Abstract
Background: Postmenopausal hormone therapy has been examined extensively in relation to cardiovascular disease. However, research relating serum levels of sex hormones to cardiovascular disease is sparse, and the results are inconclusive.
Methods: We measured sex hormones in longitudinally collected samples of 180 postmenopausal women, 91 randomized to 17β-estradiol and 89 to placebo, in the Estrogen in the Prevention of Atherosclerosis Trial. Repeated measures of sex hormone levels were tested for an association with carotid artery intima-media thickness (CIMT), which was also assessed longitudinally over 2 yr.
Results: In all women, changes in serum estrone (P = 0.02), total estradiol (P = 0.01), free estradiol (P = 0.02), and SHBG (P = 0.005) were significantly inversely associated with CIMT progression, controlling for age and body mass index. All the estrogen compounds and SHBG were significantly inversely related with low-density lipoprotein cholesterol and positively associated with high-density lipoprotein cholesterol (all P < 0.0001), whereas free testosterone was positively related with low-density lipoprotein cholesterol and inversely associated with high-density lipoprotein cholesterol (P < 0.003). Despite an increase in serum-free estradiol with estradiol therapy, women with unchanged SHBG and free testosterone levels had an average (se) progression in CIMT of 8.53 (4.72) μm/yr, whereas women with increased free estradiol and SHBG and decreased free testosterone had the largest reduction in CIMT progression [−5.45 (2.77) μm/yr; trend P = 0.03].
Conclusion: Estrogen and SHBG are associated with reduced subclinical atherosclerosis progression in healthy postmenopausal women. These associations are partially mediated by their beneficial effects on lipids. Among women taking estradiol, the most beneficial hormone profile for CIMT progression was increased free estradiol and SHBG with concomitant decreased free testosterone.
Postmenopausal women are at greater risk for cardiovascular disease (CVD) than premenopausal women. A study comparing serum levels of sex hormones in postmenopausal women with and without hormone therapy to atherosclerosis demonstrates that an increase in estrogens and SHBG levels are significantly associated with reduced progression of carotid intima media thickness, a CVD indicator.
The transition to menopause initiates a remarkable change in the endogenous sex hormone milieu in women, characterized by markedly decreased production of 17β-estradiol (estradiol), whereas androgen production from the adrenal cortex and to some extent from ovarian stromal cells remains fairly steady (1). Postmenopausal women have greater morbidity and mortality from cardiovascular disease (CVD), compared with premenopausal women (2,3). In addition to age, increased CVD may be partially attributable to the endogenous sex hormone level changes initiated by menopause.
Several studies have examined the relationship among endogenous sex hormones and atherosclerosis (4,5,6), CVD (7,8), and mortality (9,10,11) in postmenopausal women, with conflicting results. While some studies found that higher levels of androgens and SHBG were associated with a reduced level of atherosclerosis (5,12), others found a positive association between testosterone and CVD risk (7,8).
To our knowledge, no epidemiological study has demonstrated an association between serum estrogen levels and CVD in postmenopausal women. It is possible that the low levels of postmenopausal estrogen may not have an influence on atherosclerosis or CVD, relative to premenopausal levels. Another possible explanation for null findings could be use of a single measurement of serum hormones, which may not be adequate to account for the relatively high intraindividual variation in postmenopausal estradiol levels (13).
We used longitudinal data from the Estrogen in the Prevention of Atherosclerosis Trial (EPAT) to evaluate changes in sex hormone concentrations in relation to subclinical atherosclerosis progression in postmenopausal women. EPAT was a randomized, double-blind, placebo-controlled trial that showed that estradiol treatment significantly reduced progression of subclinical carotid artery atherosclerosis in postmenopausal women (14). We also examined how estradiol therapy (ET) altered levels of sex hormones and SHBG over 2 yr and whether estrogen, testosterone, and SHBG interacted with each other to exert an impact on subclinical atherosclerosis progression. This is the first study to relate sex hormone dynamics to subclinical atherosclerosis progression longitudinally.
Subjects and Methods
The EPAT study design has been described elsewhere (14). In brief, EPAT was a randomized, double-blind, placebo-controlled clinical trial designed to evaluate the impact of unopposed estradiol on the progression of atherosclerosis in postmenopausal women who had no clinical evidence of cardiovascular disease. A total of 222 women were randomized to either placebo or active treatment of unopposed oral micronized estradiol (1 mg/d). Women were eligible if their serum estradiol level was less than 20 pg/ml, low-density lipoprotein cholesterol (LDL-C) level 130 mg/dl or greater, and fasting blood glucose level less than 200 mg/dl and were not current smokers. Randomized participants were followed up with clinic visits every month for the first 6 months and then every other month for the remainder of the 2-yr trial period. Blood pressure, body weight, and body mass index (BMI) were measured at each clinic visit. Blood samples were collected in the morning at each visit after an 8-h fast. All study participants gave written informed consent. The study was approved by the Institutional Review Board of the University of Southern California.
Carotid ultrasonography and image analysis
High-resolution B-mode ultrasound images for carotid artery intima-media thickness (CIMT) measurements of the right common carotid artery were obtained, as previously described, at two prerandomization visits and every 6 months during the trial (14). Average CIMT was measured over 70–100 points covering a 1-cm length just distal to the carotid artery bulb.
