Abstract
Context: We report herein a remarkable family in which the mother of a woman with 46,XY complete gonadal dysgenesis was found to have a 46,XY karyotype in peripheral lymphocytes, mosaicism in cultured skin fibroblasts (80% 46,XY and 20% 45,X) and a predominantly 46,XY karyotype in the ovary (93% 46,XY and 6% 45,X).
Patients: A 46,XY mother who developed as a normal woman underwent spontaneous puberty, reached menarche, menstruated regularly, experienced two unassisted pregnancies, and gave birth to a 46,XY daughter with complete gonadal dysgenesis.
Results: Evaluation of the Y chromosome in the daughter and both parents revealed that the daughter inherited her Y chromosome from her father. Molecular analysis of the genes SOX9, SF1, DMRT1, DMRT3, TSPYL, BPESC1, DHH, WNT4, SRY, and DAX1 revealed normal male coding sequences in both the mother and daughter. An extensive family pedigree across four generations revealed multiple other family members with ambiguous genitalia and infertility in both phenotypic males and females, and the mode of inheritance of the phenotype was strongly suggestive of X-linkage.
Conclusions: The range of phenotypes observed in this unique family suggests that there may be transmission of a mutation in a novel sex-determining gene or in a gene that predisposes to chromosomal mosaicism.
This study reports the unprecedented case of fertility in a 46, XY woman. The ovary was: 93% 46,XY, 6% 45,X. The female menstruated regularly and had two pregnancies. The molecular analysis in the mother and daughter revealed coding sequences of nine genes known to be involved in male sexual development that are identical to the normal male reference sequences.
Normal sexual differentiation in 46,XY individuals relies on a complex cascade of numerous genes, many of which have yet to be identified (1,2,3,4,5,6,7,8,9,10,11). Defects in these genes can cause disorders of sexual development of varying severity. The external genitalia and Müllerian structures are typically female in women with complete 46,XY gonadal dysgenesis in association with streak gonads bilaterally. Because the gonads are dysgenetic and nonfunctional, spontaneous pubertal development seldom occurs in these women (12), and successful pregnancy is even more unusual; unassisted pregnancy is unheard of (1). There have been a few instances of fertility in 46,XX/46,XY true hermaphrodites (13), but no reports of fertility in a 46,XY woman. Pregnancy in Turner syndrome is reported to be possible in about 2% of cases, although it is rare for unassisted pregnancy to occur in nonmosaic Turner patients possessing only a 45,X line (14).
Herein we report the extraordinary case of a fertile woman with normal ovaries and a predominantly 46,XY ovarian karyotype, who gave birth to a 46,XY female with complete gonadal dysgenesis. The karyotype of this phenotypically normal mother was 46,XY in blood, 80% 46,XY and 20% 45,X in cultured skin fibroblasts, and 93% 46,XY, 6% 45,X, and <1% 46,XX in the ovary. The family pedigree on the mother’s side was notable for the presence of seven individuals over four generations with either sexual ambiguity, infertility, or failure to menstruate, including one individual with documented 45,X/45,XY mixed gonadal dysgenesis. Both the mother and the 46,XY daughter were screened for mutations in a number of genes known to be involved in mammalian testis determination. In all genes screened (see below), the open reading frame was found to be normal. This suggests that a mutation in a novel sex-determination gene or a gene that predisposes to chromosomal mosaicism may be responsible for the phenotype in this family.
Patients and Methods
Methods
Informed written consent was obtained from the subjects.
LHRH stimulation test
Factrel (100 μg) was given iv with sequential blood drawn at baseline and at timed intervals for 2 h.
ACTH stimulation test
Cortrosyn (0.25 mg) was given iv, and blood was drawn at baseline and 1 h after injection.
Human chorionic gonadotropin (hCG) stimulation test
hCG (5000 U) was given im daily for 3 d.
Hormone assays
Steroid hormone assays were performed by standard RIA as described (15,16,17).
Karyotypic analyses
Fluorescence in situ hybridization was performed on a paraffin section slide of the gonad using the CEP probe for Y and the chromosome X centromeric control probe (Vysis, Downer’s Grove, IL).
Molecular analysis
Maternity testing was performed by using the short tandem repeat kit AmpFlSTR Profiler Plus (PE Applied Biosystems, Foster City, CA). Nine short tandem repeats were analyzed (D3S1358, VWA, FGA, D8S1179, D21S11, D18S51, D5S818, D13S317, and D7S820) using thermal cycling conditions, capillary electrophoresis was performed according to the manufacturer’s instructions, and products were analyzed using the ABI 3100 capillary electrophoresis instrument and GeneScan software (Applied Biosystems).
The Y chromosomes of the proband and her two parents were typed using the marker Y chromosome Alu polymorphism (YAP) as described (18). The entire open reading frame of the sex-determining region Y (SRY) gene was amplified and directly sequenced as described (2,3).
Using the PCR primers (Table 1), the open reading frames of seven genes known to be involved in sexual development were sequenced.
Table 1.
