Abstract
There are at least three human diseases that are associated with germ-line mutations of the genes encoding the two essential components of telomerase, TERT and TERC. Heterozygous mutations of these genes have been described for patients with dyskeratosis congenita, bone marrow failure and idiopathic pulmonary fibrosis. In this review, we will detail the clinical similarities and difference of these diseases and review the molecular phenotypes observed. The spectrum of mutations in TERT and TERC varies for these diseases and may in part explain the clinical differences observed. Environmental insults and genetic modifiers that accelerate telomere shortening and increase cell turnover may exaggerate the effects of telomerase haploinsufficiency, contributing to the variability of age of onset as well as tissue-specific organ pathology. A central still unanswered question is whether telomerase dysfunction and short telomeres are a much more prominent factor than previously suspected in other adult-onset, age-related diseases. Understanding the biological effects of these mutations may ultimately lead to novel treatments for these patients.
INTRODUCTION
Telomeres are repetitive, non-coding DNA [TTAGGG] elements at the ends of chromosomes. Telomerase is a multimeric ribonucleoprotein complex consisting of at least a functional RNA (hTR) that contains the template region complementary to the telomeric sequence (1) and a reverse transcriptase protein component (hTERT) that catalyzes the addition of telomeric repeats to the ends of chromosomes (2,3). hTR is expressed in all tissues but hTERT is only highly expressed in specific germ line cells, proliferative stem cells of renewal tissues, and immortal cancer cells (4–9). In most human somatic cells, telomeres shorten with each cell division due to incomplete lagging DNA strand synthesis (10–12) and oxidative damage (13,14). Even cells that express telomerase (e.g. peripheral blood mononuclear cells and other stem-like cells) undergo telomere shortening with age (15,16). Telomere lengths are affected by their starting set points, the cellular activity of telomerase, the cell's history of cell division and environmental effects. Overexpression of the catalytic subunit of human telomerase (hTERT) induces telomerase activity and telomere elongation (17).
While there is substantial evidence for replicative senescence in cell culture models that is associated with telomere attrition, the evidence for in vivo telomere shortening actually contributing to human aging has been difficult to document. There is agreement that telomeres are shorter in older individuals as compared to younger individuals (12,18), but proving that telomere dysfunction causes age-related pathology has been difficult to rigorously test because it is not easy (technically or ethically) to restore telomere length in humans. Rodent animal models do not provide an optimal system for testing the importance of telomeres in human aging. In addition, there is considerable heterogeneity of telomere length between humans and also differences between tissues and cell types within an individual. This makes it very difficult to directly link telomere shortening as a proximal cause of aging. Only some cells in a given tissue may have critically shortened telomeres as a consequence of replicative exhaustion leading to cell senescence. This small fraction of senescent cells may interfere with the function of otherwise normal tissues by expression of degradative enzymes that can destroy tissue architecture and by secretion of inflammatory cytokines and growth factors that cause local activation of neighboring cells (19). Thus, central questions include what is the minimal threshold of senescent cells and what is the identity of senescent cells that can change the homeostasis of a specific tissue leading to a decline in tissue function?
The relevance of telomere shortening induced by dysfunctional telomerase to physiological aging is suggested by the phenotypes of a telomerase deficient mouse model (20,21). Early generations of mTR−/− mice do not show abnormalities presumably because laboratory mice have a much longer telomere length (20–150 kb) than humans (5–15 kb). However, late generations of mTR−/− mice have defects in cell viability of highly proliferative tissues. They have a shorter life span compared with wild-type mice and show hair loss/early graying of hair, decreased capacity for wound healing and a slight increased incidence of cancer. However, the telomerase-deficient mouse models may not completely mirror human diseases of telomerase dysfunction as they have longer telomeres (20,22), express telomerase activity in most tissues (23,24) and do not use telomere shortening as a counting mechanism (25).
The most widely accepted hypothesis is that the major function of clonal attenuation and replicative aging in humans is to form a barrier against the development of cancer. The number of permitted cell divisions may represent a balance between providing enough cell turnovers to provide maintenance and repair of tissues and limiting the number of times a cell can divide to prevent cancer. Few stone-age human ancestors lived beyond 30–40 years of age before dying, usually from infections (26). There may have been selective pressure to have a sufficient number of cell divisions to provide for reasonable fitness until the time one was likely to have died (by age 40), but additional capacity for cell division beyond this point would come at the expense of a greater risk of cancer (27). In the modern era humans live beyond their historical lifespan and, as a consequence, the limit on replicative capacity may have physiological consequences that contribute to the pathologies of aging. Rare inherited diseases described below provide additional evidence that telomerase dysfunction and short telomeres play a role in certain age-related human diseases.
During the past few years, experimental evidence has been obtained suggesting that telomere shortening may be causative for three different human disorders. These are dyskeratosis congenita (DKC), multiple different diseases collectively characterized by bone marrow failure and idiopathic pulmonary fibrosis (IPF). In this review, we will summarize the evidence for telomere dysfunction in these conditions. The review will end with speculation on the future for telomere therapy that may lead to novel interventions to slow down or even reverse disease progression.
DYSKERATOSIS CONGENITA (DKC)
DKC is a rare inherited multisystem disorder characterized by the triad of reticulated skin pigmentation, nail dystrophy and white patches (leukoplakia) in the mouth. The prevalence is approximately 1 in 1 000 000 individuals, with death occurring at a median age of 16 (28). Patients typically appear healthy at birth and develop the mucocutaneous features later in life as well as a wide range of somatic abnormalities including bone marrow, pulmonary, gastrointestinal, endocrine, skeletal, urologic, immunologic and neurologic abnormalities. Bone marrow failure is the leading cause of death, followed by pulmonary disease and cancer.
There is both clinical and genetic heterogeneity of DKC. Most affected patients in families in a large international registry of DKC are male, suggesting an X-linked pattern of inheritance (29). Almost all the patients in this group develop the mucocutaneous features of the disease during the first two decades of life. Bone marrow failure is especially common and severe. Over 85% of patients have a peripheral cytopenia of at least one lineage and 76% develop pancytopenia at a median age of 10 years. Other families exhibit an autosomal dominant or autosomal recessive pattern of inheritance.
