Abstract
Cellular senescence is the ultimate and irreversible loss of replicative capacity occurring in primary somatic cell culture. It is triggered as a stereotypic response to unrepaired nuclear DNA damage or to uncapped telomeres. In addition to a direct role of nuclear DNA double-strand breaks as inducer of a DNA damage response, two more subtle types of DNA damage induced by physiological levels of reactive oxygen species (ROS) can have a significant impact on cellular senescence: Firstly, it has been established that telomere shortening, which is the major contributor to telomere uncapping, is stress dependent and largely caused by a telomere-specific DNA single-strand break repair inefficiency. Secondly, mitochondrial DNA (mtDNA) damage is closely interrelated with mitochondrial ROS production, and this might also play a causal role for cellular senescence. Improvement of mitochondrial function results in less telomeric damage and slower telomere shortening, while telomere-dependent growth arrest is associated with increased mitochondrial dysfunction. Moreover, telomerase, the enzyme complex that is known to re-elongate shortened telomeres, also appears to have functions independent of telomeres that protect against oxidative stress. Together, these data suggest a self-amplifying cycle between mitochondrial and telomeric DNA damage during cellular senescence.
INTRODUCTION
Cellular senescence is the ultimate and irreversible loss of replicative capacity occurring in primary somatic cell culture. The discovery of replicative senescence had profound influences not only with respect to the way that ageing is studied, but also how ageing is perceived.
Initially, it was thought that cells once removed from an organism would be able to replicate indefinitely, mainly as a consequence of a long-held claim by Alexei Carrel that chicken embryonic fibroblast cultures could be kept in culture indefinitely (1). These findings lead to a widespread notion that ageing was not a consequence of an intrinsic cellular process but some characteristic inherent to the existence of cells in a ‘body environment’. In this context, it is easily understandable how Hayflick's finding of a finite lifespan of human fibroblasts had tremendous impact on our current perception of ageing. He found that embryo-derived fibroblasts can divide 50 ± 10 times before arresting irreversibly (2). This finding suggested that an intrinsic molecular process must account for this phenomenon.
Since then, a thorough characterisation of the various phenotypic changes occurring with senescence had been conducted by various laboratories in the 1970s and 1980s, but none could give unequivocal clues as to the mechanism or mechanisms behind it (3) until it was suggested that the shortening of telomeres, the ends of chromosomes, could function as a replicometer (counting the finite number of cell divisions) and as a trigger of replicative senescence in normal diploid cells (4,5). It was the Russian biologist Alexei Olovnikov who in the late 1960s, after learning about Hayflicks’ discovery, first predicted the shortening of telomeres as an explanation for finite cell division in cells grown in culture (6). This is still one of the most amazing examples of scientific foresight, since it took more than 20 years to show experimentally that the amount of telomeric DNA does decline with ageing of human fibroblasts (7). Of course, it was quite possible that this was a mere marker of senescence like many others that had been observed and no evidence of causality had been demonstrated. Later, this question was answered by showing that ectopic expression of the catalytic subunit of telomerase, an enzyme able to counteract telomere shortening, can overcome senescence and lead to cell immortalization on its own (8).
Telomere shortening was proposed as a counting mechanism, which could explain two distinct observations, namely the reproducibility of the ‘Hayflick limit’ and the fact that cells frozen at a certain population doubling level (PDL) would retain a memory of their PDL and, when thawed, undergo the expected maximum number of divisions (9). This was suggestive that a biological programme of ageing was at hand. The alternative interpretation is that telomere loss is merely a consequence of the cell's inability to synthesise new telomere sequences, and as such, a failure to mobilise resources for maintenance. As predicted by evolutionary theories of ageing, telomere shortening can thus be seen as an example of limited investment in long-term somatic maintenance and repair function (10). There are good reasons why telomere shortening is unlikely to be a counting mechanism. One is the observation that individual cells from clonally derived populations show heterogeneous division potential (11) and large heterogeneity in telomere length both between chromosome ends within individual cells and between cells (12–14). Moreover, it has been shown that the fraction of senescent cells present in a mass population increases progressively with population doublings, using BrdU labelling (15), Ki67 staining (16), p53-reporter assay (17) and staining for γ-H2AX, a marker for senescence-associated DNA damage foci (18). These senescent cells in ‘young’ cultures showed characteristics of mitochondrial dysfunction including high levels of reactive oxygen species (ROS) together with short telomeres and activation of telomere-induced DNA damage signalling (19–21). This indicates that the ‘Hayflick limit’ can only be applied to mass populations of cells, and that the lifespan of an individual cell lineage is not controlled by a defined genetic programme, but governed by stochastic factors upstream of telomere shortening, probably related to oxidative stress. Therefore, we will now examine in detail how oxidative stress does affect telomeres specifically and which role mitochondria play in this process. We will also review the evidence suggesting a role for mitochondria, in particular mitochondrial DNA, in the process of cellular senescence.
TELOMERE ATTRITION IS DEPENDENT ON MITOCHONDRIAL FUNCTION
The minimal rate of telomere shortening predicted from the end replication problem is ∼3 bp/end/cell division (22), however, human cells lacking telomerase lose on average 50–300 bp/end/cell division. How can this be explained? There is good evidence, both in Saccharomyces cerevisiae and in human cells (23–26) that telomere ends are processed by multiple nucleases to generate protruding 3′ ends as part of telomere structure. To what extent this end processing actually contributes to telomere shortening is less clear. The simplest idea of a direct correlation between overhang length and telomere shortening rate has been refused experimentally. A study involving fibroblast strains from 21 donors with 2 orders of magnitude of variation in telomere shortening rate failed to show any correlation between telomere overhang length and shortening rate, suggesting that overhang length does not correlate with telomere shortening (27).
Oxidative stress is another factor that contributes to telomere attrition (28). In normal cell culture conditions, cells are exposed to above-physiological levels of oxygen. While oxygen pressure in the environment is 137 mmHg (corresponding to 21% oxygen at sea level), cells in an organism are exposed to an oxygen partial pressure typically between 3 and 7%. Moreover, it has been demonstrated that oxygen pressure has a significant impact on the cells’ replicative lifespan. Packer and Fuehr have shown that the replicative lifespan of human diploid cells can be extended by growing them in low oxygen (29). Also, by growing telomerase-positive mice embryonic fibroblasts at 3% oxygen for at least 60 days (30), it has been shown that telomere-independent senescence at the level of a whole culture can be significantly postponed by lowering oxidative stress. However, these experiments do not exclude the possibility that individual mouse cells grown under physiological oxygen may accumulate sufficient DNA damage to become arrested (31).
