Abstract
Both TH1 and TH17 lymphocytes are implicated in inducing EAE. In mice lacking IFNγ, TH17 are assumed to be the subset responsible for inflammation induction. Here, we demonstrate that IFNγ KO mice have two additional effector subsets, one that up-regulates TH17-associated pro-inflammatory genes, but does not make IL-17 protein, and a second that utilizes IL-12-related elements of the TH1 pathway in an IFNγ-independent manner. In vivo, these two subsets induce demonstrably different disease. By using homogeneous T cell lines, we can dissect the population of autoimmune effector cells, and demonstrate the multiplicity of pro-inflammatory pathways important in disease processes.
Keywords: autoimmune encephalomyelitis, inflammation, effector T cell subsets, IL-17, IFNγ
1. Introduction
Since the initial reports on the importance of IL-23-, rather than IL-12-, driven cells in autoimmune inflammation of the central nervous system, many additional studies have supported the notion that cells of a distinct subset called TH17, rather than TH1 cells, are primarily responsible for the inflammatory pathology observed in experimental autoimmune encephalomyelitis (EAE) (Chen et al., 2006; Cua et al., 2003; Langrish et al., 2005; Park et al., 2005). This newly defined subset of CD4+ T cells is distinguished by production of the neutrophil activating cytokines, IL-17(A) and IL-17F (Bettelli et al., 2007; Gaffen et al., 2006; Langrish et al., 2005; Ley et al., 2006; McKenzie et al., 2006; Park et al., 2005); in addition, IL-6, TNF-α, IL-21, and IL-22 synthesis are associated with TH17 cells (Bettelli et al., 2007; Chung et al., 2006; Korn et al., 2007a; Langrish et al., 2005; Nurieva et al., 2007; Park et al., 2005; Zheng et al., 2007). The existence of a subset of encephalitogenic T cells utilizing an alternative to the TH1 pathway could have been predicted, due to the severity of EAE observed in mice lacking either IFNγ (Abromson-Leeman et al., 2004; Ferber et al., 1996; Krakowski and Owens, 1996), IFNγR (Willenborg et al., 1996), IL-12Rβ2 (Zhang et al., 2003), or IL-12 p35 genes (Gran et al., 2002), all of which contribute to TH1 cell development and activation. In contrast, mice lacking the IL-23 p19 gene were reported to be EAE-resistant after MOG-immunization, despite the presence of CNS-infiltrating cells producing IFNγ (Langrish et al., 2005). Comparisons of encephalitogenic potential of IL-12- versus IL-23-driven cultures of cells concluded that the latter comprised the pathogenic IL-17+ population (Langrish et al., 2005). Subsequent studies demonstrated that while IL-23 plays an important role in maintenance and expansion of this population, their differentiation from naïve cells is independent of IL-23, but requires both IL-6 and TGF-β1 (Bettelli et al., 2006; Cua and Kastelein, 2006; Veldhoen et al., 2006). More recent reports demonstrate an important autocrine role for IL-21 in generating TH17 cells (Korn et al., 2007a; Nurieva et al., 2007; Zhou et al., 2007).
Studies of EAE-inducing T cell lineages have generally utilized antigen-primed or TCR transgenic populations that are subset-enriched, but still heterogeneous, with respect to cytokine synthesis. Results have sometimes led to conflicting reports on the TH1 versus TH17 nature of the effector population. The encephalitogenic potential of the often observed IFNγ+/IL-17+ population is also unknown. For a more detailed appreciation of the spectrum of T cells that can induce autoimmune inflammation, we generated stable clones of encephalitogenic effector T cells. In earlier studies of EAE-inducing T cells derived from CNS of IFNγ-knockout (GKO) mice, we observed heterogeneity in clinical disease presentation (Abromson-Leeman et al., 2004). Two different pathologic phenotypes were described, based not only upon clinical presentation, but upon differential identification of the infiltrating inflammatory cells as being either predominantly neutrophilic or predominantly macrophage and lymphoid.
Here we dissect the molecular differences responsible for these different clinical phenotypes, analyzing gene expression by these two types of encephalitogenic CD4+ T cells derived from IFNγ-knockout mice, and revealing up-regulation of distinct sets of genes that outline different molecular pathologic pathways utilized. These results show that one pathway is similar to a TH17 pathway. Although IL-17 protein is not produced, IL-17, IL-21, and IL-22 transcripts are up-regulated, IL-6, GM-CSF, and TNFα are abundantly synthesized, and the associated downstream events, culminating in neutrophilic infiltration in vivo, are striking. The second pathway is a novel one, in that it resembles a TH1 pathway of IL-12 dependence, despite the absence of IFNγ. Inflammatory lesions consist of lymphocytes and activated macrophages. The finding of this pathway has implications for the conclusion that autoimmunity in mice lacking components of the IFNγ pathway is mediated solely by TH17 cells, and suggests the existence of at least one more subset of inflammation-inducing T cells, and a novel pathway of disease induction.
In addition to the study of GKO-derived T cells, analyses of gene expression in effector T cells from mice genotypically positive for IFNγ reveal that conventional IFNγ-secreting TH1 cells, as well as T cells with characteristics of both TH1 and TH17 subsets are also very efficient inducers of disease. These studies of cloned, stable effector T cell lines underscore the multiplicity of effector pathways utilized by T cells, and the contributions of T cell diversity to the heterogeneity of inflammatory disease.
2. Materials and methods
2.1. Mice and antigens
BALB/c By and BALB-GKO mice were purchased from Jackson Laboratories. TCR-transgenic BALB mice were generated in our laboratory; the re-arranged TCR α and β chains derive from clone 3a.56, specific for MBP exon 2 (Abromson-Leeman et al., 2004). Mice were maintained, and experiments were conducted, in accord with guidelines of the Committee on Care and Use of Animals of Harvard Medical School and those prepared by the Animal Committee on Care and Use of Laboratory Animals of the National Research Council (Department of Health and Human Services Publication NIH 85-23). MBP peptides were synthesized by Dr. Chuck Dahl, Biopolymers Facility, Harvard Medical School.