Mutations in genes involved with telomere maintenance have been identified in ∼40% of clinically diagnosed DKC cases (30). A positional cloning approach led to the identification of mutations in the gene DKC1 encoding dyskerin for the X-linked recessive form of the disease (31). Dyskerin is a nucleolar protein that copurifies with the RNA component (hTR) and protein component of telomerase (hTERT) in the catalytically active human complex (32). It is one of four proteins found to be associated with the telomerase complex (Figure 1). Cell lines from patients with X-linked DKC have reduced levels of hTR that limit telomere length (33,34). Affected males have one copy of the X-linked DKC1 gene and their cells express only the mutated form of dyskerin. Most mutations are missense changes that result in single amino acid substitutions that cluster in two regions, the N-terminal domain and the archaeosine-specific transglycosylase (PUA) domain (35). By homology with the crystal structure of a bacterial ortholog of dyskerin, both of these domains are predicted to play a role in binding of hTR (36,37).
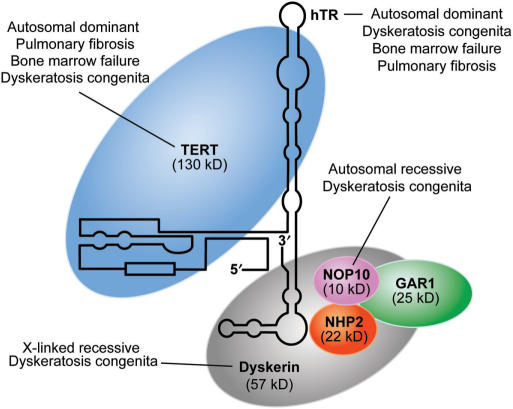
Figure 1.
Schematic structure of the telomerase complex and diseases associated with mutations in the genes encoding each protein within the complex. TERT, hTR, dyskerin, NOP10, NHP2 and GAR1 constitute the telomerase ribonucleoprotein complex. Mutations in the genes TERT or TERC (encoding hTR) are associated with autosomal dominant pulmonary fibrosis, bone marrow failure syndromes and DKC. Mutations in DKC1, encoding dyskerin, cause X-linked recessive DKC. Autosomal recessive DKC has been described in one family with a homozygous mutation in NOLA3, encoding NOP10.
Several different deletion and nucleotide substitution mutations in the TERC gene, encoding hTR, were first found in patients with the rare autosomal dominant form of DKC (38,39). Many of these mutations affect in vitro hTR accumulation and/or catalytic activity (40) but all demonstrate in vivo telomere length shortening (discussed below). Subsequently, missense mutations in the gene TERT, encoding the protein component of telomerase, have been described in patients with DKC (30,41,42). Affected individuals with mutations in either gene (TERC or TERT) have shorter telomere lengths than age-matched controls. Autosomal dominant inheritance can arise from a dominant negative effect through interaction and inhibition of the wild-type product by the mutated gene product in a multiprotein complex. Alternatively, autosomal dominant inheritance can arise from haploinsufficiency with a reduction in the normal level of telomerase due to one mutated copy of the gene. In most cases, the effect of the mutations of these two genes appears to be consistent with a mechanism of haploinsufficiency (41,43,44).
Homozygosity mapping of one large consanguineous Saudi Arabian family with autosomal recessive DKC led to the identification of a homozygous missense mutation in NOLA3 (encoding the protein NOP10) that segregated with the phenotype (45). This mutation is predicted to result in an arginine to tryptophan substitution (R34W) in a highly conserved region of the protein NOP10, one of four proteins associated with the telomerase complex (Figure 1) (46). None of the 15 other autosomal recessive families showed linkage to this region and none of the probands (index cases) of 171 uncharacterized DKC families had rare sequence variants in this gene, underscoring the rarity of mutations in this gene as a cause of DKC (45).
Regardless of the pattern of genetic inheritance, all patients with DKC have very short telomeres, implying a common pathway underlying the mechanism of this disease (33,39,41,47). When patients with DKC1 mutations are categorized by disease severity, those with the most severe phenotypes have shorter telomeres than those with the mildest phenotypes (age over 15 years with no co-existent aplastic anemia) (30). Using the telomere flow-FISH assay and a cut off of total leukocyte telomere lengths below the first percentile, DKC patients could be distinguished from their unaffected relatives with 100% sensitivity and 90% specificity (48). Interestingly, DKC families with mutations in either TERC or TERT demonstrate genetic anticipation with a worsening of disease severity and an earlier onset of symptoms with successive generations (39,41). The onset and severity of disease correlates with progressive telomere shortening in later generations. Siblings that do not inherit the mutated TERC gene do not have symptoms. Even though these siblings inherit short telomeres from the affected parent, the non-mutated telomerase preferentially acts on the shortest telomeres to normalize their lengths (49). Thus, DKC patients have to inherit both short telomeres and have a mutation in one of the components of telomerase in order to show anticipation. This is a new mechanism underlying genetic anticipation. Instead of amplification of triplet repeats as is frequently seen in neurodegenerative disorders (50), in DKC there is a ‘contraction’ of telomere repeats.
Most clinical presentations of DKC are associated with an impaired proliferative capacity of tissues. For example, skin fibroblasts from DKC patients have a longer doubling time and abnormal chromosomal rearrangements in cell culture (51). In addition, the number of hematopoietic progenitor cells is decreased in DKC patients (52). The tissues requiring constant renewal, i.e. skin, oral mucosa and bone marrow are the ones that are affected most frequently in DKC.
Connection between sporadic bone marrow failure and dyskeratosis congenita
Over 80% of patients with DKC have bone marrow failure, usually characterized by aplastic anemia or a reduction in all three blood cell lineages (53). Because of this, DKC can be considered one of the inherited bone marrow failure syndromes. After mutations in TERC were first described for patients with the rare autosomal dominant form of DKC (38), it was noted that the clinical phenotypes of mutation carriers were often only bone marrow failure without the mucocutaneous features typical of this disease (54). This led to the sequencing of TERC for patients with either the familial or sporadic forms of various bone marrow failure syndromes. Subsequently, different mutations in TERC have been found in patients with aplastic anemia (43,54–58), myelodysplastic syndrome, acute myelogenous leukemia arising from myelodysplastic syndrome (30,56,59), paroxysmal nocturnal hemoglobinuria (60) and essential thrombocytosis (43). A similar sequencing strategy led to the identification of mutations in the gene encoding the protein component of telomerase, TERT, in patients with aplastic anemia (42,58,61,62). A number of different mutations in both TERC and TERT have been found in patients with bone marrow failure syndromes (Figure 2). Again, most variants in either gene lead to reduced telomerase activity by haploinsufficiency, except for two different mutations in TERC that affect the template domain of hTR and which appear to have a dominant negative effect (58).
Figure 2.
