Abstract
Context: Chorioamnionitis (CAM)-elicited preterm delivery (PTD) is associated with elevated amniotic fluid levels of IL-1β and TNF-α. We hypothesized that IL-1β and TNF-α may induce matrix metalloproteinase (MMP)-1 and MMP-3 activity to promote PTD by degrading decidual and fetal membranes and cervical extracellular matrix.
Objective: Our objective was to evaluate: 1) MMP-1 and MMP-3 expression in decidual sections from uncomplicated term, idiopathic preterm, and CAM-complicated deliveries, and 2) the separate and interactive effects of IL-1β, TNF-α, medroxyprogesterone acetate (MPA), and a p38 MAPK inhibitor (SB203580) on MMP-1 and MMP-3 expression in term decidual cells (DCs).
Interventions and Main Outcome Measures: Decidua were immunostained for MMP-1 and MMP-3. Cultured term DCs were incubated with estradiol (E2) or E2 plus MPA with or without IL-1β or TNF-α with or without SB203580. ELISA and Western blotting assessed secreted MMP-1 and MMP-3 levels, quantitative real-time RT-PCR assessed mRNA levels, and substrate gel zymography was used to determined MMP-1 and MMP-3 proteolytic activity.
Results: MMP-1 and MMP-3 immunostaining was more prominent in CAM-complicated decidua vs. control preterm and term decidual specimens (P < 0.05). Compared with basal outputs by DCs incubated with E2, TNF-α enhanced MMP-1 and MMP-3 secretion by 14 ± 3- and 9 ± 2-fold, respectively, and IL-1β increased MMP-1 and MMP-3 secretion by 13 ± 3- and 19 ± 2-fold, respectively (P < 0.05). Addition of MPA lowered basal MMP-1 and MMP-3 outputs by 70%, whereas the TNF-α- and IL-1β-enhanced MMP-1 and MMP-3 levels were blunted by more than 50% (P < 0.05). SB203580 suppressed TNF-α- and IL-1β-induced MMP-1 and MMP-3 secretion by severalfold. Western blotting confirmed the ELISA results, and mRNA levels corresponded with MMP-1 and MMP-3 protein levels. MMP-1 and MMP-3 proteolytic activity was confirmed by substrate gel zymography.
Conclusion: Augmented DC-expressed MMP-1 and MMP-3 in CAM-complicated pregnancies may promote PTD via decidual, fetal membrane, and cervical extracellular matrix degradation. Effects of progestin-p38 MAPK signaling inhibition on cytokine-enhanced MMP-1 and MMP-3 expression in term DCs suggest alternative mechanisms to prevent CAM-induced PTD.
The mechanisms that attribute chorioamnionitis (CAM) to preterm delivery (PTD) are not fully understood. Elevated levels of inflammatory cytokines found in CAM-complicated pregnancies stimulate production of matrix metalloproteinase which may promote PTD via fetal membrane and cervical extracellular matrix degradation. Inhibition of these proteins’ main signaling pathways, via progestin and p38 MAPK, suggests an alternative mechanism to prevent CAM-induced PTD.
In human pregnancy, chorioamnionitis (CAM) is a leading contributor to inflammation of the genital tract and placenta and is a primary cause of maternal morbidity and a major antecedent of preterm premature rupture of the membranes (PPROM) and preterm delivery (PTD) (1,2). The latter complicates about 12.7% of live births in the United States and is associated with perinatal morbidity and mortality (3,4). In CAM, bacteria and mycoplasma ascend from the vagina and cervix to the uterus and provoke deciduitis; these microorganisms can then invade the chorion, amnion, amniotic fluid, and fetus even when the membranes are intact. Romero and colleagues (5) detected positive cultures in the amniotic fluid in 23% of cases of PPROM. However, no signs of overt infection were evident in the amniotic fluid of 19% of PPROM cases. These samples contained elevated levels of neutrophil collagenase [matrix metalloproteinase (MMP)-8], suggesting that the infections remained localized to the decidua (5). Neutrophil elastase, defensins, and calgranulins have been identified as proinflammatory biomarkers in PTD-associated amniotic fluid (6,7).
The amniotic fluid of cases of PTD with coexisting CAM contains elevated levels of inflammatory mediators such as IL-6, IL-8, colony-stimulating factors, and macrophage inflammatory protein 1α and the potent proinflammatory cytokines IL-1β and TNF-α (8). Bacterial-derived lipopolysaccharides enhance IL-1β and TNF-α expression in human decidual cells and decidual macrophages (9,10). Our laboratory found that both cytokines increased expression of neutrophil chemotactic and activating chemokines in term decidual cell monolayers (11). Neutrophils are the predominant immune cell type in the decidua during CAM and deciduitis. They are rich sources of extracellular matrix (ECM)-degrading proteases, neutrophil elastase, as well the MMP family members MMP-8 and gelatinase B (MMP-9) (12,13).
The underlying hypothesis of the present study is that CAM-associated IL-1β and TNF-α enhance interstitial collagenase (MMP-1) and stromelysin-1 (MMP-3) expression in decidual cells, the predominant cell type in human decidua (14). Among MMP family members, MMP-1 preferentially degrades fibrillar collagens, which maintain the tensile strength of fetal membranes, whereas MMP-3 degrades an extremely broad array of ECM substrates and can activate the secreted zymogenic form of other MMPs, including pro-MMP-1 and bacterial-derived pro-MMP-9 (15,16,17). By targeting the ECM of the decidua and fetal membranes, neutrophil-derived proteases and decidual cell-derived MMP interactions are expected to promote PTD. A clinical trial demonstrating protective actions of a progestin against PTD (18) prompted our evaluation of the separate and interactive effects of a progestin with IL-1β and TNF-α on MMP-1 and MMP-3 mRNA and protein expression in leukocyte-free term decidual cells. To elucidate a potential mechanism regulating proinflammatory cytokine-enhanced MMP-1 and MMP-3 expression, experimental incubations were expanded to include a potent inhibitor of p38 MAPK, a key member of an intracellular signal transduction pathway that is activated by microbial pathogens and proinflammatory cytokines (19,20). The in vitro studies were augmented by immunohistochemical measurements of MMP-1 and MMP-3 in the decidua of normal term, idiopathic preterm, and CAM-complicated placentas.
