Abstract
Background
The well-known immune deficiency of the chronic alcoholic dictates the need for a long-term rodent ethanol administration model to evaluate the baseline immunologic effects of chronic ethanol abuse, and investigate the genetic determinants of those effects. Much published work with rodents has shown clearly that acute ethanol administration and short-term ethanol-containing liquid diets both cause elevated corticosterone and can cause significant thymocyte, pre-B cell and peripheral lymphocyte losses. Such losses may mask more subtle alterations in immune homeostasis, and in any case are generally short-lived compared with the span of chronic ethanol abuse. Thus, it is important to have a model in which long-term immune alterations can be studied free of corticosteroid-induced cell losses.
Methods
We have utilized chronic 20% (w / v) ethanol in water administration to several mouse strains for prolonged periods of time and evaluated serum corticosterone, immunologic stress parameters, and other organ changes by standard methods.
Results
We now confirm earlier reports that chronic ethanol in water administration to mice does not produce net elevations of corticosterone, although diurnal variation is altered. Importantly, there is neither selective loss of immune cell populations known to be corticosteroid sensitive, CD4+CD8+ thymocytes and pre-B cells, nor are changes observed in the histologic appearance of the thymus. Nonetheless, there are significant chronic ethanol effects in other tissues, including reduced heart weight, mild hepatic steatosis, alterations of gut flora, increased serum peptidoglycan, and as published elsewhere, immune system abnormalities.
Conclusions
This model of ethanol administration is convenient, sustainable for up to 1 year, demonstrably feasible in several mouse strains, permits good weight gains in most strains, and results in significant changes in a number of organs. The administration method also will permit modeling of long-term steady abuse punctuated by major binges, and is suitable for supplementation studies using water soluble additives. Overall, the method is useful for a wide range of studies requiring a chronic low-stress method of ethanol administration.
Keywords: Ethanol, Corticosterone, Thymocytes, Pre-B Cells, Gut Flora
Rodent Models for Ethanol Administration
Rats, mice, and occasionally guinea pigs have been used to model various aspects of ethanol abuse. The protean effects of ethanol have encouraged substantial variations in modeling the effect of interest with respect to species and strains, and length or levels of ethanol exposure. Much of the early work was directed towards behavioral and dependency effects, and efforts were made to develop strains of rats or mice that displayed responses to ethanol that might be genetically analyzed (Collins et al., 1993). A second parameter of significance, the ethanol dosing protocol, has widely varied. Some of the main protocols used for ethanol exposure include: (i) an acute bolus given to a naïve animal by gavage (Forbes and Duncan, 1951) or by intraperitoneal injection (Ellis, 1966), (ii) complete liquid diets containing carefully adjusted nutritional components and ethanol with approximately 36% of calories from ethanol (DeCarli and Lieber, 1967), (iii) surgically implanted catheters to permit continuous or intermittent delivery of a desired level of ethanol directly to the stomach (Tsukamoto and French, 1993), and (iv) various concentrations of ethanol in water given chronically as the only water source (Abdallah et al., 1988; Blank et al., 1991; Kakihana et al., 1971; Kakihana and Moore, 1976; Sipp et al., 1993; Song et al., 2002; Sosa et al., 2005; Zhu et al., 2004). Each of these methods has advantages and disadvantages that are dependent on the rodent strain, the desired length and level of exposure, and the organ system under study.
Ethanol Administration and Adrenal Response
Models for the study of ethanol effects on immune system behavior must take into account, certain specific sensitivities of immune competent cells and their precursors. The majority of thymocytes (Blomgren and Andersson, 1969), identified as the CD4+CD8+ subset (Reichert et al., 1986), and certain other lymphocytes are glucocorticoid-sensitive. One of the major observations that arose early in ethanol research with rodent models was that various acute or short-term ethanol administration protocols caused adrenal activation and elevation of circulating corticosterone. Table 1 is a selection of articles listed by year of publication, in which rodents were administered ethanol and some subsequent measure was made of adrenal response, usually circulating corticosterone. It is clear that acute gavage or IP administration to naïve animals causes adrenal activation and elevated corticosterone (Carson and Pruett, 1996; Collier et al., 1998; Ellis, 1966; Forbes and Duncan, 1951; Guaza et al., 1983; Khisti et al., 2003; Li et al., 2005; Ogilvie et al., 1998; Patel and Pohorecky, 1988; Pruett et al., 2003b; Schwab et al., 2005; Spencer and McEwen, 1990). Accompanying this elevation is rapid loss of lymphocytes as either thymocytes (Collier et al., 1998; Santisteban, 1961), splenocytes (Pruett et al., 2003b) or both, shown to be as a result of the corticosterone elevation (Collier et al., 1998). Not all observed effects of ethanol in this model are corticosteroid-induced (Padgett et al., 2000; Pruett et al., 2003a), but the corticosterone-dependent cell losses described can be substantial. In some models, such as acute prenatal-ethanol exposure, measureable effects on the hypothalamic-pituitary-adrenal axis have been reported to be quite long-lasting (Park et al., 2004).
Table 1.
Selected Reports, by Year, of Adrenocortical and Immune Cell Responses to Ethanol in Rodents
| References | Species | Ethanol dosing | Responses |
|---|---|---|---|
| Forbes and Duncan, 1951 | Rat, guinea pig | Acute gavage | Adrenal activationa |
| Santisteban, 1961 | Mouse | Subcutaneous | Thymic involution |
| Ellis, 1966 | Rat | Acute IP | Pituitary-dependent corticosterone (cort) elevation |
| Kakihana et al., 1971 | Mouse | Chronic Et (10% v / v) | Reduced cort elevation after IP Et |
| Kakihana and Moore, 1976 | Mouse | Chronic Et (7.5% w / v) | Flattened diurnal cort variation |
| Tabakoff et al., 1978 | Mouse | 8-Days liquid diet | Weight loss, elevated cort |
| Guaza et al., 1983 | Rat | Acute IP / liquid diet | Elevated / less elevated cortb |
| Patel and Pohorecky, 1988 | Rat | Acute IP | Elevated cort |
| Spencer and McEwen, 1990 | Rat | Acute IP / chronic IP | Elevated / Less elevated cort |
| Jerrells et al., 1990 | Mouse | 8-Days liquid diet | Elevated cort, thymocyte, and splenocyte loss |
| Kruger and Jerrells, 1994 | Mouse | 7-Days liquid diet | Loss of bone marrow pre-B cells |
| Sipp et al., 1993 | Mouse | Chronic Et (20% w / v) | Flattened diurnal cort, no sustained increase |
| Carson and Pruett, 1996 | Mouse | Acute gavage | Dose-dependent elevated cort |
| Collier et al., 1998 | Mouse | Acute gavage | Splenocyte apoptosis, blocked by RU 486, reproduced by cort |
| Ogilvie et al., 1998 | Rat | Acute IP / liquid diet | Elevated ACTH and cort / reduced agonist-induced ACTH after liquid diet |
| Padgett et al., 2000 | Mouse | 7-Days liquid diet | Cort elevation, loss of thymocytes and splenocytes; partially prevented by ADX. Weight loss, both Et & pair-fed. |
| Pruett et al. (2003b) | Mouse | Acute gavage | Elevated cort, reduced thymocytes and splenocytes |
| Khisti et al., 2003 | Rat | Acute IP | Elevated plasma steroids and adrenal StAR after 60 minutes |
| Park et al., 2004 | Mouse | Acute prenatal | Adult cort increase after stress |
| Schwab et al., 2005 | Mouse | Acute gavage | Cort increase, blood PMN elevated, PBMC reduced |
| Li et al., 2005 | Rat | Acute gavage + burn | Cort elevated, T-cell activation reduced |
| Sosa et al., 2005 | Mouse | Liquid diet versus chronic Et (20%w / v) | Cort elevated, Fas-mediated liver apoptosis reduced, versus Fas-mediated apoptosis elevated |
ADX, adrenalectomy; StAR, steroidogenic acute regulatory protein; PMN, polymorphonuclear leukocytes.
Liquid diet means any 1 of several formulations of complete liquid diet with about 36% calories from ethanol and no other water source, administered for 7 days or longer. Chronic Et means ethanol in water as the only water source for several days to weeks, but with normal chow.
Measured indirectly.
Acute IP elevated cort/if liquid diet first, less elevated after IP Et.
In contrast to acute ethanol administration, persistent corti-costerone elevations were not seen with chronic low to moderate concentrations of ethanol in water (7.5–10%) as the only water source, but there were changes in the diurnal pattern of circulating corticosterone (Kakihana et al., 1971; Kakihana and Moore, 1976). Increasing the concentration of chronic ethanol in water to 20% (w/ v) also did not appear to produce net changes in corticosterone but produced a change in the diurnal pattern similar to the lower dosing (Sipp et al., 1993).
The use of complete liquid diets with up to 36% calories from ethanol, and a pair-feeding protocol has been problematic with mice. Not only has this protocol produced elevated corticosterone and thymocyte, splenocyte and bone marrow pre-B cell loss in these animals (Jerrells et al., 1990; Kruger and Jerrells, 1994; Padgett et al., 2000; Tabakoff et al., 1978), but also there may be significant weight loss in both the ethanol and pair-fed groups, and animal morbidity and mortality become substantial. Other measured effects of ethanol may also differ substantially in the liquid diet protocol as compared with the ethanol in water protocol. For example, a complete liquid ethanol diet in which corticosterone was shown to be elevated, surprisingly was shown to mediate protection against liver cell apoptosis induced by injection of anti-CD95 (Fas) monoclonal antibody. In contrast, mice provided with ethanol as chronic ethanol in water did show sensitization of the liver as demonstrated by an increased amount of Fas-mediated hepatocyte apoptosis compared with similarly treated control mice (Sosa et al., 2005).
Duration of Ethanol Exposure Required to Model Chronic Alcoholism
Increased infectious diseases (MacGregor and Louria, 1997) and immune abnormalities in alcoholics (Cook, 1998) typically occur after many years of alcohol abuse. The availability of numerous inbred, transgenic and knockout (KO) strains makes the mouse a very attractive animal for immune system modeling, but the relatively late appearance of clinically important immune abnormalities in the alcoholic suggests the protocol for ethanol administration must permit consistent exposure over a significant portion of the mouse life span. In the present work, we evaluated the suitability of ethanol in water plus normal chow as a long-term diet for studying chronic ethanol-induced changes in the mouse, and now report changes that reproduce in many ways the changes seen in human alcoholics, with no indication of net corticosterone elevation or glucocorticoid-sensitive cell loss.