Oxidative stress can induce various types of DNA damage, including oxidized bases, single- and double-strand breaks (SSBs and DSBs). DSBs trigger a DNA damage response that, if persistent, can activate senescence via the p53 and p21 tumour suppressors. This pathway has been well characterized (32). ATM and ATR are recruited to the site of damage and are activated, leading to phosphorylation of the tail of a histone protein variant called ‘H2A.X’ adjacent to the site of DNA damage. It is thought that this phosphorylation of histone H2A.X facilitates the focal assembly of checkpoint and DNA repair factors including 53BP1, MDC1/NFBD1 and Nbs1, and also promotes the activation by phosphorylation of Chk1 and Chk2, which converge the signal on p53. This response occurs when non-telomeric DNA damage is generated by various agents like oxidative stress and ionizing radiation and can lead to induction of a senescent phenotype (33–35).
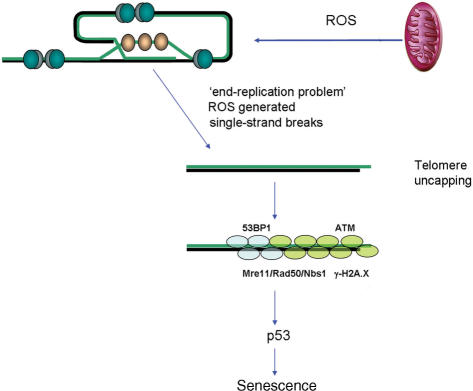
Telomeres are normally in a ‘capped’ state, i.e. unrecognizable to DNA damage response and repair enzyme complexes (36). Structurally, they form terminal loops, which are stabilised by a number of telomere binding proteins. Two of these proteins bind to double-stranded telomeric DNA (TRF1 and -2) and POT-1 binds to single-stranded telomeric DNA. This complex of proteins has been called ‘shelterin’, since it ‘shelters’ i.e. protects chromosome ends (37). It is believed that telomere shortening destabilizes telomeric loops (38) and as a consequence increases the probability of telomere uncapping. Few years ago, it was shown that uncapping of telomeres, whether by inhibition of TRF2 or telomere shortening, activates the same DNA damage response as do DSBs (18,39) (Figure 1).
Figure 1.
Telomeres shorten with cell division due to the ‘end-replication problem’ and single-strand break accumulation due to damage by ROS generated as by-product of mitochondrial respiration. This induces a DNA damage response including formation of telomeric DNA damage foci and activation of p53. Activated p53 triggers senescent growth arrest.
Thus, cellular senescence can be induced as a response to either persistent DSB (telomere-independent or stress-induced premature senescence) or uncapped telomeres (replicative senescence). Telomere-dependent, replicative senescence is not stress independent, however. The intensity and dosage of oxidative stress determine the probability of DSB generation. Intense, acute stress will generate DSBs at higher frequency and might modify these broken DNA ends to make them more resistant to repair, leading to DSB persistence and so induction of senescence via non-telomeric DNA damage response. Chronic oxidative stress of low intensity mainly generates oxidative base modifications and base excision repair intermediates, i.e. abasic sites and SSBs.
Telomeres acquire such oxidative single-strand damage faster than the bulk of the genome for two reasons: First, sequences containing guanine triplets are exquisitely sensitive to oxidative modification (40,41). For instance, a human telomere sequence inserted in a plasmid showed up to 7-fold more strand breakage than a control sequence (41). Second, repair of SSB (42), and to some extent also of UV-induced damage (43), is significantly less efficient in telomeres as compared to the bulk of the genome. This holds true even for a comparison between telomeres and interstitial guanine-rich repetitive sequence tracts (42). The cause for this telomere-specific repair deficiency has not been established with certainty yet. However, a structural basis for it is probable, since TRF2 binds to telomeric double-stranded DNA, stabilizes the telomeric loop and thus contributes to telomere capping. Overexpression of TRF2 decreases the telomere length threshold at which replicative senescence is signalled in accordance with improved capping (44). However, it also accelerates the rate of telomere shortening (44) and further decreases the efficiency of SSB repair specifically in telomeres (45). The most simplistic interpretation of these data is that telomere capping would be detrimental to free access of DNA repair complexes to telomeres. It has been shown that TRF2 (and TRF1) binding can stall the replication fork progression at telomeres (46). However, it needs to be mentioned that TRF2 plays more complex roles at telomeres as it interacts with and inhibits enzymes involved in DNA repair including ATM (47) and Polymerase β (48), while its binding to telomeres is reduced by oxidative DNA damage (49).
Irrespective of the specific mechanism causing repair inefficiency, telomeres enter DNA replication with higher frequencies of single-strand DNA damage than all the bulk of the genome. This contributes significantly to telomere shortening (50), with the frequency of single-strand damage directly correlated with the amount of telomere loss (51). Oxidative damage-dependent telomere shortening might be caused by a temporal stalling of the replication fork or might be recombination dependent (52). It might be a significant cause of telomere length heterogeneity between different chromosomes and between the same chromosomes in different clonally derived cells (12,19).
Finally, oxidative stress interferes with telomere maintenance also via its effect on telomerase activity. Telomerase is a ribonucleoprotein complex that consists of two main components – the RNA component (TER), containing the antisense template sequence for telomere synthesis and the catalytic protein, telomerase reverse transcriptase –(TERT). Telomerase re-elongates telomeres in a highly regulated fashion to counteract telomere shortening. This is sufficient to confer extended lifespan and often immortality to cells that would otherwise senesce due to continuous telomere shortening (8). Physiologically, human telomerase is expressed in germ line and stem cells and in most cancers, but its expression is low (haematopoetic cells and endothelial cells) or undetectable without sophisticated enrichment protocols (53) in somatic human cells like fibroblasts. Oxidative stress diminished telomerase activity in endothelial cells (54–56), vascular smooth muscle cells (57) or leukemic cancer cell lines (58) together with an acceleration of telomere attrition. However, oxidative stress had no effect on telomerase activity in fibroblasts overexpressing TERT ectopically (19), suggesting preferentially transcriptional regulation.
