Abstract
Context: Although racial and ethnic differences in vitamin D status and bone mineral density (BMD) are recognized, less is known about how differences in vitamin D status impact BMD, especially among men.
Objective: Our objective was to examine the relation between serum 25-hydroxyvitamin D [25(OH)D] and BMD by race and ethnic group.
Design: We conducted a population-based, observational survey.
Participants: Participants included 1114 Black, Hispanic, and White men, 30–79 yr of age.
Outcomes: We assessed 25(OH)D by a competitive protein binding assay and BMD by dual-energy x-ray absorptiometry.
Results: Mean age ± sd of the 331 Black, 362 Hispanic, and 421 White men was 48 ± 12.8 yr. Mean 25(OH)D was lower among Black (25.0 ± 14.7 ng/ml) and Hispanic (32.9 ± 13.9 ng/ml) men compared with White men (37.4 ± 14.0 ng/ml, P < 0.01). A higher percentage of both Black (44%) and Hispanic (23%) men had levels of 25(OH)D in the lowest quartile, compared with 11% of White men (P < 0.001). After adjusting for age, height, and weight, only White men showed significant positive correlation between 25(OH)D and BMD (range of correlations, 0.00–0.14). Serum 25(OH)D was not associated with BMD in Black or Hispanic men at any bone site. Results were similar when adjusted for age only.
Conclusions: Our findings confirm substantial racial and ethnic group differences in BMD and serum 25(OH)D in men. Serum 25(OH)D and BMD are significantly related to one another in White men only. This may have implications for evaluation of bone health and supplementation in men with low levels of 25(OH)D. Further understanding of the biological mechanisms for these differences between race and ethnic groups is needed.
A study among Black, Hispanic, and White men finds a positive correlation between bone mineral density and serum 25-hydroxyvitamin D in the latter group but not in the former two groups.
Vitamin D is essential for bone health (1). In recent years, concern over the burden of osteoporotic fractures in an increasingly aging population has generated much interest in the role of vitamin D in maintaining bone health. Vitamin D functions in conjunction with PTH to ensure adequate serum calcium and phosphorus to promote optimal mineralization of bone matrix (1). Although an overt deficiency of vitamin D results in rickets in children and osteomalacia in adults (2), insufficiency causes secondary hyperparathyroidism, which increases osteoclast production and has been implicated as a major risk factor for osteoporotic fracture (3,4,5).
Racial and ethnic differences in vitamin D status and risk of osteoporosis are well documented. Among men, there is very strong evidence that Black men are at elevated risk of vitamin D deficiency (6,7,8,9) but at lower risk of osteoporosis (10,11), rapid bone loss (12), and associated fractures (13) compared with White men. This apparent paradox suggests the possibility that the association between serum 25-hydroxyvitamin D [25(OH)D] and bone mineral density (BMD) or fracture risk differs by race and ethnic group, with Black men somehow protected against the negative skeletal consequences of suboptimal vitamin D levels. Few studies have addressed this paradox, and there is limited information on 25(OH)D and BMD in Hispanic populations. NHANES III data reveal significant positive associations between serum 25(OH)D and BMD regardless of age, race and ethnic group, or gender (6). However, close examination of their results clearly shows that the association between serum 25(OH)D and BMD was less pronounced in younger non-White groups, supporting the possibility of variation in the effect of 25(OH)D on BMD by race and ethnic group.
The purpose of this study was to provide descriptive data for three racial and ethnic groups of adult men living in a northeastern U.S. city (Boston, MA, latitude 42 degrees N) with respect to vitamin D status and BMD. Specifically, we examined race- and ethnic group-specific relationships between serum 25(OH)D levels and BMD among men in the Boston Area Community Health (BACH)/Bone Survey, a population-based random sample of skeletal health in adult Black, Hispanic, and White men.
Subjects and Methods
Study sample
BACH/Bone is a cross-sectional observational study of 1219 (of 1877 eligible, 65% response rate) randomly selected Black, Hispanic, and White male residents of Boston, Massachusetts, aged 30–79 yr (14). Persons of other racial or ethnic backgrounds were not enrolled. This protocol was approved by the Institutional Review Boards at New England Research Institutes and Boston University School of Medicine (BUSM). All participants gave written informed consent.