MATERIALS AND METHODS
Animals
Female-specific pathogen-free (SPF) mice, aged 6 to 7 weeks, were purchased from the National Cancer Institute (Frederick, MD) or The Jackson Laboratory (Bar Harbor, ME) and maintained under an approved protocol in barrier facilities, in the Animal Care Unit at the University of Iowa, and in the animal facility at the University of Nebraska Medical Center. The lighting schedule was 12-hour on, 12-hour off, with light from 6:00 am to 6:00 pm. Mice were maintained in quarters with sentinel mice which were tested routinely for specific pathogens. On arrival, mice were placed in filter top units and acclimated for 1 week on a diet of standard pelleted rodent chow (Harlan-Teklad NIH-31 Modified Mouse/ Rat Diet-irradiated). After the 1-week acclimation period, animals from the same shipment lot (age-, strain-, supplier-, and shipment lot-matched mice are referred to as ‘‘matched’’) were separated randomly into 2 groups, control and ethanol. Mice in the ethanol group were given, as the only water source, 10% w/ v EtOH ad libitum (95% pharmaceutical grade EtOH diluted with 18 mOhm water) for 2 days, 15% w/ v EtOH for 5 days, and 15 to 20% w/ v EtOH (according to strain tolerance) for many weeks, similar to protocols described by others (Abdallah et al., 1988; Blank et al., 1991). Mice in the matched control group were given water from the same source without ethanol, and all fluids were checked and/ or replenished daily. All durations of ethanol exposure refer to the time spent on the final tolerated ethanol concentration. All study groups were fed rodent chow ad libitum throughout the study, delivered to the bedding. In most studies, mice were caged in groups of 4 females. The strains, common names or phenotypes, and suppliers of mouse strains evaluated on the diet to date are listed in Table 2.
Table 2.
Mouse Strains Administered Chronic Ethanol
| Mouse strain | Source, Stock No. (common name or phenotype) | Age EtOH started, weeks | Number of mice testeda | Maximum No. of weeks tested on EtOHb | Growth performancec |
|---|---|---|---|---|---|
| C57BL/6Jd | Jackson 000664 (BL / 6 wild type) | 7–10 | >150 | >78 | 3+ |
| C57BL/6NCrd | National Cancer Institute (BL/6 wild type) | 8–10 | >5000 | >78 | 3+ |
| B6.CB17-Prkdcscid/SzJd | Jackson 001913 (B6 SCID) | 8 | >200 | 9 | 2+ |
| B6.SJL-Ptprca Pepcb/BoyJd | Jackson 002014 (B6 CD45.1) | 8–10 | >300 | >52 | 3+ |
| B6.129S2-Cd40lgtm1Imx/Jd | Jackson 002770 (CD40L KOh) | 10 | >100 | >78 | 2+ |
| B6.129S2-Cd28tm1Mak/Jd | Jackson 002666 (CD28 KO) | 8 | 50 | 12 | 2+ |
| BALB/cAnNCre | National Cancer Institute (BALB / c) | 10–12 | >1500 | 32 | 2+ |
| C3H/HeJf,g | Jackson 000659 (TLR4 deletion) | 10–12 | 84 | 24 | 0–1+ |
| C3H/HeOuJf,g | Jackson 000635 (TLR4 competent) | 10–12 | 84 | 24 | 0–1+ |
| C3H/HeNCrMTV-d,g | National Cancer Institute (C3H) | 10–12 | >400 | 32 | 0–1+ |
| QM κ d,i | Bred In-house | 10 | 100 | 32 | 2+ |
| B6.129S4-Cd86tm1Shr/Jd | Jackson 003609 (CD86 KO) | 8 | 6 | 9 | 2+ |
| C57BL/10Jd | Jackson 000665 | 8 | 18 | 12 | 3+ |
| C57BL/10ScNJd | Jackson 003752 (TLR-4 KO) | 8 | 8 | 9 | 3+ |
| B6.129S7-Ifngtm1Ts/Je | Jackson 002287 (IFNγ KO) | 11 | 4 | 8 | 2+ |
Total of ethanol mice and matched controls.
A wide range of times have been tested. Times listed represent the maximum times tested at the full tolerated concentration after the phase-in described in Materials and Methods.
Growth performance is expressed as the weight gain relative to the matched controls, measured between 6 and 12 weeks on ethanol. 3+, same or greater than controls; 2+, slightly less gain than controls; 0 to 1+, no or only slight weight gain.
Maximum well tolerated ethanol concentration 20% w / v.
Maximum well tolerated ethanol concentration 18% w / v.
Maximum well tolerated ethanol concentration 15% w / v.
Mouse mammary tumor virus negative.
CD40L KO. CD40 ligand knockout.
Quasi-monoclonal κ. B6 × C57BL/6F1 mice (Wolniak et al., 2006).
Hematologic Measurements
Peripheral blood cell counts and other profiles were obtained by standard automated procedures in The University of Iowa Department of Pathology Clinical Hematology Laboratory. Murine strain-specific measurements were obtained using Advia 120 multi-species software (Bayer Diagnostics, Tarrytown, NY).
Cell Preparation, Staining, and Flow Cytometry
Thymocytes and bone marrow cells (from both hind legs) were harvested and washed in balanced salt solution (BSS). Mononuclear cells were isolated by density centrifugation over FicoLite-LM (Atlanta Biologicals, Norcross, GA) followed by further washing in BSS. 5 × 105 cells were suspended in staining buffer (BSS supplemented with 5% bovine calf serum and 0.1% NaN3) and incubated with conjugated monoclonal antibodies (mAb) in the presence of 25 μg 2.4 G2 (anti-CD16/ 32 mAb) and 10 μl normal rat serum. After incubation and washing, cells were suspended in fixative [1% formaldehyde in 1.25x phosphate buffered saline (PBS)]. Stained cells were run on a FACSVantage SE flow cytometer (Becton Dickinson & Co., Mountain View, CA) with a minimum of 30,000 events collected per sample. The following reagents were utilized: 6B2, a rat IgG anti-mouse B220 (CD45R) mAb; b7-6, a rat IgG anti-mouse IgM mAb; BP-1, a mouse IgG anti-mouse surface aminopeptidase A mAb; GK1.5, a rat IgG anti-mouse CD4 mAb; 53.6.72, a rat IgG anti-mouse CD8 mAb. Antibodies were semi-purified from HB101 serum-free supernatants by 50% ammonium sulfate precipitation and conjugated to fluorescein (Sigma Chemical Co., St Louis, MO) or R-Phycoerythrin (Molecular Probes, Eugene, OR) by using standard procedures.
Quantitation of Serum Immunoglobulins
At designated time points, mice were bled and serum stored at −20° C until assayed. Total IgM, IgG1, IgG2a (C3H), IgG2b (C57BL/ 6), IgG3, IgA, and IgE levels were determined as follows: 96-well ELISA plates (Immulon 2, Thermo, Milford, MA) were coated in 0.05 M Tris–HCl buffer (pH 9.5) with goat anti-mouse IgM (10 μg/ ml), IgG1 (5 μg/ ml), IgG2a (10 μg/ ml), IgG2b (10 μg/ ml), IgG3 (5 μg/ ml), IgA (1 μg/ ml), or EM95 (10 μg/ ml), a rat anti-mouse IgE mAb. Coated plates were blocked with 5% w/ v dry milk in PBS and washed. Control immunoglobulins (for standard curves) and serum samples appropriately diluted in 5% dry milk-PBS were added. After incubation and washing, the following biotin-conjugated detection antibodies were added; b7-6, a rat anti-mouse IgM mAb (5 μg/ ml), goat anti-mouse IgG1 (5 μg/ ml), IgG2a (5 μg/ ml), IgG2b (10 μg/ ml), IgG3 (10 μg/ ml), L228.1, a rat anti-mouse IgA mAb (2 μg/ ml), and EM95 (10 μg/ ml). Following incubation and washing steps, 2 μg/ ml of substrate (Sigma Chemical Co.) diluted in substrate buffer consisting of 0.05 M Na2CO3 and 1 × 10)3 M MgCl2 · 6H2O in H2O (pH 9.8) was added to each well, and absorbance measured at a dual wavelength of 405 and 540 nm using a Microplate Autoreader EL311 (Bio-Tek Instruments, Winooski, VT). All washes between steps were performed with a 0.9% NaCl, 0.05% Tween-20 buffer (pH 7.0). The initial coating incubation was performed overnight at 4° C with all subsequent incubation steps carried out for 1 hour at 37° C. Immunoglobulin concentrations were determined from standard curves using DeltaSOFT software (Bio-Tek Instruments). Affinity purified goat-antibody preparations were purchased from Southern Biotech, Birmingham, AL, and the EM95, b7-6 and L228.1 mAbs were prepared from hybridoma supernatants as described above. The following mAbs were used as standards: 4GF8 (IgM), 1B7 (IgG1), B4D4 (IgG2a), 49.2 (IgG2b), 8–11 (IgG3), MOPC315 (IgA), and A3B1 (IgE). 49.2 was purchased from BD Pharmingen (San Jose, CA). All the standards are trinitrophenol (TNP)-specific and were affinity purified by passage of hybridoma culture supernatants over TNP-bovine γ globulin-Sepharose 6B followed by elution with TNP-glycine (Sigma Chemical Co.).
Serum Corticosterone
Serum corticosterone was analyzed by radioimmunoassay (RIA) using a solid-phase 125I RIA kit (Diagnostic Products Corporation, Los Angeles, CA) exactly according to manufacturer’s instructions. Care was taken to avoid stress of the mice during blood drawing. Mice were kept in the home cage which was partially wrapped to block the vision of the mice remaining in the cage. The same person who routinely handled the mice, withdrew the mice from the cage one at a time, drew blood from the peri-orbital sinus, and placed sampled mice in a holding pan out of sight of the remaining caged mice until all mice were drawn and returned to the cage together. The individual mouse manipulations lasted from 30 to 45 seconds.
Real Time PCR of Bacterial DNA
Bacterial DNA was isolated from cecal washes using a DNeasy kit (Qiagen, Valencia, CA) modified for additional lysis with lysozyme treatment for gram-positive bacteria such as Enterococcus and Lactobacillus. DNA was amplified with Taq DNA polymerase and quantitated by Real-Time (TaqMan) PCR using previously published primers (Frahm and Obst, 2003) purchased from Integrated DNA Technologies, Coralville, IA. Real-Time PCR products were detected with fluorogenic probes (Integrated DNA Technologies) using an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA).
Serum Peptidoglycan
Serum samples were measured by the pro-phenol-oxidase cascade catalyzed by silkworm enzymes (Wako Chemicals USA, Inc., Richmond, VA). The method was modified for serum similar to the method of Kobayashi (Kobayashi et al., 2000). The modification includes standards made up in homologous normal mouse serum, and heat inactivation to control for protein matrix effect and inhibitory serum proteins, respectively.