Materials and Methods
Tissues
After receiving written informed consent, placentas and attached fetal membranes were obtained from patients with uncomplicated pregnancies experiencing spontaneous vaginal deliveries at term (n = 10) and preterm (n = 7), and from patients with intrauterine infection-, CAM-related PTDs (n = 10). Mean gestational ages ± sem were 38.9 ± 0.3 wk for term controls, 33.1 ± 1.5 wk for preterm controls, and 30.7 ± 1.8 wk for CAM. The difference in gestational ages of the preterm control vs. the CAM specimens was not statistically significantly (P = 0.491). Each preterm control specimen was examined histologically to rule out underlying acute or chronic inflammation and decidual hemorrhage such as CAM and chronic villitis. Positive histology for CAM was defined as more than 10 neutrophils per high-power field in the subchorionic space, chorion, or placental plate, as based on the criteria reported by Naeye et al. (21). Clinical CAM was diagnosed in the presence of maternal fever (>37.8 C), uterine tenderness, foul-smelling amniotic fluid, or visualization of pus at the time of the speculum exam, maternal tachycardia (≥100 beats/min), and fetal tachycardia (160 beats/min) (22). All procedures were performed at Yale-New Haven Hospital under Yale Human Investigation Committee approval.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections were deparaffinized in xylene and rehydrated in a graded series of ethanol. For antigen retrieval, slides to be stained for MMP-3 were boiled in citrate buffer (10 mm; pH 6.0) for 20 min and then incubated with 0.05% trypsin-EDTA (Invitrogen Corp., Grand Island, NY) for 7 min; all other slides were boiled in citrate buffer for 15 min. Slides were then incubated in a humidified chamber with 5% blocking horse serum in Tris-buffered saline (TBS; Vector Laboratories, Inc., Burlingame, CA) for 30 min at room temperature to block nonspecific binding. After excess serum was removed, the sections were incubated with primary antibodies [goat polyclonal antihuman MMP-1 antibody (1:20 dilution; R&D Systems, Inc., Minneapolis, MN), mouse monoclonal antihuman anti-MMP-3 antibody (1:10 dilution; Calbiochem, Inc., San Diego, CA), and mouse monoclonal antihuman anti-vimentin antibody (1:100 dilution; DakoCytomation, Carpinteria, CA), diluted in TBS] overnight at 4 C in a humidified chamber. For negative controls, sections were treated with either normal goat or mouse or IgG isotypes at the same concentration as the primary antibodies. Sections were rinsed in TBS three times for 5 min each, and then the appropriate biotinylated horse antigoat or antimouse antibody (1.5 mg/ml; Vector) was added at a 1:400 dilution for 30 min at room temperature. After three washes in TBS, the antigen-antibody complex was detected with an avidin-biotin-alkaline phosphatase kit (Vector). Alkaline Phosphatase Substrate Kit I (Vector Red) was used as the chromogen, and sections were counterstained with Harris hematoxylin (Sigma Chemical Co., St. Louis, MO) and mounted with Permount (Fisher Chemicals, Springfield, NJ) on glass slides.
Vimentin immunoreactivity was used to distinguish decidual cells in decidual basalis sections. The intensity of MMP-1 and MMP-3 immunostaining was evaluated semiquantitatively, employing the following categories: 0 (no staining), 1+ (weak, but detectable, staining), 2+ (moderate or distinct staining), and 3+ (intense staining). For each tissue, an HSCORE value was derived by calculating the sum of the percentage of cells that stained at each intensity category and multiplying that value by the weighted intensity of the staining, using the formula HSCORE = ΣPi(i + l), where i represents the intensity score and Pi is the corresponding percentage of cells staining. In each slide, five different areas and 100 cells per field were evaluated microscopically at ×40 objective magnification. Two independent investigators blinded to the samples performed the evaluation, and their average score was then used.
Isolation of decidual cells
After receiving written informed consent, placentas and attached fetal membranes were obtained from patients with uncomplicated pregnancies undergoing repeat cesarean deliveries at term at Yale-New Haven Hospital under Human Investigation Committee approval. None of the patients from whom specimens were obtained was in labor. The decidua was separated from the amniochorionic tissues, and a small portion of the former was formalin fixed and paraffin embedded and then examined histologically for signs of underlying acute and chronic inflammation. The remainder was used for the isolation of decidual cells.
Decidual cells were isolated from minced, digested tissues as previously described (23) and passaged three times. To analyze the isolation purity, fluorescent antibody cell sorting (FACS) was used to detect the presence of CD45+ cells. Briefly, cells were washed in PBS supplemented with 2% fetal calf serum and incubated with monoclonal antibody on ice for 30 min, followed by washing twice with PBS (pH 7.2) and 2% fetal calf serum. Cell sorting and fluorescence measurements were performed on a MoFLo high-performance cell sorter (DakoCytomation). For fluorescence measurements only, data from 10,000–50,000 single-cell events were collected using a standard FACScalibur flow cytometer (Immunocytometry Systems; Becton Dickinson, San Jose, CA). Data were analyzed using CELLQuest (Becton Dickinson) or Flow Jo (Treestar, Ashland, OR) as previously described (24) and demonstrated that unpassaged cultures contained 12–15% CD45+ cells, whereas passaged cultures were more than 99% free of this common leukocyte marker. The latter were used for experimental cell incubations. Monolayers of these decidual cells were 99% vimentin positive and cytokeratin negative and displayed decidualization-related morphological and biochemical changes during incubation with medroxyprogesterone acetate (MPA). Cell aliquots were frozen in fetal calf serum/dimethylsulfoxide (9:1) (Sigma-Aldrich, St. Louis, MO) and stored in liquid nitrogen.