Data collection
Subjects were enrolled in BACH/Bone throughout the year (spring, 30.6%; summer, 20.7%; fall, 23.0%; and winter, 25.8%). Data collection was performed by trained staff in a research clinic at BUSM. Staff administered a brief bone-related risk factor questionnaire, conducted tests of physical function, collected a blood sample, and performed a bone density scan. Weight in kilograms was measured with a digital scale, and height (without shoes in centimeters) was measured with a stadiometer at the time of the BMD exam. Smoking status was defined as never smoked cigarettes, former smoker, or current smoker. The use of multivitamin and vitamin D supplements (yes/no) was also recorded.
Race and ethnicity
Race and ethnic group were determined according to modifications to the federal standard instituted by the U.S. Office of Management and Budget (15). It involved a two-step self-identification process. A subject was first asked, “Do you consider yourself to be Spanish, Hispanic, or Latino?” If the response was yes, they were asked to specify their Hispanic origin (e.g. Mexican-American, Puerto Rican, Cuban, etc.). Subjects were then asked, “What race do you consider yourself to be?” with the option of choosing multiple races. Possible responses were American-Indian or Alaskan Native, Asian, Black or African-American, Native Hawaiian or other Pacific Islander, White or Caucasian, and Other. We categorized men as Hispanic if they responded yes to the Hispanic question. Non-Hispanic respondents were categorized as Black if they stated they were Black or African-American, regardless of whether they checked more than one race, and White otherwise.
BMD
BMD (grams per square centimeter) at the hip (femoral neck, trochanter, and total hip), anteroposterior lumbar spine (L1–L4 spine) and forearm (one third distal radius and ultradistal radius) were measured by dual-energy x-ray absorptiometry (DXA) using a Hologic QDR 4500 W densitometer (Hologic, Inc., Waltham, MA). The left hip and left forearm were scanned for all subjects except those with artifacts or previous fracture; for these subjects, the right hip and right forearm was used. In this study, all DXA measurements were performed and analyzed by technicians trained by Hologic and certified by the International Society for Clinical Densitometry. The coefficients of variation for BMD at sites considered in this report are less than 1.5% (16). The DXA instrument was monitored for drift.
Serum 25(OH)D
All serum measurements were performed at The Core Laboratory, BUSM. Serum 25(OH)D [25(OH)D2 + 25(OH)D3] was measured in duplicate (the average of the two are presented) by a competitive protein binding (CPB) assay without prior chromatography as previously described (17) from serum collected at the BMD exam. The intra- and interassay coefficients of variation (CV) were 5–10 and 10–15%, respectively. The lower limit of detection for the serum 25(OH)D assay was 5 ng/ml (12.5 nmol/liter); 20 men (15 Black and five Hispanic men) with values less than the lower limit of detection were coded to 5 ng/ml. Sensitivity analyses were performed deleting these men or coding them to 2.5 ng/ml (6.25 nmol/liter); results were unchanged. The reference range was 20–100 ng/ml (50–250 nmol/liter).
Serum PTH
Serum bio-intact PTH (1–84) was measured in duplicate with the Nichols Advantage System (Nichols Institute Diagnostics, San Clemente, CA) from serum collected at the BMD exam. The sensitivity for the assay is 4.0 pg/ml, and the intra- and interassay CV are 2.2–3.6 and 5.6–8.3%, respectively.
Sampling weights
Sampling weights were used to produce estimates that are representative of the Black, Hispanic, and White Boston male population between the ages of 30 and 79 yr. Due to the stratified cluster sampling design of the BACH/Bone Survey, with oversampling of minority and older men, sampling weights were used to account for this and make the sample representative of Boston men age 30–79 yr. SUDAAN statistical analysis software (Research Triangle Institute, Research Triangle Park, NC) (18), which was designed for the analysis of complex survey data, was used for all analyses.
Analysis sample
Of the 1219 men recruited to the BACH/Bone Survey, there were 1124 men with at least one BMD site and a serum 25(OH)D measurement. When looking at the distribution of 25(OH)D, outlying observations were found where 25(OH)D was more than 80 ng/ml (200 nmol/liter). Sensitivity analyses were performed with and without the 10 observations (n = 1114 men), and the outlying observations were found to influence the relationship between BMD and serum 25(OH)D, possibly exaggerating it. Thus, to be conservative in describing the relationship between serum 25(OH)D and BMD, results of the analyses excluding the observations are presented.
