Abstract
Although exercise improves individual risk factors of the metabolic syndrome (MS), there is little research on the effect of exercise on MS as a whole. The objective of this study was to determine how much exercise is recommended to reduce the prevalence of MS. Of 334 subjects randomized, 227 finished and 171 (80 women, 91 men) had complete data for all 5 Adult Treatment Panel III-defined MS risk factors and were included in this analysis. Subjects were randomly assigned to a six-month control or 1 of 3 eight-month exercise training groups: 1) low-amount/moderate-intensity (equivalent to walking ~19 km/week); 2) low-amount/vigorous-intensity (equivalent to jogging ~19 km/week); 3) high-amount/vigorous-intensity (equivalent to jogging ~32 km/week). The low-amount/moderate-intensity exercise prescription improved MS relative to inactive controls (p<0.05). However, the same amount of exercise at a vigorous intensity was not significantly better than inactive controls, suggesting that lower intensity exercise may be more effective in improving MS. The high-amount/vigorous-intensity group improved MS relative to controls (p<0.0001), the low-amount/vigorous-intensity group (p=0.001), and the moderate intensity group (p=0.07), suggesting an exercise dose effect. In conclusion, a modest amount of moderate intensity exercise, in the absence of dietary changes, significantly improved MS and thus supports the recommendation that adults get 30 minutes of moderate intensity exercise every day. A higher amount of vigorous exercise was shown to have greater and more widespread benefits. Finally, there is an indication that moderate intensity may be better than vigorous intensity exercise for improving MS.
Keywords: exercise training, dose effects, insulin sensitivity, central adiposity
The U.S. National Cholesterol Education Program Adult Treatment Panel III (ATPIII) has defined metabolic syndrome (MS) as the presence of 3 or more of the following risk factors: increased waist circumference, low HDL-cholesterol, elevated triglycerides, hypertension and impaired fasting glucose1. These risk factors and their relationship to the development of cardiovascular disease have been well-documented while individual risk factor reduction with medical therapy and lifestyle intervention have also been examined. However, the concurrent reduction of multiple risk factors due to various exercise training regimens is under-investigated. The current study presents data from the Studies of a Targeted Risk Reduction Intervention through Defined Exercise (STRRIDE) trial on the effects of varying amounts and intensities of exercise in relation to multiple risk factors comprising the metabolic syndrome.
METHODS
Subjects
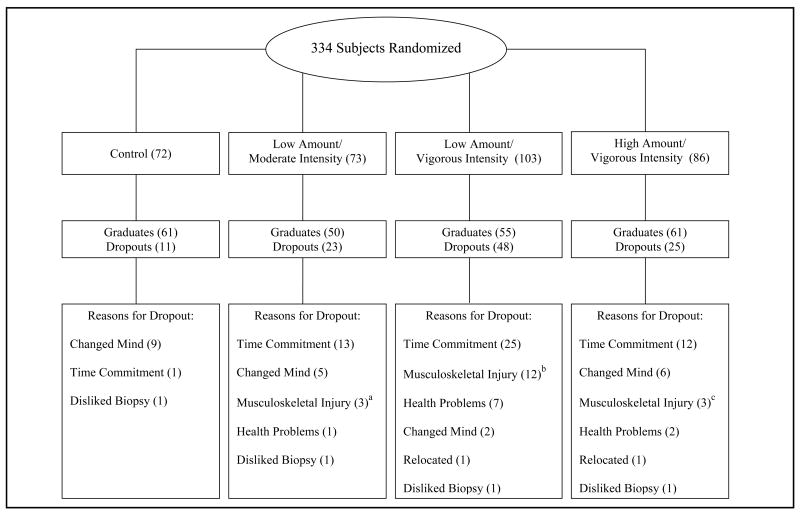
Three hundred and thirty-four subjects met inclusion criteria and were randomized to 1 of 4 groups: 3 exercise training groups and an inactive control group (Figure 1). Of these randomized subjects, 227 completed the study and 171 had complete pre- and post-training data for all 5 MS criteria and represent the study population for this analysis. A complete description of the STRRIDE study design has been described elsewhere2. All participants were overweight to mildly obese (body mass index of 25 to 35), sedentary adults, 40 to 65 years old with no known history of cardiovascular disease, diabetes or hypertension. All women were post-menopausal. Due to the original study design, inclusion criteria mandated an elevated LDL-cholesterol concentration (≥130 mg/dl and ≤190 mg/dl) or a decreased HDL-cholesterol concentration (<40 mg/dl for men and <45 mg/dl for women). Subjects were non-smokers and were not taking any lipid-altering medications. Written informed consent was obtained from each subject prior to participation.
Figure 1.
Flowchart of randomization graduates, dropouts and reasons for dropout. a 2 out of 3 due to preexisting injuries. b 4 out of 12 due to preexisting injuries. c 3 out of 3 due to preexisting injuries. Note: 56 graduates were excluded from the present analysis due to missing data for one or more of the ATPIII-defined metabolic syndrome criteria.
Intervention
The 3 exercise training groups were as follows: 1) low amount/moderate intensity [caloric equivalent of approximately 19 km (12 miles)/week at 40–55% of peak oxygen consumption], 2) low amount/vigorous intensity [caloric equivalent of approximately 19 km (12 miles)/week at 65–80% peak oxygen consumption], and 3) high amount/vigorous intensity [caloric equivalent of approximately 32 km (20 miles)/week at 65–80% peak oxygen consumption].
