Abstract
DNA repair maintains genomic stability and the loss of DNA repair capacity results in genetic instability that may lead to a decline of cellular function. Adult stem cells are extremely important in the long-term maintenance of tissues throughout life. They regenerate and renew tissues in response to damage and replace senescent terminally differentiated cells that no longer function. Oxidative stress, toxic byproducts, reduced mitochondrial function and external exposures all damage DNA through base modification or mis-incorporation and result in DNA damage. As in most cells, this damage may limit the survival of the stem cell population affecting tissue regeneration and even longevity. This review examines the hypothesis that an age-related loss of DNA damage repair pathways poses a significant threat to stem cell survival and longevity. Normal stem cells appear to have strict control of gene expression and DNA replication whereas stem cells with loss of DNA repair may have altered patterns of proliferation, quiescence and differentiation. Furthermore, stem cells with loss of DNA repair may be susceptible to malignant transformation either directly or through the emergence of cancer-prone stem cells. Human diseases and animal models of loss of DNA repair provide longitudinal analysis of DNA repair processes in stem cell populations and may provide links to the physiology of aging.
STEM CELL FUNCTION
In adult tissues, cells maintain a delicate balance between proliferation and apoptosis. Stem cells are important mediators of tissue maintenance and wound repair. Their longevity is dependent on careful control of gene expression, proliferation and cell cycle, and differentiation signals. Of note, a number of human genetic abnormalities associated with aging, and those replicated in the mouse, suggest that loss of DNA repair may contribute to the aging process. This review will provide support for the argument that maintenance of the adult stem cell genome through robust DNA repair is fundamental in the prevention of aging and disease; furthermore, that failure of genomic maintenance is a leading cause of cancer, as well as senescence.
Adult stem cells are located in a variety of locations throughout the body and provide support for a variety of tissue functions (Figure 1). Chief among these are the function of providing undifferentiated cells for the rejuvenation of tissue. In this regard, most adult stem cells function in a tissue-restricted fashion; hematopoietic stem cells (HSC) that reside in the bone marrow have long been known to mediate the production of both lymphoid and myeloid cell lineages. High-dose radiation and chemotherapy are used to ablate these cells as well as hematologic malignancies; however, single stem-cell clones either normal or cancerous in origin, are capable of repopulation (1–4). As humans age, the HSC as well as all adult stem cells must maintain a delicate balance between proliferation and tissue maintenance, senescence and oncogenesis (Figure 2). With age, disease and in humans who have a genetic loss of DNA repair capacity, recovery of hematopoietic progenitor and stem cell populations from the marrow of humans, characterized by the CD34 antigen, decrease as a function of age, while not directly measured, these data suggest that there is a decrease in the capacity for multi-lineage hematopoietic reconstitution with age (5,6). One example of this is the increasing incidence of various types of anemia and hematologic as well as most other malignancies that occurs in humans with age (7–9). Neural stem cells (NSC) reside within the olfactory lobe, spinal cord and sub-ventricular zone of the brain. These cells then migrate and differentiate to sites of damage to potentially mediate repair. Abnormalities of this function may enable the NSC pool to transform and give rise to neural cancers specifically glioblastomas, medulloblastomas and ependymomas, whereas NSC failure has been implicated in neural degenerative diseases (10,11). Adult stem cells can also be isolated from the skin, liver, gut, glandular tissue, vasculature and even muscle. Evidence suggests that there are undifferentiated adult cells capable of tissue repair in many organs (9,12,13). As noted below, defects in DNA repair affect these organs as well. The global process of aging may in fact be linked to a global decline in tissue stem cell function and this in turn may be linked to changes in DNA damage repair processes.
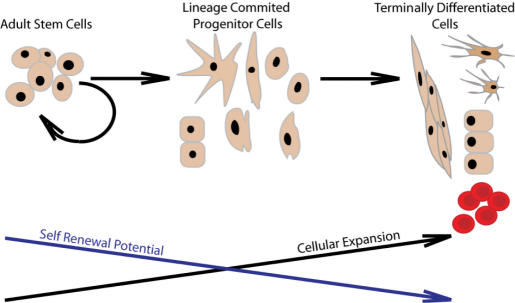
Figure 1.
The generally accepted pathway of stem cell self-renewal and differentiation.
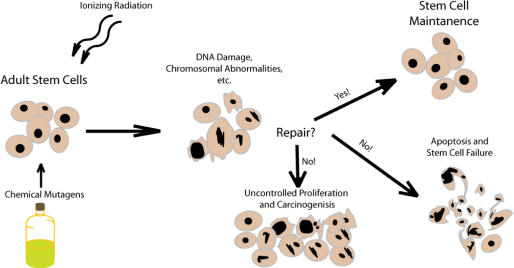
Figure 2.
An illustration depicting the role of DNA repair in stem cell maintenance and apoptotic signaling.
STEM CELL FAILURE ASSOCIATED WITH LOSS OF DNA REPAIR AND AGING
Double strand break (DSB)repair and stem cell failure
HSC deficiency in the DSB non-homologous end-joining (NHEJ) pathway has been observed in mouse models (14,15). NHEJ repairs exogenous DSBs, such as after radiation exposure or endogenous breaks such as during VDJ recombination. The complex of proteins and enzymes involved includes DNA-PK, Ku70 and Ku80, which form a complex at the break, aligning short homologies in overhand regions, and the Ligase IV/XRCC4 complex that seals the break. Nijnik et al. (15) described a mouse with hypomorphic mutations within ligase IV, LigIV, resulting in the Lig4y288c mouse. This mouse is characterized by growth retardation, immunodeficiency and pancytopenia. These mice develop a progressive, age-dependent defect in hematopoiesis. By 20–26 weeks, they have reduced multipotent hematopoietic progenitors, defined as c-Kit+, lineage –, c-SCA+ (KLS) cells, reduced total bone marrow cell count and erythroid cell count by flow cytometry. This seems to hint at reduced stem cell maintenance resulting from the loss of Ligase IV. When Lig4y288c donor stem cells were subject to competitive repopulation of lethally irradiated recipient mice that were infused with equal numbers of normal marrow cells and Lig4y288c donor marrow cells, a repopulation defect was observed. Flow cytometric analysis revealed that 8 weeks post transplant, Lig4y288c KLS donor cells have reduced contribution to all components of hematopoiesis, including whole bone marrow cell counts, pro-B-cell and granulocyte lineages. Thus this data confirms that defects in DNA DSB repair influence stem cell hematopoietic reconstitution function. However, the precise role that DSBs play during hematopoiesis and why repair of these breaks is so crucial to stem cell survival has not been elucidated.