Schematic representation of hTR (A) and TERT (B) and the location of mutations associated with DKC (red), bone marrow failure syndromes (orange) and pulmonary fibrosis (black). Mutations in TERC, encoding hTR and TERT have been described for patients with DKC (30,38,39,88) and bone marrow failure syndromes including aplastic anemia (30,43,54–58), myelodysplastic syndrome (56,59) and essential thrombocytemia (43). Rare mutations in TERC have been found in patients with familial pulmonary fibrosis (86,87). Mutations in TERT have been found in patients with DKC (30,41), familial pulmonary fibrosis (86,87) and aplastic anemia (42,58,61,62). Common genetic polymorphisms and promoter mutations are not shown. The schematic depiction of the predicted secondary structure of hTR is indicated (103); mutations in TERT are shown relative to conserved domains (3,104,105).
Telomere shortening of circulating leukocytes is observed in many of the different inherited bone marrow failure syndromes. Some patients with these disorders have short telomeres and defined mutations in TERC or TERT, as described above. For others, the connection between accelerated telomere shortening and reduced telomerase activity is not clear. Shwachman-Diamond syndrome (SDS) is an autosomal recessive syndrome characterized by bone marrow failure, pancreatic exocrine insufficiency, skeletal defects, an increased risk of hematologic cancers and abnormal telomere shortening (63,64). Mutations in the gene SBDS are found in >90% of patients with SDS (65). Indirect evidence suggests that the SBDS gene product may function in RNA metabolism (65). There is no evidence of a reduction in telomerase activity in lymphocytes from SDS patients or a physical interaction between the SBDS gene product and the telomerase complex of hTR and hTERT (65,66). Short telomeres have also been seen in subpopulations of patients with aplastic anemia (67–69), paroxysmal nocturnal hemoglobinuria (70) and Fanconi's anemia (71), suggesting that short telomeres of circulating leukocytes may be a marker of progenitor cell depletion in sporadic cases of several bone marrow failure syndromes.
IDIOPATHIC PULMONARY FIBROSIS
Idiopathic pulmonary fibrosis (IPF) is a progressive, fatal lung disease characterized by lung scarring and abnormal gas exchange (72). The disease is rare in children and typically affects individuals age 50 or more, with the incidence increasing with advanced age (73). The prevalence is 4 per 100 000 in adults aged 18–34 years but increases to over 227 per 100 000 among those 75 years and older. The disease is more common in men and in smokers (74,75). There are approximately 89 000 persons diagnosed with IPF in the United States, with about 34 000 new cases diagnosed each year (74). IPF is always fatal with no spontaneous remission. The median life span after diagnosis is usually between 2.5 and 3.5 years (76).
The clinical presentation of IPF is similar to that of all the scarring lung diseases, collectively called interstitial lung diseases, which lead to pulmonary fibrosis and symptoms of a chronic cough and shortness of breath. Patients with IPF have abnormal pulmonary function tests (PFTs) reflecting a restrictive respiratory pattern coupled with evidence of lung scarring, characteristically involving the periphery of the lower lobes of the lung. The diagnosis of IPF is supported by thorascopic lung biopsy, which reveals a histological pattern of ‘usual interstitial pneumonia’ (UIP), with fibroblastic foci at the interface between normal and scarred lung. In the absence of a surgical lung biopsy, IPF can be diagnosed after other known causes of PF are excluded and certain clinical criteria have been met using spirometry to assess lung function, high resolution computed tomography (CT) of the chest to assess lung morphology, and a transbronchial lung biopsy or bronchoalveolar fluid (BAL) to rule out alternative diagnoses (76). Over one hundred different clinical interstitial lung diseases are associated with PF, including connective tissue diseases, occupational exposures, drug toxicities and other primary diseases of the lung. If no culprit can be identified, the interstitial lung disease may represent a class of lung disease called idiopathic interstitial pneumonias (IIP). These have been further classified (77) to assist with predicting clinical course and outcome. IPF is the most common of all the IIPs and also the most deadly, with no definitive medical treatments. Lung transplantation is an option for younger patients.
A small subset of patients with IPF has a familial form of the disease. The first large collection of familial IPF families was reported just 7 years ago by Marshall and his colleagues who identified 25 families (including 67 cases) (78). They estimated that familial cases account for 0.5–2.2% of all cases of IPF, with a prevalence of 1.3 cases per million. The familial form of IPF accounts for 3.3–3.7% of all cases in Finland with geographic clustering of the familial cases suggesting a founder effect (79). In cohorts of patients with early onset disease, such as those referred for lung transplantation, the incidence of the familial form of the disease has been reported to be much higher, up to 14–19% (80,81). The pattern of inheritance in most families is consistent with an autosomal dominant pattern with incomplete penetrance (78,81–83).
The clinical presentation of familial IPF in the United Kingdom appears to be indistinguishable from sporadic IPF except that the age of onset tends to be younger (84,85). The mean age at diagnosis is 55.5 years for familial IPF compared to 67.4–69.8 years in sporadic IPF. In familial IPF, males outnumber females, with a ratio of 1.75:1. Half of the cases in families are smokers. In another collection of over 100 families with IPF, older age, male sex and smoking cigarettes were strongly associated with the development of the disease (83). An autosomal dominant pattern of inheritance was seen in 20% of these families. Within the families, affected individuals have radiographic and/or histopathologic features consistent with two or even three different types of IIP, suggesting that the inherited genetic factor(s) confer an increased risk for the development of pulmonary fibrosis in response to injury, but not an absolute risk for one particular histologic subtype of IIP.
Connections between idiopathic pulmonary fibrosis and telomere dysfunction
A clue to the molecular basis of familial IPF emerged from studies performed simultaneously by two groups using different approaches: Armanios and her colleagues at Johns Hopkins used a candidate gene approach (86) while our group used a non-biased whole genome mapping approach (87). Armanios had previously described a family with autosomal dominant DKC with haploinsufficiency of telomerase due to a missense mutation in the reverse transcriptase domain of TERT in which four of the seven affected individuals were diagnosed with idiopathic pulmonary fibrosis between 21 and 63 years of age. She and her colleagues subsequently sequenced TERT in probands of a collection of 73 kindreds with familial IPF. They identified six probands (8%) with mutations; five were heterozygous for mutations in TERT and one was heterozygous for a mutation in TERC. The telomere lengths in lymphocytes from these affected individuals were all less than the 10th percentile when compared to age-matched controls.
