Abstract
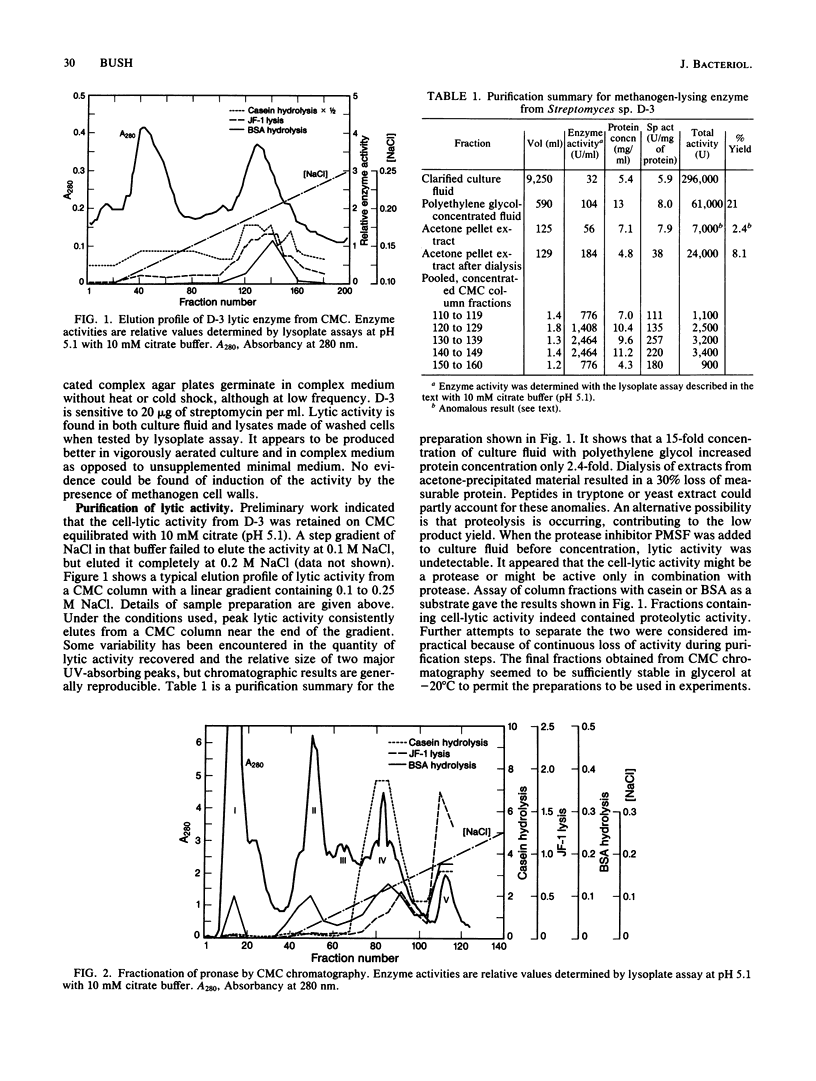
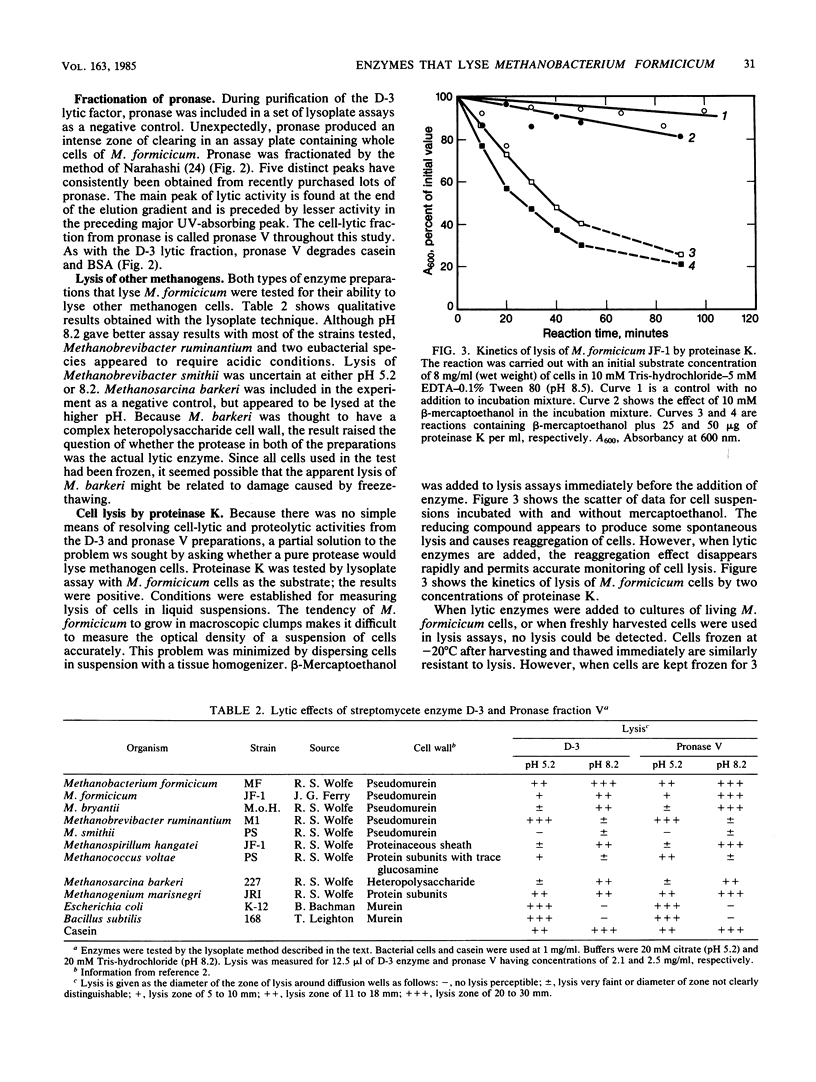
A streptomycete isolated from cow manure produces an extracellular enzyme capable of lysing the pseudomurein-containing methanogen Methanobacterium formicicum. The lytic activity has been partially purified from culture fluid and appears to be a serine protease. Similar lytic activity has been fractionated from pronase. Optimal conditions have been developed for lysis of M. formicicum by commercial preparations of proteinase K. The three lytic enzymes have been partially characterized. The results with the three enzyme preparations tend to confirm that proteolytic enzymes are capable of lysing methanogen cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker H. W., Fritz H. Enzymic characterization of rabbit blastocyst proteinase with synthetic substrates of trypsin-like enzymes. Hoppe Seylers Z Physiol Chem. 1979 Feb;360(2):107–113. doi: 10.1515/bchm2.1979.360.1.107. [DOI] [PubMed] [Google Scholar]
- Doddema H. J., van der Drift C., Vogels G. D., Veenhuis M. Chemiosmotic coupling in Methanobacterium thermoautotrophicum: hydrogen-dependent adenosine 5'-triphosphate synthesis by subcellular particles. J Bacteriol. 1979 Dec;140(3):1081–1089. doi: 10.1128/jb.140.3.1081-1089.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Ebeling W., Hennrich N., Klockow M., Metz H., Orth H. D., Lang H. Proteinase K from Tritirachium album Limber. Eur J Biochem. 1974 Aug 15;47(1):91–97. doi: 10.1111/j.1432-1033.1974.tb03671.x. [DOI] [PubMed] [Google Scholar]
- Herzig R. H., Ratnoff O. D., Shainoff J. R. Studies on a procoagulant fraction of southern copperhead snake venom: the preferential release of fibrinopeptide B. J Lab Clin Med. 1970 Sep;76(3):451–465. [PubMed] [Google Scholar]