In related work by Rossi et al. (14) Ku80−/− mice, also defective in NHEJ, were examined for stem cell defects. They found that these mice are significantly retarded in macrophage and granulocyte reconstitution after transplantation. In addition, after competitive repopulation with wild-type marrow cells, the Ku80−/− donor derived HSC reconstituted poorly and were lost over time (14).
Recent data from our laboratory also implicate a role for Ku70 in hematopoiesis. Multilineage hematopoietic failure is seen in Ku70−/− mice leading to severe immunodeficiency of both T and B cells and poor hematopoietic reconstitution after transplantation (16). These cells also have a competitive repopulation defect compared to wild-type marrow cells. Together, these mouse models provide evidence that NHEJ deficiencies result in reduced HSC activity, defined by reduced capacity for hematopoietic reconstitution and failure to compete against normal reconstituting stem cells after transplantation. In these mice, the hematopoietic defect is exacerbated with age. Whether a qualitative loss of NHEJ contributes to accumulated abnormalities of normal hematopoietic capacity with age has not been evaluated.
Of note, NHEJ proteins are also involved in VDJ recombination, a process in T and B cells of generating diversity in the T-cell receptor and B-cell heavy chain loci. During this process, initiated by Rag1 and Rag2, a strand break at the VDJ loci is initiated, followed by formation of a hairpin recognized by DNA-PK and Ku70/80 and closed by Ligase IV. In the process, and to increase genetic diversity, terminal deoxytransferase fills in the overhang, creating mismatches in the double strand that are then repaired by the mismatch repair (MMR) pathway (17,18). Given the activity of VDJ recombination in early lymphoid progenitors, it is possible that in the absence of well-regulated NHEJ, a similar process is ongoing in more primitive HSC populations.
Another component of the DSBs repair response, telomere maintenance and DNA replication is the MRE11, Rad50 and NBS1 (MRN) complex. This complex is also a factor in hematopoietic maintenance. The MRN complex participates in repair of DSB by sensing and unwinding the region of the break. The complex also participates in DNA-strand break scaffold formation and signaling of the repair response, including ATM (see below). MRN complex partner null mutants are not viable (19–22), and Bender et al. (23) identified that a hypomorphic Rad50k22m mutation in mice results in partial embryonic lethality. At birth, Rad50k22m mice are underweight compared to wild type and had an average survival of about 2.5 months. In 20% cases the cause of death was a B-cell lymphoma with splenic hyperplasia. The death of the other 80% of Rad50k22m mice could be attributed to sever anemia, a form of hematopoietic failure. HSC were present at 2 weeks, however, complete ablation of bone marrow HSC was observed by 4–8 weeks. The authors observed that the HSC ablation was mirrored by loss of macrophage, T cell and pro- and pre- B cells when compared to wild type at Week 4. Co-transplant experiments involving mixing wild-type and Rad50k22m mutant marrow cells in a 1:1 ratio and transplantation into a normal but lethally irradiated host, failed to demonstrate any Rad50k22m mutant HSC survival. Rather, all recipient cells detected were derived from the wild-type donor cells.
This indicates that there is a stem cell defect caused by the reduced function of Rad50 either alone, or implicating the entire MRN complex. It appears that the MRN complex, by mediating DNA strand break repair, maintains stem cell function (23).
A partial rescue of the Rad50k22m phenotype occurs with loss of p53 function, indicating that cell cycle and apoptotic signaling from the unrepaired DSB through p53 results in stem cell death so that loss of function allows stem cell survival. The p53−/− Rad50k22m mice did not have severe anemia or HSC ablation, and had an extended lifespan. However, the stem cell pool is not normal because these animals died of lymphoma suggesting that loss of genomic stability contributed to malignant transformation (23). This evidence illustrates the importance of DNA damage repair to the self-renewal capacity of the HSC and indicates that stem cell death is mediated by p53-dependent apoptotic signaling.
These results raise the question of whether there is evidence that DNA repair capacity decreases with age and if so under what circumstances. This has been studied in mice. Goodell et al. (24) evaluated a number of genes in mouse kit+sca+lin- (KLS) HSC isolated from aged mice, 21–28 months of age or young mice aged 2, 8 or 12 months. While a number of stress response, protein folding and inflammatory genes were upregulated, these authors also identified a number of DNA repair associated genes that were downregulated. Although these results were not associated with a phenotype in the mice analyzed, they do suggest that there is a trend toward, if not a substantial reduction in, DNA repair capacity associated with aging in mice. The specific genes noted to be reduced in expression with age include Xab2 [XPA-binding protein 2, involved in the nucleotide excision repair (NER) pathway], Rad52 (a ssDNA-binding protein involved in homologous recombination) (25), XRCC1 [involved in coordination of single-strand break repair and base excision repair (BER) pathways] (26) and Blm (a req helicase involved in DSB repair) (27). It is feasible that reduced expression of these genes could disrupt DNA repair performance and lead to genomic instability. Since this is likely to occur in single somatic cells, the immediate impact on a stem or progenitor cell population would be difficult to assess. However, over time, these changes may cumulatively lead to decreased stem cell function with age.
There are data in human HSC from Prall et al. (28) that these stem cells acquire NHEJ repair deficiency with age. Using immuno-magnetic separation to isolate CD34+ cell fractions of human umbilical cord bloods and bone marrow samples from donors ranging in age from 18 to 101 years, HSC were obtained. cDNA from these samples was then synthesized and amplified on arrays. The resulting gene cluster analysis revealed a number of genes that were up- or downregulated. Ku70, the heterodimeric partner of Ku80, was shown to be downregulated. Further BIK or BCL2-interacting killer protein was upregulated in HSC from older individuals. The authors postulate that BIK and Ku70 have roles in the apoptotic response. Ku70 interacts with a promoter of apoptosis, Bax, while BIK (29) interacts with the anti-apoptotic protein Blc2. Thus, reduced Ku70 expression leads to reduced NHEJ repair efficiency as well as priming the apoptosis signaling pathway in stem cells of elderly individuals.
In DNA ligase 1 (Lig1) knockout mice (30), several phenotypic characteristics also indicate a role for this enzyme in stem cell maintenance. Lig1(−/−) is an embryonic lethal, with abnormalities observed after embryonic Day 10.5 due to increasingly anemic and death by E 15.5 (30) due to a failure to develop fetal liver erythropoiesis. Fetal liver cells from the Lig(−/−) embryos were unable to repopulate lethally irradiated recipients, resulting in bone marrow failure by 10 weeks. Surviving mice exhibited anemia, enlarged erythrocytes and reticulocytosis (30). This indicates the reliance of HSC on proficient and reliable genomic replication and maintenance to function, even without overt external DNA damage.