Experimental incubations
Decidual cells were grown and passaged three times as previously described (23) and grown to confluence in serum-containing medium and incubated in parallel in either 0.1% ethanol as vehicle control or 10−8 mol/liter estradiol (E2) with or without 10−7 mol/liter MPA. After 7 d, the cultures were then switched to a serum-free defined medium (DM) with either vehicle or steroids or 0.01–10 ng/ml IL-1β or TNF-α (R&D Systems, Minneapolis, MN). In a separate set of experiments, the cultured decidual cells were preincubated in DM with or without a p38 MAPK inhibitor [SB203580 (SB); Calbiochem] for 30 min before initiating the standard experimental treatment in DM. After the test period, cells, harvested cell lysates, and conditioned medium supernatants were stored at −70 C. Total RNA was extracted from parallel incubations with Tri-Reagent (Sigma-Aldrich).
ELISA
After a 24-h incubation, immunoreactive MMP-1 and MMP-3 levels in conditioned DM were measured using specific ELISAs according to the manufacturer’s instructions (R&D Systems). The sensitivities of the ELISAs for MMP-1 and MMP-3 were 0.021 and 0.009 ng/ml, respectively. For MMP-1, the intra- and interassay coefficients of variation were 4.6 and 9.4, respectively; for MMP-3, they were 6.1 and 7.8. According to the manufacturer, there is no significant cross-reactivity or interference with other known MMPs in these assays. Levels of MMP-1 and MMP-3 were normalized to the total cell culture protein content, as measured by a modified Lowry protein assay (Bio-Rad Laboratories, Hercules, CA).
Substrate gel zymography
Conditioned DM from term decidual cell cultures was centrifuged and the supernatant mixed 1:1 with nonreducing Zymogram sample buffer (Bio-Rad) and then incubated for 10 min at room temperature. Samples were loaded onto a 12% casein Zymogram gel (Bio-Rad) and then electrophoresed for 2 h in Tris-glycine SDS running buffer. To enable the enzymes to renature, the gel was incubated for 45 min in 25% Triton X-100 at room temperature and incubated in Zymogram development buffer (Bio-Rad) for 30 min and then placed in fresh Zymogram development buffer overnight at 37 C. The gel was stained with 0.5% Coomassie Blue (Sigma) solution of methanol/acetic acid/water (40:10:50 vol/vol) for 1 h at room temperature and then destained with methanol/acetic acid/water (30:10:60 vol/vol) for 4 h at room temperature. Zymograms from three separate and unrelated term decidual cell preparations were used, and the band intensities were quantified using computer-based densitometry analysis (ImageJ; National Institutes of Health, Bethesda, MD).
Real-time quantitative RT-PCR
At the end of the 6-h experimental incubations, RNA was isolated from cultures with Tri-Reagent (Sigma-Aldrich Corp.). To verify that the MMP-1, MMP-3, and β-actin probes yielded the correct bands, extracted RNA was subjected to semiquantitative RT-PCR using a kit from Invitrogen Life Technologies, Inc. (Carlsbad, CA), which was carried out for 35 cycles with a MasterCycler (Eppendorf, Westbury, NY). To perform quantitative real-time RT-PCR, RT was initially carried out with avian myeloblastosis virus reverse transcriptase (Invitrogen Life Technologies). A quantitative standard curve was created between 1 and 40 ng cDNA with a Roche LightCycler by monitoring increasing fluorescence of PCR products during amplification. Quantification of the unknowns was then determined with the LightCycler 3 data analysis software and adjusted to the quantitative expression of β-actin from the corresponding unknowns. Melting curve analysis determined the specificity of the amplified products and the absence of primer-dimer formation. All products obtained yielded correct melting temperatures. The following primers were synthesized and gel purified at the Yale DNA Synthesis Laboratory, Critical Technologies: β-actin sense, 5′-CGTACCACTGGCATCGTGAT-3′, and antisense, 5′-GTGTTGGCGTACAGGTCTTTG-3′ (452 bp); MMP-1 sense, 5′-ACTACGATTCGGGGAG-3′, and antisense, 5′-CTTGGGGTATCCGTGT-3′ (225 bp); and MMP-3 sense, 5′-CCTGCTTTGTCCTTTGATGC-3′, and antisense, 5′-TGAGTCAATCCCTGGAAAGTC-3′ (432 bp).
Western blot analysis
At the end of the 24-h treatment, conditioned DM supernatants were diluted 1:1 in reducing sample buffer composed of Laemmli sample buffer and 2-mercaptoethanol (Bio-Rad) and then boiled for 3 min at 95 C. Soluble proteins were separated by SDS-PAGE on a 10% linear gradient Tris-HCl gel (Bio-Rad) under reducing conditions and electroblotted onto a 0.2-μm pore size nitrocellulose membrane (Bio-Rad) in transfer buffer. The membrane was blocked for 2 h at room temperature in TBS (Sigma) with 4.5% nonfat dry milk and then incubated overnight at 4 C with a 1:1000 dilution of a goat polyclonal antihuman MMP-1 antibody (R&D Systems). The blot was washed in PBS and 0.2% Tween 20 (PBS-T) and then incubated with horseradish peroxidase-conjugated antigoat IgG (ICN Biomedicals, Inc., Aurora, OH) diluted in 4.5% nonfat dry milk in TBS for 1 h and then washed in PBS-T. Chemiluminescence was detected with ECL reagents (Perkin-Elmer Life Sciences, Boston, MA) and visualized by light emission on film (Amersham Pharmacia, Little Chalfont, UK). The membrane was then stripped for 20 min at 50 C in buffer containing dH2O, 1 m Tris-HCl (pH 6.7), 10% SDS, and β-mercaptoethanol. The blot was washed in PBS-T and blocked for 2 h in TBS with 4.5% nonfat dry milk and then incubated overnight at 4 C with a 1:1000 dilution of a mouse monoclonal anti-MMP-3 antibody (Calbiochem). The membrane was rinsed in PBS-T before and after incubation with horseradish peroxidase-conjugated antimouse IgG in 4.5% nonfat dry milk in TBS for 1 h. Chemiluminescence was detected as indicated above.
Statistical analysis
Gestational ages were normally distributed and analyzed by Student’s t test. The HSCOREs of immunostaining in control vs. CAM cases were normally distributed as determined by the Kolmogorov-Smirnov test, and therefore results were analyzed with one-way ANOVA, followed by post hoc Holm-Sidak testing. The ELISA and quantitative RT-PCR results were not normally distributed, and therefore, pair-wise multiple comparisons were analyzed with nonparametric ANOVA on ranks (Kruskal-Wallis test), followed by post hoc Student-Newman-Keuls test. Statistical calculations were performed using SigmaStat version 3.0 (Jandel Scientific Corp., San Rafael, CA). Statistical significance was defined as P < 0.05.