The actual amount of exercise prescribed was calculated as a weekly expenditure of 14 kcal per kilogram of body weight for the 2 low amount groups and 23 kcal/kg for the high amount group. Table 1 details the exercise prescription for each group and the actual exercise amount performed based on subject compliance. Adherence was calculated for each subject as the number of minutes completed within the assigned heart rate range each week divided by the minutes prescribed. No subjects were excluded from the analysis based on their adherence.
TABLE 1.
Exercise Prescription and Adherence by Group
| Variable | Low Amount/Moderate Intensity | Low Amount/Vigorous Intensity | High Amount/Vigorous Intensity |
|---|---|---|---|
| Exercise Prescription | |||
| Intensity (% peak oxygen consumption) | 40–55% | 65–80% | 65–80% |
| Prescription amount (km/week)a | 19.3 | 19.3 | 32.2 |
| Prescription amount (Kcal/week) | 1221 (222) | 1201 (181) | 2024 (313) |
| Prescription time (minutes/week) | 205 (43) | 128 (29) | 200 (38) |
| Actual Exercise Dose | |||
| Adherence | 88 (14%) | 92 (10%) | 86 (11%) |
| Actual amount (km/week)b | 17.0 | 17.8 | 27.7 |
| Actual time (minutes/week)c | 179 (37) | 114 (29) | 175 (36) |
| Frequency (sessions/week) | 3.5 (0.6) | 3.0 (0.5) | 3.7 (0.7) |
Data are shown as mean (SD)
Prescription amount is presented as the approximate number of km/week that are calorically equivalent to the prescribed kcal/week of 14 kcal/kg/week for the low dose groups and 23 kcal/kg/week for the high dose group.
Actual amount = Prescription amount × Adherence for each group (therefore no SD).
Actual time = Prescription time × Adherence for each subject.
The intensity of prescribed exercise was based on the individual results of maximal cardiopulmonary exercise (CPE) tests. All exercise groups underwent a 2 to 3 month initial ramp period, whereby amount and intensity of exercise were gradually increased to minimize the chance of injury. This period was followed by 6 months of training at the assigned exercise prescription. Maximal CPE tests were repeated at the end of the ramp period to adjust the exercise prescription for improvements in peak oxygen consumption during these initial exercise sessions. Appropriate intensity for the remaining six-month training period was maintained by an increase in work rate as needed to exercise within a specific target heart rate range. All exercise sessions were verified by direct supervision or by heart rate monitors that supplied recorded heart rate files (Polar Electro, Inc.). Approved modalities included treadmills, elliptical machines and cycle ergometers. The unmonitored control group was instructed to maintain an inactive lifestyle for 6 months. All groups were counseled to maintain dietary intake as unchanged throughout the trial and dietary measures were collected to confirm there were no significant differences or changes between or within study groups.
Anthropometric and Blood Pressure Measurements
Height and weight were determined for each subject while wearing light clothing and no shoes. Waist circumference was taken at the minimal waist, the narrowest portion of the torso between the umbilicus and the xiphoid process. Blood pressure readings were taken prior to each CPE test.
Lipids and Insulin Sensitivity
HDL-cholesterol and triglycerides were determined from fasting plasma samples using nuclear magnetic resonance spectroscopy (LipoScience; Raleigh, NC)3. Insulin sensitivity was determined with a 3-hour intravenous glucose tolerance test4, which was performed 16 to 24 hours after the final exercise bout. Blood samples from pre- and post-intervention were assayed simultaneously and included samples from all study groups. An insulin sensitivity index was calculated using the minimal model computer algorithm4.
Metabolic Syndrome
The revised ATPIII criteria for MS are: 1) increased waist circumference (≥102 cm in men; ≥88 cm in women), 2) elevated triglycerides (≥150 mg/dl), 3) reduced HDL-cholesterol (<40 mg/dl in men, <50 mg/dl in women), 4) elevated blood pressure (≥130 mmHg systolic or ≥85 mmHg diastolic), and 5) elevated fasting glucose (≥100 mg/dl)5.
Statistical Analyses
Analysis of variance was used to test for differences between groups and to evaluate any gender differences at baseline. Paired t-tests were performed to determine any significant changes within the groups for each of the MS variables and scores. When examining differences between the 4 groups, a two-way ANOVA was used to account for any gender differences. As no significant gender-group interactions (p>0.05) were observed, all gender interactions were removed from the model and the analysis repeated (SAS; Cary, NC).
The ATPIII criteria for MS are the current clinical standard5 and are necessarily dichotomous for clinical purposes, whereby if 3 or more of the 5 metabolic variables are above a certain threshold an individual is considered as having MS. For the purpose of measuring the effects of an intervention, there are several reasons a continuous score, rather than a series of dichotomous scores, might more accurately represent and detect overall metabolic changes. We propose that this continuous score would: 1) be more sensitive to small and large changes that do not meet the criteria of the ATPIII score (e.g. a triglyceride value that substantially improves from 250 to 151 mg/dl would have no effect on the ATPIII score, but would be reflected in the continuous MS score) and 2) be less sensitive to small changes that occur in the vicinity of diagnostic criteria for any one variable (e.g. a triglyceride change from 152 to 149 mg/dl would have a significant effect on the ATPIII score, but little effect on a continuous MS score). To test the effect of such a score on the responses in this study, we designed a continuous MS Z-score.