Two properties of oxidative stress-dependent telomere attrition are important. First, it is dependent on DNA replication. The probability of generating DSBs directly at a frequency high enough to result in a measurable telomere loss without DNA replication is negligible for physiologically and pathologically obtainable stress levels. Second, oxidative damage can be a major, and often actually the main, contributor to telomere loss in cell culture under standard conditions. This is shown by the fact that telomere shortening rates in cultured human fibroblasts not only accelerate if oxidative stress is increased, but that they can be diminished if oxidative stress levels are reduced below those in standard conditions by using free radical scavengers (59), enzymatic (60) or non-enzymatic (61) antioxidants or low ambient oxygen concentrations (62,63), in some cases to levels similar to the minimal values predicted by the end replication problem alone. For instance, we measured telomere shortening rates of 7 ± 9 bp/PD in BJ fibroblasts, a strain with very high antioxidant capacity (28,60), and of 9 ± 29 bp/PD in MRC5 fibroblasts treated with Dinitrophenol, an agent that reduces mitochondrial ROS generation by mild uncoupling (20)
Now, the important question is: Is oxidative stress-dependent telomere length regulation merely a response to more or less artificial changes in cell culture environments (i.e. a ‘cell culture artefact’)? or is it part of an intrinsic mechanism governing the ageing process? Correlative evidence from human population studies collectively suggests an association of short telomeres with conditions of increased oxidative stress, including smoking, obesity, various cardiovascular diseases, psychological and socio-economic stress (64–66). Cells in telomere-dependent senescence accumulate in aging baboon skin (67,68). We propose mitochondrial dysfunction as a major mechanistic link between stress-dependent and telomere-dependent physiological ageing processes in cells. Mitochondria are the major source of oxygen-free radicals in cells. It has been revealed that mitochondrial function changes as cells reach the end of their replicative lifespan, leading to increased generation of ROS and metabolic inefficiency (20,69–72). Increased ROS leads to accumulation of oxidation products, such as protein carbonyls and lipofuscin, which have been shown to occur in senescent fibroblasts (73,74).
In addition to correlative evidence showing mitochondrial dysfunction in telomere-dependent senescence, there is also good evidence supporting a causal role for mitochondrial dysfunction in the process. Selective targeting of antioxidants directly to the mitochondria has been shown to counteract telomere shortening and increase lifespan in fibroblasts under mild oxidative stress (61). Continuous treatment with nicotinamide, which affects mitochondrial function and ROS generation, has been reported to extend lifespan (an amazing 1.6-fold increase) and decelerate telomere shortening (75). Also, mild chronic uncoupling of mitochondria that reduced the production of superoxide anion, improved telomere maintenance and extended telomere-dependent lifespan (20).
On the other hand, mitochondrial dysfunction generated by severe mitochondrial depolarization using an uncoupling agent FCCP led to an increased production of ROS, telomere loss and chromosome fusions in mouse embryos (76). Moreover, Oexle and Zwirner showed that patients with mitochondrial diseases MELAS and LHON had, on average, 1.5 kb shorter telomeres in white blood cells than those of age-matched controls (77).
This set of data is suggestive of a novel role for telomeres as sensors of mitochondrial function in a cell (78). When mitochondrial dysfunction occurs, with concomitant increased ROS generation, the specific susceptibility of telomeres to oxidative damage (42) leads to accelerated telomere shortening, increased probability of uncapping, activation of a DNA damage response and finally irreversible cell cycle arrest (Figure 1). Thus, as previously suggested, the replicative lifespan of a cell could be determined by a network of processes involving mitochondrial dysfunction, oxidative stress and telomere shortening (20,78,79).
However, one important question remains: What is the cause of mitochondrial dysfunction and ROS generation? Is it dependent on damage to the mitochondrial genome?
MTDNA DAMAGE AND SENESCENCE: IS THERE A LINK?
Mitochondrial DNA has for a long time been implicated in the ageing process. The idea was first proposed by Harman, who predicted the involvement of free radicals in the ageing process (80,81). The concept was that the close proximity between the sites of ROS production and the mtDNA would render the latter more susceptible to damage than the nuclear genome, and lead to defects in mitochondrial metabolism. The long-held view that the mitochondrial genome is not protected by histones and mtDNA repair is inefficient has been challenged in recent years by showing that TFAM acts like a histone covering mtDNA (82) and that there exist functional repair mechanisms for different types of DNA damage within mitochondria with the possible exception of nucleotide excision repair (83). Even so, it has been shown that mtDNA damage is more extensive and persistent than nuclear DNA damage in cultured human fibroblasts following treatment with hydrogen peroxide (84) and this correlates with functional impairment in mitochondria (85). Treatment of normal human fibroblasts with sublethal levels of oxidative stress has also been associated with accumulation of the mtDNA common deletion, a specific 4977 bp deletion often found in mtDNA (33).
An age-dependent increase of mtDNA mutation frequency was first observed in post-mitotic cells such as neurons and muscle cells (86–89), but has since been described in highly proliferative cells such as the epithelial stem cells of the gut wall as well (90). mtDNA mutations are responsible for deficient activity of respiratory chain enzymes such as Cytochrome C oxidase, which is a mitochondrial membrane–bound enzyme composed of subunits that are encoded in both the mitochondria (COX subunits I, II and III) and the nucleus (all others). The frequency of COX-deficient human heart muscle cells was shown to increase significantly with age, but remained always below 1% (88). In situ hybridization studies using mtDNA probes showed accumulation of mtDNA deletions in COX negative cells (91). Using microdissection to isolate muscle fibres, it was shown that all electron transport chain–deficient fibres in rat skeletal muscle contained mtDNA deletion mutations (92,93). In single neurons from substantia nigra of humans showing COX deficiency, an age-dependent increase in mtDNA deletions was found in two independent studies (94,95).
Consequently, an attractive hypothesis is that the increase of mutational load in mtDNA with age contributes causally to mitochondrial dysfunction and increased ROS production, so creating a circulus vitiosus. However, new data question the causal link between mtDNA mutations and ROS generation. Homozygous knock-in mice expressing a proof reading–deficient version of the nucleus-encoded catalytic subunit of mtDNA polymerase γ (PolgA) have been generated that showed an extremely high level of mtDNA mutations and deletions and a significant decrease in lifespan (96). However, even though mitochondrial function was affected, no evidence for increased oxidative stress was found in these animals (97,98). Most strikingly, mice that are heterozygous for PolgA function showed no significant reduction in lifespan despite a mtDNA point mutation burden 30 times higher than in old wild-type animals (99). These studies suggested that mtDNA point mutation load does not limit lifespan of wild-type mice and that mtDNA point mutations, even at very high levels, do not necessarily lead to increased mitochondrial ROS generation. It remains to be seen whether there is a more sinister role for mtDNA deletions in aging.