Taking an unbiased genetic approach, we performed a whole genome linkage scan in two large families with familial IPF. We found evidence of linkage to the short arm of chromosome 5 in a small region that contained the TERT gene. Since a mutation in this gene had been previously found in a DKC family in which many of the affected individuals had pulmonary fibrosis (41), we considered this to be an excellent candidate gene. Heterozygous mutations in TERC or TERT are seen in ∼12% of our cohort of families; TERT mutations were found not only in the two large families used for the linkage analysis but also in four other kindreds with familial pulmonary fibrosis, and a TERC mutation was found in an additional family (87). The mutations in TERT segregated not only with individuals that met the strict diagnosis of IPF, but also several with pulmonary fibrosis favoring the upper lobes of the lung and others with unclassified pulmonary disease. Several family members of these kindreds with no self-reported lung problems were identified to be heterozygous for a mutant TERT allele; thus, the pulmonary disease was not completely penetrant. We have found that almost 70% of telomerase mutation carriers over the age of 40 have pulmonary disease of some kind. More than one-third has osteoporosis or osteopenia affecting the axial skeleton. While this condition may have been exacerbated by corticosteroid treatment that is frequently given to patients with pulmonary fibrosis, in half the cases this diagnosis either preceded steroid treatment or occurred independently of a diagnosis of pulmonary fibrosis. A mild to moderate anemia (hematocrit of 23–32%) and several cancers (lymphoma, breast, skin) were occasionally found among the telomerase mutations carriers in the kindreds with familial pulmonary fibrosis. Thus, our preliminary findings indicate that the clinical phenotypes of anemia and osteoporosis may provide additional clinical indices of disease in IPF families due to telomerase mutations.
The mutations in TERT found in kindreds with familial IPF are different from those previously found in patients with DKC or bone marrow failure (Figure 2B). Three identified mutations are nucleotide deletions that are predicted to create frameshifts and truncated proteins. As predicted, the V747fs frameshift mutations missing half the reverse transcriptase domain had undetectable in vitro telomerase activity. Co-translation of different ratios of plasmids encoding the V747fs protein and the wild-type TERT protein did not negatively affect the activity of the wild-type protein, suggesting a mechanism of haploinsufficiency (87). The other two identified deletion mutants, P122fs and E1116fs, are predicted to delete most of the protein or just the terminal 17 amino acids of the protein containing the conserved E-IV domain, respectively. The latter mutation has almost undetectable in vitro telomerase activity (87).
The other identified mutations in TERT in patients with familial pulmonary fibrosis are missense and splice site mutations. The missense mutations demonstrate a range of 30–100% wild-type telomerase activity in conventional rabbit reticulocyte in vitro assays (87). The missense mutations span all the different functional domains of the protein. There is a cluster of three independent mutations in the motif C region of the reverse transcriptase domain. We found the R865H mutation in motif C that segregated in one of the large families used in the original linkage scan. Sequencing TERT in 44 individuals with sporadic IIPs identified one individual with a mutation involving the same amino acid residue, R866C. Armanios et al. also identified a mutation that results in selective skipping of exon 10, which encodes motif C (86). These three independent mutations in motif C in IPF patients suggest a potential genotype–phenotype relationship.
Only two different mutations in TERC have been described in kindreds with familial IPF. One mutation, 37a > g, affecting the terminal residue of the P1b helix that functions in the hTR template boundary definition, was previously identified in a patient with DKC and severe aplastic anemia who is a compound heterozygote for mutations in TERC (88). It was not reported whether family members with the same mutation had any signs or symptoms of pulmonary fibrosis. A second mutation in TERC was found in a family with pulmonary fibrosis and aplastic anemia. Mutations in TERC thus appear more likely associated with DKC and bone marrow failure syndromes than familial pulmonary fibrosis.
When compared with age-matched normal controls, individuals heterozygous for all the identified mutations in TERT or TERC had shorter telomere lengths of circulating white blood cells (86,87). When these lengths were compared between individuals belonging to the same large family with the R865H mutation in TERT, the younger heterozygous mutation carriers had telomere lengths similar to age-matched controls. In contrast, many of the mutation carriers older than 40 had telomeres with a shorter mean length and an increased proportion of short telomeres. Haploinsufficiency of telomerase due to TERT mutations may lead to the clinical phenotype of pulmonary fibrosis only after sufficient time has elapsed and after multiple rounds of cell division have occurred to shorten telomeres to critical lengths. This may explain why individuals present with this disease later in life, as adults. IPF is an age-related disease, with markedly increased prevalence with advanced age, especially after the fifth decade of life. Some fraction of sporadic cases is likely due to telomere attrition from age and environmental insults independent of specific genetic mutations.
Comparisons between DKC, bone marrow failure syndromes and IPF
Table 1 compares the clinical characteristics of these diseases associated with mutations in genes encoding the telomerase complex. Is telomere shortening a common molecular mechanism underlying them all? With rare exceptions, telomere lengths of circulating cells, cultured fibroblasts and lymphoblasts, or of genomic DNA isolated from leukocytes are all abnormally short for patients with DKC, bone marrow failure and IPF with mutations in genes associated with the telomerase complex. In general, the shorter telomeres are associated with earlier onset and more severe phenotypes. This is seen most dramatically in families with genetic anticipation.
Table 1.
Characteristics of diseases associated with mutations in genes encoding proteins in the telomerase complex: dyskeratosis congenita (DKC), various bone marrow failure syndromes and Idiopathic Pulmonary Fibrosis
| DKC | Bone marrow failure syndromes | Idiopathic pulmonary fibrosis | |
|---|---|---|---|
| Inheritance patterns | XLR, AD, AR | AD, AR, sporadic | AD, sporadic |
| Age of onset | Birth—60 (usually 10–30) | All ages | Adult (usually >40) |
| Skin abnormalities | Yes | No | No |
| Bone marrow failure | Yes (>80%) | Yes | Anemia? |
| Somatic abnormalities | Yes | Rare | Osteoporosis? |
| Cancer | Yes | Yes | ? |
| Chromosomal instability | Yes | Yes | ? |
| Short telomeres in patients with disease | All | Some | ? |
| Genes in which mutations have been described | DKC1, TERC, TERT, NOLA3 | TERC, TERT | TERT, TERC |
XLR, X-linked recessive; AD, autosomal dominant and AR, autosomal recessive.
But how can mutations in the same genes lead to such different clinical presentations? We believe that the resulting phenotype is likely determined by genotype, time and tissue-specific environmental effects. As seen in Figure 2, different mutations in TERC and TERT are found in patients with different clinical diseases. Mutations in TERC are usually found in patients with DKC or bone marrow failure. Mutations in TERT have been found more often in patients with IPF or bone marrow failure than those with the classic skin features of DKC. In addition to the effect of the mutations upon telomere length, these mutations may have other cellular effects. For example, DKC patients with NOLA3, DKC1 and TERC mutations show significantly reduced levels of hTR RNA expression in whole blood than controls or patients with TERT mutations (45). In addition, hTR has been shown to have functions that are separable from its role in telomerase (89,90). In particular, hTR has recently been implicated as a negative regulator of ATR activity (86). It is possible that some classes of stem cells are programmed to leave the stem cell compartment in response to sub-lethal DNA damage signals, in order to reduce the likelihood of cancer. The increased hematopoietic/mucocutaneous prominence in DKC versus IPF may reflect a preferential exhaustion of progenitors in the marrow and skin due to this non-telomeric effect of hTR. There is no evidence of loss of heterozygosity in affected lung tissue to explain the lung-specific fibrosis seen with germline TERT mutations (data not shown).