The MS Z-score used for the present study is a continuous score of the 5 MS variables. A modified Z-score was calculated for each variable using individual subject data, the ATPIII criteria and standard deviations (denominator of each factor in the formulae) using data from the entire STRRIDE cohort at baseline (n=334). Gender-specific Z-scores were used to account for variations in the ATPIII criteria for men and women. The equations used were: Z-score = [(50-HDL)/14.1] + [(TG-150)/81.0] + [(FBG-100)/11.3] + [(WC-88)/9.0] + [(MAP-100)/9.1] for women, and Z-score = [(40-HDL)/9.0] + [(TG-150)/81.0] + [(FBG-100)/11.3] + [(WC-102)/7.7] + [(MAP-100)/9.1] for men. The concept of a continuous score to evaluate MS has also been utilized by Wijndaele et al.6 and Franks and colleagues7 elsewhere.
An ATPIII score was also calculated for each subject as a sum of the ATPIII criteria met at baseline and end-of-study. Prevalence data were then evaluated pre- and post-intervention. To assess the significance of these changes, we applied McNemar’s test, which uses as its inputs the 2×2 table cross-classifying the ATPIII criteria (i.e., present/absent) pre- and post-intervention. Exact p-values were obtained from applying the binomial distribution to those subjects whose status differed between pre- and post-intervention.
RESULTS
Baseline characteristics are depicted in Table 2. Women displayed more desirable risk profiles than the men as the mean HDL-cholesterol was significantly higher and the triglycerides, diastolic blood pressure, and fasting plasma glucose were all significantly lower than that of the men. There were no significant differences between groups for any of the variables at baseline.
TABLE 2.
Descriptive Characteristics by Group
| Variable | All | Control | Low Amount/Moderate Intensity | Low Amount/Vigorous Intensity | High Amount/Vigorous Intensity | ||||
|---|---|---|---|---|---|---|---|---|---|
| n=171 (91 men; 80 women) | n=41 (21 men; 20 women) | n=41 (20 men; 21 women) | n=45 (24 men; 21 women) | n=44 (26 men; 18 women) | |||||
| Baseline | Baseline | Change | Baseline | Change | Baseline | Change | Baseline | Change | |
| Age (years) | |||||||||
| All | 53 ± 7 | 53 ± 8 | 54 ± 5 | 53 ± 7 | 51 ± 6 | ||||
| Men | 51 ± 7 § | 50 ± 8 | 53 ± 6 | 51 ± 7 | 50 ± 6 | ||||
| Women | 55 ± 5 | 56 ± 6 | 55 ± 4 | 56 ± 6 | 53 ± 5 | ||||
| Body mass index (kg/m2) | |||||||||
| All | 29.7 ± 2.9 | 30.2 ± 3.1 | 0.3 ± 0.9 | 29.9 ± 3.2 | −0.1 ± 0.7 | 29.7 ± 3.0 | −0.2 ± 0.8 | 29.2 ± 2.4 | −0.8 ± 0.9 ††† |
| Men | 29.9 ± 2.8 | 29.8 ± 2.9 | 0.5 ± 0.8 †† | 30.1 ± 3.1 | −0.3 ± 0.8 | 29.9 ± 3.3 | −0.3 ± 0.8 | 29.9 ± 2.0 | −0.8 ± 0.8 ††† |
| Women | 29.5 ± 3.1 | 30.5 ± 3.3 | 0.0 ± 1.0 | 29.7 ± 3.3 | 0.0 ± 0.6 | 29.5 ± 2.7 | −0.1 ± 0.8 | 28.2 ± 2.6 | −0.7 ± 1.0 †† |
| Minimal waist circumference (cm) | |||||||||
| All | 95.3 ± 9.7 | 95.6 ± 9.5 | 0.6 ± 3.9 | 95.8 ± 10.5 | −1.1 ± 2.8 † | 94.5 ± 9.1 | −1.1 ± 2.6 †† | 95.3 ± 9.8 | −2.6 ± 3.3 ††† |
| Men | 101.1 ± 7.1 § | 100.6 ± 7.5 | 1.7 ± 2.6 †† | 102.3 ± 8.7 | −1.5 ± 2.9 † | 100.6 ± 6.3 | −1.8 ± 2.7 †† | 101.0 ± 6.2 | −2.7 ± 3.0 ††† |
| Women | 88.7 ± 7.8 | 90.4 ± 8.5 | −0.6 ± 4.6 | 89.7 ± 8.2 | −0.7 ± 2.7 | 87.5 ± 6.4 | −0.4 ± 2.4 | 87.0 ± 8.1 | −2.4 ± 3.8 † |
| HDL cholesterol (mg/dl) | |||||||||