2.2. Generation of T cell clones and T cell line
Clones 8-4.G6 (specific for MBP 59–76) and BC.D9 (specific for MBP exon 2 peptide) were derived from draining lymph nodes of BALB/c mice immunized with myelin basic protein, and have been previously described (Abromson-Leeman et al., 1995b; Abromson-Leeman et al., 2004). Line 173M10 derives from draining lymph node of an immunized T cell receptor (TCR) transgenic BALB mouse. M10.1 was cloned from line 173M10 by limiting dilution. Clones 3--8 and X2.51 were each cloned from independent lines derived by isolating T cells from CNS of BALB-IFNγ-knockout (BALB-GKO) mice that developed EAE after immunization with MBP exon 2 peptide; clone X2.51 has been previously described (Abromson-Leeman et al., 2004). T cells have been continually maintained in culture as previously described (Abromson-Leeman et al., 1995a; Abromson-Leeman et al., 2004).
2.3. In vitro activation of T cells for preparation of supernatants, mRNA and intracellular flow cytometry
T cells were activated by co-culture with 5 × 106 irradiated BALB/c spleen cells and 10μg/ml peptide in complete DME medium. Supernatants for ELISA were collected from 24–72 hours. For mRNA, peptide-pulsed splenic adherent cells were cocultured with T cells, and harvested at 24 hours. For intracellular flow cytometry, T cells were incubated 16–18 hours before addition of GolgiPlug for 2 hours; staining was done in accordance with manufacturer’s protocol (BD Biosciences). For ELISA quantitation of secreted cytokines, BD Biosciences capture and biotinylated antibodies were used; recombinant cytokines were used to construct standard curves. Manufacturer’s protocols were followed.
2.4. EAE induction
For in vivo injections, T cells were activated by co-culture with peptide and irradiated spleen cells for three days; 10 × 106 cells were injected i.v. into BALB/c recipients irradiated with 350 R. No pertussis was used. Mice were monitored daily, and sacrificed at first onset of clinical signs of EAE. All clones tested had similar kinetics of EAE induction, with disease onset at 7–9 days.
2.5. RT-PCR
At first onset of clinical signs of disease, mice were euthanized; brain and spinal cord were used for mRNA preparation. Tri-reagent (MRC) was used according to manufacturer’s protocol. cDNA was prepared using Qiagen Quantitect RT kit. Primer sequences were chosen using Primer3 software on-line, and are available upon request. Housekeeping genes were 18S rRNA for in vitro samples, and GAPDH for in vivo samples. Quantitative real-time PCR analyses were done using the Roche Light Cycler 480 in a 96-well format, with manufacturer’s reagents for SYBR green detection. Standard curves were used to ensure linearity between Ct values and relative gene levels. Pfaffl equations were used to calculate fold up-regulation. For in vitro samples, antigen and spleen activated T cells were compared to T cells co-cultured in the absence of antigen. For in vivo samples, CNS tissue of diseased animals was compared with normal CNS tissue.
Levels of mRNA expression, both under basal conditions and under conditions of activation, vary widely for different genes; the Ct value reflects the expression level. Thus some genes may be expressed at relatively high basal levels (reflected in low Ct values) but may not be up-regulated under conditions of activation or in disease (as reflected in a low “fold-upregulation”). Conversely, a gene with a low basal level of expression (i.e. high Ct value) may be highly up-regulated as compared with its basal level, reflected in a high “fold-upregulation”. Thus in Figures 2, 3, and 5, we have presented the quantitative PCR data in two forms, with both a number reflecting “fold up-regulation” in the experimental condition as compared with control condition, as well as with a Ct value, reflected by the level of shading in each box. Higher levels of mRNA expression are depicted by more intense shading.
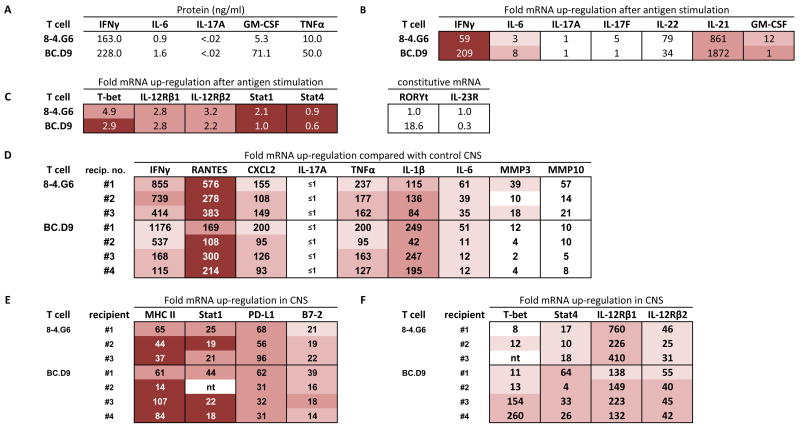
Figure 2.
Expression profiles of TH1 cells in vitro (A, B, C) and genes up-regulated in CNS tissue of TH1-induced EAE (D, E, F). (A) ELISA quantitation of supernatants collected 40 hours after activation of T cell clones with MBP peptides (10 μg/ml) and irradiated BALB/c spleen. None of the cytokines were detectable without addition of antigen. (B) Real time PCR analysis of cytokine gene expression 24 hours after activation with antigen and irradiated spleen cells in vitro. mRNA expression is given as fold up-regulation by T cells with antigen compared with T cells incubated in the absence of antigen (both with irradiated antigen presenting cells); a value of 1 indicates that expression after antigen stimulation is the same as expression without antigen. For in vitro cultured cells, this calculated value of fold-up-regulation uses 18S rRNA as housekeeping gene to normalize values. For expression levels, actual Ct values are shown (cDNA from in vitro cultured cells was used at 1/10; Ct of housekeeping gene is nearly identical for each sample shown, within a value of 0.5, and therefore not subtracted). Intensity of shading correlates with intensity of expression level. Lowest Ct values (16–22) are most deeply shaded; three intermediate levels of decreasing shading indicate, respectively, Ct values of 22–26, 26–28, and 28–30. No shading indicates Ct >30. (C) Realtime PCR analysis of up-regulation (after antigen-specific activation, compared with no antigen addition, normalized with 18S rRNA) and expression level (Ct value) of T-bet, IL-12R chains, Stat1, and Stat4. Levels of RORγt and IL-23R did not increase after activation, therefore relative constitutive levels are arbitrarily compared between each T cell and clone 8-4.G6, which had a basal Ct value of 40. (D,E,F) Realtime PCR analysis of gene expression level (Ct value) and fold up-regulation of each gene in CNS of sick mice, compared with expression levels in normal CNS tissue. GAPDH is used as the housekeeping gene for normalization of in vivo tissue. Brain and spinal cord were harvested at first onset of clinical signs of EAE, and cDNA combined in 1:1 ratios for PCR analysis of each BALB/c recipient. Combined cDNA was used at 1/100 dilution.