Histologic Analysis
Livers, pancreata, and thymi were obtained from mice provided with either 20% ethanol in water or water only, after various times of consumption and fixed with 10% buffered formalin and paraffin embedded. Sections (5 μM) were cut and stained routinely with hematoxylin and eosin. Special stains for fibrous tissue were Masson Trichrome for heart and Klatskin Trichrome for liver. Hepatic glycogen was stained as diastase-sensitive periodic-acid Schiff positive granules. Sections were evaluated microscopically for parameters of ethanol-mediated damage to include steatosis, inflammation, fibrosis, cell loss, and apoptosis.
Statistical Analysis
All results are expressed as mean ± standard error (S.E.). Because comparisons of each parameter contain only 2 groups (water controls and ethanol), significance was estimated by standard T-test with adjustment for equal or unequal variance as appropriate, or by the Mann–Whitney nonparametric method. In some cases, multiple data groupings were evaluated by 1-way ANOVA followed by a post test as described in the figure legends. All calculations were performed using the InStat program version 3.0a (GraphPad Software, Inc., San Diego, CA). All p-values are expressed as the 2-tailed value.
RESULTS
Mouse Weights and Management
Utilizing sole water sources containing lower ethanol concentrations (up to 12% v/ v) than employed here, C57BL/ 10 mice have been previously shown to exhibit good weight gains and longevity with only mild reductions in life span at the highest ethanol concentration (Schmidt et al., 1987). Our laboratory also showed that C57BL/ 6 wild-type (WT) mice have normal weight gains for 13 weeks after being phased up to 20% (w/ v) ethanol (Song et al., 2002), in general agreement with others who used 20% (w/ v) ethanol in water (Blank et al., 1991; Meadows et al., 1989). In our experience with chronic 20% ethanol, such mice have obvious signs of intoxication and peak blood alcohol levels up to 400 mg/ dl at 6:00 am, with much lower levels down to and including zero late in the day after the nonfeeding and drinking period, as we have previously published (Song et al., 2002). We have now maintained such mice in satisfactory condition for over 70 weeks on 20% ethanol. Between 24 and 32 weeks or beyond, 20% ethanol C57BL/6 mice may begin to lose weight almost imperceptibly, such that if the ethanol mice were slightly heavier than the lot-matched controls at 24 weeks, the small relative weight loss may not be reliably measured until well beyond 32 weeks ethanol administration (data not shown). BALB/ c mice, in spite of initial ethanol aversion, do well after a longer adaptation period than required by C57BL/6 mice. In most cases, we reduced the ethanol concentration to 18% (w/ v) for administration to BALB/ c mice. In long-term protocols, ethanol consuming BALB/ c mice gain weight more slowly than controls and are slightly lighter at the time of euthanasia (Song et al., 2002). Other strains have been evaluated as shown in Table 2. CD40 ligand knock-out mice (CD40 L KO) on the C57BL/ 6 background do well on the ethanol diet in our hands, but similar to all mice used in our studies, they are kept in a barrier facility for the entire feeding period. CD28 KO mice also can be fed in this way, but are variably less tolerant than the mice of the CD40 L KO strain. Severe combined immunodeficiency (SCID) mice and interferon (IFN)γ KO mice (both C57BL/ 6 background) also do remarkably well, but we have not taken them beyond 8 to 9 weeks of 20% ethanol at this time. The C3H mouse is ethanol averse, and gains little if any weight, once on the ethanol diet. Accordingly, C3H mice are only placed on ethanol phase-in once they weigh at least 20 g. However, they maintain excellent appearance with normal grooming and activity and actually appear physically more vigorous than do the C3H water controls which become quite obese and inactive. Strains listed in Table 2, which have not been tested in numbers of 50 or more, may require additional testing to determine their ethanol tolerance and growth response. Note that the growth responses listed in Table 2 refer only to weights relative to matched controls measured in the 6 to 12-week period of ethanol administration.
Standard rodent chow pellets are offered ad libitum to all mice throughout the feeding trials. It has been found necessary to place some of the chow in the bedding to enable the most inebriated mice to eat well. Otherwise, some mice will eat too little and can lose weight if the chow pellets are in overhead bins. It is important for experienced members of the laboratory group while replenishing the daily ethanol formulations, to observe the mice for signs of fighting, or piling up bedding to manipulate the bottle spouts. We have typically been successful in this protocol with caging mice in groups of 4 females, but this has been unsuccessful in unrelated trials with ethanol males which require individual cages to prevent fighting. All mice used in the present study (and represented in Table 2) were female.
Peripheral Blood Parameters
No statistically significant differences were found between water and ethanol C57BL/6 mice in total white cell count, platelets, neutrophils (polymorphonuclear leukocytes, PMN), and lymphocytes (Fig. 1). There were trends towards reduced PMN and increased lymphocytes. There were small but statistically significant increases in red cell mean corpuscular volume, and in hematocrit.
Fig. 1.
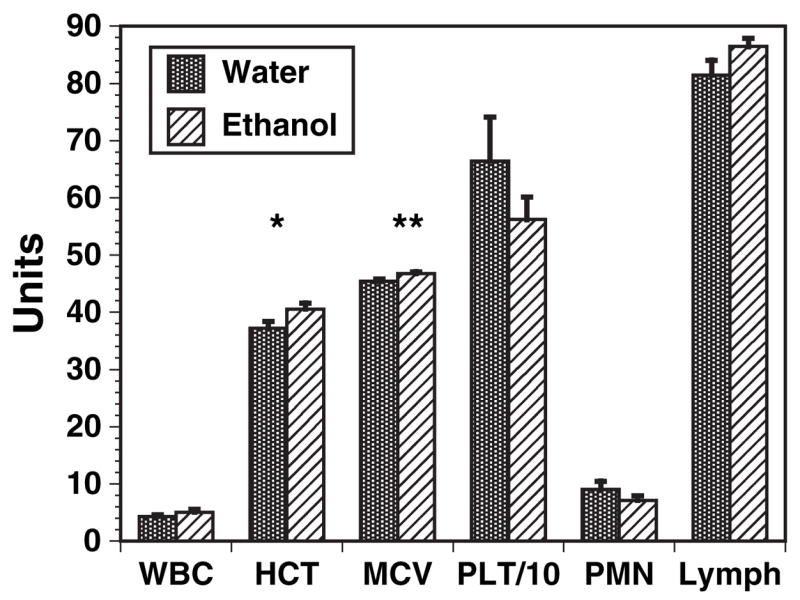
Peripheral blood parameters of C57BL / 6 mice. Peripheral blood samples of ethanol mice and matched controls were obtained after 4, 8, 16, 24 and 32 weeks of 20% ethanol ingestion. Samples were evaluated by routine automated methods with appropriate corrections. Mean erythrocyte corpuscular volume (MCV) values (femtoliters) were significantly different at all times tested, and hematocrit (HCT, as percent) was marginally significant at the various individual times. There were no time points in which significant differences were present in the other parameters, and all times are shown combined. n = 20 each ethanol and control C57BL / 6 mice. *p < 0.05; **p < 0.01. WBC, total white cell count (thousands / microliter); PLT / 10, platelet count in thousands divided by 10; PMN, polymorphonuclear leukocytes as percentage of WBC; lymph, lymphocytes as percentage of WBC.
Serum Immunoglobulins
Total serum IgM, IgG1, IgG3, IgA, IgE, IgG2b (C57BL/ 6), and IgG2a (C3H) were measured after 8, 16, 24 and 32 weeks in ethanol and matched water controls, in both C57BL/ 6 (Fig. 2A) and C3H (Fig. 2B) mice. The individual time points did not yield any statistically significant differences between water and ethanol groups, and all times were combined to maximize the number of data points. Although there were trends for increased IgA and IgM and decreased IgG2b (C57BL/ 6), and increased IgM and decreased IgG3 (C3H), none of these was statistically significant. IgE was statistically elevated in the ethanol C57BL/ 6 mice (Fig. 2A), but the total levels were low and the biologic significance of this elevation is unclear.
Fig. 2.
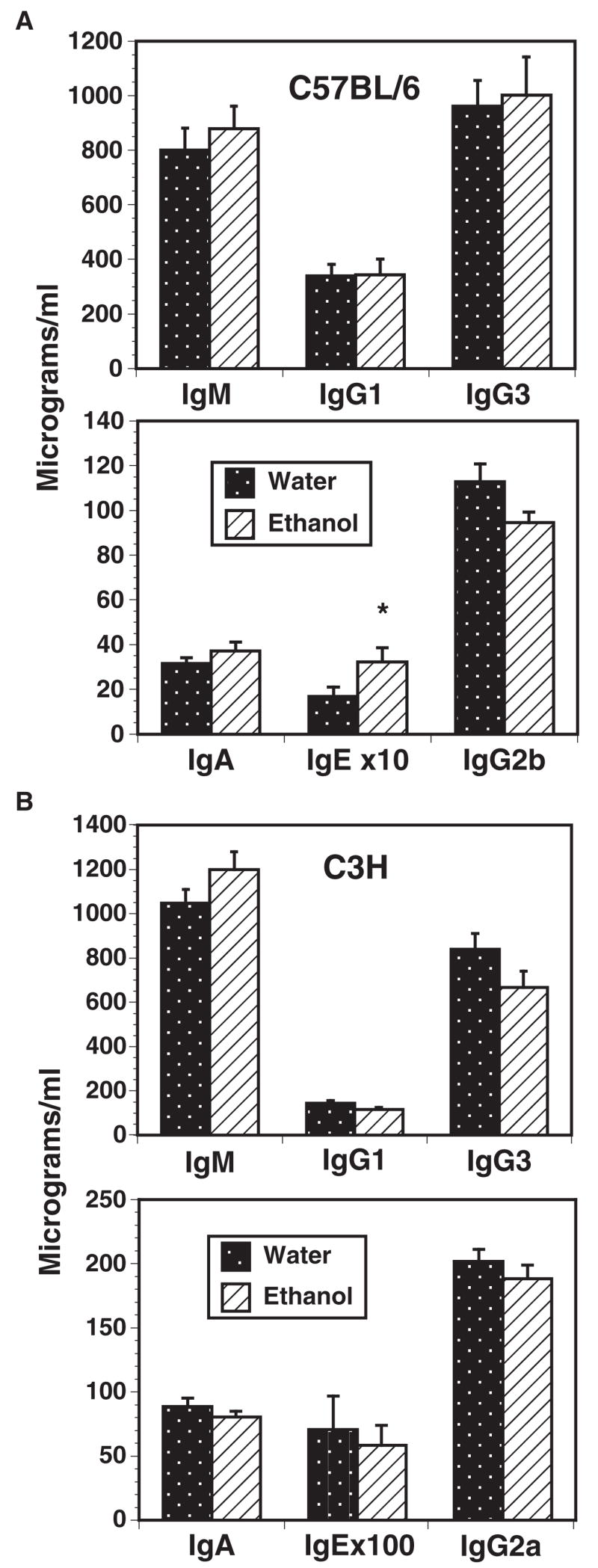
Serum immunoglobulin values of control and chronic ethanol C57BL / 6 (A) and C3H (B) mice. Serum immunoglobulin levels were measured using sandwich ELISA after 8, 16, 24 and 32 weeks ethanol and in matched water controls. IgG2b levels were measured in (A) because C57BL / 6 mice do not have the IgG2a constant region gene. Values shown are the composite of all time points. n = 27 to 33 per group. *p < 0.05.