Laboratory measurements
Sex hormone levels were measured at the end of the study from stored samples frozen at −70 C. Serum levels of androstenedione, dehydroepiandrosterone (DHEA), testosterone, estrone, and estradiol were quantified by validated, previously described RIA methods (15). Before RIA, steroids were extracted from serum with hexane-ethyl acetate (3:2). Androstenedione, DHEA, and total testosterone were then separated by Celite column partition chromatography using increasing concentrations of toluene in trimethylpentane. Estrone and estradiol were separated in a similar fashion by use of ethyl acetate in trimethylpentane. SHBG was quantified by direct immunoassays using the Immulite analyzer (Diagnostic Products Corp., Inglewood, CA). Free testosterone was calculated using total testosterone and SHBG concentrations and an assumed constant for albumin in a validated algorithm (16,17). Free estradiol was calculated in a similar manner.
All immunoassay methods were shown to be reliable. Specificity of the RIAs were achieved by use of highly specific antisera and use of organic solvent extraction and chromatography before quantification of the analytes. Assay accuracy was established by demonstrating parallelism between measured concentrations of the serially diluted analyte in serum with the corresponding standard curve. Intra- and interassay coefficients of variation ranged from 4 to 8% and 8 to 13%, respectively. All assay methods were found to be sensitive. The sensitivity of a RIA method was determined by the smallest amount of analyte that reduced the number of counts per minute of the radiolabeled analyte at zero mass by 2 sd.
Fasting plasma total cholesterol and total triglyceride (TG) levels were measured using an enzymatic method under the standardization program of the Centers for Disease Control and Prevention. High-density lipoprotein cholesterol (HDL-C) levels were measured after apolipoprotein B-containing lipoproteins were precipitated in whole plasma with heparin manganese chloride. LDL-C levels were estimated using the Friedewald equation.
Fasting insulin levels were measured by direct RIA. Fasting serum glucose levels were measured by the glucose oxidase technique using the Beckman Glucose II analyzer (Beckman Instruments, Brea, CA). Hemoglobin A1C levels were measured by HPLC (Diamat, Bio-Rad Corp., Hercules, CA).
Statistical analysis
Baseline, average on-trial, and changes from baseline to average on-trial levels of sex hormones were compared between the treatment groups using independent t tests. Spearman correlation coefficients were used to assess the correlations among the changes in the hormone levels.
Associations between the CIMT progression rate and changes in sex hormone levels were tested using a mixed-effects model in which the serial follow-up measures of CIMT were the dependent variable and the primary explanatory variables were follow-up time in years, changes in the serum hormone levels, and their interaction with follow-up time. Changes in the sex hormone measures were modeled as continuous variables. The average annual rate of CIMT progression among women with no change in sex hormones from baseline was estimated from the regression coefficient associated with the main effect of follow-up time. The rate of CIMT progression associated with a per unit change in the sex hormone levels was estimated from the regression coefficient associated with the interaction terms of change in sex hormone levels × follow-up time. Each sex hormone was modeled separately for its association with CIMT progression. Hormones were first modeled unadjusted for any factors. Subsequent models included age at randomization and change in BMI (baseline to on trial) as covariates. Race, former smoking, and alcohol intake were not significantly associated with CIMT progression, and including them in the multivariate model did not alter the impact of sex hormones on CIMT progression. Therefore, these variables were not included in the multivariate model.
Repeated measures of sex hormones and serum lipids (HDL-C, LDL-C, TG), and carbohydrate-related factors (glucose, insulin, hemoglobin A1C) were correlated using generalized estimating equations.
Changes in lipids and carbohydrate-related factors were then included in the models correlating sex hormone levels and CIMT progression to evaluate whether these factors were significant intermediate mediators. These factors were introduced one at a time in addition to age and BMI. Only those lipids or factors related to carbohydrate metabolism that substantially (>10% change in the β-estimates) altered the relationship between sex hormones and CIMT progression were considered intermediate mediators.
Analyses were also performed stratified by treatment groups and lipid-lowering medication intake within each treatment group. Four women in the placebo group who used exogenous estrogens during the trial were excluded from stratified analysis performed among the placebo group.
To examine the patterns of sex hormone changes in relation to CIMT progression among ET users, we categorized the change in each sex hormone from baseline for each subject into three groups: increase, no change, or decrease. We used the within-subject sd (over repeated measures) in the placebo group to define the change categories (increase = > 1 sd change; decrease = < 1 sd change, no change = between ± 1 sd). Categorical hormone change variables were then tested in different combinations for an association with CIMT progression using the mixed-effects model. All analyses used SAS (version 8.01; Cary, NC); two-sided P values are reported.
Results
Baseline and follow-up data on CIMT and serum levels of sex hormones and SHBG were available on 180 women, 89 in the placebo and 91 in the estradiol group. The average (sd) age of the women was 61.5 (6.8) yr, predominantly non-Hispanic white (61%), and moderately overweight with an average (sd) BMI of 28.8 (5.4) kg/m2 (Table 1). Average LDL-C level at baseline was above the normal range [164.1 (27.9) mg/dl] because only women with LDL-C 130 mg/dl or greater were included in the trial by design. Of the 42 women not included in the analysis, 23 did not have repeat CIMT measure, and 19 did not have a repeat sex hormone measure. Demographic and clinical characteristics of the 42 women not included in the analysis were not statistically different from the 180 women included in the analysis.