Telomere lengths are decreased not only in IPF patients with TERT mutations, but also in mutation carriers within the same family without lung disease (87), suggesting that other factors influence the clinical expression of IPF associated with TERT mutations. Environmental factors may influence cell proliferation or the rate of telomere loss in an organ-specific manner. Cigarette smoking has been associated with a dose-dependent shortening of telomere lengths of circulating leukocytes (91), and this effect may be magnified in lung tissue. In our study, mutation carriers of TERT or TERC with a past history of smoking died on average 10 years earlier than non-smokers (58 years versus 68 years) (87). In family F11, one individual who was a non-smoker with liver cirrhosis of unknown etiology had the same inherited TERT mutation as the proband with IPF who was a previous cigarette smoker (87). The full spectrum of phenotypes associated with TERT mutations is likely to grow as more heterozygous mutation carriers are identified. As discussed above, IPF associated with heterozygous TERT mutations is always diagnosed in adults, not children, suggesting that age is important in the pathogenesis of disease. Older adults have a greater magnitude of cumulative environmental exposures than children. With the passage of time, there is further telomere shortening with cell proliferation and replicative exhaustion.
A key feature of the disease process in DKC is that there is a failure of proliferative tissues that are dependent upon renewal by stem cell activity. In a model initially proposed by Mason et al. (35), germline mutations in the genes encoding the telomerase complex cause progressive telomere shortening, resulting in the senescence and perhaps death of stem cells (Figure 3). An increased number of quiescent cells with replicative potential must then be recruited into the cell cycle. These new stem cells proliferate and undergo further telomere shortening, followed by senescence. The premature exhaustion of the pool of stem cells leads to clinical disease. If mutations in cell cycle checkpoint genes such as p53 have occurred, cells with very short telomeres can continue to divide until their telomeres become more dysfunctional and lose their ability to protect the ends of chromosomes. These can then initiate breakage-fusion-bridge cycles and generate genomic instability, which in turn can lead to cancer. In contrast to the bone marrow, the adult lung has a much slower turnover of cells. The different regions of the lung house different stem cell populations that contribute to repair and maintenance (92). The observations of TERT mutations and shortened telomere lengths in patients with IPF suggest that the lung is among the tissues that are dependent upon renewal by telomerase-expressing stem cells, with disruption of this process leading to fibrosis. Similarly, in different rodent models of pulmonary fibrosis, injury of the lung epithelial cells leads to increased expression of telomerase suggesting its protective role in this disease process (93,94).
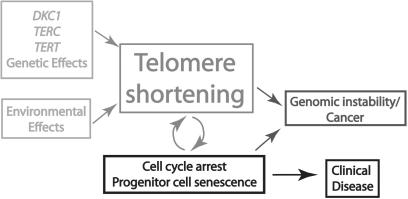
Figure 3.
A proposed model underlying the pathogenesis of inherited human diseases of telomere dysfunction. Inherited genetic effects and certain environmental effects can affect telomere lengths. With each round of cell division telomeres shorten until a critical telomere length is reached. Additional quiescent cells with replicative potential are then recruited into the cell cycle. These new stem cells proliferate and undergo further telomere shortening, followed by senescence.The premature exhaustion of the pool of stem cells leads to the clinical diseases of pulmonary fibrosis, bone marrow failure and failure of other organs associated with dyskeratosis congenita. If mutations in cell cycle checkpoint genes such as p53 have occurred, the senescent growth arrest is blocked, cells divide and telomeres continue to shorten until they lose their ability to protect the ends of chromosomes. These can initiate breakage-fusion-bridge cycles and generate genomic instability, which can lead to cancer.
Several correlative studies lend support to the idea that individuals with shorter than age-matched control telomeres are prone to disease. For example, patients with atherosclerotic heart disease have significantly shorter telomeres compared to healthy aged-matched controls (95–97). Independent of age and mean arterial pressure, arterial stiffness and pulse pressure are inversely correlated with TRF length in men (95). In a retrospective study of archival cryopreserved blood cells, telomere length was a major independent predictor for overall mortality. Patients (60–97 years of age) with the shortest quartile of blood telomeres had an 8-fold higher mortality due to infectious disease compared to those in the other quartiles (98). Shorter telomere lengths have also been associated with reduced bone mineral density and osteoporosis (99), obesity (100) and cigarette smoking (91,100). Whether mutations in TERT or TERC or the genes that encode telomerase-associated proteins underlie these more common age-related diseases remains to be seen.
Can we slow down the progressive telomeres shortening in DKC, bone marrow failure or IPF?
There is now strong circumstantial evidence that inherited defects in the telomerase pathway cause some cases of DKC, bone marrow failure and IPF. Collectively, these studies lend support to the hypothesis that short telomeres caused by various insults (increases in cell turnover associated with chronic age-associated diseases, inflammatory processes, oxidative damage, genetic alterations in telomerase components) correlate with disease. Showing cause and effect will require demonstrating that slowing down the rate of telomere loss or resetting the telomere clock reverses or delays the onset of disease. Are DKC, bone marrow failure and IPF the appropriate diseases to test the therapeutic value of telomere rejuvenation?
We have expressed TERT to immortalize a variety of human cell types including skin keratinocytes, dermal fibroblasts, muscle satellite (stem) cells, endothelial cells, retinal-pigmented epithelial cells, breast epithelial cells, and both corneal fibroblasts (keratocytes) and corneal epithelial cells. Human corneal and skin cells expressing TERT can be used to form organotypic cultures. Such bioengineered tissues express differentiation-specific proteins, suggesting that TERT does not inhibit the normal differentiation of cells. TERT-immortalized myoblasts form apparently normal muscle in vivo following injection into immunoincompetent mice (101). Even the overexpression of TERT, cdk4 and mutant Ras does not prevent the differentiation of normal skin keratinocytes in organotypic culture (unpublished data). The production of engineered cells and tissues thus offers the possibility of treating a variety of age-related medical diseases that are due to telomere-based replicative senescence.