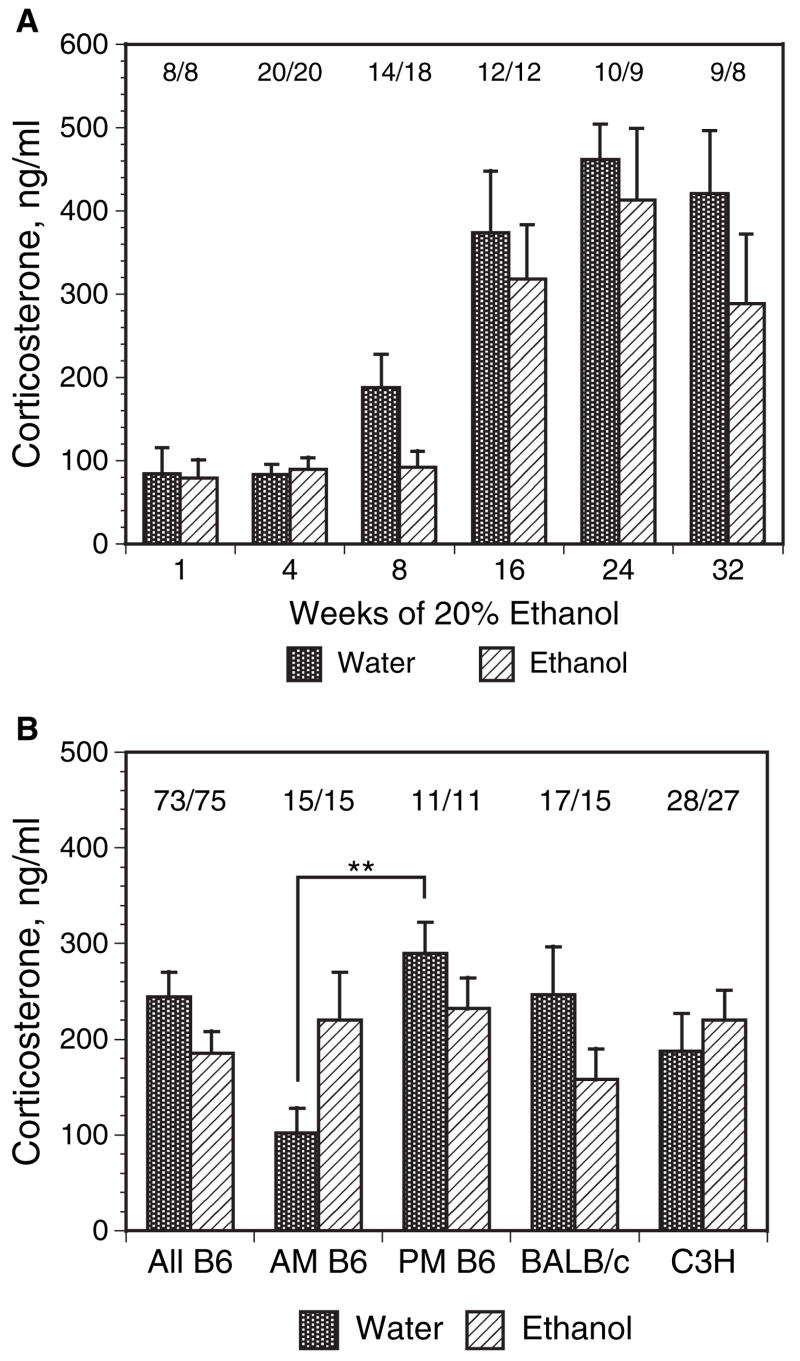
Serum Corticosterone Levels
Serum corticosterone levels were measured in ethanol and matched water control mice after 1, 4, 8, 16, 24, and 32 weeks ethanol administration, at different times of day, and in different strains (Figs. 3A and 3B). There was a trend in C57BL/ 6 mice to have lower AM (9:00 AM to 11:00 AM) corticosterone levels after some periods of ethanol administration, but most such reductions did not reach statistical significance (Fig. 3A). At short times of ethanol administration (1 to 4 weeks, mouse ages 11 to 14 weeks), there was a lower serum corticosterone level in both water and ethanol mice than at later time points. This result is consistent with reports showing that both in rats (Bandyopadhyay and Poddar, 1998; de Almeida et al., 1998; Kizaki et al., 2000; Sapolsky, 1992) and in mice (Ferrandez and De la Fuente, 1999; Kizaki et al., 1998) there is a significant increase in the basal glucocorticoid level with aging. Increases in serum corticosterone levels with age have been previously reported to be over 2-fold when comparing young and aged mice (Kizaki et al., 1998), but we are unaware of any reports in which the levels at intermediate ages were systematically measured. In the present data set, control C57BL/ 6 mice exhibited a partial increase in corticosterone levels by 16 to 17 weeks of age (Fig. 3A, 8-week treatment group). Of interest, ethanol mice at the same time point (8 weeks on protocol) did not show increased corticosterone levels. However, the lower corticosterone levels in ethanol mice after 8-weeks ethanol (compared with control mice) were only marginally significant (p = 0.0462 by T-test and not normally distributed, but not significant by nonparametric methods) and not further evaluated. The absolute levels of corticosterone shown for the 1 and 4-week ethanol- and control mice are consistent with somewhat higher basal levels reported in females compared with males (Ferrandez and De la Fuente, 1999). The diurnal variation of corticosterone level was measured in separate studies with comparison of 6:00 am and 6:00 pm times in C57BL/ 6 mice after 16 weeks on protocol. Similar to earlier reports (Kakihana and Moore, 1976; Sipp et al., 1993) using chronic ethanol in water administration, the ethanol mice displayed loss of the normal diurnal variation exhibited by the water mice (Fig. 3B). The aggregate results from BALB/ c and C3H mice are also shown in Fig. 3B. Similar to C57BL/ 6 mice, there was again no significant net difference between water and ethanol mice in the mid-morning corticosterone levels measured between 9:00 am to 11:00 AM (Fig. 3B).
Fig. 3.
Serum corticosterone values after chronic ethanol ingestion. (A) Serum corticosterone values in C57BL / 6 mice after ingestion of 20% ethanol for 1 to 32 weeks. Mice were phased on to 20% ethanol in water as described in Materials and Methods, starting from 8 to 9 weeks of age. At the indicated ethanol exposure times, serum corticosterone was measured in serum samples obtained at 9 to 10 AM. Samples from mice at widely differing exposure times were routinely measured in the same assay kit to avoid possible distortion by interassay variability. There were no significant differences in values from water versus ethanol mice at any time point, nor in the aggregated values. All 1 and 4-week mice had significantly lower values (0.01 > p > 0.001) than that of the corresponding groups at 16 to 32 weeks, consistent with published data showing increasing corticosterone levels with age. The values above the bars represent the number of water / ethanol mice tested at each time point. (B) Altered diurnal pattern of corticosterone in ethanol C57BL / 6 mice, and aggregate corticosterone values in several mouse strains. Serum corticosterone was measured in C57BL / 6 (All B6 = the aggregate of all times represented in A for C57BL / 6, and as AM and PM samples in a separate feeding trial), BALB / c, and C3H mice. The BALB / c mice ranged from 6 to 21-weeks ethanol exposures and were chosen as matched groupings from several exposure times. C3H mice were measured in groups of 6 to 8 water and ethanol mice at 8, 16, 24 and 32 weeks and are shown in aggregate here; the difference is not significant. The numbers for each group were as shown: water / ethanol mice. Diurnal variation was measured in C57BL / 6 mice (B6) after 16 weeks ethanol versus matched water controls. Samples were obtained at 6:00 AM and 6:00 PM. Asterisks indicate the PM water controls were significantly different from the AM water controls, p < 0.01 when comparing the four AM and PM water and ethanol groups either by parametric ANOVA, followed by Bonferroni post test, or by nonparametric ANOVA and Dunn’s test.
Double Positive Thymocytes and Bone Marrow B Cells
CD4, CD8 double positive (DP) thymocytes and bone marrow pre- and immature B cells are prone to apoptosis and thus preferentially depleted during systemic stress. These losses can be duplicated by glucocorticoid administration or elevation (Carson and Pruett, 1996; Compton and Cidlowski, 1986; Garvy et al., 1993a,b; Merino et al., 1994; Nieto et al., 1992; Pruett et al., 2000; Reichert et al., 1986; Sabbele et al., 1987; Schwartzman and Cidlowski, 1994; Wyllie, 1980), although such losses are not necessarily seen in all forms of stress. In the chronic model of ethanol administration, DP thymocytes were not affected at any time point. Figure 4A illustrates the flow cytometric staining pattern of thymocytes from both ethanol and water consuming C57BL/ 6 mice after 24 weeks, and shows normal expression of CD4 and CD8. In Fig. 4B, total thymocyte recoveries and DP frequencies are listed for C57BL/6 and C3H mice. When examining C57BL/ 6 and C3H total thymocyte numbers, diminished recovery is observed in both ethanol and water control mice after 32 weeks, an expected finding given the process of thymic atrophy with increasing age. Of interest, ethanol mice exhibited significantly lower cell recoveries compared with control mice at 24 weeks, raising the possibility that ethanol may accelerate the aging process in this organ. Most importantly, the percentage of DP thymocytes remained constant at all times (approximately 80%) regardless of treatment group, duration on protocol, mouse strain and total cell recovery, indicating chronic ethanol intake to have no specific effect on this corticoid sensitive DP subset. In BALB/ c mice, total thymocytes were enumerated after 12 and 16 weeks of ethanol ingestion, and were not different than matched water controls (not shown). Further, histologic evaluation of thymi obtained from BALB/ c and C57BL/ 6 mice provided 20% ethanol in water for 12 weeks showed no differences in the thymus architecture (i.e., medullary and cortical region cellularity) as compared with thymi obtained from the paired control group (Cachine M, Burrows MP, and Jerrells TR, unpublished observations).
Fig. 4.