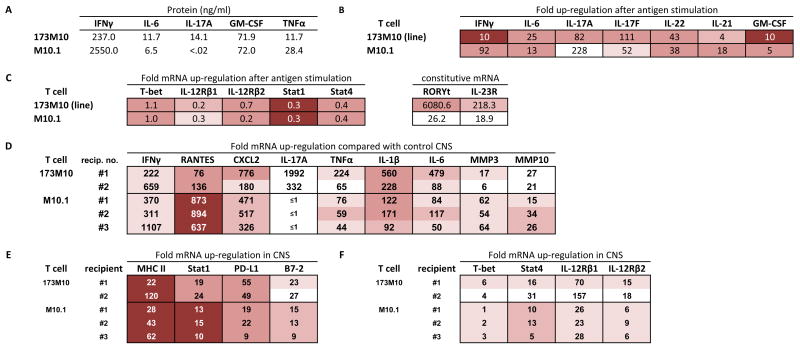
Figure 3.
Expression profiles of T cells from a TCR transgenic, IFNγ+ mouse. (A–C) In vitro cultured T cells; see legend for Fig. 2. (D–F) CNS tissue from T cell recipients with EAE; see legend for Fig. 2.
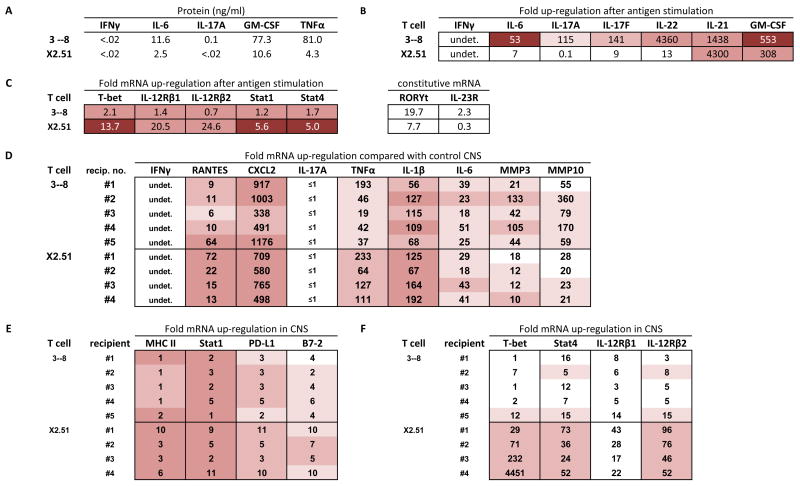
Figure 5.
Expression profiles of BALB-GKO-derived T cells. (A-C) In vitro cultured cells; see legend for Fig. 2. (A) Levels of IL-17 protein were undetectable (<.02 ng/ml) in 6 of 9 independently generated supernatants, and present at .08, 0.25, and 0.31 ng/ml in three of nine supernatants (D–F) CNS tissue from sick T cell clone recipients; see legend for Figure 2.
3. Results
3.1. TH1 subset-induced EAE
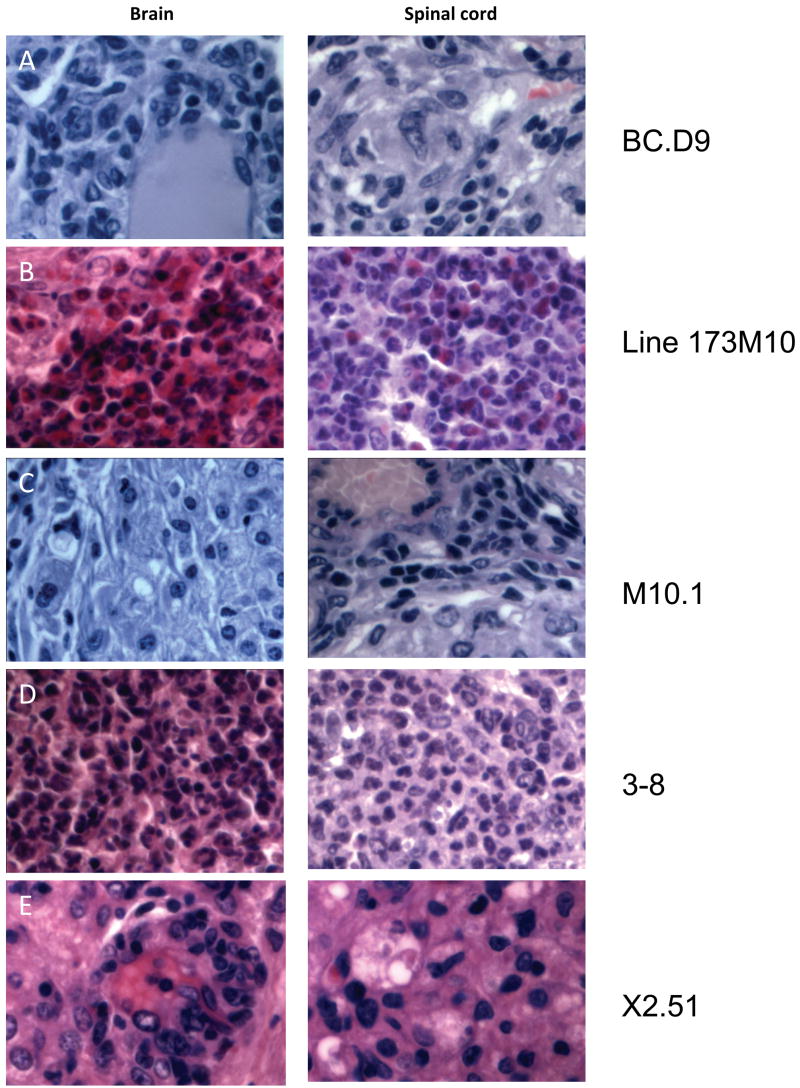
We have previously reported on clones 8-4.G6 and BC.D9 (Abromson-Leeman et al., 1995b; Abromson-Leeman et al., 2004), two independently-derived TH1 clones obtained initially by immunizing wild-type BALB/c mice with myelin basic protein (MBP) and repeatedly stimulating the draining lymph node lymphocytes with MBP in vitro. The two clones recognize different epitopes of MBP -- 8-4.G6 is specific for residues 59–76 (Abromson-Leeman et al., 1995b), while BC.D9 recognizes a sequence in exon 2 of MBP (Abromson-Leeman et al., 2004). Both clones proliferate and produce cytokines, including IFNγ, in response to MBP presented by MHC Class II. When injected in vivo into naïve recipient mice, both these TH1 clones induce severe EAE in 100% of recipients, with the classical clinical signs of ascending paralysis beginning 7–10 days after transfer of cells, and proceeding to severe disease with complete paralysis within another 1–2 days. Infiltrating inflammatory cells typically consist of macrophages and lymphoid cells, which are seen in meningeal and perivascular areas in the CNS. Representative sections of brain and spinal cord from a BC.D9 recipient are shown (Figure 1A).