| All | 46.7 ± 14.0 | 43.8 ± 12.3 | 0.5 ± 4.8 | 48.6 ± 13.4 | −0.2 ± 5.2 | 47.5 ± 14.9 | 1.1 ± 5.6 | 46.8 ± 15.0 | 3.7 ± 6.8 ††† |
| Men | 39.7 ± 8.4 § | 36.7 ± 6.6 | −0.4 ± 4.1 | 42.6 ± 7.4 | −1.6 ± 4.8 | 39.7 ± 8.9 | 1.4 ± 4.8 | 39.9 ± 9.4 | 3.9 ± 4.8 ††† |
| Women | 54.6 ± 14.8 | 51.3 ± 12.6 | 1.5 ± 5.3 | 54.3 ± 15.4 | 1.2 ± 5.2 | 56.3 ± 15.6 | 0.7 ± 6.5 | 56.8 ± 16.2 | 3.4 ± 9.1 |
| Triglycerides (mg/dl) | |||||||||
| All | 151.6 ± 81.6 | 155.8 ± 75.7 | −3.7 ± 42.2 | 161.6 ± 96.4 | −36.2 ± 75.8 †† | 139.3 ± 69.6 | −6.1 ± 51.7 | 151.0 ± 84.6 | −19.2 ± 35.4 ††† |
| Men | 168.9 ± 92.7 § | 172.7 ± 84.0 | −2.5 ± 44.0 | 180.6 ± 117.9 | −49.4 ± 96.4 † | 153.2 ± 70.2 | −8.7 ± 60.4 | 171.5 ± 99.1 | −27.4 ± 41.6 †† |
| Women | 131.9 ± 61.7 | 138.1 ± 63.0 | −5.0 ± 41.2 | 143.5 ± 68.2 | −23.7 ± 48.1 † | 123.5 ± 67.1 | −3.1 ± 40.9 | 121.3 ± 45.5 | −7.3 ± 19.1 |
| Systolic blood pressure (mmHg) | |||||||||
| All | 130.3 ± 14.4 | 129.4 ± 16.2 | −4.5 ± 12.2 † | 131.8 ± 11.9 | −0.4 ± 12.4 | 130.0 ± 15.5 | −1.1 ± 13.9 | 130.2 ± 14.1 | −4.2 ± 13.8 † |
| Men | 130.6 ± 12.2 | 127.3 ± 14.7 | −4.6 ± 9.8 † | 132.0 ± 11.3 | −0.9 ± 12.9 | 129.5 ± 10.4 | 1.1 ± 11.4 | 133.1 ± 12.4 | −4.2 ± 11.5 |
| Women | 130.1 ± 16.6 | 131.6 ± 17.7 | −4.4 ± 14.5 | 131.6 ± 12.8 | 0.0 ± 12.2 | 130.5 ± 20.1 | −3.7 ± 16.2 | 126.1 ± 15.8 | −4.3 ± 16.9 |
| Diastolic blood pressure (mmHg) | |||||||||
| All | 84.1 ± 7.6 | 81.9 ± 6.7 | 0.2 ± 7.7 | 84.2 ± 7.8 | −1.9 ± 8.5 | 84.5 ± 7.8 | −0.6 ± 8.2 | 85.7 ± 7.5 | −4.0 ± 8.5 †† |
| Men | 85.8 ± 7.2 § | 81.0 ± 6.4 | 1.6 ± 7.5 | 86.1 ± 8.7 | −1.8 ± 8.9 | 86.5 ± 6.5 | 0.1 ± 7.3 | 88.7 ± 5.5 | −6.2 ± 9.3 § †† |
| Women | 82.2 ± 7.5 | 82.7 ± 7.1 | −1.2 ± 7.9 | 82.5 ± 6.7 | −1.9 ± 8.4 | 82.2 ± 8.8 | −1.3 ± 9.2 | 81.3 ± 8.0 | −0.8 ± 6.1 |
| Mean blood pressure (mmHg) | |||||||||
| All | 99.5 ± 8.6 | 97.7 ± 8.9 | −1.4 ± 8.4 | 100.1 ± 8.5 | −1.4 ± 8.5 | 99.7 ± 9.2 | −0.7 ± 8.8 | 100.5 ± 8.9 | −4.1 ± 8.8 †† |
| Men | 100.7 ± 7.9 | 96.5 ± 8.0 | −0.5 ± 7.5 | 101.4 ± 8.9 | −1.5 ± 9.1 | 100.8 ± 6.6 | 0.4 ± 7.5 | 103.5 ± 6.8 | −5.5 ± 8.8 †† |
| Women | 98.1 ± 9.7 | 99.0 ± 9.7 | −2.3 ± 9.4 | 98.8 ± 8.1 | −1.3 ± 8.0 | 98.3 ± 11.5 | −2.1 ± 10.2 | 96.2 ± 9.8 | −2.0 ± 8.7 |
| Fasting glucose (mg/dl) | |||||||||
| All | 93.5 ± 9.6 | 92.3 ± 8.8 | 2.1 ± 7.8 | 94.6 ± 9.8 | −1.3 ± 8.0 | 94.3 ± 9.6 | 0.9 ± 7.7 | 92.9 ± 10.2 | −0.7 ± 8.6 |
| Men | 94.9 ± 8.9 § | 92.6 ± 7.5 | 3.0 ± 8.3 | 96.8 ± 9.5 | −1.7 ± 9.3 | 95.2 ± 8.7 | 1.0 ± 6.8 | 95.0 ± 9.6 | −0.5 ± 8.6 |
| Women | 92.0 ± 10.1 | 92.1 ± 10.1 | 1.1 ± 7.2 | 92.4 ± 9.8 | −1.0 ± 6.8 | 93.2 ± 10.6 | 0.8 ± 8.8 | 89.9 ± 10.5 | −0.9 ± 8.8 |
Data expressed as mean ± SD
p ≤ 0.05
p < 0.01
p < 0.001 indicates significant change score within a group
p ≤ 0.05 indicates significant gender difference
In examining the 171 participants at baseline, it was clear that 69 (40%) of the subjects met 3 or more ATPIII criteria. Furthermore, a greater percentage of the men (46%) presented with MS than did women (34%) despite having met the same study inclusion and exclusion criteria. Table 3 details the baseline MS scores for all subjects by group and gender. The Z-score ranged from −7.51 to 6.25 for all subjects, whereby a negative number indicates lower risk. Again, the women show a more desirable pattern as both the Z-score (p<0.01) and the ATPIII score (p<0.05) are significantly better than that of men at baseline.