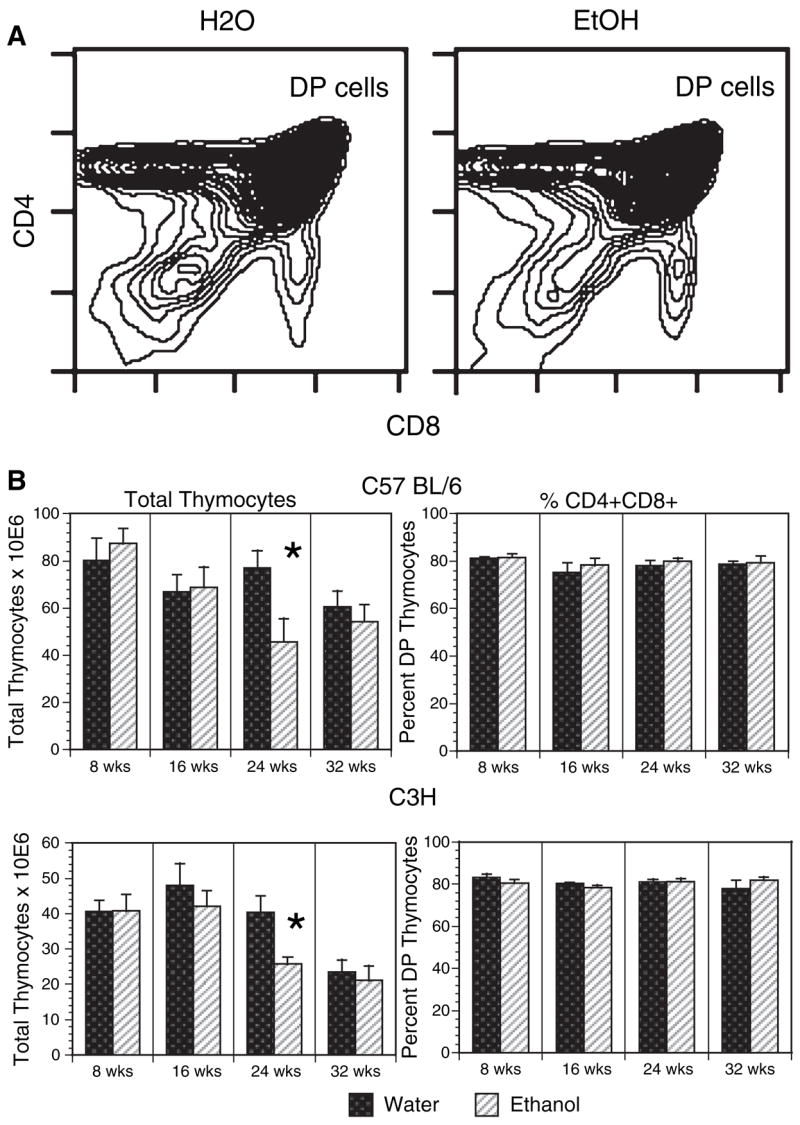
Double positive (DP) thymocytes are unaffected by long-term ethanol intake. C57BL / 6 and C3H mice were provided 20% ethanol or water alone for 8 to 32 weeks, after which thymocytes were harvested, stained with anti-CD4 and anti-CD8 and analyzed by flow cytometry. Representative contour plots of 24 weeks C57BL / 6 chronic ethanol and control thymi are shown in panel A. In panel B, total recovered thymocytes from both C57BL / 6 and C3H mice at each time are summarized, as are the percentages of CD4+CD8+ cells. Percentages of DP thymocytes were determined by software gating. n = 6 to 8 mice per group at each time point. *24 weeks C57BL / 6 ethanol versus water total cell recoveries, p < 0.03 using an unpaired t-test. 24-weeks C3H ethanol versus water total cell recoveries, p < 0.015 using an unpaired t-test with Welch correction.
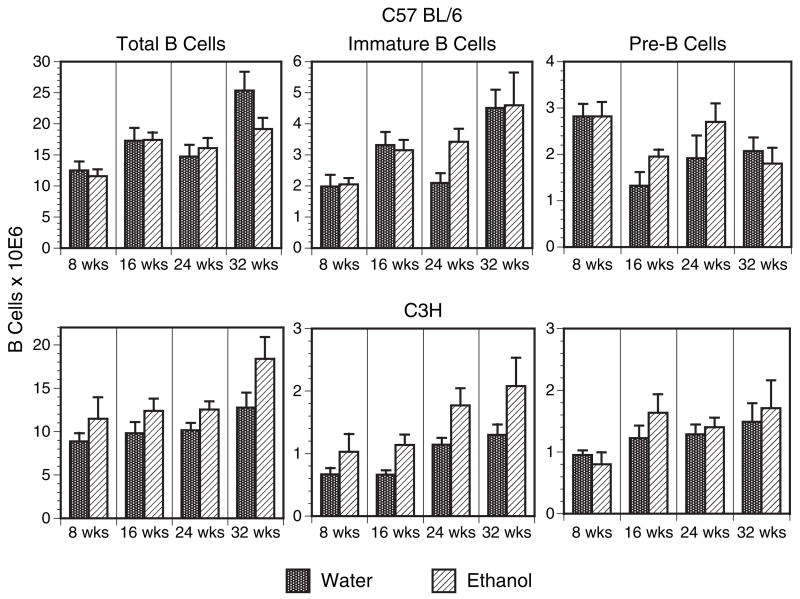
Examination of the stress sensitive pre- and immature B cells in the bone marrow yielded similar results. Bone marrow pre-B cells express the BP-1 marker and immature B cells display high levels of IgM. Figure 5 summarizes the findings from both C57BL/6 and C3H mice and demonstrates that ethanol-treated mice experienced no loss of total (B220+) B cells, immature (B220+IgM+) B cells, or pre-(B220+BP-1+) B cells in the bone marrow. Taken together, the thymocyte and bone marrow B cell recovery data support the conclusion that the ethanol in water model does not lead to corticoid-induced depletion of the early T and B cell populations examined here, even after long periods of administration.
Fig. 5.
Bone marrow pre- and immature B cells are unaffected by long-term ethanol intake. C57BL / 6 and C3H mice were provided 20% ethanol or water alone for 8 to 32 weeks, after which bone marrow cells were harvested, stained with anti-B220 and either anti-BP-1 or anti-IgM and analyzed by flow cytometry. Total recovered bone marrow B cells (B220+), total recovered pre-B cells (B220+BP-1+) and total recovered immature B cells (B220+ IgM+) are summarized. n = 6 to 8 mice per group at each time point.
Other Organs
Liver
Livers of ethanol mice were normal in gross appearance, although typically 10 to 15% heavier than controls. Light microscopy did not reveal any obvious inflammation, necrosis or increase in collagen (not shown). There were increases in microvesicular fat and small areas of macrovesicular fat in livers of mice examined after 8 weeks or longer of ethanol consumption; moderate glycogen depletion was also present in such livers. We reported previously that serum samples from mice on 20% ethanol in water diets up to 12 weeks did not have any elevations of alanine transaminase activity (Song et al., 2002), and similar results were obtained for times of consumption up to 21 weeks (Sosa et al., 2005).
Heart
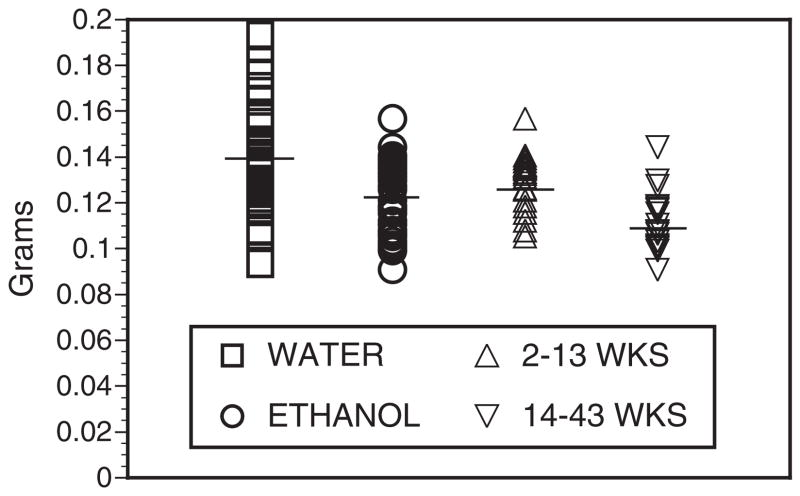
The mean and median weights of the hearts of chronic ethanol mice were significantly lower than those of the matched water-fed control cohort (Fig. 6). Long-term ethanol hearts were lighter than shorter-term ethanol hearts. Light microscopy of the myocardium neither revealed any focal inflammatory infiltrates nor any evidence of necrosis. Special stains of the hearts for collagen and elastin were unchanged in the ethanol hearts as compared with controls (not shown).
Fig. 6.
Heart weights of C57BL / 6 mice after 2 to 43 weeks of 20% ethanol. After euthanasia, hearts were collected and carefully trimmed of excess tissue, washed, blotted dry, and weighed by the same person over the course of many separate feeding trials. Length of ethanol exposures varied from 2 to 43 weeks, weighted average 13.4 weeks. In each case, equal numbers of water control and ethanol hearts were collected in any given feeding trial. Total n was 39 water and 39 ethanol hearts. Mean weights (bars) were 0.1380 (water controls) versus 0.1213 g (all ethanol), a 12% mean decrease in the ethanol heart weights, p = 0.0002. Median weights were 0.1347 (controls) versus 0.1186 g, p = 0.0007. The ethanol hearts were further analyzed by comparing those weighed after 2 to 13 weeks ethanol, mean weights 0.1230, n = 21, versus those on ethanol for 14 to 43 weeks, mean weights 0.1120, n = 18, p = 0.0002.
Pancreas
Pancreata from C57BL/6 mice provided ethanol in drinking water for up to 21 weeks did not differ in appearance, weight, or histologic characteristics as compared with pancreata from the age- and sex-matched control mice. Further, sera from mice provided with 20% ethanol in water for up to 21 weeks did not show statistically significant elevations of amylase and lipase as compared with concentrations in sera from control mice (not shown).
Colonic flora
We observed increased weight of the large bowel from ethanol mice (Fig. 7). This increase appeared to be due largely to increased fecal material suggesting an increased transit time. We did not examine the bowel wall or mucosa microscopically, but the bowel walls appeared grossly normal. The cecal contents of water control and ethanol mice from several different feeding trials were suspended in cell culture medium, diluted equally, and aliquots cultured both aerobically and anaerobically for standard colony counts and isolation of colony types for further analysis. Aliquots were saved at the same time and frozen for later DNA extraction and quantitative PCR for Escherichia coli β-glucosidase and Enterococcus 23S rRNA specific sequences.
