Abstract
Autoimmune autonomic ganglionopathy (AAG) is an antibody-mediated form of severe autonomic failure. AAG is associated with serum antibodies against ganglionic acetycholine receptors (AChR) and appears to result from impaired synaptic transmission in autonomic ganglia. The rabbit model of experimental AAG (EAAG), induced by immunization, reproduces the cardinal features of the human disease. Pupillary dysfunction is a prominent and defining feature in both AAG and EAAG. We adapted infrared computer-assisted video pupillometry to record direct pupil light responses in control and EAAG rabbits. Offline analysis algorithms were used to determine latency, velocity and amplitude of the constriction and redilation phases of the light reflex. Following immunization, pupillary abnormalities were the earliest sign of evolving autonomic failure. EAAG rabbits showed significant reduction in velocity and amplitude of pupil constriction while redilation parameters were only mildly affected. Fatigue in pupillary constriction, evidenced by premature redilation of the pupil prior to termination of the light stimulus, was observed only in seropositive rabbits. The severity of pupillary abnormalities was significantly correlated with ganglionic AChR antibody level. In chronic EAAG, treatment with pyridostigmine produced a partial recovery of pupil function. We conclude that pupillometry is a robust and sensitive diagnostic tool to assess autonomic dysfunction, distinguish AAG from other disorders, and assess responses to therapy. In EAAG, pupillary dysfunction is partially reversible, parasympathetic pupil function is more severely compromised than sympathetic function, and fatigue of pupillary constriction may be seen. These characteristic abnormalities of the pupillary light reflex may prove to have diagnostic value.
Introduction
Autoimmune autonomic ganglionopathy (AAG, also known as autoimmune autonomic neuropathy, acute pandysautonomia, or idiopathic autonomic neuropathy) is a form of severe diffuse autonomic failure that is usually acute or subacute in onset (Vernino et al., 2000). This syndrome affects previously healthy individuals and may occur following symptoms of a viral illness (Suarez et al., 1994). Less commonly, a similar presentation occurs in association with cancer, especially thymoma or small cell carcinoma of the lung. Patients with AAG present with symptoms of both sympathetic dysfunction (orthostatic hypotension and anhidrosis) and parasympathetic dysfunction (dry mouth, dry eyes, and urinary retention). Gastrointestinal symptoms due to bowel hypomotility are prominent, including nausea and early satiety due to gastroparesis and severe constipation. Severe gastrointestinal pseudoobstruction and orthostatic hypotension can be life-threatening. Some patients show spontaneous, but incomplete, recovery. Others may respond to treatment with immunosuppression, intravenous immunoglobulin (Heafield et al., 1996, Modoni et al., 2007), or plasma exchange (Schroeder et al., 2005). It is important to differentiate this potentially treatable disorder from degenerative forms of autonomic failure. Reduced or absent pupillary light reflex is seen in many patients and may help distinguish AAG from other autonomic disorders (Klein et al., 2003, Sandroni et al., 2004, Suarez et al., 1994, Vernino et al., 2000).
A majority of patients with AAG have serum antibodies against the neuronal acetylcholine receptor in autonomic ganglia (ganglionic nAChR). Higher levels of these antibodies are correlated with greater severity of autonomic signs and symptoms. A decrease in antibody levels is associated with clinical improvement (Modoni et al., 2007, Schroeder et al., 2005, Vernino et al., 2000). The ganglionic AChR mediates fast synaptic transmission in all peripheral autonomic ganglia and is absolutely necessary for normal autonomic function (Xu et al., 1999). Animal models of experimental autoimmune autonomic ganglionopathy (EAAG) have been developed. Rabbits immunized with a recombinant fusion protein corresponding to the extracellular domain of the ganglionic AChR produce ganglionic AChR antibodies and develop autonomic failure that recapitulates most of its clinical features of AAG (Lennon et al., 2003). Rabbits with EAAG have gastroparesis, intestinal dysmotility (Lennon et al., 2003), reduced lacrimation, hypotension, reduced heart rate variability, and low circulating catecholamines (Vernino et al., 2003). As in the human condition, the severity of cardiovascular autonomic dysfunction correlates with the level of serum ganglionic AChR antibody. Pupil abnormalities are a hallmark of both AAG and EAAG. Impaired pupil responses to light and accommodation help to differentiate AAG from other autonomic disorders (Klein et al., 2003, Sandroni et al., 2004).
Pupillometry is a simple, relatively inexpensive, non-invasive technique that can be used to assess the functioning of both branches of the autonomic nervous system with minimal patient cooperation (Fotiou et al., 2000, Gavriysky, 1995, Piha and Halonen, 1994, Wachler and Krueger, 1999). Modern pupillometry systems use infrared digital video systems that automatically detect and measure pupil diameter with high temporal resolution. This technique has been increasingly used in research and clinical applications, including preoperative assessment before ocular surgery (Rosen et al., 2002, Wickremasinghe et al., 2005), clinical assessment of coma after closed head injury (Larson and Muhiudeen, 1995), studies of drug metabolism (Bitsios et al., 1999, Knaggs et al., 2004), psychology (Fukuda et al., 2005, Steinhauer and Hakerem, 1992) and behavioral disorders (Granholm et al., 2003). Pupillometry can detect subtle abnormalities when the pupil response appears normal or may demonstrate a small pupillary light reflex when no pupil response is apparent clinically. Pupillometry may also be utilized in assessment of primary autonomic disorders, and in some instances; it is more sensitive than cardiovascular function tests to investigate autonomic dysfunction (Maguire et al., 2007, Micieli et al., 1995, Pena et al., 1995).