- Jarrell K. F., Colvin J. R., Sprott G. D. Spontaneous protoplast formation in Methanobacterium bryantii. J Bacteriol. 1982 Jan;149(1):346–353. doi: 10.1128/jb.149.1.346-353.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. B., Bowers B., Stadtman T. C. Methanococcus vannielii: ultrastructure and sensitivity to detergents and antibiotics. J Bacteriol. 1977 Jun;130(3):1357–1363. doi: 10.1128/jb.130.3.1357-1363.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O., Hippe H. Lack of peptidoglycan in the cell walls of Methanosarcina barkeri. Arch Microbiol. 1977 May 13;113(1-2):57–60. doi: 10.1007/BF00428580. [DOI] [PubMed] [Google Scholar]
- Kandler O., König H. Chemical composition of the peptidoglycan-free cell walls of methanogenic bacteria. Arch Microbiol. 1978 Aug 1;118(2):141–152. doi: 10.1007/BF00415722. [DOI] [PubMed] [Google Scholar]
- König H., Kandler O. 2-amino-2-deoxytaluronic acid and 2-amino-2-deoxyglucose from the pseudomurein of Methanobacterium termoautotrophicum possess the L- and D-configurations, respectively. Hoppe Seylers Z Physiol Chem. 1980;361(6):981–983. [PubMed] [Google Scholar]
- König H., Kandler O., Jensen M., Rietschel E. T. The primary structure of the glycan moiety of pseudomurein from Methanobacterium thermoautotrophicum. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):627–636. doi: 10.1515/bchm2.1983.364.1.627. [DOI] [PubMed] [Google Scholar]
- König H., Kandler O. The amino acid sequence of the peptide moiety of the pseudomurein from Methanobacterium thermoautotrophicum. Arch Microbiol. 1979 Jun;121(3):271–275. doi: 10.1007/BF00425067. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langenberg K. F., Bryant M. P., Wolfe R. S. Hydrogen-oxidizing methane bacteria. II. Electron microscopy. J Bacteriol. 1968 Mar;95(3):1124–1129. doi: 10.1128/jb.95.3.1124-1129.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrum L. J., Luehrsen K. R., Woese C. R. Are extreme halophiles actually "bacteria"? J Mol Evol. 1978 May 12;11(1):1–8. doi: 10.1007/BF01768019. [DOI] [PubMed] [Google Scholar]