So far, there is very little data to assess the role, if any, of mtDNA mutations in cellular replicative senescence. Accumulation of mtDNA point mutations in fibroblasts isolated from old individuals has been reported (100). Most strikingly, a T414G transversion was found in a high proportion (up to 50%) of mtDNA molecules from individuals above 65 years of age. However, when several fibroblasts populations carrying the heteroplasmic T414G mutation were grown in vitro, outgrowth of the mutant cells by wild-type cells was observed (101). This led to the suggestion that accumulation of mtDNA point mutations is a phenomenon occurring exclusively in vivo.
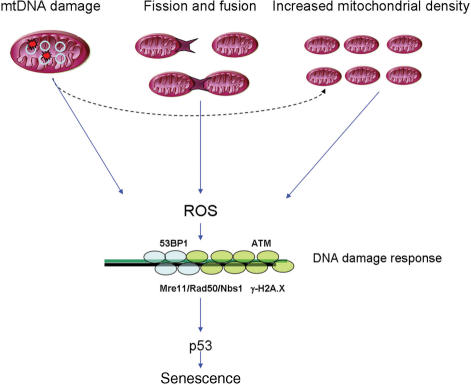
Using a real-time PCR-based method (102), we have recently found evidence of increased mtDNA damage in senescent human fibroblasts (20). However, these are correlative data and it is not possible to discern whether mtDNA damage caused mitochondrial dysfunction or was a consequence of it. It could well be that other factors besides mtDNA damage and/or mutation might be more important for the generation of ROS. Such factors could include the regulation of mitochondrial turnover or mitochondrial fusion and fission (Figure 2) in addition to extra-mitochondrial ones.
Figure 2.
Possible mechanisms influencing mitochondrial ROS generation: (1) mtDNA damage could lead to dysfunctional respiratory chain activity and increased ROS, (2) mitochondrial elongation by decreased fission has been shown to increase ROS generation, (3) increased mitochondrial biogenesis has been shown to correlate with ROS generation and induce cellular senescence. ROS-generated telomere shortening as well as double-stranded breaks at non-telomeric DNA activate a DNA damage response and cellular senescence.
Accordingly, a recent study has shown that mitochondrial elongation by knock down of the mitochondrial fission protein hFis1 led to induction of senescence, possibly through increased ROS generation and activation of a DNA damage response (103). Moreover, there is some evidence suggesting that mitochondrial ROS generation in senescence might be due to increased mitochondrial density, which can occur as a consequence of mitochondrial dysfunction (20,104). In fact, it has been shown that overexpression of PGC-1α, the master regulator of mitochondrial biogenesis, leads to induction of senescence in human fibroblasts (105). The association between increased mitochondrial density and ROS generation might be due to amplification of damaged mtDNA molecules. In summary, the role of mtDNA damage and mutation in senescence is still unclear.
Telomerase and oxidative stress: a role beyond telomeres
In recent years, indications for additional functions of telomerase independent of telomere maintenance are accumulating. If telomerase is inhibited in tumour cells, there is either a delayed response that depends on telomere shortening or a rapid effect on cell viability without any measurable effect on telomere length (106–110). These data suggest that telomerase promotes cell survival and stress resistance by mechanisms that appear to be largely independent on telomere length maintenance. Zhang et al. and Fu et al. (111,112) showed an increased resistance of hTERT overexpressing cells to apoptotic stimuli at an early, premitochondrial step. Sharma et al. (113) found an increased repair capacity of hTERT overexpressing cells. We and others demonstrated an enhanced sensitivity of tumour cells to certain DNA damaging agents when telomerase was impaired (107,113–117). We showed that overexpression of TERT conferred increased stress resistance, improved antioxidant defence and differentiation capacity to mouse embryonic stem cells (118).
Ectopic hTERT expression in normal cells as well as hTERT inhibition/depletion in telomerase positive cancer cells and even in yeast S. cerevisiae caused significant changes to the transcriptome and global gene expression patterns (106,108,119,120) that are largely unrelated to telomere maintenance. Interestingly, among a wide range of functional classes, large groups of genes with functions in metabolism, specifically mitochondrial metabolism, have repeatedly been reported to appear dependent of telomerase (106,119). Bagheri et al. (106) found that telomerase modulated glucose consumption and appeared to control the glycolytic pathway in tumour cells, thereby potentially altering the energy state. Recently, telomerase has been added to a growing list of proteins, such as p53, HMGA1, VHL, APP, prohibitins, Lon-protease, etc., that can shuttle between the nucleus and the different subcellular compartments including mitochondria. Importantly, it has been shown that telomerase that normally is located in the nucleus can shuttle to cytoplasm and/or mitochondria upon oxidative challenge (55,121–123).
Santos et al. (117) described a specific mitochondrial import sequence at the N-terminus of hTERT. Oxidative stress activates nuclear export and mitochondrial import for both ectopically expressed and endogenous telomerase (55,121–123). Haendeler et al. (55) found that nuclear export of telomerase occurs during in vitro senescence of human endothelial cells and can be delayed by treatment with antioxidants. One possible mechanism connecting oxidative stress and nuclear exclusion of telomerase is the activation of Src kinases by ROS (124). It has been shown that phosphorylation of tyrosine 707 by Src kinase is necessary for nuclear exclusion of hTERT following oxidative stress (121). A similar process has been described for T-lymphocytes where telomerase function is regulated via phosphorylation and nuclear translocation (125). These data suggest that subcellular shuttling is not an artefact of ectopic hTERT expression. Rather, transport of hTERT to mitochondria is a directed, naturally occurring process that is regulated by either exogeneously or endogenously generated oxidative stress.
The biological significance of the translocation of hTERT into mitochondria under oxidative stress is still elusive. Santos et al. (122,123) found that ectopic overexpression of hTERT led to higher levels of mtDNA damage under acute oxidative stress. In contrast, there is an increasing number of papers showing the telomerase protects mitochondrial function and displays an anti-apoptotic function. Massard et al. (109) characterised hTERT as an endogenous inhibitor of mitochondrial apoptosis induced by different agents including oxidative stress in cancer cells. Neurons with decreased hTERT levels exhibited increased levels of oxidative stress and mitochondrial dysfunction after exposure to amyloid beta, whereas overexpression of hTERT in the same system led to an improved mitochondrial function and a decreased oxidative stress level (126). Kang et al. (127) found that after ischemic brain injury TERT is induced in postmitotic neurons in TERT transgenic mice and prevents NMDA neurotoxicity via shifts of free cytosolic free Ca2+ to the mitochondria. The authors found an enhanced basal level of mitochondrial membrane potential and a higher Ca2+ storage capacity of the mitochondria due to TERT overexpression. Our own data indicate protection of mtDNA and mitochondrial function under oxidative stress by ectopically expressed hTERT (unpublished).