Results
Immunostaining for MMP-1 and MMP-3 in specimens of human deciduas
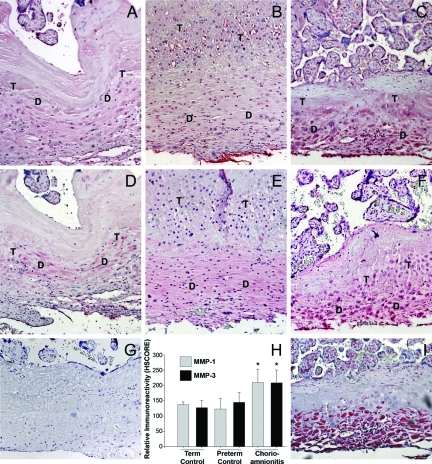
In sections of decidua basalis shown in Fig. 1, the decidual cells are distinguished by positive immunostaining for vimentin (Fig. 1I). Immunohistochemical staining reveals that decidual cells in term, preterm, and CAM-complicated tissues express both MMP-1 (Fig. 1, A–C, respectively) and MMP-3 (Figs. 1, D–F, respectively). There was no significant difference in HSCORE values for either MMP-1 or MMP-3 immunoreactivity between preterm (mean ± sem, 123 ± 14 and 145 ± 12, respectively; Fig. 1H) and term control decidua (137 ± 3 and 127 ± 7; Fig. 1H). In contrast, the HSCOREs for MMP-1 and MMP-3 in decidual cells of CAM-complicated tissues (210 ± 14 and 209 ± 13, Fig. 1H) were significantly higher than those of control preterm and term decidua (P < 0.001).
Figure 1.
Immunoreactive MMP-1 and MMP-3 expression in decidual cells in placental sections from term, preterm, and CAM-complicated deliveries. Representative photomicrographs of immunohistochemical staining for MMP-1 and MMP-3 in term decidua (A and D, respectively), preterm decidua (B and E, respectively), and CAM-complicated decidua (C and F, respectively) with positive signals are in red. Decidual cell-enriched areas (D) and trophoblast-enriched areas (T) are noted. Vimentin staining (I) was used to identify decidual cells. Staining with isotype-matched control antibody (G) revealed no positive staining. HSCORE analysis (H) revealed significantly higher levels of MMP-1 and MMP-3 staining in CAM-complicated tissues (n = 10), compared with tissues from term (n = 10) and preterm (n = 7) deliveries (*, P < 0.001 vs. term and preterm). Magnifications, ×200.
Regulation of MMP-1 and MMP-3 protein expression in cultured term decidual cells
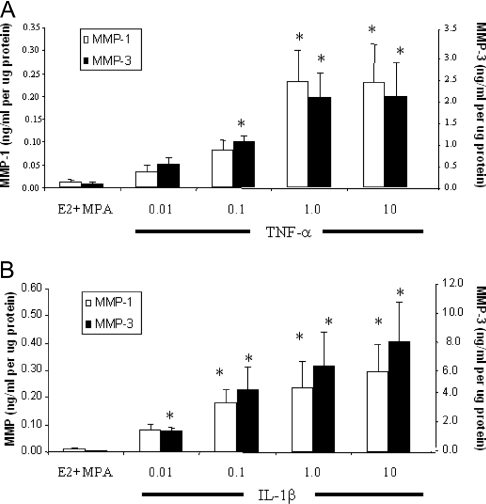
As indicated by Fig. 2, addition of 0.1–10 ng/ml TNF-α or IL-1β to the cultures elicited dose-dependent increases in MMP-1 and MMP-3 secreted levels with MMP-3 output exceeding MMP-1 output by about one log over the range of cytokine concentrations studied (n = 3; P < 0.05).
Figure 2.
Concentration-dependent effects of TNF-α and IL-1β on MMP-1 and MMP-3 output by E2- plus MPA-treated term decidual cell monolayers. Confluent, leukocyte-free decidual cells were incubated for 7 d in 10−8 m E2 plus 10−7 m MPA and then switched to DM with steroids alone or together with TNF-α (A) or IL-1β (B) for 24 h. The abscissa indicates cytokine concentrations in nanograms per milliliter. Levels of MMP-1 and MMP-3 were measured by ELISAs in conditioned DM and normalized to cell protein (mean ± sem; n = 3). Note the different values on the ordinates corresponding to MMP-1 and MMP-3. *, P < 0.05 vs. E2 plus MPA.
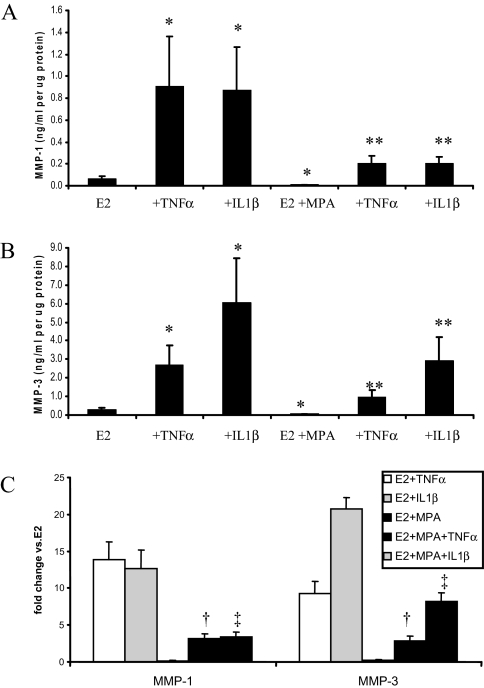
In term decidual cells incubated with E2 alone, basal MMP-1 output [0.065 ± 0.03 ng/ml/μg protein (mean ± sem, n = 7)] was increased to 0.91 ± 0.45 and to 0.87 ± 0.39 ng/ml/μg protein by TNF-α and IL-1β, respectively (P < 0.05) (Fig. 3A). The addition of MPA significantly reduced basal MMP-1 output to 0.007 ± 0.002 ng/ml/μg protein as well as blunted the increase in MMP-1 output elicited by both TNF-α and IL-1β to 0.20 ± 0.07 and 0.20 ± 0.06 ng/ml/μg protein, respectively. Figure 3B indicates that MMP-3 levels conformed to the same pattern as MMP-1. Specifically, in term decidual cell monolayers incubated with E2 alone, basal MMP-3 output [0.28 ± 0.11 ng/ml/μg protein (mean ± sem, n = 8)] was increased to 2.70 ± 1.02 and to 6.06 ± 2.38 ng/ml/μg protein in incubations with TNF-α and IL-1β, respectively (P < 0.05.) The addition of MPA significantly inhibited basal MMP-3 output (0.05 ± 0.01 ng/ml/μg protein as well as blunted the enhanced MMP-3 output elicited by TNF-α and IL-1β (0.97 ± 0.36 and 2.90 ± 1.27 ng/ml/μg protein, respectively; P < 0.05).