Table 1.
Baseline characteristics of EPAT women (n = 180)a
| Characteristics | |
|---|---|
| Age | 61.5 (6.8)b |
| Race | |
| Non-Hispanic white | 109 (61%) |
| African-American | 19 (11%) |
| Hispanic | 35 (19%) |
| Others | 17 (9%) |
| Smoking | |
| Never smoker | 93 (52%) |
| Ex-smoker | 87 (48%) |
| Alcohol intake (at least weekly) | 132 (73%) |
| CIMT (μm) | 763.5 (133.3) |
| BMI (kg/m2) | 28.8 (5.4) |
| Blood pressure (mm Hg) | 127/77 (15.4/8.7) |
| HDL-C (mg/dl) | 53.8 (12.1) |
| LDL-C (mg/dl) | 164.1 (27.9) |
| Glucose (mg/dl) | 89.7 (11.9) |
| Insulin (μIU/ml) | 9.7 (6.1) |
Sample includes 180 women with baseline and at least one on-trial measure of CIMT and serum level of sex hormones.
Mean (sd) or n (%).
Table 2 compares the mean (sd) levels of sex hormones at baseline, on-trial, and on-trial changes from baseline between the treatment groups. Compared with placebo, women randomized to estradiol had significant increases in total estradiol, free estradiol, estrone, and SHBG and significant decreases in free testosterone, androstenedione, and DHEA levels (P < 0.0001). On-trial changes in total testosterone did not differ between groups (P = 0.17).
Table 2.
Serum levels of sex hormones and SHBG by treatment group
| Hormones | Placebo (n = 89) | Estradiol (n = 91) | P valuea |
|---|---|---|---|
| Estrone (pg/ml) | |||
| Baseline | 39.8 (13.3)b | 47.0 (31.2) | 0.05 |
| Follow-up | 48.1 (42.0) | 310.3 (167.2) | <0.0001 |
| Change | 8.4 (41.9) | 263.4 (165.6) | <0.0001 |
| Estradiol (pg/ml) | |||
| Baseline | 19.1 (5.3) | 21.4 (19.7) | 0.27 |
| Follow-up | 21.1 (10.2) | 68.2 (16.2) | <0.0001 |
| Change | 2.0 (9.4) | 46.8 (28.0) | <0.0001 |
| Free estradiol (pg/ml) | |||
| Baseline | 0.5 (0.2) | 0.6 (0.5) | 0.25 |
| Follow-up | 0.6 (0.2) | 1.6 (0.6) | <0.0001 |
| Change | 0.05 (0.2) | 0.9 (0.7) | <0.0001 |
| Total testosterone (ng/dl) | |||
| Baseline | 22.0 (9.1) | 21.5 (10.8) | 0.73 |
| Follow-up | 22.3 (9.0) | 22.8 (10.8) | 0.72 |
| Change | 0.3 (3.6) | 1.3 (6.2) | 0.17 |
| Free testosterone (pg/ml) | |||
| Baseline | 4.0 (1.7) | 4.1 (2.1) | 0.93 |
| Follow-up | 4.1 (1.8) | 3.1 (1.6) | 0.0001 |
| Change | 0.05 (0.9) | −0.9 (1.4) | <0.0001 |
| Androstenedione (pg/ml) | |||
| Baseline | 535 (228) | 544 (250) | 0.09 |
| Follow-up | 522 (189) | 492 (180) | <0.0001 |
| Change | −13 (167) | −51 (175) | <0.0001 |
| DHEA (ng/ml) | |||
| Baseline | 2.37 (1.57) | 2.23 (1.45) | 0.09 |
| Follow-up | 2.14 (1.34) | 1.77 (0.78) | <0.0001 |
| Change | −0.23 (0.98) | −0.46 (1.05) | <0.0001 |
| SHBG (nmol/liter) | |||
| Baseline | 35.2 (14.5) | 35.2 (18.5) | 0.90 |
| Follow-up | 36.5 (18.2) | 58.2 (23.6) | <0.0001 |
| Change | 1.2 (12.9) | 23.2 (17.7) | <0.0001 |
P values from t test for independent samples.
Mean (sd).
In the total sample (n = 180), on-trial changes in estrone (P = 0.03), total estradiol (P = 0.01), free estradiol (P = 0.02), and SHBG (P = 0.007) were significantly inversely related to CIMT progression, whereas total testosterone had a borderline significant association (P = 0.06) (Table 3). Changes in free testosterone, androstenedione, and DHEA did not have any significant association with CIMT progression. Similar associations were observed adjusting for age and BMI.
Table 3.