These results show that there is an interaction between mitochondrially localised hTERT and mitochondrial function that seems to be complex and can involve different physiological and signalling pathways. It is not yet clear whether mitochondrial localisation of hTERT is necessary to improve mitochondrial function and/or to protect cells from stress-induced apoptosis.
CONCLUSIONS
It appears highly probable that damage to two specific subsets of cellular DNA, namely telomeres and mtDNA, plays an important role in cellular senescence. These two types of DNA damage are functionally interrelated at various levels. mtDNA damage, especially deletions, might contribute to mitochondrial dysfunction and ensuing ROS production, which is a major causal factor for telomere damage and shortening, resulting eventually in senescence signalling. Conversely telomerase, the central enzyme in telomere length maintenance, can translocate to mitochondria under stress and impacts on mtDNA protection and mitochondrial function. It is possible that there is a self-amplifying cycle between mitochondrial and telomeric DNA damage driving cellular senescence.
ACKNOWLEDGEMENTS
Work leading to this review was supported by programme grant 252 from Research into Ageing, UK and by a BBSRC/EPSRC systems biology grant (CISBAN). Funding to pay the Open Access publication charges for this article was provided by BBSRC.
Conflict of interest statement. None declared.
REFERENCES
- 1.Witkowski JA. Dr. Carrel's immortal cells. Med. History. 1980;24:129–142. doi: 10.1017/s0025727300040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 3.Holliday R. Understanding Ageing. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 4.Olovnikov AM. [Principle of marginotomy in template synthesis of polynucleotides] Dokl Akad Nauk SSSR. 1971;201:1496–1499. [PubMed] [Google Scholar]
- 5.Watson JD. Origin of concatemeric T7 DNA. Nat. New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 6.Olovnikov AM. Telomeres, telomerase, and aging: origin of the theory. Exp. Gerontol. 1996;31:443–448. doi: 10.1016/0531-5565(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 7.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 9.Hayflick L. The illusion of cell immortality. Br. J. Cancer. 2000;83:841–846. doi: 10.1054/bjoc.2000.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Smith JR, Whitney RG. Intraclonal variation in proliferative potential of human diploid fibroblasts: stochastic mechanism for cellular aging. Science. 1980;207:82–84. doi: 10.1126/science.7350644. [DOI] [PubMed] [Google Scholar]
- 12.Baird DM, Rowson J, Wynford-Thomas D, Kipling D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat. Genet. 2003;33:203–207. doi: 10.1038/ng1084. [DOI] [PubMed] [Google Scholar]
- 13.Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, Dirks RW, Raap AK, Tanke HJ. Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- 14.Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol. Biol. Cell. 2004;15:3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kill IR, Faragher RG, Lawrence K, Shall S. The expression of proliferation-dependent antigens during the lifespan of normal and progeroid human fibroblasts in culture. J. Cell Sci. 1994;107:571–579. doi: 10.1242/jcs.107.2.571. [DOI] [PubMed] [Google Scholar]
- 16.Thomas E, Al-Baker E, Dropcova S, Denyer S, Ostad N, Lloyd A, Kill IR, Faragher RGA. Different kinetics of senescence in human fibroblasts and peritoneal mesothelial cells. Exp. Cell Res. 1997;236:355–358. doi: 10.1006/excr.1997.3760. [DOI] [PubMed] [Google Scholar]
- 17.Bond JA, Wyllie FS, Wynford-Thomas D. Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene. 1994;9:1885–1889. [PubMed] [Google Scholar]
- 18.Fagagna FdAd, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Ruiz C, Saretzki G, Petrie J, Ladhoff J, Jeyapalan J, Wei W, Sedivy J, von Zglinicki T. Stochastic variation in telomere shortening rate causes heterogeneity of human fibroblast replicative life span. J. Biol. Chem. 2004;279:17826–17833. doi: 10.1074/jbc.M311980200. [DOI] [PubMed] [Google Scholar]
- 20.Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, Wappler I, Birkett M, Harold G, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Zglinicki T, Petrie J, Kirkwood TB. Telomere-driven replicative senescence is a stress response. Nat. Biotechnol. 2003;21:229–230. doi: 10.1038/nbt0303-229b. [DOI] [PubMed] [Google Scholar]
- 22.de Lange T. In: Telomeres. de Lange T, Lundblad V, Blackburn EH, editors. New York: Cold Spring Harbour Laboratory Press; 2006. pp. 387–431. [Google Scholar]
- 23.Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997;88:657–666. doi: 10.1016/s0092-8674(00)81908-x. [DOI] [PubMed] [Google Scholar]
- 24.Maringele L, Lydall D. EXO1-dependent single-stranded DNA at telomeres activates subsets of DNA damage and spindle checkpoint pathways in budding yeast yku70Delta mutants. Genes Dev. 2002;16:1919–1933. doi: 10.1101/gad.225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sfeir AJ, Chai W, Shay JW, Wright WE. Telomere-end processing: the terminal nucleotidesof human chromosomes. Mol. Cell. 2005;18:131–138. doi: 10.1016/j.molcel.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Zubko MK, Guillard S, Lydall D. Exo1 and Rad24 differentially regulate generation of ssDNA at telomeres of Saccharomyces cerevisiae cdc13–1 mutants. Genetics. 2004;168:103–115. doi: 10.1534/genetics.104.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keys B, Serra V, Saretzki G, von Zglinicki T. Telomere shortening in human fibroblasts is not dependent on the size of the telomeric-3'-overhang. Aging Cell. 2004;3:103–109. doi: 10.1111/j.1474-9728.2004.00094.x. [DOI] [PubMed] [Google Scholar]
- 28.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 29.Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267:423–425. doi: 10.1038/267423a0. [DOI] [PubMed] [Google Scholar]
- 30.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell. Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell. Biol. 2004;6:168–170. doi: 10.1038/ncb1095. [DOI] [PubMed] [Google Scholar]
- 32.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 33.Dumont P, Burton M, Chen QM, Gonos ES, Frippiat C, Mazarati J-B, Eliaers F, Remacle J, Toussaint O. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Rad. Biol. Med. 2000;28:361–373. doi: 10.1016/s0891-5849(99)00249-x. [DOI] [PubMed] [Google Scholar]