TABLE 3.
Metabolic Syndrome Characteristics by Group
| All | Control | Low Amount/Moderate Intensity | Low Amount/Vigorous Intensity | High Amount/Vigorous Intensity | |||||
|---|---|---|---|---|---|---|---|---|---|
| n=171 (91 men; 80 women) | n=41 (21 men; 20 women) | n=41 (20 men; 21 women) | n=45 (24 men; 21 women) | n=44 (26 men; 18 women) | |||||
| Baseline | Baseline | Change | Baseline | Change | Baseline | Change | Baseline | Change | |
| Z-scorea | |||||||||
| All | −0.8 ± 2.5 | −0.7 ± 2.1 | 0.0 ± 1.5 | −0.5 ± 2.4 | −0.8 ± 1.6 †† | −1.0 ± 2.5 | −0.3 ± 1.4 | −0.9 ± 3.0 | −1.4 ± 1.7 ††† |
| Men | −0.2 ± 2.2 § | −0.6 ± 2.1 | 0.5 ± 1.3 | 0.0 ± 2.3 | −0.9 ± 1.6 † | −0.4 ± 1.8 | −0.4 ± 1.4 | 0.1 ± 2.5 | −1.8 ± 1.7 ††† |
| Women | −1.4 ± 2.8 | −0.8 ± 2.2 | −0.4 ± 1.5 | −1.0 ± 2.5 | −0.7 ± 1.6 | −1.6 ± 3.0 | −0.3 ± 1.5 | −2.3 ± 3.3 | −0.9 ± 1.6 † |
| ATPIII score | |||||||||
| All | 2.2 ± 1.2 | 2.1 ± 1.1 | 0.1 ± 0.9 | 2.4 ± 1.0 | −0.5 ± 1.1 †† | 2.2 ± 1.3 | −0.2 ± 1.1 | 2.2 ± 1.4 | −0.5 ± 1.1 †† |
| Men | 2.4 ± 1.1 § | 2.2 ± 1.2 | 0.4 ± 0.9 § † | 2.4 ± 1.0 | −0.7 ± 0.9 †† | 2.5 ± 1.1 | −0.2 ± 1.3 | 2.6 ± 1.2 | −0.7 ± 1.2 †† |
| Women | 2.0 ± 1.2 | 2.1 ± 1.0 | −0.2 ± 0.9 | 2.3 ± 1.1 | −0.4 ± 1.3 | 1.9 ± 1.4 | −0.2 ± 0.8 | 1.7 ± 1.5 | −0.2 ± 1.0 |
| Insulin sensitivity index | |||||||||
| All | 3.5 ± 2.3 | 3.3 ± 1.8 | −0.1 ± 1.7 | 3.0 ± 2.3 | 1.6 ± 2.1 ††† | 3.9 ± 2.2 | 0.5 ± 1.7 | 3.7 ± 2.7 | 1.5 ± 2.2 ††† |
| Men | 3.1 ± 2.2 § | 3.2 ± 1.7 | −0.2 ± 1.9 | 2.7 ± 2.4 | 1.6 ± 2.4 † | 3.7 ± 2.7 | 0.6 ± 1.9 | 2.8 ± 1.8 | 1.4 ± 1.5 ††† |
| Women | 3.9 ± 2.3 | 3.4 ± 1.9 | −0.1 ± 1.6 | 3.3 ± 2.2 | 1.6 ± 1.7 †† | 4.1 ± 1.5 | 0.3 ± 1.5 | 5.1 ± 3.4 | 1.7 ± 3.0 † |
Data expressed as mean ± SD
p ≤ 0.05 indicates gender difference
p ≤ 0.05
p < 0.01
p < 0.001 indicates significant change score within a group
Negative score indicates lower risk
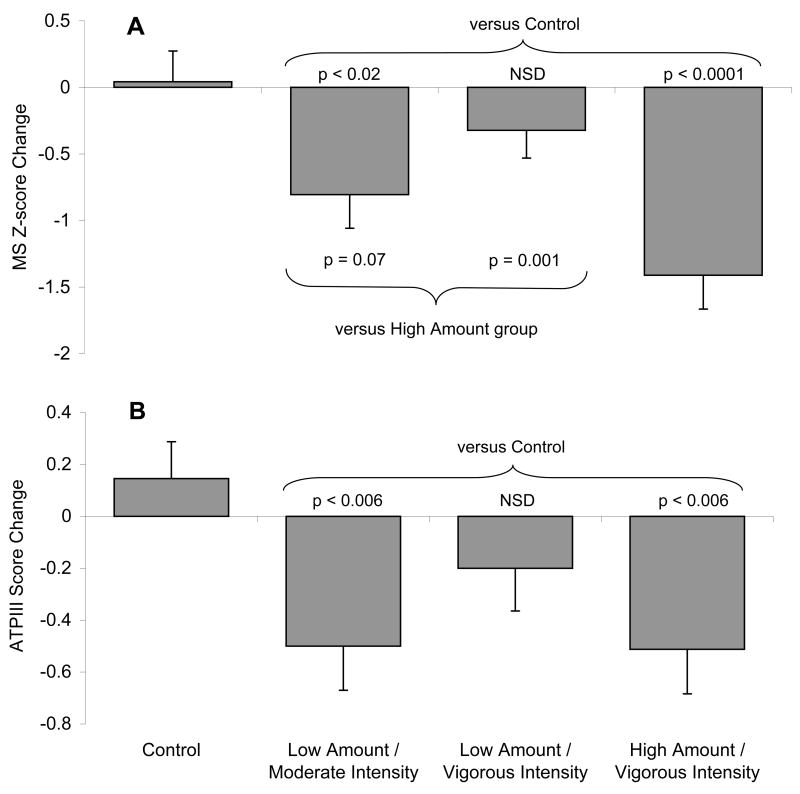