Primer pairs used for the amplification of genes involved in mammalian sex determination
| Sequence | |
|---|---|
| DMRT1 exon 1 | |
| Forward | GGCAGACCTCGCCACTCCAG |
| Reverse | AAGGCTGAACCCGGGCTCCC |
| DMRT1 exon 2 | |
| Forward | TCTGTGTTTTGGCAAAGCTG |
| Reverse | CTGCTTCTGTGGCTGCAA |
| DMRT1 exon 3 | |
| Forward | GCAGGTCTTGGGTAGGAAGG |
| Reverse | CATGTGGCTTTCACACAACC |
| DMRT1 exon 4 | |
| Forward | CAAGGTGTCGGGAACATAGG |
| Reverse | CTCTCTCAACCCCAAATCCA |
| DMRT1 exon 5 | |
| Forward | GGAGAGCGTCACTTTCTTTGTT |
| Reverse | CCATGCAGATGGTAGTCACG |
| DMRT3 exon 1 | |
| Forward | CGGAGCACACACGACCAC |
| Reverse | GTCCTCCCAAGTGGAGCTG |
| DMRT3 exon 2 | |
| Forward 1 | TGCATTTGCTCTTCCAAAA |
| Reverse 1 | AGAGTCGGCAGAAAACCTCA |
| Forward 2 | AACTTCCGCAGAACCTGAGA |
| Reverse 2 | AGATGTGGCCTCTCCTCAGA |
| BPESC1 | |
| Forward | AAGGTGACTTAAGGGCAGAGC |
| Reverse | GCCTGTCTCCAGACAAGAGTG |
| WNT4 exon 1 | |
| Forward | CCCAGGTAACCCCATCCT |
| Reverse | GGTGTGCAGAGGGACGTT |
| WNT4 exon 2 | |
| Forward | ACAGCATTTCCACTCCCTTG |
| Reverse | TCCTTTATGCCCTCACTTGG |
| WNT4 exons 3/4 | |
| Forward | GGGTGCCTAGCACATGATTT |
| Reverse | TGAGAGCCTGCACAAATGTT |
| WNT4 exon 5 | |
| Forward | CACAACGGCAAATCTGACTG |
| Reverse | TGAGGACCCAAAAACCAAAC |
| DAX1 exon 1 | |
| Forward 1 | ACAGCATCCAGGACATAGTGG |
| Reverse 1 | TGCCTCCTGGGACCTATTTAT |
| Forward 2 | CGTGCGCGCTAGGTATAAAT |
| Reverse 2 | AAGCAGCAGCGGTACAGAAG |
| Forward 3 | ACTAGCTCAAAGCAAACGCAC |
| Reverse 3 | TCCTCTTGGCTGAGTTTCTGA |
| DAX1 exon 2 | |
| Forward 1 | AGCAAAGGACTCTGTGGTGAG |
| Reverse 1 | GCAGGTTCCATGAAATTGCTA |
| TSPYL | |
| Forward | GCCGCTGAAATGTTAGTGAGA |
| Forward | GGAAACAGGGTFCAGAAAAG |
| SOX9 exon1 | |
| Forward | GCGCCTTCCTAAGTGCTC |
| Reverse | GCAAATCAGCCCTGACCA |
| SOX9 exon 2 | |
| Forward | TGACCCCTCTCCCTCTTTTT |
| Reverse | TGCCTCTTAGGCTCTGGGTA |
| SOX9exon3 | |
| Forward 1 | GCACAGCCCTTGTTGATTTT |
| Reverse 1 | CTCAGCTGCTCCGTCTTGAT |
| Forward 2 | ATCAAGACGGAGCAGCTGAG |
| Reverse 2 | AGCGAACGCACATCAAGAC |
The DMRT1 and DMRT3 genes were sequenced using lymphocyte DNA isolated from the patient. The conditions of amplification were as follows: for DMRT1 exon 1, incubation at 95 C for 5 min followed by 40 cycles of 95 C for 1 min, 68 C for 1 min, and 72 C for 30 sec; for DMRT1 exons 2 and 4, incubation at 95 C for 5 min followed by 40 cycles of 95 C for 1 min, 57 C for 30 sec, and 72 C for 1 min; for DMRT1 exon 3, incubation at 95 C for 5 min followed by 40 cycles of 95 C for 1 min, 62 C for 1.30 min, and 72 C for 30 sec; for DMRT1 exon 5, incubation at 95 C for 5 min followed by 40 cycles of 95 C for 1 min, 50 C for 30 sec, and 72 C for 30 sec. For DMRT3 exon 1, the PCR conditions were incubation at 95 C for 5 min followed by 40 cycles of 95 C for 1 min and 62 C for 1.30 min with no extension time. For DMRT3 exon 2, two amplicons were used to amplify the entire exon for direct sequencing. Both primer pairs of each amplicon were used at the conditions of incubation at 95 C for 5 min followed by 35 cycles of 95 C for 1 min and 60 C for 1 min with no extension time.
Amplification of the coding region of the BPESC1 gene was performed using PCR for 35 cycles at 95 C for 30 sec, 56 C for 30 sec, and 72 C for 30 sec. PCR products were directly sequenced using the forward primer for each amplicon.
The reaction conditions for amplifying and sequencing the WNT4 open reading frame were identical to that described for the BPESC1 gene above. PCR products were sequenced using the forward primer of each amplicon.
Exon 1 of the DAX1 open reading frame was amplified using three amplicons, and exon 2 was amplified in one step. PCR amplification was performed as indicated for the BPESC1 gene as described above with the exception of the primer pairs DAX1 exon1 F2/DAX1 exon 1 R2 where the annealing temperature was 58 C and the annealing time was 45 sec. Direct sequencing of all amplicons was performed using both the forward and reverse primers.
Amplification and sequencing of the DHH gene was performed as described by Umehara et al. (19).
The TSPYL gene was sequenced using the conditions of 95 C for 5 min, followed by 35 cycles of 95 C for 1 min, 60 C for 1 min, and 72 C for 1 min. A final extension of 72 C for 5 min was included.
