Abstract
Although it has been suggested that presynaptic active zone (AZ) may be preassembled, it is still unclear which entities carry the various proteins to the AZ during synaptogenesis. Here, I propose that aggregates of dense core vesicles (DCV) and small clear vesicles in the axons of young hippocampal cultures are carriers containing preformed AZ and synaptic vesicle (SV) components on their way to developing synapses. The aggregates were positively labeled with antibodies against Bassoon and Piccolo (two AZ cytomatrix proteins), VAMP, SV2, synaptotagmin (three SV membrane proteins), and synapsin I (a SV-associated protein). Bassoon and Piccolo labeling were localized at dense material both in the aggregates and at the AZ. In addition to the SV at the synapses, the SV membrane proteins labeled the clear vesicles in the aggregate as well as many other SV-like and pleiomorphic vesicular structures in the axons, and synapsin I labeling was associated with the vesicles in the aggregates. In single sections, these axonal vesicle aggregates were ~0.22 by 0.13 μm in average dimensions and contain 1-2 DCV and 5-6 small clear vesicles. Serial sections confirmed that the aggregates were not synaptic junctions sectioned en face. Labeling intensities of Bassoon and Piccolo measured from serially sectioned transport aggregates and AZ were within range with each other, suggesting that one or a few aggregates, but not individual DCV, can carry sufficient Bassoon and Piccolo to form an AZ. The present findings provide the first ultrastructural evidence localizing various AZ and SV proteins in a preassembled multi-vesicle transport aggregate that has the potential to quickly form a functional active zone.
Keywords: Bassoon, Piccolo, VAMP, SV2, synaptotagmin, synapsin I
Synaptic transmission takes place at the active zone (AZ) where synaptic vesicles (SV) fuse with the plasma membrane and release their transmitter contents. The process of synaptogenesis, including AZ formation, remains a subject of intense interest (reviewed in Matteoli et al., 2004; Zhen and Jin, 2004). Ahmari et al., (2000) conducted a salient study combining live imaging of GFP-VAMP [a SV membrane protein fused with green fluorescent protein (GFP)], and double labeling of other presynaptic proteins (including SV markers, calcium channel, and endocytosis-related protein). They proposed that these presynaptic proteins may be preassembled in a packet and transported together. Their retrospective EM analysis showed that GFP-VAMP transport packets coincide with a mixture of entities including pleiomorphic vesicles, tubulo-vesicular structures, and dense core vesicles (DCV), but few SV. Some of the mobile GFP-VAMP transport packets became endo/exocytosis-competent within 1 hr of contact between axon and dendrite, consistent with the advantage offered by a pre-assembled presynaptic packet — poised for action and functional in a short time. However, they did not identify which particular structures in the packet carried VAMP or other synaptic proteins. Immunolabeling at the EM level for the various proteins could provide more specific answers at the ultrastructural level.
In addition to the SV proteins and various other presynaptic proteins, the AZ has a specialized cytomatrix structure (Phillips et al, 2001) consisting of numerous proteins (reviewed in Schoch and Gundelfinger, 2006), with Bassoon and Piccolo being two stable components of the AZ cytomatrix (Tao-Cheng, 2006). Immunolabeling of Piccolo at light microscopy (LM) and EM levels (Zhai et al., 2001) as well as live imaging of GFP-Bassoon (Shapira et al., 2003) combined with fractionation/immunolabeling on antibody-coated magnetic beads, identified a type of DCV which is ~80 nm in size and termed as Piccolo/Bassoon transport vesicle (PTV). It was suggested that PTVs carry a comprehensive set of AZ materials, and that AZ is formed by unitary assembly of two or three PTVs (Shapira et al., 2003). Furthermore, it was also proposed that DCV in the GFP-VAMP packet (Ahmari et al., 2000) may be the same as the PTV identified as the precursor vesicle for AZ (reviewed in Bonanomi et al., 2006). However, due to the challenging nature of EM immunolabeling, the micrographs of Piccolo-labeled neuronal cultures were of limited resolution (Zhai et al., 2001).
The aim of the present study was to identify the specific cargo carriers for Bassoon and Piccolo in developing axons by EM immunolabeling. Different antibodies were tested with different fixation conditions in order to improve structural preservation and signal levels. One particular type of vesicle aggregate, rather than individual DCV, was identified to be the carrier of Bassoon and Piccolo. Measurements of labeling intensities of aggregates were compared to those from AZ of immature synapses to see if one aggregate is sufficient to form an AZ. Since these aggregates also contain SV-like vesicles, three SV membrane proteins (VAMP, SV2 and synaptotagmin) and one SV-associated protein (synapsin I; reviewed in Bonanomi et al., 2006) were also selected for EM immunolabeling analysis. The Bassoon/Piccolo transport aggregates identified here are smaller than the GFP-VAMP packet (Ahmari et al., 2000), but more complex than the PTV (Zhai et al., 2001). These aggregates that contain various AZ and SV proteins may represent a preassembled transport unit that is sufficient to form a stable presynaptic active zone.
EXPERIMENTAL PROCEDURES
Antibodies
Mouse monoclonal antibody (mAb) against Bassoon (1:100, clone SAP7F407) and synaptotagmin (p65, 1:250, clone ASV30) were from Stressgen (Victoria, BC, Canada). Rabbit polyclonal antibody (pAb) against Piccolo (1:400) and mouse mAb against synapsin I (1:250, clone 46.1) were from Synaptic Systems (Gottingen, Germany). Guinea pig polyclonal antibody against Piccolo (1:100) was a gift from Dr. Eckart Gundelfinger (Leibniz Institute for Neurobiology, Magdeburg, Germany). Mouse mAb against VAMP (1:100, clone SP10) and SNAP-25 (1:250, clone SP14) were from Chemicon (Temecula, CA). Mouse mAb against SV2 (1:500, clone 10H3) was a gift from Dr. Erik S. Schweitzer (UCLA, Los Angeles, CA). Rabbit pAb against chromogranin A (CGA, 1:250) was a gift from Dr. Lee Eiden (NIMH, Bethesda, MD). A summary of labeling patterns for each antibody is listed in supplementary materials.