Following the exercise intervention, both the low amount/moderate intensity and high amount/vigorous intensity groups had significantly reduced their ATPIII and Z-scores compared with controls (Figure 2). Further, the Z-score for the high amount/vigorous group was significantly better than the vigorous group exercising at a lower amount. Table 2 clearly demonstrates that the high amount/vigorous intensity group had the greatest number of metabolic variables improve. Waist circumference significantly improved for all 3 exercise groups. Also note that triglycerides were significantly lower in the low amount/moderate intensity group, but not the low amount/vigorous intensity group.
Figure 2.
The effects of exercise amount and intensity on mean changes in MS Z-score (panel A) and ATPIII Score (panel B) are shown. Error bars show SE. Two-way ANOVA for a main group effect was significant (p<0.0005). Post hoc tests for group differences are shown. ANOVA results for gender effect (p=0.70) and gender by group interaction effect (p=0.085) were not significant.
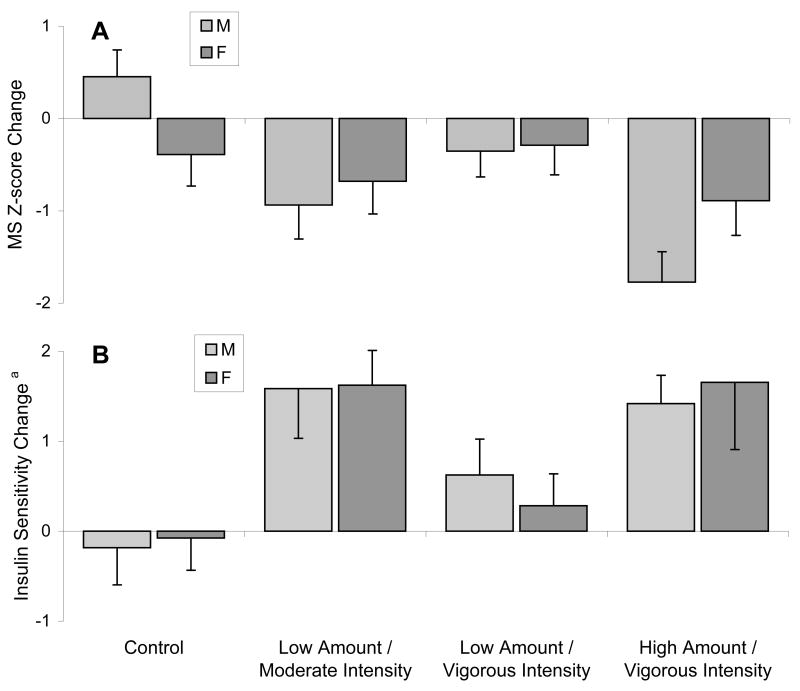
As the present study was also designed to examine gender responses, we have presented data for both men and women separately (Table 3). The Z-score dropped significantly for men in the moderate intensity and high amount/vigorous intensity groups. Women in the high amount/vigorous group also had a significant reduction in Z-score while the women in the moderate intensity group approached significance (p=0.07). Men in the moderate intensity group and the high amount/vigorous intensity group also had a significant reduction in their ATPIII score, while the ATPIII score for men in the control group got significantly worse. As the women in this study had lower ATPIII scores at baseline there is less room for their score to improve. These gender patterns are evident in Figure 3 where the men and women display similar responses in all 3 exercise groups to change in Z-score.
Figure 3.
The effects of exercise amount and intensity on mean changes in MS Z-score (panel A) and Insulin Sensitivity (panel B) are shown for men and women separately. Error bars show SE. Two-way ANOVA results for a main group effect were significant (p<0.0005) and the post hoc tests for specific group differences are the same as shown in Figure 2. The test for gender by group interaction was not significant (p=0.085). The test for a main gender effect (p=0.70) was also not significant. aUnits for insulin sensitivity index were mU/L/min.