- Morihara K., Oka T., Tsuzuki H. Subtilisin BPN': kinetic study with oligopeptides. Arch Biochem Biophys. 1970 Jun;138(2):515–525. doi: 10.1016/0003-9861(70)90376-0. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pähler A., Banerjee A., Dattagupta J. K., Fujiwara T., Lindner K., Pal G. P., Suck D., Weber G., Saenger W. Three-dimensional structure of fungal proteinase K reveals similarity to bacterial subtilisin. EMBO J. 1984 Jun;3(6):1311–1314. doi: 10.1002/j.1460-2075.1984.tb01968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. Isolation of Streptomyces spp. capable of decomposing preparations of cell walls from various micro-organisms and a comparison of their lytic activities with those of certain actinomycetes and myxobacteria. J Gen Microbiol. 1955 Feb;12(1):25–30. doi: 10.1099/00221287-12-1-25. [DOI] [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955 Apr;16(4):570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- Sauer F. D., Mahadevan S., Erfle J. D. Methane synthesis by membrane vesicles and a cytoplasmic cofactor isolated from Methanobacterium thermoautotrophicum. Biochem J. 1984 Jul 1;221(1):61–69. doi: 10.1042/bj2210061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sprott G. D., Colvin J. R., McKellar R. C. Spheroplasts of Methanospirillum hungatii formed upon treatment with dithiothreitol. Can J Microbiol. 1979 Jun;25(6):730–738. doi: 10.1139/m79-106. [DOI] [PubMed] [Google Scholar]
- Svendsen L., Blombäck B., Blombäck M., Olsson P. I. Substrates for determination of trypsin, thrombin and thrombin-like enzymes. Folia Haematol Int Mag Klin Morphol Blutforsch. 1972;98(4):446–454. [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Fox G. E. Archaebacteria. J Mol Evol. 1978 Aug 2;11(3):245–251. doi: 10.1007/BF01734485. [DOI] [PubMed] [Google Scholar]
- Wood A. G., Redborg A. H., Cue D. R., Whitman W. B., Konisky J. Complementation of argG and hisA mutations of Escherichia coli by DNA cloned from the archaebacterium Methanococcus voltae. J Bacteriol. 1983 Oct;156(1):19–29. doi: 10.1128/jb.156.1.19-29.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Wählby S. Studies on Streptomyces griseus protease. I. Separation of DFP-reacting enzymes and purification of one of the enzymes. Biochim Biophys Acta. 1968 Feb 5;151(2):394–401. doi: 10.1016/0005-2744(68)90106-x. [DOI] [PubMed] [Google Scholar]