Statistical analysis
Characteristics of the study participants are presented using ANOVA to compare continuous variables and χ2 tests of independence to compare categorical variables across racial and ethnic groups. In the case where the overall test yielded P < 0.05, individual t tests and χ2 tests were used to assess pairwise differences by race and ethnicity (e.g. Black vs. Hispanic, Black vs. White, Hispanic vs. White). Locally weighted linear regression (LOESS) was used to examine the relationship between BMD and serum 25(OH)D. The LOESS fit is nonparametric, does not require assumptions about the functional form of the relationship between two variables, and is resistant to the influence of outliers. Partial correlation coefficients (rp), adjusted first for age, and then for age, height, and weight, were used to estimate the linear relation between BMD at each bone site and 25(OH)D. To test whether the linear correlation between BMD site and 25(OH)D was significantly different from zero, regression models incorporating sampling weights to account for the oversampling of minority and older men were fit in SUDAAN. An interaction term, 25(OH)D × race and ethnic group, was used to examine race and ethnic group variation in the effect of 25(OH)D on BMD. Because the purpose of our analysis was descriptive, all results are presented without adjusting for multiple comparisons. Statistical significance was judged by P < 0.05. All statistical tests were two-tailed.
Results
The 1114 respondents were, as designed, roughly equally distributed by racial and ethnic group (331 Black, 362 Hispanic, and 421 White) with no race and ethnic difference in season of measurement (P = 0.76). As shown in Table 1, age, weight, and height varied by racial and ethnic group with Hispanic men generally being younger, thinner, and shorter in stature compared with the Black and White men. Mean PTH levels were slightly but not significantly higher in Black and Hispanic compared with White men. Median (25th, 75th percentiles) PTH values were as follows: Black, 27.4 (20.3, 40.3) pg/ml; Hispanic, 26.3 (19.9, 38.0) pg/ml; and White 27.1 (19.3, 36.2) pg/ml. Smoking status varied significantly among racial and ethnic groups with 37% of Black men reporting current smoking compared with 22% of Hispanic men and 22% of White men. Hispanic men were less likely to take vitamin supplements (19%) compared with one third of Black men (33%) and over 41% in White men. Vitamin D supplement use was low, and there were no racial or ethnic differences in use. As reported previously (14), Black men had significantly higher BMD levels than Hispanic and White men at all BMD sites considered. BMD levels in Hispanic men were found to differ significantly from White men only at the femoral neck.
Table 1.
Sample characteristics by race/ethnicity (n = 1114), BACH/Bone Survey, 2002–2005
| Variable | Race/ethnicity
|
P value | ||
|---|---|---|---|---|
| Black (n = 331) | Hispanic (n = 362) | White (n = 421) | ||
| Age, mean ± sd (yr) | 48.0 ± 12.5a | 44.4 ± 10.9b | 48.3 ± 13.1 | <0.001 |
| Weight, mean ± sd (kg) | 87.6 ± 17.0a | 82.1 ± 14.8b | 89.3 ± 15.1 | <0.001 |
| Height, mean ± sd (cm) | 175.0 ± 7.4a,b | 169.6 ± 6.2b | 177.2 ± 6.9 | <0.001 |
| Mean PTH level ± sd (pg/ml) | 33.9 ± 23.2 | 31.4 ± 21.5 | 29.9 ± 15.2 | 0.111 |
| PTH level > 50 pg/ml (%) | 12.5% | 11.9% | 8.2% | 0.35 |
| Smoking status (%) | 0.009 | |||
| Never | 38.5a,b | 52.4 | 46.2 | |
| Former | 24.6 | 25.6 | 31.4 | |
| Current | 36.9 | 22.1 | 22.4 | |
| Vitamin supplement user (%) | 32.7a | 19.4b | 41.4 | <0.001 |
| Vitamin D supplement user (%) | 2.1 | 1.7 | 3.7 | 0.44 |
| BMD ± sd (g/cm2) | ||||
| Hip: femoral neck | 0.94 ± 0.15a,b | 0.88 ± 0.14b | 0.84 ± 0.12 | <0.001 |
| Hip: trochanter | 0.81 ± 0.14a,b | 0.76 ± 0.12 | 0.75 ± 0.12 | <0.001 |
| Hip: total | 1.09 ± 0.15a,b | 1.02 ± 0.15 | 1.00 ± 0.14 | <0.001 |
| Spine: L1-L4 | 1.10 ± 0.15a,b | 1.00 ± 0.13 | 1.03 ± 0.15 | <0.001 |
| Forearm: distal radius | 0.80 ± 0.07a,b | 0.75 ± 0.06 | 0.76 ± 0.06 | <0.001 |
| Forearm: ultradistal radius | 0.56 ± 0.08a,b | 0.52 ± 0.07 | 0.52 ± 0.07 | <0.001 |
Means, sd, and percentages were adjusted inversely to the probability of selection. P values are from F-test for βBlack = βHispanic = 0 (continuous variables) where White men serve as the reference group or χ2 test of independence (categorical variables).