Figure 3.
Effects of E2, MPA, TNF-α, and IL-1β on MMP-1 and MMP-3 output by term decidual cell monolayers. Confluent, leukocyte-free decidual cells were incubated for 7 d in 10−8 m E2 or E2 plus 10−7 m MPA and then switched to DM with corresponding steroid(s) with or without 1 ng/ml TNF-α or IL-1β for 24 h. Levels of MMP-1 and MMP-3 were measured by ELISA in conditioned DM and normalized to cell protein (mean ± sem). P < 0.05: *, vs. E2; **, vs. E2 plus MPA; †, vs. corresponding fold change for E2 plus TNF-α; ‡, vs. corresponding fold change for E2 plus IL-1β (mean ± sem). A, MMP-1 (n = 7); B, MMP-3 (n = 8); C, MMP-1 and MMP-3 (n = 7 or 8).
Figure 3C presents the results in Fig. 3, A and B, as fold change from E2 alone and indicates that for MMP-1, the 13.8 ± 2.5-fold and 12.6 ± 2.6-fold elicited by TNF-α and IL-1β, respectively, were significantly reduced to 3.2 ± 0.6-fold and 3.4 ± 0.7-fold in parallel incubations with E2 plus MPA. Similarly, for MMP-3, the 8.5 ± 1.6-fold and 19.4 ± 1.9-fold increase elicited by TNF-α and IL-1β, respectively, were significantly reduced in parallel incubations with E2 plus MPA to 3.3 ± 0.7-fold and 8.8 ± 1.3-fold, respectively.
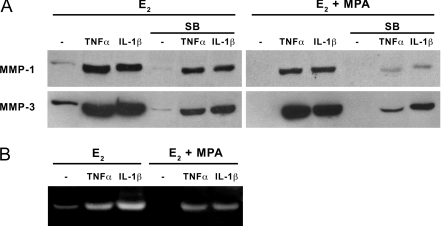
The Western blot displayed in Fig. 4A demonstrates that conditioned DM supernatants from incubations of term decidual cells with E2 alone contains a single band at the appropriate molecular mass of 59/57 kDa identified by specific antibodies as MMP-1 (upper panel) and MMP-3 (lower panel). Consistent with the ELISA results shown in Fig. 3, both the MMP-1 and MMP-3 bands were enhanced by TNF-α and IL-1β, and MPA reduced both basal output and cytokine-enhanced outputs for both MMPs. Figure 4A also indicates that a specific p38 MAPK inhibitor (SB) reduced the increase by TNF-α and IL-1β of the MMP-1 and MMP-3 bands in incubations with E2 or E2 plus MPA.
Figure 4.
A, Western blot analysis of E2, MPA, TNF-α, IL-1β, and p38 MAPK inhibitor SB effects on MMP-1 and MMP-3 output by term decidual cell monolayers. Confluent, leukocyte-free decidual cells were incubated for 7 d in 10−8 m E2 or E2 plus 10−7 m MPA and then switched to DM with corresponding steroid(s) in the presence or absence of 1 ng/ml TNF-α or IL-1β for 24 h with or without exposure to 10−5 m SB 30 min before treatment. Aliquots of conditioned DM were subjected to Western blot analysis. Results of a representative experiment are shown. B, Substrate gel zymography of E2, MPA, TNF-α, and IL-1β effects on MMP activity by term decidual cell monolayers. Confluent, leukocyte-free decidual cells were primed for 7 d in DM containing vehicle control (C), 10−8 m E2, or E2 plus 10−7 m MPA and then incubated for 24 h in DM with corresponding steroid(s) in the presence or absence of 1 ng/ml TNF-α or IL-1β. Conditioned DM was run on a 12% casein gel zymogram and then subjected to renaturing and staining procedures. Proteolytic activity of the active MMPs can be seen as clear bands around 45 kDa in the darkly stained gel (representative zymogram shown).
The results of substrate gel zymography shown in Fig. 4B indicate that term decidual cell-conditioned DM displays caseinolytic activity in the molecular mass range corresponding to the active forms of MMP-1 and MMP-3 (approximately 45 kDa). Proteolytic activity of MMP-1/3 was markedly inhibited by E2 plus MPA in comparison with E2 alone; basal E2-only stimulation had very weak levels of MMP-1/3 proteolytic activity, and no MMP-1/3 activity was seen upon addition of MPA. TNF-α and IL-1β enhanced the levels of the active forms of MMP-1/3 (14.7 ± 4.6- and 20.1 ± 7.4-fold increase vs. E2 alone, respectively), which were decreased upon addition of MPA (7.9 ± 2.7- and 11.7 ± 4.4-fold vs. E2 alone, respectively).