The amplification conditions for all SOX9 amplicons were 95 C for 5 min, followed by 35 cycles of 95 C for 1 min, 61 C for 1 min, and 72 C for 1 min. A final extension of 72 C for 10 min was also performed.
The open reading frame of the SF1 gene was amplified and directly sequenced using conditions described (20).
Case histories
Patient 1 (daughter)
This 17-yr-old woman from Croatia was the product of a 39-wk gestation, delivered by cesarean section due to a maternal hip fracture. Birth weight was 3.8 kg, and length was 52 cm. She was breastfed for 1 yr. She sought medical attention at age 17 yr because of lack of breast development and primary amenorrhea. Intelligence was normal, determined by her standing as a top student in her class.
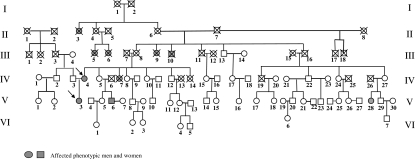
On exam, she was an articulate, tall, thin woman. The height was 187 cm (>95th percentile) with a mid-parental target height of 181 cm. Her weight was 68 kg. She had a normal frontal hairline. There was mild facial acne but no facial hair. She had Tanner stage I breasts and Tanner stage IV pubic hair. External female genitalia were normal, without clitoromegaly or labial fusion. The vaginal introitus was normal. Pelvic examination revealed a hypoplastic uterus with no palpable gonads. Bone age was 14 yr at a chronological age of 17 yr. Karyotype on peripheral blood (performed twice) was 46,XY. Further investigation revealed that multiple family members on the mother’s side had ambiguous genitalia, infertility, or problems with sexual identity (see Fig. 2). This led to the decision to karyotype the mother.
Figure 2.
Family members: II-3, woman with masculine appearance, no breasts, infertile, moved away from hometown because of unacceptable appearance and died during World War II at age of 62 yr; II-4, woman who died at age 76 yr; II-6, woman who died at age 68 yr; II-7, man who died at age 64 yr; III-5,6, woman with hirsutism who died around age 60 yr; III-9, ambiguous genitalia with hirsutism (beard), raised as female, family was ashamed of her and hid her from public, died at age 55 yr; III-10, man with confused gender identity, infertile, committed suicide at age 24 yr; III-13, normal man who died at age 70 yr; III-14, normal woman; *IV-3, 46,XY fertile man; *IV-4, fertile woman with a predominantly 46,XY ovary (patient 2, mother); *IV-5, 46,XX fertile woman; IV-7, woman with absent uterus and ovaries [established outside of Zagreb, and history was obtained from patient 1 (daughter)], on hormone replacement, died at age 42 yr from multiple sclerosis; *IV-8, normal fertile woman; *IV-9, normal fertile man; *IV-10, 46,XX fertile woman; *IV-11, normal fertile man; *IV-12, 46,XY normal fertile man; *IV-27, 46,XX fertile woman; *V-3, 46,XY complete gonadal dysgenesis; *V-5, 46,XX fertile woman; *V-6, 46,XY male with ambiguous genitalia, bifid scrotum, and hypospadias, hypoplastic testes in scrotum; stretched penile length 5 cm; high gonadotropins (LH 20.9 IU/liter, FSH 59.1 IU/liter), infertile, testosterone 15.4 nmol/liter, estradiol 0.04 nmol/liter, prolactin 4.3 ng/ml; *V-7, normal woman; *V-8, normal man; *V-9, normal woman; *V-10, normal man; *V-11, 46,XX normal woman with normal hormones; *V-12, normal woman; *V-13, normal man; *V-16, normal woman; *V-20, normal man; V-21, infertile woman, tried in vitro fertilization without success; V-23, normal male; *V-28, 46,XY/45,X mixed gonadal dysgenesis, gonadoblastoma; *VI-1, normal woman; *VI-2, normal woman; *VI-3, normal woman; *VI-4, normal woman; *VI-5, normal man. *, Personal examination by M. Dumic.
Patient 2 (mother)
This 52-yr-old phenotypically normal woman underwent normal pubertal development and reached spontaneous menarche at age 11 yr. She had a history of two pregnancies, the first of which resulted in a spontaneous miscarriage. Her second pregnancy was uneventful, except that her daughter was delivered by cesarean section due to a recent hip fracture in the mother from a motor vehicle accident. She breastfed the daughter for 1 yr. She continued to have regular menses until menopause at age 49, after which time she received hormone replacement therapy for 2 yr.
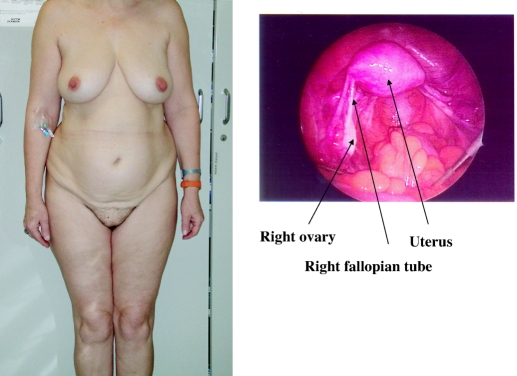
Physical examination revealed a feminine-appearing woman with a normal body habitus (Fig. 1). The height was 177 cm. There was no receding hairline or balding of the scalp and no acne or facial hair. Breasts and pubic hair were Tanner stage V, although pubic hair was sparse. The external genitalia were normal with no clitoromegaly or labial fusion. The vaginal introitus was normal. Pelvic examination revealed a uterus in retroverted position with no adnexal masses. The karyotype in peripheral blood was 46,XY (20 cells).