Disassociated hippocampal cultures
The animal protocol used in this study was approved by the NIH Animal Use and Care Committee and conforms to NIH guidelines. Hippocampal cells from 21-day embryonic Sprague-Dawley rats were dissociated and grown either on top of a feeder layer of glial cells (for details, see Lu et al., 1998) or without the feeder glial cells for 3-11 days. No difference in labeling pattern was observed between the two types of cultures for any of the antibodies. Cultures older than 7 days already had numerous mature looking synapses. Thus, most of the sampling of immature synapses and developing axons are from 3-6 day old cultures. Samples of 3 wk old cell cultures from a previous study (Tao-Cheng, 2006) were also examined for comparison.
Fixation and Pre-embedding immunocytochemistry
Cells were fixed with different fixatives for optimal immunolabeling for different antibodies (assessment of optimal fixation conditions for each antibody is listed in supplemental materials): (1) 4% paraformaldehyde in phosphate buffered saline (PBS) for 45-60 min for all antibodies, (2) 4% paraformaldehyde and 0.02-0.1% glutaraldehyde for 30-60 min for the SV2 antibody, and for 30-45 min for the guinea pig Piccolo antibody, (3) 2% acrolein in PBS for 1 min followed by 4% paraformaldehyde in PBS for 30-60 min for the guinea pig Piccolo and the SV2 antibodies, and (4) 4% glutaraldehyde in 0.1 M sodium cacodylate buffer for 1 hr for samples without immonolabeling. Fixed cells were washed and permeabilized/blocked with 0.1% saponin/5% normal goat serum in PBS for 1 hr, incubated with primary antibody for 1-2 hr, incubated with secondary antibody conjugated to 1.4 nm gold particles (1:250, Nanogold from Nanoprobes, Yaphand, NY) for 1 hr, silver enhanced (HQ silver enhancement kit,Nanoprobes) for 10-15 min, treated with 0.2% OsO4 in phosphate buffer for 30 min, followed by 0.25% uranyl acetate at 4°C overnight, dehydrated in ethanol and embedded in epoxy resin (Tanner et al., 1996). Controls for specificity of immunolabeling include omitting the primary antibody and using the different primary antibodies as controls for each other.
Morphometry
Sampling
Every encountered synapse, labeled vesicle aggregates, and individual DCV within randomly selected grid openings was photographed at 40,000 X on the microscope with a CCD digital camera system (XR-100 from AMT, Danvers, MA) and measured at a final magnification of 150,000X. Synapses are characterized by at least two of the following three features: the uniform synaptic gap between the apposed pre-and post-synaptic membranes, the postsynaptic density (PSD), and the presence of clustered SV. Labeled vesicle aggregates are characterized by a mixture of vesicles closely clustered and are associated with more than two labeling grains.
Synaptic AZ measurement
Length of AZ was measured by tracing the presynaptic membrane opposite the PSD. An area at the AZ, 67 nm from the presynaptic membrane (cf. Tao-Cheng, 2006), was shown to preferentially contain ~80% and 55% of Bassoon and Piccolo (the rabbit antibody) labeling, respectively. This area was marked to measure all the AZ-associated labeling grains.
Measurements of transport vesicle aggregates
Each labeled vesicle aggregate was traced with a pen to mark the border defined by the outer edge of the associated vesicles. The aggregates are typically irregular in shape, and their size was expressed as two linear dimensions: the length of the marked area along its long axis, and a second measurement perpendicular to the first line at mid point. Within each marked aggregate, the number of DCV, clear vesicles and labeling grains were counted. The mean diameter of DCV and small clear vesicles was measured as the average of two measurements – the maximum diameter and a second measurement taken at mid point of the first.
Statistical analysis (KaleidaGraph by Synergy Software) was carried out by Student’s t test (2-sided, unpaired data with unequal variance) with confidence level set at P <0.01 unless otherwise indicated.
RESULTS
Bassoon localization at AZ and vesicle aggregates
Among all of the antibodies used here, Bassoon had the most consistent and selective localization at the AZ of immature synapses (double arrow in Fig. 1A, enlarged in 1B) and at a particular type of vesicle aggregate (arrow in Fig. 1A, enlarged in 1C). Thus, Bassoon labeling offered an exceptional opportunity to examine developing synapses in these young (3-6 days in vitro) neuronal cultures, and to unequivocally characterize this novel vesicle aggregate.
Fig. 1.
