Abstract
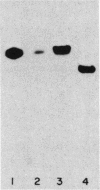
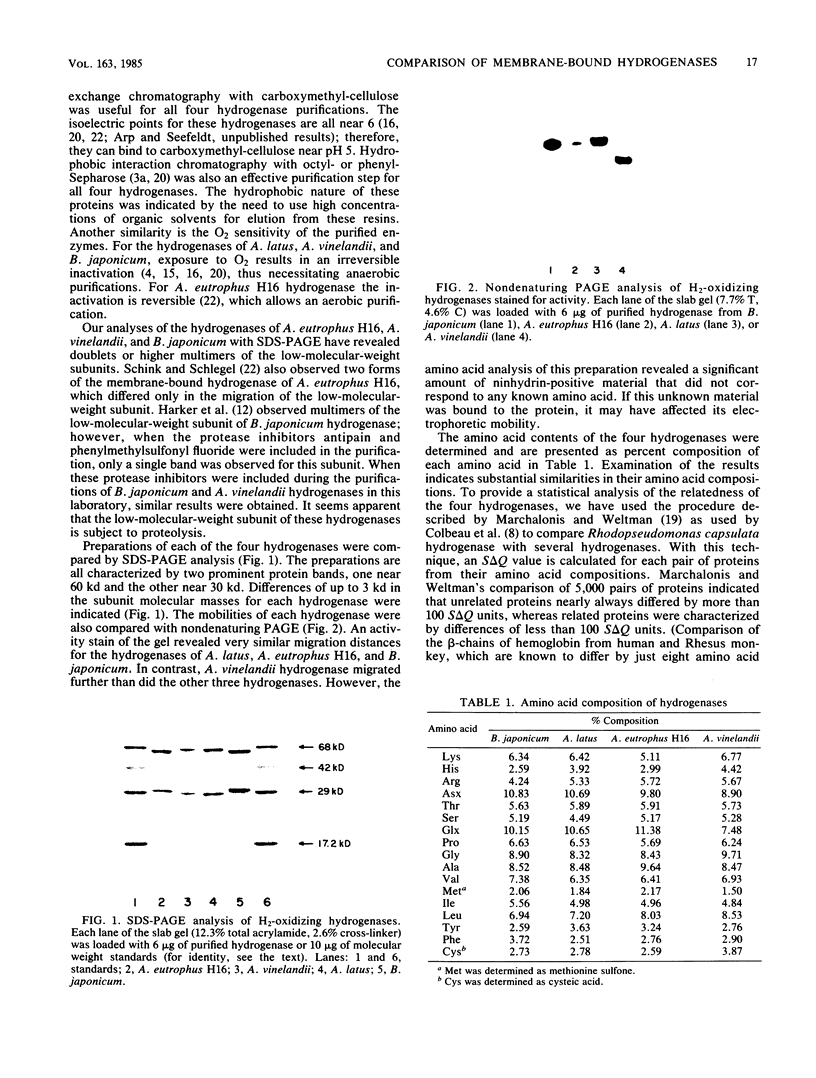
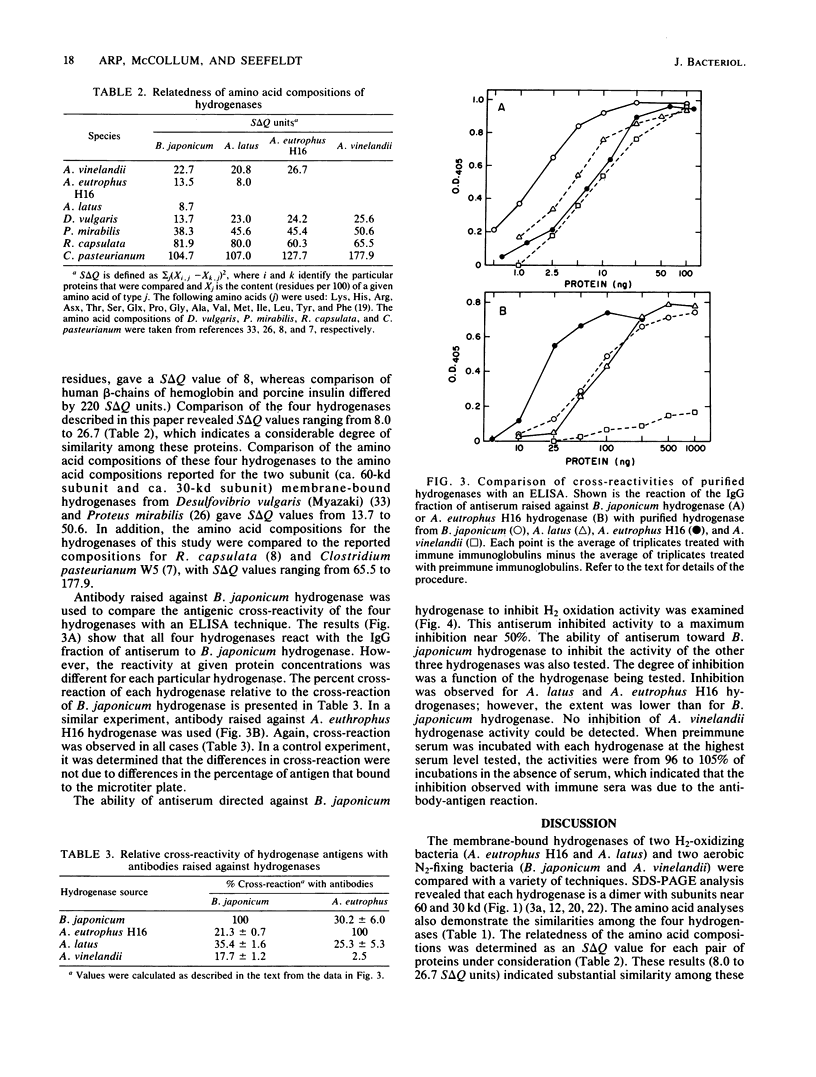
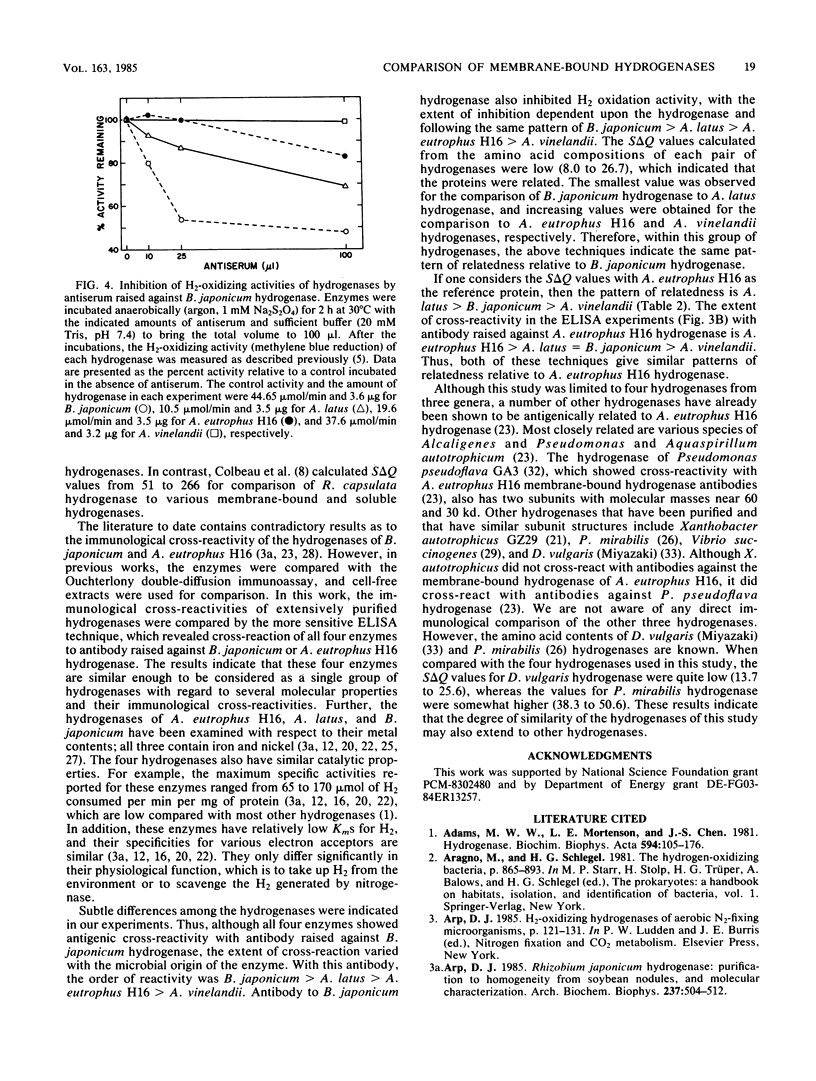
The membrane-bound hydrogenases of Bradyrhizobium japonicum, Alcaligenes eutrophus, Alcaligenes latus, and Azotobacter vinelandii were purified extensively and compared. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of each hydrogenase revealed two prominent protein bands, one near 60 kilodaltons and the other near 30 kilodaltons. The migration distances during nondenaturing polyacrylamide gel electrophoresis were similar for all except A. vinelandii hydrogenase, which migrated further than the other three. The amino acid composition of each hydrogenase was determined, revealing substantial similarity among these enzymes. This was confirmed by calculation of S delta Q values, which ranged from 8.0 to 26.7 S delta Q units. S delta Q is defined as sigma j(Xi,j-Xk,j)2, where i and k identify the proteins compared and Xj is the content (residues per 100) of a given amino acid of type j. The hydrogenases of this study were also compared with an enzyme-linked immunosorbent assay. Antibody raised against B. japonicum hydrogenase cross-reacted with all four hydrogenases, but to various degrees and in the order B. japonicum greater than A. latus greater than A. eutrophus greater than A. vinelandii. Antibody raised against A. eutrophus hydrogenase also cross-reacted with all four hydrogenases, following the pattern of cross-reaction A. eutrophus greater than A. latus = B. japonicum greater than A. vinelandii. Antibody raised against B. japonicum hydrogenase inhibited B. japonicum hydrogenase activity to a greater extent than the A. eutrophus and A. latus activities; no inhibition of A. vinelandii hydrogenase activity was detected. The results of these experiments indicated remarkable homology of the hydrogenases from these four microorganisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Arp D. J., Burris R. H. Kinetic mechanism of the hydrogen-oxidizing hydrogenase from soybean nodule bacteroids. Biochemistry. 1981 Apr 14;20(8):2234–2240. doi: 10.1021/bi00511a025. [DOI] [PubMed] [Google Scholar]