Fig. 7.
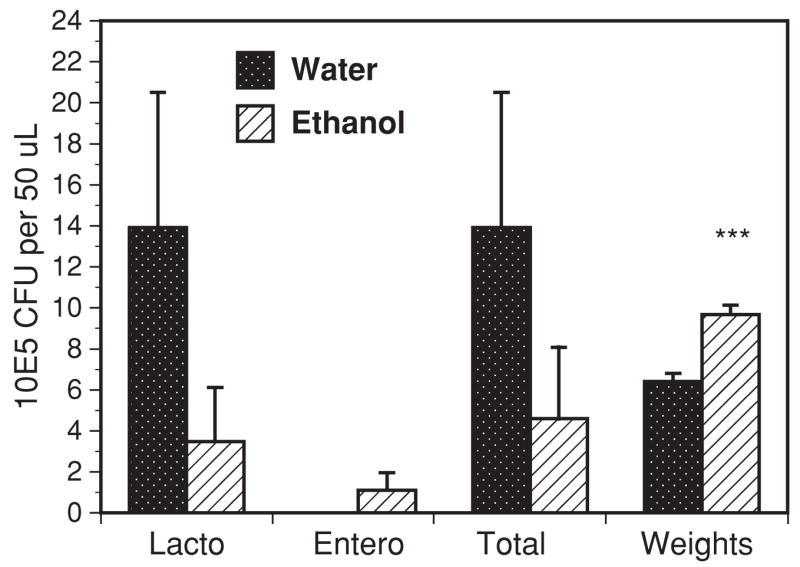
Bacterial flora and colon weights of C57BL / 6 mice. Colons of euthanized mice were carefully ligated to preserve the contents, weighed and the cecal contents extracted, diluted and cultured under standard aerobic conditions on blood agar plates. Cultures under anaerobic conditions and in both MRS and Peptone broths (Remel) were also carried out but did not add significantly to the results shown. Results displayed are the mean and SEM of cecal counts for 3 separate experiments after feeding trials of 6 or more weeks. ***Colon weights in grams × 10, p = 0.0002. Lacto, Lactobacillus species; Entero, Enterococcus species, Total, total colony counts.
The dominant cecal bacterium in control water SPF C57BL/ 6 mice was Lactobacillus. The population density of these bacteria dropped dramatically in the ethanol mice (Fig. 7). Other bacteria identified in the water mice included rare colonies of both E. coli and Enterococcus species. In the ethanol mice, E. coli was still too low to reliably count, but a few colonies were seen. There was a consistent appearance in the ethanol mice of significant numbers of Enterococcus colonies as shown. DNA extraction of the cecal washes and PCR were consistent with the culture results and showed a substantial increase in the Enterococcus 23S rRNA gene, Table 3, and a small increase in the E. coli β-glucosidase gene (not shown).
Table 3.
Enterococcal DNA in Cecal Fluids
| Feeding trial | Group | Ct | Log10 counts | Fold increase |
|---|---|---|---|---|
| 103–4 weeks | Water | 26.14 | 5.69 | – |
| Ethanol | 21.76 | 7.27 | 38 (47) | |
| 114–14 weeks | Water | 33.67 | 2.98 | – |
| Ethanol | 25.97 | 5.75 | 587 |
Cecal fluid of C57BL/6 mice was carefully harvested and diluted for cultures as described in Materials and Methods, after 4 or 14 weeks of 20% ethanol feeding. Aliquots of the diluted fluid were processed for PCR detection and quantitation of DNA for Escherichia coli β-glucosidase and Enterococcus 23S rRNA, exactly as described by Frahm and Obst, 2003. Standard curves of log10 of bacterial number in control cultures processed for PCR versus Ct were constructed; the slopes of the curves were essentially identical to the control curves published by Frahm and Obst. Ct represents the threshold cycle in which the fluorescence signal exceeds background. There was a dramatic increase in Enterococcus DNA in ethanol mice (38 to over 500-fold in separate determinations), consistent with the culture results. The parenthetic result shown for fold increase in trial 103–4 represents a separate PCR determination of another sample from the same feeding trial. E. coli was isolated in very few colonies by culture, and specific DNA was only marginally increased in the cecal fluid of ethanol mice as compared with water controls (data not shown).
Serum Peptidoglycan
We have evaluated over 80 serum samples obtained from ethanol and control feeding trials of variable lengths in C57BL/ 6 mice (Fig. 8). There was no significant increase in peptidoglycan levels with age in the water control mice, but the aggregate data demonstrate a significant elevation in ethanol mice overall, and a strong trend towards higher peptidoglycan with increased length of ethanol exposure. The range of values in the ethanol mice was substantial with some levels over 100 ng/ ml.
Fig. 8.
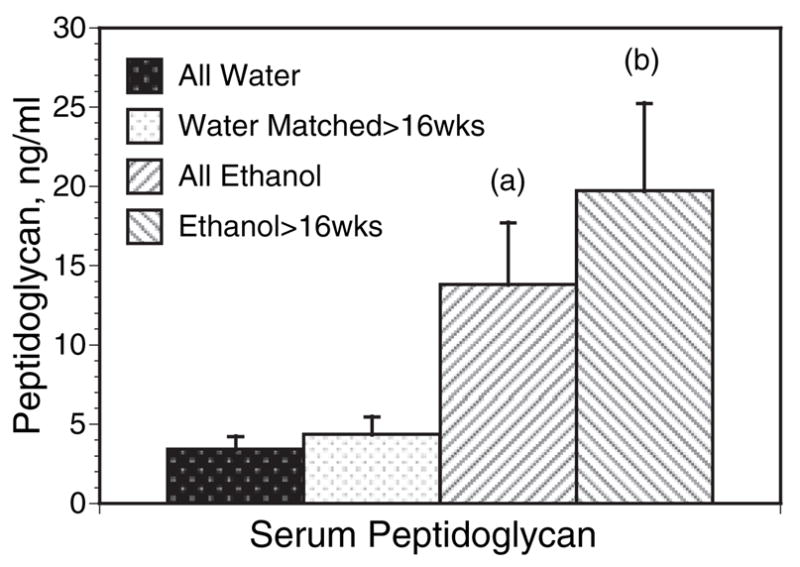
Serum peptidoglycan in water and ethanol C57BL / 6 mice. Serum samples from 40 water control mice and 42 chronic ethanol mice were measured by the prophenol-oxidase cascade catalyzed by silkworm enzymes as described in the Materials and Methods. Mice tested varied in age from 14 to 68 weeks and the ethanol groups had received 20% ethanol in water for 7 to 58 weeks, 28 of these for over 16 weeks. The over 16-week ethanol mice and the n = 26 matched water mice ranged in age from 35 to 68 weeks. There was no consistent difference between the young and older water mice. ANOVA for all groups, p = 0.0005. Student-Neumann post test: (a) p < 0.05 versus all water; (b) p < 0.001 versus all water, and p < 0.05 versus the older water group matched with the over 16 week ethanol mice. There were no overlaps in the 95% confidence intervals between all water and all ethanol, nor between ethanol over 16 weeks and matched controls.
DISCUSSION
We demonstrated previously (Song et al., 2002) that both C57BL/ 6 and BALB/ c mice administered 20% ethanol in water (w/ v) develop activated splenic T cells, which after in vitro stimulation have increased rapid production of both TH1 and TH2 cytokines (Song et al., 2002). Both mouse strains also have altered splenic macrophages that have increased expression of the CD80 and CD86 co-stimulatory molecules, and increased production of inflammatory cytokines after in vitro activation (Zhu et al., 2004). In the present work, we analyzed several additional features of the chronic ethanol mouse model, with special attention to indicators of systemic stress. In acute or short-term ethanol models, it has been widely reported that corticosterone levels rise as referenced earlier in this report. A previous study, using chronic 20% ethanol in water administration, reported that corticosterone levels are not persistently elevated (Sipp et al., 1993). This result is confirmed by the present analysis. We regard stress mechanisms to be of importance in attempts to model the immunodeficiency of the chronic alcoholic. Study results of immune system functional parameters could be significantly skewed if stress sensitive precursor populations were depleted by abnormally elevated corticosteroids. The absolute basal levels of corticosterone reported by different investigators for both mice and rats vary widely with the analytical method employed as well as other conditions in the protocol, so it is primarily the relationships between the water and ethanol groups that must be evaluated once a satisfactory baseline has been established for the conditions of the specific protocol and the analytical method employed. In the present data, the corticosterone changes in chronic ethanol mice might be considered ambiguous due to the loss of diurnal variation reported here and in the publications cited earlier for the ethanol in water model; however, it is clear that there is no increase relative to matched controls at mid-morning measurement times, when the mice are on a standard 12-hour off and 12-hour on dark–light schedule. Procedure-related stress, as an explanation for any of the results reported here, appears to be unlikely because of the precautions described under Materials and Methods, and the fact that young mice of an age typically used for stress studies had much lower basal levels of corticosterone than did older mice in our protocol (Fig. 3A, 1 to 4 weeks ethanol, 11 to 14 weeks of age).
To further evaluate the parameters that would be affected by overt stress in this model, we enumerated CD4+CD8+ thymocytes as well as bone marrow pre- and immature B cell populations after 8, 16, 24 and 32 weeks on protocol. These populations are well known to be susceptible to glucocorticoid-induced cell death and hence are sensitive indicators of systemic stress (Carson and Pruett, 1996; Compton and Cidlowski, 1986; Garvy et al., 1993a,b; Merino et al., 1994; Nieto et al., 1992; Pruett et al., 2000; Reichert et al., 1986; Sabbele et al., 1987; Schwartzman and Cidlowski, 1994; Wyllie, 1980). At no time point were any of these populations affected by ethanol intake. Taken together, these results encourage us to consider this method of ethanol administration to be a low-stress model that can be used efficiently for long-term studies of the baseline effects of chronic ethanol abuse. However, it is obvious that in long-term administration protocols age-matched controls are imperative; serum corticosterone levels increase with age (Fig. 3A) in agreement with previously published studies by others in rats (Bandyopadhyay and Poddar, 1998; de Almeida et al., 1998; Kizaki et al., 2000; Sapolsky, 1992) and in mice (Ferrandez and De la Fuente, 1999; Kizaki et al., 1998). After baseline chronic ethanol effects on specific parameters are established, the model also will permit the superimposition of periodic binges (administered for example, by gavage) as may be seen in the chronic alcoholic; we have not explored this dosing variation.