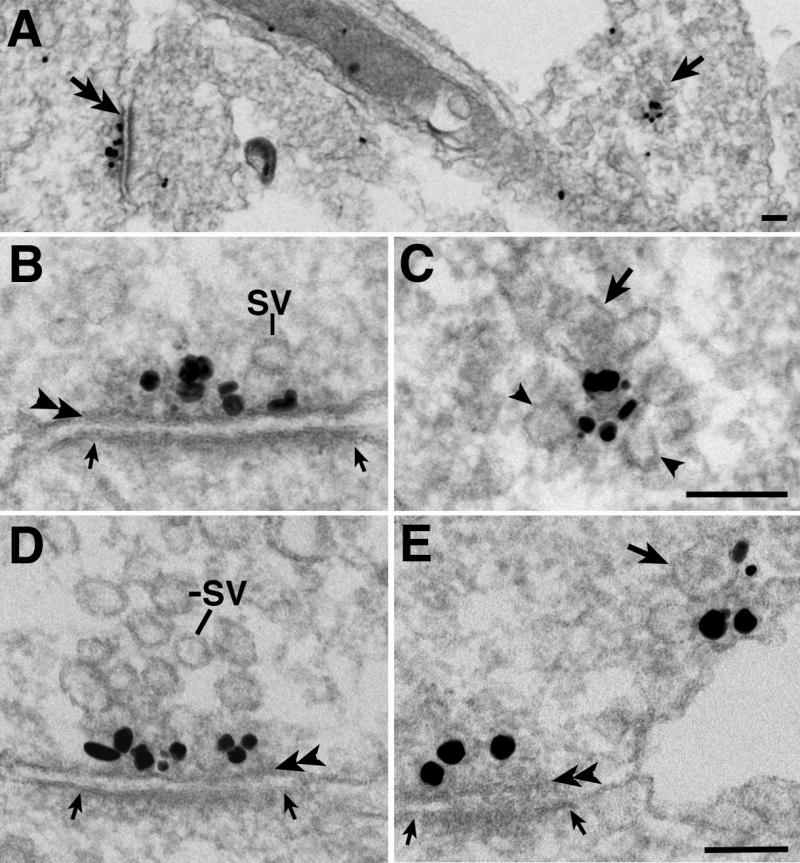
Immunogold labeling of Bassoon, an active zone (AZ) cytomatrix protein, in 4 day old dissociated hippocampal neuronal cultures. Black dots represent silver enhanced grains. Immature synapses (double arrow on the left in A, enlarged in B with a 90° rotation) have few synaptic vesicles (SV), but were reliably identified by the synaptic gap, a uniform apposition ~ 20 nm wide between the presynaptic membrane (double arrowheads in B, D, E) and the postsynaptic density (demarcated by two small arrows in B, E, D). Bassoon also labeled a second structure: a vesicle aggregate (arrow on the right in A, enlarged in C) with one dense core vesicle (DCV, arrow in C), several SV-like small clear vesicles (arrowheads in C) and Bassoon labeling concentrated in the center. (D) A more mature looking synapse with many more SV. (E) A labeled vesicle aggregate (arrow at upper right points to the DCV) is near an immature synapse at lower left. Scale bar = 0.1 μm (B and C share the same bar, D and E share the same bar).
Bassoon labeling at immature synapses was concentrated within 70 nm from the presynaptic membrane (double arrowheads in Fig. 1B, D, E), a pattern similar to mature synapses (cf. Tao-Cheng, 2006). Some of the young synapses at 4 days in vitro already have many SVs (Fig. 1D), and in synapses where SVs were scarce (Fig. 1B, E) it was apparent that Bassoon signals were associated with dense material at the AZ. Regardless of the amount of SV present, in 3-4 day old samples, only 17% (14/84) of the synapses had any DCV within 200 nm of the AZ. The frequency of seeing DCV within 200 nm of the AZ decreased to 3% (1 out of 35 synapses) in 6-8 day old cultures.
The second type of Bassoon-labeled entities is aggregates of heterogeneous vesicles (arrow in 1A and enlarged in Fig. 1C). In single thin sections, these aggregates typically consisted of a mixture of 1-2 DCV (arrow in Fig. 1C) located at the edge of the aggregate, and 5-6 smaller clear vesicles (arrowheads in Fig. 1C), with labeling (black grains in Fig. 1C) concentrated toward the center of the aggregates where dense material was located. Although the number of DCV per aggregate is low, the frequency of seeing at least one DCV is ~4 times higher [69% (68 out of 99 aggregates)] in aggregates than at AZ of synapses in 3-4 day old cultures.
Identification of a Bassoon transport aggregate
Serial thin sections were analyzed to determine if these vesicle aggregates are part of synaptic AZ sectioned en face. Figs. 2A1-A4 show serial sections through a synapse where the AZ was cross-sectioned. Bassoon labeling was consistently within 70 nm of the presynaptic membrane (double arrowheads in Fig. 2A3). These serial sections also demonstrate that Bassoon labeled material at the AZ is flat in shape, consistent with the finding that the AZ cytomatrix material was disc-like (cf. Phillips et al., 2001). Thus, due to the close association of the labeling with the presynaptic membrane (within 70 nm), if a Bassoon-labeled structure is an AZ sectioned en face, the labeling would not have lasted more than two consecutive thin sections ~80-100 nm in thickness. In contrast, Fig. 2B shows a low magnification view of a Bassoon-labeled vesicle aggregate (enlarged in Fig 2B1) which is situated more than 100 nm away from the plasma membrane (arrowhead in Fig. 2B). Figs. 2B1-B3 show three consecutive thin sections of this aggregate without catching any synaptic specializations. Thus, this vesicle aggregate cannot be an en face sectioned synapse, and these serial sections confirmed that Bassoon-labeled vesicle aggregates and AZ are distinctly different from each other in their distance to the plasma membrane as well as in their composition and shape. Furthermore, en face sections of the AZ are rare occurrences, thus, the chance of misidentifying an AZ to be a vesicle aggregates is very low even in single sections.
Fig. 2.

Serial thin sections of a Bassoon-labeled synapse (A1-A4) and a vesicle aggregate (B1-B3). AZ is demarcated by two arrows in A1-A3. Arrowheads in B point to plasma membrane. Number of grains per AZ or aggregate in each section is marked on the upper right corner of each panel. No DCV was observed in this synapse and one distinctive DCV (arrow in B1) is seen in this aggregate. ER – endoplasmic reticulum. Scale bar = 0.1 μm (all panels share the same bar in B3 except for B).
Analysis of serial sections also demonstrated that all of such Bassoon-labeled aggregates were located in axons and not in dendrites. Thus, Bassoon is selectively sorted into axons at an early stage, consistent with LM findings (Friedman et al., 2000). Even though some labeled-aggregates were found to be near axon-dendrite contact points, many aggregates were in axons that are not in contact with any nearby dendrites, and therefore, not part of any synapses. One such serial is shown in Supplemental Fig. 1A1-A4. This finding is consistent with previous live LM observations that many mobile GFP-Bassoon punctas are non-synaptic (Shapira et al., 2003). Thus, these vesicle aggregates most likely represent cargo carriers in transit between soma and axon terminals.