- Arp D. J., Burris R. H. Purification and properties of the particulate hydrogenase from the bacteroids of soybean root nodules. Biochim Biophys Acta. 1979 Oct 11;570(2):221–230. doi: 10.1016/0005-2744(79)90142-6. [DOI] [PubMed] [Google Scholar]
- Arp D. J. Rhizobium japonicum hydrogenase: purification to homogeneity from soybean nodules, and molecular characterization. Arch Biochem Biophys. 1985 Mar;237(2):504–512. doi: 10.1016/0003-9861(85)90303-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Mortenson L. E. Purification and properties of hydrogenase from Clostridium pasteurianum W5. Biochim Biophys Acta. 1974 Dec 18;371(2):283–298. doi: 10.1016/0005-2795(74)90025-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- HYNDMAN L. A., BURRIS R. H., WILSON P. W. Properties of hydrogenase from Azotobacter vinelandii. J Bacteriol. 1953 May;65(5):522–531. doi: 10.1128/jb.65.5.522-531.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker A. R., Xu L. S., Hanus F. J., Evans H. J. Some properties of the nickel-containing hydrogenase of chemolithotrophically grown Rhizobium japonicum. J Bacteriol. 1984 Sep;159(3):850–856. doi: 10.1128/jb.159.3.850-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway G. M., Lundak T. S., Tahara S. M., Traugh J. A. Isolation of protein kinases from reticulocytes and phosphorylation of initiation factors. Methods Enzymol. 1979;60:495–511. doi: 10.1016/s0076-6879(79)60047-2. [DOI] [PubMed] [Google Scholar]
- Howard J., Shannon L. A rapid, quantitative, and highly specific assay for carbohydrate-binding proteins. Anal Biochem. 1977 May 1;79(1-2):234–239. doi: 10.1016/0003-2697(77)90398-0. [DOI] [PubMed] [Google Scholar]