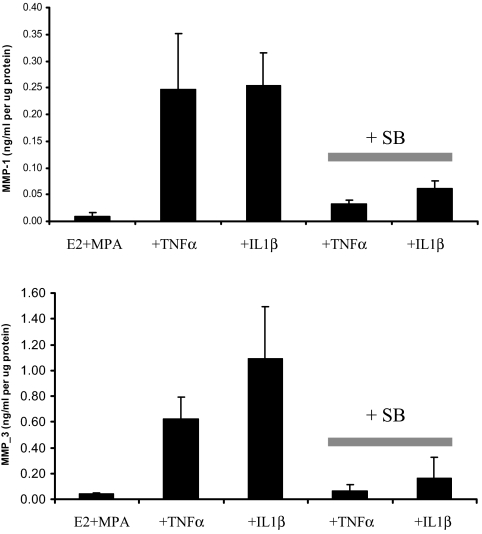
Figure 5 confirms that the inhibitory effects of SB observed by Western blotting are also demonstrable by subjecting the conditioned DM supernatants to ELISA measurements. Specifically, in E2- plus MPA-primed decidual cells for the two experiments depicted in Fig. 5, preincubation with SB markedly inhibited the severalfold up-regulation induced by TNF-α and IL-1β for both MMP1 (Fig. 5A) and MMP-3 (Fig. 5B).
Figure 5.
ELISA analysis of E2, MPA, TNF-α, and IL-1β, and p38 MAPK inhibitor SB effects on MMP-1 and MMP-3 output by term decidual cell monolayers. Confluent, leukocyte-free decidual cells were incubated for 7 d in 10−8 m E2 plus 10−7 m MPA and then switched to DM with corresponding steroids in the presence or absence of 1 ng/ml TNF-α or IL-1β for 24 h with or without exposure to 10−5 m SB 30 min before treatment. MMP-1 and MMP-3 levels were quantified by ELISA in conditioned DM and normalized to cell protein (mean ± sd; n = 2).
MMP-1 and MMP-3 mRNA expression in cultured term decidual cells
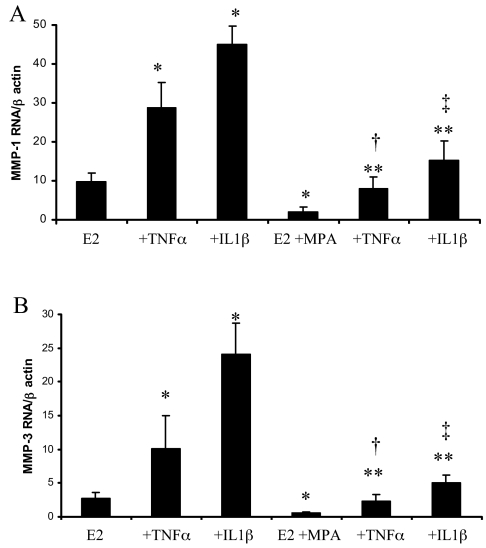
Figure 6 demonstrates that steady-state MMP-1 and MMP-3 mRNA levels corresponded to the ELISA findings of Fig. 3. Specifically, in cultures maintained in E2 alone, 1 ng/ml TNF-α or IL-1β increased expression of MMP-1 mRNA (3.7 ± 1.4-fold and 5.7 ± 1.8-fold, respectively (n = 4, mean ± sem, P < 0.05) and MMP-3 mRNA (4.6 ± 1.9 and 11.2 ± 2.7-fold, respectively, P < 0.05). Moreover, MPA significantly reduced basal levels of both MMP-1 and MMP-3 mRNA by more than 60% and inhibited the up-regulatory effects of TNF-α and IL-1β on the expression of MMP-1 (about 60%) and MMP-3 (about 80%).
Figure 6.
Effects of E2, MPA, TNF-α, and IL-1β on MMP-1 and MMP-3 mRNA levels in term decidual cell monolayers. Confluent, leukocyte-free decidual cells were incubated for 7 d in 10−8 m E2 plus 10−7 m MPA and then switched to DM with E2 plus 10−7 m MPA alone or together with TNF-α or IL-1β for 6 h. Quantitative real-time RT-PCR was performed on extracted RNA for MMP-1 and MMP-3 and normalized to β-actin mRNA (mean ± sem, n = 4). P < 0.05: *, vs. E2; **, vs. E2 plus MPA; †, vs. corresponding E2 plus TNF-α; ‡, vs. corresponding E2 plus IL-1β. A, MMP-1 mRNA/β-actin mRNA; B, MMP-3 mRNA/β-actin mRNA.
Discussion
Infections of the cervix, decidual chorionic tissues, or amniotic cavity by such microorganisms as group B streptococcus, Escherichia coli, Neisseria gonorrhoeae, and Chlamydia trachomatis elicit CAM (9). Neutrophils are key mediators of innate immunity. They arrive initially at sites of local injury and inflammation where they phagocytose and kill invading pathogens to promote acute inflammation via release of proteases and reactive oxygen species (25). Contact between bacteria or bacteria-derived products such as lipopolysaccharide, lipid A, or lipoteichoic acid initiates a signaling cascade in both immune and nonimmune cells that furthers the inflammatory response and is associated with a reduction in chorioamniotic membrane integrity and tensile strength (26). The primary role played by the decidua in responding to infections has been demonstrated in experiments with full-thickness amnion-choriodecidual membranes, which showed much greater enhancement of IL-1β and TNF-α expression when exposure to lipopolysaccharide and group B streptococcus was initiated at the choriodecidua than at the amnion sides of these membranes (10).
The current study demonstrated that decidual cells of placental sections from CAM patients exhibit significantly elevated MMP-1 and MMP-3 immunohistochemical levels when compared with term or idiopathic preterm control specimens. In the decidual cells, immunostaining for both MMPs was localized primarily to the cytoplasm. The mechanism underlying CAM-related up-regulation of MMP-1 and MMP-3 expression in the decidua was then assessed using monolayer cultures of leukocyte-free term decidual cells. The in vitro results demonstrated that mRNA and protein levels for both MMPs were markedly augmented in incubations with either IL-1β or TNF-α. By contrast, MPA significantly inhibited both basal and proinflammatory cytokine-enhanced MMP-1 and MMP-3 mRNA and protein expression. As expected from the well-established role played by p38 MAPK in mediating intracellular signal transduction in response to microbial pathogens and proinflammatory cytokines (19,20), SB, a potent p38 MAPK inhibitor, blunted enhancement of secreted MMP-1 and MMP-3 levels elicited by IL-1β and TNF-α. Other intracellular signaling pathways such as nuclear factor-κB (27,28) and protein kinase C (29,30) are likely to contribute to enhanced MMP-1 and MMP-3 expression elicited by IL-1β and TNF-α and are worth investigating in this regard.