Bassoon-labeled vesicle aggregates, termed as “Bassoon transport aggregates” in this study, were much more readily seen in young cultures than in 3 wk old samples. For example, 99 labeled aggregates were identified in 3-4 day old cultures from 4 experiments, while extensive examination in 3 wk old cultures from 5 experiments did not yield any such aggregates. The ratio of encountered synapses to aggregates increased as the cultures matured: it was 0.85 (84 synapses/99 aggregates) at 3-4 day (pooled from 4 experiments), and 1.52 (41synapses/27 aggregates) at 6-8 day (pooled from 2 experiments). These results suggest that proportionally fewer aggregates are present as synaptogenesis progresses.
Piccolo is co-localized with Bassoon in transport aggregates
Piccolo labeling was also distinctive at the AZ of immature synapses (Fig. 3A, C) and at vesicle aggregates (Fig. 3 B, D) that resembled the Bassoon transport packet. This finding is not surprising since previous LM studies have shown co-localization of these two proteins at both synaptic and non-synaptic locations (Zhai et al., 2001; Shapira et al., 2003).
Fig. 3.

AZ of immature synapses (A, C) and vesicle aggregates (B, D) are specifically labeled by two different Piccolo antibodies (a rabbit antibody in A, B, and a guinea pig antibody in C, D). (C) Double arrowhead marks the presynaptic membrane. (D) One DCV (arrow), and two SV-like clear vesicles (arrowheads) are visible in this aggregate.
The two different antibodies against Piccolo used here showed similar, but not identical labeling patterns at the AZ. The rabbit antibody labeling (Fig. 3A) was very close to the presynaptic membrane, and the guinea pig antibody labeling (Fig. 3C) was further away from the membrane, but still approximately within 70 nm of the presynaptic membrane. Due to the extraordinary large size of the Piccolo protein (Cases-Langhoff et al., 1996), it is not surprising that two different epitope sites could be situated ~30 nm apart. On the other hand, the two antibodies labeled the vesicle aggregates in a very similar pattern (Fig. 3B, D). One Piccolo-labeled vesicle aggregate in serial sections is shown in supplemental Fig. 1B1-B4.
Measurements from both Bassoon-and Piccolo-labeled aggregates confirmed the similarity in size and in the number of different vesicles in these aggregates (Table 1). Thus, the aggregates appear to be the same entity and they most likely transport Bassoon and Piccolo together.
Table 1.
Mean dimensions and number of vesicles and grains of Bassoon-or Piccolo-labeled transport aggregates in young axons 3-6 day old* measured from single sections.
| proteins | Mean# dimensions of aggregates (μm) | Number of DCV | Number of clear vesicles | Number of grains |
|---|---|---|---|---|
| Bassoon (47 aggregates from 2 experiments**) | 0.217 ± 0.007 by 0.128 ± 0.003 | 1.4 ± 0.2 | 4.9 ± 0.3 | 7.0 ± 0.4 |
| Piccolo (rabbit antibody, 21 aggregates from 3 experiments) | 0.216± 0.010 by 0.129 ± 0.005 | 1.2 ± 0.2 | 5.6 ± 0.5 | 8.2 ± 0.9 |
| P value | 0.97, 0.89 | 0.48 | 0.23 | N. A.ˆ |
Quantitative measurements were taken from 3-6 day old cultures because aggregates were relatively scarce in older cultures and tend to have more SV-like vesicles (cf. Supplemental Fig. 1A1-A4) than those in younger cultures.
Mean ± SEM
Measurements from different experiments were only pooled when the labeling intensities were similar.
Labeling intensities were not compared among different antibodies.
Size measurements and labeling counts for Bassoon/Piccolo transport aggregates are comparable to those for AZ
In 3-6 day old cultures, the aggregates were ~0.22 by 0.13 μm in average dimensions (Table 1, ranged from 0.13 - 0.30 by 0.09 - 0.20 μm), whereas the average length of AZ was 0.26 ± 0.01 μm (n= 74 synapses from 8 experiments, ranged from 0.12 – 0.47 μm). Although a direct size comparison for these two entities is difficult due to their different shape and composition, their size dimensions are within range of each other. As expected, the size of AZ increased as the synapses matured. The average length of AZ significantly increased at 3 weeks (0.35 ± 0.01 μm, n=154 from 4 experiments, ranged from 0.15 – 0.84 μm, P<0.0001). This age-related increase represents a 1.8 fold difference in average area of the AZ, and could come from addition of transport aggregates to existing AZ over time. Interestingly, Bassoon-labeled aggregates (Fig. 1E, upper right) were occasionally seen near the AZ of immature synapses (Fig. 1E, lower left), consistent with the possibility that these aggregates are in transit to the synapse.
Furthermore, the average number of grains in the aggregates in single thin sections was also within range of that of the immature AZ. For the Bassoon antibody, there were 7.0 ± 0.4 grains per aggregate (Table 1) and 7.9 ± 0.7 grains per AZ (n=36 synapses from 4 experiments, P > 0.25). For the Piccolo antibody, there were 8.2 ± 0.9 grains per aggregate (Table 1) and 6.5 ± 0.4 grains per AZ (n=38 synapses from 4 experiments, P ~ 0.1). The total number of grains combined from serial sections was also compared between aggregates and AZ. For the Bassoon antibody, the totals are 11, 13, 14, 15, 19, and 29 grains (Figs. 2B1-B3) in six serially sectioned vesicle aggregates (averaged at 16.8 ± 2.7 grains); and 18, 19, 25, 30, 43, and 44 grains (Figs. 2A1-A4) in six serially sectioned AZ (averaged at 29.8 ± 4.7 grains). For the Piccolo antibody, the totals are 10, 17 and 46 grains (supplemental Fig 1B1-B4) in three serially sectioned aggregates, and 20 and 29 grains in two serially sectioned AZ. The variable range of the total number of grains in Bassoon-labeled aggregates suggests that transport aggregates carry variable amounts of Bassoon. This variation in amount of Bassoon per aggregate is also consistent with the observation that mobile GFP-Bassoon punctas are variable in size and can split or coalesce (Shapira et al., 2003). Most importantly, these numbers are compatible with the possibility that one or a few transport aggregates can bring a sufficient amount of Bassoon/Piccolo to form an AZ, and that the size of AZ can increase by addition of new aggregates.