These various lines of evidence exhibit a delicate balance between DNA replication and repair in cell proliferation, self-renewal and quiescence in stem cell maintenance. While DNA repair is essential for stem cell maintenance, there appears to be a decline in DNA repair function with age. While not proven, we propose that this sets the stage for a programmed loss of stem cell capacity with age. Adult stem cells must serve as long-term reservoirs of undifferentiated tissue that can be called upon to repair, and yet they prime themselves to undergo apoptosis as they age. These results also indicate a relationship between long-term stem cell survival and cancer. For instance, one response to limited cell pool and a decline in function is selection of surviving stem cells for properties of increased proliferation and resistance to apoptosis, two requisites for malignant transformation. This is facilitated by emergence of genomic instability in the face of decreased DNA repair capacity. Alternatively, these defects in stem cells may lead to adult stem cell failure syndromes, either aplastic anemia, osteoporosis or myelodysplastic syndrome, a defect in hematopoiesis.
MMR DEFECTS IN STEM CELLS AND CANCER STEM CELLS
A number of malignancies occur in the setting of genomic instability and these tumors may be linked to DNA repair defects at the level of the tissue stem cell. Hereditary non-polyposis colorectal cancer (HNPCC) was discovered in patients who had functional heterozygous mutations in a gene for MMR. Receiving a second hit in the single functional copy due to gene deletion, promoter hypermethylation or other epigenetic events leads to the formation of a hypermutator phenotype in the intestinal stem cell. This hypermutator adult stem cell exhibits increased rates of mutation at microsatellite loci, described as microsatellite instability (MSI) (31). One of the results of high levels of MSI in the intestinal stem cell is the resulting mutation of a particular microsatellite loci located within the TNF-β receptor gene, which results in a nonfunctional truncated TNF-β receptor. Haploinsufficiency then reduces TNF-β anti-tumor signaling and leads to the formation of aberrant crypt foci, and later cancer (32,33). Thus, the consequences of loss of MMR are progressive over time and with age.
It appears that similar processes occur with loss of MMR in HSC. In mice with a knockout of many of the MMR genes—Pms2, Msh2 and Mlh1, lymphomagenesis is observed. MMR also plays a broad role in the survival and function of the HSC. Using Msh2−/− mice by our laboratory, Reese et al. 2003 (34) found that HSC from mice lacking MMR were defective in hematopoiesis. Serial-transplant experiments revealed that these MMR-defective HSC possess a severe repopulation defect characterized by loss of HSC in recipient mice, decrease in hematopoiesis and inability to rescue tertiary transplant mice that received lethal irradiation prior to cell infusion. Rather, these mice die of stem cell failure. In hematopoietic competitive reconstitution experiments, co-transplanting HSC mixtures of wild-type and Msh2−/− marrow cells in a 1:1 ratio results in loss of the Msh2 knockout HSC in the recipient, indicating a severe repopulation defect. Progenitor colony-forming cells from the Msh2 marrow recipient mice also showed evidence of MSI in the Msh2−/− cells. This occurs as a function of age, since no evidence of microsatellites is seen in young Msh2−/− mice.
In humans, acquired defects in MMR have been observed in both childhood and adult leukemias and lymphomas. While a family with a germline defect in MLH1 is predisposed to leukemias (35) and neurofibromatosis (36,37), sporadic leukemias and lymphomas (38) are also associated with MSI and with defects in MMR. Human leukemia cell lines also show evidence of loss of MMR protein expression (39). Secondary leukemias, those induced in patients previously receiving chemotherapy also have a high incidence of MSI, suggesting both selection in favor of leukemic clones from the stem cell population, and possibly the impact of drug selection of MMR-defective drug-resistant cells (40–42). Cumulatively, these data suggest that maintenance of genomic stability retains stem cells in their normal physiologic composition whereas the presence of MMR defects allows expansion of stem cell clones with acquired mutations leading to malignant transformation (43). Given the fact that families with HNPCC also are at risk for ovarian cancers—Geisler et al. (44) report 16.8% of ovarian tumor samples tested showed high-frequency MSI—and that other patients with ovarian cancer demonstrate MSI, and have no expression at the mRNA level of hMLH1, due to promoter hypermethylation, the overall impact of MMR on maintenance of stem cells from multiple organs and their tendency towards malignant transformation is relatively clear. Thus, there is evidence that MMR is important in HSC preservation, human carcinogenesis, and perhaps with the overall aging process of multiple tissues and their respective stem cells.
STEM CELL DEFECTS DUE TO LOSS OF INTERSTRAND DNA CROSS-LINK REPAIR
Fanconi's anemia (FA) is characterized by early onset aplastic anemia due to HSC exhaustion, increased incidence of cancer—particularly AML—as well as heterogeneous birth defects. The hematologic defects include pancytopenia, low marrow reserve, poor cytokine responsiveness, increased apoptosis of hematopoietic progenitors and poor stem cell transplantation capabilities (45). The molecular phenotypes of FA are hypersensitivity to DNA cross-linking agents, mild sensitivity to ionizing radiation and reactive oxygen species, chromosomal instability, growth arrest, apoptosis and G2/M phase delay (45–48). Patients with FA are also hypersensitive to cancer therapies that cause DNA damage. Thus, DNA repair function appears to link the phenotypes of both cancer and cancer stem cells when repair function is disrupted and maintenance of normal stem cells when DNA repair is intact.
Early descriptions of FA found that cell lines from FA patients could be divided into 12 complementation groups and later, 11 gene mutations. These have now been clarified as FANC[A–C, D1(BRCA2),D2-F,G, BRIP1, J, L, M] (48). Additionally, a 13th complementation group has been reported to be associated with mutations in the partner and localizer of BRCA2, or PALB2 (49,50). Five of the FA proteins (FANCA, C, E, F and G) form a nuclear complex that monoubiquinate FANCD2. One protein is a ubiquitin ligase (FANCL), one has helicase function, (FANCJ) and one is homologous with the breast cancer susceptibility gene BRCA1 (48). While still not fully characterized, these proteins bind to other complexes involved in DSB repair and at DNA replication forks (51) and in this way appear to mediate these processes.
While the FA protein complex includes BRCA 1 and 2, the FA protein complex is not the primary functional group for either DSB repair or homologous recombination. Conversely, hematopoietic defects are not recognized in carriers of BRCA1 or BRCA2. It is clear that individuals possessing a single mutated copy of the BRCA2 gene have an increased risk of breast and ovarian cancer (52). However, possessing two mutated copies of BRCA2 results in the HSC exhaustion associated with FA. Loss of function leads to chromosomal translocations, rearrangements and deletions. Disruptions of the FANC pathway alter the response to DSB in DNA. Thus it is possible that complete loss of FA-associated gene products results in adult stem cell failure due to senescence and apoptosis; however, single allele mutations in the FA core complex and associated proteins lead to a requirement for a second hit to initiate a mutator phenotype in adult stem cells that potentially escape cell cycle and apoptotic controls, leading to immortalization, transformation and carcinogenesis (48).