Figure 1.
Inflammatory infiltrates in CNS tissues of T cell recipients. Representative hematoxylin and eosin stained sections from brain and spinal cord of indicated T cell clone or line recipients are shown, magnified x600. All mice received 10 × 106 in vitro activated T cells intravenously. Mice were sacrificed within 1–2 days of showing clinical signs of disease. (A) Brain and spinal cord of BCD9 recipient sacrificed 10 days after transfer. Infiltrates consist primarily of macrophages and lymphoid cells, and are concentrated in perivascular and meningeal regions. (B) Brain and spinal cord of line 173M10 recipient 10 days after transfer. Abcesses of neutrophils and eosinophils are present in both brain and spinal cord tissues. (C) Tissues from clone M10.1 recipient 8 days after transfer; macrophages dominate among infiltrating cells. (D) Lateral medulla is completely taken over by infiltrating neutrophils in 3–8 recipients, 10 days after transfer. Spinal cord lesions are rare and isolated, but also consist of very large foci of neutrophils. (E) Tissues from X2.51 recipient 10 days post transfer, dominated by foamy macrophages and lymphoid cells.
Quantitation of a panel of cytokines synthesized and secreted by clones 8-4.G6 and BC.D9 confirms the TH1 nature of these two clones (Figure 2A and 2B). After in vitro activation of T cells with their cognate peptides presented by syngeneic BALB/c spleen cells, significant levels of IFNγ are secreted in supernatants (Figure 2A). Cytokines are undetectable in the absence of antigen. mRNA for IFNγ is highly up-regulated after antigen activation of cells, as shown by real-time PCR studies (Figure 2B). Very low levels of IL-6 mRNA and protein are detectable (Figure 2A and 2B). Neither protein nor mRNA for IL-17 is detectable (Figure 2A and 2B). Both GM-CSF and TNFα are secreted in response to antigen (Figure 2A). mRNA for IL-22 is up-regulated, although the expression levels (Ct values ≥ 32) are still relatively low (Figure 2B). mRNA for IL-21, in contrast, is both highly up-regulated and is present at relatively high levels after activation of these TH1 cells (Ct values go from 35 in resting TH1 cells to 23 after antigen-induced activation). The TH1 lineage of these cells is also demonstrable in the high level of T-bet expression; because constitutive levels are relatively high as judged by Ct values (21–23), up-regulation after antigen stimulation may not appear as dramatic -- 4.9 and 2.9-fold. Similarly, mRNA for IL-12Rβ1 and β2 are constitutively high and are modestly up-regulated following antigenic stimulation (Figure 2C).
We next prepared cDNA from CNS tissues harvested at the onset of disease from recipients of each of these TH1 cells. Real-time PCR was used to quantitate relative levels of gene expression in diseased tissue as compared with levels in control, healthy CNS tissue. Data (Figure 2D) depict the expression of a panel of pro-inflammatory molecules that are generally secreted – cytokines, chemokines, and metalloproteinases. The data show both fold-upregulation of gene expression in CNS of diseased T cell recipients as compared with normal CNS tissue (a calculated value taking into account housekeeping gene levels), as well as depicting the relative levels of gene expression, as judged by the cycle crossing the background threshold in real-time PCR (an earlier crossing point, or smaller Ct value signifies higher level of gene expression than a later crossing point, with a higher Ct value; shading is used to depict Ct values). Results are shown for individual recipients of each clone, to illustrate the range of observed values.
Despite mouse-to-mouse variability, distinct patterns can be discerned. IFNγ is highly up-regulated (ranging from 115- to 1176-fold; Ct values range from 27–29) in CNS of TH1 clone recipients (Figure 2D). The chemokine RANTES/CCL5 is highly upregulated, from 108- to 576-fold (Ct values range from 20–24), as is the chemokine CXCL2/MIP-2 (93–200 fold; Ct values 27–29). There is no detectable IL-17 mRNA in the CNS of TH1 clone recipients (Ct ≥40). The levels of three pro-inflammatory cytokines TNFα, IL-1β, and IL-6 are all up-regulated in all recipients as compared with control CNS. With the exception of one 8-4.G6 recipient (#1), levels of MMP3 and MMP10 are modestly up-regulated (2–18 fold, and 5–21 fold, respectively).
Expression of four molecules known to be influenced by IFNγ were highly up-regulated in CNS of TH1 recipients (Figure 2E); MHC Class II, Stat1, PD-L1, and B7-2 were highest in CNS of recipients of IFNγ+ T cells, and highly expressed. Finally, transcription factors and receptors related to the IFNγ/IL-12 pathway are also up-regulated in CNS of TH1 recipients. These include T-bet and Stat4, as well as the heterodimeric IL-12R chains, β1 and β2 (Figure 2F).
3.2. EAE induced by IFNγ+ TCR transgenic T cells
Line 173M10 is a stable T cell line (in continuous culture > 2 years) of MBP exon 2-primed lymph node cells from a TCR transgenic BALB/c mouse. Clone M10.1 is a clone derived by limiting dilution cloning of the line. Both line and clone induce EAE upon adoptive transfer into naïve BALB recipients. Early clinical signs of disease induced by both the line and its clone include hind leg weakness and cachexia. These presenting signs of disease develop slowly in recipients of line 173M10, but with accelerated severity in clone M10.1 recipients. Thus recipients of the line generally develop score 2 EAE by about 2 weeks, whereas most recipients of the same number of clone M10.1 cells have score 4–5 EAE by day 10. Slides of CNS tissue from diseased recipients show very different pictures of inflammatory infiltrates (Figure 1B and Figure 1C). Characteristic of line 173M10-induced disease are large abcesses comprised predominantly of neutrophils and eosinophils (Figure 1B), present in brain and spinal cord. Clone M10.1, in contrast, induces primarily a macrophage infiltrate, with lymphocytes also visible (Figure 1C). Cells tend to be more meningeal and perivascular in M10.1 recipients.