SV proteins are also localized in the vesicle aggregates but to different components than Bassoon and Piccolo
The appearance and the uniform size (45 ± 1 nm in average diameter, n=53) of the small clear vesicles in these aggregates resembled those of synaptic vesicles (SV). Indeed, vesicle aggregates also labeled for three SV integral membrane proteins: VAMP, SV2 and p65 (Fig. 4F, G, H), and one SV-associated protein, synapsin I (Fig. 4I).
Fig. 4.
Labeling of AZ and SV proteins in vesicle aggregates. Bassoon (A, B) and Piccolo (C, D) signals are typically clustered in the center of the aggregates surrounded by vesicles. (B, C) -Arrows point to DCVs that are not labeled. (E) - An aggregate from a sample fixed with 4% glutaraldehyde without any immuno-labeling. Arrow in E indicates dense material among the clustered vesicles, and arrowheads point to filamentous material among vesicles. VAMP (F), SV2 (G) and synaptotagmin (p65, H) labeling are mostly associated with SV-like vesicles and not confined to the center of the aggregate where dense material is present (arrowhead in G). Synapsin I labeling is associated with the vesicles (I). SNAP-25 labeling is not detected in aggregates (J). Scale bar = 0.1 μm.
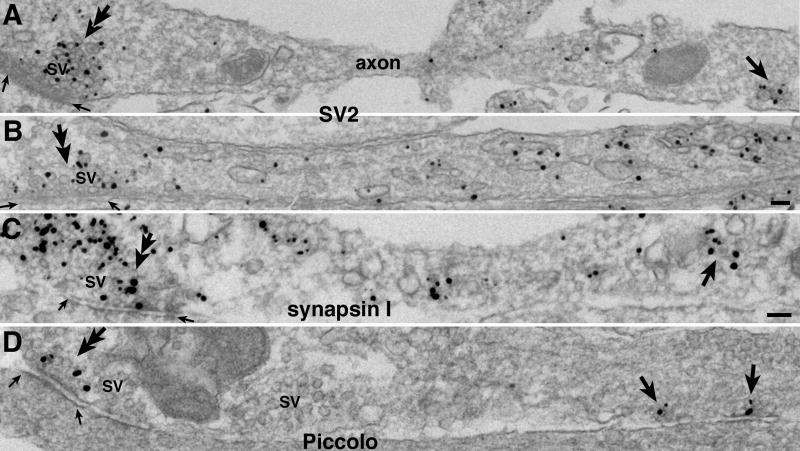
A comparison of different antibody labeling patterns showed that labeling for Bassoon (Fig. 4A, B) and Piccolo (Fig. 4C, D) were typically in the center of the aggregate surrounded by vesicles. The clustered grains often obscured the presence of dense material among the vesicles. Fig. 4E shows an aggregate fixed with glutaraldehyde and with no immunolabeling. A distinct patch of dense material was present near the center of the aggregate (arrow in Fig. 4E), and filamentous structures also appeared to connect the vesicles together (arrowheads in 4E). In contrast, VAMP (Fig. 4F), SV2 (Fig. 4G, a low magnification view of this aggregate is shown in Fig. 5A, arrow at right) and p65 (Fig. 4H) labeling did not concentrate in the center of the aggregate, but was associated with SV-like vesicles. SV2 and p65 also labeled DCV as expected (cf Lowe et al., 1988). Synapsin I labeling (Fig. 4I, a low magnification view of this aggregate is shown in Fig. 5C, arrow at right) was associated with the vesicles, consistent with its role in tethering SV together (reviewed in Hilfiker et al., 1999).
Fig. 5.
The labeling patterns of SV2 (A, B), synapsin I (C) and Piccolo (D, the guinea pig antibody) are very different in young axons. Long segments of axons are shown in these fortuitous sections. In each panel, double arrow on left points to synapses with cluster of synaptic vesicles (SV), and two small arrows mark the area of the synaptic junction. The numerous SVs at the synapses are intensely labeled for SV2 and synapsin I, but not for Piccolo. Arrows on right point to positively labeled transport aggregates in A (enlarged in Fig. 4G) and C (enlarged in Fig. 4I). Arrows in D may be labeling immature AZ or two transport aggregates. Scale bars = 0.1 μm (A and B share the same bar, C and D share the same bar).
No labeled vesicle aggregates were seen in control samples where no primary antibody was used. These control samples were fixed under various conditions used here and extensively searched to verify the specificity of the immunolabeling of each antibody. Furthermore, two antibodies served as additional controls for specificity of the vesicle aggregate labeling. SNAP-25 (a member of the SNARE complex, and a mouse antibody) labeling was distinctive on plasma membranes of the axon (doublearrows in supplemental Fig. 2, cf. Tao-Cheng et al., 2000) but was not detected on vesicle aggregates (Fig. 4J). Chromogranin A (CGA, a DCV marker, and a rabbit antibody) labeling was specifically localized inside the DCV (arrows in supplemental Fig. 3) and not at other vesicles in the aggregates, a pattern very different from those of the various AZ (Fig. 4A-D) and SV (Fig. 4F-I) proteins. Thus, all of these controls demonstrated that labeling of the transport aggregates are indeed specific and not random associations caused by particular fixation conditions or the secondary antibodies or other immunolabeling reagents used here. The present finding illustrates that structures resembling Bassoon transport aggregates also carry SV proteins, and that both AZ and SV components are in close association in these aggregates.