Mixed models relating changes in sex hormones/SHBG and CIMT progression
| On-trial changes in hormones | Unadjusted
|
Adjusted for age and BMI
|
Adjusted for age, BMI, LDL-C, and HDL-C
|
|||
|---|---|---|---|---|---|---|
| Estimate (se)a | P value | Estimate (se)a | P value | Estimate (se)a | P value | |
| Estrone (pg/ml) | −0.018 (0.008) | 0.03 | −0.018 (0.008) | 0.02 | −0.003 (0.008) | 0.73 |
| Total E2 (pg/ml) | −0.118 (0.05) | 0.01 | −0.119 (0.05) | 0.01 | −0.01 (0.05) | 0.82 |
| Free E2 (pg/ml) | −4.97 (2.17) | 0.02 | −4.95 (2.18) | 0.02 | −0.32 (2.2) | 0.88 |
| Total T (ng/dl) | −0.526 (0.28) | 0.06 | −0.531 (0.28) | 0.06 | −.51 (0.25) | 0.04 |
| Free T (pg/ml) | 0.384 (1.1) | 0.73 | 0.403 (1.12) | 0.72 | −.72 (1.04) | 0.50 |
| Androstenedione (pg/ml) | 0.0045 (0.008) | 0.59 | −0.005 (0.008) | 0.55 | −0.01 (0.01) | 0.32 |
| DHEA (ng/ml) | 0.234 (1.4) | 0.87 | 0.102 (1.43) | 0.94 | −0.62 (1.34) | 0.64 |
| SHBG (nmol/liter) | −0.205 (0.08) | 0.007 | −0.212 (0.08) | 0.005 | −0.08 (0.08) | 0.29 |
E2, Estradiol; T, testosterone.
CIMT progression rate (micrometers per year) per unit change in the sex hormone levels.
Estrogen and SHBG were significantly inversely associated with LDL-C and positively related to HDL-C (Table 4). In contrast, free testosterone and DHEA were significantly positively related to LDL-C and inversely associated with HDL-C. A moderate positive relationship was observed between estrogens and TG.
Table 4.
Relationship of serum sex hormones/SHBG levels to serum lipids and triglycerides
| Sex hormones | LDL-C (mg/dl)
|
HDL-C (mg/dl)
|
TG (mg/dl)
|
|||
|---|---|---|---|---|---|---|
| β-Estimate | P value | β-Estimate | P value | β-Estimate | P value | |
| Estrone (pg/ml) | −0.06 (0.01) | <0.0001 | 0.02 (0.002) | <0.0001 | 0.02 (0.01) | 0.10 |
| Total E2 (pg/ml) | −0.34 (0.03) | <0.0001 | 0.09 (0.01) | <0.0001 | 0.13 (0.08) | 0.10 |
| Free E2 (pg/ml) | −14.1 (1.4) | <0.0001 | 3.82 (0.57) | <0.0001 | 5.74 (3.1) | 0.06 |
| Total testosterone (ng/dl) | −0.12 (0.13) | 0.34 | 0.11 (0.06) | 0.07 | −0.40 (0.36) | 0.28 |
| Free testosterone (pg/ml) | 2.36 (0.77) | 0.002 | −1.36 (0.34) | <0.0001 | 0.20 (1.6) | 0.93 |
| Androstenedione (pg/ml) | 0.001 (0.005) | 0.83 | −0.007 (0.002) | 0.0005 | 0.004 (0.01) | 0.79 |
| DHEA (ng/ml) | 2.5 (0.82) | 0.002 | −1.58 (0.38) | <0.0001 | −1.15 (1.8) | 0.52 |
| SHBG (nmol/liter) | −0.41 (0.05) | <0.0001 | 0.15 (0.02) | <0.0001 | −0.10 (0.13) | 0.44 |
Generalized estimating equation models adjusted for age, BMI, and treatment group. E2, Estradiol.
The addition of LDL-C and HDL-C as independent variables in the models substantially attenuated (>10%) the relationship between CIMT progression and levels of estrogens and SHBG (Table 3). Total testosterone remained significantly inversely associated with CIMT progression after controlling for LDL-C and HDL-C (P = 0.05, Table 3). Adjustment for fasting glucose, insulin and hemoglobin A1C levels did not substantially alter the estimates of association of sex hormones and SHBG with CIMT progression.
Among women randomized to estradiol, only changes in SHBG were significantly associated with CIMT progression (P = 0.05, Table 5). In the placebo group, total testosterone (P = 0.05) was the single significant inverse correlate of CIMT progression adjusted for age and BMI.
Table 5.
Mixed models relating changes in the sex hormones/SHBG and CIMT progression, stratified by treatment group
| On-trial changes in hormones | Placebo (n = 85)a
|
Estradiol (n = 91)
|
||
|---|---|---|---|---|
| CIMT progression rate/yr (se)b | P value | CIMT progression rate/yr (se)b | P value | |
| Estrone (pg/ml) | −0.03 (0.08) | 0.75 | −0.10 (0.06) | 0.19 |
| Total E2 (pg/ml) | −0.70 (0.46) | 0.13 | −0.09 (0.07) | 0.18 |
| Free E2 (pg/ml) | −22.9 (15.8) | 0.15 | −3.29 (2.9) | 0.26 |
| Total T (ng/dl) | −1.23 (0.64) | 0.05 | −0.29 (0.28) | 0.30 |
| Free T (pg/ml) | −4.58 (2.7) | 0.09 | 0.61 (1.2) | 0.61 |
| Androstenedione (pg/ml) | 0.003 (0.01) | 0.83 | −0.02 (0.01) | 0.12 |
| DHEA (ng/ml) | 1.23 (2.38) | 0.60 | −1.1 (1.65) | 0.51 |
| SHBG (nmol/liter) | −0.12 (0.26) | 0.65 | −0.20 (0.10) | 0.046 |
Each model adjusted for age and BMI. E2, Estradiol; T, testosterone.