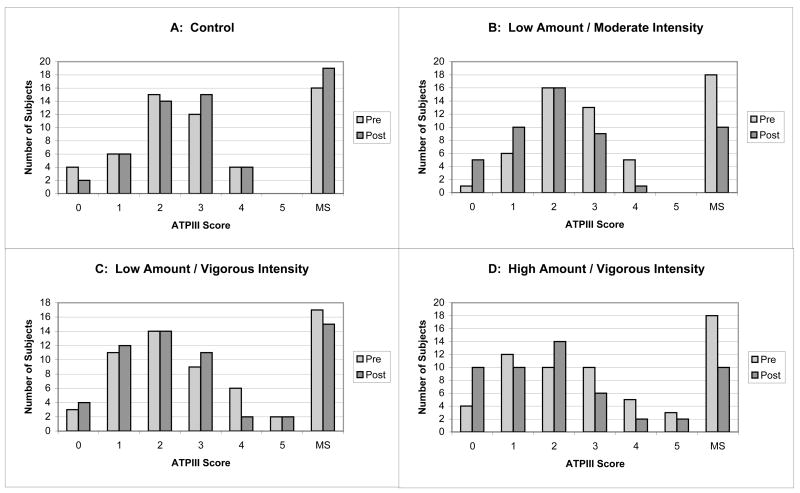
Figure 4 displays the ATPIII score distribution by group at baseline and end-of-study. Clearly, the number of subjects with ATPIII-defined MS dropped in all 3 exercise groups, while the number of subjects with MS in the inactive control group increased.
Figure 4.
The number of subjects with 0, 1, 2, 3, 4, or 5 of the ATPIII criteria and with ATPIII defined metabolic syndrome for Controls (panel A), Low Amount/Moderate Intensity (panel B), Low Amount/Vigorous Intensity (panel C), and High Amount/Vigorous Intensity (panel D) are shown. The percentage change in ATPIII prevalence was +19% in Controls, −44% in the Low Amount/Moderate Intensity, −12% in the Low Amount/Vigorous Intensity, and −44% in the High Amount/Vigorous Intensity groups.
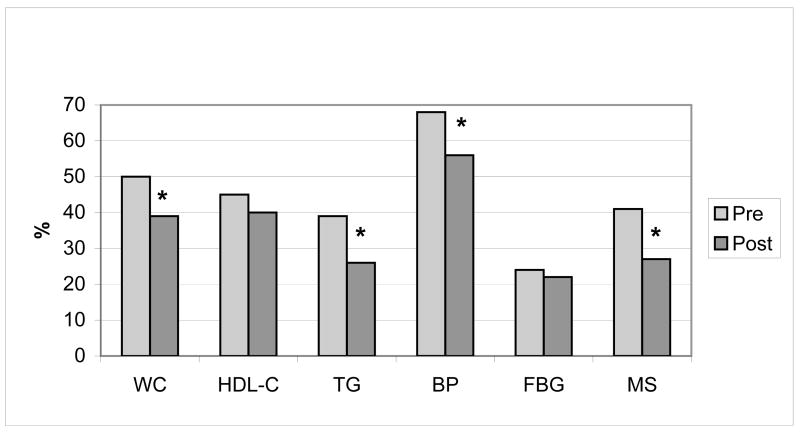
Figure 5 represents the percentage of exercisers that meet ATPIII criteria for each variable and have MS pre- and post-intervention. There was a significant reduction in the prevalence of MS, as well as the number of subjects who met the criteria for waist circumference, triglycerides and blood pressure.
Figure 5.
Prevalence of risk factors in all exercisers (n=130) before and after exercise training. * p≤0.05 indicates significant change from pre to post. WC = waist circumference; HDL-C = HDL-cholesterol; TG = triglycerides; FBG = fasting blood glucose; MS = metabolic syndrome based on ATPIII-defined criteria.
DISCUSSION
One major finding in this study was that, compared to the inactive controls, moderate intensity exercise – at an amount calorically equivalent to walking approximately 17 km (10 to 11 miles) over an average of 170 minutes per week – resulted in a significant improvement in MS (Figure 2). This is an important finding, as the data directly addresses the question of how much and what intensity of exercise is needed to obtain metabolic health benefits. Our findings clearly indicate that a modest amount of moderate intensity exercise is adequate for obtaining significant health benefits. This is an exercise prescription that is likely to be perceived by the general public and clinicians alike as an obtainable goal. Further, these data lend support to the 1995 CDC/ACSM position stand, which states “all adults should accumulate 30 minutes of moderate intensity activity most, preferably all, days of the week”8. At the same time, this study also shows that for the group that did a higher amount of more vigorous exercise – an energy expenditure calorically equivalent to approximately 28 km (17 miles) of jogging over 170 minutes per week – greater and more widespread benefits were realized. The beneficial effect obtained by the high amount exercise group was not only significantly better than observed in the inactive control group (p<0.0001), but was also better than that experienced by the low amount/vigorous intensity group (p<0.01).
In addressing the current controversy of whether 30 or 60 minutes of exercise per day should be the national recommendation, experts commonly agree that: 1) physical inactivity is detrimental to long term health, 2) some exercise is better than none, and 3) more exercise, up to a point, leads to greater and more widespread health benefits. The data from the current report together with other published data from STRRIDE, add strong support to these previously already well founded concepts8–11.