Overall distribution of SV proteins in young axons is very different from that of Bassoon and Piccolo
It should be noted that in addition to labeling SVs at synapses (Fig. 5A, B, double arrows at left) and the SV-like clear vesicles in the aggregates (cf. Fig. 4F, G, H), the SV membrane proteins also labeled many other structures in young axons, including numerous SV-like and/or pleiomorphic vesicles and tubulo-vesicular structures. An example of SV2 labeling of such an axon is shown in Fig. 5B. How various SV proteins are transported from soma to synapses has been reviewed in detail (Bonanomi et al., 2006), and their specific cargo carriers will be further examined and reported in a subsequent study. Nevertheless, Fig. 5 shows that the bulk of the SV proteins are not carried by Bassoon/Piccolo transport aggregates. In Piccolo-labeled axon (Fig. 5D), only the AZ (double arrow at left) and two other structures (arrows at right) are labeled. This Piccolo labeling pattern is similar to that of Bassoon (Fig. 1A) but vastly different form those of SV2 (Fig. 5 A, B) and synapsin I (Fig. 5C) where the great majority of the grains are localized to SVs and other structures.
Are DCV specifically involved in Bassoon/Piccolo transport?
The consistent presence of DCV in Bassoon/Piccolo transport aggregates is intriguing. DCVs have been termed as PTVs (Piccolo/Bassoon transport vesicles, Zhai et al., 2001), and it has been proposed that the fusion of two to three PTV with the axolemma can form an AZ (Shapira et al., 2003). However, DCVs (cf. arrows in Fig. 4B, C) in Bassoon/Piccolo transport aggregates often did not appear to contain enough grains to account for the labeling seen at the AZ. These DCVs in aggregates had an average diameter of ~70 nm (70 ± 1 nm, n=104), which is within range of that reported for PTV (~80 nm, Zhai et al., 2001), and are likely the same entity as PTVs.
DCVs were more readily detected in young than 3 wk old cultures. While DCVs were typically not found at the AZ of mature synapses, in young cultures on rare occasions, several DCVs were seen at contact points between axon and dendrite (Fig. 6A, B), sites that may represent developing AZ. However, the majority of grains were preferentially associated with dense material among the vesicles and not with DCV itself (arrows in 6A, B).
Fig. 6.
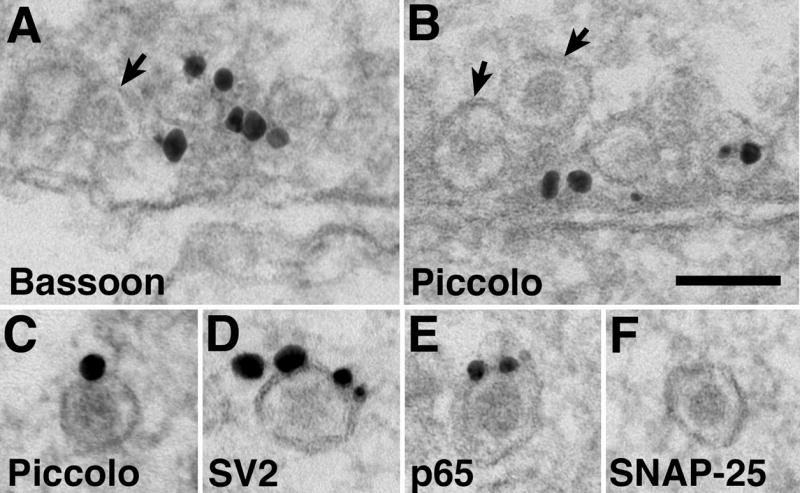
Bassoon (A) and Piccolo (B) labeling at contact points between axon (upper processes in A and B) and dendrite (lower processes in A and B). Grains are preferentially associated with dense material, but not specifically with DCV (arrows). In the lower panels, individual DCV are labeled to different degrees for Piccolo (C), SV2 (D), synaptotagmin (p65, E), but not for SNAP-25 (F). Scale bar = 0.1 μm.
In order to quantify the relative amounts of the proteins associated with each DCV, individual DCVs not in close association with other vesicles were scored for labeling of Bassoon and Piccolo. SV2 and p65, two SV proteins that are also present on DCV membranes (Lowe et al., 1988) were scored as well for comparison. Only a very low percentage of individual DCV was labeled for Bassoon (5%, 2/40, from 7 experiments) and for Piccolo (17%, 12/72, from 6 experiments). In contrast, the percentages were higher for SV2 (88%, 21/24, from 5 experiments) and p65 (56%, 15/27, from 5 experiments). Also, the labeling intensities were very low for Bassoon and Piccolo (Fig. 6C), typically with only one grain associated when detected, whereas the labeling intensities were much higher for SV2 (3.2 ± 0.4 grains/labeled DCV, Fig. 6D) and p65 (1.6 ± 0.3 grains/labeled DCV, Fig. 6E). SNAP-25, which was purported to be associated with PTV (Zhai et al., 2001; Shapira et al., 2003), did not label any DCV (0/46 from 3 experiments, Fig. 6F, Supplemental Fig. 2A insert). These results indicate that individual DCVs are not the major carrier of Bassoon/Piccolo, and by itself is not likely to form an AZ.
How are Bassoon and Piccolo packaged into the transport aggregates?
Examination of the neuronal soma could provide additional information on Bassoon and Piccolo’s association with the transport aggregates. The Golgi complex was diffusely labeled for both Bassoon (Fig. 7A) and Piccolo (Fig. 7B), consistent with membrane association of these proteins in young brains (Sanmarti-Vila et al., 2000; Zhai et al., 2001) and these proteins going through the Golgi complex (Dresbach et al., 2006). Serial thin section analysis confirmed that grains at the Golgi complex were dispersed and not clustered as in transport aggregates. This lack of clustering of labeling at the Golgi complex is not due to poor penetration of immunolabeling reagents, as intense labeling of chromogranin A (Fig. 7C) and SV2 (data not shown) were readily detected at DCV in the Golgi complex. Interestingly, Bassoon and Piccolo-labeled structures similar to transport aggregates were occasionally seen in the neuronal soma, at times near the Golgi complex (Fig. 7B, enlarged in 7D), but never in the Golgi complex itself. Thus, Bassoon and Piccolo may be carried at low levels by various clear vesicles and/or DCV, and only become aggregated after they have left the Golgi complex. These vesicles coalesce to form the aggregates, with Bassoon and Piccolo located toward the center as part of the dense material, and DCV at the edge of the aggregate.
Fig. 7.