- 34.Herskind C, Rodemann HP. Spontaneous and radiation-induced differentiation of fibroblasts. Exp. Gerontol. 2000;35:747–755. doi: 10.1016/s0531-5565(00)00168-6. [DOI] [PubMed] [Google Scholar]
- 35.Robles S, Adami G. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- 36.Blackburn EH, Chan S, Chang J, Fulton TB, Krauskopf A, McEachern M, Prescott J, Roy J, Smith C, et al. Molecular manifestations and molecular determinants of telomere capping. Cold Spring Harb Symp Quant Biol. 2000;65:253–263. doi: 10.1101/sqb.2000.65.253. [DOI] [PubMed] [Google Scholar]
- 37.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 38.Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 39.Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- 40.Oikawa S, Tada-Oikawa S, Kawanishi S. Site-specific DNA damage at the GGG sequence by UVA involves acceleration of telomere shortening. Biochemistry. 2001;40:4763–4768. doi: 10.1021/bi002721g. [DOI] [PubMed] [Google Scholar]
- 41.Henle E, Han Z, Tang N, Rai P, Luo Y, Linn S. Sequence-specific DNA cleavage by Fe2+-mediated fenton reactions has possible biological implications. J. Biol. Chem. 1999;274:962–971. doi: 10.1074/jbc.274.2.962. [DOI] [PubMed] [Google Scholar]
- 42.Petersen S, Saretzki G, von Zglinicki T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Exp. Cell Res. 1998;239:152–160. doi: 10.1006/excr.1997.3893. [DOI] [PubMed] [Google Scholar]
- 43.Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. PNAS. 1995;92:258–262. doi: 10.1073/pnas.92.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karlseder J, Smogorzewska A, de Lange T. Senescence induced by altered telomere state, not telomere loss. Science. 2002;295:2446–2449. doi: 10.1126/science.1069523. [DOI] [PubMed] [Google Scholar]
- 45.Richter T, Saretzki G, Nelson G, Melcher M, Olijslagers S, von Zglinicki T. TRF2 overexpression diminishes repair of telomeric single-strand breaks and accelerates telomere shortening in human fibroblasts. Mech. Ageing Dev. 2007;128:340–345. doi: 10.1016/j.mad.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Ohki R, Ishikawa F. Telomere-bound TRF1 and TRF2 stall the replication fork at telomeric repeats. Nucleic Acids Res. 2004;32:1627–1637. doi: 10.1093/nar/gkh309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlseder J, Hoke K, Mirzoeva OK, Bakkenist C, Kastan MB, Petrini JHJ, Lange TD. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. 2004;2:e240. doi: 10.1371/journal.pbio.0020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fotiadou P, Henegariu O, Sweasy JB. DNA polymerase {beta} interacts with TRF2 and induces telomere dysfunction in a murine mammary cell line. Cancer Res. 2004;64:3830–3837. doi: 10.1158/0008-5472.CAN-04-0136. [DOI] [PubMed] [Google Scholar]
- 49.Opresko PL, Fan J, Danzy S, Wilson DM, III, Bohr VA. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33:1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 51.Sitte N, Saretzki G, von Zglinicki T. Accelerated telomere shortening in fibroblasts after extended periods of confluency. Free Rad. Biol. Med. 1998;24:885–893. doi: 10.1016/s0891-5849(97)00363-8. [DOI] [PubMed] [Google Scholar]
- 52.Richter T, von Zglinicki T. In: Oxidative DNA Damage and Telomere Shortening. Evans MD, Cooke MS, editors. Austin, TX: Landes Bioscience; 2006. pp. 100–108. [Google Scholar]
- 53.Masutomi K, Possemato R, Wong JMY, Currier JL, Tothova Z, Manola JB, Ganesan S, Lansdorp PM, Collins K, et al. The telomerase reverse transcriptase regulates chromatin state and DNA damage responses. PNAS. 2005;102:8222–8227. doi: 10.1073/pnas.0503095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furumoto K, Inoue E, Nagao N, Hiyama E, Miwa N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci. 1998;63:935–948. doi: 10.1016/s0024-3205(98)00351-8. [DOI] [PubMed] [Google Scholar]
- 55.Haendeler J, Hoffmann J, Diehl JF, Vasa M, Spyridopoulos I, Zeiher AM, Dimmeler S. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ. Res. 2004;94:768–775. doi: 10.1161/01.RES.0000121104.05977.F3. [DOI] [PubMed] [Google Scholar]
- 56.Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 2004;117:2417–2426. doi: 10.1242/jcs.01097. [DOI] [PubMed] [Google Scholar]
- 57.Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ. Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 58.Pizzimenti S, Briatore F, Laurora S, Toaldo C, Maggio M, De Grandi M, Meaglia L, Menegatti E, Giglioni B, et al. 4-Hydroxynonenal inhibits telomerase activity and hTERT expression in human leukemic cell lines. Free Rad. Biol. Med. 2006;40:1578–1591. doi: 10.1016/j.freeradbiomed.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 59.von Zglinicki T, Pilger R, Sitte N. Accumulation of single-strand breaks is the major cause of telomere shortening in human fibroblasts. Free Rad. Biol. Med. 2000;28:64–74. doi: 10.1016/s0891-5849(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 60.Serra V, von Zglinicki T, Lorenz M, Saretzki G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J. Biol. Chem. 2003;278:6824–6830. doi: 10.1074/jbc.M207939200. [DOI] [PubMed] [Google Scholar]
- 61.Saretzki G, Murphy MP, von Zglinicki T. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell. 2003;2:141–143. doi: 10.1046/j.1474-9728.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 62.Forsyth NR, Evans AP, Shay JW, Wright WE. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell. 2003;2:235–243. doi: 10.1046/j.1474-9728.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 63.Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Exp. Gerontol. 2007 doi: 10.1016/j.exger.2007.08.005. DOI: 10.1016/j.expger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 64.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. From the cover: accelerated telomere shortening in response to life stress. PNAS. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. The Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 66.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr. Mol. Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 67.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257–1258. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 68.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allen RG, Tresini M, Keogh BP, Doggett DL, Cristofalo VJ. Differences in electron transport potential, antioxidant defenses, and oxidant generation in young and senescent fetal lung fibroblasts (WI-38) J. Cell Physiol. 1999;180:114–122. doi: 10.1002/(SICI)1097-4652(199907)180:1<114::AID-JCP13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 70.Hutter E, Renner K, Pfister G, Stockl P, Jansen-Durr P, Gnaiger E. Senescence-associated changes in respiration and oxidative phosphorylation in primary human fibroblasts. Biochem. J. 2004;380:919–928. doi: 10.1042/BJ20040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hutter E, Unterluggauer H, Uberall F, Schramek H, Jansen-Durr P. Replicative senescence of human fibroblasts: the role of Ras-dependent signaling and oxidative stress. Exp. Gerontol. 2002;37:1165–1174. doi: 10.1016/s0531-5565(02)00136-5. [DOI] [PubMed] [Google Scholar]