STEM CELL DEFECTS ASSOCIATED WITH ABNORMALITIES IN NER
Premature aging diseases are rare and multivariate in cause; however, many seem to be related in some way to abnormalities in genomic maintenance. Many of these diseases also have evidence of defects in stem cell maintenance. Table 1 depicts a number of the recognized premature aging diseases, phenotypes and corresponding genetic mutations in DNA damage repair and response genes, particularly NER genes.
Table 1.
Premature aging syndromes, associated genetic mutations and affected DNA damage repair pathway
| Disease | Affected DNA repair pathway | Gene(s) mutated |
|---|---|---|
| Xeroderma Pigmentosa | Nucleotide excision repair | XP(A-G) |
| Cockayne syndrome | Nucleotide excision repair | CSA & CSB |
| Trichothiodystrophy | Nucleotide excision repair | Various XP and CS related |
| Werner syndrome | DSB repair homologous Rec. | WRN (a RECQ family member) |
| Rothmund–Thomson syndrome | DSB repair homologous Rec. | RECQ4 |
| Bloom | DSB repair homologous Rec. | BLM (a RECQ family member) |
| Ataxia Telangiectasia | Global DSB repair | ATM (a PI3K) |
The NER pathway removes DNA lesions caused by a wide variety of chemical alterations of the DNA bases including pyrimidine dimers produced by exposure to UV radiation. NER functions in two major pathways, the global genome NER pathway (GG-NER) and the transcription coupled NER pathway (TC-NER). As its name implies, GG-NER is involved in the global surveillance and repair of the genome, while TC-NER repairs lesions that block elongation of transcription complexes.
Ten gene products of the NER pathway have been associated with human disease—these are XPA-G, named for their association with Xeroderma Pigmentosa (XP), CSA, CSB and ERCC-1 (53,54). XP, Cockayne Syndrome (CS) and Trichothiodystrophy (TTD) diseases are characterized by solar hypersensitivity and all share mutations in the NER pathway. TTD patients have also been observed with mutations in or lowered expression of TFIIH a general transcription factor complex with helicase activity that is required for transcriptional elongation as well as NER but also mutations in XPD and XPB (55,56). This illustrates very clearly the multifunctional nature of NER gene products. Those mutations that lead to XP are related to GG-NER and more specifically the XP proteins that function to recognize and bind DNA lesions and participate in apoptotic signaling. CS and TTD patients have mutations in NER genes associated with TC-NER and retain apoptotic signaling as well as DNA lesion recognition but lack the ability to rapidly restore stalled transcription due to base alterations. Thus, the CS or TTD mutant cell is unable to restore transcription during important transcriptionally regulated developmental signaling. On the other hand, increased rates of apoptosis due to DNA lesion accumulation and recognition, could account for neural degenerative phenotypes as well as the segmental progeroid phenotype due to tissue-specific stem cell failure (53).
The stem cell defect of these conditions is best characterized in mouse knockout models. First, mouse models of TTD, in which the Xpd helicase is mutated, show striking similarities to the human disease (14,57). In addition, hematopoietic marrow transplant studies using competitive transplantation with 26 week wt or Xpd helicase mutant mice show that Xpd mutant donor cells have a significant repopulation defect compared to wild-type cells with reductions in whole-bone marrow engraftment of B cell, T cell, myeloid and granulocytes (14). In Ercc1−/− mice, a premature aging phenotype is observed by 3 weeks of age that is associated with hematopoietic failure (58). Normal Ercc1 forms a heterodimer with XPF to crease an endonuclease that functions in NER and interstrand cross-link repair. These mice have a progressive peripheral blood cytopenia, marrow fatty infiltrate, progressive reduced bone marrow cellularity, decreased progenitor potential and decreased lymphoid lineage commitment (58). In humans, the phenotype of stem cell failure is subtler, with an increased risk of leukemogenesis but only minimal evidence of hematopoietic failure.
Thus, loss of NHEJ, MMR, DSB repair and NER in mice are all associated with progressive severe hematopoietic repopulation defects, many have a primary engraftment defect, with loss of stem cell pools, and many are also associated with a tendency to develop genomic instability among stem cell pools giving rise to carcinogenesis. In each case, the defects manifest themselves with progressive loss of hematopoietic function over time, suggesting that age contributes to the severity of hematopoietic failure. Whether acquired defects in these pathways contribute to loss of hematopoietic function during the normal aging process is not yet clear.
TELOMERASE: A NONESSENTIAL ENZYME FOR HEMATOPOIESIS LEADING TO LATE STEM CELL DYSFUNCTION
Despite the recognized need to maintain telomere length in proliferating cells especially stem cells, and the recognition that aging might be associated with loss of telomere length leading to senescence, hematopoiesis in telomere knockout mice is surprisingly normal. However, examining late generation telomerase-deficient mice [mTerc(−/−)], hematopoietic reserve is indeed limited and is accompanied by short telomeres in the progenitor population (59). This is pronounced under forced stem cell proliferation either in repopulation assays as described above or in short term proliferation assays of hematopoietic progenitors—the colony forming unit (CFU)—granulocyte-macrophage or CFU-spleen (CFU-S). In serial repopulation transplantation experiments, stem cell failure is seen (59) and p21 is activated, leading to checkpoint activation and cell cycle arrest. This can be partially rescued by loss of p21 (Cdkn1a, Cip1/Waf) because DNA strand break sensing which occurs in cells with short telomeres does not occur, limiting the apoptotic signaling and cell cycle arrest that normally occurs as a consequence of telomere shortening in proliferating cells (60). Not only is hematopoiesis restored, but also marrow cells retain multilineage repopulation capacity.
DISORDERS OF HELICASE FUNCTION AND STEM CELL DEFECTS
An essential feature of homologous recombination is the role of RECQ family helicases. Abnormalities of three genes are associated with premature aging: Werner (WS), Rothmund–Thomson (RTS) and Bloom (BL) syndromes (61,62), caused by mutations in the WRN, RECQ4 and BLM genes, respectively. Each of these syndromes shares some common traits and yet is characterized by distinct pathologies. To understand the differences between these diseases, a more complete picture of the functional overlap and specificities of these gene products must be formed. All five of the RECQ family of gene products are 3′–5′ DNA helicases; and all are associated with resolution of DNA DSB. Furthermore, each of those associated with human disease have unique as well as overlapping functions (62).