Bassoon (A) and Piccolo (B) labeling at the Golgi complex are diffuse compared to that of Chromogranin A (C, arrows indicate labeled DCV). A Piccolo labeled aggregate (arrow in B, enlarged in D) is near but not at the Golgi complex (star). Scale bar = 0.1 μm. A, B share the same bar as in C.
DISCUSSION
In young axons, Bassoon and Piccolo, two active zone (AZ) cytomatrix proteins, are transported in multi-vesicle aggregates containing dense material surrounded by a mixture of dense core vesicles (DCV) and SV-like small clear vesicles. The size and labeling intensities of these aggregates are within the same range as immature synaptic AZ. Quantitative measurements suggest that one or a few aggregates, but not DCV by itself, can provide sufficient Bassoon/Piccolo to form an AZ. Although DCVs are not likely to be the precursor vesicle for AZ formation, their constant presence and peripheral location in these aggregates do suggest a DCV involvement in the formation and/or transportation of these aggregates during early stages of development. However, the role of DCV in aggregate formation and transportation awaits further studies to identify their contents and functions.
The present finding that Bassoon and Piccolo are carried by vesicle aggregates rather than individual DCV disagrees with the PTV hypothesis (Zhai et al., 2001) that the 80 nm DCVs (termed as PTV in that study) are the vehicles that carry a comprehensive set of AZ materials. This discrepancy could be explained by differences in EM fixation and immunolabeling procedures resulting in different degrees of sample preservation and labeling intensities, which in turn, led to different interpretations. One of the reasons why this particular vesicle aggregate escaped detection by conventional EM until now may be because these aggregates were only prevalent in very young (3-6 days) axons and most EM reports on older cultures would have missed them. A second reason may be due to the fact that the individual components of the aggregates, DCV and SV-like vesicles, are common throughout young axons. Thus, the resulting aggregates would hardly be detected as a novel entity without the distinctive immunolabeling of Bassoon or Piccolo. However, EM immunolabeling technique is handicapped by the limited choices of fixatives that preserve the ultrastructure as well as the antigenicity of the sample. The Bassoon antibody and the rabbit Piccolo antibody used here were not compatible with fixatives that contained glutaraldehyde, even for a concentration as low as 0.02%. These antibodies also did not give high intensity of signals in samples that were fixed for more than a few hours of 4% paraformaldehyde. Under these restricted fixation conditions, structural preservation is understandably compromised, and DCVs are more readily detectible than SV-like vesicles due to their larger size and the distinctive presence of their proteinaceus dense core. Thus, an aggregate of vesicles may be perceived as individual DCVs because the SV-like vesicles did not stand out.
A second discrepancy between the present finding and the PTV hypothesis is that, here, DCV is devoid of SNAP-25 labeling, but PTV fraction is reported to contain SNAP-25 (Zhai et al., 2001; Shapira et al., 2003). However, in immunolabeled hippocampal neuronal cultures, brain tissues or PC12 cells, SNAP-25 was specifically localized on the plasma membranes of the axons (Garcia et al., 1996) and at the Golgi complex, but not on DCV (Tao-Cheng et al., 2000). Thus, the presence of SNAP-25 in the PTV fraction may represent contamination of other membranous materials in the PTV fraction. Nevertheless, the present report is in close agreement with many of the observations on the mobile GFP-Bassoon punctas (Shapira et al., 2003), especially in the estimated number of Bassoon/Piccolo transport vehicles required to form an AZ. By EM serial section analysis, the present report estimates that one or a few transport aggregates are sufficient to form an immature AZ; whereas by quantitative measurements of LM immunolabeling, Shapira et al (2003) calculated that AZs are assembled from 2-3 PTVs.
The finding that the SV-like vesicles in Bassoon/Piccolo transport aggregates label for SV proteins raises the question about their similarity to SV both in structure and in function. It has been shown that SV proteins go through exo-endocytotic recycling along the axons during development and that SV-like vesicles are present in young axons prior to synaptogenesis (Matteoli et al., 1992; Kraszeqski et al., 1995). Thus it is possible that the SV-like vesicles in the present Bassoon/Piccolo transport aggregates are also recycling-competent. However, they are often distant (greater than 100 nm) from the plasma membrane, and thus, unlikely to be undergoing ready or frequent recycling with the plasma membrane as are in the case of the SVs at synapses. This speculation is consistent with the report that mobile GFP-Bassoon punctas in young axons do not take up FM dyes until they become immobilized (Shapira et al., 2003). Interestingly, in a study of older neuronal cultures (10-21 day old), among the “orphan” Bassoon punctas that were not apposed to dendrites, about two third took up FM dyes and only about a third did not (Krueger et al., 2003). Thus, as the cultures grow older, more non-synpatic Bassoon punctas become recycling-efficient even without a synaptic partner. This is consistent with the present observation that in older cultures, the Bassoon transport aggregates appear to have more SV-like vesicles. Since older cultures have many more synapses and SV-like vesicles, and fewer DCV, it is more difficult to unequivocally identify transport aggregates from synaptic AZ without serial section analysis in older samples. Thus, measurements of vesicle aggregates in the present study are focused on samples form 3-6 day old cultures where chances of misidentifying immature AZs for transport aggregates are very low.
It should be noted that Bassoon/Piccolo transport aggregates only carry a very small portion of the SV proteins, and that the bulk of SV proteins are transported by the numerous pleiomorphic or SV-like vesicles (Matteoli et al., 1992; Kraszeqski et al., 1995) and tubulo-vesicular structures (Nakata et al., 1998) in developing axons. These observations are consistent with the retrospective EM analysis that mobile GFP-VAMP punctas (Ahmari et al., 2000) contain many pleiomorphic and tubulo-vesicular structures. Thus, based on ultrastructural analysis of their components, mobile GFP-VAMP packet, a SV protein carrier, is different from the Bassoon transport aggregate presented here. Furthermore, the two entities are vastly different in size (~1 μm for GFP-VAMP packet vs. ~0.22 by 0.13 μm for Bassoon/Piccolo transport aggregate). However, since co-localization of Piccolo and p65 or VAMP was sometimes seen (Zhai et al., 2001), it is possible that some Bassoon/Piccolo transport aggregates may be intermingled with the bulk SV carriers as a sub-structure of the larger packet.