- 72.Zwerschke W, Mazurek S, Stockl P, Hutter E, Eigenbrodt E, Jansen-Durr P. Metabolic analysis of senescent human fibroblasts reveals a role for AMP in cellular senescence. Biochem. J. 2003;376:403–411. doi: 10.1042/BJ20030816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sitte N, Merker K, von Zglinicki T, Grune T. Protein oxidation and degradation during proliferative senescence of human MRC-5 fibroblasts. Free Rad. Biol. Med. 2000;28:701–708. doi: 10.1016/s0891-5849(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 74.Sitte N, Merker K, Grune T, von Zglinicki T. Lipofuscin accumulation in proliferating fibroblasts in vitro: an indicator of oxidative stress. Exp. Gerontol. 2001;36:475–486. doi: 10.1016/s0531-5565(00)00253-9. [DOI] [PubMed] [Google Scholar]
- 75.Kang HT, Lee HI, Hwang ES. Nicotinamide extends replicative lifespan of human cells. Aging Cell. 2006;5:423–436. doi: 10.1111/j.1474-9726.2006.00234.x. [DOI] [PubMed] [Google Scholar]
- 76.Liu L, Trimarchi JR, Smith PJ, Keefe DL. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell. 2002;1:40–46. doi: 10.1046/j.1474-9728.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 77.Oexle K, Zwirner A. Advanced telomere shortening in respiratory chain disorders. Hum. Mol. Genet. 1997;6:905–908. doi: 10.1093/hmg/6.6.905. [DOI] [PubMed] [Google Scholar]
- 78.Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp. Gerontol. 2005;40:466–472. doi: 10.1016/j.exger.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 79.Sozou PD, Kirkwood T. A stochastic model of cell replicative senescence based on telomere shortening, oxidative stress, and somatic mutations in nuclear and mitochondrial DNA. J. Theor. Biol. 2001;213:573–586. doi: 10.1006/jtbi.2001.2432. [DOI] [PubMed] [Google Scholar]
- 80.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 81.Harman D. The biologic clock: the mitochondria? J. Am. Geriatr. Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 82.Wiesner RJ, Zsurka G, Kunz WS. Mitochondrial DNA damage and the aging process; facts and imaginations. Free Radical Res. 2006;40:1284–1294. doi: 10.1080/10715760600913168. [DOI] [PubMed] [Google Scholar]
- 83.Berneburg M, Kamenisch Y, Krutmann J, Rocken M. To repair or not to repair – no longer a question’: repair of mitochondrial DNA shielding against age and cancer. Exp. Dermatol. 2006;15:1005–1015. doi: 10.1111/j.1600-0625.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 84.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. PNAS. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santos JH, Hunakova L, Chen Y, Bortner C, Van Houten B. Cell sorting experiments link persistent mitochondrial DNA damage with loss of mitochondrial membrane potential and apoptotic cell death. J. Biol. Chem. 2003;278:1728–1734. doi: 10.1074/jbc.M208752200. [DOI] [PubMed] [Google Scholar]
- 86.Brierley EJ, Johnson MA, Lightowlers RN, James OF, Turnbull DM. Role of mitochondrial DNA mutations in human aging: implications for the central nervous system and muscle. Ann. Neurol. 1998;43:217–223. doi: 10.1002/ana.410430212. [DOI] [PubMed] [Google Scholar]
- 87.Cottrell DA, Turnbull DM. Mitochondria and ageing. Curr. Opin. Clin. Nutr. Metab. Care. 2000;3:473–478. doi: 10.1097/00075197-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 88.Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart–an age-related phenomenon. A histochemical ultracytochemical study. Am. J. Pathol. 1989;134:1167–1173. [PMC free article] [PubMed] [Google Scholar]
- 89.Muller-Hocker J, Seibel P, Schneiderbanger K, Kadenbach B. Different in situ hybridization patterns of mitochondrial DNA in cytochrome c oxidase-deficient extraocular muscle fibres in the elderly. Virchows Arch. A Pathol. Anat. Histopathol. 1993;422:7–15. doi: 10.1007/BF01605127. [DOI] [PubMed] [Google Scholar]
- 90.Taylor RW, Barron MJ, Borthwick GM, Gospel A, Chinnery PF, Samuels DC, Taylor GA, Plusa SM, Needham SJ, et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J. Clin. Inv. 2003;112:1351–1360. doi: 10.1172/JCI19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee CM, Lopez ME, Weindruch R, Aiken JM. Association of age-related mitochondrial abnormalities with skeletal muscle fiber atrophy. Free Rad. Biol. Med. 1998;25:964–972. doi: 10.1016/s0891-5849(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 92.Cao Z, Wanagat J, McKiernan SH, Aiken JM. Mitochondrial DNA deletion mutations are concomitant with ragged red regions of individual, aged muscle fibers: analysis by laser-capture microdissection. Nucleic Acids Res. 2001;29:4502–4508. doi: 10.1093/nar/29.21.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]
- 94.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 95.Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- 96.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 97.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 98.Trifunovic A, Hansson A, Wredenberg A, Rovio AT, Dufour E, Khvorostov I, Spelbrink JN, Wibom R, Jacobs HT, et al. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. PNAS. 2005;102:17993–17998. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, Loeb LA. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet. 2007;39:540–543. doi: 10.1038/ng1988. [DOI] [PubMed] [Google Scholar]