WS is characterized by several premature aging phenotypes, which occur by the end of the third decade of life. These include diabetes, increased cancer incidence, gray hair, as well as myocardial and cerebral vascular diseases (61,62). The pathologies associated with RTS are primarily skin disorders; including pigment and hair follicle growth, osteosarcoma and skeletal defects (63). Bloom patients suffer from dwarfism, hair and nail malformations and an elevated risk of malignancies and rare tumors that is especially increased in hematologic malignancies (27,63). These distinct relationships with the various RECQ genes and tissue-specific pathology seem to distinguish specific roles for the various RECQ enzymes in development and genomic surveillance.
WRN has been associated with telomere maintenance in mouse models (64). A mouse double knockout model of Wrn and TerC, results in successive shortening of the telomeres (65,66) and subsequent failure to protect chromosome ends from being recognized and ‘repaired’ as DSB, giving rise to premature aging, stem cell and marrow failure and shortened lifespan.
BLM seems to have a more global genomic surveillance role. Normal BLM has also been shown to interact with TOPIIIα (67) suggesting that this interaction plays a role in Holliday junction resolution and suppression of aberrant recombination (27,68). The association of BLM mutation with nearly 100-fold greater incidence of nearly all forms of cancer as well as numerous chromosomal abnormalities supports this statement. While these cells have abnormalities in sister chromatid exchange and chromosomal rearrangements and translocations, actual HSC defects, if present, are more subtle.
Mutations of RECQ4 associated with RTS on the other hand are less well studied. In fact, ∼33% of RTS cases lack a recognized RECQ4 mutation (69). A mouse model however confirms that RecQ4 disruption causes at least similar pathology as that seen in RTS (70) but no obvious HSC or other stem cell defect. Thus, despite the importance of helicases in maintaining hematopoiesis is in humans, mouse models have not confirmed a linkage between these processes.
STEM CELL DEFECTS ACCOMPANYING LOSS OF DSB SENSING PROTEINS
Ataxia Telangiectasia (AT) is a disease associated with premature aging, particularly of the skin, progressive loss of cerebellar architecture and neuromotor dysfunction, immunodeficiency including thymic involution, low T-cell numbers, recurrent respiratory infections, low antibody production as well as increased incidence of lymphoma and leukemia and acquisition of chromosomal abnormalities of the skin and hematopoietic system. AT patients are sensitive to ionizing radiation and DSB-inducing compounds. AT is caused by a mutation in phosphatidylinositol 3-kinase (PI3K)-like protein kinase, ATM (71,72) which senses DNA damage and induces Chk2, a cell cycle checkpoint protein, phosphorylation and cell cycle arrest. Following DSBs, ATM and ATM-related PI3Ks (ATR) signal the presence of DSBs and initiate DSB repair and cell cycle control through a series of protein phosphorylation events (73). ATM undergoes auto-phosphorylation and activates mediators of the DSB pathway including H2AX, Nbs1 and MRE11. These then localize to a DSB forming a complex and recruiting the necessary repair proteins, such as DNA-PK and the Ku70/80 heterodimer for NHEJ or Rad51 for homologous recombination. Furthermore, ATM activates checkpoint proteins to control the cell cycle during repair, including both Chk2 and the mediator of DNA damage checkpoint (MDC1). Upon its activation, MDC1 seems to be involved in further Chk2 signaling activation (73). Thus, ATM sits at the cross roads of DSB repair and cell signaling. This tight relationship between cell cycle control and DSB repair seems to be the cause of developmental abnormalities and accelerated aging observed in AT patients.
In ATM knockout mice, aging is associated with a progressive loss of lymphoid function, both B- and T-cell loss and a fundamental defect in HSC function, with decreased stem cell numbers, loss of repopulation capacity and increased apoptosis (74). Clear loss of hematopoietic activity is age-dependent, apparent by 24 weeks of age, and the mice have an increased incidence of T-cell lymphomas. The mice are unable to adequately mount a DNA repair response in the face of reactive oxygen species, initiating the caspase apoptotic cascade (74). Ito et al. (74) found that reactive oxygen species are critical to maintenance of hematopoiesis since treatment with anti-oxidants reduced the oxidative stress and maintained stem cell function in ATM−/− mice.
The ATM HSC defect can also be ameliorated by loss of other cell cycle check point genes including the proteins of the DSB recognition complex, mre11/Rad50/Nbs1. As noted above, the Rad50 hypomorph has evidence of severe hematopoietic deficiency ameliorated by p53 (23). In regards to rescue of the hematopoietic defect of ATM, double knockout of ATM and Rad50 results in better HSC survival (75). Since increased reactive oxygen species and increased reactive oxidative stress accompanies aging, these pathways may be critical for stem cell maintenance. How these processes impact physiologic aging and contribute to a decline in hematopoietic function are not yet well defined.
Other Causes of Stem Cell Failure
Aplastic anemia can arise following viral infection, immune reaction, drug associated toxicity and overwhelming DNA damage. Parvovirus B19 (76), influenza virus (77) and CMV (78) can directly infect HSC inducing stem cell loss or infect stromal elements that alter the viral response and impede hematopoiesis. Other viral processes including EBV (79) and HIV often with coinfection with Mycobacterium avium intracellulare (80) are associated with infection of other cells within the stroma that alter hematopoietic signaling and induce immune responses that can directly target HSC leading to stem cell failure. Hepatitis C infection can give rise to a severe immune mediated aplastic anemia (81). In some instances, either aplastic anemia or myelodysplastic syndrome appears to occur as part of an immune response, either as primary or secondary events. Is has been proposed that abnormal stem cells present altered surface peptides that induce a T-cell-mediated immune response (82–84) or that drugs bind to the surface of these cells eliciting a similar T-cell response, leading to cell-mediated stem cell death. Finally, for over 60 years we have recognized that agents and exposures that cause DNA damage to the stem cell genome induces stem cell failure such as high-dose radiation and chemotherapy.
Dna Repair During Aging, Stem Cell Survival and Carcinogenesis
Stem cells continually renew and rejuvenate tissues as these tissues age and become injured. Reactive oxygen species from mitochondria, exposure to solar and other forms of radiation, chemical exposures and extensive rounds of replication can damage the genome and for long-lived cells require authentic repair to insure that there is fidelity in stem cell progeny. Since stem cell survival through maintenance of pools and self-renewal are essential for tissue homeostasis, it can be inferred that DNA repair and genome maintenance may be key factors in stem cell maintenance and survival of the organism. If adult stem cells respond to damage with increasing apoptotic signaling, an organism faces stem cell ablation, and without further tissue rejuvenation, senescent death. On the other hand, if an adult stem cell fails to recognize and repair DNA damage and fails to signal apoptosis, then damaged adult stem cells will incur mutations over time, eventually leading to either stem cell loss due to altered gene expression and loss of stem cell phenotypic properties or loss of stem cell homeostasis and carcinogenesis.