The present study is the first ultrastructural identification of a Bassoon/Piccolo transport aggregate that also carries many SV proteins. These aggregates may represent a pre-assembled unit that has the potential to quickly form a new functional active zone. It is possible that these aggregate are the first entity to become immobilized at certain predefined sites along the axons to form presynaptic terminals (Sabo et al., 2006). The exocytosis of these few DCV and SV-like clear vesicles may leave the Bassoon/Piccolo-containing dense material behind, stabilizing these sites that may anchor for the bulk SV cargo carriers to follow and develop into a mature synapse.
Supplementary Material
Acknowledgments
I thank Christine A. Winters for hippocampal neuronal cultures, Virginia Crocker and Rita Azzam for expert EM technical support, Drs. Catherine Galbraith, Jim Galbraith and Ayse Dosemeci for critical reading of the manuscript; and Dr. Tom Reese for support and helpful discussions. Supported by the Intramural Research Program of the NIH, NINDS.
List of abbreviations
- AZ
active zone
- SV
synaptic vesicle
- DCV
dense core vesicle
- PTV
Piccolo/Bassoon transport vesicles
- p65
synaptotagmin
- CGA
chromogranin A
- LM
light microscopy
- GFP
green fluorescence protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Bonanomi D, Benfenati F, Valtorta F. Protein sorting in the synaptic vesicle life cycle. Prog Neurobiol. 2006;80:177–217. doi: 10.1016/j.pneurobio.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Cases-Langhoff C, Voss B, Garner AM, Appeltauer U, Takei K, Kindler S, Veh RW, De Camilli P, Gundelfinger ED, Garner CC. Piccolo, a novel 420 kDa protein associated with the presynaptic cytomatrix. Eur J Cell Biol. 1996;69:214–223. [PubMed] [Google Scholar]
- Dresbach T, Torres V, Wittenmayer N, Altrock WD, Zamorano P, Zuschratter W, Nawrotzki R, Ziv NE, Garner CC, Gundelfinger ED. Assembly of active zone precursor vesicles: Obligatory trafficking of presynaptic cytomatrix proteins bassoon and piccolo via a trans-golgi compartment. J Biol Chem. 2006;281:6038–6047. doi: 10.1074/jbc.M508784200. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Garcia EP, McPherson PS, Chilocote TJ, Takei K, De Camilli P. rbSec1A and B colocalize with synataxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszewski K, Mundigl O, Daniell L, Verderio C, Matteoli M, De Camilli P. Synaptic vesicle dynamics in living cultured hippocampal neurons visualized with CY3-conjugated antibodies directed against the lumenal domain of synaptotagmin. J Neurosci. 1995;15:4328–4342. doi: 10.1523/JNEUROSCI.15-06-04328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger SR, Kolar A, Fitzsimonds RM. The presynaptic release apparatus is functional in the absence of dendritic contact and highly mobile within isolated axons. Neuron. 2003;40:945–957. doi: 10.1016/s0896-6273(03)00729-3. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Takei K, Perin MS, Sudhof TC, De Camilli P. Exo-endocytotic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. J Cell Biol. 1992;117:849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli M, Coco S, Schenk U, Verderio C. Vesicle turnover in developing neurons: how to build a presynaptic terminal. Trends Cell Biol. 2004;14:133–140. doi: 10.1016/j.tcb.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Nakata T, Terada S, Hirokawa N. Visualization of the dynamics of synaptic vesicle and plasma membrane proteins in living axons. J Cell Biol. 1998;140:659–674. doi: 10.1083/jcb.140.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AW, Madeddu L, Kelly RB. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. J Cell Biology. 1988;106:51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, McLaren RS, Winters CA, Ralston E. Ribosome association contributes to restricting mRNAs to the cell body of hippocampal neurons. Mol Cell Neurosci. 1998;12:363–375. doi: 10.1006/mcne.1998.0723. [DOI] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Sabo SL, Gomes RA, McAllister AK. Formation of presynaptic terminals at predefined sites along axons. J Neurosci. 2006;26:10813–10825. doi: 10.1523/JNEUROSCI.2052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmarti-Vila L, tom Dieck S, Richter K, Altrock W, Zhang L, Volknandt W, Zimmermann H, Garner CC, Gundelfinger ED, Dresbach T. Membrane association of presynaptic cytomatrix protein bassoon. Biochem Biophys Res Commun. 2000;275:43–46. doi: 10.1006/bbrc.2000.3256. [DOI] [PubMed] [Google Scholar]
- Schoch S, Gundelfinger ED. Molecular organization of the presynaptic active zone. Cell Tissue Res. 2006;326:379–391. doi: 10.1007/s00441-006-0244-y. [DOI] [PubMed] [Google Scholar]
- Shapira M, Zhai RG, Dresbach T, Bresler T, Torres VI, Gundelfinger ED, Ziv NE, Garner CC. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- Tanner VA, Ploug T, Tao-Cheng JH. Subcellular localization of SV2 and other secretory vesicle components in PC12 cells by an efficient method of preembedding EM immunocytochemistry for cell cultures. J Histochem Cytochem. 1996;44:1481–1488. doi: 10.1177/44.12.8985140. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Du J, McBain CJ. SNAP-25 is polarized to axons and abundant throughout the axolemma : an immunogold study. J Neurocytol. 2000;29:67–77. doi: 10.1023/a:1007168231323. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH. Activity-related redistribution of presynaptic proteins at the active zone. Neuroscience. 2006;141:1217–1224. doi: 10.1016/j.neuroscience.2006.04.061. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active zone precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- Zhen M, Jin Y. Presynaptic terminal differentiation: transport and assembly. Curr Opin Neurobiol. 2004;14:280–287. doi: 10.1016/j.conb.2004.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.