Figure 1.
Patient 2 (mother) and laparascopic photograph of right ovary of patient 2.
Results
Patient 1 (daughter)
Repeat karyotype revealed the following: blood, 46,XY (100%) (20 nuclei); skin, 46,XY (100%) (50 nuclei); gonad, 46,XY (99.25%), 45,X (0.75%) (400 nuclei).
Hypothalamic-pituitary-gonadal axis (Table 2)
Table 2.
LHRH stimulation test
| Time (min) | LH (mIU/ml) | FSH (mIU/ml) | T (ng/dl) | E2(ng/dl) | Δ4A (ng/dl) | DHEA (ng/dl) |
|---|---|---|---|---|---|---|
| Daughter | ||||||
| 0 | 135.7 | 194.6 | 91 | 1.1 | 78 | 574 |
| 15 | 162.5 | 118.6 | ||||
| 30 | 232.7 | 133.6 | ||||
| 45 | 241.5 | 156.1 | ||||
| 60 | 229.7 | 143.7 | ||||
| 90 | 193.9 | 147.6 | ||||
| 120 | 156.1 | 147.3 | 72 | 0.5 | 91 | 718 |
| Mother | ||||||
| 0 | 22.9 | 48.9 | 22 | 0.6 | 42 | 96 |
| 15 | 101.7 | 70.7 | ||||
| 30 | 114.5 | 81.8 | ||||
| 45 | 115.5 | 86.7 | ||||
| 60 | 109.2 | 84.0 | ||||
| 90 | 96.8 | 84.5 | ||||
| 120 | 75.1 | 77.5 | 20 | 0.3 | 37 | 102 |
| Normal values (mean ± sd) | ||||||
| XY | 329 ± 166 | 2.5 ± 2.7 | 49 ± 20 | 226 ± 110 | ||
| XX | 31 ± 17 | 9.5 ± 5.8 | 87 ± 26 | 296 ± 218 |
Δ4A, Androstenedione; DHEA, dehydroepiandrosterone; E2, estradiol; T, testosterone.
LHRH stimulation test was consistent with gonadal failure/absent gonads.
Gonadal function (Table 3)
Table 3.
hCG stimulation test
| Time (h) | T (ng/dl) | E2(ng/dl) | Δ4A (ng/dl) | DHEA (ng/dl) |
|---|---|---|---|---|
| Daughter | ||||
| Baseline | 90 | 0.5 | 29 | 255 |
| 24 h | 110 | 0.3 | 76 | 424 |
| 48 h | 85 | 0.3 | 53 | 207 |
| Mother | ||||
| Baseline | 25 | 2.7 | 44 | 157 |
| 24 h | 46 | 0.5 | 33 | 157 |
| 48 h | 30 | 0.3 | 38 | 98 |
Times (24 and 48 h) indicate blood was drawn 24 or 48 h after last dose of hCG. Δ4A, Androstenedione; DHEA, dehydroepiandrosterone; E2, estradiol; T, testosterone.
hCG stimulation test revealed high baseline testosterone with little response to hCG.
Adrenal function (Table 4)
Table 4.
ACTH stimulation test
| Time (min) | 17OHP (ng/dl) | 17Δ5P (ng/dl) | Δ4A (ng/dl) | DHEA (ng/dl) | T (ng/dl) | E2 (ng/dl) | DOC (ng/dl) | B (μg/dl) | F (μg/dl) | Aldo (ng/dl) | DHT (ng/dl) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Daughter | |||||||||||
| 0 | 36 | 321 | 78 | 574 | 91 | 1.1 | 25 | 0.48 | 12.5 | 5 | 7 |
| 60 | 73 | 886 | 98 | 821 | 94 | 1.5 | 56 | 3.36 | 24.7 | 9 | 10 |
| Mother | |||||||||||
| 0 | 19 | 78 | 44 | 157 | 25 | 2.7 | 19 | 0.24 | 9.0 | 10 | 5 |
| 60 | 159 | 797 | 118 | 415 | 34 | 3.2 | 69 | 2.92 | 31.1 | 11 | 7 |
| Normal baseline values (mean ± sd) | |||||||||||
| 46,XY | 210 ± 110 | 180 ± 180 | 10 ± 6 | 0.65 ± 0.34 | 15.6 ± 6.5 | 15 ± 9 | 38 ± 15 | ||||
| 46,XX | 150 ± 120 | 196 ± 187 | 18 ± 11 | 0.35 ± 0.39 | 12.0 ± 6.7 | 11 ± 7 | 13 ± 8 | ||||
Aldo, Aldosterone; B, corticosterone; DHT, dihydrotestosterone; DOC, deoxycorticosterone; F, cortisol; 17OHP, 17-hydroxyprogesterone; 17Δ5P, 17-hydroxypregnenolone; Δ4A, Androstenedione; DHEA, dehydroepiandrosterone; E2, estradiol; T, testosterone.
No evidence of a steroidogenic defect was demonstrated.
Pelvic ultrasound
Normal kidneys and a left extrarenal pelvis were noted. The uterus was hypoplastic on ultrasound. A small left gonad was present. No gonad was noted on the right.
Surgical pathology
The patient underwent laparoscopic gonadectomy. No gonadal tissue was identified on the right, although there was a normal fallopian tube on that side.
Histology
On the left, she had a small fragment of fibrous tissue with a small focus of ovarian stroma. Inhibin staining was positive.