Current evidence indicates that AAG and EAAG are caused by an antibody-mediated impairment of synaptic transmission in autonomic ganglia (Lennon et al., 2003, Vernino et al., 2003). Assessment of pupil responses (with or without pharmacological treatment) may allow identification of a specific pattern of abnormalities diagnostic of AAG. Detailed assessment of pupil responses has been reported in a few patients with AAG (Low et al., 1983, Maki et al., 2006). In most cases, the pupil constriction to light showed a prolonged latency to onset and reduced constriction amplitude. Patients often also showed pupillary constriction to dilute pilocarpine suggesting cholinergic denervation. However, autonomic deficits, including pupillary reflexes, in patients with AAG may rapidly improve or even normalize with treatment indicating intact innervation of the pupil (Heafield et al., 1996, Schroeder et al., 2005)(S. Vernino, unpublished observations).
In this study, we used quantitative pupillometry as a tool to study autonomic dysfunction in the rabbit EAAG model. A pattern of abnormalities in the pupil response to light and to pharmacological agents emerged as a potentially specific pattern for AAG.
Materials and Methods
Experimental protocols were approved by the Institutional Animal Care and Use Committee. Female New Zealand white rabbits (6 to 8 weeks of age at the start of the protocol) were used. The animals had unrestricted access to food and water and were observed daily and weighed weekly during the protocol. Nutritional supplements were provided as needed. During pupil recordings, rabbits were gently restrained in a soft “Bunny-Snuggle” (Lomir Biomedical, Malone, NY) and were not sedated. They were accommodated to this short period of restraint and to the testing environment for several weeks before collecting data. Recordings were carried out in a quiet, temperature-controlled room (22 ± 2 deg C) with ambient fluorescent white light.
Immunization
Rabbits were immunized with a recombinant fusion protein corresponding to the N-terminal extracellular domain of the human neuronal nAChR α3 subunit using methods previously described (Lennon et al., 2003). For primary immunization, 0.8 mg of purified antigen was emulsified in complete Freund’s adjuvant (CFA) and injected intradermally at multiple sites, on the dorsal flanks. A second subcutaneous booster immunization of antigen in incomplete Freund’s adjuvant was administered 3 weeks later. Control rabbits were not immunized.
One to two ml of blood was collected weekly from the central ear artery. The serum level of ganglionic nAChR binding antibodies was quantified using a previously described radioimmunoprecipitation assay (Vernino et al., 2000). Ganglionic AChR antibodies were first detectable about 4 weeks after primary immunization. The antibody levels increased to reach a stable level about 12 weeks after primary immunization. Rabbits were classified as non-responders if their serum antibody level remained below 0.2 nmol/L. Non-responders were indistinguishable from control rabbits in appearance, weight, heart rate, blood pressure and pupil responses. Seropositive rabbits were subdivided into low and high antibody groups based on their maximum antibody titer. Low Ab EAAG rabbits had serum antibody levels between 0.2 and 2.0 nmol/L, and high Ab EAAG rabbits with serum antibody levels above 2.0 nmol/L. The description of the EAAG rabbit phenotype has been reported previously (Lennon et al., 2003, Vernino et al., 2003).
Pupillometry
Weekly assessments of pupil responses were performed in control and immunized rabbits. Measurement of pupillary responses to light was carried out using an automated pupillometry system (ISCAN Inc., Burlington, MA). All recordings were made from the right pupil with the eye positioned at a fixed distance from the camera. The eye was gently held open during recording. Two infrared light sources were used to illuminate the pupil. The data acquisition software (ISCAN) automatically tracked pupil movement, discriminated the pupil, and determined pupil diameter and area. Pupil diameter was sampled at 60 Hz, acquired using Axoscope v.9.0 (Molecular Devices, Sunnyvale, CA), and stored for offline analysis. An array of 8 white light-emitting diodes driven by a standard pulse generator (World Precision Instruments, Sarasota, FL) provided a standard light stimulus.
Based on pilot studies with different sequences of light stimuli, we elected to use a train of nine light flashes, one flash every seven seconds. The duration of each light flash was 2 seconds. We found that the response of the dark-adapted pupil to the first light stimulus was variable and often contaminated by movement artifact. After four conditioning light stimuli, the pupillary light response stabilized (figure 1A), an observation also seen in previous studies with human subjects (Fotiou et al., 2000). The last three pupillary light responses in the sequence were reproducible, and we used the average of these three responses for further analysis.
Figure 1.