Supernatants collected after in vitro stimulation of line 173M10 and clone M10.1 with their cognate peptide (MBP exon 2) on BALB/c spleen cells show that high levels of IFNγ are secreted by the line (237 ngs/ml), and extremely high levels (2550 ng/ml) are secreted by the clone, M10.1. Both also secrete IL-6 (11.7 and 6.5 ng/ml, respectively), GM-CSF (both the same, 72 ng/ml), and TNFα (11.7 and 28.4 ng/ml, respectively). However, only line 173M10 secretes detectable IL-17 protein – 14.1 ng/ml; no IL-17 protein is detectable in supernatants of clone M10.1 (Figure 3A). Surprisingly, however, real-time PCR studies show that clone M10.1 does up-regulate mRNA for the TH17-associated cytokines IL-17A (228-fold), IL-17F (52-fold), and IL-22 (38-fold) after antigen stimulation (Figure 3B), although the Ct values for IL-17A and IL-17F are significantly less (30 and 29, respectively, compared with Ct values of 37 and 34 without antigen) than those for line 173M10 (Ct values of 24 and 25, respectively, compared with Ct values of 31 and 32 without antigen), which secretes detectable IL-17 protein. mRNA for IL-21 is also up-regulated after activation of both line 173M10 (Ct values of 29 and 27 before and after activation, respectively) and clone M10.1 (Ct values of 30 and 26 before and after activation, respectively). Confirming the protein data, mRNA for IFNγ, IL-6, and GM-CSF are all up-regulated (Figure 3B). While TGFβ1 mRNA is present at relatively high levels in all samples (Ct values in the range of 20±1), it is neither differentially expressed between different T cell clones nor up-regulated after activation (data not shown).
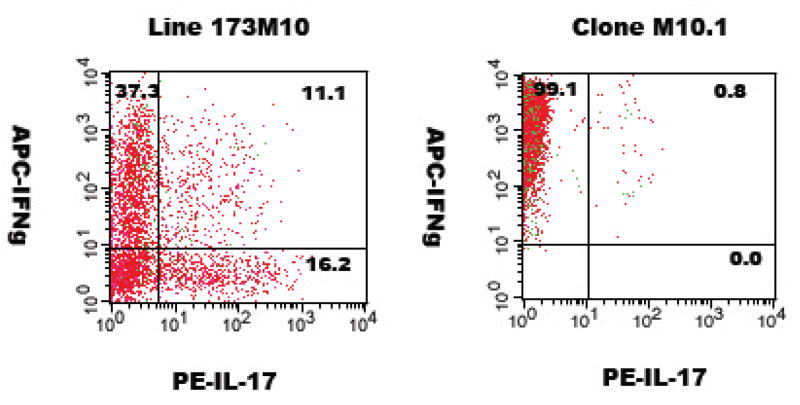
To investigate further the TH1 versus TH17 nature of these two T cells, they were activated in vitro with their cognate peptide (MBP exon 2) and antigen-presenting spleen cells, followed by intracellular flow cytometry. Results of this analysis (Figure 4) show that line 173M10 appears to consist of distinct populations, with 37% IFNγ+/IL-17−, 16% IFNγ−/IL-17+, and 11% IFNγ+/IL-17+ when gated on CD4+ cells. Double positive, i.e. IFNγ+/IL-17+cells, have been observed by others (Acosta-Rodriguez et al., 2007; Suryani and Sutton, 2007). In contrast to the heterogeneity of line 173M10, clone M10.1 is homogeneous, and consists exclusively of cells making IFNγ (>99%); the level of intracellular IFNγ was an order of magnitude higher than that of line 173M10, in accordance with results observed by ELISA analysis of supernatants (Figure 3A).
Figure 4.
Cytokine analysis by intracellular flow cytometry. T cells were activated by 18 hour incubation with splenic adherent cells and antigen, followed by intracellular cytokine staining for IFNγ and IL-17. Plots shown are gated on CD4+ cells.
Line 173M10 expresses mRNA for both the TH1- and TH17- associated transcription factors, T-bet and RORγt (Ct values of 24.4 and 25.6, respectively). This level of RORγt is >6,000 fold higher than the background level (Ct of 40) of the TH1 clone (8-4.G6) described earlier (Figure 3C). Likewise, mRNA for IL-23R, another marker of TH17 lineage, is 218-fold higher for line 173M10 (Ct, 26.8) than for the aforementioned TH1 clone, 8-4.G6 (Figure 3C). Clone M10.1, in contrast, has levels of RORγt and IL-23R mRNA that are higher than the TH1 clone (26-fold and 19-fold), but still at relatively low expression levels, as judged by the high Ct values (34 and 31, respectively).
Expression of chemokines and cytokines in the CNS of individual recipients of line 173M10 or clone M10.1 at the time of disease onset is shown (Figure 3D). IFNγ expression is highly up-regulated in recipients of both line 173M10 and clone M10.1. Both RANTES/CCL5 and CXCL2 chemokines are up-regulated in CNS of all recipients as well. CNS tissues of clone M10.1 recipients have notably high levels of both these chemokines; the TH1 clone recipients shown earlier (Figure 2D) also have high levels of RANTES/CCL5 but have lower CXCL2 expression than M10.1 recipients. IL-17A mRNA is highly up-regulated in CNS of line 173M10 recipients, but is not detectable in M10.1 CNS tissues. As with CNS of TH1 clone recipients, the three pro-inflammatory cytokines TNFα, IL-1β, and IL-6 are all significantly up-regulated in all recipients. Finally, both MMP3 and MMP10 are up-regulated in all recipients (Figure 3D), but MMP3 levels are notably higher in clone M10.1 recipients (54-fold to 64-fold) than they are in CNS of either line 173M10 recipients (6-17-fold), or in CNS of the TH1 clones (Figure 2D).
mRNA for molecules up-regulated by IFNγ – MHC Class II, Stat1, PD-L1, and B7-2 –are all up-regulated in CNS of recipients of both line 173M10, and clone M10.1 (Figure 3E). Finally, mRNA for IL-12Rβ1 is upregulated in both line 173 and M10.1 recipient CNS, although expression levels are less than for TH1 clones. Transcripts of the IL-12R-specific chain, IL-12Rβ2, are only minimally up-regulated in these recipients (Figure 3F) as compared with recipients of TH1 cells shown (Figure 2F).