TREATMENT OPTIONS
We propose that there are likely to be many different diseases in which replicative aging due to excess telomere shortening contributes to tissue-specific or cell-specific disease. For these pathologic processes, rejuvenating cells by increasing telomere lengths may be beneficial. We already have the ability to express telomerase, leaving cells with long telomeres and a greatly extended lifespan. Advances in gene therapy, particularly the development of ‘fail-safe’ constructs that encode inducible cell-suicide products, will reduce the risk of cancerous transformation associated with telomerase expression for therapeutic purposes. To test if telomere rejuvenation could impact on the progression of disease such as bone marrow failure, one approach would be to isolate hematopoietic stem cells from patients with short telomeres, expand the cells in the laboratory, transfect them with adenoviral hTERT (that does not integrate into nuclear DNA), characterize telomere lengths of the cells in vitro and return the rejuvenated stem cells to the patient. The obvious advantages of this approach over allogenic bone marrow transplants are that using the patient's own cells would avoid problems of rejection and there would be no need for bone marrow ablation. Clearly, safety and efficacy standards, as well as quality and control assurances will need to be carefully considered prior to initiating these studies. Several reports have suggested that small molecule activators of telomerase can extend the lifespan of lymphocytes, and such molecules might potentially work with hematopoietic stem cells as well (102).
Treatment of pulmonary fibrosis may present an especially difficult clinical challenge because fibrosis of the lung parenchyma is generally considered an end-stage manifestation of an irreversible pathologic process. For this reason, effective treatment of pulmonary fibrosis due to telomerase insufficiency may require early recognition of preclinical disease, modification of environmental exposures and strategies for early intervention with telomerase activation. Since oxidative damage has been shown to shorten telomeres in tissue culture models (13,14), systemic administration of antioxidants may be beneficial at preserving telomere lengths and delaying the onset of disease in susceptible individuals. Strategies towards activation of telomerase should be targeted to cells of replicative potential in the lung, ideally before the onset of disease. The goal would be to maintain a balance of enough telomerase activity to lengthen critically short telomeres without allowing pre-malignant lesions to progress. Since this may be difficult, the most promising approach may involve gene therapy including several conditional replication-dependent suicide genes. Under these conditions, any tumors that develop could have built-in therapeutic targets for their control.
CONCLUSIONS
The identification of mutations in multiple components of the telomerase complex in IPF, DKC and sporadic bone marrow failure patients underscores the importance of telomerase in the pathogenesis of these disorders. These mutations may decrease the stability of the telomerase complex, reduce the recruitment or activation of telomerase at the telomere itself, or directly affect the enzymatic activity of telomerase. An insufficient level of telomerase results in progressive telomere shortening, most notably in tissues that require continuous renewal. Progress has been slow in DKC since it is such a rare disease. However, the discovery that other more common diseases, such as bone marrow failure syndromes and IPF, are also associated with telomerase mutations and telomere shortening opens up more opportunities so that we may learn a great deal about the mechanisms and consequences of telomere maintenance in humans.
ACKNOWLEDGEMENTS
We would like to thank Angela Diehl for drawing the figure illustrations. This work was supported by the Ted Nash Foundation (J.W.S. and W.E.W), Lung Cancer SPORE P50 CA75907, NSCOR NNJ05HD36G (J.W.S.) and NIH K23 RR020632 (C.K.G.). Funding to pay the Open Access publication charges for this article was provided by Ted Nash Foundation.
Conflict of interest statement. None.
REFERENCES
- 1.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 4.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Harle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc. Natl Acad. Sci. USA. 1996;93:6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broccoli D, Young JW, de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc. Natl Acad. Sci. USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, Shay JW, Ishioka S, Yamakido M. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J. Immunol. 1995;155:3711–3715. [PubMed] [Google Scholar]
- 8.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 9.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur. J. Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 10.de Lange T, Shiue L, Myers RM, Cox DR, Naylor SL, Killery AM, Varmus HE. Structure and variability of human chromosome ends. Mol. Cell. Biol. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 12.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 13.von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp. Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 14.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 15.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc. Natl Acad. Sci. USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwama H, Ohyashiki K, Ohyashiki JH, Hayashi S, Yahata N, Ando K, Toyama K, Hoshika A, Takasaki M, et al. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum. Genet. 1998;102:397–402. doi: 10.1007/s004390050711. [DOI] [PubMed] [Google Scholar]
- 17.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 18.Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- 19.Krtolica A, Campisi J. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int. J. Biochem. Cell Biol. 2002;34:1401–1414. doi: 10.1016/s1357-2725(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 20.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, II, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 22.Zijlmans JM, Martens UM, Poon SS, Raap AK, Tanke HJ, Ward RK, Lansdorp PM. Telomeres in the mouse have large inter-chromosomal variations in the number of T2AG3 repeats. Proc. Natl Acad. Sci. USA. 1997;94:7423–7428. doi: 10.1073/pnas.94.14.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl Acad. Sci. USA. 1995;92:4818–4822. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Rivera L, Herrera E, Albar JP, Blasco MA. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc. Natl Acad. Sci. USA. 1998;95:10471–10476. doi: 10.1073/pnas.95.18.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat. Med. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- 26.Finch CE. Longevity, Senescence, and the Genome. Chicago: University of Chicago Press; 1990. [Google Scholar]
- 27.Shay JW, Wright WE. Hallmarks of telomeres in ageing research. J. Pathol. 2007;211:114–123. doi: 10.1002/path.2090. [DOI] [PubMed] [Google Scholar]