SUMMARY
A number of diseases of DNA repair are associated with premature aging, stem cell failure and carcinogenesis. Animal knockout models of these processes similarly show evidence of premature aging, loss of competitive hematopoiesis, stem cell failure and carcinogenesis. Recent data suggests that age dependent and acquired loss of DNA repair may contribute to physiologic aging and could contribute to the increase in cancer incidence with age.
Whether these acquired defects are programmed, subject to genetic polymorphisms or due to environmental factors remains to be determined. Likewise, interventions into this decline in DNA repair function may improve stem cell function and reserve, delay aging and reduce the incidence of stem cell failure, leukemia and lymphomas.
ACKNOWLEDGEMENTS
This work was supported by P30 CA043703-18 and R01 AG024916-03. Funding to pay the Open Access publication charges for this article was provided by University Hospitals Of Cleveland Research funds.
Conflict of interest statement. None declared.
REFERENCES
- 1.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 2.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 3.Bellantuono I. Haemopoietic stem cells. Int. J. Biochem. Cell Biol. 2004;36:607–620. doi: 10.1016/j.biocel.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Tam W.-L, Ang Y.-S, Lim B. The molecular basis of ageing in stem cells. Mech. Ageing Dev. 2007;128:137–148. doi: 10.1016/j.mad.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 5.French RA, Broussard SR, Meier WA, Minshall C, Arkins S, Zachary JF, Dantzer R, Kelley KW. Age-associated loss of bone marrow hematopoietic cells is reversed by GH and accompanies thymic reconstitution. Endocrinology. 2002;143:690–699. doi: 10.1210/endo.143.2.8612. [DOI] [PubMed] [Google Scholar]
- 6.Morris CL, Siegel E, Barlogie B, Cottler-Fox M, Lin P, Fassas A, Zangari M, Anaissie E, Tricot G. Mobilization of CD34+ cells in elderly patients (>/= 70 years) with multiple myeloma: influence of age, prior therapy, platelet count and mobilization regimen. Br. J. Haematol. 2003;120:413–423. doi: 10.1046/j.1365-2141.2003.04107.x. [DOI] [PubMed] [Google Scholar]
- 7.Perryman SV, Sylvester KG. Repair and regeneration: opportunities for carcinogenesis from tissue stem cells. J. Cell. Mol. Med. 2006;10:292–308. doi: 10.1111/j.1582-4934.2006.tb00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea–a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 9.Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 10.Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat. Rev. Cancer. 2006;6:425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Martin LJ. The adult neural stem and progenitor cell niche is altered in amyotrophic lateral sclerosis mouse brain. J. Comp. Neurol. 2006;497:468–488. doi: 10.1002/cne.21012. [DOI] [PubMed] [Google Scholar]
- 12.Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J. Cell. Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machida S, Narusawa M. The roles of satellite cells and hematopoietic stem cells in impaired regeneration of skeletal muscle in old rats. Ann. NY Acad. Sci. 2006;1067:349–353. doi: 10.1196/annals.1354.049. [DOI] [PubMed] [Google Scholar]
- 14.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 15.Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 16.Qing Y, Gerson SL. Loss of Ku70 results in a HSC repopulating defect. Blood. 2007;110:378A–379A. [Google Scholar]
- 17.Larson ED, Duquette ML, Cummings WJ, Streiff RJ, Maizels N. MutSalpha binds to and promotes synapsis of transcriptionally activated immunoglobulin switch regions. Curr. Biol. 2005;15:470–474. doi: 10.1016/j.cub.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 18.Longo NS, Lipsky PE. Somatic hypermutation in human B cell subsets. Springer Semin. Immunopathol. 2001;23:367–385. doi: 10.1007/s281-001-8165-0. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Y, Weaver DT. Conditional gene targeted deletion by Cre recombinase demonstrates the requirement for the double-strand break repair Mre11 protein in murine embryonic stem cells. Nucleic Acids Res. 1997;25:2985–2991. doi: 10.1093/nar/25.15.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo G, Yao MS, Bender CF, Mills M, Bladl AR, Bradley A, Petrini JHJ. Disruption of mRad50 causes embryonic stem cell lethality, abnormal embryonic development, and sensitivity to ionizing radiation. Proc. Natl Acad. Sci. USA. 1999;96:7376–7381. doi: 10.1073/pnas.96.13.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi-Iwai Y, Sonoda E, Sasaki MS, Morrison C, Haraguchi T, Hiraoka Y, Yamashita YM, Yagi T, Takata M, et al. Mre11 is essential for the maintenance of chromosomal DNA in vertebrate cells. EMBO J. 1999;18:6619–6629. doi: 10.1093/emboj/18.23.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu J, Petersen S, Tessarollo L, Nussenzweig A. Targeted disruption of the Nijmegen breakage syndrome gene NBS1 leads to early embryonic lethality in mice. Curr. Biol. 2001;11:105–109. doi: 10.1016/s0960-9822(01)00019-7. [DOI] [PubMed] [Google Scholar]
- 23.Bender CF, Sikes ML, Sullivan R, Huye LE, Le Beau MM, Roth DB, Mirzoeva OK, Oltz EM, Petrini JHJ. Cancer predisposition and hematopoietic failure in Rad50S/S mice. Genes Dev. 2002;16:2237–2251. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugiyama T, New JH, Kowalczykowski SC. DNA annealing by Rad52 Protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc. Natl Acad. Sci. USA. 1998;95:6049–6054. doi: 10.1073/pnas.95.11.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazarkina ZK, Khodyreva SN, Marsin S, Lavrik OI, Radicella JP. XRCC1 interactions with base excision repair DNA intermediates. DNA Repair. 2007;6:254–264. doi: 10.1016/j.dnarep.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Chester N, Babbe H, Pinkas J, Manning C, Leder P. Mutation of the Murine Bloom's syndrome gene produces global genome destabilization. Mol. Cell. Biol. 2006;26:6713–6726. doi: 10.1128/MCB.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prall WC, Czibere A, Jager M, Spentzos D, Libermann TA, Gattermann N, Haas R, Aivado M. Age-related transcription levels of KU70, MGST1 and BIK in CD34+ hematopoietic stem and progenitor cells. Mech. Ageing Dev. 2007;128:503–510. doi: 10.1016/j.mad.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Li GC, Ouyang H, Li X, Nagasawa H, Little JB, Chen DJ, Ling CC, Fuks Z, Cordon-Cardo C. Ku70: a candidate tumor suppressor gene for murine T cell lymphoma. Mol. Cell. 1998;2:1–8. doi: 10.1016/s1097-2765(00)80108-2. [DOI] [PubMed] [Google Scholar]