Leukocytes are recruited into inflammatory sites by migrating down a gradient of chemotactic agents and then interacting with endothelial cell-expressed adhesion molecules (31,32). Bacteria, immune cells, and resident cells of the inflamed tissue are documented sources for these chemoattractants, which include bacterial chemotactic peptide, platelet-activating factor, leukotriene B4, and an array of chemokines. Among the chemokines, members of the CXC chemokine superfamily are highly specific neutrophil chemoattractants and activators (33,34). Previous studies from our laboratory observed elevated immunohistochemical levels of the key neutrophil chemoattractant and activator IL-8 in the decidua of women with CAM vs. control deciduas (11). In leukocyte-depleted first-trimester decidual cells, IL-1β and TNF-α markedly enhanced the expression of IL-8 as well as three other CXC chemokine superfamily members, growth-related oncogene-α, epithelial-cell-derived neutrophil attractant-78, and granulocyte chemotactic protein-2, that also recruit neutrophils to inflammatory sites (34). The concerted actions of these chemokines are likely to contribute significantly to the characteristic neutrophil infiltration of the CAM-associated decidua.
Human fetal membranes contain a fibrillar collagen-enriched ECM that plays an integral role in maintaining its structural integrity and tensile strength (35,36,37). This ECM is susceptible to degradation by neutrophil-derived proteases and inflammatory cytokine-enhanced MMPs expressed by the resident decidual cell population. Neutrophils are rich sources of neutrophil elastase, a serine protease that degrades phagocytosed and ECM proteins (6), as well as MMP-8 and MMP-9, which respectively degrade fibrillar collagens and basement membrane-associated collagens IV and V (12,38). The MMPs that were found in the current study to be overexpressed by decidual cells during CAM can also effectively degrade the decidual and fetal membrane ECM. Specifically, MMP-1, a member of the gelatinase subfamily, degrades basement membrane-associated collagens as well as denatured fibrillar collagens (gelatins). Cleavage of the helical structure of fibrillar collagens by MMP-1 initiates their denaturation to gelatins, which are then processed further by MMP-3 and MMP-3-activated MMP-9 (39,40,41). An integral member of the stromelysin subfamily, MMP-3 can degrade such ECM components as proteoglycans, glycoproteins, fibronectin, laminin, type IV and V collagen, and both the nonhelical amino- and carboxyl-terminal peptides of type II collagen as well as the globular but not the triple-helical regions of interstitial collagens (39,40). That MMP-3 can also degrade gelatins formed by MMP-1 and activate the secreted, zymogenic forms of MMP-1 and neutrophil-expressed pro-MMP-9 (15,17) places MMP-3 in a pivotal position in the proteolytic cascade that targets the ECM of the decidua and fetal membranes to promote PPROM.
In summary, the current study indicates that MMP-1 and MMP-3 expression is significantly up-regulated in CAM-complicated decidual specimens. We further found that inflammatory cytokines associated with CAM, IL-1β, and TNF-α strongly enhance MMP-1 and MMP-3 expression in decidual cells via involvement of the p38 MAPK pathway. This co-up-regulation is consistent with the breakdown of the ECM of the decidua and fetal membranes leading to PPROM and PTD. The additional observation that MPA inhibits basal and cytokine-enhanced MMP-1 and MMP-3 expression in the decidual cells is consistent with the results of a multicenter clinical trial demonstrating protection against PTD by the progestin metabolite 17α-hydroxyl-progesterone caproate (18). These findings suggest that MMP-1 and MMP-3 are important mediators of CAM-related membrane rupture and that targeting their expression through the use of progestin or p38 MAPK inhibitor may have significant treatment potential for the prevention of PTD.
Footnotes
This work was supported by National Institutes of Health (2 R01HD33937-05 and 1 R01 HL070004-04, both to C.J.L.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 16, 2007
Abbreviations: CAM, Chorioamnionitis; DM, defined medium; E2, estradiol; ECM, extracellular matrix; MMP, matrix metalloproteinase; MPA, medroxyprogesterone acetate; PBS-T, PBS and 0.2% Tween 20; PPROM, preterm premature rupture of the membranes; PTD, preterm delivery; SB, SB203580; TBS, Tris-buffered saline.