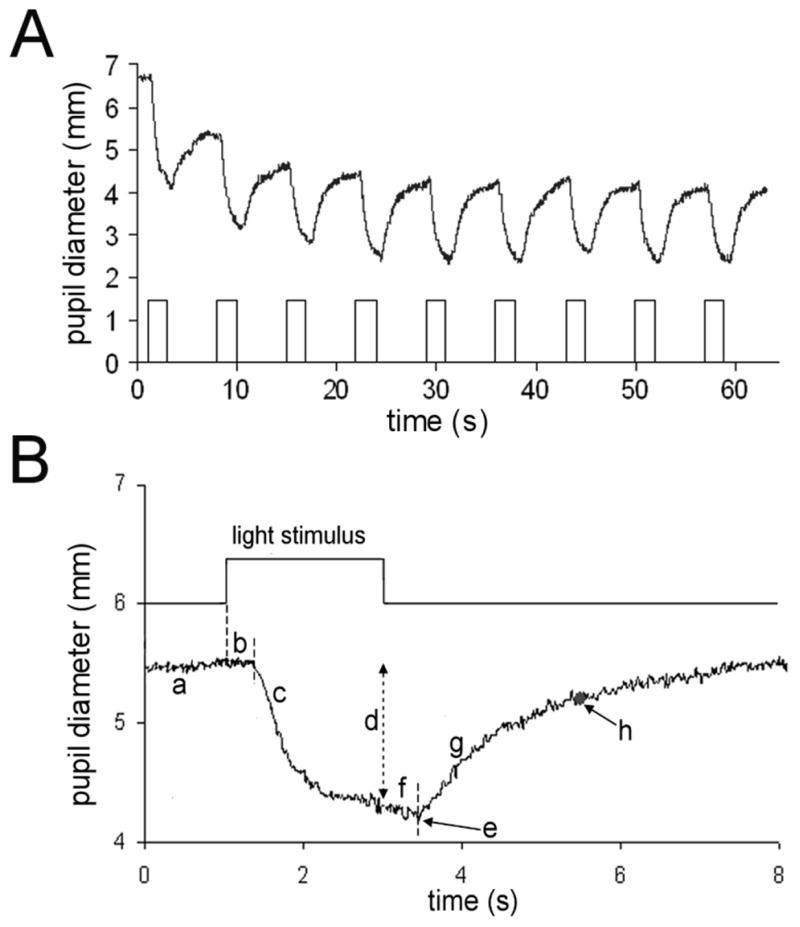
(A) Typical response of a control rabbit pupil to repetitive light stimuli. After the fourth flash, the response is reproducible. (B) Response of a normal rabbit pupil to light stimulus (average of three responses). Pupil response parameters measured are a) initial pupil diameter, b) constriction latency, c) constriction velocity (slope), d) constriction amplitude, e) peak constriction amplitude, f) redilation latency, g) redilation velocity (slope), and h) time to 75 % redilation.
Determination of static and dynamic pupil parameters
Rabbits were adapted to the dark for five minutes prior to testing. The pupil diameter prior to light stimulus was recorded for all rabbits. Using the averaged pupil light response waveform (figure 1B), the following response parameters were automatically determined using computer algorithms: (1) initial pupil diameter, the initial diameter of the pupil just prior to the light stimuli; (2) constriction latency, the time between the onset of light stimulus and the initation of pupillary constriction; (3) maximum constriction velocity, the maximum rate of change in pupil size during constriction; (4) constriction amplitude, the difference between initial pupil diameter and pupil diameter at the end of the 2-second light stimulus; (5) peak constriction amplitude, the difference between initial pupil diameter and the minimum pupil diameter during the recording; (6) time to peak constriction, the time from onset of light stimulus to the minimum pupil diameter; (7) redilation latency, the time between cessation of light stimulus and initiation of pupil redilation (a negative redilation latency indicates that the pupil began to redilate before the end of the light stimulus); (8) maximum redilation velocity, the maximum rate of change in pupil size during redilation; (9) time to 75% redilation, the time required after cessation of light stimulus for the pupil to redilate to 75% of initial pupil diameter. Figure 1B provides a graphical representation of the parameters studied.
Since the absolute magnitude of the change in pupil diameter depends, in part, on the initial pupil diameter, we also calculated the ratio between the constriction amplitudes and the initial pupil diameter. This ratio, or relative constriction amplitude, is expressed as a percent. Likewise the ratio between the initial pupil diameter and the peak constriction is expressed as the relative peak constriction amplitude. Relative peak constriction amplitude is the most commonly used single quantitative measure of the pupillary light reflex (Fotiou et al., 2000).
Pharmacological experiments
Pharmacological experiments were performed in some rabbits to better define the nature of the pupillary abnormalities. Pilocarpine and pyridostigmine experiments were separated by at least one week. In the dark, control and EAAG rabbits were treated with dilute (0.125%) pilocarpine or saline applied topically to the eye. The pupil diameter was recorded at baseline and 10 minutes after topical application. Some rabbits were treated with the acetylcholinesterase inhibitor, pyridostigmine. The drug was administered by subcutaneous injection (0.2 mg/kg) twice a day for 5 days. The drugs in aqueous solution were obtained from the clinical pharmacy at our institution.