Placebo women (n = 4) who took exogenous estrogens during the trial were excluded.
CIMT progression rate (micrometers per year) per unit change in the sex hormone levels.
Stratified analysis by lipid-lowering medication use did not reveal any significant interaction such that the hormone associations with CIMT progression did not differ by lipid-lowering medication use (all interaction P > 0.10; data not shown).
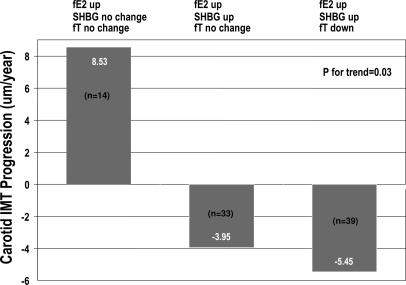
Despite the increased free estradiol levels with ET, women with no change in SHBG and free testosterone had an average progression in CIMT over the trial [mean (se) 8.53 (4.72) μm/yr] (Fig. 1). Women with increased free estradiol and SHBG but no change had reduction in CIMT progression of −3.95 (3.03) μm/yr. However, women receiving ET with increased free estradiol and SHBG and decreased free testosterone had the largest reduction of −5.45 (2.77) μm/yr. The decreasing trend in CIMT progression rate across the three aforementioned groups was statistically significant (P = 0.03). When baseline clinical parameters were compared across the three aforementioned groups, age, BMI, and serum levels of glucose, insulin, free estradiol, and total and free testosterone were significantly higher in the group of women with the greatest reduction in CIMT progression (Table 6). Serum SHBG levels were significantly lower in this group of women, compared with the other two groups.
Figure 1.
Change in sex hormones combined with ET and CIMT progression. For each subject, the change in each sex hormone from baseline was categorized using the within-subject sd (over repeated measures) in the placebo group (up = > 1 sd change; down = < 1 sd change; no change = between ± 1 sd). fE2, Free estradiol; fT, free testosterone.
Table 6.
Comparison of baseline clinical parameters across three groups of estradiol-treated women categorized by sex hormone changes in response to ET
| Clinical factors | Free E2 increased, free T not changed, SHBG not changed (n = 14) | Free E2 increased, free T not changed, SHBG increased (n = 33) | Free E2 increased, free T decreased, SHBG increased (n = 39) | P value for difference |
|---|---|---|---|---|
| Age (yr) | 57.4 (1.7) | 59.9 (1.1) | 62.7 (1.0) | 0.02 |
| BMI (kg/m2) | 29.6 (1.5) | 27.3 (1.0) | 30.5 (0.9) | 0.05 |
| IMT (μm) | 758.8 (29.3) | 740.3 (19.1) | 761.6 (17.6) | 0.70 |
| LDL-C (mg/dl) | 168.3 (7.9) | 165.1 (5.2) | 167.4 (4.7) | 0.92 |
| HDL-C (mg/dl) | 54.3 (3.2) | 57.0 (2.1) | 51.5 (1.9) | 0.17 |
| TG (mg/dl) | 144.7 (17.8) | 147.2 (11.6) | 180.6 (10.6) | 0.07 |
| Glucose (mg/dl) | 87.7 (2.9) | 85.2 (1.8) | 92.6 (1.7) | 0.01 |
| Insulin (mg/dl) | 7.8 (1.3) | 8.0 (0.8) | 10.8 (0.7) | 0.03 |
| Hemoglobin A1C (%) | 4.9 (0.2) | 4.9 (0.1) | 5.1 (0.1) | 0.41 |
| E2 (pg/ml) | 17.8 (1.7) | 17.6 (1.1) | 21.0 (1.0) | 0.07 |
| Free E2 (pg/ml) | 0.5 (0.06) | 0.5 (0.04) | 0.6 (0.03) | 0.02 |
| T (ng/dl) | 17.3 (2.5) | 16.6 (1.6) | 25.5 (1.5) | 0.0002 |
| Free T (pg/ml) | 3.2 (0.5) | 2.9 (0.32) | 5.2 (0.3) | <0.0001 |
| Androstenedione (pg/ml) | 589.3 (68.3) | 500.5 (44.5) | 581.9 (40.9) | 0.34 |
| DHEA (ng/ml) | 2.6 (0.4) | 2.1 (0.3) | 2.4 (0.3) | 0.59 |
| SHBG (nmol/liter) | 30.8 (4.1) | 38.7 (2.7) | 29.0 (2.4) | 0.03 |
E2, Estradiol; T, testosterone.
When changes in SHBG (P = 0.16) and total estradiol (P = 0.47) were included in a single model, neither had significant association with CIMT progression. However, SHBG changes (P = 0.01) still had significant association with CIMT progression after adjusting for total testosterone (P = 0.17).
Change in total estradiol (P = 0.02) remained significantly inversely associated with CIMT progression after controlling for change in total testosterone (P = 0.09). Total estradiol (P = 0.41), total testosterone (P = 0.15), and SHBG (P = 0.27) were not significantly associated with CIMT progression when tested in a single multivariate model (data not shown).