A somewhat surprising finding was that although the exercise at the lower intensity was found to be effective compared to the inactive control group, the same amount of exercise at a more vigorous intensity was not significantly different from the inactive group (Figure 2). It is important to point out that this same amount of exercise at a lower intensity requires more total minutes per week, which generally leads to a greater weekly exercise frequency (Table 1). As a result, we cannot rule out that greater weekly frequency and/or duration are important factors in achieving the health effects studied here. We have observed similar responses for other health outcomes in the STRRIDE study. For example, we observed that the moderate intensity group was significantly better at improving insulin sensitivity than the same amount of exercise at a higher intensity12. This same pattern is also evident in the triglyceride response (Table 2). Also, in the lower intensity group triglycerides (Table 2), the MS Z-score and insulin sensitivity index (Table 3) are improved for both men and women (the improvement in Z-score just failed significance for women, p<0.07).
The exact mechanism whereby lower intensity exercise might confer greater health benefits than does higher intensity exercise is not clear. The same amount of exercise at a lower exercise intensity increases the percentage of energy coming from fat oxidation. Conversely, the higher intensity exercise group gets more of its energy from carbohydrate oxidation13. While caution is certainly warranted and additional confirmatory studies are needed, taken together these data suggest that for metabolic health there may be some real advantages of moderate intensity exercise and the consumption of fat oxidation as a fuel.
While it is known that men and women differ in their clinical presentation of cardiovascular disease and risk factors, an additional purpose of this analysis was to examine the relationship between MS and exercise volume and intensity in men and women separately. Although men and women were recruited with the same criteria and the women were significantly older, it is clear that women generally displayed more desirable cardiovascular risk profiles at baseline. Of note, national survey studies report that MS prevalence is almost identical for men and women, at about 23%14. The prevalence of MS in women from the present study is 34%, whereas 46% of men in this study met MS criteria at baseline.
Given these consistent, strong gender differences at baseline, attempts to study the differences statistically between men and women in their responses to exercise are likely to be complicated. When baseline MS Z-score (or ATPIII score) is added to the model, this variable is highly significant, indicating that baseline values explain an important part of the exercise response. These findings suggest that if there is a gender difference in the effect of exercise on MS, it is likely found in the fact that in this study the women had better risk profiles at baseline.
In addition to the randomized, controlled design, other major strengths of this study include: 1) verification of time and intensity of exercise - not just attendance - for nearly all training sessions; 2) carefully defined and controlled exercise amounts and intensities, 3) a significant proportion of women and minorities in the study sample. Two limitations should be noted. While the findings should be applicable to the general population, they may not be as appropriate for special populations, such as those with hypertension, diabetes, or cardiovascular disease. Due to practical reasons, we could not compare the effects of a high amount/moderate intensity intervention. It was felt that the amount of time necessary for this group (up to 8 hours/week for low fitness subjects) would seriously hinder recruitment efforts.
In sum, in a middle-aged, overweight to obese, at-risk physically inactive population of men and women, moderate intensity exercise consistent with the current ACSM/CDC exercise recommendations for health effects had a significant impact on metabolic syndrome in both men and women.
Acknowledgments
Supported by National Institutes of Health Grant HL-57354.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Panel E. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Kraus W, Torgan C, Duscha B, Norris J, Brown S, Cobb F, Bales C, Annex B, Samsa G, Houmard J, Slentz C. Studies of a targeted risk reduction intervention through defined exercise. Med Sci Sports Exerc. 2001;33:1774–1784. doi: 10.1097/00005768-200110000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Otvos J, Jeyarajah E, Bennett D, Krauss R. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem. 1992;38:1632–1638. [PubMed] [Google Scholar]
- 4.Bergman R, Finegood D, Ader M. Assessment of insulin sensitivity in vivo. Endocrine Reviews. 1985;6:45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- 5.Grundy S, Cleeman J, Daniels S, Donato K, Eckel R, Franklin B, Gordon D, Krauss R, Savage P, Smith S, Spertus J, Costa F. Diagnosis and management of the metabolic syndrome: an AHA/NHLBI scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 6.Wijndaele K, Beunen G, Duvigneaud N, Matton L, Duquet W, Thomis M, Lefevre J, Philippaerts R. A continuous metabolic syndrome risk score: utility for epidemiological analyses. Diabetes Care. 2006;29:2329. doi: 10.2337/dc06-1341. [DOI] [PubMed] [Google Scholar]
- 7.Franks P, Ekelund U, Brage S, Wong M, Wareham N. Does the association of habitual physical activity with the metabolic syndrome differ by level of cardiorespiratory fitness? Diabetes Care. 2004;27:1187–1193. doi: 10.2337/diacare.27.5.1187. [DOI] [PubMed] [Google Scholar]
- 8.Pate R, Pratt M, Blair S, Haskell W, Macera C, Bouchard C, Buchner D, Ettinger W, Heath G, King A. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 9.Blair S, Kohl H, Paffenbarger R, Clark D, Cooper K, Gibbons L. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 10.Blair S, Kohl H, Barlow C, Paffenbarger R, Gibbons L, Macera C. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 11.Paffenbarger R, Hyde R, Wing A, Hsieh C. Physical activity, all-cause mortality, and longevity of college alumni. New Engl J Med. 1986;314:605–613. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 12.Houmard J, Tanner C, Slentz C, Duscha B, McCartney J, Kraus W. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96:101–106. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 13.Brooks G, Fahey T, White T. Exercise Physiology: Human Bioenergetics and Its Applications. Mountain View: Mayfield Publishing Company; 1996. p. 749. [Google Scholar]
- 14.Ford E, Giles W, Dietz W. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]