3.3. EAE induced by BALB-GKO T cells
Two clones were independently derived from CNS of two different BALB-IFNγ-knockout (GKO) mice that developed EAE after immunization with MBP exon 2. The first, clone 3–8, was isolated from a mouse with axial-rotatory EAE; it consistently induces axial-rotatory EAE when transferred back into naïve BALB or BALB-GKO recipients. Early clinical signs of disease are tilting of the head and spasticity, which are rapidly (often within hours) followed by uncontrollable axial rotation of the body (Abromson-Leeman et al., 2004; Muller et al., 2000; Sobel, 2000). Clone 3–8 was passaged through mice and re-isolated to obtain a number of subclones, all of which induce generally the same patterns of disease and gene expression as the parent clone 3–8. Histological analysis of CNS from recipients of 3–8 or its subclones shows massive neutrophilic infiltrates, primarily in lateral medulla, but with an occasional spinal cord focus as well (Figure 1D).
The second BALB-GKO CNS-derived cell, clone X2.51, also transfers EAE upon injection into naïve BALB or BALB-GKO recipients. Disease induced by X2.51 generally develops between 10–18 days after T cell transfer. Induced disease presents as a combination of tilting, spasticity, leg weakness and cachexia. Clone X2.51 induces an infiltrate in which macrophages and lymphocytes are prominent; neutrophils are rarely observed (Figure 1E).
Cytokines secreted after in vitro activation of these two GKO-derived T cells with their cognate peptide (MBP exon 2) and BALB antigen presenting cells are shown (Figure 5A). Predictably, there is no detectable IFNγ. Clone 3–8 supernatants contain fairly high levels of IL-6 (11.6 ng/ml), GM-CSF (77.3 ng/ml), and TNFα (81 ng/ml), all characteristic of TH17, yet there is little to no IL-17A protein (see legend to Figure 5A). Addition of IL-23 to cultures did not result in any change in IL-17 levels (data not shown). The second GKO clone, X2.51, has a very different profile of secreted cytokines, with significantly lower levels of IL-6 (2.5 ng/ml), GM-CSF (10.6 ng/ml) and TNFα (4.3 ng/ml). This second clone, X2.51, made no IL-17.
Analysis by real-time PCR of cytokine gene expression by these two GKO-derived clones is shown (Figure 5B). Following in vitro activation with antigen, mRNA’s for both IL-17A and IL-17F are up-regulated by clone 3–8, 115-fold and 141-fold, respectively (with before and after activation Ct values going from 36 to 29 for IL-17A, and 34 to 27 for IL-17F). Neither IL-17 mRNA is up-regulated by the second GKO clone, X2.51. mRNA for IL-22, another TH17-associated cytokine, is highly up-regulated by clone 3–8 (Ct of 34 before activation, Ct of 22 after activation). IL-21 is also highly up-regulated and expressed at high levels, with Ct values of 35 before activation and 23 after activation. There is very little expression of IL-22 by the second clone, X2.51 (Ct values of 34 and 32 before and after activation), although it does highly up-regulate IL-21, with Ct values of 36 and 25, respectively, before and after activation).
Clone 3–8 has a very low constitutive level of RORγt (although almost 20-fold higher than the TH1 clone 84G6 shown in Figure 2C, the expression level is still extremely low, with a Ct value of 32). Similarly, although the level of IL-23R is 2-fold higher than clone 84G6, its Ct value of 32 indicates a very low mRNA expression level. The second GKO clone, X2.51, has even lower expression levels of these two TH17-associated genes (Figure 5C). On the other hand, clone X2.51 up-regulates and expresses relatively high levels of genes associated with the IL-12-signalling pathway; these include T-bet, IL-12Rβ1 and IL-12Rβ2 (both of which are up-regulated >20 fold after antigen activation, with Ct values of 25 and 22, respectively), and Stat1 and Stat4 (up-regulated 5-fold, with Ct values of 20 and 21, respectively). Clone 3–8 does not significantly up-regulate mRNA for genes of the IL-12 pathway (Figure 5C).
Finally, we looked in vivo at gene expression in CNS of recipients of these two GKO clones. As expected, there is no detectable IFNγ. Recipients in both these groups up-regulated RANTES, although levels were far lower than those observed for recipients of IFNγ-producing clones shown earlier. CXCL2 was highly expressed in both groups of recipients. There was no detectable up-regulation of IL-17(A or F) mRNA; Ct values for IL-17A in all mice in both groups were ≥40 (Figure 5D). Levels of the three pro-inflammatory cytokines TNFα, IL-1β, and IL-6 were elevated in recipients of both groups. Notably different between the two groups, however, were levels of MMP3 and MMP10, which were up-regulated significantly more in clone 3–8 recipients than in clone X2.51 recipients (21–133-fold and 55–360-fold, respectively, for clone 3–8 recipients, and 10–18-fold and 20–28-fold, respectively, in clone X2.51 recipients). Not surprisingly, genes regulated by IFNγ, i.e. MHC Class II, Stat1, PD-L1, and B7-2, were up-regulated to a lower degree in recipients of these two GKO clones as compared with recipients of IFNγ-producing clones shown earlier (Figure 5E). Significant differences between the two groups of recipients did exist, however, in CNS expression of genes related to the IL-12 pathway (Figure 5F), as noted in the in vitro studies. Levels of T-bet, Stat4, IL-12Rβ1, and IL-12Rβ2 were highly up-regulated and expressed in recipients of clone X2.51, but only minimally up-regulated and expressed in recipients of clone 3–8. Taken together with in vitro data (Figure 5C), these results strongly suggest that the GKO clone X2.51, although having no IFNγ, induces a pathway of inflammation that involves IL-12-signalling through Stat4, and none of the elements of the IL-17 pathway. The striking differences in gene expression between these two GKO-derived clones are reflected in the nature of the inflammatory infiltrates observed in CNS lesions (Figure 1).