Discussion
Our results suggest that increases in estrogens, total testosterone, and SHBG levels over 2 yr in postmenopausal women with and without ET are significantly associated with reduced progression of CIMT after controlling for age and BMI. Possible mechanisms for these associations could be modulation of atherosclerosis risk factors including: increasing HDL-C and insulin sensitivity and decreasing LDL-C, glucose, insulin, and hemoglobin A1C. Our data support the mediating role of lipids but not carbohydrate-related factors in this association.
Although lower circulating estrogens have been found to be significantly associated with more severe angiographically determined coronary artery disease in premenopausal women (18), no study has been able to find such a relationship in postmenopausal women (19). Two prospective studies investigating the impact of sex hormones on cardiovascular mortality did not find any of the sex hormones to be a significant predictor of cardiovascular death in postmenopausal women (9,10). This null finding may be in part explained by lack of prospective data measuring serum estrogens more than one time because postmenopausal estradiol levels show relatively high intraindividual variation (13). In addition, the low serum estrogen levels (<25 pg/ml) in postmenopausal women result in low interindividual variability at these physiological levels than evident in premenopausal women, making it difficult to determine relationships between estrogen and CVD. Our data support this fact because we did not observe any effect of estrogens on CIMT progression among the women not receiving ET (although the regression estimates indicated an inverse association, Table 5).
Most of the previous studies evaluating the relationship between estrogens and CVD in postmenopausal women excluded women taking hormone therapy (HT). A nested case-control study performed among women in the Women’s Health Study included both HT users and nonusers to examine the relationship between sex hormone levels and risk of cardiovascular events (8). Results were reported stratified by HT use showing no association between estradiol and cardiovascular events in either of the groups, which is consistent with the null finding in the placebo and estradiol groups of our study. However, the authors did not report results in the combined population, which could depict the impact of a wide range of estrogen levels (physiological and pharmacological) on CVD.
In addition to estrogens, our results indicate that total testosterone and SHBG are also inversely related to progression of carotid atherosclerosis. This finding is consistent with the existing literature. A case-control study from the Atherosclerosis Risk in Communities cohort reported that postmenopausal women (not on HT) in the highest quartiles of total testosterone and SHBG had significantly lower odds of atherosclerosis measured by a single CIMT greater than the 95th percentile adjusted for a multitude of cardiovascular risk factors (4). The inverse association between SHBG and CVD among postmenopausal women has also been reported (5,8). At physiologic concentrations, total testosterone has been found to be protective against carotid atherosclerosis and cardiovascular disease in postmenopausal women (12,20). Our longitudinal results extend these data and indicate that a higher total testosterone level is protective against subclinical atherosclerosis progression at physiological concentrations (in the placebo group) as well as in the total sample, half of whom were on HT. This association was independent of age, BMI, HDL-C, and LDL-C (P = 0.04, Tables 3 and 5).
Total testosterone also showed a beneficial association with HDL-C, whereas free testosterone had a detrimental association with serum cholesterol (Table 4). It is interesting to note that at least two epidemiological studies in postmenopausal women with established CVD found a positive association between free testosterone and angiographically determined coronary artery disease (7,8). Although we did not observe any significant association of free testosterone with CIMT progression, we did observe a diverging relationship of total vs. free testosterone on serum lipids. It is known that unbound or free testosterone is the functionally active form that can bind with the androgen receptor, whereas the bound form is not functionally active. Higher levels of total testosterone, 98% of which is bound with SHBG or albumin, could be an indicator of increased SHBG concentration. SHBG was significantly inversely correlated with free testosterone concentration as well as CIMT progression and had a beneficial association with serum cholesterols.
SHBG regulates the concentration of bioavailable estrogens and androgens by binding with testosterone and estradiol proportionately (21). Because exogenous estrogen induces SHBG production, evidenced in this study and by others (22), and testosterone binds to SHBG with a greater affinity than estrogen, there may be a relative reduction in the concentration of free testosterone (Table 2). Therefore, any detrimental effect of free testosterone on CVD and/or risk factors, as reported by some investigators (7,8), may also be reduced. However, this hypothesis is yet to be examined in a properly designed study.
To explore possible interactive dynamics among estrogen, testosterone, and SHBG, we modeled the changes in these three components of the sex hormone milieu among estradiol-treated women. We found that women who had increased free estradiol and SHBG and decreased free testosterone showed the largest regression in CIMT, compared with women who had increased free estradiol only (Fig. 1). There was a significant trend (P = 0.03) in the reduction of CIMT progression among the three groups of women shown in Fig. 1. However, the interaction term (free estradiol × SHBG × free testosterone) was not statistically significant (P = 0.17), with small sample size being an issue in this analysis. It is interesting to note that older, overweight women with higher pretreatment levels of both estrogens and testosterone and lower levels of SHBG benefited the most from ET (Table 6). CIMT was not significantly different across these three groups before taking ET, indicating that higher free testosterone levels was not related with CIMT when estradiol levels were low without ET. A ratio of free testosterone to estradiol and to SHBG could explain these interactions, but we did not find any association between CIMT progression and the ratios mentioned above either at baseline or over the follow-up period (data not shown). It is not clear from the pretreatment clinical profile of these three groups of women as to why they responded differently to ET in terms of attaining serum sex hormone levels. Although their serum glucose and insulin levels were relatively higher, compared with the other women, those values were not out of the normal range. These results need to be examined in larger samples of postmenopausal women.