P < 0.05 vs. Hispanic from an individual test comparing the mean/frequency in Black men with the mean/frequency in Hispanic men.
P < 0.05 vs. White from an individual test comparing the mean/frequency in Black men or Hispanic men with the mean/frequency in White men.
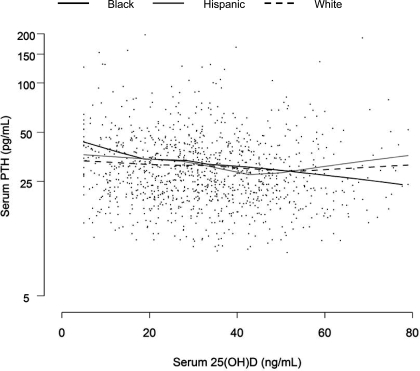
Serum 25(OH)D values also differed considerably by racial and ethnic group (Table 2); 25(OH)D was 50 and 14% higher in White compared with Black and Hispanic men, respectively (P < 0.01). Hispanic men had 32% higher 25(OH)D values than Black men (P < 0.01). Consistent with this, a higher percentage of Black and Hispanic subjects had 25(OH)D levels in the lowest quartile. Higher PTH values were observed in men with lower 25(OH)D. Interestingly, there were modest racial and ethnic differences in PTH according to vitamin D status (see Table 3), with relative differences in median PTH between men in the lowest vs. the highest quartile of 25(OH)D varying by race and ethnicity. Relative to men in the highest quartile of 25(OH)D, median PTH levels among men in the lowest quartile were 40, 22, and 29% higher among Black, Hispanic, and White men, respectively. This is further detailed in Fig. 1, which shows PTH concentration as a function of 25(OH)D concentration by race and ethnic group. Maximal suppression of PTH seemed to occur at a 25(OH)D level approximately 40–50 ng/ml among Hispanic and White men, whereas PTH decreased across the range of 25(OH)D concentrations in Black men.
Table 2.
Distributions of vitamin D status by race/ethnicity (n = 1114), BACH/Bone Survey, 2002–2005
| Variable | Race/ethnicity
|
P value | ||
|---|---|---|---|---|
| Black | Hispanic | White | ||
| Mean 25(OH)D ± sd (ng/ml) | 25.0 ± 14.7a,b | 32.9 ± 13.9b | 37.4 ± 14.0 | <0.001 |
| 25(OH)D quartiles (ng/ml) | <0.001 | |||
| Q1: 25(OH)D ≤ 20.8 | 44.4a,b | 23.1b | 11.4 | |
| Q2: 20.8 < 25(OH)D ≤ 31.3 | 25.6 | 24.5 | 27.2 | |
| Q3: 31.3 < 25(OH)D ≤ 42.7 | 18.2 | 30.8 | 28.5 | |
| Q4: 25(OH)D > 42.7 | 11.7 | 21.6 | 32.9 | |
Means, sd, and percentages were adjusted inversely to the probability of selection. P values are from F-test for βBlack = βHispanic = 0 (continuous variables) where White men serve as the reference group or χ2 test of independence (categorical variables). To convert 25(OH)D to nmol/liter, multiply values by 2.496.
P < 0.05 vs. Hispanic from an individual test comparing the mean/frequency in Black men to the mean/frequency in Hispanic men.
P < 0.05 vs. White from an individual test comparing the mean/frequency in Black men or Hispanic men to the mean/frequency in White men.
Table 3.