- 100.Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–779. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- 101.Michikawa Y, Laderman K, Richter K, Attardi G. Role of nuclear background and in vivo environment in variable segregation behavior of the aging-dependent T414G mutation at critical control site for human fibroblast mtDNA replication. Somat. Cell Mol. Genet. 1999;25:333–342. doi: 10.1023/a:1019972500785. [DOI] [PubMed] [Google Scholar]
- 102.Santos JH, Mandavilli BS, Van Houten B. Measuring oxidative mtDNA damage and repair using quantitative PCR. Methods Mol. Biol. 2002;197:159–176. doi: 10.1385/1-59259-284-8:159. [DOI] [PubMed] [Google Scholar]
- 103.Lee S, Jeong S-Y, Lim W-C, Kim S, Park Y-Y, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J. Biol. Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 104.Passos JF, Von Zglinicki T, Kirkwood TB. Mitochondria and ageing: winning and losing in the numbers game. BioEssays. 2007;29:908–917. doi: 10.1002/bies.20634. [DOI] [PubMed] [Google Scholar]
- 105.Xu D, Finkel T. A role for mitochondria as potential regulators of cellular life span. Bioch. Biophy. Res. Com. 2002;294:245–248. doi: 10.1016/S0006-291X(02)00464-3. [DOI] [PubMed] [Google Scholar]
- 106.Bagheri S, Nosrati M, Li S, Fong S, Torabian S, Rangel J, Moore DH, Federman S, LaPosa RR, et al. Genes and pathways downstream of telomerase in melanoma metastasis. PNAS. 2006;103:11306–11311. doi: 10.1073/pnas.0510085103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kondo Y, Kondo S, Tanaka Y, Haqqi T, Barna BP, Cowell JK. Inhibition of telomerase increases the susceptibility of human malignant glioblastoma cells to cisplatin-induced apoptosis. Oncogene. 1998;16:2243–2248. doi: 10.1038/sj.onc.1201754. [DOI] [PubMed] [Google Scholar]
- 108.Li S, Crothers J, Haqq CM, Blackburn EH. Cellular and gene expression responses involved in the rapid growth inhibition of human cancer cells by RNA interference-mediated depletion of telomerase RNA. J. Biol. Chem. 2005;280:23709–23717. doi: 10.1074/jbc.M502782200. [DOI] [PubMed] [Google Scholar]
- 109.Massard C, Zermati Y, Pauleau AL, Larochette N, Métivier D, Sabatier L, Kroemer G, Soria JC. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene. 2006;25:4505–4514. doi: 10.1038/sj.onc.1209487. [DOI] [PubMed] [Google Scholar]
- 110.Saretzki G, Ludwig A, von Zglinicki T, Runnebaum IB. Ribozyme-mediated telomerase inhibition induces immediate cell loss but not telomere shortening in ovarian cancer cells. Cancer Gene Ther. 2001;8:827–834. doi: 10.1038/sj.cgt.7700383. [DOI] [PubMed] [Google Scholar]
- 111.Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J. Biol. Chem. 1999;274:7264–7271. doi: 10.1074/jbc.274.11.7264. [DOI] [PubMed] [Google Scholar]
- 112.Zhang P, Chan SL, Fu W, Mendoza M, Mattson MP. TERT suppresses apoptotis at a premitochondrial step by a mechanism requiring reverse transcriptase activity and 14-3-3 protein binding ability. FASEB J. 2003;17:767–769. doi: 10.1096/fj.02-0603fje. [DOI] [PubMed] [Google Scholar]
- 113.Sharma GG, Gupta A, Wang H, Scherthan H, Dhar S, Gandhi V, Iliakis G, Shay JW, Young CS, et al. hTERT associates with human telomeres and enhances genomic stability and DNA repair. Oncogene. 2003;22:131–146. doi: 10.1038/sj.onc.1206063. [DOI] [PubMed] [Google Scholar]
- 114.Cao Y, Li HE, Mu F-T, Ebisui O, Funder JW, Liu J-P. Telomerase activation causes vascular smooth muscle cell proliferation in genetic hypertension. FASEB J. 2002;16:96–98. doi: 10.1096/cj.01-0447fje. [DOI] [PubMed] [Google Scholar]
- 115.Lee K-H, Rudolph KL, Ju Y-J, Greenberg RA, Cannizzaro L, Chin L, Weiler SR, DePinho RA. Telomere dysfunction alters the chemotherapeutic profile of transformed cells. PNAS. 2001;98:3381–3386. doi: 10.1073/pnas.051629198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ludwig A, Saretzki G, Holm PS, Tiemann F, Lorenz M, Emrich T, Harley CB, von Zglinicki T. Ribozyme cleavage of telomerase mRNA sensitizes breast epithelial cells to inhibitors of topoisomerase. Cancer Res. 2001;61:3053–3061. [PubMed] [Google Scholar]
- 117.Tentori L, Portarena I, Barbarino M, Balduzzi A, Levati L, Vergati M, Biroccio A, Gold B, Lombardi ML, et al. Inhibition of telomerase increases resistance of melanoma cells to temozolomide, but not to temozolomide combined with poly(ADP-Ribose) polymerase inhibitor. Mol. Pharmacol. 2003;63:192–202. doi: 10.1124/mol.63.1.192. [DOI] [PubMed] [Google Scholar]
- 118.Armstrong L, Saretzki G, Peters H, Wappler I, Evans J, Hole N, von Zglinicki T, Lako M. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells. 2005;23:516–529. doi: 10.1634/stemcells.2004-0269. [DOI] [PubMed] [Google Scholar]
- 119.Nautiyal S, DeRisi JL, Blackburn EH. The genome-wide expression response to telomerase deletion in Saccharomyces cerevisiae. PNAS. 2002;99:9316–9321. doi: 10.1073/pnas.142162499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith LL, Coller HA, Roberts JM. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat. Cell Biol. 2003;5:474–479. doi: 10.1038/ncb985. [DOI] [PubMed] [Google Scholar]
- 121.Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of Tyrosine 707. Mol. Cell. Biol. 2003;23:4598–4610. doi: 10.1128/MCB.23.13.4598-4610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell. 2004;3:399–411. doi: 10.1111/j.1474-9728.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 123.Santos JH, Meyer JN, Van Houten B. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum. Mol. Genet. 2006;15:1757–1768. doi: 10.1093/hmg/ddl098. [DOI] [PubMed] [Google Scholar]
- 124.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regulatory Peptides. 2000;91:21–27. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 125.Liu K, Schoonmaker MM, Levine BL, June CH, Hodes RJ, Weng N-P. Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. PNAS. 1999;96:5147–5152. doi: 10.1073/pnas.96.9.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu H, Fu W, Mattson MP. The catalytic subunit of telomerase protects neurons against amyloid β (β)-peptide-induced apoptosis. J. Neurochem. 2000;75:117–124. doi: 10.1046/j.1471-4159.2000.0750117.x. [DOI] [PubMed] [Google Scholar]
- 127.Kang HJ, Choi YS, Hong S-B, Kim K-W, Woo R-S, Won SJ, Kim EJ, Jeon HK, Jo S-Y, et al. Ectopic expression of the catalytic subunit of telomerase protects against brain injury resulting from ischemia and NMDA-induced neurotoxicity. J. Neurosci. 2004;24:1280–1287. doi: 10.1523/JNEUROSCI.4082-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]