Cardiovascular effects of estrogen can be classified as indirect and direct (23). Indirect effects include increased HDL and reduced LDL, whereas direct effects include genomic and nongenomic mechanisms. In this study we measured only the indirect effects. In EPAT, women treated with estradiol had significant reductions in LDL-C and insulin and increases in HDL-C, compared with placebo-treated subjects (14). In the current study, inclusion of LDL-C and HDL-C in the multivariate models attenuated the relationship between serum levels of estrogens and SHBG and CIMT progression (Table 3), indicating that the effect of estrogen and SHBG levels on CIMT progression can be partially mediated by alteration in serum lipid levels, particularly LDL-C and HDL-C (Table 3). In a previous publication, we have shown that reduction in carotid atherosclerosis progression among women treated with estradiol in EPAT was explained by decreases in LDL-C and increases in HDL-C (24). In that analysis, we also observed that alterations in glucose and insulin did not explain the beneficial effect of estradiol on subclinical atherosclerosis progression despite significant reduction in these metabolites among women randomized to estradiol treatment. These carbohydrate-related factors also did not play a role in the effect of serum sex hormone levels on subclinical atherosclerosis progression in the current analysis.
An important strength of our study is the longitudinal data collection with serial measurements of sex hormones and CIMT over 2 yr. The intraindividual variability of the sex hormone levels, particularly estrogens, are expected to be better captured by multiple measurements over time than a single measurement. According to our data, among women not taking exogenous estradiol, within-subject variability of total estradiol over the 2-yr trial was 39% and free estradiol was 33%, whereas that of total testosterone and free testosterone was 15 and 18%, respectively. Among women taking exogenous estradiol, the within-subject variability of total estradiol, free estradiol, total testosterone, and free testosterone was 36, 37, 18, and 25%, respectively. Estrogen concentrations had much greater variability than testosterone, indicating that a single measurement of estrogen in postmenopausal women may not be sufficient for epidemiologic studies. These data are consistent with previous reports showing lower reliability for estradiol, compared with testosterone (13,25).
Because half of our study participants were treated with estradiol, we were able to observe the influence of sex steroid hormones and SHBG on subclinical atherosclerosis at pharmacologic levels (estradiol treated group) as well as physiological levels (placebo treated group). Combining these two groups, we also had the capability to study the effect of a wide range of sex hormone levels on atherosclerosis progression.
A limitation of the analysis is that 62% of the women were on lipid-lowering medication. However, stratified analysis by lipid-lowering medication intake within each treatment group did not show significant variation in the sex hormones and atherosclerosis association (all P values for interactions > 0.10). However, the relatively small sample size to detect such interactions is another limitation of this analysis. The power was particularly limited in stratified analysis by treatment group and in analysis of the interaction of changes in sex hormones and SHBG among the estradiol-treated women. These data need to be reproduced in a larger group of women. Progesterone was not measured in this study because women were treated with an estradiol-only regimen in EPAT. It has been suggested that progesterone counteracts the beneficial effects of estrogen on the vascular wall and lipid metabolism (26,27). Clinical trials using a combination of estrogen and progesterone have not shown any significant impact on CVD. In the Postmenopausal Estrogen/Progestin Intervention trial, increase in HDL-C was significantly greater in women taking estradiol-only regimens, compared with estradiol and medroxyprogesterone acetate combination regimen users. It will be of interest to determine how serum estrogen and progesterone levels relate to atherosclerosis among women taking combination HT.
The current uncertainty regarding the effect of HT on CVD has underscored the importance of research evaluating the association between serum sex hormone levels and atherosclerosis in postmenopausal women. To our knowledge, no prior study has used multiple measurements of sex hormones, SHBG, and CIMT to examine their relationship over time in postmenopausal women. We examined an extensive panel of sex steroid hormones for their association with subclinical atherosclerosis. Further longitudinal studies are required to confirm and extend the findings of this study.
Footnotes
First Published Online October 9, 2007
Abbreviations: BMI, Body mass index; CIMT, carotid artery intima-media thickness; CVD, cardiovascular disease; DHEA, dehydroepiandrosterone; EPAT, Estrogen in the Prevention of Atherosclerosis Trial; estradiol, 17β-estradiol; ET, estradiol therapy; HDL-C, high-density lipoprotein cholesterol; HT, hormone therapy; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride.
This work was supported by National Institute of Aging, National Institutes of Health Grant H RO1 AG-18798.
Disclosure Statement: R.K., H.N.H., and W.J.M. have nothing to declare. F.Z.S. served on the advisory board of Novo Nordisk. R.A.L. has research support from Kronos and Wyeth, was expert witness for Wyeth, and served on the advisory boards of Berlex and Organon.