Median (25th, 75th percentiles) PTH by vitamin D status and race/ethnicity (n = 1113), BACH/Bone Survey, 2002–2005
| 25(OH)D quartiles (ng/ml) | PTH, median (25th, 75th percentiles) (pg/ml)
|
||
|---|---|---|---|
| Black | Hispanic | White | |
| Q1: 25(OH)D ≤ 20.8 | 30.9 (20.8, 41.6) | 28.8 (23.2, 38.0) | 31.0 (22.2, 41.0) |
| Q2: 20.8 < 25(OH)D ≤ 31.3 | 27.5 (20.5, 42.0) | 27.0 (19.8, 43.1) | 30.0 (21.5, 37.5) |
| Q3: 31.3 < 25(OH)D ≤ 42.7 | 31.0 (20.4, 34.9) | 24.8 (19.1, 36.4) | 27.0 (19.0, 38.0) |
| Q4: 25(OH)D > 42.7 | 22.1 (16.7, 30.4) | 23.6 (15.9, 34.0) | 24.1 (18.0, 32.1) |
Medians and 25th, 75th percentiles were adjusted inversely to the probability of selection. To convert 25(OH)D to nmol/liter, multiply values by 2.496.
Figure 1.
Serum PTH (pg/ml) as a function of serum 25(OH)D by race/ethnic group, natural log scale. Fitted lines are generated using LOESS (smoothing parameter = 0.75) (BACH/Bone 2002–2005).
Figure 2 shows scatterplots of site-specific BMD as a function of serum 25(OH)D by race and ethnic group. With the exception of the distal radius, these plots suggest a stronger association between BMD and 25(OH)D among White as compared with Black and Hispanic men. Note that we also examined the possibility of nonlinear associations between 25(OH)D and BMD, particularly evident among Whites. Evidence in support of this was very weak, with marginal significance (P = 0.05) found for a possible threshold effect of 25(OH)D on femoral neck BMD at approximately 47 ng/ml among White men (it should be noted that this is approximately the 75th percentile in Whites). Given the general lack of support for nonlinearity, we estimated race- and ethnic group-specific correlations between 25(OH)D and BMD. Consistent with the figure, we observed stronger correlations between 25(OH)D and BMD among White men (Table 4). After adjusting for age, weight, and height, there were no significant correlations between serum 25(OH)D and BMD among Black or Hispanic men. Among White men, 25(OH)D showed significant positive correlations with BMD at five of the six skeletal sites. We also stratified the sample by age (<50 and ≥50 yr) and observed the same pattern of results in the young and old men (data not shown), with formal testing of the interaction between race and ethnic group (Black, Hispanic, and White) and age (<50 and ≥ 50 yr) showing nonsignificance in all instances (all interaction P values > 0.28). In addition, statistical adjustment for season of measurement had no impact on the results, mainly due to similar distributions of season of measurement among the racial and ethnic groups and similar associations between race and ethnic group and 25(OH)D across season. We conducted a parallel analysis of race- and ethnic group-specific associations between PTH and BMD, and only one of the 18 correlations between PTH and BMD within race and ethnic group was significant (data not shown).
Figure 2.
Site-specific BMD (g/cm2) as a function of serum 25(OH)D by race/ethnic group, natural log scale: (A) femoral neck; (B) total hip; (C) lumbar spine; (D) distal radius. Fitted lines are generated using LOESS (smoothing parameter = 0.5) (BACH/Bone 2002–2005).
Table 4.
Age-, weight-, and height-adjusted partial correlation coefficients (rp) for serum 25-hydroxyvitamin D in relation to BMD measures by race/ethnicity, BACH/Bone Survey, 2002–2005
| Black
|
Hispanic
|
White
|
||||
|---|---|---|---|---|---|---|
| rp | P value | rp | P value | rp | P value | |
| Hip: femoral neck | −0.03 | 0.653 | 0.07 | 0.360 | 0.12 | 0.044 |
| Hip: trochanter | −0.01 | 0.853 | 0.06 | 0.375 | 0.13 | 0.009 |
| Hip: total | −0.06a | 0.387 | 0.07 | 0.301 | 0.11 | 0.034 |
| Spine: L1–L4 | −0.10a | 0.106 | −0.05a | 0.445 | 0.12 | 0.024 |
| Forearm: distal radius | −0.01 | 0.867 | 0.07 | 0.325 | 0.00 | 0.951 |
| Forearm: ultradistal radius | −0.02 | 0.766 | 0.02 | 0.799 | 0.14 | 0.008 |
P values shown test whether the correlation between BMD outcome and 25(OH)D within each race and ethnic group is 0.