Statistical Analysis
Data are expressed as mean ± standard deviation. The distribution of the data was determined using the Kolmogorov-Smirnov and Shapiro-Wilk tests of normality. Antibody levels were log transformed to approximate a normal distribution. In order to estimate the reliability of each of the pupil response parameters, we calculated coefficient of variance (CV), and intra-class correlation (ICC) using the two-way mixed effects model. Based on the results of repeated testing in control rabbits, we selected the most reliable pupil parameters to use in comparing rabbits in different study groups. Comparison between control and EAAG groups was carried out using repeated measures ANOVA, with time as the within-subject factor and group as between-subjects factor. In cases where significant differences were identified, a post hoc Bonferroni’s analysis was carried out. In EAAG rabbits, we used a non-parametric Spearman’s rank order correlation to examine the association between antibody levels and pupil parameters.
For pilocarpine experiments, a non-parametric Mann-Whitney U test was performed to compare control and EAAG rabbits. Pyridostigmine treatment data was analyzed groupwise using a paired non-parametric Friedman’s test. In all instances, analyses were carried out using SPSS (version 13), and p ≤ 0.05 was considered significant.
Results
Resting pupil changes in EAAG rabbits
In the dark, control rabbits had a mean pupil diameter of 8.0 ± 0.7 mm (n=7). EAAG rabbits (with ganglionic AChR antibodies) showed various signs of autonomic failure including impaired pupillary response to light (figure 2). However, the mean dark-adapted pupil diameter in rabbits with ganglionic AChR antibodies (8.3 ± 0.9 mm, n=10) was not different compared to control rabbits.
Figure 2.

Pupil images from control and EAAG rabbit. The pupil diameters are similar in the dark and in ambient room light, but the EAAG pupil constrict poorly in bright light.
We investigated the sensitivity of the EAAG pupil to the direct cholinergic agonist pilocarpine. On topical application of dilute (0.125%) pilocarpine to the eye, EAAG rabbits exhibited a significantly larger and more prolonged constriction in pupil diameter. Ten minutes after installation of pilocarpine, the EAAG pupil diameter had decreased by an average of 3.1 ± 1.8 mm (p = 0.005 compared to control) while the decrease in pupil size in controls was 1.2 ± 0.6 mm. At the time of pilocarpine studies, the mean antibody level in EAAG rabbits was 0.85 nmol/L. Topical installation of saline produced no effect on pupil size.
Reliability of pupillometry in control rabbits
We adapted quantitative pupillometry to better assess the pupil light reflex in rabbits. Pupil responses are recognized to have large inter-and intra-subject variability (Wilhelm and Wilhelm, 2003). To assess the reliability of pupillometry in rabbits, we collected data weekly for seven consecutive weeks from five control rabbits. A total of 11 different parameters were extracted from the data, and the reliability of these measures was assessed (Table 1). The coefficient of variation (CV), intra-class correlation coefficient (ICC) and p values are shown as an estimate of test-retest reliability. Initial pupil diameter, relative constriction amplitudes, and redilation latency were the most reliable measures of pupil response. Five parameters were chosen for use in further studies: initial pupil diameter, maximum constriction velocity, peak relative constriction amplitude, redilation latency and time to 75% redilation. Although the redilation latency showed a high CV, it was a significantly reliable measure (intra-class correlation 0.797, p 0.005). Our observations in EAAG rabbits indicated that redilation latency is a useful parameter to detect early pupil abnormalities. The time to 75% redilation was less reliable than other measures but was included as a measure of sympathetic pupil function (Fotiou et al., 2000).
Table 1.
Reproducibility of pupil responses in control rabbits
| Measured Parameter | Mean ± SD | CV | ICC | P value |
|---|---|---|---|---|
| Initial pupil diameter | 4.09 ± 0.55 mm | 0.103 | 0.914 | < 0.001 |
| Constriction latency | 387 ± 39 ms | 0.220 | 0.442 | 0.163 |
| Maximum constriction velocity | −2.69 ± 0.25 mm/s | 0.151 | 0.533 | 0.107 |
| Constriction amplitude | 1.38 ± 0.13 mm | 0.117 | 0.491 | 0.132 |
| Relative constriction amplitude | 34.0 ± 2.9 % | 0.109 | 0.749 | 0.013 |
| Peak constriction amplitude | 1.65 ± 0.15 mm | 0.114 | 0.437 | 0.166 |
| Peak relative constriction amplitude | 40.9 ± 4.2 % | 0.121 | 0.813 | 0.003 |
| Latency to peak constriction | 2.50 ± 0.11 s | 0.079 | 0.497 | 0.128 |
| Redilation latency | 543 ± 179 ms | 0.390 | 0.797 | 0.005 |
| Maximum redilation velocity | 1.79 ± 0.13 mm/s | 0.378 | −2.821 | 0.900 |
| Time to 75% redilation | 1948 ± 264 ms | 0.283 | 0.051 | 0.401 |
Pupillary light responses in EAAG rabbits
We performed weekly measurement of the pupillary light reflex in EAAG rabbits. As the rabbits began to produce antibodies, the first measurable sign of autonomic dysfunction was a reduction in the amplitude of pupillary constriction to light. An impaired pupillary light reflex could be detected at the same time or, in a few cases, one week prior to the first detection of ganglionic AChR antibodies in the serum. As the antibody levels rose, the severity of pupil abnormalities and other autonomic deficits increased.