- 30.Bentley DJ, Harrison C, Ketchen A-M, Redhead NJ, Samuel K, Waterfall M, Ansell JD, Melton DW. DNA ligase I null mouse cells show normal DNA repair activity but altered DNA replication and reduced genome stability. J. Cell. Sci. 2002;115:1551–1561. doi: 10.1242/jcs.115.7.1551. [DOI] [PubMed] [Google Scholar]
- 31.Umar A, Boland CR, Terdiman JP, Syngal S, Chapelle ADL, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J. Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markowitz SD, Roberts AB. Tumor suppressor activity of the TGF-[beta] pathway in human cancers. Cytokine Growth Factor Rev. 1996;7:93–102. doi: 10.1016/1359-6101(96)00001-9. [DOI] [PubMed] [Google Scholar]
- 33.Markowitz S. TGF-[beta] receptors and DNA repair genes, coupled targets in a pathway of human colon carcinogenesis. Biochim. Biophys. Acta Rev. Cancer. 2000;1470:M13–M20. doi: 10.1016/s0304-419x(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 34.Reese JS, Liu L, Gerson SL. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood. 2003;102:1626–1633. doi: 10.1182/blood-2002-10-3035. [DOI] [PubMed] [Google Scholar]
- 35.Gu L, Cline-Brown B, Zhang F, Qiu L, Li GM. Mismatch repair deficiency in hematological malignancies with microsatellite instability. Oncogene. 2002;21:5758–5764. doi: 10.1038/sj.onc.1205695. [DOI] [PubMed] [Google Scholar]
- 36.Whiteside D, McLeod R, Graham G, Steckley JL, Booth K, Somerville MJ, Andrew SE. A homozygous germ-line mutation in the human MSH2 gene predisposes to hematological malignancy and multiple cafe-au-lait spots. Cancer Res. 2002;62:359–362. [PubMed] [Google Scholar]
- 37.Bandipalliam P. Syndrome of early onset colon cancers, hematologic malignancies and features of neurofibromatosis in HNPCC families with homozygous mismatch repair gene mutations. Familial Cancer. 2005;4:323–333. doi: 10.1007/s10689-005-8351-6. [DOI] [PubMed] [Google Scholar]
- 38.Morimoto H, Tsukada J, Kominato Y, Tanaka Y. Reduced expression of human mismatch repair genes in adult T-cell leukemia. Am. J. Hematol. 2005;78:100–107. doi: 10.1002/ajh.20259. [DOI] [PubMed] [Google Scholar]
- 39.Taverna P, Liu L, Hanson AJ, Monks A, Gerson SL. Characterization of MLH1 and MSH2 DNA mismatch repair proteins in cell lines of the NCI anticancer drug screen. Cancer Chemother. Pharmacol. 2000;46:507–516. doi: 10.1007/s002800000186. [DOI] [PubMed] [Google Scholar]
- 40.Offman J, Opelz G, Doehler B, Cummins D, Halil O, Banner NR, Burke MM, Sullivan D, Macpherson P, et al. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004;104:822–828. doi: 10.1182/blood-2003-11-3938. [DOI] [PubMed] [Google Scholar]
- 41.Casorelli I, Offman J, Mele L, Pagano L, Sica S, D’Errico M, Giannini G, Leone G, Bignami M, et al. Drug treatment in the development of mismatch repair defective acute leukemia and myelodysplastic syndrome. DNA Repair. 2003;2:547–559. doi: 10.1016/s1568-7864(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 42.Olipitz W, Hopfinger G, Aguiar RC, Gunsilius E, Girschikofsky M, Bodner C, Hiden K, Linkesch W, Hoefler G, et al. Defective DNA-mismatch repair: a potential mediator of leukemogenic susceptibility in therapy-related myelodysplasia and leukemia. Genes Chromosomes Cancer. 2002;34:243–248. doi: 10.1002/gcc.10059. [DOI] [PubMed] [Google Scholar]
- 43.Vaish M. Mismatch repair deficiencies transforming stem cells into cancer stem cells and therapeutic implications. Mol. Cancer. 2007;6:26. doi: 10.1186/1476-4598-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geisler JP, Goodheart MJ, Sood AK, Holmes RJ, Hatterman-Zogg MA, Buller RE. Mismatch repair gene expression defects contribute to microsatellite instability in ovarian carcinoma. Cancer. 2003;98:2199–2206. doi: 10.1002/cncr.11770. [DOI] [PubMed] [Google Scholar]
- 45.Butturini A, Gale RP, Verlander PC, Adler-Brecher B, Gillio AP, Auerbach AD. Hematologic abnormalities in Fanconi anemia: an International Fanconi Anemia Registry study [see comments] Blood. 1994;84:1650–1655. [PubMed] [Google Scholar]
- 46.Grompe M, D’Andrea A. Fanconi anemia and DNA repair. Hum. Mol. Genet. 2001;10:2253–2259. doi: 10.1093/hmg/10.20.2253. [DOI] [PubMed] [Google Scholar]
- 47.Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, Hanenberg H, Auerbach AD. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101:1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi T, D’Andrea AD. Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006;107:4223–4233. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 49.Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol. Cell. 2006;22:719–729. doi: 10.1016/j.molcel.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Xia B, Dorsman JC, Ameziane N, de Vries Y, Rooimans MA, Sheng Q, Pals G, Errami A, Gluckman E, et al. Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat. Genet. 2007;39:159–161. doi: 10.1038/ng1942. [DOI] [PubMed] [Google Scholar]
- 51.Hussain S, Wilson JB, Medhurst AL, Hejna J, Witt E, Ananth S, Davies A, Masson JY, Moses R, et al. Direct interaction of FANCD2 with BRCA2 in DNA damage response pathways. Hum. Mol. Genet. 2004;13:1241–1248. doi: 10.1093/hmg/ddh135. [DOI] [PubMed] [Google Scholar]
- 52.Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br. J. Cancer. 2007;96:11–15. doi: 10.1038/sj.bjc.6603535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Laat WL, Jaspers NGJ, Hoeijmakers JHJ. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999;13:768–785. doi: 10.1101/gad.13.7.768. [DOI] [PubMed] [Google Scholar]
- 54.Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Vermeulen W, Bergmann E, Auriol J, Rademakers S, Frit P, Appeldoorn E, Hoeijmakers JHJ, Egly J-M. Sublimiting concentration of TFIIH transcription/DNA repair factor causes TTD-A trichothiodystrophy disorder. Nat. Genet. 2000;26:307–313. doi: 10.1038/81603. [DOI] [PubMed] [Google Scholar]
- 56.Lehmann AR. The xeroderma pigmentosum group D (XPD) gene: one gene, two functions, three diseases. Genes Dev. 2001;15:15–23. doi: 10.1101/gad.859501. [DOI] [PubMed] [Google Scholar]