References
- Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG 2004 The menopausal transition: a 9-year prospective population-based study. The Melbourne Women’s Midlife Health Project. Climacteric 7:375–389 [DOI] [PubMed] [Google Scholar]
- Kannel WB, Gordan T 1978 Evaluation of cardiovascular risk in the elderly: the Framingham study. Bull NY Acad Med 54:573–591 [PMC free article] [PubMed] [Google Scholar]
- Kannel WB 1987 Metabolic risk factors for coronary heart disease in women: perspective from the Framingham Study. Am Heart J 114:413–419 [DOI] [PubMed] [Google Scholar]
- Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M 2002 Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol 155:437–445 [DOI] [PubMed] [Google Scholar]
- Reinecke H, Bogdanski J, Woltering A, Breithardt G, Assmann G, Kerber S, von Eckardstein A 2002 Relation of serum levels of sex hormone binding globulin to coronary heart disease in postmenopausal women. Am J Cardiol 90:364–368 [DOI] [PubMed] [Google Scholar]
- Cauley JA, Gutai JP, Glynn NW, Paternostro-Bayles M, Cottington E, Kuller LH 1994 Serum estrone concentrations and coronary artery disease in postmenopausal women. Arterioscler Thromb 14:14–18 [DOI] [PubMed] [Google Scholar]
- Phillips GB, Pinkernell BH, Jing TY 1997 Relationship between serum sex hormones and coronary artery disease in postmenopausal women. Arterioscler Thromb Vasc Biol 17:695–701 [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, Buring JE 2003 Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation 108:1688–1693 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D 1995 Dehydroepiandrosterone sulfate does not predict cardiovascular death in postmenopausal women. The Rancho Bernardo Study. Circulation 91:1757–1760 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D 1995 Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. BMJ 311:1193–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman-Gruen D, Barrett-Connor E 1997 Sex hormone-binding globulin and glucose tolerance in postmenopausal women. The Rancho Bernardo Study. Diabetes Care 20:645–649 [DOI] [PubMed] [Google Scholar]
- Bernini GP, Sgro M, Moretti A, Argenio GF, Barlascini CO, Cristofani R, Salvetti A 1999 Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab 84:2008–2012 [DOI] [PubMed] [Google Scholar]
- Cauley JA, Gutai JP, Kuller LH, Powell JG 1991 Reliability and interrelations among serum sex hormones in postmenopausal women. Am J Epidemiol 133:50–57 [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, Selzer RH, Liu CR, Liu CH, Azen SP 2001 Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 135:939–953 [DOI] [PubMed] [Google Scholar]
- Goebelsmann U, Horton R, Mestman JH, Arce JJ, Nagata Y, Nakamura RM, Thorneycroft IH, Mishell Jr DR 1973 Male pseudohermaphroditism due to testicular 17-hydroxysteroid dehydrogenase deficiency. J Clin Endocrinol Metab 36:867–879 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Goemaere S, Kaufman JM 1999 Testosterone, body composition and aging. J Endocrinol Invest 22:110–116 [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Bairey Merz CN, Johnson BD, Sharaf BL, Bittner V, Berga SL, Braunstein GD, Hodgson TK, Matthews KA, Pepine CJ, Reis SE, Reichek N, Rogers WJ, Pohost GM, Kelsey SF, Sopko G 2003 Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol 41:413–419 [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E 2003 Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 138:1–9 [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Georgiadis EI, Young R, Evagelopoulou C, Khoury S, Katsilambros N, Sowers JR 1995 Relative androgenicity, blood pressure levels, and cardiovascular risk factors in young healthy women. Am J Hypertens 8:606–614 [DOI] [PubMed] [Google Scholar]
- Rosner W 1991 Plasma steroid-binding proteins. Endocrinol Metab Clin North Am 20:697–720 [PubMed] [Google Scholar]
- Tazuke S, Khaw KT, Barrett-Connor E 1992 Exogenous estrogen and endogenous sex hormones. Medicine (Baltimore) 71:44–51 [DOI] [PubMed] [Google Scholar]
- Skafar DF, Xu R, Morales J, Ram J, Sowers JR 1997 Clinical review 91: female sex hormones and cardiovascular disease in women. J Clin Endocrinol Metab 82:3913–3918 [DOI] [PubMed] [Google Scholar]
- Karim R, Mack WJ, Lobo RA, Hwang J, Liu CR, Liu CH, Sevanian A, Hodis HN 2005 Determinants of the effect of estrogen on the progression of subclinical atherosclerosis: Estrogen in the Prevention of Atherosclerosis Trial. Menopause 12:366–373 [DOI] [PubMed] [Google Scholar]
- Toniolo P, Koenig KL, Pasternack BS, Banerjee S, Rosenberg C, Shore RE, Strax P, Levitz M 1994 Reliability of measurements of total, protein-bound, and unbound estradiol in serum. Cancer Epidemiol Biomarkers Prev 3:47–50 [PubMed] [Google Scholar]
- Hanke H, Hanke S, Finking G, Muhic-Lohrer A, Muck AO, Schmahl FW, Haasis R, Hombach V 1996 Different effects of estrogen and progesterone on experimental atherosclerosis in female versus male rabbits. Quantification of cellular proliferation by bromodeoxyuridine. Circulation 94:175–181 [DOI] [PubMed] [Google Scholar]
- Miller VM, Vanhoutte PM 1991 Progesterone and modulation of endothelium-dependent responses in canine coronary arteries. Am J Physiol 261:R1022–R1027 [DOI] [PubMed] [Google Scholar]