- 28.Drachtman RA, Alter BP. Dyskeratosis congenita. Dermatol. Clin. 1995;13:33–39. [PubMed] [Google Scholar]
- 29.Dokal I. Dyskeratosis congenita in all its forms. Br. J. Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 30.Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 31.Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat. Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 32.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 34.Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes. Dev. 2006;20:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason PJ, Wilson DB, Bessler M. Dyskeratosis congenita–a disease of dysfunctional telomere maintenance. Curr. Mol. Med. 2005;5:159–170. doi: 10.2174/1566524053586581. [DOI] [PubMed] [Google Scholar]
- 36.Rashid R, Liang B, Baker DL, Youssef OA, He Y, Phipps K, Terns RM, Terns MP, Li H. Crystal structure of a Cbf5-Nop10-Gar1 complex and implications in RNA-guided pseudouridylation and dyskeratosis congenita. Mol. Cell. 2006;21:249–260. doi: 10.1016/j.molcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Ye K. Crystal structure of an H/ACA box ribonucleoprotein particle. Nature. 2006;443:302–307. doi: 10.1038/nature05151. [DOI] [PubMed] [Google Scholar]
- 38.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 39.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat. Genet. 2004;36:447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 40.Fu D, Collins K. Distinct biogenesis pathways for human telomerase RNA and H/ACA small nucleolar RNAs. Mol. Cell. 2003;11:1361–1372. doi: 10.1016/s1097-2765(03)00196-5. [DOI] [PubMed] [Google Scholar]
- 41.Armanios M, Chen JL, Chang YP, Brodsky RA, Hawkins A, Griffin CA, Eshleman JR, Cohen AR, Chakravarti A, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc. Natl Acad. Sci. USA. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vulliamy TJ, Walne A, Baskaradas A, Mason PJ, Marrone A, Dokal I. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol. Dis. 2005;34:257–263. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Ly H, Calado RT, Allard P, Baerlocher GM, Lansdorp PM, Young NS, Parslow TG. Functional characterization of telomerase RNA variants found in patients with hematologic disorders. Blood. 2005;105:2332–2339. doi: 10.1182/blood-2004-09-3659. [DOI] [PubMed] [Google Scholar]
- 44.Marrone A, Stevens D, Vulliamy T, Dokal I, Mason PJ. Heterozygous telomerase RNA mutations found in dyskeratosis congenita and aplastic anemia reduce telomerase activity via haploinsufficiency. Blood. 2004;104:3936–3942. doi: 10.1182/blood-2004-05-1829. [DOI] [PubMed] [Google Scholar]
- 45.Walne AJ, Vulliamy T, Marrone A, Beswick R, Kirwan M, Masunari Y, Al-Qurashi FH, Aljurf M, Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum. Mol. Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564–579. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- 47.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol. Dis. 2001;27:353–357. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- 48.Alter BP, Baerlocher GM, Savage SA, Chanock SJ, Weksler BB, Willner JP, Peters JA, Giri N, Lansdorp PM. Very short telomere length by flow FISH identifies patients with Dyskeratosis Congenita. Blood. 2007 doi: 10.1182/blood-2007-02-075598. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldman F, Bouarich R, Kulkarni S, Freeman S, Du HY, Harrington L, Mason PJ, Londono-Vallejo A, Bessler M. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc. Natl Acad. Sci. USA. 2005;102:17119–17124. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashley CT, Jr, Warren ST. Trinucleotide repeat expansion and human disease. Annu. Revi. Genet. 1995;29:703–728. doi: 10.1146/annurev.ge.29.120195.003415. [DOI] [PubMed] [Google Scholar]
- 51.Dokal I, Bungey J, Williamson P, Oscier D, Hows J, Luzzatto L. Dyskeratosis congenita fibroblasts are abnormal and have unbalanced chromosomal rearrangements. Blood. 1992;80:3090–3096. [PubMed] [Google Scholar]
- 52.Marsh JC, Will AJ, Hows JM, Sartori P, Darbyshire PJ, Williamson PJ, Oscier DG, Dexter TM, Testa NG. “Stem cell” origin of the hematopoietic defect in dyskeratosis congenita. Blood. 1992;79:3138–3144. [PubMed] [Google Scholar]
- 53.Dokal I, Vulliamy T. Dyskeratosis congenita: its link to telomerase and aplastic anaemia. Blood Rev. 2003;17:217–225. doi: 10.1016/s0268-960x(03)00020-1. [DOI] [PubMed] [Google Scholar]
- 54.Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand E, Zeng WS, Read EJ, Lansdorp PM, Young NS. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 55.Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002;359:2168–2170. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, Young NS. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 57.Ortmann CA, Niemeyer CM, Wawer A, Ebell W, Baumann I, Kratz CP. TERC mutations in children with refractory cytopenia. Haematologica. 2006;91:707–708. [PubMed] [Google Scholar]
- 58.Xin ZT, Beauchamp AD, Calado RT, Bradford JW, Regal JA, Shenoy A, Liang Y, Lansdorp PM, Young NS, Ly H. Functional characterization of natural telomerase mutations found in patients with hematologic disorders. Blood. 2007;109:524–532. doi: 10.1182/blood-2006-07-035089. [DOI] [PubMed] [Google Scholar]
- 59.Field JJ, Mason PJ, An P, Kasai Y, McLellan M, Jaeger S, Barnes YJ, King AA, Bessler M, et al. Low frequency of telomerase RNA mutations among children with aplastic anemia or myelodysplastic syndrome. J Pediatr. Hematol. Oncol. 2006;28:450–453. doi: 10.1097/01.mph.0000212952.58597.84. [DOI] [PubMed] [Google Scholar]
- 60.Keith WN, Vulliamy T, Zhao J, Ar C, Erzik C, Bilsland A, Ulku B, Marrone A, Mason PJ, et al. A mutation in a functional Sp1 binding site of the telomerase RNA gene (hTERC) promoter in a patient with Paroxysmal Nocturnal Haemoglobinuria. BMC Blood Disord. 2004;4:3. doi: 10.1186/1471-2326-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, Lansdorp PM, Young NS. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 62.Liang J, Yagasaki H, Kamachi Y, Hama A, Matsumoto K, Kato K, Kudo K, Kojima S. Mutations in telomerase catalytic protein in Japanese children with aplastic anemia. Haematologica. 2006;91:656–658. [PubMed] [Google Scholar]
- 63.Thornley I, Dror Y, Sung L, Wynn RF, Freedman MH. Abnormal telomere shortening in leucocytes of children with Shwachman-Diamond syndrome. Br. J. Haematol. 2002;117:189–192. doi: 10.1046/j.1365-2141.2002.03371.x. [DOI] [PubMed] [Google Scholar]
- 64.Dror Y. Shwachman-Diamond syndrome. Pediatr. Blood Cancer. 2005;45:892–901. doi: 10.1002/pbc.20478. [DOI] [PubMed] [Google Scholar]
- 65.Boocock GR, Morrison JA, Popovic M, Richards N, Ellis L, Durie PR, Rommens JM. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat. Genet. 2003;33:97–101. doi: 10.1038/ng1062. [DOI] [PubMed] [Google Scholar]
- 66.Calado RT, Graf SA, Wilkerson KL, Kajigaya S, Ancliff PJ, Dror Y, Chanock SJ, Lansdorp PM, Young NS. Mutations in the SBDS gene in acquired aplastic anemia. Blood. 2007;110:1141–1146. doi: 10.1182/blood-2007-03-080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC. Progressive telomere shortening in aplastic anemia. Blood. 1998;91:3582–3592. [PubMed] [Google Scholar]