Serum testosterone
Repeat testosterone after gonadectomy was 51.8 ng/dl (normal range, 23.0–69.1 ng/dl).
Patient 2 (mother)
Repeat karyotype revealed the following: blood, 46,XY (100%) (20 nuclei); skin, 46,XY (80%)/45,X (20%) (50 nuclei); gonad, 46,XY (92.9%), 45,X (5.9%), 46,XX (0.6%), 47,XXY (0.6%) (1000 nuclei).
Hypothalamic-pituitary-gonadal axis (Table 2)
LHRH stimulation test was consistent with menopause or absent gonads.
Gonadal function (Table 3)
hCG stimulation test revealed no evidence of testicular function.
Adrenal function (Table 4)
No evidence of a steroidogenic defect was demonstrated.
Pelvic ultrasound
The uterus measured 8.8 cm × 4.5 cm × 5.5 cm and was normal in echotexture. The endometrial stripe measured 4 mm. The right ovary measured 3.3 cm × 2.5 cm × 2.7 cm with normal venous and arterial flow. The left ovary measured 3.0 cm × 1.6 cm × 2.5 cm with normal flow. There was no free fluid.
MRI of pelvis
The uterus appeared mildly atrophic and had a mildly thickened endometrial stripe. There were probable small myomas in the lower uterine segment. Both ovaries were atrophic with no follicles or masses noted.
Surgical pathology
The mother agreed to undergo gonadectomy because of the increased risk of gonadoblastoma in gonads containing a Y chromosome. The internal structures were those of a normal woman (see Fig. 1). No Wolffian remnants were seen. The uterus and fallopian tubes were left intact, and the ovaries were removed.
Histology
Pathology revealed a histologically unremarkable right ovary with several corpora albicans, suggestive of previous ovulation. The left ovary contained fibromuscular tissue with ovarian hilar cells, and an immunohistochemical stain for inhibin showed a focus of positive cells confirming the presence of ovarian stromal elements.
Family history and genetic studies
Family history
There is a remarkable family history of ambiguous genitalia and infertility affecting both phenotypic men and women across four generations in the mother’s family (Fig. 2). The daughter inherited her Y chromosome from the father (see below), thereby excluding involvement of the Y chromosome in the development of sex reversal in this family. The pedigree is strongly suggestive of X-linked inheritance of the phenotype, although autosomal dominant sex-limited transmission cannot be excluded.
Genetic evaluation
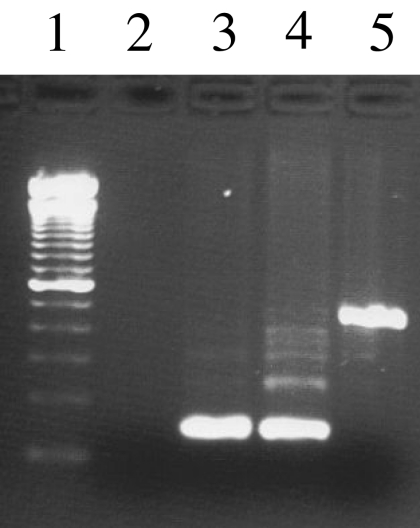
Maternity was established at a probability exceeding 99.15%. The analysis of the Y chromosome polymorphism YAP revealed that this insertion was present in the Y chromosome of the mother (defining her Y chromosome haplogroup as D/E), and the insertion was not present in the Y chromosome of the daughter or her father (Fig. 3). These data indicate that the daughter inherited the Y chromosome from her father, and the sequence was identical to that of a normal male. The molecular analysis of the coding sequences of nine genes known to be involved in sexual development (SOX9, SF1, DMRT1, DMRT3, TSPYL, BPESC1, DHH, WNT4, SRY, and DAX1) revealed coding sequences in both the mother and daughter that are identical to the normal reference sequences. Polymorphisms were not identified in any of the analyzed genes.
Figure 3.
PCR amplification of the YAP insertion polymorphism on the Y chromosome. Lane 1, 100-bp molecular mass marker; lane 2, water negative control; lane 3, father of patient 2; lane 4, patient 2 (daughter); lane 5, patient 1 (mother). The Y chromosome of the mother has the YAP insertion, which is absent from the daughter and father.
Discussion
Although there have been reports of fertility in 46,XX/46,XY true hermaphrodites with ovotestes (13) and in patients with mosaic and nonmosaic Turner syndrome (21), we believe this to be the first report of fertility in a woman with a predominantly 46,XY karyotype in the ovary. The fact that this mother had normal functioning ovaries, menstruated regularly, and achieved unassisted pregnancy twice is remarkable. Additionally, her hormonal findings are compatible with a normal menopausal woman. Of course, it should be noted that the incidence of normal fertile females who have a 46,XY karyotype is not known because it is not routine to check the karyotype in fertile women. Although the demonstration of 5.9% 45,X cells in the ovary is difficult to interpret, most cytogeneticists agree that 5% does not indicate mosaicism. The finding of 20% 45,X cells in fibroblasts cultured from skin indicates that she is a 46,XY/45,X mosaic, at least in the skin. Individuals with a karyotype of 46,XY/45,X usually have ambiguous genitalia or a male phenotype, although occasionally they can have a Turner female phenotype (21). Our case is unique, however, because the presence of bilateral ovaries or unassisted pregnancy has not previously been reported in this form of mosaicism. Moreover, ovarian cells were predominately 46,XY; the small percentage of X (5.9%) out of 1000 cells counted in the gonad might be due to artifact or technical error. Pregnancy is believed to occur in about 2% of women with Turner syndrome (14). Although fertility did occur in a woman with mosaicism of an isodicentric Y chromosome (22), we believe that our case of fertility in a female with a predominantly 46,XY karyotype in the ovary is unprecedented. Of note, XY female wood lemmings (Myopus shisticolor), carrying an Xp mutation, are fertile and produce X-containing oocytes (23). There have also been reports of potential fertility in XY sex-reversed female mice. In the B6.YDOM sex-reversed female mouse, almost all of the XY female mice, although they lack estrous cyclicity, are able to mate and ovulate after treatment with gonadotropins (24). Likewise, fertility has been described in XY female horses (25).
