Parton R.G., Chavrier P., Valencia A., Huber L.A., Lehtonen E., Zerial M. Rab17, a novel small GTPase, is specific for epithelial cells and is induced during cell polarization. J. Cell Biol. 1993;121:553–564. doi: 10.1083/jcb.121.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutcke A., Parton R., Murphy C., Olkannen V., Dupree P., Valencia A., Simons K., Zerial M. Cloning and subcellular localization of novel rab proteins reveals polarized and cell type-specific expression. J. Cell Sci. 1994;107:3437–3448. doi: 10.1242/jcs.107.12.3437. [DOI] [PubMed] [Google Scholar]
- Mallard F., Antony C., Tenza D., Salamero J., Goud B., Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 1998;143:973–990. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez O., Schmidt A., Salamero J., Hoflack B., Roa M., Goud B. The small GTP-binding protein rab6 functions in intra-Golgi transport. J. Cell Biol. 1994;127:1575–1588. doi: 10.1083/jcb.127.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez O., Antony C., Pehau-Arnaudet G., Berger E.G., Salamero J., Goud B. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 1997;94:1828–1833. doi: 10.1073/pnas.94.5.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S., Presley J., Maxfield F.R. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H.T., Ushkaryov Y.A., Edelmann L., Link E., Binz T., Niemann H., Jahn R., Sudhof T.C. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Niehrs C., Huttner W.B. Purification and characterization of tyrosylprotein sulfotransferase. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:35–42. doi: 10.1002/j.1460-2075.1990.tb08077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Zerial M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- Nuoffer C., Davidson H.W., Matteson J., Meinkoth J., Balch W.E. A GDP-bound of rab 1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J. Cell Biol. 1994;125:225–237. doi: 10.1083/jcb.125.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A.D., Tesh V.L., Donohue-Rolfe A., Jackson M.P., Olsnes S., Sandvig K., Lindberg A.A., Keusch G.T. Shiga toxinbiochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 1992;180:65–94. doi: 10.1007/978-3-642-77238-2_4. [DOI] [PubMed] [Google Scholar]
- Rabouille C., Hui N., Hunte F., Kieckbusch R., Berger E.G., Warren G., Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci. 1995;108:1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- Ren M., Xu G., Zeng J., De Lemos-Chiarandini C., Adesnic M., Sabatini D. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B., Horn M., Banting G. TGN38/41 recycles between the cell surface and the TGNbrefeldin A affects its rate of return to the TGN. Mol. Biol. Cell. 1993;4:93–105. doi: 10.1091/mbc.4.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riberdy J.M., Avva R.R., Geuze H.J., Cresswell P. Transport and intracellular distribution of MHC class II molecules and associated invariant chain in normal and antigen-processing mutant cell lines. J. Cell Biol. 1994;125:1225–1237. doi: 10.1083/jcb.125.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer M.A., Soldati T., Shapiro A.D., Lin J., Pfeffer S.R. Lysosome biogenesis requires rab9 function and receptor recycling from endosomes to the trans-Golgi network. J. Cell Biol. 1994;125:573–582. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Taatjes D.J., Lucocq J.M., Weinstein J., Paulson J.C. Demonstration of an extensive trans-tubular network continuous with the Golgi apparatus stack that may function in glycosylation. Cell. 1985;43:287–295. doi: 10.1016/0092-8674(85)90034-0. [DOI] [PubMed] [Google Scholar]
- Salamero J., Humbert M., Cosson P., Davoust J. Mouse B lymphocyte specific endocytosis and recycling of MHC class II molecules. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:3489–3496. doi: 10.1002/j.1460-2075.1990.tb07557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamero J., Le Borgne R., Saudrais C., Goud B., Hoflack B. Expression of major histocompatibility complex class II molecules in HeLa cells promotes the recruitment of AP-1 Golgi-specific assembly proteins on Golgi membranes. J. Biol. Chem. 1996;271:30318–30321. doi: 10.1074/jbc.271.48.30318. [DOI] [PubMed] [Google Scholar]
- Sandvig K., van Deurs B. Endocytosis, intracellular transport, and cytotoxic action of Shiga toxin and ricin. Physiol. Rev. 1996;76:949–966. doi: 10.1152/physrev.1996.76.4.949. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Prydz K., Ryd M., van Deurs B. Endocytosis and intracellular transport of the glycolipid-binding ligand Shiga toxin in polarized MDCK cells. J. Cell Biol. 1991;113:553–562. doi: 10.1083/jcb.113.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Garred O., Prydz K., Kozlov J.V., Hansen S.H., van Deurs B. Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature. 1992;358:510–512. doi: 10.1038/358510a0. [DOI] [PubMed] [Google Scholar]
- Saudrais C., Spenher D., de la Salle H., Bohbot A., Cazenave J.P., Goud B., Hanau D., Salamero J. Intracellular pathway for the generation of functional MHC class II peptide complexes in immature human dendritic cells. J. Immunol. 1998;160:2597–2607. [PubMed] [Google Scholar]
- Sheff D.R., Daro E.A., Hull M., Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider M.D., Rogers O.C. Intracellular movement of cell surface receptors after endocytosisresialylation of asialo-transferrin receptor in human erythroleukemia cells. J. Cell Biol. 1985;100:826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sönnichsen B., De Renzis S., Nielson E., Rietdorf J., Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter K., Chandy G., Quinones B., Pereyra K., Machen T., Moore H.P. Cellubrevin-targeted fluorescence uncovers heterogeneity in the recycling endosomes. J. Biol. Chem. 1998;273:19625–19633. doi: 10.1074/jbc.273.31.19625. [DOI] [PubMed] [Google Scholar]
- Tisdale E.J., Balch W.E. Rab2 is essential for the maturation of pre-Golgi intermediates. J. Biol. Chem. 1996;271:29372–29379. doi: 10.1074/jbc.271.46.29372. [DOI] [PubMed] [Google Scholar]
- Ullrich O., Reinsch S., Urbe S., Zerial M., Parton R. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbe S., Huber L.A., Zerial M., Tooze S.A., Parton R.G. Rab11, a small GTPase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett. 1993;334:175–182. doi: 10.1016/0014-5793(93)81707-7. [DOI] [PubMed] [Google Scholar]
- Van der Sluijs P., Hull M., Zahraoui A., Tavitian A., Goud B., Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc. Natl. Acad. Sci. USA. 1991;88:6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluijs P., Hull M., Webster P., Male P., Goud B., Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- Wei M.L., Bonzelius F., Scully R.M., Kelly R.B., Herman G.A. GLUT4 and transferrin receptor are differentially sorted along the endocytic pathway in CHO cells. J. Cell Biol. 1998;140:565–575. doi: 10.1083/jcb.140.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.H., Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localization. J. Biol. Chem. 1993;268:22853–22862. [PubMed] [Google Scholar]
- Yamashiro D.J., Tycko B., Fluss S.R., Maxfield F.R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Yoshimori T., Keller P., Roth M.G., Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J. Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]