- 68.Brummendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PM. Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood. 2001;97:895–900. doi: 10.1182/blood.v97.4.895. [DOI] [PubMed] [Google Scholar]
- 69.Lee JJ, Kook H, Chung IJ, Na JA, Park MR, Hwang TJ, Kwak JY, Sohn SK, Kim HJ. Telomere length changes in patients with aplastic anaemia. Br. J. Haematol. 2001;112:1025–1030. doi: 10.1046/j.1365-2141.2001.02669.x. [DOI] [PubMed] [Google Scholar]
- 70.Karadimitris A, Araten DJ, Luzzatto L, Notaro R. Severe telomere shortening in patients with paroxysmal nocturnal hemoglobinuria affects both GPI- and GPI+ hematopoiesis. Blood. 2003;102:514–516. doi: 10.1182/blood-2003-01-0128. [DOI] [PubMed] [Google Scholar]
- 71.Leteurtre F, Li X, Guardiola P, Le Roux G, Sergere JC, Richard P, Carosella ED, Gluckman E. Accelerated telomere shortening and telomerase activation in Fanconi's anaemia. Br. J. Haematol. 1999;105:883–893. doi: 10.1046/j.1365-2141.1999.01445.x. [DOI] [PubMed] [Google Scholar]
- 72.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 73.Conkright JJ, Na CL, Weaver TE. Overexpression of surfactant protein-C mature peptide causes neonatal lethality in transgenic mice. Am. J. Respir. Cell Mol. Biol. 2002;26:85–90. doi: 10.1165/ajrcmb.26.1.4686. [DOI] [PubMed] [Google Scholar]
- 74.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 75.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 76.American Thoracic Society/European Respiratory Society. International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. [DOI] [PubMed] [Google Scholar]
- 77.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am. J. Respir. Crit. Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 78.Marshall RP, Puddicombe A, Cookson WO, Laurent GJ. Adult familial cryptogenic fibrosing alveolitis in the United Kingdom. Thorax. 2000;55:143–146. doi: 10.1136/thorax.55.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax. 2002;57:338–342. doi: 10.1136/thorax.57.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nadrous HF, Myers JL, Decker PA, Ryu JH. Idiopathic pulmonary fibrosis in patients younger than 50 years. Mayo. Clin. Proc. 2005;80:37–40. doi: 10.1016/S0025-6196(11)62955-8. [DOI] [PubMed] [Google Scholar]
- 81.Loyd JE. Pulmonary fibrosis in families. Am. J. Respir. Cell Mol. Biol. 2003;29:S47–S50. [PubMed] [Google Scholar]
- 82.Lee HL, Ryu JH, Wittmer MH, Hartman TE, Lymp JF, Tazelaar HD, Limper AH. Familial idiopathic pulmonary fibrosis: clinical features and outcome. Chest. 2005;127:2034–2041. doi: 10.1378/chest.127.6.2034. [DOI] [PubMed] [Google Scholar]
- 83.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA, III, et al. Clinical and pathologic features of familial interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2005;172:1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnston I, Britton J, Kinnear W, Logan R. Rising mortality from cryptogenic fibrosing alveolitis. BMJ. 1990;301:1017–1021. doi: 10.1136/bmj.301.6759.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnston ID, Prescott RJ, Chalmers JC, Rudd RM. British Thoracic Society study of cryptogenic fibrosing alveolitis: current presentation and initial management. Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. Thorax. 1997;52:38–44. doi: 10.1136/thx.52.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 87.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc. Natl Acad. Sci. USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ly H, Schertzer M, Jastaniah W, Davis J, Yong SL, Ouyang Q, Blackburn EH, Parslow TG, Lansdorp PM. Identification and functional characterization of 2 variant alleles of the telomerase RNA template gene (TERC) in a patient with dyskeratosis congenita. Blood. 2005;106:1246–1252. doi: 10.1182/blood-2005-01-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blasco MA, Rizen M, Greider CW, Hanahan D. Differential regulation of telomerase activity and telomerase RNA during multi-stage tumorigenesis. Nat. Genet. 1996;12:200–204. doi: 10.1038/ng0296-200. [DOI] [PubMed] [Google Scholar]
- 90.Kedde M, le Sage C, Duursma A, Zlotorynski E, van Leeuwen B, Nijkamp W, Beijersbergen R, Agami R. Telomerase-independent regulation of ATR by human telomerase RNA. J. Biol. Chem. 2006;281:40503–40514. doi: 10.1074/jbc.M607676200. [DOI] [PubMed] [Google Scholar]
- 91.Morla M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. Eur. Respir. J. 2006;27:525–528. doi: 10.1183/09031936.06.00087005. [DOI] [PubMed] [Google Scholar]
- 92.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development (Cambridge,England) 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 93.Fridlender ZG, Cohen PY, Golan O, Wallach-Dayan S, Breuer R. Telomerase activity in bleomycin-induced epithelial cell apoptosis and lung fibrosis. Eur. Respir. J. 2007 doi: 10.1183/09031936.00009407. [DOI] [PubMed] [Google Scholar]
- 94.Driscoll B, Buckley S, Bui KC, Anderson KD, Warburton D. Telomerase in alveolar epithelial development and repair. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1191–1198. doi: 10.1152/ajplung.2000.279.6.L1191. [DOI] [PubMed] [Google Scholar]
- 95.Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 96.Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152:391–398. doi: 10.1016/s0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- 97.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 98.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 99.Valdes AM, Richards JB, Gardner JP, Swaminathan R, Kimura M, Xiaobin L, Aviv A, Spector TD. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos. Int. 2007 doi: 10.1007/s00198-007-0357-5. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 100.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 101.Zhu CH, Mouly V, Cooper RN, Mamchaoui K, Bigot A, Shay JW, Di Santo JP, Butler-Browne GS, Wright WE. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell. 2007;6:515–523. doi: 10.1111/j.1474-9726.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 102.Effros RB. Telomerase induction in T cells: a cure for aging and disease? Exp. Gerontol. 2007;42:416–420. doi: 10.1016/j.exger.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen JL, Blasco MA, Greider CW. Secondary structure of vertebrate telomerase RNA. Cell. 2000;100:503–514. doi: 10.1016/s0092-8674(00)80687-x. [DOI] [PubMed] [Google Scholar]
- 104.Jacobs SA, Podell ER, Cech TR. Crystal structure of the essential N-terminal domain of telomerase reverse transcriptase. Nat. Struct. Mol. Biol. 2006;13:218–225. doi: 10.1038/nsmb1054. [DOI] [PubMed] [Google Scholar]
- 105.Banik SS, Guo C, Smith AC, Margolis SS, Richardson DA, Tirado CA, Counter CM. C-terminal regions of the human telomerase catalytic subunit essential for in vivo enzyme activity. Mol. Cell Biol. 2002;22:6234–6246. doi: 10.1128/MCB.22.17.6234-6246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]