For group comparisons, a total of 16 rabbits were assessed, and analysis for each rabbit was carried out on data from a seven week period when the serum ganglionic AChR antibody level was stable. In this analysis, there were no significant changes over time (within-subjects) in any of the study groups. Low Ab EAAG rabbits (n=5) had a mean antibody level of 0.81 nmol/L and pupillary responses were analyzed starting 12–16 weeks following primary immunization. High Ab EAAG (n=6) had a mean antibody level of 4.09 nmol/L and were studied 11–15 weeks after primary immunization. Group comparisons are shown in Table 2.
Table 2.
Comparison of pupil function in experimental groups.
| P values
|
||||||
|---|---|---|---|---|---|---|
| Group | Control (N=5) | Low Ab (N=5) | High Ab (N=6) | Control vs. Low Ab | Control vs. High Ab | Low Ab vs. High Ab |
| Initial pupil diameter (mm) | 4.18 ± 0.14 | 4.67 ± 0.20 | 5.78 ± 0.26 | 0.662 | 0.002 | 0.026 |
| Maximum constriction velocity (mm/s) | −2.74 ± 0.48 | −2.62 ± 0.62 | −2.00 ± 0.44 | 1.0 | 0.009 | 0.029 |
| Peak relative constriction amplitude | 41.4 ± 2.3 % | 33.2 ± 1.9 % | 15.2 ± 1.5 % | 0.037 | <0.001 | <0.001 |
| Redilation latency (ms) | 578 ± 21 | 460 ± 221 | − 502 ± 92 | 1.000 | <0.001 | <0.001 |
| Time to 75% redilation (ms) | 1882 ± 589 | 1888 ± 535 | 2049 ± 1220 | 1.000 | 0.693 | 0.717 |
Compared to control and low Ab EAAG rabbits, high Ab EAAG rabbits showed significantly larger initial pupil diameter, slower constriction velocity, and diminished peak constriction amplitude to light stimuli. All high Ab EAAG rabbits also showed a remarkable phenomenon in which the pupil started to redilate before the termination of the light flash (figure 3). This premature redilation is reflected as negative redilation latency (Table 2). Premature redilation was never seen in seronegative controls or non-responders. The constriction latency, redilation velocity and time to 75% redilation did not differ significantly between control and high Ab EAAG rabbits.
Figure 3.

A comparison of pupillary light reflex in control and EAAG rabbits. The upper trace indicates the timing of the light stimulus. The EAAG pupil has a larger initial pupil diameter, slower constriction velocity, and reduced constriction amplitude. Unlike the control pupil, the EAAG pupil does not continue to constrict throughout the light stimulus and shows premature redilation (arrow).
Low Ab EAAG rabbits showed less severe abnormalities of the pupillary light reflex. The constriction amplitude was significantly reduced, but initial pupil diameter, constriction velocity and redilation latency were not significantly different from control rabbits (Table 2). Premature redilation of the pupil or failure to constrict throughout the entire light stimulus was seen in some recordings but was not as consistently seen as in high Ab EAAG rabbits.
Correlation of ganglionic AChR antibody level and pupil dysfunction
We have previously shown that the severity of autonomic dysfunction in AAG and EAAG is correlated with antibody levels (Klein et al., 2003, Lennon et al., 2003, Vernino et al., 2000, Vernino et al., 2003). We examined the correlation of pupil response parameters with antibody levels in this EAAG study. Eight of the 11 pupil parameters showed a significant correlation with antibody levels (table 3, figure 4), supporting the utility of pupillometry as a valid tool for measuring severity in EAAG. In all cases, higher antibody levels were associated with more severe autonomic impairment. Notably, the two measures associated with sympathetic function (redilation velocity and time to 75% redilation) did not strongly correlate with antibody levels. Higher antibody levels tended to associate with slower maximum redilation velocity, but the correlation did not reach statistical significance (p=0.09). Either the sympathetic parameters are not sensitive enough to detect abnormalities in EAAG rabbits or, more likely, the sympathetic innervation of the pupil is relatively spared compared to parasympathetic pupil function.
Table 3.
Correlation of ganglionic AChR antibody with pupil function in EAAG rabbits
| Measured Parameter | Spearmann rho | p-value |
|---|---|---|
| Initial pupil diameter | 0.673 | 0.023 |
| Constriction latency | −0.018 | 0.958 |
| Maximum constriction velocity | 0.773 | 0.005 |
| Constriction amplitude | −0.855 | 0.001 |
| Relative constriction amplitude | −0.882 | < 0.001 |
| Peak constriction amplitude | −0.818 | 0.002 |
| Peak relative constriction amplitude | −0.882 | < 0.001 |
| Latency to peak constriction | −0.782 | 0.004 |
| Redilation latency | −0.855 | 0.001 |
| Maximum redilation velocity | −0.536 | 0.089 |
| Time to 75% redilation | 0.291 | 0.385 |
Figure 4.