4. Discussion
The effector phase of EAE is widely studied as a potential model for the inflammation associated with multiple sclerosis. Activated, myelin-recognizing, pro-inflammatory TH1 T cells were long thought to be the primary cells responsible for initiating the cascade of inflammation in the CNS, but doubts were raised when it was shown that IL-12 p35 knockout mice were still susceptible to disease, while mice deficient in IL-23 p19 were no longer susceptible (Cua et al., 2003). Since IL-23 contributes to expansion of the TH17 subset of cells, and IL-23-driven TH17 cell populations were shown to be efficient inducers of EAE, they soon came to be thought of as the sole population capable of inducing this inflammatory disease. Furthermore, because EAE is inducible in IFNγ-knockout mice which lack conventional TH1 cells, it was assumed that a TH17 population dominated, especially in the absence of IFNγ, and therefore could explain the severity of disease in GKO mice.
Because previous studies utilized populations of cells, whether from cytokine knockout strains, ‘driven’ in culture with recombinant cytokines and/or derived from TCR transgenic mice, the heterogeneous nature of the population may preclude an accurate assessment of the capability of a given T cell subset and the contribution of its genetic program to the disease process. Additionally, cell interactions that invariably occur may add a level of complexity to interpretations. Using stable monoclonal populations of defined T cells, we show in this report that there is a spectrum of genetically distinct T cell subsets that utilize multiple, distinct pathways for mediating tissue inflammation and destruction. These include cells that are classical IFNγ+TH1 cells, others that combine characteristics of IFNγ+TH1 cells with some characteristics of TH17 cells, cells that are IFNγ-negative with TH17 characteristics but that do not make IL-17, and IFNγ-negative cells that appear to mediate strong IL-12-signalling that is IFNγ-independent. Ultimately, inflammation and serious destruction of CNS white matter occurs in all these cases, but because the gene programs differ, the nature of the inflammatory infiltrate differs, and clinical presentation varies as well. When mixed populations of cells induce disease, many of these individual contributions would be obscured.
Prototypical TH1 cells (such as those shown in Figure 2) are able to induce EAE upon adoptive transfer to naïve recipient mice, characterized by the classical ascending paralysis associated with EAE. TH1 cells are characterized by their high levels of secreted IFNγ, but make other pro-inflammatory proteins such as TNFα and GM-CSF (Figure 2A). These cells neither secrete IL-17 nor synthesize mRNA for IL-17, and although they up-regulate mRNA for IL-22, expression levels are very minimal, with Ct values 32 (Figure 2A and 2B). IL-21 mRNA is, in contrast, highly up-regulated and expressed by the TH1 clones studied (Figure 2B). This finding was somewhat unexpected, since IL-21 expression has previously been associated primarily with TH17-enriched populations of cells (Korn et al., 2007b; Nurieva et al., 2007). These recently published reports examined IL-21 mRNA expression by heterogeneous naïve populations driven in vitro with IL-6 and TGFβ1 and activated with anti-CD3 and anti-CD28 ((Korn et al., 2007b; Nurieva et al., 2007). Our findings, in contrast, pertain to effector T cells derived by cloning cells from mice immunized in vivo.
Upon transfer to naïve recipients, they induce up-regulation of additional pro-inflammatory cytokines (IL-1β and IL-6) and chemokines (especially CCL5/RANTES) (Figure 2D), leading to disruption of the blood-brain barrier and infiltration of macrophages into the CNS (Figure 1A). IFNγ contributes to significant up-regulation of MHC Class II, Stat1, PD-L1, and B7-2 in inflamed CNS tissue (Figure 2E). T-bet is constitutively expressed at high levels (Ct values of ≤23), for the two TH1 clones (Figure 2C).
Line 173M10 is a mixed population; intracellular flow cytometry shows distinct populations based on synthesis of IFNγ and/or IL-17 (Figure 4). This diversity exists in spite of the fact that all cells in this line have the same TCR for antigen. It is interesting that these diverse sub-populations have co-existed for well over two years, since there are suggestions in the literature that IFNγ-producing TH1 regulate or preclude the expansion of TH17 cells in vitro. Cells in this line consistently produce both of these cytokines after activation with antigen (Figure 3A), and there does not appear to be a diminution of IL-17 production over time. In addition to significant levels of IFNγ and IL-17, supernatants of line 173M10 also contain high levels of IL-6, GM-CSF, and TNFα after activation of cells (Figure 3A), and mRNA for both IL-21 and IL-22 are up-regulated (Figure 3B). Transcription factors associated with both TH1 and TH17, i.e. T-bet and RORγt, are both present (Figure 3C).
Clone M10.1 also expresses high levels of T-bet, but in contrast with the line from which it derives, minimal RORγt (Figure 3C). Proteins present in supernatants of M10.1 are consistent with a TH1 profile – very high levels of IFNγ, and no detectable IL-17 (Figures 3A and 4). Yet, M10.1 is not an entirely typical TH1; it has ‘echoes’ of a TH17 lineage. Thus mRNA for IL-17A and IL-17F are up-regulated after antigen activation of M10.1 (228-fold and 52-fold, respectively) (Figure 3B). Other similarities to TH17 lineage cells that differ from TH1 cells include production of higher levels of IL-6 and GM-CSF (Figure 3A), higher mRNA levels for IL-22 (Ct of 23.5) (Figure 3B) and induction of higher levels of CXCL2 and MMP3 in CNS of recipients (Figure 3D).
Although both clones 3–8 and X2.51 originated in the CNS of IFNγ-knockout diseased mice, they share no similarities other than their lack of IFNγ. Clone 3–8 has many similarities to TH17 cells, but makes little to no IL-17 (Figure 5A). It does, however, up-regulate mRNA for both IL-17A and IL-17F (Figure 5B), in a manner similar to the IFNγ+ clone M10.1 just described. The mRNA levels for IL-17A and IL-17F increase over two logs after addition of antigen. IL-21 mRNA is highly expressed by clone 3–8 after activation (Figure 5B). Other characteristics of TH17 cells include high IL-22 expression (up-regulated over 1000-fold after activation) (Figure 5B), as well as high levels of IL-6, GM-CSF, and TNFα at the protein level (Figure 5A), and at the mRNA level (for IL-6 and GM-CSF, Figure 5B). In vivo, clone 3–8 induces very high expression of CXCL2, and significantly higher levels of MMP3 and MMP10 up-regulation than other T cell clones studied (Figure 5D). No up-regulation of mRNA for IL-17 is detectable in CNS of 3–8 recipients. Like M10.1, there is only minimal RORγt expression (Ct 32, Figure 5C).