P < 0.05 vs. White from an individual test comparing correlations in Black men or Hispanic men with those in White men.
Discussion
In this study of Black, Hispanic, and White men, we found modest, positive correlations between BMD at most sites and serum 25(OH)D in White men but not among Black or Hispanic men. After accounting for the effects of age, height, and weight, the magnitude of these correlations in White men are mostly greater than 0.10 and about twice the size of the correlations observed in Hispanic and Black men.
The role of vitamin D in maintaining bone health is well documented in White populations. Lips et al. (19) showed that low serum 25(OH)D was associated with low BMD in a large group of White postmenopausal osteoporotic women. Furthermore, low serum levels of 25(OH)D have been correlated with low BMD in men living in Finland and Austria (20,21). Most recently, Saquib et al. (22) also reported a positive association between serum 25(OH)D and BMD of the hip and spine in men from the Rancho Bernardo cohort, which is mainly White.
Data from the racially diverse NHANES III (6) are consistent with these previous studies. However, the strength of the association between 25(OH)D and BMD in NHANES III showed some variation by race and age group. Notably, consistent with our results, the association between 25(OH)D and BMD was strongest in White adults. The relatively weaker correlations between 25(OH)D and BMD observed among non-Whites in NHANES III and the current study are quite consistent with recent data showing that vitamin D supplementation had no impact on bone loss or bone turnover markers in a randomized controlled trial of calcium-replete, postmenopausal African-American women (23). This points to the possibility that the detrimental effects of low serum 25(OH)D are in some way mitigated in Black and Hispanic men.
Findings from previous studies suggest that the apparent disparity in the 25(OH)D-BMD relation among racial and ethnic groups might be explained by differences in rates of bone turnover. Markers of bone resorption are lower in Black men and women compared with White men and women, suggesting that Blacks may be more resistant to the bone-resorbing action of PTH (7,24,25), observations generally confined to studies comparing Black and White populations. In fact, a separate investigation in this sample comparing bone turnover markers by race and ethnic group shows significantly lower bone formation and resorption in Black as compared with White men and lower bone formation among Black compared with Hispanic men (26). To determine whether the differences in the 25(OH)D-BMD relation among racial and ethnic groups could be explained by differences in rates of bone turnover, we conducted additional analyses examining how serum markers of bone formation (osteocalcin) or resorption (C-terminal telopeptides of type-1 collagen) affected the association between 25(OH)D and BMD. Adjustment for osteocalcin or C-terminal telopeptides of type-1 collagen had no impact on the correlation coefficients reported in Table 4, and therefore, our results are not explained by racial and ethnic differences in bone turnover. Furthermore, our data on racial and ethnic differences in median PTH according to vitamin D status and the general lack of correlation between PTH and BMD within race and ethnic groups are not necessarily in support of the hypothesis that race and ethnic differences in the 25(OH)D-BMD relation is explained by differences in PTH action. Finally, another potential factor driving the racial and ethnic disparities in the vitamin D-bone relation may relate to polymorphisms or differences in expression of the vitamin D receptor (VDR) gene (27). Both the BsmI and FokI polymorphisms in the VDR gene are associated with BMD, and there is a large variation in allele frequencies of these genotypes among different racial and ethnic groups (28,29,30). Although there has been no evidence of effect modification by race and ethnicity on the relation of these VDR polymorphisms with BMD, the variation in genotype frequencies may account for differences in peak bone mass among racial and ethnic groups.