The fact that this mother gave birth to a 46,XY female is even more remarkable. However, the daughter’s clinical picture, unlike that of her mother, is more typical of 46,XY complete gonadal dysgenesis, in which spontaneous puberty is rare and fertility is unreported. The significant family history of ambiguous genitalia and sex reversal across several generations presents a unique opportunity to explore the genetics of sexual differentiation and perhaps identify a novel gene involved in gonadal determination.
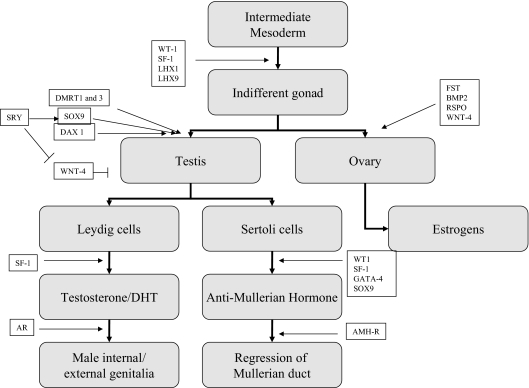
The mother and daughter were both screened for mutations in a number of genes that are either known to be necessary or are excellent candidate genes for males in human testicular development. Some of the genes known to be involved in gonadal differentiation are outlined in Fig. 4 (4,5,6,7,26). Both the mother and daughter had normal SRY (sex-determining region of the Y chromosome) sequences, and the daughter inherited the Y chromosome from her father, thereby excluding involvement of the nonrecombining male-specific portion of the Y chromosome, including the SRY gene, in the development of sex reversal in this family.
Figure 4.
Genes involved in sexual differentiation: WT-1, Wilms’ tumor 1; SF-1, steroidogenic factor 1; LHX1, LIM homeobox 1; LHX9, LIM homeobox 9; DMRT1 and -3, doublesex and Mab3-related transcription factors 1 and 3 (located on chromosome 9p24); SRY, sex-determining region of Y chromosome; SOX9, SRY-box-related 9; DAX1, dosage-sensitive sex reversal locus-adrenal hypoplasia congenita-critical region on the X, gene 1; WNT-4, wingless-type MMTV integration site family, member 4 (member of Wnt family of locally secreted growth factors); RSPO, R-spondins; FST, follistatin; BMP2, bone morphogenetic protein 2; GATA-4, GATA-binding protein 4 (codes for a zinc finger transcription factor); AR, androgen receptor; AMH-R, anti-Mullerian hormone receptor.
Included in the screen were the normal DMRT1 and DMRT3 genes. When deleted, these genes are associated with male-to-female sex reversal. The analysis of another gene that is associated with gonadal dysgenesis (TSPYL) also revealed a normal sequence. Mutations in this gene are also associated with sudden infant death and testicular dysgenesis in an Amish family (27).
The BPESC1 gene, which exhibits testis-specific expression and is located within the homologous region in the human at 3q23 (28), was normal in both mother and daughter. The DAX1 gene is located on Xp22 and appears to be necessary for correct testis determination and, in the mouse at least, necessary for the up-regulation of Sox9 expression (29). The gene WNT4 is critical for normal ovarian and female sexual development. A mutation in WNT4 leads to Mullerian duct regression and virilization in a 46,XX female (8), whereas duplication of the locus containing WNT4 leads to 46,XY sex reversal (30). In the XY mouse, the absence of Wnt4 is associated with a lack of Sertoli cell differentiation, suggesting that the gene is involved in mammalian testis determination (31). Sequence analysis of both DAX1 and WNT4 genes revealed normal wild-type male coding sequences in both the mother and daughter.
The Desert hedgehog gene (Dhh) is a member of a family of signaling genes that play an important role in regulating morphogenesis. Mutations in the human DHH gene have been reported in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy (19) and in women with complete gonadal dysgenesis and the absence of somatic anomalies (32).
Other possible genes to explore in this remarkable family are the follistatin (Fst) and the bone morphogenetic protein 2 (Bmp2) genes, which are both expressed in the mouse embryonic ovary and appear to be important for ovary organogenesis. Fst acts downstream of Wnt4 to inhibit the formation of the XY-specific coelomic vessel and to maintain germ cell survival in the cortical domain of the ovary. Bmp2 appears to also act downstream of Wnt4 but independently of Fst (9,33). Mutations in Gata4 or Fog2 can also cause sex reversal in mice (10,34,35) and are potential candidate genes to be explored.
Alternatively, because at least one other member of this extended family has 46,XY/45,X mixed gonadal dysgenesis (V-28), disorders of sexual development in this family may be due to a mutant gene that predisposes to chromosomal mosaicism and mixed gonadal dysgenesis. In either scenario, the transmission of the phenotype is strongly suggestive of X-linked inheritance, although an autosomal dominant sex-limited mutation cannot be formally excluded. Various studies of familial 46,XY gonadal dysgenesis have suggested the existence of an X chromosome locus that is necessary for testis determination (36,37,38,39), perhaps on proximal Xp (40). The latter finding is consistent with observations of deletions of Xp associated with 46,XX SRY-negative true hermaphroditism (11).