Correlation between peak relative constriction amplitude and antibody levels in 11 EAAG rabbits. Antibody level is plotted on a logarithmic scale. There is a significant negative correlation (p < 0.001). Higher antibody levels are associated with reduced constriction amplitude.
Response to pyridostigmine
Deficits in the pupillary light response in EAAG rabbits indicate a deficit in parasympathetic innervation of the pupil. The failure to maintain constriction (premature redilation) could be explained by fatigue in autonomic pathways, perhaps secondary to impaired synaptic transmission in the ciliary ganglia. We administered pyridostigmine, a reversible acetylcholinesterase inhibitor, as a way to enhance cholinergic autonomic neurotransmission. The drug was administered subcutaneously twice daily for 5 days. Administration of pyridostigmine resulted in dramatically increased defecation, salivation and lacrimation in the high Ab EAAG rabbits, consistent with stimulation of the autonomic nervous system. At the dose used, the drug produced no apparent effects on control rabbits. Pupillometry was performed before treatment, 30 minutes after the first dose of pyridostigmine and 30 minutes after the final dose of pyridostigmine (on treatment day 5). The response to this drug in the three animal groups is summarized in Figure 5A. In high Ab EAAG rabbits, there was improvement in relative peak constriction amplitude (15.2 ± 6.5 % at baseline compared to 20.4 ± 5.0 % at day 5, p < 0.05). There was no significant change in initial pupil diameter, constriction velocity or redilation latency although each of these parameters tended to improve.
Figure 5.
Response of pupil light reflex to pyridostigmine (A) Changes in peak relative constriction amplitude in control and EAAG rabbits after one dose of pyridostigmine and after 5 days of treatment. Control rabbits and low Ab EAAG rabbits did not show significant changes, although the pupil responses in 2 of the 4 low Ab EAAG rabbits improved. All three high Ab EAAG rabbits improved with pyridostigmine. (B) Representative tracings of pupil responses in a low Ab EAAG rabbit in which there was improvement of pupillary function with pyridostigmine treatment. After pyridostigmine (bottom trace), the initial pupil diameter was smaller, the constriction amplitude increased, and the pupil constriction continued throughout the stimulus (arrow).
In low Ab EAAG rabbits, there was clear improvement in constriction amplitude in two rabbits (figure 5), but the average change in constriction amplitude (25.8% at baseline to 29.8% at day 5) was not significant. Prior to treatment, two of the low Ab EAAG rabbits showed premature pupil redilation (mean redilation latency for low Ab EAAG rabbits of −13 ± 723 ms). After treatment, the pupil constriction was more sustained (figure 5B), reflected as a significant increase in redilation latency to 302 ± 580 ms (p< 0.05).
Discussion
Computer-assisted infrared pupillometry is a reliable and accessible tool to measure the autonomic function of the eye. Abnormalities of the pupil light reflex are prominent in AAG and help distinguish this disorder from other causes of dysautonomia (Klein et al., 2003). In EAAG, a disease caused by antibody-mediated impairment of cholinergic neurotransmission in autonomic ganglia, a typical pattern of pupillary dysfunction was seen. Impairment in the constriction phase of the pupil light reflex (reduced amplitude and impersistence of constriction) was one of the earliest signs. The severity of parasympathetic pupillary dysfunction correlated well with antibody levels. Parasympathetic autonomic function of the pupil was more impaired than sympathetic function consistent with our previous findings in autonomic studies in the rabbit (Vernino et al., 2003).
In high Ab EAAG, the pupils became nearly unresponsive to light. Loss of pupillary constriction could be explained by impairment in afferent pathways of the pupil light reflex, impaired neurotransmission through the ciliary ganglia, loss of postganglionic fibers innervating the pupil, or insensitivity of the iris to acetylcholine (as with muscarinic antagonists). The EAAG rabbits showed an exaggerated pupil constriction after topical application of dilute pilocarpine, which is usually suggestive of parasympathetic denervation of the pupil. However, in the case of EAAG, supersensitivity to pilocarpine may be misleading. Pupil responses significantly improved after pyridostigmine injection, signifying that the postganglionic neurons were intact. In EAAG, we believe that cholinergic supersensitivity of the pupil can be explained by a functional parasympathetic denervation due to a synaptic transmission blockade in the ciliary ganglia.
Another novel finding of this study was the impersistence of pupillary constriction in EAAG rabbits. Unlike control rabbits, the pupils in EAAG rabbits consistently began to redilate before the cessation of light stimuli. This inability to maintain constriction could be explained by fatigue of cholinergic synaptic transmission in the ciliary ganglia during high levels of parasympathetic activity. Subtle pupillary abnormalities have been reported in patients with myasthenia gravis (MG), a disorder associated with antibodies against muscle AChR, and it has been suggested that these abnormalities represent fatiguability of the pupillary constrictor muscles (Bryant, 1980, Dutton et al., 1982, Lepore et al., 1979). In MG patients, a slight improvement in pupillary function has been reported after administration of the acetylcholinesterase inhibitor edrophonium (Yamazaki and Ishikawa, 1976). However, the obvious pupillary abnormalities that we observe in the EAAG model and those seen qualitatively in AAG patients are quite distinct from those reported in MG. Many EAAG rabbits showed improvement in constriction amplitude and a correction of the premature redilation indicating that the pupillary deficits are partially reversible.