Limitations and strengths
The limitations and strengths of this study should be considered. First, only cross-sectional data on men spanning a broad age range were available to examine the relation between serum 25(OH)D and BMD in the cohort. This could be problematic if our observations are driven by racial and ethnic differences in peak bone mass as opposed to rates of bone loss with aging. To address this concern, we stratified the sample into two age groups and observed that the race and ethnic differences in the relationship between 25(OH)D and BMD that were observed in the whole sample also applied to both younger as well as older subjects. Second, we excluded 105 members of the participating cohort, mainly due to refusals with respect to blood draws. Nevertheless, these men did not differ with respect to BMD compared with study participants. Third, the serum 25(OH)D values in this study may appear to be high, but this is likely due in part to the fact that most of our subjects were otherwise healthy and active and, therefore, likely had significant cutaneous synthesis of vitamin D3. The CPB assay that was used in this study and RIAs in general often overestimate serum 25(OH)D levels by 15–20% because the CPB and RIAs recognize not only 25(OH)D but also 24,25-dihydroxyvitamin D as well as other metabolites in the serum sample. Typically, these metabolites circulate at a level of about 10–20% of the 25(OH)D levels, which could cause a small overestimation that is within the CV for the assay. Our CPB assay was compared with liquid chromatography-tandem mass spectrometry with a correlation coefficient of 0.70 (31). Furthermore, Lips et al. (32) have observed that routine assays for serum 25(OH)D can discriminate well between low, average, and high values. It is unlikely that bias in the 25(OH)D measurements, if present, would impact the association between 25(OH)D and BMD differentially by race and ethnicity. Finally, this sample is composed solely of men of either Black, Hispanic, or White racial and ethnic groups in the Boston area, and the results may not be generalizable to women or to other minority groups, e.g. Asian men, or to residents in other geographic regions.
Despite these limitations, this study has several unique strengths. This study sample represents one of the few population-based studies of a large, ethnically diverse group of men to evaluate the association between serum 25(OH)D and BMD. Second, direct serum measures of serum 25(OH)D were used instead of indirect measures such as questionnaire-based scales or food frequency intakes of vitamin D-rich foods. Third, our study design resulted in estimates of 25(OH)D concentrations that are not influenced by latitude because all subjects reside in Boston. Furthermore, they were not influenced by season of measurement, because an equal proportion of racial and ethnic groups were measured in each season of the year. As a result, the data more accurately reflect the impact of vitamin D status on BMD by race and ethnic group. Finally, we had multiple bone sites to allow us to confirm findings at the different axial and appendicular sites.
Conclusions
The paucity of studies on BMD in racially and ethnically diverse populations of men explains why we know little about the relationship between serum 25(OH)D and bone health in minority groups. Identification of important determinants of bone health in minority men is the first step in understanding more about the possible impact of bone fracture and interventions in older men. Given increasing numbers of elderly men in the United States, especially those of minority descent, it is important to identify modifiable risk factors of consequence because the public health impact may be quite substantial. In conclusion, despite much lower 25(OH)D levels in Black compared with White men, as we and others (6,8) have observed, BMD is higher in Black populations (11,14,33). This paradox may be explained, at least in part, by race and ethnic differences in the association between 25(OH)D and BMD.
Acknowledgments
We appreciate the statistical support of Gretchen R. Esche, M.S., and the technical support of Jeffery Mathieu.
Footnotes
First Published Online November 6, 2007
Abbreviations: BACH, Boston Area Community Health; BMD, bone mineral density; CPB, competitive protein binding; CV, coefficients of variation; DXA, dual-energy x-ray absorptiometry; 25(OH)D, 25-hydroxyvitamin D; VDR, vitamin D receptor.
The BACH/Bone Survey was supported by Grant AG-20727 from the National Institute on Aging. The parent study (BACH) was supported by Grant DK-56842 from the National Institute of Diabetes and Digestive and Kidney Diseases. Additional support came from MO RR00533.
This work was presented in part as an abstract at the 28th Annual Meeting of the American Society for Bone and Mineral Research, Philadelphia, Pennsylvania, June 2006.
Disclosure Summary: The authors have nothing to disclose.