References
- Mueller-Heubach E, Rubinstein DN, Schwarz SS 1990 Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol 75:622–626 [PubMed] [Google Scholar]
- Lockwood CJ, Kuczynski E 1999 Markers of risk for preterm delivery. J Perinat Med 27:5–20 [DOI] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, Ventura SJ 2006 Births: preliminary data for 2005. Natl Vital Stat Rep 55:1–18 [PubMed] [Google Scholar]
- Newton ER 2005 Preterm labor, preterm premature rupture of membranes, and chorioamnionitis. Clin Perinatol 32:571–600 [DOI] [PubMed] [Google Scholar]
- Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, Yoon BH 2004 Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 191:1339–1345 [DOI] [PubMed] [Google Scholar]
- Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, Ohlsson K, Uldbjerg N 2002 Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med 12:237–246 [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Christner R, Buhimschi CS 2005 Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. BJOG 112:173–181 [DOI] [PubMed] [Google Scholar]
- Saji F, Samejima Y, Kamiura S, Sawai K, Shimoya K, Kimura T 2000 Cytokine production in chorioamnionitis. J Reprod Immunol 47:185–196 [DOI] [PubMed] [Google Scholar]
- Singh U, Nicholson G, Urban BC, Sargent IL, Kishore U, Bernal AL 2005 Immunological properties of human decidual macrophages: a possible role in intrauterine immunity. Reproduction 129:631–637 [DOI] [PubMed] [Google Scholar]
- Zaga V, Estrada-Gutierrez G, Beltran-Montoya J, Maida-Claros R, Lopez-Vancell R, Vadillo-Ortega F 2004 Secretions of interleukin-1β and tumor necrosis factor α by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod 71:1296–1302 [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Arcuri F, Toti P, Felice CD, Krikun G, Guller S, Buchwalder LF, Schatz F 2006 Tumor necrosis factor-α and interleukin-1β regulate interleukin-8 expression in third trimester decidual cells: implications for the genesis of chorioamnionitis. Am J Pathol 169:1294–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, Yoon BH 2000 Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol 183:94–99 [DOI] [PubMed] [Google Scholar]
- Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L 2000 Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest 80:617–653 [DOI] [PubMed] [Google Scholar]
- Dunn CL, Kelly RW, Critchley HO 2003 Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online 7:151–161 [DOI] [PubMed] [Google Scholar]
- Ogata Y, Enghild JJ, Nagase H 1992 Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem 267:3581–3584 [PubMed] [Google Scholar]
- Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G 1996 Biochemical characterization of human collagenase-3. J Biol Chem 271:1544–1550 [DOI] [PubMed] [Google Scholar]
- He CS, Wilhelm SM, Pentland AP, Marmer BL, Grant GA, Eisen AZ, Goldberg GI 1989 Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl Acad Sci USA 86:2632–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spong CY, Meis PJ, Thom EA, Sibai B, Dombrowski MP, Moawad AH, Hauth JC, Iams JD, Varner MW, Caritis SN, O’Sullivan MJ, Miodovnik M, Leveno KJ, Conway D, Wapner RJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S 2005 Progesterone for prevention of recurrent preterm birth: impact of gestational age at previous delivery. Am J Obstet Gynecol 193:1127–1131 [DOI] [PubMed] [Google Scholar]
- Herlaar E, Brown Z 1999 p38 MAPK signalling cascades in inflammatory disease. Mol Med Today 5:439–447 [DOI] [PubMed] [Google Scholar]
- Ono K, Han J 2000 The p38 signal transduction pathway: activation and function. Cell Signal 12:1–13 [DOI] [PubMed] [Google Scholar]
- Naeye RL, Maisels MJ, Lorenz RP, Botti JJ 1983 The clinical significance of placental villous edema. Pediatrics 71:588–594 [PubMed] [Google Scholar]
- Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D, Sehgal P 1993 The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol 169:805–816 [DOI] [PubMed] [Google Scholar]
- Cakmak H, Schatz F, Huang ST, Buchwalder L, Rahman M, Arici A, Lockwood CJ 2005 Progestin suppresses thrombin- and interleukin-1β-induced interleukin-11 production in term decidual cells: implications for preterm delivery. J Clin Endocrinol Metab 90:5279–5286 [DOI] [PubMed] [Google Scholar]
- Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL 2003 Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med 198:1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ 1989 Tissue destruction by neutrophils. N Engl J Med 320:365–376 [DOI] [PubMed] [Google Scholar]
- Schoonmaker JN, Lawellin DW, Lunt B, McGregor JA 1989 Bacteria and inflammatory cells reduce chorioamniotic membrane integrity and tensile strength. Obstet Gynecol 74:590–596 [PubMed] [Google Scholar]
- Lauder SN, Carty SM, Carpenter CE, Hill RJ, Talamas F, Bondeson J, Brennan P, Williams AS 2007 Interleukin-1β induced activation of nuclear factor-κB can be inhibited by novel pharmacological agents in osteoarthritis. Rheumatology (Oxford) 46:752–758 [DOI] [PubMed] [Google Scholar]
- Bondeson J, Lauder S, Wainwright S, Amos N, Evans A, Hughes C, Feldmann M, Caterson B 2007 Adenoviral gene transfer of the endogenous inhibitor IκBα into human osteoarthritis synovial fibroblasts demonstrates that several matrix metalloproteinases and aggrecanases are nuclear factor-κB-dependent. J Rheumatol 34:523–533 [PubMed] [Google Scholar]
- Alexander JP, Acott TS 2001 Involvement of protein kinase C in TNFα regulation of trabecular matrix metalloproteinases and TIMPs. Invest Ophthalmol Vis Sci 42:2831–2838 [PubMed] [Google Scholar]
- Yokoo T, Kitamura M 1996 Opposite, binary regulatory pathways involved in IL-1-mediated stromelysin gene expression in rat mesangial cells. Kidney Int 50:894–901 [DOI] [PubMed] [Google Scholar]
- Janeway C 2005 Immunobiology: the immune system in health and disease. 6th ed. New York: Garland Science; 37–100 [Google Scholar]
- Baggiolini M 1995 Activation and recruitment of neutrophil leukocytes. Clin Exp Immunol 101(Suppl)1:5–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck KA 1999 Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-κB. Int J Mol Med 4:223–230 [DOI] [PubMed] [Google Scholar]
- Thomson AW 1998 The cytokine handbook. 3rd ed. San Diego: Academic Press; 271–311 [Google Scholar]
- Malak TM, Ockleford CD, Bell SC, Dalgleish R, Bright N, Macvicar J 1993 Confocal immunofluorescence localization of collagen types I, III, IV, V and VI and their ultrastructural organization in term human fetal membranes. Placenta 14:385–406 [DOI] [PubMed] [Google Scholar]
- Bryant-Greenwood GD, Yamamoto SY 1995 Control of peripartal collagenolysis in the human chorion-decidua. Am J Obstet Gynecol 172:63–70 [DOI] [PubMed] [Google Scholar]
- Qin X, Chua PK, Ohira RH, Bryant-Greenwood GD 1997 An autocrine/paracrine role of human decidual relaxin. II. Stromelysin-1 (MMP-3) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1). Biol Reprod 56:812–820 [DOI] [PubMed] [Google Scholar]
- Parks WC, Mecham RP 1998 Matrix metalloproteinases. San Diego: Academic Press; 15–148 [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA 1993 Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4:197–250 [DOI] [PubMed] [Google Scholar]
- Nagase H 1995 Human stromelysins 1 and 2. Methods Enzymol 248:449–470 [DOI] [PubMed] [Google Scholar]
- McDonnell S, Wright JH, Gaire M, Matrisian LM 1994 Expression and regulation of stromelysin and matrilysin by growth factors and oncogenes. Biochem Soc Trans 22:58–63 [DOI] [PubMed] [Google Scholar]