In a clinical setting, an objective assessment of autonomic failure is generally made based on laboratory autonomic function tests which focus on cardiovascular and sudomotor function. On such studies, AAG with an insidious onset may be indistinguishable from pure autonomic failure or other degenerative autonomic disorders (Klein et al., 2003, Sandroni et al., 2004). One of the key differences is the presence of pupillary dysfunction in AAG. Based on our findings in the EAAG rabbit model, pupillometry may prove to be an important addition to the clinical autonomic testing laboratory. Pupillometry would allow detection of subtle abnormalities such as slowed constriction velocity or specific abnormalities such as fatigue of pupillary constriction during longer light stimuli. Improvement in the pupillary light reflex after administration of acetylcholinesterase inhibitor also needs to be evaluated as a diagnostic finding in AAG. Since pupillary dysfunction correlates with ganglionic AChR antibody levels, quantitative pupillometry can be further used to assess the severity of AAG and monitor the response to treatment.
Acknowledgments
This study was supported by NIH grant NS48077 and UT Southwestern Medical Center. Steve Hopkins and Janice Nhan provided excellent technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bitsios P, Szabadi E, Bradshaw CM. Comparison of the effects of venlafaxine, paroxetine and desipramine on the pupillary light reflex in man. Psychopharmacology (Berl) 1999;143:286–92. doi: 10.1007/s002130050949. [DOI] [PubMed] [Google Scholar]
- Bryant RC. Asymmetrical pupillary slowing and degree of severity in myasthenia gravis. Ann Neurol. 1980;7:288–9. doi: 10.1002/ana.410070317. [DOI] [PubMed] [Google Scholar]
- Dutton GN, Garson JA, Richardson RB. Pupillary fatigue in myasthenia gravis. Trans Ophthalmol Soc U K. 1982;102:510–3. [PubMed] [Google Scholar]
- Fotiou F, Fountoulakis KN, Goulas A, Alexopoulos L, Palikaras A. Automated standardized pupillometry with optical method for purposes of clinical practice and research. Clinical Physiology. 2000;20:336–47. doi: 10.1046/j.1365-2281.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Stern JA, Brown TB, Russo MB. Cognition, blinks, eye-movements, and pupillary movements during performance of a running memory task. Aviat Space Environ Med. 2005;76:C75–85. [PubMed] [Google Scholar]
- Gavriysky VS. Human pupillary light reflex during and after two-fold Valsalva maneuver. J Auton Nerv Syst. 1995;54:247–52. doi: 10.1016/0165-1838(95)00017-r. [DOI] [PubMed] [Google Scholar]
- Granholm E, Morris S, Galasko D, Shults C, Rogers E, Vukov B. Tropicamide effects on pupil size and pupillary light reflexes in Alzheimer's and Parkinson's disease. Int J Psychophysiol. 2003;47:95–115. doi: 10.1016/s0167-8760(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Heafield MT, Gammage MD, Nightingale S, Williams AC. Idiopathic dysautonomia treated with intravenous gammaglobulin. Lancet. 1996;347:28–29. doi: 10.1016/s0140-6736(96)91559-7. [DOI] [PubMed] [Google Scholar]
- Klein CM, Vernino S, Lennon VA, Sandroni P, Fealey RD, Benrud-Larson L, Sletten D, Low PA. The spectrum of autoimmune autonomic neuropathies. Ann Neurol. 2003;53:752–758. doi: 10.1002/ana.10556. [DOI] [PubMed] [Google Scholar]
- Knaggs RD, Crighton IM, Cobby TF, Fletcher AJ, Hobbs GJ. The pupillary effects of intravenous morphine, codeine, and tramadol in volunteers. Anesth Analg. 2004;99:108–12. doi: 10.1213/01.ANE.0000116924.16535.BA. [DOI] [PubMed] [Google Scholar]
- Larson MD, Muhiudeen I. Pupillometric analysis of the 'absent light reflex'. Arch Neurol. 1995;52:369–72. doi: 10.1001/archneur.1995.00540280051018. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Ermilov LG, Szurszewski JH, Vernino S. Immunization with neuronal nicotinic acetylcholine receptor induces neurological autoimmune disease. J Clin Invest. 2003;111:907–13. doi: 10.1172/JCI17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore FE, Sanborn GE, Slevin JT. Pupillary dysfunction in myasthenia gravis. Ann Neurol. 1979;6:29–33. doi: 10.1002/ana.410060107. [DOI] [PubMed] [Google Scholar]