References
- Holick MF 2004 Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80:1678S–1688S [DOI] [PubMed] [Google Scholar]
- DeLuca HF 1988 The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J 2:224–236 [PubMed] [Google Scholar]
- Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, Delmas PD, Meunier PJ 1992 Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327:1637–1642 [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Krall EA, Dallal GE, Falconer G, Green CL 1995 Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. Am J Clin Nutr 61:1140–1145 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martinez MA, Garcia-Cohen EC 2002 Role of Ca2+ and vitamin D in the prevention and treatment of osteoporosis. Pharmacol Ther 93:37–49 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B 2004 Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 116:634–639 [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B 2004 Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr 80:1763S–1766S [DOI] [PubMed] [Google Scholar]
- Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B 2000 Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab 85:4125–4130 [DOI] [PubMed] [Google Scholar]
- Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR 2002 Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30:771–777 [DOI] [PubMed] [Google Scholar]
- Looker AC, Orwoll ES, Johnston Jr CC, Lindsay RL, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP 1997 Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 12:1761–1768 [DOI] [PubMed] [Google Scholar]
- Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston Jr CC, Lindsay R 1998 Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489 [DOI] [PubMed] [Google Scholar]
- Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC 2005 Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res 20:1228–1234 [DOI] [PubMed] [Google Scholar]
- Baron JA, Barrett J, Malenka D, Fisher E, Kniffin W, Bubolz T, Tosteson T 1994 Racial differences in fracture risk. Epidemiology 5:42–47 [DOI] [PubMed] [Google Scholar]
- Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB 2007 Race/ethnic differences in bone mineral density in men. Osteoporos Int 18:943–953 [DOI] [PubMed] [Google Scholar]
- Wallman K, Evinger S, Schechter S 2000 Measuring our nation’s diversity: developing a common language for data on race/ethnicity. Am J Public Health 90:1704–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran D 1995 Precision in fan beam densitometry: a multi-site validation. Manufacturer’s specifications. Bedford, MA: Hologic [Google Scholar]
- Chen TC, Turner AK, Holick MF 1990 Methods for the determination of the circulating concentration of 25-hydroxyvitamin D. J Nutr Biochem 1:315–319 [DOI] [PubMed] [Google Scholar]
- Research Triangle Institute 2004 SUDAAN Language Manual Release 9.0. Research Triangle Park, NC: Research Triangle Institute [Google Scholar]
- Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T 2001 A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 86:1212–1221 [DOI] [PubMed] [Google Scholar]
- Lamberg-Allardt CJ, Outila TA, Karkkainen MU, Rita HJ, Valsta LM 2001 Vitamin D deficiency and bone health in healthy adults in Finland: could this be a concern in other parts of Europe? J Bone Miner Res 16:2066–2073 [DOI] [PubMed] [Google Scholar]
- Kudlacek S, Schneider B, Peterlik M, Leb G, Klaushofer K, Weber K, Woloszczuk W, Willvonseder R 2003 Assessment of vitamin D and calcium status in healthy adult Austrians. Eur J Clin Invest 33:323–331 [DOI] [PubMed] [Google Scholar]
- Saquib N, von Muhlen D, Garland CF, Barrett-Connor E 2006 Serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in men: the Rancho Bernardo study. Osteoporos Int 17:1734–1741 [DOI] [PubMed] [Google Scholar]
- Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK 2006 Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr 84:602–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell NH, Greene A, Epstein S, Oexmann MJ, Shaw S, Shary J 1985 Evidence for alteration of the vitamin D-endocrine system in blacks. J Clin Invest 76:470–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, Lindsay R, Parisien M 1997 Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res 12:958–966 [DOI] [PubMed] [Google Scholar]
- Leder BZ, Araujo AB, Travison TG, McKinlay JB 2007 Racial and ethnic differences in bone turnover markers in men. J Clin Endocrinol Metab 92:3453–3457 [DOI] [PubMed] [Google Scholar]
- Fang Y, van Meurs JB, d’Alesio A, Jhamai M, Zhao H, Rivadeneira F, Hofman A, van Leeuwen JP, Jehan F, Pols HA, Uitterlinden AG 2005 Promoter and 3′-untranslated-region haplotypes in the vitamin D receptor gene predispose to osteoporotic fracture: the Rotterdam study. Am J Hum Genet 77:807–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Umbach DM 1996 Are vitamin D receptor polymorphisms associated with bone mineral density? A meta-analysis. J Bone Miner Res 11:1841–1849 [DOI] [PubMed] [Google Scholar]
- Harris SS, Eccleshall TR, Gross C, Dawson-Hughes B, Feldman D 1997 The vitamin D receptor start codon polymorphism (FokI) and bone mineral density in premenopausal American black and white women. J Bone Miner Res 12:1043–1048 [DOI] [PubMed] [Google Scholar]
- Nelson DA, Vande Vord PJ, Wooley PH 2000 Polymorphism in the vitamin D receptor gene and bone mass in African-American and white mothers and children: a preliminary report. Ann Rheum Dis 59:626–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE 2005 Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224 [DOI] [PubMed] [Google Scholar]
- Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF 1999 An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int 9:394–397 [DOI] [PubMed] [Google Scholar]
- Cauley JA, Fullman RL, Stone KL, Zmuda JM, Bauer DC, Barrett-Connor E, Ensrud K, Lau EM, Orwoll ES 2005 Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int 16:1525–1537 [DOI] [PubMed] [Google Scholar]