The serendipitous discovery of a predominantly 46,XY karyotype in this fertile mother of a 46,XY daughter suggests that perhaps all mothers of 46,XY (SRY+) females with complete gonadal dysgenesis should be carefully examined for an XY karyotype as well. This extraordinary family affords an exceptional opportunity to investigate potential factors that can induce ovarian differentiation and function without the Xq critical region. Meanwhile, DNA has been obtained on 19 family members (three affected, 16 unaffected) across three generations in the hopes of identifying the etiology of sex reversal in this interesting family. Linkage analysis is a potential next step as efforts are being made to obtain DNA from more affected members. A genome-wide search for deletions or duplications in this mother and her daughter may serve to uncover novel gene mutations responsible for sex reversal or may even reveal a genetic cause for chromosomal mosaicism and mixed gonadal dysgenesis.
Acknowledgments
We thank Dr. M. Lita Alonso for performing the fluorescence in situ hybridization studies on the gonadal tissue of both patients. We also acknowledge Dr. Dix Poppas and Dr. Isaac Kligman, who performed the surgical procedures for these patients.
Footnotes
This work was supported by the GIS-Institut des Malade Rares, National Institutes of Health Grant HD-00072, a National Institutes of Health Rare Diseases grant, the Children’s Hormone Foundation, and the Children’s Clinical Research Center at Weill Medical College of Cornell University.
Disclosure Statement: The authors have nothing to declare.
First Published Online November 13, 2007
Abbreviations: hCG, Human chorionic gonadotropin; YAP, Y chromosome Alu polymorphism.
References
- Kucheria K, Mohapatra I, Ammini AC, Bhargava VL, McElreavey K 1996 Clinical and DNA studies on 46,XY females with gonadal dysgenesis. A report of six cases. J Reprod Med 41:263–266 [PubMed] [Google Scholar]
- Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M 1990 Genetic evidence equating SRY and the testis-determining factor. Nature 348:448–450 [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN 1990 A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346:240–244 [DOI] [PubMed] [Google Scholar]
- McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M 1993 A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci USA 90:3368–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, Chinwalla A, Delehaunty A, Delehaunty K, Du H, Fewell G, Fulton L, Fulton R, Graves T, Hou SF, Latrielle P, Leonard S, Mardis E, Maupin R, McPherson J, Miner T, Nash W, Nguyen C, Ozersky P, Pepin K, Rock S, Rohlfing T, Scott K, Schultz B, Strong C, Tin-Wollam A, Yang SP, Waterston RH, Wilson RK, Rozen S, Page DC 2003 The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 423:825–837 [DOI] [PubMed] [Google Scholar]
- Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R 2004 SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol 274:271–279 [DOI] [PubMed] [Google Scholar]
- Sarafoglou K, Ostrer H 2000 Clinical review 111: familial sex reversal: a review. J Clin Endocrinol Metab 85:483–493 [DOI] [PubMed] [Google Scholar]
- MacLaughlin DT, Donahoe PK 2004 Sex determination and differentiation. N Engl J Med 350:367–378 [DOI] [PubMed] [Google Scholar]
- Yao HH, Matzuk MM, Jorgez CJ, Menke DB, Page DC, Swain A, Capel B 2004 Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev Dyn 230:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH 2002 Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development 129:4627–4634 [DOI] [PubMed] [Google Scholar]
- Ergun-Longmire B, Vinci G, Alonso L, Matthew S, Tansil S, Lin-Su K, McElreavey K, New MI 2005 Clinical, hormonal and cytogenetic evaluation of 46,XX males and review of the literature. J Pediatr Endocrinol Metab 18:739–748 [DOI] [PubMed] [Google Scholar]
- Villanueva AL, Benirschke K, Campbell J, Wachtel SS, Rebar RW 1984 Complete development of secondary sex characteristics in a case of 46,XY pure gonadal dysgenesis. Obstet Gynecol 64:68S–72S [DOI] [PubMed] [Google Scholar]
- Verp MS, Harrison HH, Ober C, Oliveri D, Amarose AP, Lindgren V, Talerman A 1992 Chimerism as the etiology of a 46,XX/46,XY fertile true hermaphrodite. Fertil Steril 57:346–349 [DOI] [PubMed] [Google Scholar]
- Cools M, Rooman RP, Wauters J, Jacqemyn Y, Du Caju MV 2004 A nonmosaic 45,X karyotype in a mother with Turner’s syndrome and in her daughter. Fertil Steril 82:923–925 [DOI] [PubMed] [Google Scholar]
- Abraham GE, Swerdloff RS, Tulchinsky D, Hopper K, Odell WD 1971 Radioimmunoassay of plasma 17-hydroxyprogesterone. J Clin Endocrinol Metab 33:42–46 [DOI] [PubMed] [Google Scholar]
- Abraham GE, Manlimos FS, Solis M, Wickman AC 1975 Combined radioimmunoassay of four steroids in one ml of plasma. II. Androgens. Clin Biochem 8:374–378 [DOI] [PubMed] [Google Scholar]