- 57.de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GTJ, van Leeuwen W, et al. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- 58.Prasher JM, Lalai AS, Heijmans-Antonissen C, Ploemacher RE, Hoeijmakers JHJ, Touw IP, Niedernhofer LJ. Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1-/- mice. EMBO J. 2005;24:861–871. doi: 10.1038/sj.emboj.7600542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samper E, Fernandez P, Eguia R, Martin-Rivera L, Bernad A, Blasco MA, Aracil M. Long-term repopulating ability of telomerase-deficient murine hematopoietic stem cells. Blood. 2002;99:2767–2775. doi: 10.1182/blood.v99.8.2767. [DOI] [PubMed] [Google Scholar]
- 60.Choudhury AR, Ju Z, Djojosubroto MW, Schienke A, Lechel A, Schaetzlein S, Jiang H, Stepczynska A, Wang C, et al. Cdkn1a deletion improves stem cell function and lifespan of mice with dysfunctional telomeres without accelerating cancer formation. Nat. Genet. 2007;39:99–105. doi: 10.1038/ng1937. [DOI] [PubMed] [Google Scholar]
- 61.Oshima J. The Werner syndrome protein: an update. BioEssays. 2000;22:894–901. doi: 10.1002/1521-1878(200010)22:10<894::AID-BIES4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 62.Killoran MP, Keck JL. Sit down, relax and unwind: structural insights into RecQ helicase mechanisms. Nucleic Acids Res. 2006;34:4098–4105. doi: 10.1093/nar/gkl538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larizza L, Magnani I, Roversi G. Rothmund–Thomson syndrome and RECQL4 defect: splitting and lumping. Cancer Lett. 2006;232:107–120. doi: 10.1016/j.canlet.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 64.Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, Lombard D, Pathak S, Guarente L, DePinho RA. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat. Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 65.Martin GM. Genetic modulation of senescent phenotypes in homo sapiens. Cell. 2005;120:523–532. doi: 10.1016/j.cell.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 66.Crabbe L, Jauch A, Naeger CM, Holtgreve-Grez H, Karlseder J. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc. Natl Acad. Sci. USA. 2007;104:2205–2210. doi: 10.1073/pnas.0609410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu L, Davies SL, North PS, Goulaouic H, Riou J-F, Turley H, Gatter KC, Hickson ID. The Bloom's syndrome gene product interacts with topoisomerase III. J. Biol. Chem. 2000;275:9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- 68.Wu L, Hickson ID. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 69.Wang LL, Gannavarapu A, Kozinetz CA, Levy ML, Lewis RA, Chintagumpala MM, Ruiz-Maldanado R, Contreras-Ruiz J, Cunniff CRP, et al. Association between osteosarcoma and deleterious mutations in the RECQL4 gene in Rothmund–Thomson syndrome. J. Natl Cancer Inst. 2003;95:669–674. doi: 10.1093/jnci/95.9.669. [DOI] [PubMed] [Google Scholar]
- 70.Hoki Y, Araki R, Fujimori A, Ohhata T, Koseki H, Fukumura R, Nakamura M, Takahashi H, Noda Y, et al. Growth retardation and skin abnormalities of the Recql4-deficient mouse. Hum. Mol. Genet. 2003;12:2293–2299. doi: 10.1093/hmg/ddg254. [DOI] [PubMed] [Google Scholar]
- 71.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, Tagle DA, Smith S, Uziel T, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 72.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 73.Lavin MF, Birrell G, Chen P, Kozlov S, Scott S, Gueven N. ATM signaling and genomic stability in response to DNA damage. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005;569:123–132. doi: 10.1016/j.mrfmmm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 74.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 75.Morales M, Theunissen JW, Kim CF, Kitagawa R, Kastan MB, Petrini JH. The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor. Genes Dev. 2005;19:3043–3054. doi: 10.1101/gad.1373705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young NS, Abkowitz JL, Luzzatto L. New insights into the pathophysiology of acquired cytopenias. Hematology (Am. Soc. Hematol. Educ. Prog.) 2000:18–38. doi: 10.1182/asheducation-2000.1.18. [DOI] [PubMed] [Google Scholar]
- 77.Curzon PG, Muers MF, Rajah SM. Aplastic anaemia associated with influenza A infection. Scand. J. Haematol. 1983;30:232–234. doi: 10.1111/j.1600-0609.1983.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 78.Mayer A, Podlech J, Kurz S, Steffens HP, Maiberger S, Thalmeier K, Angele P, Dreher L, Reddehase MJ. Bone marrow failure by cytomegalovirus is associated with an in vivo deficiency in the expression of essential stromal hemopoietin genes. J. Virol. 1997;71:4589–4598. doi: 10.1128/jvi.71.6.4589-4598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Revoltella RP, Vigneti E, Fruscalzo A, Park M, Ragona G, Rocchi G, Calef E. Epstein-Barr virus DNA sequences in precursor monocyte-macrophage cell lines established from the bone marrow of children with maturation defects of haematopoiesis. J. Gen. Virol. 1989;70(Pt 5):1203–1215. doi: 10.1099/0022-1317-70-5-1203. [DOI] [PubMed] [Google Scholar]
- 80.Meira DG, Lorand-Metze I, Toro AD, Silva MT, Vilela MM. Bone marrow features in children with HIV infection and peripheral blood cytopenias. J. Trop. Pediatr. 2005;51:114–119. doi: 10.1093/tropej/fmh096. [DOI] [PubMed] [Google Scholar]
- 81.Ramos-Casals M, Garcia-Carrasco M, Lopez-Medrano F, Trejo O, Forns X, Lopez-Guillermo A, Munoz C, Ingelmo M, Font J. Severe autoimmune cytopenias in treatment-naive hepatitis C virus infection: clinical description of 35 cases. Medicine. 2003;82:87–96. doi: 10.1097/00005792-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Lu J, Basu A, Melenhorst JJ, Young NS, Brown KE. Analysis of T-cell repertoire in hepatitis-associated aplastic anemia. Blood. 2004;103:4588–4593. doi: 10.1182/blood-2003-11-3959. [DOI] [PubMed] [Google Scholar]
- 83.Young NS. Acquired aplastic anemia. Ann. Intern. Med. 2002;136:534–546. doi: 10.7326/0003-4819-136-7-200204020-00011. [DOI] [PubMed] [Google Scholar]
- 84.Zeng W, Nakao S, Takamatsu H, Yachie A, Takami A, Kondo Y, Sugimori N, Yamazaki H, Miura YS, et al. Characterization of T-cell repertoire of the bone marrow in immune-mediated aplastic anemia: evidence for the involvement of antigen-driven T-cell response in cyclosporine-dependent aplastic anemia. Blood. 1999;93:3008–3016. [PubMed] [Google Scholar]