Since both M10.1 and clone 3–8 have a number of characteristics of TH17 cells but make little to no IL-17 (and are therefore merely ‘TH17-like’) and have little to no expression of RORγt, it is likely that RORγt is a necessary transcription factor for IL-17 itself, but not necessarily the one responsible for transcription of many of the other genes that have characterized “TH17”. The abundant expression of IL-6 and IL-21 mRNA (Figure 5B), and the presence of TGFβ1 mRNA (data not shown), suggest that it is not an absence of these cytokines (important for TH17 lineage differentiation from naïve populations), that is responsible for the TH17-like nature of these cells (rather than being bona fide TH17 cells). Production of IL-17 initially defined this “TH17 lineage, but subsequently expanded to include other neutrophil-chemotactic and activating cytokines, either made or induced by these T cells. Notable among these are IL-17F, IL-22 (Liang et al., 2006), IL-21 ((Korn et al., 2007b; Nurieva et al., 2007), MMP3 (Park et al., 2005), VEGF and other angiogenic factors, CXCL2/IL-8-like chemokines KC and MIP2 (Numasaki et al., 2004; Takahashi et al., 2005), and CCL11/eotaxin (Rahman et al., 2006), together with higher than usual levels of TNFα, IL-1β, IL-6 and GM-CSF (Kolls and Linden, 2004; Langrish et al., 2005). Results reported here dissociate IL-17 from the other cytokines and chemokines that orchestrate the process of tissue infiltration and inflammation, and indicate that non-IL-17-producing cells can also be efficient inducers of organ-specific inflammatory reactions.
Finally, clone X2.51, also isolated from CNS of a GKO mouse, is neither a TH1 (since it lacks IFNγ), nor TH17 or TH-17-like, but appears to utilize a pathway involving IL-12-signalling to induce inflammation. T-bet, already at a high level (Ct 24) is up-regulated (to a Ct of 21) after activation (Figure 5C), and both IL-12Rβ1 and IL-12Rβ2 levels are up-regulated as well. In CNS of X2.51 recipients, there is also a notable up-regulation of genes related to the IL-12-signalling pathway, including T-bet, Stat4, IL-12Rβ1 and IL-12Rβ2 (Figure 5F). Many of these same changes are observed in CNS of TH1 clone recipients (shown in Figure 2E), where an IL-12-signalling pathway is predictable. Unlike TH1 cell recipients, clone X2.51 recipients show minimal up-regulation of MHC Class II, Stat1, PD-L1, and B7-2 (Figure 5E).
In summary, by studying monoclonal populations of EAE-inducing T cells, we are able to directly address the issue of which subset of T cells and which sets of genes are critical in the effector phase of EAE. We found that there is a spectrum of T cells that can induce disease, and that disease characteristics vary depending on the set of genes expressed and induced. Although the heterogeneous T cell line produced IL-17 protein, none of the five encephalitogenic clones secreted IL-17. The neutrophil-dominated inflammatory reaction mediated by clone 3—8 is perhaps the most striking example of dissociation of IL-17 from a “TH17-like” reaction, while the TH1 clones and the (IFNγ-independent) IL-12-associated pathway utilized by GKO clone X2.51 represent inflammatory pathways unrelated to TH17 and IL-17. These results emphasize the multiplicity of inflammatory pathways that contribute to autoimmune disease.
Acknowledgments
This work is supported by research grants NMSS RG 3871A4 and NIH NS 042900.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abromson-Leeman S, Alexander J, Bronson R, Carroll J, Southwood S, Dorf M. Experimental autoimmune encephalomyelitis-resistant mice have highly encephalitogenic myelin basic protein (MBP)-specific T cell clones that recognize a MBP peptide with high affinity for MHC class II. J Immunol. 1995a;154:388–398. [PubMed] [Google Scholar]
- Abromson-Leeman S, Bronson R, Dorf ME. Experimental autoimmune peripheral neuritis induced in BALB/c mice by myelin basic protein-specific T cell clones. J Exp Med. 1995b;182:587–592. doi: 10.1084/jem.182.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abromson-Leeman S, Bronson R, Luo Y, Berman M, Leeman R, Leeman J, Dorf M. T-cell properties determine disease site, clinical presentation, and cellular pathology of experimental autoimmune encephalomyelitis. Am J Pathol. 2004;165:1519–1533. doi: 10.1016/S0002-9440(10)63410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Kastelein RA. TGF-beta, a ‘double agent’ in the immune pathology war. Nat Immunol. 2006;7:557–559. doi: 10.1038/ni0606-557. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Gaffen SL, Kramer JM, Yu JJ, Shen F. The IL-17 cytokine family. Vitam Horm. 2006;74:255–282. doi: 10.1016/S0083-6729(06)74010-9. [DOI] [PubMed] [Google Scholar]
- Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007a doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007b;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Smith E, Stark MA. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunol Res. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Muller DM, Pender MP, Greer JM. A neuropathological analysis of experimental autoimmune encephalomyelitis with predominant brain stem and cerebellar involvement and differences between active and passive induction. Acta Neuropathol (Berl) 2000;100:174–182. doi: 10.1007/s004019900163. [DOI] [PubMed] [Google Scholar]
- Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor-alpha-induced elaboration of proangiogenic factors from fibroblasts. Immunol Lett. 2004;93:39–43. doi: 10.1016/j.imlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007 doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MS, Yamasaki A, Yang J, Shan L, Halayko AJ, Gounni AS. IL-17A induces eotaxin-1/CC chemokine ligand 11 expression in human airway smooth muscle cells: role of MAPK (Erk1/2, JNK, and p38) pathways. J Immunol. 2006;177:4064–4071. doi: 10.4049/jimmunol.177.6.4064. [DOI] [PubMed] [Google Scholar]
- Sobel RA. Genetic and epigenetic influence on EAE phenotypes induced with different encephalitogenic peptides. J Neuroimmunol. 2000;108:45–52. doi: 10.1016/s0165-5728(99)00270-2. [DOI] [PubMed] [Google Scholar]
- Suryani S, Sutton I. An interferon-gamma-producing Th1 subset is the major source of IL-17 in experimental autoimmune encephalitis. J Neuroimmunol. 2007;183:96–103. doi: 10.1016/j.jneuroim.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol Lett. 2005;98:189–193. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]