- Korth-Schutz S, Levine LS, New MI 1976 Serum androgens in normal prepubertal and pubertal children and in children with precocious adrenarche. J Clin Endocrinol Metab 42:117–1124 [DOI] [PubMed] [Google Scholar]
- Hammer M 1994 A recent insertion of an alu element on the Y chromosome is a useful marker for human population studies. Mol Biol Evol:749–761 [DOI] [PubMed] [Google Scholar]
- Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, Osame M 2000 A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am J Hum Genet:1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Ramayya MS, Chrousos GP, Driggers PH, Parker KL 1996 Cloning and sequence analysis of the human gene encoding steroidogenic factor 1. J Mol Endocrinol 17:139–147 [DOI] [PubMed] [Google Scholar]
- Rosenberg C, Frota-Pessoa O, Vianna-Morgante A, Chu T 1987 Phenotypic spectrum of 45,X/46,XY individuals. Am J Med Genet 27:553–559 [DOI] [PubMed] [Google Scholar]
- Landin-Wilhelmsen K, Bryman I, Hanson C, Hanson L 2004 Spontaneous pregnancies in a Turner syndrome woman with Y-chromosome mosaicism. J Assist Reprod Genet 21:229–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WS, Eriksson L, Fredga K 1998 XY sex reversal in the wood lemming is associated with deletion of Xp21–23 as revealed by chromosome microdissection and fluorescence in situ hybridization. Chromosome Res 6:379–383 [DOI] [PubMed] [Google Scholar]
- Amleh A, Ledee N, Saeed J, Taketo T 1996 Competence of oocytes from the B6.YDOM sex-reversed female mouse for maturation, fertilization, and embryonic development in vitro. Dev Biol 178:263–275 [DOI] [PubMed] [Google Scholar]
- Sharp AJ, Wachtel SS, Benirschke K 1980 H-Y antigen in a fertile XY female horse. J Reprod Fertil 58:157–160 [DOI] [PubMed] [Google Scholar]
- Kwok C, Goodfellow PN, Hawkins JR 1996 Evidence to exclude SOX9 as a candidate gene for XY sex reversal without skeletal malformation. J Med Genet 33:800–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffenberger E, Hu-Lince D, Parod JM, Craig DW, Dobrin SE, Conway AR, Donarum EA, Strauss KA, Dunckley T, Cardenas JF, Melmed KR, Wright CA, Liang W, Stafford P, Flynn CR, Morton DH, Stephan DA 2004 Mapping of a sudden infant death with dysgenesis of the testes syndrome (SIDDT) by a SNP genome scan and identification of TSPYL loss of function. Proc Natl Acad Sci USA 101:11689–11694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G 2001 The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166 [DOI] [PubMed] [Google Scholar]
- Bouma G, Albrecht KH, Washburn LL, Recknagel AK, Churchill GA, Eicher EM 2005 Gonadal sex reversal in mutant Dax1 XY mice: a failure to upregulate Sox9 in pre-Sertoli cells. Development 132:3045–3054 [DOI] [PubMed] [Google Scholar]
- Jordan BK, Mohammed M, Ching ST, Delot E, Chen XN, Dewing P, Swain A, Rao PN, Elejalde BR, Vilain E 2001 Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet 68:1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeays-Ward K, Dandonneau M, Swain A 2004 Wnt4 is required for proper male as well as female sexual development. Dev Biol 276:431–440 [DOI] [PubMed] [Google Scholar]
- Canto P, Soderlund D, Reyes E, Mendez JP 2004 Mutations in the desert hedgehog (DHH) gene in patients with 46,XY complete pure gonadal dysgenesis. J Clin Endocrinol Metab [Erratum (2004) 89:5453] 89:4480–4483 [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC 2003 Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 262:303–312 [DOI] [PubMed] [Google Scholar]
- Ketola I, Pentikainen V, Vaskivuo T, Ilvesmaki V, Herva R, Dunkel L, Tapanainen JS, Toppari J, Heikinheimo M 2000 Expression of transcription factor GATA-4 during human testicular development and disease. J Clin Endocrinol Metab 85:3925–3931 [DOI] [PubMed] [Google Scholar]
- Viger RS, Mertineit C, Trasler JM, Nemer M 1998 Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Mullerian inhibiting substance promoter. Development 125:2665–2675 [DOI] [PubMed] [Google Scholar]
- Espiner EA, Veale AM, Sands VE, Fitzgerald PH 1970 Familial syndrome of streak gonads and normal male karyotype in five phenotypic females. N Engl J Med 283:6–11 [DOI] [PubMed] [Google Scholar]
- Sternberg WH, Barclay DL, Kloepfer HW 1968 Familial XY gonadal dysgenesis. N Engl J Med 278:695–700 [DOI] [PubMed] [Google Scholar]
- German J, Simpson JL, Chaganti RS, Summitt RL, Reid LB, Merkatz IR 1978 Genetically determined sex-reversal in 46,XY humans. Science 202:53–56 [DOI] [PubMed] [Google Scholar]
- Mann JR, Corkery JJ, Fisher HJ, Cameron AH, Mayerova A, Wolf U, Kennaugh AA, Woolley V 1983 The X linked recessive form of XY gonadal dysgenesis with a high incidence of gonadal germ cell tumors: clinical and genetic studies. J Med Genet 20:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajender S, Thangaraj K, Gupta NJ, Leelavathy N, Rani DS, Nambiar RG, Kalavathy V, Santhiya ST, Rajangam S, Gopinath PM, Chakravarty B, Singh L 2006 A novel human sex-determining gene linked to Xp11.21–11.23. J Clin Endocrinol Metab 91:4028–4036 [DOI] [PubMed] [Google Scholar]