- Low PA, Dyck PJ, Lambert EH, Brimijoin WS, Trautmann JC, Malagelada JR, Fealey RD, Barrett DM. Acute panautonomic neuropathy. Ann Neurol. 1983;13:412–7. doi: 10.1002/ana.410130407. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Craig ME, Craighead A, Chan AK, Cusumano JM, Hing SJ, Silink M, Howard NJ, Donaghue KC. Autonomic nerve testing predicts the development of complications: a 12-year follow-up study. Diabetes Care. 2007;30:77–82. doi: 10.2337/dc06-0793. [DOI] [PubMed] [Google Scholar]
- Maki T, Nakamura M, Nakamura M, Suenaga T. [A case of autoimmune autonomic neuropathy with marked orthostatic hypotension, decreased salivation, constipation, and Adie pupil] Rinsho Shinkeigaku - Clinical Neurology. 2006;46:218–22. [PubMed] [Google Scholar]
- Micieli G, Tassorelli C, Martignoni E, Marcheselli S, Rossi F, Nappi G. Further characterization of autonomic involvement in multiple system atrophy: a pupillometric study. Functional Neurology. 1995;10:273–80. [PubMed] [Google Scholar]
- Modoni A, Mirabella M, Madia F, Sanna T, Lanza G, Tonali P, Silvestri G. Chronic autoimmune autonomic neuropathy responsive to immunosuppressive therapy. Neurology. 2007;68:161–2. doi: 10.1212/01.wnl.0000251194.82212.75. [DOI] [PubMed] [Google Scholar]
- Pena MM, Donaghue KC, Fung AT, Bonney M, Schwingshandl J, Howard NJ, Silink M. The prospective assessment of autonomic nerve function by pupillometry in adolescents with type 1 diabetes mellitus. Diabetic Medicine. 1995;12:868–73. doi: 10.1111/j.1464-5491.1995.tb00388.x. [DOI] [PubMed] [Google Scholar]
- Piha SJ, Halonen JP. Infrared pupillometry in the assessment of autonomic function. Diabetes Res Clin Pract. 1994;26:61–6. doi: 10.1016/0168-8227(94)90140-6. [DOI] [PubMed] [Google Scholar]
- Rosen ES, Gore CL, Taylor D, Chitkara D, Howes F, Kowalewski E. Use of a digital infrared pupillometer to assess patient suitability for refractive surgery. J Cataract Refract Surg. 2002;28:1433–8. doi: 10.1016/s0886-3350(01)01350-5. [DOI] [PubMed] [Google Scholar]
- Sandroni P, Vernino S, Klein CM, Lennon VA, Benrud-Larson L, Sletten D, Low PA. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neurol. 2004;61:44–8. doi: 10.1001/archneur.61.1.44. [DOI] [PubMed] [Google Scholar]
- Schroeder C, Vernino S, Birkenfeld AL, Tank J, Heusser K, Lipp A, Benter T, Lindschau C, Kettritz R, Luft FC, Jordan J. Plasma exchange for primary autoimmune autonomic failure. N Engl J Med. 2005;353:1585–90. doi: 10.1056/NEJMoa051719. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Hakerem G. The pupillary response in cognitive psychophysiology and schizophrenia. Ann N Y Acad Sci. 1992;658:182–204. doi: 10.1111/j.1749-6632.1992.tb22845.x. [DOI] [PubMed] [Google Scholar]
- Suarez GA, Fealey RD, Camilleri M, Low PA. Idiopathic autonomic neuropathy: Clinical, neurophysiologic, and follow-up studies on 27 patients. Neurology. 1994;44:1675–1682. doi: 10.1212/wnl.44.9.1675. [DOI] [PubMed] [Google Scholar]
- Vernino S, Low PA, Fealey RD, Stewart JD, Farrugia G, Lennon VA. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343:847–855. doi: 10.1056/NEJM200009213431204. [DOI] [PubMed] [Google Scholar]
- Vernino S, Low PA, Lennon VA. Experimental autoimmune autonomic neuropathy. J Neurophysiol. 2003;90:2053–9. doi: 10.1152/jn.00408.2003. [DOI] [PubMed] [Google Scholar]
- Wachler BS, Krueger RR. Agreement and repeatability of infrared pupillometry and the comparison method. Ophthalmology. 1999;106:319–23. doi: 10.1016/s0161-6420(99)90070-2. [DOI] [PubMed] [Google Scholar]
- Wickremasinghe SS, Smith GT, Stevens JD. Comparison of dynamic digital pupillometry and static measurements of pupil size in determining scotopic pupil size before refractive surgery. J Cataract Refract Surg. 2005;31:1171–6. doi: 10.1016/j.jcrs.2004.10.049. [DOI] [PubMed] [Google Scholar]
- Wilhelm H, Wilhelm B. Clinical applications of pupillography. J Neuroophthalmol. 2003;23:42–9. doi: 10.1097/00041327-200303000-00010. [DOI] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, Patrick J, Role L, De Biasi M, Beaudet AL. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 1999;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki A, Ishikawa S. Abnormal pupillary responses in myasthenia gravis. A pupillographic study. Br J Ophthalmol. 1976;60:575–80. doi: 10.1136/bjo.60.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]