A number of mouse strains, including several KO strains that are known to be more susceptible to infectious diseases than are WT, were successfully phased on to 20% ethanol as described here, so there is some flexibility of the model for examining the role of specific gene function in the response to chronic ethanol ingestion. The diet used contained severalfold higher zinc and vitamin A levels than the standard NRC recommendations, so reductions in micronutrient availability seem unlikely for these constituents. However, the broader questions of nutrient absorption and cell or tissue-specific concentrations of micronutrients in the ethanol mouse were not addressed in our current work.
There are several similarities in the immunologic and hematologic changes in human chronic alcoholics and the corresponding parameters of the mouse after chronic ethanol in water. The human alcoholic and chronic ethanol mouse both have activated CD4+ and CD8+ T cells (Cook et al., 1994; Song et al., 2002, 2001). Accompanying the T-cell changes in both species are changes in T-cell cytokine responses, primarily in the increased rapid TH1 response characteristic of the memory subset. In both humans and mice, there is clear evidence also of innate immune cell activation (McClain and Cohen, 1989; Zhu et al., 2004). The peripheral B cells of the human alcoholic are altered in number and subset distribution (Cook et al., 1996; Laso et al., 1996). Although as shown here, there is no corticosterone-induced preferential loss of immature and pre-B cells in the chronic mouse model, there are clearly peripheral B cell abnormalities that are under evaluation in our laboratories (Tygrett LT, Coleman RA, Cook RT, and Waldschmidt TJ, manuscript in preparation). In the peripheral erythroid cells, both the human alcoholic (Sillanaukee, 1996) and the mice in this report have increased mean erythrocyte corpuscular volume values. In spite of the above cited similarities in the immune responses to chronic ethanol, there appear to be some differences. For example, there was little or no change in the serum level of polyclonal immunoglobulins observed in the present data on chronic ethanol mice. At present, it is not possible to state whether this is because of the relatively stringent isolation conditions under which our mice are kept, as compared with the constant exposure of most humans to new viral and bacterial agents, or whether this is simply a species difference in response to ethanol.
Although we have been especially interested in validating the chronic ethanol in water method for examination of ethanol-induced changes in the immune system, other organs as described here deserve some comment. The reduction in heart weights of ethanol mice was not predicted, as there was a premeasurement expectation of hypertension with some degree of resulting ventricular hypertrophy. No physiologic studies of cardiac function were done, but the hearts were clearly lighter in the ethanol mice, and the comparison of weights after 2 to 13 weeks ethanol versus 14 to 43 weeks exposure indicates that there is a continuing loss of cardiac mass with extended ethanol exposure times. Examination of the hearts by routine light microscopy including special stains did not reveal changes such as inflammation or fibrosis, and it seems that the weight loss is probably due to a loss of muscle mass. As reviewed recently, modeling studies with rats showed that chronic ethanol ingestion decreases the cardiac and skeletal muscle contents of myofibrillar and sarcoplasmic proteins, apparently by inhibition of translational but not transcriptional steps in protein synthesis (Lang et al., 2005). In humans it was shown that the development of alcoholic cardiomyopathy is functionally reduced or reversed if ethanol consumption is restricted to 60 g/ day or less (Nicolas et al., 2002). Although difficult to compare precisely, the 20% ethanol in water model used here provides approximately 30% of calories (Blank et al., 1991) and appears to be calorically higher than the 60 g/ day ethanol in an average human diet. Further study of the long-term cardiac effects of the 20% ethanol diet in mice appears warranted as a potentially useful model of cardiomyopathy with a large diversity of genetically characterized mouse strains available for study.
Liver changes in the chronic ethanol mice are not dramatic, but are clearly present including glycogen depletion and microvesicular and limited macrovesicular steatosis. However, we failed to find any areas with inflammatory cell infiltrates, and the changes so far identified appear likely to be entirely reversible. Nonetheless, there is widespread microvesicular steatosis; this is considered by some to have the same basic pathogenic significance as macrovesiclar steatosis (Reddy and Rao, 2006). Thus, in this chronic ethanol mouse model it might be expected that livers are sensitized to further damage, but under the conditions employed in the present study additional insults were not sufficient to produce frank inflammation.
Finally, the changes observed in the bacterial flora of the cecum are of interest because of the increased total colon weight and the increase in cecal Enterococcus numbers. The increased total colon weight appears to be due to increased transit time with a resulting increase in fecal contents. Thus, the total bacterial counts may be higher than implied by the reduction in culturable cecal bacteria. The appearance of increased Enterococcus in the ethanol mice may be of significance; Enterococcus is known to be relatively ethanol resistant in vitro (Benachour et al., 2005), and can be highly pathogenic. We measured serum peptidoglycan as one possible indicator of increased translocation of this or other bacteria or fragments thereof, and found higher levels with increasing time on ethanol (Fig. 8). This result is consistent with the well known increase in gut permeability and endotoxemia in alcoholic humans (Bjarnason et al., 1984; Bode et al., 1987), and in experimental animal models (Enomoto et al., 2001; Tabata et al., 2002). Although the Toll-like receptor (TLR) 4 ligand lipopolysaccharide is usually reported, the TLR2 ligand peptidoglycan has been observed to be elevated in plasma up to 24 hours after acute ethanol gavage (Tabata et al., 2002). Immune stimulation thus might occur after ethanol exposure as a result of either or both TLR2 and TLR4 signaling. As shown recently, such signaling may involve not only cells of the innate immune system but also may occur in TLR expressing T cells, thus modulating T-cell regulation directly by altering the activity of regulatory T cells (Liu et al., 2006).
Acknowledgments
We thank Ling Hu, Betty Young, Lucas Turner, Susan Wiechert and Teresa Duling for their excellent continuing assistance in this work. We also thank Mike Burrows, Department of Pathology and Microbiology, University of Nebraska Medical Center, for providing some of the BALB/ c corticosterone data points.
This work was supported by NIH AA-09598, AA-014405 (RTC), AA-014400 (TJW), AA-014406 (AJS), AA-014418 (ZKB), AA-012450, AA-013841 (TRJ) and AA-013275 (NBR), The Department of Pathology and the University of Iowa Carver College of Medicine, and the Department of Pathology and Microbiology of the University of Nebraska Medical Center.
References
- Abdallah RM, Starkey JR, Meadows GG. Toxicity of chronic high alcohol intake on mouse natural killer cell activity. Res Commun Chem Pathol Pharmacol. 1988;59:245–258. [PubMed] [Google Scholar]
- de Almeida H, Magalhaes MC, Magalhaes MM. Age-related changes in the inner zone of the adrenal cortex of the rat—a morphologic and biochemical study. Mech Ageing Dev. 1998;105:1–18. doi: 10.1016/s0047-6374(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay BC, Poddar MK. Dietary protein-induced change in mammalian corticosterone status (index of immune response) during aging. Mech Ageing Dev. 1998;103:57–68. doi: 10.1016/s0047-6374(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Benachour A, Muller C, Dabrowski-Coton M, LeBreton Y, Giard JC, Rince A, Auffray Y, Hartke A. The Enterococcus faecalis sigV protein is an extracytoplasmic function sigma factor contributing to survival following heat, acid, and ethanol treatments. J Bacteriol. 2005;187:1022–1035. doi: 10.1128/JB.187.3.1022-1035.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason IK, Ward K, Peters TJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- Blank SE, Duncan DA, Meadows GG. Suppression of natural killer cell activity by ethanol consumption and food restriction. Alcohol Clin Exp Res. 1991;15:16–22. doi: 10.1111/j.1530-0277.1991.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Blomgren H, Andersson B. Evidence for a small pool of immunocompetent cells in the mouse thymus. Exp Cell Res. 1969;57:185–192. doi: 10.1016/0014-4827(69)90140-2. [DOI] [PubMed] [Google Scholar]
- Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- Carson EJ, Pruett SB. Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Collier SD, Wu WJ, Pruett SB. Endogenous glucocorticoids induced by a chemical stressor (ethanol) cause apoptosis in the spleen in B6C3 F1 female mice. Toxicol Appl Pharmacol. 1998;148:176–182. doi: 10.1006/taap.1997.8324. [DOI] [PubMed] [Google Scholar]
- Collins AC, Wehner JM, Wilson WR. Animal models of alcoholism: genetic strategies and neurochemical mechanisms. [Review] Biochem Soc Symp. 1993;59:173–191. [PubMed] [Google Scholar]
- Compton MM, Cidlowski JA. Rapid in vivo effects of glucocorticoids on the integrity of rat lymphocyte genomic deoxyribonucleic acid. Endocrinology. 1986;118:38–45. doi: 10.1210/endo-118-1-38. [DOI] [PubMed] [Google Scholar]
- Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- Cook RT, Waldschmidt TJ, Ballas ZK, Cook BL, Booth BM, Stewart BC, Garvey MJ. Fine T-cell subsets in alcoholics as determined by the expression of L-selectin, leukocyte common antigen, and beta-integrin. Alcohol Clin Exp Res. 1994;18:71–80. doi: 10.1111/j.1530-0277.1994.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Cook RT, Waldschmidt TJ, Cook BL, Labrecque DR, McLatchie K. Loss of the CD5+ and CD45RAhi B cell subsets in alcoholics. Clin Exp Immunol. 1996;103:304–310. doi: 10.1046/j.1365-2249.1996.d01-621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli LM, Lieber CS. Fatty liver in the rat after prolonged intake of ethanol with a nutritionally adequate new liquid diet. J Nutr. 1967;91:331–336. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- Ellis FW. Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther. 1966;153:121–127. [PubMed] [Google Scholar]
- Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, Takei Y, Sato And N, Thurman RG. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001;25:51S–54S. doi: 10.1097/00000374-200106001-00012. [DOI] [PubMed] [Google Scholar]
- Ferrandez MD, De la Fuente M. Effects of age, sex and physical exercise on the phagocytic process of murine peritoneal macrophages. Acta Physiol Scand. 1999;166:47–53. doi: 10.1046/j.1365-201x.1999.00535.x. [DOI] [PubMed] [Google Scholar]