- Kow Y. W., Burris R. H. Purification and properties of membrane-bound hydrogenase from Azotobacter vinelandii. J Bacteriol. 1984 Aug;159(2):564–569. doi: 10.1128/jb.159.2.564-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pinkwart M., Schneider K., Schlegel H. G. Purification and properties of the membrane-bound hydrogenase from N2-fixing Alcaligenes latus. Biochim Biophys Acta. 1983 Jun 29;745(3):267–278. doi: 10.1016/0167-4838(83)90058-4. [DOI] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. Biochim Biophys Acta. 1979 Apr 12;567(2):315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus: II. Localization and immunological comparison with other hydrogenase systems. Antonie Van Leeuwenhoek. 1980;46(1):1–14. doi: 10.1007/BF00422224. [DOI] [PubMed] [Google Scholar]
- Schoenmaker G. S., Oltmann L. F., Stouthamer A. H. Purification and properties of the membrane-bound hydrogenase from Proteus mirabilis. Biochim Biophys Acta. 1979 Apr 12;567(2):511–521. doi: 10.1016/0005-2744(79)90137-2. [DOI] [PubMed] [Google Scholar]
- Stults L. W., O'Hara E. B., Maier R. J. Nickel is a component of hydrogenase in Rhizobium japonicum. J Bacteriol. 1984 Jul;159(1):153–158. doi: 10.1128/jb.159.1.153-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Böcher R., Knecht J., Kröger A. Hydrogenase from Vibrio succinogenes, a nickel protein. FEBS Lett. 1982 Aug 23;145(2):230–234. doi: 10.1016/0014-5793(82)80173-7. [DOI] [PubMed] [Google Scholar]
- Weeden N. F., Higgins R. C., Gottlieb L. D. Immunological similarity between a cyanobacterial enzyme and a nuclear DNA-encoded plastid-specific isozyme from spinach. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5953–5955. doi: 10.1073/pnas.79.19.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T., Kimura K., Daidoji H., Sakai F., Tamura S. Properties of purified hydrogenase from the particulate fraction of Desulfovibrio vulgaris, Miyazaki. J Biochem. 1976 Mar;79(3):661–671. doi: 10.1093/oxfordjournals.jbchem.a131111. [DOI] [PubMed] [Google Scholar]