- Forbes JC, Duncan GM. The effect of acute alcohol intoxication on the adrenal glands of rats and guinea pigs. Q J Stud Alcohol. 1951;12:355–359. [PubMed] [Google Scholar]
- Frahm E, Obst U. Application of the fluorogenic probe technique (Taq-Man PCR) to the detection of Enterococcus spp. and Eschericia coli in water samples. J Microbiol Methods. 2003;52:123–131. doi: 10.1016/s0167-7012(02)00150-1. [DOI] [PubMed] [Google Scholar]
- Garvy BA, King LE, Telford WG, Morford LA, Fraker PJ. Chronic elevation of plasma corticosterone causes reductions in the number of cycling cells of the B lineage in murine bone marrow and induces apoptosis. Immunology. 1993a;80:587–592. [PMC free article] [PubMed] [Google Scholar]
- Garvy BA, Telford WG, King LE, Fraker PJ. Glucocorticoids and irradiation-induced apoptosis in normal murine bone marrow B-lineage lymphocytes as determined by flow cytometry. Immunology. 1993b;79:270–277. [PMC free article] [PubMed] [Google Scholar]
- Guaza C, Torrellas A, Borrell S. Adrenocortical response to acute and chronic ethanol administration in rats. Psychopharmacology. 1983;79:173–176. doi: 10.1007/BF00427806. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Smith W, Eckardt MJ. Murine model of ethanol-induced immunosuppression. Alcohol Clin Exp Res. 1990;14:546–550. doi: 10.1111/j.1530-0277.1990.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Kakihana R, Butte JC, Hathaway A, Noble EP. Adrenocortical response to ethanol in mice: modification by chronic ethanol consumption. Acta Endocrinol (Copenh) 1971;67:653–664. doi: 10.1530/acta.0.0670653. [DOI] [PubMed] [Google Scholar]
- Kakihana R, Moore JA. Circadian rhythm of corticosterone in mice: the effect of chronic consumption of alcohol. Psychopharmacologia. 1976;46:301–305. doi: 10.1007/BF00421118. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Kumar S, Morrow AL. Ethanol rapidly induces steroidogenic acute regulatory protein expression and translocation in rat adrenal gland. Eur J Pharmacol. 2003;473:225–227. doi: 10.1016/s0014-2999(03)01969-1. [DOI] [PubMed] [Google Scholar]
- Kizaki T, Ookawara T, Iwabuchi K, Onoe K, Day NK, Good RA, Maruyama N, Haga S, Matsuura N, Ohira Y, Ohno H. Age-associated increase of basal corticosterone levels decreases ED2high, NF-kappaBhigh activated macrophages. J Leuk Biol. 2000;68:21–30. [PubMed] [Google Scholar]
- Kizaki T, Ookawara T, Oh-Ishi S, Itoh Y, Iwabuchi K, Onoe K, Day NK, Good RA. An increase in basal glucocorticoid concentration with age induces suppressor macrophages with high-density FcgRII / III. Immunology. 1998;93:409–414. doi: 10.1046/j.1365-2567.1998.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Tani T, Yokota T, Kodama M. Detection of peptidoglycan in human plasma using the silkworm larvae plasma test. FEMS Immunol Med Microbiol. 2000;28:49–53. doi: 10.1111/j.1574-695X.2000.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Kruger TE, Jerrells TR. Effects of ethanol consumption and withdrawal on B cell subpopulations in murine bone marrow. Clin Exp Immunol. 1994;96:521–527. doi: 10.1111/j.1365-2249.1994.tb06060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol. 2005;37:2180–2195. doi: 10.1016/j.biocel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Laso FJ, Madruga JI, San Miguel JF, Ciudad J, Lopez A, Alvarez Mon M, Orfao A. Long lasting immunological effects of ethanol after withdrawal. Cytometry. 1996;26:275–280. doi: 10.1002/(SICI)1097-0320(19961215)26:4<275::AID-CYTO6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Li X, Rana SN, Kovacs EJ, Gamelli RL, Chaudry IH, Choudhry MA. Corticosterone suppresses mesenteric lymph node T cells by inhibiting p38 / ERK pathway and promotes bacterial translocation after alcohol and burn injury. Am J Physiol Regul Integr Comp Physiol. 2005;289:R37–44. doi: 10.1152/ajpregu.00782.2004. [DOI] [PubMed] [Google Scholar]
- Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor RR, Louria DB. Alcohol and infection. [Review] Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic patients. Hepatology. 1989;9:349. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- Meadows GG, Blank SE, Duncan DD. Influence of ethanol consumption on natural killer cell activity in mice. Alcohol Clin Exp Res. 1989;13:476–479. doi: 10.1111/j.1530-0277.1989.tb00359.x. [DOI] [PubMed] [Google Scholar]
- Merino R, Ding L, Veis DJ, Korsmeyer SJ, Nunez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994;13:683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas JM, Fernandez-Sola J, Estruch R, Pare JC, Sacanella E, Urbano-Marquez A, Rubin E. The effect of controlled drinking in alcoholic cardiomyopathy. Ann Intern Med. 2002;136:192–200. doi: 10.7326/0003-4819-136-3-200202050-00007. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Gonzalez A, Gambon F, Diaz-Espada F, Lopez-Rivas A. Apoptosis in human thymocytes after treatment with glucocorticoids. Clin Exp Immunol. 1992;88:341–344. doi: 10.1111/j.1365-2249.1992.tb03084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, Rivier C. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. [Review] Alcohol Clin Exp Res. 1998;22:243S–247S. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- Padgett EL, Sibley DA, Jerrells TR. Effect of adrenalectomy on ethanol-associated changes in lymphocyte cell numbers and subpopulations in thymus, spleen, and gut-associated lymphoid tissues. Int J Immunopharmacol. 2000;22:285–298. doi: 10.1016/s0192-0561(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Park E, Dumas R, Schuller-Levis G, Rabe A. Exposure to alcohol on E9 raises poststress corticosterone in mature but not old mice. Neurosci Lett. 2004;368:345–348. doi: 10.1016/j.neulet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Patel VA, Pohorecky LA. Interaction of stress and ethanol: effect on beta-endorphin and catecholamines. Alcohol Clin Exp Res. 1988;12:785–788. doi: 10.1111/j.1530-0277.1988.tb01346.x. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Myers LP, Wu WJ, Collier S. Quantitative analysis of the neuroendocrine-immune axis: linear modeling of the effects of exogenous corticosterone and restraint stress on lymphocyte subpopulations in the spleen and thymus in female B6C3 F1 mice. Brain Behav Immun. 2000;14:270–287. doi: 10.1006/brbi.2000.0605. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q. Acute ethanol administration profoundly alters poly I:C-induced cytokine expression in mice by a mechanism that is not dependent on corticosterone. Life Sci. 2003a;72:1825–1839. doi: 10.1016/s0024-3205(02)02507-9. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Myers LP, Hebert P. Modeling and predicting immunological effects of chemical stressors: characterization of a quantitative biomarker for immunological changes caused by atrazine and ethanol. Toxicol Sci. 2003b;75:343–354. doi: 10.1093/toxsci/kfg200. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- Reichert RA, Weissman IL, Butcher EC. Dual immunofluorescence studies of cortisone-induced thymic involution: Evidence for a major cortical component to cortisone-resistant thymocytes. J Immunol. 1986;136:3529–3534. [PubMed] [Google Scholar]
- Sabbele NR, Van Oudenaren A, Hooijkaas H, Benner R. The effect of corticosteroids upon murine B cells in vivo and in vitro as determined in the LPS-culture system. Immunology. 1987;62:285–290. [PMC free article] [PubMed] [Google Scholar]
- Santisteban GA. The response of the thymolymphatic system to graded doses of ethyl alcohol and its relationship to adrenocortical activity. G J Stud Alcohol. 1961;22:1–13. [PubMed] [Google Scholar]
- Sapolsky RM. Do glucocorticoid concentrations rise with age in the rat? Neurobiol Aging. 1992;13:171–174. doi: 10.1016/0197-4580(92)90025-s. [DOI] [PubMed] [Google Scholar]
- Schmidt W, Popham RE, Israel Y. Dose-specific effects of alcohol on the lifespan of mice and the possible relevance to man. Brit J Addiction. 1987;82:775–788. doi: 10.1111/j.1360-0443.1987.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Schwab CL, Fan R, Zheng Q, Myers LP, Hebert P, Pruett SB. Modeling and predicting stress-induced immunosuppression in mice using blood parameters. Toxicol Sci. 2005;83:101–113. doi: 10.1093/toxsci/kfi014. [DOI] [PubMed] [Google Scholar]
- Schwartzman RA, Cidlowski JA. Glucocorticoid-induced apoptosis of lymphoid cells. [Review] Int Arch Allergy Immunol. 1994;105:347–354. doi: 10.1159/000236781. [DOI] [PubMed] [Google Scholar]
- Sillanaukee P. Laboratory markers of alcohol abuse. Alcohol Alcohol. 1996;31:613–616. doi: 10.1093/oxfordjournals.alcalc.a008199. [DOI] [PubMed] [Google Scholar]
- Sipp TL, Blank SE, Lee EG, Meadows GG. Plasma corticosterone response to chronic ethanol consumption and exercise stress. Proc Soc Exp Biol Med. 1993;204:184–190. doi: 10.3181/00379727-204-43650. [DOI] [PubMed] [Google Scholar]
- Song K, Coleman RA, Alber C, Ballas ZK, Waldschmidt TJ, Mortari F, LaB-recque DR, Cook RT. TH1 cytokine response of CD57+ T-cell subsets in healthy controls and patients with alcoholic liver disease. Alcohol. 2001;24:155–167. doi: 10.1016/s0741-8329(01)00146-x. [DOI] [PubMed] [Google Scholar]
- Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leuk Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- Sosa L, Vidlak D, Strachota JM, Pavlik J, Jerrells TR. Rescue of in vivo FAS-induced apoptosis of hepatocytes by corticosteroids either associated with alcohol consumption by mice or provided exogenously. Int Immunopharmocal. 2005;5:301–314. doi: 10.1016/j.intimp.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Spencer RL, McEwen BS. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990;52:481–489. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Jafee RC, Ritzmann RF. Corticosterone concentrations in mice during ethanol drinking and withdrawal. J Pharm Pharmacol. 1978;30:371–374. doi: 10.1111/j.2042-7158.1978.tb13259.x. [DOI] [PubMed] [Google Scholar]
- Tabata T, Tani T, Endo Y, Hanasawa K. Bacterial translocation and peptidoglycan translocation by acute ethanol administration. J Gastroenterol. 2002;37:726–731. doi: 10.1007/s005350200118. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, French SW. Evolution of intragastric ethanol infusion model. Alcohol. 1993;10:437–441. doi: 10.1016/0741-8329(93)90060-2. [DOI] [PubMed] [Google Scholar]
- Wolniak KL, Noelle RJ, Waldschmidt TJ. Characterization of (4-hydroxy-3- nitrophenyl)acetyl (NP)-specific germinal center B cells and antigen-binding B220- cells after primary NP challenge in mice. J Immunol. 2006;177(4):2072–2079. doi: 10.4049/jimmunol.177.4.2072. [DOI] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Zhu X, Coleman RA, Alber C, Ballas ZK, Waldschmidt TJ, Ray NB, Krieg AM, Cook RT. Chronic ethanol ingestion by mice increases expression of CD80 and CD86 by activated macrophages. Alcohol. 2004;32:91–100. doi: 10.1016/j.alcohol.2004.01.004. [DOI] [PubMed] [Google Scholar]